Abstract
We detail the structure and dynamics of a synthetic peptide corresponding to transmembrane helix 6 (TMH6) of human cannabinoid receptor 2 (hCB2) in biomembrane mimetic environments. The peptide’s NMR structural biology is characterized by two α-helical domains bridged by a flexible, nonhelical hinge region containing a highly-conserved CWFP motif with an environmentally sensitive, Pro-based conformational switch. Buried within the peptide’s flexible region, W258 may hydrogen-bond with L255 to help stabilize the Pro-kinked hCB2 TMH6 structure and position C257 advantageously for interaction with agonist ligands. These characteristics of hCB2 TMH6 are potential structural features of ligand-induced hCB2 activation in vivo.
Keywords: agonist ligand, conformational switch, G protein-coupled receptor, high-resolution NMR, peptide helix, proline, spectrofluorometry, structural biology, transmembrane protein, tryptophan
Introduction
G protein-coupled receptors (GPCRs) comprise a superfamily of cell-surface proteins whose iconic structural signature includes seven hydrophobic transmembrane helices (TMHs) [1]. As components of the information-transducing machinery of eukaryotic cells, GPCRs are activated by exogenous agonists whose binding elicits GPCR structural changes that transition the receptor from inactive to active state(s). A “rotamer toggle switch” involving the bend angle or “kink” around a conserved proline in TMH6 and an “ionic lock” involving salt-bridge formation between charged amino acids in TMH3 and TMH6 have been implicated in the structural changes upon ligand-induced GPCR activation, largely from data on those few GPCRs whose high-resolution crystal structures have been solved [2–4]. Isolated successes with GPCR crystallization notwithstanding, direct experimental information on GPCR structural biology remains very limited.
Cannabinoid (CB) receptors are class-A, rhodopsin-like GPCRs that are activated by intrinsic lipid mediators (endocannabinoids) and exogenous cannabimimetic agents [5]. The (patho)physiological importance of cannabinergic signaling has prompted the pharmacotherapeutic evaluation of designer ligands (agonists/antagonists) selective for one of the two CB receptor subtypes, CB1 and CB2, which have been cloned, expressed, and sequenced [6]. Because of CB2’s largely peripheral localization, CB2-targeted ligands as potential drugs for pain and inflammatory and neurodegenerative disorders have an inherently low risk of inducing centrally-mediated side effects, which mainly reflect CB1 activation [7]. Although CB1 and CB2 have been the focus of mutation and homology-modeling studies, their crystal structures are lacking [8,9]. Consequently, CB-receptor structural properties critical to ligand docking and pharmacological activation remain experimentally ill-defined. Our previous work has implicated a flexible TMH6 CWxP motif in the structural changes critical to agonist-induced CB1 activation [10]. Molecular dynamics simulations suggest the existence of at least two CB1 TMH6 conformations differing in the degree of Pro-kink in that flexible-hinge region [11]. Such findings cannot be extrapolated readily to CB2, however, for human CB1 and CB2 (hCB2) differ considerably in their primary sequence, modeled tertiary conformations, and agonist structure-activity profiles [12,13].
The present work is focused on defining experimentally the structural features of CB2 TMH6 and identifying motifs potentially involved in the conformational switching of CB2 TMH6 upon ligand-induced activation. We have utilized a synthetic peptide representing hCB2 TMH6 [9], high-resolution solution nuclear magnetic resonance (NMR) spectroscopy (including 2D-1H TOCSY and NOESY experiments), and spectroflurometry. This approach is well validated for complete 1H assignment with small nonlabeled peptides [14,15]. For our NMR experiments, the hCB2 TMH6 peptide was studied in a compatible solvent system that simulates a biomembrane environment [16]. Tryptophan is considered to play a special role in helping anchor and stabilize protein α-helical regions [17]. For our spectroflurometry studies, we have exploited the single hCB2 TMH6 tryptophan residue (W258) modeled as being centrally disposed within the plasma membrane in situ [9] to monitor local structural responses of hCB2 TMH6 when the peptide is reconstituted in micelle membrane mimetics [18]. To our knowledge, this study provides the first direct experimental characterization of hCB2 TMH6 structure and implicates a motif around P260 in agonist-induced TMH6 conformational switching.
