Abstract
Novel monocyclic analogues of 2-arachidonoylglycerol (2-AG) were designed in order to explore the pharmacophoric conformations of this endocannabinoid ligand at the key cannabinergic proteins. All 2-arachidonoyl esters of 1,2,3-cyclohexanetriol [meso-7 (AM5504), (±)-8 (AM5503), and meso-9 (AM5505)] were synthesized by regioselective acylation of 2,3-dihydroxycyclohexanone followed by selective reductions. The optically active isomers (+)-8 (AM4434) and (−)-8 (AM4435) were synthesized from (2S,3S)- and (2R,3R)-2,3-dihydroxycyclohexanone, respectively, via a chemoenzymatic route. These head group constrained and conformationally restricted analogues of 2-AG as well as the 1-keto precursors were evaluated as substrates for the endocannabinoid deactivating hydrolytic enzymes monoacylglycerol lipase (MGL) and fatty acid amide hydrolase (FAAH), and also were tested for their affinities for CB1 and CB2 cannabinoid receptors. The observed biochemical differences between these ligands can help define the conformational requirements for 2-AG activity at each of the above endocannabinoid protein targets.
Keywords: 2-arachidonoylglycerol, 2-AG, monoacylglycerol lipase, MGL, fatty acid amide hydrolase, FAAH, cannabinoid, endocannabinoid
2-Arachidonoylglycerol (2-AG, 1) (Figure 1) is a monoacylglycerol identified as an endogenous ligand which binds to both CB1 and CB2 cannabinoid receptors.1,2 The other key endocannabinoid is N-arachidonoylethanolamine (AEA, 2), although it has been postulated that 2-AG is the primary endocannabinoid agonist ligand for CB13,4 as well as CB25 receptors. For example, 2-AG binds to the CB1 cannabinoid receptors on presynaptic axons during the duration of its existence in the extracellular space and functions as a retrograde synaptic neurotransmitter,6,7 where it elicits a variety of cannabinergic effects in vitro and in vivo.8 2-AG is primarily inactivated by an efficient transporter system-mediated cellular uptake followed by intracellular enzymatic hydrolysis to arachidonic acid and glycerol by monoacylglycerol lipase (MGL).9–12 In addition to the hydrolysis of this metabolically labile molecule by MGL, hydrolysis by fatty acid amide hydrolase (FAAH),10,12 phosphorylation,13 as well as metabolism by lipoxogenases (LOX)14 and cyclooxygenase 2 (COX 2)15 also occur, although the biological relevance of these other mechanisms for 2-AG deactivation have not yet been fully established. Thus, 2-AG interacts not only with the CB receptors, but with a transporter system, intracellular MGL and FAAH, as well as other enzymes. We postulated that the conformations of 2-AG required for interactions with each of these targets may be different and that probing its bioactive conformation in each target could lead to information useful in the design of selective inhibitors which have the potential to be therapeutic drugs.16–18
Figure 1.
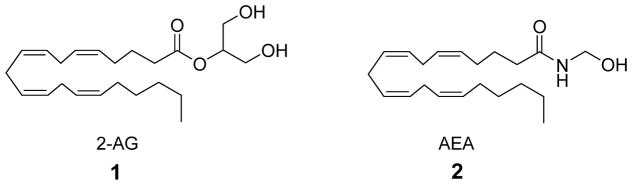
Endocannabinoids 2-arachidonoylglycerol (2-AG, 1) and N-arachidonoylethanolamine (AEA, 2).
Our approach for the design of conformationally defined 2-AG analogues involved constraining the conformation of the glycerol moiety by incorporation of its key pharmacophoric features into a six-membered carbocyclic ring system. This series of analogs includes all the 2-arachidonoyl esters of 1,2,3-cyclohexanetriol as well as the corresponding keto analogs. These ligands were synthesized and assayed for their affinities for the two known cannabinoid receptors and evaluated as substrates for MGL and FAAH, as we have been particularly interested in identifying the structural features of the 2-AG molecule which can discriminate between these two endocannabinoid deactivating enzymes.
