Abstract
Hippocampal function and plasticity differ with gender, but the regulatory mechanisms underlying sex differences remain elusive and may be established early in life. The present study sought to elucidate sex differences in hippocampal plasticity under normal developmental conditions and in response to repetitive, predictable versus varied, unpredictable prenatal stress (PS). Adult male and diestrous female offspring of pregnant rats exposed to no stress (control), repetitive stress (PS-restraint), or a randomized sequence of varied stressors (PS-random) during the last week of pregnancy were examined for hippocampal proliferation, neurogenesis, cell death, and local microenvironment using endogenous markers. Regional volume was also estimated by stereology. Control animals had comparable proliferation and regional volume regardless of sex, but females had lower neurogenesis compared to males. Increased cell death and differential hippocampal precursor kinetics both appear to contribute to reduced neurogenesis in females. Reduced local interleukin-1beta (IL-Iβ immunoreactivity (IR) in females argues for a mechanistic role for the anti-apoptotic cytokine in driving sex differences in cell death. Prenatal stress significantly impacted the hippocampus, with both stress paradigms causing robust decreases in actively proliferating cells in males and females. Several other hippocampal measures were feminized in males such as precursor kinetics, IL-Iβ-IR density, and cell death, reducing or abolishing some sex differences. The findings expand our understanding of the mechanisms underlying sex differences and highlight the critical role early stress can play on the balance between proliferation, neurogenesis, cell death, and hippocampal microenvironment in adulthood.
Keywords: Restraint and random stress, subgranular zone, Ki-67, doublecortin, apoptosis, interleukin-1 beta, sex differences
Introduction
The generation of new neurons (neurogenesis) in the adult hippocampal subgranular zone (SGZ) is hypothesized to maintain plasticity in the hippocampus, supporting both structure and function (Gould et al. 1999; Hastings and Gould 2003; Kempermann and Gage 1999; Markakis et al. 2004; van Praag et al. 2002). Adult hippocampal neurogenesis and hippocampus-dependent behaviors differ in males and females of various species (Einon 1980; Falconer and Galea 2003; Galea and McEwen 1999; Perfilieva et al. 2001; Roof and Stein 1999; Sutcliffe et al. 2007; Tanapat et al. 1998; Westenbroek et al. 2004; Williams et al. 1990). Mechanisms underlying sex differences in hippocampal neurogenesis are not fully understood, but increasing evidence indicates that both adrenal (stress) and gonadal (sex) hormones are involved (for a recent reviews see (Galea 2007; Hajszan et al. 2007)). For example, stress experienced in adulthood decreases proliferation and neurogenesis in males, but not females (Falconer and Galea 2003; Westenbroek et al. 2004). Estrogen is thought to protect the female hippocampus against stress-induced changes in hippocampal neurogenesis, indicating the importance of estrous status in hippocampal plasticity (Falconer and Galea 2003; Lee and McEwen 2001; Tanapat et al. 1999).
Sex differences in hippocampal neurogenic capacity and environment in adulthood may underlie sex differences in neurogenesis in this region. Microenvironment factors can modulate neurogenesis through direct or indirect regulation of SGZ precursors. For example, females have higher hippocampal corticosteroid receptor labeling compared to males (Liu et al. 2001; Richardson et al. 2006). A subset of SGZ precursors express GR (Garcia et al. 2004), and corticosteroid activation of hippocampal GR is pro-apoptotic via activation of factors such as p53 and BcL-2 (Almeida et al. 2000; Crochemore et al. 2002; Hassan et al. 1996; Wang and Garabedian 2003). Conversely, glucocortiocoids also interact with interleukin-1beta (IL-1β; (MacPherson et al. 2005; Schmidt et al. 1999)), a cytokine thought to be anti-apoptotic and, unlike other cytokines, does not impair neurogenesis (Monje et al. 2002). The above findings altogether hint at a potential interplay between stress hormones and neuroprotective cytokines in sex differences in hippocampal proliferation, neurogenesis, and cell death (McEwen 2002).
Regulatory mechanisms underlying sex differences in hippocampal plasticity may be established early in life. Adult males and females have differential sensitivity to stress experienced during prenatal development (Weinstock 2007). Exposure to repeated restraint stress during the last week of pregnancy in rats differentially alters the hypothalamic pituitary adrenal (HPA) axis responses to a novel stressor in both male and female adulthood offspring, enhancing the sex difference observed in control animals (Richardson et al. 2006; Szuran et al. 2000). This same treatment elicits increases in anxiety-like behavior only in females (Bowman et al. 2004; Richardson et al. 2006; Roussel et al. 2005). Furthermore, prenatal restraint stress decreases hippocampal factors involved in learning and memory, perhaps by promoting factors that produce oxidative stress, favoring a greater change in female offspring (Li et al. 2006; Schmitz et al. 2002; Zhu et al. 2004). Thus, the physiological and behavioral consequences of exposure to early stress differ greatly depending on the sex of the offspring. While studies have demonstrated the negative effects of prenatal stress on the development and survival of hippocampal neural precursors, this work has primarily focused on males (Coe et al. 2003; Fujioka et al. 2006; Lemaire et al. 2000; Lemaire et al. 2006; Van den Hove et al. 2005).
The present study explored whether hippocampal neurogenic capacity and microenvironment differed in adult males and females under normal developmental conditions or in response to stress experienced during late prenatal development. Sex differences were observed in several hippocampal measures in adult offspring of unstressed dams including, neurogenesis, cell death, and key aspects of hippocampal microenvironment. Prenatal stress significantly impacted hippocampal neurogenic capacity, with a robust vulnerability in males. Feminization of hippocampal measures was predominant in adult male offspring, thereby resulting in the elimination or reduction of some sex differences observed in controls.
Materials and Methods
Animals and tissue preparation
As previously described (Richardson et al. 2006), timed-pregnant rat dams (Crl:CD(SD) rats; Charles River, Wilmington, MA) were assigned to one of the following three treatment conditions for the last week of pregnancy (gestational days 14-21); (a) Control, which received no stress, (b) Prenatal stress via repeated restraint (PS-restraint; three daily 45 min sessions at 0900 h, 1200 h, and 1700 h under illumination by two 150 W bulbs;(Ward and Weisz 1980), and (c) Prenatal stress via randomized stressors (PS-random; daily exposure to either restraint stress (three 45 min sessions), foot shock stress, or saline injection stress). Litters were culled to a maximum of 14 pups and left undisturbed after culling until weaning at 21 d, when females and males were separated from their mothers and group housed with same-sex littermates until adulthood (21-23 weeks old). To avoid litter effects, which can interfere with data interpretation (Zorrilla 1997), only one to two animals (of each sex) per litter were used in the present study. Sex ratio and litter size did not differ between groups.