Materials and methods
Peptide synthesis and purification
The 33-mer [240DVRLAKTLGLVLAVLLICWFPVLALMAHSLATT272] corresponding to hCB2 TMH6 [9] was synthesized by a standard 9H-fluoren-9-ylmethoxycarbonyl (Fmoc)–polyamide method at the Molecular Biology Core Facility, Dana-Farber Cancer Institute (Boston, MA, USA). The peptide was isolated by reverse-phase LC to >95% purity according to LC and MALDI-TOF mass spectrometry (MS) analyses.
Sample preparation and NMR experiments
For 1-D and 2-D NMR, the hCB2 TMH6 peptide was dissolved (1.0 mM final conc.) in 30% (v/v) aqueous trifluoroethanol-d2 (TFE). 2-Dimethyl-2-silapentane-5-sulfonic acid was added as reference standard. All experiments were conducted at 27 or 37 °C on a 700-MHz NMR spectrometer (Bruker BioSpin, Billerica, MA, USA). TOCSY (with 70, 80 and 90 ms mixing times) and NOESY (with 200 and 250 ms mixing times) spectra were acquired in phase-sensitive mode. Virtually complete proton chemical shift assignments have been obtained for this TFE/H2O mixture [16,19].
Circular dichroism (CD) spectropolarimetry
Small micelles composed of either 1,2-dihexanoyl-sn-glycero-3-phosphocholine (D-6-PC) or dodecylphosphocholine (DPC) were prepared in 10 mM Tris buffer, pH ~ 7.5, containing 0.5 mM EDTA and 10 mM NaCl. The hCB2 TMH6 peptide was dissolved in 30% TFE/H20 or incorporated into micelles at a 100:1 lipid-to-peptide molar ratio. Analysis of peptide secondary structure was then performed with a nitrogen-flushed J-810 spectropolarimeter controlled by Spectra Manager (version 1.15.00) (Jasco Instruments, Easton, MD, USA) [20]. Spectra were recorded from 280 to 185 nm at 23 °C using a 1-cm cuvette, a scan rate of 1 nm/min, and an average of three scans per sample. Percent α-helical content was calculated as described [21].
Fluorescence spectroscopy
Spectrofluorometry experiments were performed at 30° C using a QuantaMaster QM-1/2005 spectrofluorometer (Photon Technology International, Birmingham, NJ, USA) in a quartz cuvette. The samples were excited at 295 nm, and emission spectra were collected between 300 and 400 nm. The bandwidth for both excitation and emission monochromators was 5 nm. Samples were prepared by mixing D-6-PC or DPC with hCB2 TMH6 peptide in TFE/chloroform at a molar lipid-to-peptide ratio of 100:1. The mixture was dried to a film that was rehydrated with 25 mM Tris buffer, pH 7.4, containing 100 mM NaCl to give a stock solution of 1 mM peptide, which was then suitably diluted with the Tris-NaCl solution to a final peptide concentration of 2.5 μM in the analyzed sample.
Collisional quenching experiments with acrylamide were performed by adding increasing amounts of a 10 M acrylamide stock solution in 25 mM Tris buffer (pH 7.4) to hCB2 TMH6 peptide samples reconstituted either in 30% TFE/H20 or in D-6-PC or DPC micelles up to a maxial acrylamide concentration of 5 mM. The difference between the peptide’s (i.e., the peptide’s sole tryptophan residue, W258) net fluorescence in the presence and absence of a given acrylamide concentration in each respective membrane-mimetic environment was determined. Quenching of the fluorescence intensities at maximum emission was calculated with the Stern-Volmer equation,
where: F0 is the unquenched fluorescence intensity; F is the fluorescence intensity at [acrylamide]; Ksv is the Stern-Volmer quenching constant, which was determined as a function of [acrylamide].