As shown in Scheme 1, 2,3-dihydroxycyclohexanone (4) was an intermediate in the synthesis of all possible isomeric 2-AG analogues. Commercially available cyclohexenone (3) was treated with osmium tetroxide and N-methylmorpholine N-oxide (NMO) to give the racemic ketodiol (±)-4, which was acylated at the more acidic α-hydroxyl group using arachidonic acid in the presence of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide (EDCI) and 4-dimethylaminopyridine (DMAP) at −20 °C to give (±)-5 (AM5501) and a minor amount of the epimerized byproduct (±)-6 (AM5502). Both (±)-5 and (±)-6 were reduced with sodium borohydride in methanol at 0 °C to give the corresponding 2-AG analogues meso-7 (AM5504), (±)-8 (AM5503), and meso-9 (AM5505). These 2-arachidonoyl esters of 1,2,3-cyclohexanetriol were free of acyl migration byproducts usually observed for 2-acyl glycerols such as 2-AG4,19–21 and have been characterized.22
Scheme 1.
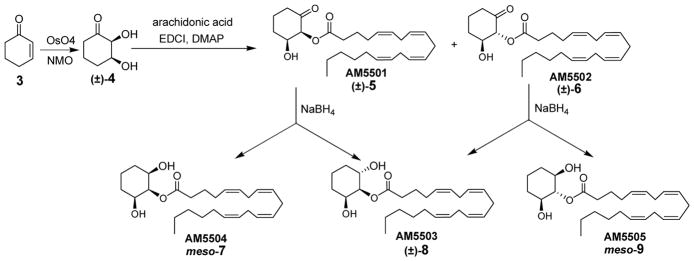
Syntheses of 1,2,3-cyclohexanetriol ester analogues of 2-AG.
Scheme 2.
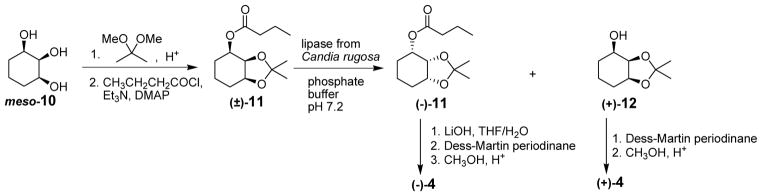
Chemoenzymatic syntheses of optically active starting 2,3-dihydroxycyclohexanones (−)-4 and (+)-4.
Racemic compound (±)-8 (AM5503) was not resolvable by chiral HPLC (CHIRALPAK AD). Therefore, enzymatic resolution of the chiral ketodiol (±)-4 was carried out (Scheme 2) to give the required crucial intermediates (−)-4 and (+)-4 in optically active forms. The synthetic intermediates (−)-11 and (+)-12 were previously reported from an enzymatic method which utilized lipase from Pseudomonas sp. (SAM-II, Amano).23 We utilized a different lipase from Candida rugosa (lipase L1754, Sigma) to selectively hydrolyze one enantiomer of ester (±)-11 and give the corresponding alcohol (+)-12 which was readily chromatographically separable from the unreactive enantiomeric ester (−)-11. Both ester (−)-11 ([α]D −48.1° (c 1.00, CH3OH). Lit.23 [α]D −41.2° (c 0.7, CHCl3)) and alcohol (+)-12 ([α]D = +10.8° (c = 1.00, CH3OH), Lit.23 [α]D = +12.3° (c = 0.6, CHCl3)) were obtained in good yields and high optical purities. Ketodiol (−)-(2R,3R)-4 was then prepared from (−)-11 and converted to (−)-8 (AM4435, [α]D = −16.4° (c = 1.2, CHCl3)), while ketodiol (+)-(2S,3S)-4 was prepared from (+)-12 and converted to (+)-8 (AM4434, [α]D = + 17.0° (c = 0.9, CHCl3)).