Brains from adult male and female offspring (21-23 weeks old) were used for the present study (n=5/Sex/Prenatal Treatment). Tissue was obtained from animals in which anxiety-related behavior and gonadal steroids are reported elsewhere (Richardson et al. 2006). It should be noted that the brains used in the present study were from a different subset of animals from those used for glucocorticoid receptor expression in the previous report (Richardson et al. 2006). Estrous cycles were synchronized in adult female offspring by giving two doses of 2 μg of the potent gonadotropin releasing hormone (GnRH) agonist ([DTrp6,Pro9,Net]GnRH (sc) synthesized by solid phase methodology (Rivier et al. 1974), generously provided by Dr. Jean Rivier, the Salk Institute, La Jolla, CA) at 0900 h and 1400h eleven days prior to perfusions (Rivier and Vale 1990). This procedure is used to simulate the proestrous GnRH surge (Sisk et al. 2001), and females then proceed through the rest of the cycle (estrus, diestrus I, diestrus II, proestrus…) and subsequent cycles thereafter (Rivier and Vale 1990). Thus the treatment with the GnRH agonist does not induce persistent diestrus, and in our experience and that of others, the females continue to cycle regularly after this treatment. The advantage of this method is that females can be investigated at the same stage of the cycle, which provides a much more robust comparison with males by decreasing estrous status variability. Although males were not given the GnRH analog because they do not naturally experience high levels of GnRH, it is unlikely that synchronization of females significantly impacted hippocampal labeling by the various endogenous markers or accounts for observed sex differences, as injections occurred 11 days (almost 3 cycles) prior to perfusions. The day of synchronization was timed so that females were in diestrus on the day of tissue collection, which was confirmed by vaginal smears. Rats were deeply anesthetized with chloral hydrate (35%, 2 ml/kg, a drug that does not affect stress-related immediate early genes or peptides mRNA levels (C. Rivier unpublished results), and intracardially perfused with 4% paraformaldehyde/0.1 M borate buffer, pH 9.5. All perfusions were done between 0830-1130h. Brains were post-fixed for 4 h, submerged in 20% sucrose solution for 24-48 h at 4°C, snap frozen in Isopentane (2-Methylbutane, Sigma), and stored at -80°C until sectioning on a microtome into 30 μ coronal sections. Tissue sections were then stored at -20°C in a cryoprotectant solution (50% 0.1 M phosphate buffered saline, 30% ethylene glycol, and 20% glycerol) until immuohistochemistry (IHC). All protocols were approved by the Institutional Animal Care and Use Committee of The Salk Institute.
Immunohistochemistry
To investigate whether prenatal stress altered actively proliferating cells, adult generated neurons and cell death in the hippocampal SGZ, left and right hemisphere of every eighteenth coronal section through the rat brain was slide mounted and dried overnight prior to IHC. Slides were coded prior to IHC and the code was not broken until after analysis was complete. All IHC incubations were performed at room temperature unless otherwise indicated. Slide mounted sections were subjected to three pretreatment steps as described previously (Mandyam et al. 2004). Slides were incubated with 0.3% H2O2 for 30 min to remove endogenous peroxidase activity. Non-specific binding was blocked with 5% serum and 0.5% Triton-X in 1XPBS for 30 min. Sections were then incubated with primary antibody (in 5% serum and 0.5% Tween-20) for 18-20 h. For single labeling colorimetric IHC (Ki-67, DCX, AC-3, and IL-1β), sections were then incubated with biotinylated secondary IgG for 1 h (Vector Laboratories, Burlingame, CA; 1:200) and incubated in ABC for 1 h (cat# PK-6100; Vector Laboratories, Burlingame, CA). Staining was visualized with DAB (cat# 34065; Pierce Laboratories, Rockford, IL) and sections were counterstained with Nuclear FastRed (Vector Laboratories, Burlingame, CA). For double labeling IHC (Ki-67/DCX), in lieu of DAB staining was visualized via tyramide signal amplification (cat# SAT704A; PerkinElmer Life Sciences, Boston, MA) and sections were counterstained with DAPI. Omission or dilution of primary antibody resulted in lack of specific staining, thus serving as a negative control for antibody experiments.
The following primary antibodies were used for IHC. To examine proliferation, we used an antibody against Ki-67, an endogenous proliferation marker expressed in S, G2 and M phases of the cell cycle (Gerdes et al. 1984). Rabbit polyclonal anti-Ki-67 (cat# NCL-Ki-67p; Novocastra Laboratories, Norwell, MA; 1:500) was raised against prokaryotic recombinant fusion protein corresponding to a 1086bp Ki-67 motif-containing cDNA fragment. The pattern of Ki-67 staining was similar to that previously reported (Mandyam et al. 2007). To examine neurogenesis, we used an antibody against doublecortin (DCX; (Brown et al. 2003). Goat polyclonal anti-doublecortin (cat# sc-8066; Santa Cruz, Santa Cruz, CA; 1:700) was raised against an 18 amino acid peptide 384-401 of human doublecortin. This antiserum stains a single 40 kDa band on Western blot and the pattern of staining was similar to that previously reported with almost all immunoreactive cells in the SGZ (Brown et al. 2003). Two approaches were used to examine cell death: an antibody against cleaved caspase-3 (AC-3; (Cooper-Kuhn and Kuhn 2002) and the presence of pyknotic cells. Rabbit polyclonal anti-AC-3 (cat# 9661; Cell Signaling Technology, Beverly, MA; 1:500) prepared against a synthetic peptide recognizing amino acids 167-175 of human caspase-3 was used to quantify apoptosis. The antiserum recognizes bands in the 17-19 kDa range on Western blot, representing cleaved, but not full-length, caspase-3 (Olney et al. 2002). The pattern of AC-3 staining was similar to that previously reported, with most immunoreactive cells in the SGZ (Donovan et al. 2006). The presence and quantification of pyknotic cells was performed on sections counterstained with FastRed (Vector Laboratories, Burlingame, CA). Pyknotic cells were identified by their lack of a nuclear membrane, pale or lucent cytoplasm, and condensed, darkly stained, spherical chromatin visualized with nuclear FastRed staining (Harburg et al. 2007; Jortner 2006; Mandyam et al. in press). To examine stress-induced alterations in factors that influence cell death, we used a goat polyclonal antibody generated against IL-1β (cat# 500-P21BG; PeproTech Inc, Rocky Hill, NJ; 1:500) prepared against human IL-1β. The pattern of IL-1β staining was similar to that previously reported, with most immunoreactive cells in the hilus of the hippocampal dentate gyrus (Cunningham et al. 2005).
Microscopic analysis and quantification
To determine how prenatal stress alters proliferation, adult generated neurons and cell death, the number of cells in the SGZ immunoreactive (IR) for Ki-67, DCX, AC-3 and pyknotic cells was quantified via brightfield or epifluorescent microscopy using a Zeiss Axiophot photomicroscope. Immunoreactive cells from the left and right hemispheres of each bregma were quantified in the SGZ of the hippocampal dentate gyrus (bregma -1.8 to -6.3; (Paxinos and Watson 1997) as previously described (Eisch et al. 2000). We used the optical fractionator method in which every 18th section through the hippocampus was examined. The total number of IR cells in the SGZ was multiplied by 18 and are reported as total number of cells. Raw data for cell counts were subjected to statistical analysis.
DCX is a marker for young neurons, and the developmental stages of young neurons can be further delineated using morphological and co-labeling strategies. Specifically immature (early phase) DCX-IR cells can be differentiated from the mature (late phase) DCX-IR cell types with careful morphological analysis, immature cells having short processes and mature cells having long processes that extend into the molecular layer of the dentate gyrus (Fig 2d; (Brown et al. 2003; Couillard-Despres et al. 2005; Kempermann et al. 2003; Kuhn et al. 2005; Rao and Shetty 2004; Seri et al. 2004)). The immature DCX-IR cells can be further divided into two phenotypes: transiently amplifying (cycling) cells (“type I”) and non-cycling cells (“type II”) by the presence or absence, respectfully, of colabeling with Ki-67 (Jessberger et al. 2007; Jessberger et al. 2005). To determine the phenotype of DCX-IR cells, confocal analysis was performed on sections co-labeled with fluorescent tagged Ki-67 and DCX. Using a confocal microscope (BioRad LaserSharp 2000, version 5.2; emission wavelengths 488, 568, and 647), cells were scanned and optically sectioned in the Z plane as described previously (Mandyam et al. 2004). All analysis was done at a magnification of 1000X. For Ki-67/DCX analysis, every 27th section (3 coronal sections per rat) through the hippocampus was mounted and all DCX cells in the SGZ from each rat were subjected to confocal z scanning to determine colocalization of DCX and Ki-67. The total number of DCX-IR cells that were Ki-67-IR was subjected to statistical analysis. Fluorescently labeled confocal images presented here were taken from one 0.45 μm optical slice and imported into Photoshop (Adobe Systems) for composition purposes.