Structure determination
Observed nuclear Overhauser effects (NOEs) were classified according to three categories: short, medium, and long range. A total of 830 inter-residue distance restraints were used in the structure calculations. All the spectra were processed with Topspin (Bruker BioSpin) and visualized using CARA software (http://www.nmr.ch/). NOE assignments were improved by a KNOWNOE protocol [22]. Structure calculations were performed by Xplor-NIH [23]. Fifteen lowest-energy conformations were subjected to molecular dynamics simulations in explicit water using the Crystallography and NMR System software suite [24–26]. Structures were validated by PROCHECK-NMR and visualized with MOLMOL [27,28].
Results
hCB2 TMH6 solution structure
Superimposition of the fifteen lowest-energy hCB2 TMH6 NMR conformers in membrane-mimetic solvent (30% TFE/H20) is depicted in Fig. 1A. The α-helical secondary elements of the averaged hCB2 TMH6 structure are depicted as the solid ribbon in Fig. 1B. The ensemble statistics are presented in Table 1. The solution structure of hCB2 TMH6 is characterized by two helical segments of different lengths separated by a discrete unstructured region with a pronounced Pro-based kink. The kink, well defined by numerous NOEs, seems quite rigid in nature and orients the two helical segments at almost 45° degrees with respect to each another. The two hCB2 TMH6 terminal regions, i.e., from the N-terminus to K245 and from A264 to the C-terminus, are dynamically unstructured.
Fig. 1.

(A) Fifteen lowest-energy hCB2 TMH6 peptide structures are displayed. Only backbone atoms are shown. In each case, the structures were calculated using NOE and hydrogen bond restraints. (B) The α-helical secondary elements of the mean TMH6 structure are depicted as a solid ribbon.
Table 1.
Structural statistics for the fifteen best hCB2 TMH6 NMR conformers
| Parameter | Ensemblea | |
|---|---|---|
| Distance restrains | ||
| All | 830 | |
| Short range (| i−j| ≤ 1 ) | 477 | |
| Medium range (1 < | i−j| < 5 ) | 339 | |
| Long range (| i−j| ≥ 5 ) | 4 | |
| Violations | ||
| NOE (> 0.5 Å) | 0 | |
| r.m.s.d.b (residues 6 to 27) | ||
| Average backbone r.m.s.d. to mean | 0.46 ± 0.15c | 0.64 ± 0.17d |
| Average heavy atom r.m.s.d. to mean | 0.81 ± 0.21c | 1.09 ± 0.23d |
| Van der Waals energy | − 68.41 ± 3.72 | |
| Ramachandran plote | ||
| Residues in most favored regions | 55.6% c | 67.1%d |
| Residues in additional allowed regions | 33.6%c | 27.4%d |
| Residues in generously allowed regions | 9.0%c | 5.5% d |
| Residues in disallowed regions | 1.8%c | 0% d |
| r.m.s.d. from idealized covalent geometry | ||
| Bonds (Å) | 0.00318 ± 0.00025 | |
| Angles (°) | 0.43285 ± 0.0265 | |
| Impropers (°) | 1.21733 ± 0.21031 | |
Values given are means ± S.E.M., wherever applicable.
Residues 1 to 5 and 28 to 33 were excluded from r.m.s.d. calculations due to the dynamic disorder in these regions.
Before water refinement
After water refinement
Calculated with PROCHECK-NMR [27]
CD structural analysis of hCB2 TMH6
In order to assess the structural responsiveness of hCB2 TMH6 to its immediate environment, we conducted CD studies of the peptide as reconstituted in three biomembrane-mimetic environments: the 30% TFE/H20 mixture used in the NMR analysis (above) and detergent micelles composed of either D-6-PC or DPC [18]. The shapes and intensities of representative CD spectra (Fig. 2) are indicative of the peptide’s having significant α-helical content in all three membrane mimetics. The average α-helical content of hCB2 TMH6 was 44, 48, and 36% in D-6-PC micelles, DPC micelles, and 30% TFE/H20, respectively. The convergence (isodichroic) point among the three traces reflects a two-state system associated with environmentally-sensitive changes in hCB2 TMH6 conformation.