Compounds were tested for their affinities for the CB1 and CB2 receptors using membrane preparations from rat brain or mouse spleen, respectively, as previously described24–27 via competition-equilibrium binding with [3H]CP55940 as the radioligand. The results were analyzed using nonlinear regression to determine the actual IC50 of the ligand (Prizm by GraphPad Software, Inc.) and the Ki values were calculated from the IC50.28 All data were in duplicate with IC50 and Ki values determined from single experiments. This series of rigid 2-AG analogues had affinities for the CB receptors that were comparable to the endogenous cannabinoid 2-AG (Ki CB1 472,1 2400,2 100,20 538;29 Ki CB2 1400,1 100,20 1100;30 review8) even in the presence of the bulky -(CH2)3- methylenes which were used to constrain the glycerol headgroup portion of the ligands. For example, the Ki’s for (+)-8 (AM4434, CB1 970 nM, CB2 370 nM) and (−)-8 (AM4435, CB1 4200 nM, CB2 1200 nM)) were determined, though some ester hydrolysis did occur during the course of the binding assays, as evidenced by the examples of the CB1 Ki’s for (+)-8 (AM4434) and (−)-8 (AM4435), which were 360 nM and 770 nM, respectively, when the CB1-containing rat brain membrane preparations were pretreated27 with phenylmethylsulfonyl fluoride (PMSF). All other compounds exhibited CB1 and CB2 Ki values larger than 1000 nM without substantial differences except for (±)-5 (AM5501, CB2 Ki 410 nM).
FAAH and MGL enzymes were partially purified from adult Sprague-Dawley rat brains purchased from Pel-Freeze Biologicals according to a previously reported procedure.31,32 The pellet from the last centrifugation step (microsomal fraction) was resuspended in 25 mM Tris-HCl, 5 mM MgCl2, 1 mM EDTA, pH 7.4 (TME) buffer for the FAAH assay (plus 0.1% BSA), and the supernatant from the last centrifugation step (cytosol fraction without BSA) was used for the MGL assay. All compound stock solutions used were 10 mM in DMSO, and the chemical stabilities of the compounds were first checked under the assay conditions (100 μM substrate) without any added FAAH or MGL enzymes. To screen compounds as substrates for FAAH, assays were carried out according to our previously reported procedures.32,33 Samples (100 μL) were taken at the start of the assay to obtain the background concentration of arachidonic acid in the biological sample, and then after 15 minutes of FAAH hydrolysis. Samples were diluted 1:5 with acetonitrile and centrifuged (20,000g, 5 minutes, room temperature) to precipitate the proteins. The resulting supernatant was analyzed by HPLC to determine the percentage of compound hydrolyzed in the reaction time. The assays with cytosolic MGL were carried out in similar fashion to the FAAH assay described above, except that TME buffer was used without BSA, the MGL enzyme preparation used 30 μg of protein, and the reaction time was 20 minutes.
These raw data in Table 1 from the enzyme susceptibility screenings have not been adjusted to reflect the stability of the substrates in the absence of enzyme, where (+)-5 (AM5501) showed the most chemical instability (12% and 11% hydrolyses) and meso-7 (AM5504) showed the least instability (2% and 1% hydrolyses) under control conditions for FAAH and MGL screenings, respectively. However, the data clearly indicated that the stereochemical features of the triol ester headgroup of some analogues could be used to distinguish the active sites for ester hydrolysis at the MGL and FAAH enzyme active sites. The preferred conformations of the individual analogs are represented in Figure 2. In general, FAAH was very effective in hydrolyzing all equatorial arachidonoyl esters. However, with the energy difference of 2.0 kcal/mol between the axial and equatorial conformations (see Figure 3), meso-7 (cis,cis-7, AM5504) exists primarily (97% at 25 °C) in a conformation where the arachidonoyl ester is axial, and it was not a good substrate for FAAH. The arachidonoyl ester of meso-9 (trans,trans-9, AM5505) was only somewhat less susceptible to FAAH hydrolysis than (±)-8 (AM5503), which represents a conformation of 2-AG that is readily hydrolyzable by FAAH. Interestingly, no difference was seen between the two enantiomers (+)-8 (AM4434) and (−)-8 (AM4435) for this observed excellent FAAH specificity, but it should be noted that both endocannabinoid substrates (AEA and 2-AG) are achiral. FAAH also readily hydrolyzes ketone (±)-6, which like 8, has one hydroxyl group adjacent and in the plane of the arachidonoyl ester.
Table 1.
Substrate hydrolysis by FAAH and MGL enzymes with standard deviations.
| Compound | Name | Substrate Assay (% Hydrolysis) | |
|---|---|---|---|
| FAAH | MGL | ||
| 1 | 2-AG | 100±0 | 74±4 |
| (±)-5 | AM5501 | 91±1 | 48±3 |
| (±)-6 | AM5502 | 100±0 | 8±3 |
| meso-7 | AM5504 | 2±3 | 2±2 |
| (±)-8 | AM5503 | 100±0 | 1±1 |
| (+)-8 | AM4434 | 88±2 | 10±3 |
| (-)-8 | AM4435 | 88±3 | 13±2 |
| meso-9 | AM5505 | 78±6 | 19±4 |
Figure 2.