Figure 2.
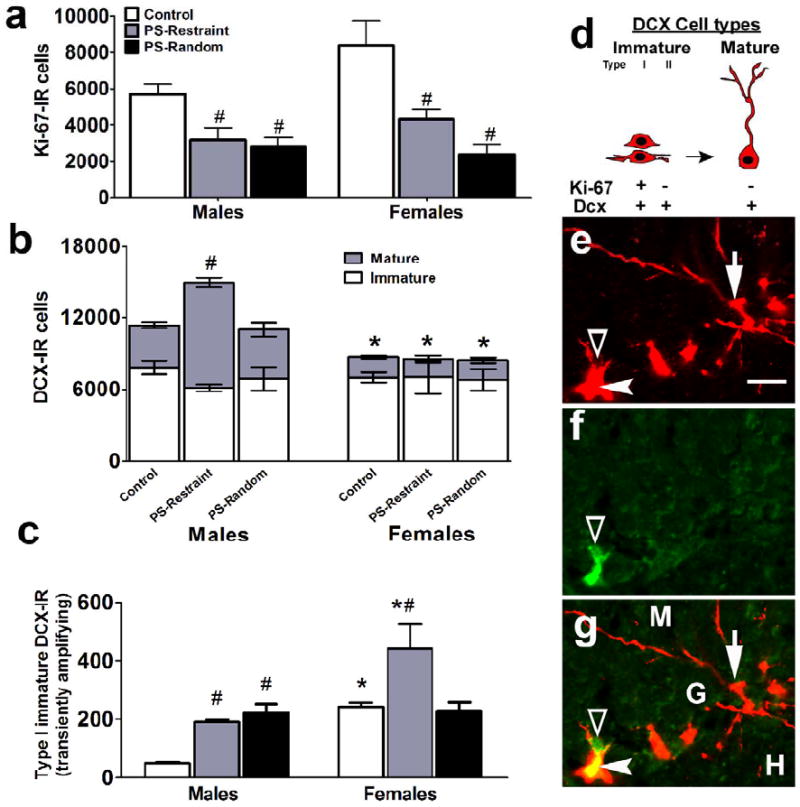
PS-restraint and -random alter the endogenous levels of actively proliferating cells (Ki-67-IR) and young neurons (DCX-IR). (a) Quantitative analysis of prenatal stress (both PS-restraint and -random)-induced decreased the number of Ki-67-IR cells in the subgranular zone of the dentate gyrus. Data are indicated as mean ± SEM (n = 5 per treatment group and sex). (b-c) Prenatal restraint and random stress differentially influence the number of doublecortin (DCX)-IR neurons. (b) Quantitative analysis of immature and mature DCX-IR cells. (c) Quantitative analysis of number of immature DCX-IR cells that are Ki-67 positive (“transiently amplifying young neurons,”. (d) Schematic representation of DCX cell types. Open arrowhead (e, f, g) points to Ki-67-IR cells (green, FITC), closed arrowhead (e, g) points to immature DCX-IR cells (red, CY3) that are along the SGZ with short processes. Some immature cells are Ki-67 positive (indicated as type I in (d)) most others are Ki-67 negative (indicated as type II in (d)). Arrow (e, g) points to a mature DCX-IR cell in the SGZ, these cells are Ki-67 negative. (e-g) Single Z scan (0.5um) of a confocal Z stack; Ki-67 in green (FITC) and DCX in red (CY3). Open arrowhead in (g) points to a Ki-67+/DCX- cell, closed arrowhead points to a Ki-67+/immature DCX+ (“transiently amplifying neuroblasts”) cell, short arrow points to a Ki-67-/mature DCX+ cell. Scale bar in (e) = 10 um, applies (e-g.) *sex difference compared to males, Ps<.05; #prenatal stress difference compared to control group of same sex; Ps<.01. Data are shown as mean ± SEM (n = 5 per treatment group and sex).
IL-1β densitometry analysis
For analysis of IL-1β densitometry, every 27th section (3 coronal sections per rat) through the hippocampus containing both the left and right hippocampal DG regions (including the molecular layer, granule cell layer and the hilus) were captured at 200X magnification with a Zeiss Axiophot photomicroscope fitted with a Zeiss ZVS video camera. Captured sections were sorted by bregma according to Paxinos and Watson (Paxinos and Watson 1997). The hilus was analyzed to determine qualitative changes in IL-1β IR by NIH Scion Image 4.03 Software. Density slice function was first applied to the captured image such that IL-1β IR cells and fibers were highlighted, mean density values were then obtained from the hilus and background region (molecular layer), and background levels were subtracted out to achieve a final density of IL-1β IR expressed as pixels/μm2.
Stereological assessment of hippocampal, dentate gyrus, and granule cell volume
Sections analyzed for Ki-67 IR were then analyzed to assess volume of the hippocampus, dentate gyrus and granule cell layer. Volumes were estimated based on surface area measurements made from 7-8 coronal brain sections per rat. Sections were coded so the experimenter was blind to the group of the animal until completion of analyses. All measurements were obtained using StereoInvestigator software (MicroBrightField Inc., Williston, VT) with a Zeiss Axiophot photomicroscope. Cell proliferation and neurogenesis were not quantitatively different between hemispheres in control and treatment groups. Therefore, unilateral (left hemisphere) volume estimates were measured according to the Cavalieri principle (West and Gundersen 1990; West et al. 1991). All contours were drawn at X50 magnification and a grid spacing of 150 μm was used to determine the X and Y spacing between neighboring points in the array. A randomized rotation was used for superimposing the grid on the contours to perform volume estimates according to the Cavalieri principle. In each section the following three structures were outlined: the hippocampus (including the CA regions and the dentate gyrus), the dentate gyrus (including the molecular layer, granule cell layer and the hilus), or just the granule cell layer. The number of sampling sites ranged from 25 to 280 (bregma -1.8 to -6.3) in the dentate gyrus and granule cell layer and 40 to 600 (bregma-1.8 to -6.3) in the hippocampus. Mounted section thickness of 18 μm (cut section thickness of 30 μm) was used for all three groups, and every 18th section (7-8 coronal sections per rat) was used for the analysis.
Measurement of hippocampal dentate gyrus granule cell number
Quantitative analysis to obtain unbiased estimates of the total number of granule cells was performed on a Zeiss Axiophot Microscope equipped with MicroBrightField Stereo Investigator software, (MicroBrightField Inc, Colchester Vermont), a 3 axis Mac 5000 motorized stage (Ludl Electronics Products Ltd Hawthorne NY), a digital CCCD ZVS video camera (Zeiss Inc, New York), PCI color frame grabber and PC workstation. Every 18th section (cut section thickness 30 μm; measured mounted section thickness 18 μm) through the dentate gyrus of the hippocampus counter stained with Nuclear FastRed were saved in strict anatomical order for quantitative analysis. Systematic random sampling of the dentate granule cell layer consisted of a 1/18 section analysis and 7-8 sections were analyzed for each rat. Live video images were used to draw contours delineating the granule cell layer. All contours were drawn at low magnification (Zeiss Plan-Apochromat X100, N/A 0.32) and the contours were realigned at high magnification X630 oil objective N/A 1.4. Following determination of mounted section thickness, Z plane values and selection of contours, an optical fractionator analysis was used to determine unilateral estimates (left hemisphere) of granule cell neuron number per granule cell layer of each dentate. A counting frame of appropriate dimensions, denoting forbidden and non-forbidden boundaries, was superimposed on the video monitor, and the optical fractionator analysis was performed at X630 and a 1.4 auxillary condenser lens. Cells were identified as granule cell neurons based on standard morphology and only neurons with a focused nucleus within the non-forbidden regions of the counting frame were counted.