Fig. 2.
CD spectra of hCB2 TMH6 peptide reconstituted in 30% TFE/H20 or in D-6-PC or DPC micelles.
Involvement of W258 in hCB2 TMH6 structural dynamics
To determine whether W258 is involved in any local conformational changes of hCB2 TMH6 when the peptide interacts with lipid, we conducted comparative spectrofluorometric studies of that 33-mer reconstituted in D-6-PC or DPC micelles or in 30% TFE/H20. As shown in Fig. 3, the fluorescence emission maxima (λmax) of hCB2 TMH6 in D-6-PC, DPC, and TFE/H20 are 340, 343, and 347 nm, respectively. The 3-nm blue shift between the D-6-PC and DPC samples indicates that the W258 residue resides in a more polar environment when the hCB2 TMH6 peptide is in a D-6-PC vs. DPC micelle. These fluorescence data further suggest that TMH6 is flexed such that W258 within the Pro-kinked region is better protected when associated with a D-6-PC micelle, D-6-PC hydrocarbon chains being shorter than DPC. The 7-nm blue shift and the 60% increase in the fluorescence intensity at λmax of hCB2 TMH6 in D-6-PC micelles as compared to the peptide in aqueous TFE is indicative of the highly hydrophobic environment of W258 when hCB2 TMH6 is reconstituted in D-6-PC micelles.
Fig. 3.
Fluorescence emission spectra of the hCB2 TMH6 peptide in TFE/H20 mixture (dashed line), D-6-PC (dotted line) and DPC (solid line). The lipid-to-peptide molar ratio of TMH6 in D-6-PC or DPC was 100:1.
W258 is centrally located within the hCB2 TMH6 peptide sequence and is presumed to be buried within the plasma membrane in situ [9] where, by analogy with other GPCRs [11,17,29], it may help stabilize the Pro-kinked hCB2 structure. Preliminary analysis with 3-D molecular visualization software (DA Visualizer Pro; Accelrys Inc., San Diego, CA, USA) suggested that the steady-state water accessibility of W258 in hCB2 TMH6 reconstituted in 30% TFE/H20 is limited, W258 being well-protected within the static Pro-kinked hCB2 TMH6 structural matrix (data not shown). To gain experimental insight into the degree of dynamic exposure of W258 within the various membrane-mimetic entities studied, fluorescence quenching studies were next conducted [30]. Linear plots of Fo/F vs. acrylamide quencher concentration were obtained for all peptide environments examined (data not shown). For peptide reconstituted in D-6-PC or DPC, the respective Stern-Volmer quenching constant (Ksv) was 3.3 ± 0.1 M−1 and 2.3 ± 0.2 M−1. The Ksv for the hCB2 TMH6 peptide in TFE/H20 was 10.7 ± 1.2 M−1, a comparatively high value indicating that a detergent micelle environment restricts the local structural oscillations of hCB2 TMH6 that allow penetration of acrylamide quencher to W258.
Discussion
We have used high-resolution NMR spectroscopy to define experimentally the structural features of a peptide representing hCB2 TMH6 in a membrane-mimetic TFE/H20 solution. To our knowledge, this is the initial report of an NMR-derived structure for the TMH6 of any GPCR. Contrary to popular conceptualizations of transmembrane receptor domains as ideally linear α-helices, emerging data suggest that such regions may have nonhelical and/or highly flexible components that contribute decisively to receptor structural character and dynamics [31]. The deviation observed in the hCB2 TMH6 from an ideally linear, continuous α-helix implicates its CWFP region as a flexible hinge that could help accommodate hCB2 TMH6 conformational changes associated with, for example, ligand-induced hCB2 activation. This feature of hCB2 TMH6 is functionally reminiscent of the highly-conserved CWxP motif found in other GPCRs [3,32]. Indeed, the NMR solution structure of hCB2 TMH6 proposed herein is generally consistent with the character of the TMH6’s of the few GPCRs whose crystal structures are known [33–36].