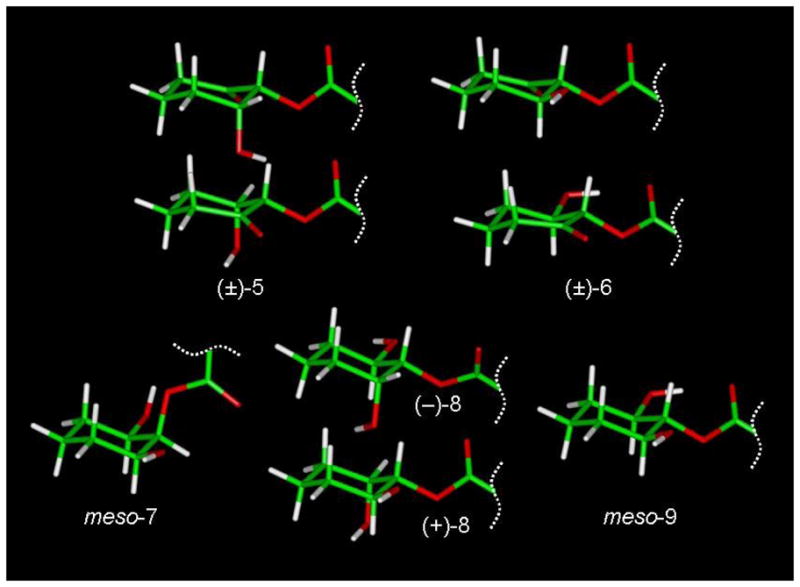
Molecular models for (±)-5 (AM5501), (±)-6 (AM5502), meso-7 (AM5504), the enantiomers (−)-8 (AM4435) and (+)-8 (AM4434), and meso-9 (AM5505) were obtained on a Silicon Graphics Fuel workstation using Insight II (2000). The O-arachidonoyl groups were modeled in extended conformations34,35 but are not displayed. All structures were subject to molecular mechanics calculations using the steepest descent method for the first 1000 iterations, then the conjugate gradient method until the maximum derivative was less than 0.001 kcal/mol. Only for meso-7 (AM5504) was 2-O-arachidonoyl group found to be axial in the lowest energy conformation (see Figure 3).
Figure 3.
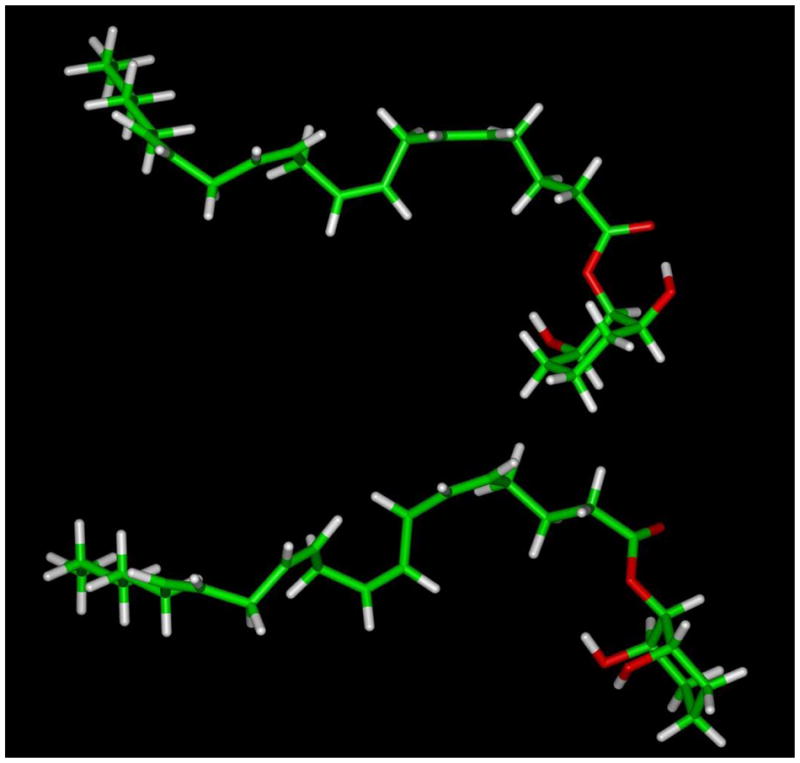
Molecular models of both possible chair conformations of meso-7 (AM5504) were obtained on a Silicon Graphics Fuel workstation using Insight II (2000). The O-arachidonoyl groups were modeled in extended conformations as reported by Reggio, et al.34 for 2-AG and were constrained between C1 and C15. A restraint file for the cyclohexyl ring was also incorporated during the dynamics run in order to prevent possible isomerization and/or racemization at high temperature. The energy-minimized structures underwent constrained molecular dynamics performed by heating it to 1200 °K and recording 100 atomic coordinate trajectories every 10,000 iterations (1 fs per iteration). Next, each trajectory was subjected to simulated annealing followed by energy minimization using the steepest descent method for 100 iterations followed by conjugate gradient method until the maximum derivative was less than 0.001 kcal/mol. Two families of low-energy conformers were identified, and the conformer with the axial 2-O-arachidonoyl group (top) was found to be 2.0 kcal/mol lower in energy than the corresponding equatorial 2-O-arachidonoyl conformer (bottom).
The best access to the ester linkage by MGL were in the cases of ketone (±)-5, and to a much lesser extent, meso-9 (trans,trans-9, AM5505). Thus, the combination of a carbonyl which is adjacent and in the plane of the arachidonoyl ester with a hydroxyl group axial as in (±)-5 resulted in the highest MGL activity for 2-O-arachidonoylglycerol analog hydrolysis.