The total number of granule cells was calculated by multiplying the average density of the granule cells and total volume of the granule cell layer of the hippocampal dentate gyrus (Donovan et al. 2006). Volume was estimated on tissue counter stained with FastRed using StereoInvestigator software that employs the Cavalieri method (West and Andersen 1980; West et al. 1991). The average density of granule cells was determined by examining 7-8 sections from every rat, from rostral to caudal portions of the granule cell layer. Over 400 cells (X630) were counted at a 10- X 10- X 2-μm counting grid, and a 2-μm top and bottom guard zone. The total number of cells were determined by multiplying the average density of cells (cells/μm3) by the total volume of the granule cell layer (West et al. 1991). Granule cell layer volume and cell number estimates were made by an observer blind to the study.
Data analysis and image presentation
Ki-67, DCX, AC-3, pyknotic, and IL-1β data were analyzed using between-subjects 2 (Sex) X 3 (Prenatal Treatment) two-way ANOVAs, followed by Bonferroni post-hoc tests. Bonferroni correction for multiple pairwise comparisons is generally used for a small number of comparisons (5 or fewer is recommended (Altman 1991)). The correction is appropriate for the present analyses, as alpha levels were adjusted only for the comparisons made (no more than 3). For clarity purposes, sex differences in control animals are first presented separately as bar graphs in Fig 1, even though statistical analyses included all groups as described above. Unless otherwise stated, differences were considered significant when P<.05. Images presented here were collected on a Zeiss Axiophot photomicroscope with a Zeiss ZVS video camera and imported into Photoshop (version, CS2, Adobe, Carlsbad CA). Only the gamma adjustment in the Levels function was used.
Figure 1.
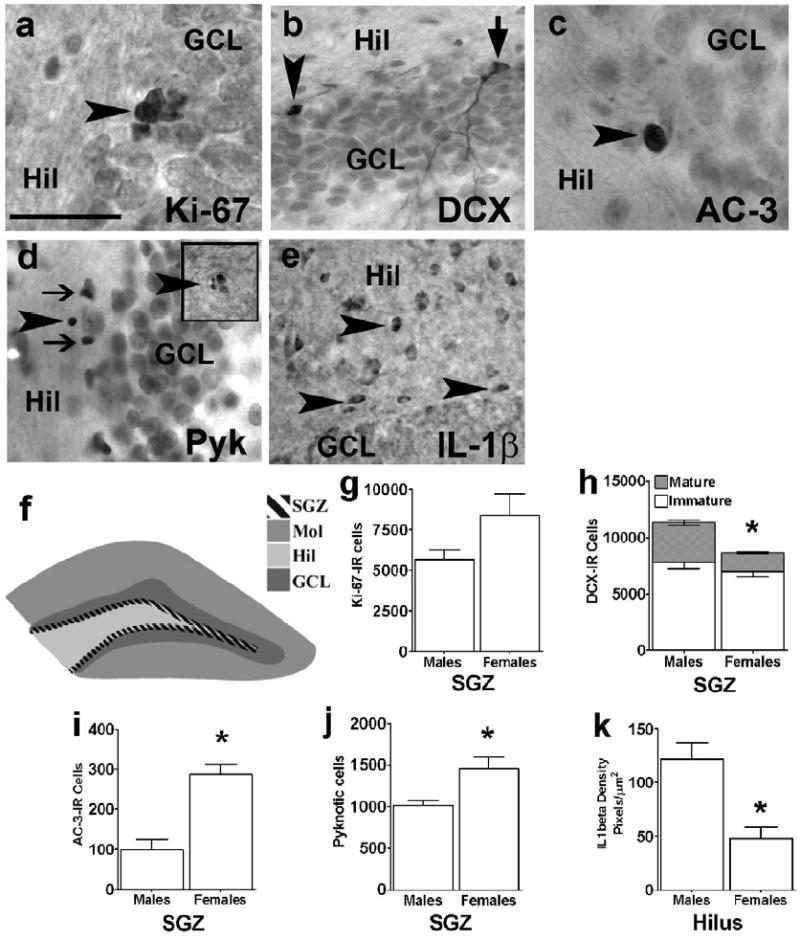
Sex differences in cell proliferation, immature neurons, immature neuron types, cell death, and hippocampal microenvironment under control conditions. Photomicrographs of (a) Ki-67, (b) DCX, (c) apoptotic (AC-3), (d) pyknotic cell (Pyk; main panel: arrowhead points to a pyknotic cell with single blob of chromatin, inset: arrowhead points to a pyknotic cell with many blobs of chromatin; arrow in main panel points to dark neurons that were not included as pyknotic cells), (e) IL-1β – labeling in the dentate gyrus of a control male rat. Arrowhead in each panel points to an immunoreactive cell. Ki-67-IR cells are seen as clusters of cells. Two types of DCX-IR are observed: arrowhead in (b) points to an immature DCX-IR cell, and arrow in (b) points to mature DCX-IR cell. Scale bar in (a) = 50 μm, applies to (a-d); = 100 μm, applies to (e). (f) Schematic of bregma -3.8 (Paxinos and Watson 1997) depicting the subgranular zone (SGZ), hilus (Hil), granule cell layer (GCL) and molecular layer (Mol) used for analysis (see Methods). (g-k) Quantitative analysis of male and female differences in (g) cell birth via Ki67-IR cells counts, (h) immature neurons via DCX-IR cell counts, (i, j) cell death via AC-3-IR or pyknotic cell counts, and (k) hippocampal microenvironment via densitometry of IL-1β. *sex difference, as indicated by post-hoc pairwise comparisons between control males and females, Ps<.05. Data are shown as mean ± SEM (n = 5 in each group).
Results
Sex differences in hippocampal neurogenesis, cell death, and microenvironment
The first objective was to determine whether hippocampal neurogenic capacity and microenvironment differed in males and diestrous females under control conditions. Data from all groups were first analyzed by two-way (Sex X Prenatal Treatment) ANOVAs, followed by post-hoc pairwise comparisons between control males and females. Graphs specifically from control animals are presented in Fig 1 to focus on sex differences in untreated animals.
The proliferating population was evaluated by counting Ki-67-IR cells (Fig 1a), an endogenous marker specific for precursors actively cycling in the cell division cycle (Bacchi and Gown 1993; Endl et al. 2001; Kee et al. 2002; Peissner et al. 1999). The number of Ki-67-IR cells was not significantly different in males and females, though there was a trend towards an increase in females (Fig 1g; effect of Sex, F(1,23)=3.2, P=0.08, post-hoc pairwise comparison between control males and females, P=0.14).
The number of young neurons was evaluated by counting DCX-IR cells, an endogenous marker for cell differentiation and neurogenesis (Brown et al. 2003; Rao and Shetty 2004). Consistent with previous studies (Couillard-Despres et al. 2005; Kuhn et al. 2005), morphological analysis of DCX-IR cells revealed two distinct types of cells: 1) DCX-IR cell bodies without processes or with very short processes extending parallel to the granule cell layer (Fig 1b and Fig 2e-g, arrowhead); and 2) DCX-IR cell bodies with elongated processes extending perpendicular from the granule cell layer into the molecular layer (Figs 1b, 2e, and 2g arrows). The distinction between these two morphological cell types is thought to reflect immature (early phase) and mature (late phase) young neurons, respectively. The immature population of DCX-IR cells consists of both transiently amplifying cells (co-localize with Ki-67 (type I, (Jessberger et al. 2007; Jessberger et al. 2005)) and cells that are no longer dividing (do not co-localize Ki-67) but have not yet developed processes (type II). The older, more mature DCX-IR cells have processes and proportion of these cells co-label with the mature neuronal marker NeuN (Brown et al. 2003).