Similar to the homolog in TMH6 of the rhodopsin and β-adrenergic receptor high-resolution structures [33,34] and in models of some other GPCRs [3,11,37], W258 of the hCB2 TMH6 is localized within the nonhelical Pro-kinked region. The spectrofluorometric blue shift of the emission λmax we observe confirms that W258 experiences a more hydrophobic environment in D-6-PC or DPC relative to that in TFE/H20. The distinct fluorescence spectral signatures of hCB2 TMH6 when the peptide is in a D-6-PC vs. DPC bicelle can be conceptualized in terms of a dynamic response of W258 to its local environment such that TMH6 assumes an enhanced Pro-kinked structure in the shorter-chain D-6-PC detergent. Initial molecular dynamics simulations conducted in our laboratories suggest that the effective hydrophobic length of the shorter-chain D-6-PC vs. DPC micelles induces more α-helical peptide content, perhaps by restricting the flexible hCB2 TMH6 peptide regions so as to avoid hydrophobic mismatch (data not shown). This view of hCB2 TMH6 structural accommodation is supported by demonstrations that biomembrane thickness itself influences protein (including GPCR) TMH conformation and signal transmission [38,39]. It is tempting to speculate that Pro-based conformational switching may represent a structural feature of ligand (agonist)-induced hCB2 activation in situ.
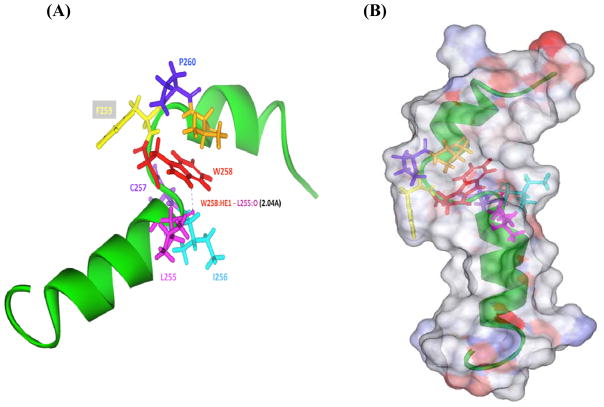
As schematized in Fig. 4A, we propose that the stability of the Pro-kinked hCB2 TMH6 structure derived from our NMR data is controlled by a H-bonding network involving conserved polar residues in the vicinity of TMH6’s flexible region. In TFE/H20 solution, we envision that the side chain of W258 resides in the concave face of the Pro-kink, favorably oriented to H-bond with neighboring residues. Our proposed structure features a 2-Å i to i-3 H-bond between the W258 imino (-NH) and L255 carbonyl (C=O) groups. Another H-bond (4 Å) may exist between W258 and I256 i to i- 2. We have demonstrated that C257 plays a critical role in agonist-induced hCB2 activation [40]. C257 is part of the highly conserved CWxP motif, a putative molecular hinge generally implicated in GPCR agonist recognition [36,37]. It is therefore noteworthy in our NMR structure of hCB2 TMH6 that the C257 side-chain is oriented away from the Pro-kinked region toward the aqueous phase, suggesting that H-bonding within the CWFP motif of the Pro-kinked structure advantageously positions C257 for accessibility during agonist docking [11,37]. Surface presentation of the atomic charge (Fig. 4B) of our proposed hCB2 TMH6 solution structure shows that the positive and negative charges are oriented toward the hydrophilic medium, whereas the neutral surface (i.e., side chain) is buried within the helical structure.
Fig. 4.
(A) The H-bond network between the conserved W258 with the carbonyl (C=O) of L255, which is some 2 Å away. Another H-bond (4 Å distance) between W258 and I256 is depicted. W258 resides in the concave region of the Pro-kinked region and contributes to structural stability. (B) Surface presentation of the atomic charge of hCB2 TMH6 peptide. Blue denotes positive charges; red, negative charges; white, neutral surface.