This screening of conformationally restricted 2-AG analogues has identified key structural features which distinguish the endocannabinoid hydrolytic enzymes MGL and FAAH, and the Km values for these compounds are being determined. We are also interested in evaluating this series of 2-AG analogs as competitive inhibitors of MGL and FAAH, and for their effects on signal transduction via the cannabinoid receptors in both the forskolin-stimulated cyclic AMP accumulation assay and the [35S]GTPγS binding assay. Future work on constrained analogs of 2-AG will involve, not only modifying the triol ester headgroups through the use of different-sized rings, but also modifications of the 2-arachidonoyl group. These conformationally well-defined 2-AG analogs can now be used to develop lead compounds which are selective inhibitors of MGL or FAAH.
Acknowledgments
The authors are grateful to Ying Pei, Yan Peng, Alexander A. (Sasha) Zvonok, and Pusheng Fan for the biochemical assays of these compounds and to Lakshmipathi Pandarinathan for helpful discussions. This work was supported by grants from the National Institute on Drug Abuse DA03801, DA09158 and DA07215.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Mechoulam R, Ben-Shabat S, Hanuš L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, Pertwee RG, Griffin G, Bayewitch M, Barg J, Vogel Z. Biochem Pharmacol. 1995;50:83. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- 2.Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. Biochem Biophys Res Commun. 1995;215:89. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- 3.Sugiura T, Kodaka T, Nakane S, Miyashita T, Kondo S, Suhara Y, Takayama H, Waku K, Seki C, Baba N, Ishima Y. J Biol Chem. 1999;274:2794. doi: 10.1074/jbc.274.5.2794. [DOI] [PubMed] [Google Scholar]
- 4.Savinainen JR, Järvinen T, Laine K, Laitinen JT. Br J Pharmacol. 2001;134:664. doi: 10.1038/sj.bjp.0704297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sugiura T, Kondo S, Kishimoto S, Miyashita T, Nakane S, Kodaka T, Suhara Y, Takayama H, Waku K. J Biol Chem. 2000;275:605. doi: 10.1074/jbc.275.1.605. [DOI] [PubMed] [Google Scholar]
- 6.Wilson RI, Nicoll RA. Science. 2002;296:678. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- 7.Diana MA, Marty A. Br J Pharmacol. 2004;142:9. doi: 10.1038/sj.bjp.0705726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lambert DM, Fowler CJ. J Med Chem. 2005;48:5059. doi: 10.1021/jm058183t. [DOI] [PubMed] [Google Scholar]
- 9.Gulyas AI, Cravatt BF, Bracey MH, Dinh TP, Piomelli D, Boscia F, Freund TF. Eur J Neurosci. 2004;20:441. doi: 10.1111/j.1460-9568.2004.03428.x. [DOI] [PubMed] [Google Scholar]
- 10.Ligresti A, Cascio MG, Di Marzo V. Curr Drug Targets - CNS Neurol Disord. 2005;4:615. doi: 10.2174/156800705774933104. [DOI] [PubMed] [Google Scholar]