The first analysis indicated that the total number (mature + immature) DCX-IR cells was greater in males compared to females (Figs 1h and 2b, total DCX-IR cells, males: 11331 ± 835, females: 8676 ± 532; effect of Sex, F(1,24)=17.2, P<0.01). Morphological analyses indicated that the sex difference in total DCX-IR is coming from a greater number of mature young neurons in males (Figs 1h and 2b, males: 3519 ± 259, females: 1701 ± 104; effect of Sex, F(1,24)=188.5, P<0.01, post-hoc pairwise comparison between control males and females, P<0.01, following a Sex X Prenatal Treatment interaction, F(2,24)=37.5, P<0.01). Phenotypic analyses were next used to estimate sex differences on transiently amplifying immature DCX-IR cell types (i.e., DCX/Ki67-IR or type I cells, Figs 1b and 2d). Females had a higher number of type I transiently amplifying DCX-IR cells compared to males (Fig 2c control; effect of Sex, F(1,24)=21.4, P<0.01, post-hoc pairwise comparison between control males and females, P<0.01, following a Sex X Prenatal Treatment interaction, F(2,24)=5.2, P<0.01), indicating a sex difference in the time frame of SGZ precursors residing in the cell cycle.
Cell death in the hippocampal SGZ was evaluated by two approaches: pyknotic morphology (Fig 1d), which reflects earlier and later stages of the apoptotic cascade, (Faherty et al. 1999) and apoptosis (AC-3 immunoreactivity; Fig 1c). While pyknotic cells were identified by their lack of a nuclear membrane, pale or lucent cytoplasm, and condensed, darkly stained, spherical chromatin visualized with nuclear FastRed staining (Harburg et al. 2007; Jortner 2006; Mandyam et al. in press), apoptotic AC-3-IR cells were easily detected by the dense small circle of immunoreactive staining (Donovan et al. 2006; Harburg et al. 2007). Cell death in the hippocampus SGZ differed with sex. Pyknotic cell counts were significantly higher in females (Figs 1j and 3a, post-hoc pairwise comparison between control males and females, P<0.01, following a Sex X Prenatal Treatment interaction, F(2,20)=17.4, P<0.0001). Apoptotic cell counts were also higher in females (Figs 1i and 3b, effect of sex on apoptosis: F(1,24)=11.8, P=0.002; post-hoc pairwise comparison between control males and females, P<0.01), suggesting an upsurge in cell death by apoptotic pathways in the SGZ of females.
Figure 3.
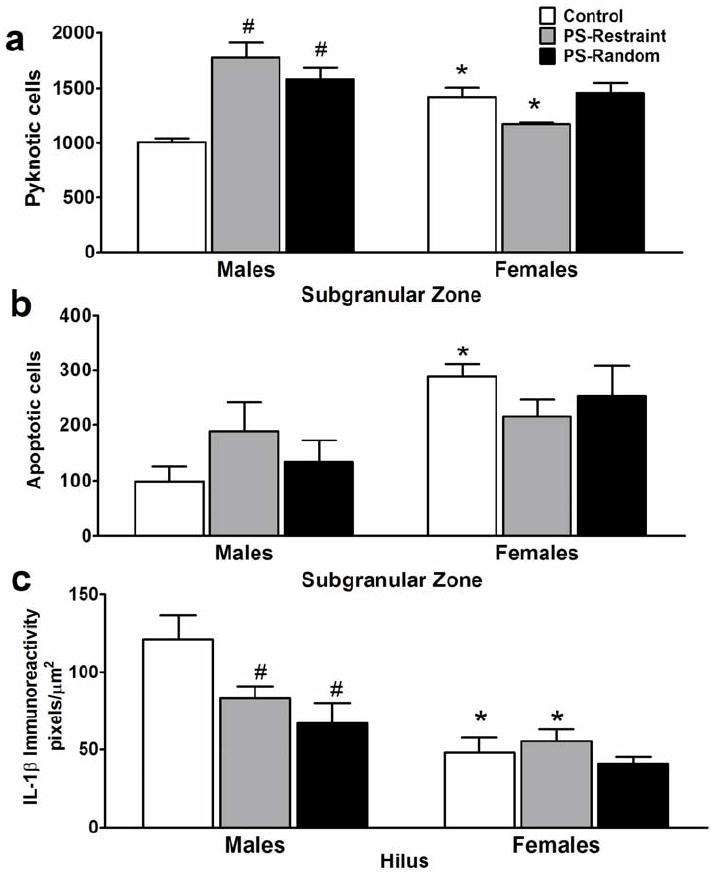
Prenatal stress alters cell death and hippocampal microenvironment in adult male offspring. Quantitative analysis of the number of pyknotic cells (a), and apoptotic (AC-3-IR) cells (b) in the subgranular zone of the dentate gyrus. (c) Densitometry analysis of IL-1β immunoreactivity. *sex difference compared to males, Ps<.05; #prenatal stress difference compared to control group of same sex; Ps<.01. Data are shown as mean ± SEM (n = 5 per treatment group and sex).
In vivo and in vitro evidence suggests that glucocorticoids act to stimulate apoptosis in part by decreasing levels of IL-1β, a cytokine with anti-apoptotic actions (Almeida et al. 2000; Crochemore et al. 2002; Hassan et al. 1996; Schmidt et al. 1999; Wang and Garabedian 2003). Thus, IL-1β density in the hippocampal dentate gyrus was next examined. IL-1β-IR was mostly seen in the hilar region of the hippocampus, with immunoreactive cells evenly distributed throughout the hilus and a few seen in the SGZ (Fig 1e). IL-1β density in the hilus was significantly lower in females compare to males (Figs 1k and 3c; effect of Sex (F(1,41)=31.9, P<0.0001; post-hoc pairwise comparison between control males and females, P<0.01, following a Sex X Prenatal Treatment interaction, F(2,41)=3.3, P=0.04). This finding in conjunction with the fact that females have higher basal and stress-induced corticosterone levels and greater hippocampal GR-IR density in the granule cell neurons of the hippocampal DG compared to males (Richardson et al. 2006), makes a strong argument for decreased IL-1β as one mechanism for increased apoptosis in females.
We also estimated volume of the hippocampus, dentate gyrus, and granule cell layer, along with the number of granule cells in the granule cell layer (GCL) of the dentate gyrus to address the possibility that greater cell death in females may result in decreased volume or cell number. No sex differences in volume or granule cells (Table 1) were detected suggesting that an upsurge in hippocampal cell death in females does not yield measurable changes in hippocampal structure.
Table 1.