In summary, this report provides the initial, direct experimental data on the conformation and dynamics of a 33-mer representing TMH6 of hCB2 in membrane-mimetic environments. The peptide is characterized as a discontinuous, Pro-kinked α-helix with a hydrophobic core flanked by unstructured nonhelical regions. We have identified in hCB2 TMH6 a Pro-based conformational switch and a stabilizing effect of W258 on the structure of the Pro-kinked peptide. These characteristics of hCB2 TMH6 are likely structural features of ligand-induced hCB2 activation in vivo.
Acknowledgments
This study was supported by grants from National Institute on Drug Abuse grants DA009158-10S2 (EKT) and DA3801 (AM).
Footnotes
Atomic coordinates and structure factors will be deposited in the Research Collaboratory for Structural Bioinformatics Protein Data Bank, Rutgers University, Department of Chemistry and Chemical Biology, Piscataway, New Jersey.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hanson MA, Stevens RC. Discovery of new GPCR biology: one receptor structure at a time. Structure. 2009;17:8–14. doi: 10.1016/j.str.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballesteros JA, Jensen AD, Liapakis G, Rasmussen SG, Shi L, Gether U, Javitch JA. Activation of the β2-adrenergic receptor involves disruption of an ionic lock between the cytoplasmic ends of transmembrane segments 3 and 6. J Biol Chem. 2001;276:29171–29177. doi: 10.1074/jbc.M103747200. [DOI] [PubMed] [Google Scholar]
- 3.Shi L, Liapakis G, Xu R, Guarnieri F, Ballesteros JA, Javitch JA. β2 adrenergic receptor activation. Modulation of the proline kink in transmembrane 6 by a rotamer toggle switch. J Biol Chem. 2002;277:40989–40996. doi: 10.1074/jbc.M206801200. [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharya S, Hall SE, Vaidehi N. Agonist-induced conformational changes in bovine rhodopsin: insight into activation of G-protein-coupled receptors. J Mol Biol. 2008;382:539–555. doi: 10.1016/j.jmb.2008.06.084. [DOI] [PubMed] [Google Scholar]
- 5.Pertwee RG. The pharmacology of cannabinoid receptors and their ligands: an overview. Int J Obesity. 2006;30(Suppl 1):13–18. doi: 10.1038/sj.ijo.0803272. [DOI] [PubMed] [Google Scholar]
- 6.Vemuri VK, Janero DR, Makriyannis A. Pharmacotherapeutic targeting of the endocannabinoid signaling system: drugs for obesity and the metabolic syndrome. Physiol Behav. 2008;93:671–686. doi: 10.1016/j.physbeh.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janero DR, Vadivel SK, Makriyannis A. Pharmacotherapeutic modulation of the endocannabinoid signaling system in psychiatric disorders: drug-discovery strategies. Int Rev Psychiatry. 2009;21:122–133. doi: 10.1080/09540260902782778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kapur A, Hurst DP, Fleischer D, Whitnell R, Thakur GA, Makriyannis A, Reggio PH, Abood ME. Mutation studies of Ser7.39 and Ser2.60 in the human CB1 cannabinoid receptor: evidence for a serine-induced bend in CB1 transmembrane helix 7. Mol Pharmacol. 2007;71:1512–1524. doi: 10.1124/mol.107.034645. [DOI] [PubMed] [Google Scholar]
- 9.Nebane NM, Hurst DP, Carrasquer CA, Qiao Z, Reggio PH, Song ZH. Residues accessible in the binding-site crevice of transmembrane helix 6 of the CB2 cannabinoid receptor. Biochemistry. 2008;47:13811–13821. doi: 10.1021/bi8007802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Picone RP, Khanolkar AD, Xu W, Ayotte LA, Thakur GA, Hurst DP, Abood ME, Reggio PH, Fournier DJ, Makriyannis A. (−)-7′-Isothiocyanato-11-hydroxy-1′,1′-dimethylheptylhexahydrocannabinol (AM841), a high-affinity electrophilic ligand, interacts covalently with a cysteine in helix six and activates the CB1 cannabinoid receptor. Mol Pharmacol. 2005;68:1623–1635. doi: 10.1124/mol.105.014407. [DOI] [PubMed] [Google Scholar]