- 11.Vandevoorde S, Lambert DM. Curr Pharm Des. 2005;11:2647. doi: 10.2174/1381612054546914. [DOI] [PubMed] [Google Scholar]
- 12.Bari M, Battista N, Fezza F, Gasperi V, Maccarrone M. Mini-Rev Med Chem. 2006;6:257. doi: 10.2174/138955706776073466. [DOI] [PubMed] [Google Scholar]
- 13.Sugiura T, Kobayashi Y, Oka S, Waku K. Prostaglandins, Leukotrienes Essent Fatty Acids. 2002;66:173. doi: 10.1054/plef.2001.0356. [DOI] [PubMed] [Google Scholar]
- 14.Kozak KR, Gupta RA, Moody JS, Ji C, Boeglin WE, DuBois RN, Brash AR, Marnett LJ. J Biol Chem. 2002;277:23278. doi: 10.1074/jbc.M201084200. [DOI] [PubMed] [Google Scholar]
- 15.Kozak KR, Crews BC, Morrow JD, Wang LH, Ma YH, Weinander R, Jakobsson PJ, Marnett LJ. J Biol Chem. 2002;277:44877. doi: 10.1074/jbc.M206788200. [DOI] [PubMed] [Google Scholar]
- 16.Di Marzo V, Bifulco M, De Petrocellis L. Nature Rev Drug Disc. 2004;3:771. doi: 10.1038/nrd1495. [DOI] [PubMed] [Google Scholar]
- 17.Makriyannis A, Mechoulam R, Piomelli D. Neuropharmacology. 2005;48:1068. doi: 10.1016/j.neuropharm.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 18.Pertwee RG. AAPS J. 2005;7:E625. doi: 10.1208/aapsj070364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rouzer CA, Ghebreselasie K, Marnett LJ. Chem Phys Lipids. 2002;119:69. doi: 10.1016/s0009-3084(02)00068-3. [DOI] [PubMed] [Google Scholar]
- 20.van der Stelt M, van Kuik JA, Bari M, van Zadelhoff G, Leeflang BR, Veldink GA, Finazzi-Agrò A, Vliegenthart JFG, Maccarrone M. J Med Chem. 2002;45:3709. doi: 10.1021/jm020818q. [DOI] [PubMed] [Google Scholar]
- 21.Saario SM, Savinainen JR, Laitinen JT, Järvinen T, Niemi R. Biochem Pharmacol. 2004;67:1381. doi: 10.1016/j.bcp.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 22.(±)-5 (CDCl3, 500 MHz) δ 5.35–5.44 (m, 8H), 5.25 (d, J = 2.9 Hz, 1H), 4.48 (br s, 1H), 2.82 (m, 6H), 2.41–2.55 (m, 4H), 2.14–2.25 (m, 4H), 2.09–2.10 (m, 2H), 1.96–2.09 (m, 2H), 1.65–1.83 (m, 2H), 1.22–1.42 (m, 6H), 0.91 (t, J = 6.6 Hz, 3H); (±)-6 (CDCl3, 500 MHz) δ 5.28–5.33 (m, 8H), 4.98 (d, J = 9.9 Hz, 1H), 3.85-3.92 (dt, J = 10.4, 4.7 Hz, 1H), 2.82 (m, 6H), 2.47–2.55 (m, 4H), 2.14–2.25 (m, 4H), 2.09–2.10 (m, 2H), 1.96–2.09 (m, 2H), 1.78–1.83 (m, 2H), 1.22–1.42 (m, 6H), 0.91 (t, J = 6.6 Hz, 3H); meso-7 (CDCl3, 400 MHz) δ 5.30–5.43 (m, 8H), 4.95 (br s, 1H), 3.95 (br s, 2H), 2.79–2.85 (m, 6H), 2.43 (t, J = 7.6 Hz, 2H), 2.14–2.15 (m, 2H), 2.04–2.06 (m, 2H), 1.73–1.77 (m, 4H), 1.60–1.67 (m, 2H), 1.27–1.37 (m, 8H), 0.89 (t, J = 7.0 Hz, 3H); (±)-8 (CDCl3, 400 MHz) δ 5.30–5.43 (m, 8H), 4.72–4.82 (m, 1H), 4.04–4.05 (m, 1H), 3.68–3.71 (m, 1H), 2.84–2.88 (m, 6H), 2.38 (t, J = 7.6 Hz, 2H), 2.06–2.20 (m, 4H), 1.67–1.78 (m, 8H), 1.28–1.39 (m, 6H), 0.89 (t, J = 7.0 Hz, 3H); meso-9 (CDCl3, 400 MHz) δ 5.30–5.43 (m, 8H), 4.63–4.65 (m, 1H), 3.48–3.53 (m, 1H), 3.34–3.40 (dt, J = 3.4, 9.1 Hz, 1H), 2.79–2.85 (m, 6H), 2.35 (t, J = 7.4 Hz, 2H), 2.00–2.17 (m, 4H), 1.68–1.75 (m, 4H), 1.19–1.39 (m, 10H), 0.88 (t, J = 6.9 Hz, 3H).
- 23.Dumortier L, Van der Eycken J, Vandewalle M. Tetrahedron Lett. 1989;30:3201. [Google Scholar]
- 24.Guo Y, Abadji V, Morse KL, Fournier DJ, Li X, Makriyannis A. J Med Chem. 1994;37:3867. doi: 10.1021/jm00049a002. [DOI] [PubMed] [Google Scholar]
- 25.Morse KL, Fournier DJ, Li X, Grzybowska J, Makriyannis A. Life Sci. 1995;56:1957. doi: 10.1016/0024-3205(95)00176-7. [DOI] [PubMed] [Google Scholar]
- 26.Lan R, Liu Q, Fan P, Lin S, Fernando SR, McCallion D, Pertwee R, Makriyannis A. J Med Chem. 1999;42:769. doi: 10.1021/jm980363y. [DOI] [PubMed] [Google Scholar]
- 27.Li C, Wei X, Vadivel SK, Makriyannis A. J Med Chem. 2005;48:6423. doi: 10.1021/jm050272i. [DOI] [PubMed] [Google Scholar]
- 28.Cheng YC, Prusoff WH. Biochem Pharmacol. 1973;22:3099. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 29.Ghafouri N, Tiger G, Razdan RK, Mahadevan A, Pertwee RG, Martin BR, Fowler CJ. Br J Pharmacol. 2004;143:774. doi: 10.1038/sj.bjp.0705948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mukherjee S, Adams M, Whiteaker K, Daza A, Kage K, Cassar S, Meyer M, Yao BB. Eur J Pharmacol. 2004;505:1. doi: 10.1016/j.ejphar.2004.09.058. [DOI] [PubMed] [Google Scholar]
- 31.Lang W, Qin C, Hill WAG, Lin S, Khanolkar AD, Makriyannis A. Anal Biochem. 1996;238:40. doi: 10.1006/abio.1996.0247. [DOI] [PubMed] [Google Scholar]
- 32.Lang W, Qin C, Lin S, Khanolkar AD, Goutopoulos A, Fan P, Abouzid K, Meng Z, Biegel D, Makriyannis A. J Med Chem. 1999;42:896. doi: 10.1021/jm980461j. [DOI] [PubMed] [Google Scholar]
- 33.Qin C, Lin S, Lang W, Goutopoulos A, Pavlopoulos S, Mauri F, Makriyannis A. Anal Biochem. 1998;261:8. doi: 10.1006/abio.1998.2713. [DOI] [PubMed] [Google Scholar]
- 34.Barnett-Norris J, Guarnieri F, Hurst DP, Reggio PH. J Med Chem. 1998;41:4861. doi: 10.1021/jm9803471. [DOI] [PubMed] [Google Scholar]
- 35.Tian X, Guo J, Yao F, Yang DP, Makriyannis A. J Biol Chem. 2005;280:29788. doi: 10.1074/jbc.M502925200. [DOI] [PubMed] [Google Scholar]