The effects of sex and prenatal stress on hippocampal volume and granule cell neurons.
| Analysis | Males | Females | ||||
|---|---|---|---|---|---|---|
| Control | PS-Restraint | PS-Random | Control | PS-Restraint | PS-Random | |
| Volume mm3 (Hippocampus) | 28.1 ± 1.0 | 29.1 ± 1.8 | 22.3 ± 0.9# | 26.3 ± 0.6 | 28.3 ± 3.7 | 25.6 ± 0.8# |
| Volume mm3 (Dentate Gyrus) | 8.7 ± 0.7 | 10.7 ± 1.4 | 6.8 ± 0.4# | 8.6 ± 0.9 | 9.9 ± 0.7 | 7.5 ± 0.6# |
| Volume mm3 (GCL) | 1.1 ± 0.4 | 1.4 ± 0.16 | 1.2 ± 0.07 | 2.2 ± 0.43 | 1.6 ± 0.28 | 1.6 ± 0.05 |
| Granule cell neurons per GCL (×106) | 0.9 ± 0.03 | 1.0 ± 0.14 | 1.1 ± 0.06 | 1.0 ± 0.07 | 1.0 ± 0.02 | 1.1 ± 0.06 |
PS-random decreased hippocampal and dentate gyrus volume (Ps<.05, main effects of Prenatal Treatment, no indicators). No other measures were significantly altered by stress or sex. Data are indicated as mean ± SEM (n = 5 per treatment group and sex).
Prenatal stress decreases cell proliferation in the hippocampal SGZ
The next goal of the study was to determine the impact of prenatal stress on actively proliferating cells in the adult male and female hippocampal SGZ, a process regulated by corticosterone levels (Gould et al. 1992; Rodriguez et al. 1998). Both PS-restraint and -random significantly decreased Ki-67-IR cell counts in males and females (Fig 2a, effect of prenatal treatment, F(2,23)=16.96, P<0.01). There was no interaction between Prenatal Treatment and Sex on proliferating population of cells in the SGZ.
Prenatal stress alters DCX-IR cells in the hippocampal SGZ
Prenatal stress did not alter the number of total (immature + mature type) DCX-IR cells, but significantly affected mature and immature type DCX-IR cells in a sex- and stress paradigm-specific manner. Prenatal exposure to repeated restraint stress significantly increased the number of mature DCX-IR cells in the hippocampal SGZ only in males (Fig 2b, post-hoc pairwise comparisons between PS-restraint and control males, P<.0.01 following a significant Sex and Prenatal Treatment interaction, F(2,24)=37.5, P<0.01). Furthermore, this same prenatal treatment increased immature DCX-IR cells that were transiently amplifying (DCX+/Ki-67+ or type I immature DCX-IR cells) in both males and females (Fig 2c, post-hoc pairwise comparisons between PS-restraint animals and same sex controls, Ps<.05, following a significant Sex X Prenatal Treatment interaction, F(2,24)=5.2, P<0.01). PS-random treatment also elevated the number of DCX-IR cells that were transiently amplifying, but only in males (Fig 2c, P<0.05), thereby eliminating the sex difference in this measure. Thus, prenatal stress had highly specific effects on differentiating cells depending on the type of prenatal stress and the sex of the offspring.
Prenatal stress alters cell death in the hippocampal SGZ
There was a main effect of Prenatal Treatment (F(2,20)=7.67, P=0.003) and a Sex X Prenatal Treatment interaction (F(2,20)=17.4, P<0.0001) on the number of pyknotic cells in the SGZ (Fig 3a, b). Post-hoc analyses indicated that prenatal stress increased pyknotic cells only in males (Ps<0.001 compared to control males). PS-random eliminated the sex difference in cell death, primarily due to increases in males (Fig 3a, b). PS-restraint resulted in such a robust increase in pyknotic cells in males that the sex difference was reversed (males > females, P=0.005, Fig 3a, b). Prenatal stress elicited no measurable change in pyknotic cells in females. Although there was not significant effect of Prenatal Treatment on apoptosis, the pattern of change in AC-3 cells was similar to that of pyknotic cells (increase in males exposed to prenatal stress). Consistent with observation, there was only a significant sex difference in control animals (post-hoc pairwise comparisons between males and female: control animals, P<0.01, PS-restraint and –random animals, Ps>.05).
Prenatal stress alters hippocampal microenvironment
Prenatal stress significantly reduced hilar IL-1β-IR densitometry in males (F(2,41)=4.9, P=0.01), with a significant interaction between Sex and Prenatal Treatment on IL-1β immunoreactivity (F(2,41)=3.3, P=0.04). Post-hoc analyses indicated that IL-1β was significantly reduced in PS-restraint (P<0.05) and PS-random (P<0.001) males compared to controls, eliminating the sex difference observed in controls. Prenatal stress had no measurable effect on hilar IL-1β-IR densitometry in females.
Prenatal stress alters hippocampal and dentate gyrus volume
Because actively proliferating cells were decreased by PS-restraint and -random in both males and females (Fig 2a), and cell death was increased in male PS-restraint and -random groups (Fig 3a), we evaluated whether these changes were associated with decreased hippocampal, dentate gyrus or granule cell layer volume (Table 1). While PS-restraint had no effect on these hippocampal measure, PS-random decreased both hippocampal (Table 1, effect of prenatal treatment, F(2,24)=3.3, P=0.05) and dentate gyrus volume (Table 1, effect of prenatal treatment, F(2,24)=6.3, P=0.006). Thus, exposure to varied stress elicits long-term changes in hippocampal structure.
Discussion
The current report demonstrates that under normal developmental conditions, male and diestrous female adult rats have comparable levels of SGZ proliferation and hippocampal, dentate gyrus and granule cell layer volumes, but males have a higher level of neurogenesis and a lower level of cell death. Endogenous markers for proliferation and neurogenesis identified possible mechanisms underlying sex differences in hippocampal neurogenic capacity. Differential microenvironment, cytokine-cell death pathways, and kinetics of proliferating and differentiating cells may all contribute to sex differences in hippocampal neurogenesis. We also provide evidence for high sensitivity of the developing hippocampus to early stress, dependent on both the sex of the offspring and type of stress experienced during prenatal development. Prenatal stress (PS-restraint and –random) significantly impacted the hippocampus, with both stress paradigms causing robust decreases in proliferation in males and females. Several other hippocampal measures were feminized specifically in males, reducing or abolishing some sex differences. The findings augment previously reported morphometric and physiological differences in the adult male and female rat hippocampus (Bethus et al. 2005; Bowman et al. 2004; Falconer and Galea 2003; Franklin and Perrot-Sinal 2006; Galea et al. 1996; Krasnoff and Weston 1976; Schmitz et al. 2002; Westenbroek et al. 2004). The data may also help explain differential effects of stress on hippocampal-dependent behavior and neuroendocrine function in males and females (Li et al. 2006; Richardson et al. 2006; Roussel et al. 2005; Zhu et al. 2004).
Gonadal steroids influence hippocampal plasticity and may contribute to sex differences observed in the present study (for recent reviews see (Galea 2007; Hajszan et al. 2007)). Sex differences in SGZ proliferation are robust when female rodents in the proestrous phase of the estrous cycle (high estrogen; (Falconer and Galea 2003; Tanapat et al. 1999)). Proestrous females have higher proliferation (2hr survival after injection of exogenous marker BrdU; (Tanapat et al. 1999)), though the increase in proliferation does not extend to increases in migration and differentiation of SGZ neural precursors (24hr-14d survival after injection of exogenous marker BrdU; (Falconer and Galea 2003; Tanapat et al. 1999). Somewhat consistent with the earlier findings, the current study shows that proliferation is not significantly higher (albeit a trend) in females on diestrus, when estrogen levels are low. Furthermore, diestrous females in the present study also had elevated pyknotic cell counts compared to males, consistent with a trend observed in an earlier report (Tanapat et al. 1999). Proestrous females, on the other hand, have lower cell death than males (Falconer and Galea 2003; Tanapat et al. 1999). Thus, estrogen appears to both enhances proliferation and protects against cell death ((Falconer and Galea 2003; Tanapat et al. 1999), and present report). The findings altogether demonstrate the proliferative environment fluctuates in females depending on estrous status, which can have considerable influence on the extent to which sex differences are observed in hippocampal plasticity, structure, and function.