- 11.Singh R, Hurst DP, Barnett-Norris J, Lynch DL, Reggio PH, Guarnieri F. Activation of the cannabinoid CB1 receptor may involve a W6.48/F3.36 rotamer toggle switch. J Pept Res. 2002;60:357–370. doi: 10.1034/j.1399-3011.2002.21065.x. [DOI] [PubMed] [Google Scholar]
- 12.Montero C, Campillo NE, Goya P, Páez JA. Homology models of the cannabinoid CB1 and CB2 receptors: a docking analysis study. Eur J Med Chem. 2005;40:75–83. doi: 10.1016/j.ejmech.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Ahston JC, Wright JL, McPartland JM, Tyndall JD. Cannabinoid CB1 and CB2 receptor ligand specificity and the development of CB2-selective agonists. Curr Med Chem. 2008;15:1428–1443. doi: 10.2174/092986708784567716. [DOI] [PubMed] [Google Scholar]
- 14.Gong XM, Franzin CM, Thai K, Yu J, Marassi FM. Nuclear magnetic resonance structural studies of membrane proteins in micelles and bilayers. Methods Mol Biol. 2007;400:515–529. doi: 10.1007/978-1-59745-519-0_35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang G. NMR of membrane-associated peptides and proteins. Curr Protein Pept Sci. 2008;9:50–69. doi: 10.2174/138920308783565714. [DOI] [PubMed] [Google Scholar]
- 16.Roccatano D, Colombo G, Fioroni M, Mark AE. Mechanism by which 2,2,2-trifluoroethanol/water mixtures stabilize secondary-structure formation in peptides: a molecular dynamics study. Proc Natl Acad Sci USA. 2002;99:12179–12184. doi: 10.1073/pnas.182199699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roth CB, Hanson MA, Stevenson RC. Stabilization of the human β2-adrenergic receptor TM4-TM3-TM5 helix interface by mutagenesis of Glu122(3.41), a critical residue on GPCR structure. J Mol Biol. 2008;376:1305–1319. doi: 10.1016/j.jmb.2007.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garavito RM, Ferguson-Miller S. Detergents as tools in membrane biochemistry. J Biol Chem. 2001;276:2403–32406. doi: 10.1074/jbc.R100031200. [DOI] [PubMed] [Google Scholar]
- 19.Buck M. Trifluoroethanol and colleagues: cosolvents come of age. Recent studies with peptides and proteins. Q Rev Biophys. 1998;31:297–355. doi: 10.1017/s003358359800345x. [DOI] [PubMed] [Google Scholar]
- 20.Chang CT, Wu CS, Yang JT. Circular dichroism analysis of protein conformation inclusion of the β-turns. Anal Biochem. 1978;91:13–31. doi: 10.1016/0003-2697(78)90812-6. [DOI] [PubMed] [Google Scholar]
- 21.Martin SR, Schilstra MJ. Circular dichroism and its application to the study of biomolecules. Methods Cell Biol. 2008;84:263–293. doi: 10.1016/S0091-679X(07)84010-6. [DOI] [PubMed] [Google Scholar]
- 22.Gronwald W, Moussa S, Elsner R, Jung A, Ganslmeier B, Trenner J, Kremer W, Neidig KP, Kalbitzer HR. Automated assignment of NOESY NMR spectra using a knowledge based method (KNOWNOE) J Biomol NMR. 2002;23:271–287. doi: 10.1023/a:1020279503261. [DOI] [PubMed] [Google Scholar]
- 23.Schwieters CD, Kuszewski JJ, Tjandra N, Clore GM. The Xplor-NIH NMR molecular structure determination package. J Magn Reson. 2003;160:65–73. doi: 10.1016/s1090-7807(02)00014-9. [DOI] [PubMed] [Google Scholar]
- 24.Linge JP, Williams MA, Spronk CA, Bonvin AM, Nilges M. Refinement of protein structures in explicit solvent. Proteins. 2003;50:496–506. doi: 10.1002/prot.10299. [DOI] [PubMed] [Google Scholar]
- 25.Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, Read RJ, Rice LM, Simonson T, Warren GL. Crystallography and NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr B Biol Crystallogr. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 26.Jung JW, Yee A, Wu B, Arrowsmith CH, Lee W. Solution structure of YKR049C, a putative redox protein from Saccharomyces cerevisiae. J Biochem Mol Biol. 2005;38:550–554. doi: 10.5483/bmbrep.2005.38.5.550. [DOI] [PubMed] [Google Scholar]