Stress hormones may also contribute significantly to sex differences in hippocampal plasticity either directly or indirectly through regulation of the local environment (Li et al. 2005). The hippocampal microenvironment may be reshaped by chronically elevated HPA axis activity and partly responsible for both the sex difference in cell death (higher in females). For example, cytokines such as IL-1β can enhance the hippocampal proliferative environment and conversely elevated corticosterone activates hippocampal GR to induce cell death (Almeida et al. 2000; Crochemore et al. 2002; Hassan et al. 1996; Wang and Garabedian 2003), thereby decreasing the proliferative capacity of the hippocampus. Furthermore, in vitro studies evidence indicates that the two pathways are interlinked (Monje et al. 2002; Schmidt et al. 1999). Low to moderate levels of acute or chronic glucocorticoid exposure are thought to be anti-inflammatory by decreasing IL-1β levels (MacPherson et al. 2005), resulting in decreased proliferation and increased apoptosis. The present data suggest this pathway contributes to higher cell death in females. Males have higher IL-1β, supporting lower levels of cell death and increased neurogenic environment in the male SGZ compared to females.
Sex differences in hippocampal neurogenesis may be partially attributable to differential precursor kinetics. Morphological analysis and phenotype of DCX-IR cells show that males have more DCX-IR cells that reached maturity compared to diestrous females, but females had more transiently amplifying neuroblasts (Ki-67-DCX double labeled cells) compared to males. Expression patterns of Ki-67 protein or kinetics of SGZ precursors signify delayed exit from the cell cycle in females, potentially interfering with migration and differentiation into a mature neuron and/or making the cells vulnerable to cell death. Either outcome could lead to decreased neurogenesis in females.
Prenatal stress significantly impacted a number of hippocampal measures, eliminating some sex differences primarily by feminizing actions in males. Precursor kinetics, IL-1β density, and cell death were all feminized in males by one or both prenatal stress paradigms. Greater sensitivity of the hippocampus to prenatal stress in males differs from what is observed in stress-related neuroendocrine and behavioral responses, where prenatal stress elicits the most robust changes in females (McCormick et al. 1995; Palanza 2001; Richardson et al. 2006; Weinstock et al. 1992). The present data illustrate the critical influence early environment has on establishing sex differences in certain neural structures provides insight into possible mechanisms for the changes observed in males. During perinatal development, gonadal hormones have organizational effects on the developing brain that lead to sexual dimorphisms in neurochemistry, synaptic connections, and behavior in adulthood (for a recent review, see (Weinstock 2007)). On gestational days 18-19 (the last third of pregnancy in the rodent), a testosterone surge occurs in males that is critical for masculinization of sexually dimorphic neural circuitry and behavior (McEwen et al. 1977; Ward and Weisz 1980; Ward and Weisz 1984). Prenatal restraint stress in the last week of pregnancy is known to suppress the testosterone surge during prenatal development (Ward and Weisz 1980; Ward and Weisz 1984) and in the present study both PS-restraint and -random reduced adult levels of testosterone (Richardson et al. 2006). Testosterone is known to enhance hippocampal neurogenesis (Galea et al. 2006). Therefore, reduced organizational (prenatal) and/or activational (adulthood) actions of testosterone may have lead to the feminization of certain hippocampal measures in males in the present study, eliminating some sex differences. Feminization of differentiation processes might translate into changes in hippocampal function via PS-induced feminization of hippocampus-dependent behavior in males (Bowman et al. 2004).
Prenatal stress reduced proliferation of SGZ precursors (Ki-67) in both males and females, similar to what has been shown in males following restraint stress experienced in utero (Coe et al. 2003; Fujioka et al. 2006; Lemaire et al. 2000). Stress hormones could contribute significantly to altering proliferation capacity either by action early in development or in adulthood. It is unlikely, however, that stress hormones in adulthood underlie the prenatal stress effects observed here. Activity of the HPA axis (under basal and stressed conditions) differs greatly in males and females and in response to the different prenatal treatments (Richardson et al. 2006), but the pattern of change in proliferation does not reflect this (present report). Instead, exposure to elevated glucocorticoid levels during prenatal development more likely contributed to reduced proliferation in adulthood. Gestational stress can affect developing pups despite enhanced protection against maternal glucocorticoids by the enzyme β-hydroxysteroid dehydrogenase (Holmes et al. 2006; Welberg et al. 2000). In rodents, corticosterone levels are elevated in maternal and fetal blood several hours following restraint stress (Montano et al. 1991). In addition, a small but significant number of actively dividing SGZ precursors express GR (Garcia et al. 2004), and high concentrations of corticosterone can decrease proliferation in a glucocorticoid receptor-dependent manner in prenatal day 16 hippocampal cultures and neonatal rat brain (Van den Hove et al. 2006; Yu et al. 2004). Thus, exposure to elevated stress hormones early in development may cause long-term changes in proliferative environment, resulting in reduced neurogenic capacity in the hippocampus in adulthood.
The impact of prenatal treatment on neurogenesis (DCX-IR cells) was multifaceted. PS-restraint resulted in increased DCX-IR cells that reached maturity in the SGZ in males. While increased DCX-IR cell number indicates increased neurogenesis, this does not necessarily result in more granule cell neurons. In fact, an earlier report shows that survival of BrdU labeled cells is not increased, but rather decreased, following a similar treatment (Lemaire et al. 2000). Furthermore, in the present report granule cell numbers were not higher in PS-restraint males despite having higher DCX-IR cell numbers. Thus, instead of simply altering the number of neurons in the granule cell layer, prenatal stress may be eliciting more specific changes in the differentiating cell population. For example, prenatal stress specifically impacted the number of immature DCX-IR cells that were transiently amplifying in the present study. Both PS-restraint and -random had feminizing effects in males, prolonging the amount of time immature DCX-IR cells spend in active division. Transiently amplifying DCX-IR cell number was also increased by prenatal stress in females, but only with PS-restraint. Thus, stress experienced in utero may delay or prevent hippocampal neuroblasts exiting from the cell cycle in a timely fashion, which could result in a number of different outcomes such as alteration of the maturation process, vulnerability to cell death, or synaptic integration and plasticity within the hippocampus.
The relationship between hippocampal cell death and stress is complex (Heine et al. 2004; Lucassen et al. 2006; Montaron et al. 2003). Both prenatal stress treatments increased pyknosis in the male SGZ, but caused no measurable changes in females. IL-1β was also reduced in the two prenatal stress male groups, which may have contributed to increased cell death. However, this should be reflected in a higher number of AC-3 IR cells (increased apoptosis), which was not the case. This suggests perturbation in the GR- IL-1β-apoptotic pathway (evidenced by an inverse correlation between AC-3 IR cell counts and IL-1β IR in control animals but not prenatal stress groups, data not shown). Thus, elevated cell death is likely driven by other mechanisms, such as reduced testosterone in adulthood (Galea et al. 2006; Richardson et al. 2006), as mentioned earlier.
Other environmental factors experienced throughout the lifespan could also contribute to altered hippocampal neurogenesis following prenatal stress. For example, maternal behavior can influence stress-related neural and behavioral systems in adult offspring, and adoption in rats has been shown to reverse some of the effects of prenatal stress (Maccari et al. 1995; Moore and Power 1986). Thus, while gross abnormalities in maternal behavior were not observed in dams, it is possible that subtle changes in maternal behavior resulting from stress during pregnancy may have also contributed to the changes in hippocampal proliferative capacity in adult offspring.