- 27.Laskowski RA, Moss DS, Thornton JM. Main-chain bond lengths and bond angles in protein structures. J Mol Biol. 1993;231:1049–1067. doi: 10.1006/jmbi.1993.1351. [DOI] [PubMed] [Google Scholar]
- 28.Koradi R, Billeter M, Wuthrich K. MOLMOL: a program for display and analysis of macromolecular structures. J Mol Graph. 1996;14:51–5. 29–32. doi: 10.1016/0263-7855(96)00009-4. [DOI] [PubMed] [Google Scholar]
- 29.Landolt-Marticorena C, Williams KA, Deber CM, Reithmeier RA. Non-random distribution of amino acids in the transmembrane segments of human type I single span membrane proteins. J Mol Biol. 1993;229:602–608. doi: 10.1006/jmbi.1993.1066. [DOI] [PubMed] [Google Scholar]
- 30.Walters J, Milam SJ, Clark AC. Practical approaches to protein folding and assembly: spectroscopic strategies in thermodynamics and kinetics. Methods Enzymol. 2009;455:1–39. doi: 10.1016/S0076-6879(08)04201-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma D, Liu Z, Li P, Tang P, Xu Y. Structure and dynamics of the second and third transmembrane domains of human glycine receptor. Biochemistry. 2005;44:8790–8800. doi: 10.1021/bi050256n. [DOI] [PubMed] [Google Scholar]
- 32.Yao X, Parnot C, Deupi X, Ratnala VR, Swaminath G, Farrens D, Kobilka B. Coupling ligand structure to specific conformational switches in the β2-adrenoceptor. Nat Chem Biol. 2006;2:417–422. doi: 10.1038/nchembio801. [DOI] [PubMed] [Google Scholar]
- 33.Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, Yamamoto M, Miyano M. Crystal structure of rhodopsin: a G protein-coupled receptor. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 34.Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Kuhn P, Weis WI, Kobilka BK, Stevens RC. High-resolution crystal structure of an engineered human β2-adrenergic G protein-coupled receptor. Science. 2007;318:1258–1265. doi: 10.1126/science.1150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Warne T, Serrano-Vega MJ, Baker JG, Moukhametzianov R, Edwards PC, Henderson R, Leslie AG, Tate CG, Schertler GF. Structure of a β1-adrenergic G-protein-coupled receptor. Nature. 2008;454:486–491. doi: 10.1038/nature07101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lagerstrom MC, Schioth HB. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat Rev Drug Discov. 2008;7:339–357. doi: 10.1038/nrd2518. [DOI] [PubMed] [Google Scholar]
- 37.Reggio PH. Computational methods in drug design: modeling G protein-coupled receptor monomers, dimers, and oligomers. AAPS J. 2006;8:E322–336. doi: 10.1007/BF02854903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marsh D. Protein modulation of lipids, and vice-versa, in membranes. Biochim Biophys Acta. 2008;1778:1545–1575. doi: 10.1016/j.bbamem.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 39.Tiburu E, Bowman AL, Struppe JO, Janero DR, Avraham HK, Makriyannis A. Solid-state and molecular dynamics characterization of cannabinoid receptor-1 (CB1) helix 7 conformational plasticity in model membranes. Biochim Biophys Acta. 2009 doi: 10.1016/j.bbamem.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pei Y, Mercier RW, Anday JK, Thakur GA, Zvonok AM, Hurst D, Reggio PH, Janero DR, Makriyannis AA. Ligand-binding architecture of human CB2 cannabinoid receptor: evidence for receptor subtype-specific binding motif and modeling of GPCR activation. Chem Biol. 2008;15:1207–1219. doi: 10.1016/j.chembiol.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]