In summary, the data described herein provide possible mechanisms for sex differences in hippocampal plasticity and vulnerability of the hippocampus to early stress. Much remains unknown regarding the interplay between microenvironment, cell cycle kinetics, and neurogenesis. Future investigation of the vulnerability of the actively proliferating cells in the adult brain to stress-induced alterations in adrenal and gonadal hormones, cytokine levels, and gender-specificity of cellular events that maintain adult hippocampal microenvironment could serve useful enhancing our understanding of neural plasticity and behavior.
Acknowledgments
This work was supported by NIH grants MH51774 to Catherine L. Rivier and DA010072 to George F. Koob. The authors wish to thank Geneviève Dubé, Krisha Begalla, and Hanan Jammal for assistance with tissue processing, Ron Smith for assistance with the stereology volumetric analysis, Dr. Jean Rivier for the gift of [DTrp6,Pro9,Net] GnRH, Drs. Ronald Evans and Wylie Vale for the gift of glucocorticoid receptor antiserum, Drs. Caroline Lanigan and Eric Zorrilla for assistance with statistical analysis, Drs. George F. Koob and Olivier George for helpful comments on this manuscript, and Mike Arends for editorial assistance. This is publication number 18249 from The Scripps Research Institute.
References
- Almeida OF, Conde GL, Crochemore C, Demeneix BA, Fischer D, Hassan AH, Meyer M, Holsboer F, Michaelidis TM. Subtle shifts in the ratio between pro- and antiapoptotic molecules after activation of corticosteroid receptors decide neuronal fate. Faseb J. 2000;14(5):779–790. doi: 10.1096/fasebj.14.5.779. [DOI] [PubMed] [Google Scholar]
- Altman D. Practical statistics for medical research. Chapman and Hall; 1991. [Google Scholar]
- Bacchi CE, Gown AM. Detection of cell proliferation in tissue sections. Braz J Med Biol Res. 1993;26(7):677–687. [PubMed] [Google Scholar]
- Bethus I, Lemaire V, Lhomme M, Goodall G. Does prenatal stress affect latent inhibition? It depends on the gender. Behav Brain Res. 2005;158(2):331–338. doi: 10.1016/j.bbr.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Bowman RE, MacLusky NJ, Sarmiento Y, Frankfurt M, Gordon M, Luine VN. Sexually dimorphic effects of prenatal stress on cognition, hormonal responses, and central neurotransmitters. Endocrinology. 2004;145(8):3778–3787. doi: 10.1210/en.2003-1759. [DOI] [PubMed] [Google Scholar]
- Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467(1):1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- Coe CL, Kramer M, Czeh B, Gould E, Reeves AJ, Kirschbaum C, Fuchs E. Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile rhesus monkeys. Biol Psychiatry. 2003;54(10):1025–1034. doi: 10.1016/s0006-3223(03)00698-x. [DOI] [PubMed] [Google Scholar]
- Cooper-Kuhn CM, Kuhn HG. Is it all DNA repair? Methodological considerations for detecting neurogenesis in the adult brain. Brain Res Dev Brain Res. 2002;134(1-2):13–21. doi: 10.1016/s0165-3806(01)00243-7. [DOI] [PubMed] [Google Scholar]
- Couillard-Despres S, Winner B, Schaubeck S, Aigner R, Vroemen M, Weidner N, Bogdahn U, Winkler J, Kuhn HG, Aigner L. Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci. 2005;21(1):1–14. doi: 10.1111/j.1460-9568.2004.03813.x. [DOI] [PubMed] [Google Scholar]
- Crochemore C, Michaelidis TM, Fischer D, Loeffler JP, Almeida OF. Enhancement of p53 activity and inhibition of neural cell proliferation by glucocorticoid receptor activation. Faseb J. 2002;16(8):761–770. doi: 10.1096/fj.01-0577com. [DOI] [PubMed] [Google Scholar]
- Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci. 2005;25(40):9275–9284. doi: 10.1523/JNEUROSCI.2614-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan MH, Yazdani U, Norris RD, Games D, German DC, Eisch AJ. Decreased adult hippocampal neurogenesis in the PDAPP mouse model of Alzheimer’s disease. J Comp Neurol. 2006;495(1):70–83. doi: 10.1002/cne.20840. [DOI] [PubMed] [Google Scholar]
- Einon D. Spatial memory and response strategies in rats: age, sex and rearing differences in performance. Q J Exp Psychol. 1980;32(3):473–489. doi: 10.1080/14640748008401840. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Barrot M, Schad CA, Self DW, Nestler EJ. Opiates inhibit neurogenesis in the adult rat hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(13):7579–7584. doi: 10.1073/pnas.120552597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endl E, Kausch I, Baack M, Knippers R, Gerdes J, Scholzen T. The expression of Ki-67, MCM3, and p27 defines distinct subsets of proliferating, resting, and differentiated cells. Journal of Pathology. 2001;195(4):457–462. doi: 10.1002/path.978. [DOI] [PubMed] [Google Scholar]
- Faherty CJ, Xanthoudakis S, Smeyne RJ. Caspase-3-dependent neuronal death in the hippocampus following kainic acid treatment. Brain Res Mol Brain Res. 1999;70(1):159–163. doi: 10.1016/s0169-328x(99)00143-6. [DOI] [PubMed] [Google Scholar]
- Falconer EM, Galea LA. Sex differences in cell proliferation, cell death and defensive behavior following acute predator odor stress in adult rats. Brain Res. 2003;975(1-2):22–36. doi: 10.1016/s0006-8993(03)02542-3. [DOI] [PubMed] [Google Scholar]
- Franklin TB, Perrot-Sinal TS. Sex and ovarian steroids modulate brain-derived neurotrophic factor (BDNF) protein levels in rat hippocampus under stressful and non-stressful conditions. Psychoneuroendocrinology. 2006;31(1):38–48. doi: 10.1016/j.psyneuen.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Fujioka A, Fujioka T, Ishida Y, Maekawa T, Nakamura S. Differential effects of prenatal stress on the morphological maturation of hippocampal neurons. Neuroscience. 2006;141(2):907–915. doi: 10.1016/j.neuroscience.2006.04.046. [DOI] [PubMed] [Google Scholar]
- Galea LA. Gonadal hormone modulation of neurogenesis in the dentate gyrus of adult male and female rodents. Brain Res Rev. 2007 doi: 10.1016/j.brainresrev.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Galea LA, Kavaliers M, Ossenkopp KP. Sexually dimorphic spatial learning in meadow voles Microtus pennsylvanicus and deer mice Peromyscus maniculatus. J Exp Biol. 1996;199(Pt 1):195–200. doi: 10.1242/jeb.199.1.195. [DOI] [PubMed] [Google Scholar]
- Galea LA, McEwen BS. Sex and seasonal differences in the rate of cell proliferation in the dentate gyrus of adult wild meadow voles. Neuroscience. 1999;89(3):955–964. doi: 10.1016/s0306-4522(98)00345-5. [DOI] [PubMed] [Google Scholar]
- Galea LA, Spritzer MD, Barker JM, Pawluski JL. Gonadal hormone modulation of hippocampal neurogenesis in the adult. Hippocampus. 2006;16(3):225–232. doi: 10.1002/hipo.20154. [DOI] [PubMed] [Google Scholar]
- Garcia A, Steiner B, Kronenberg G, Bick-Sander A, Kempermann G. Age-dependent expression of glucocorticoid- and mineralocorticoid receptors on neural precursor cell populations in the adult murine hippocampus. Aging Cell. 2004;3(6):363–371. doi: 10.1111/j.1474-9728.2004.00130.x. [DOI] [PubMed] [Google Scholar]
- Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. Journal of Immunology. 1984;133(4):1710–1715. [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2(3):260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Gould E, Cameron HA, Daniels DC, Woolley CS, McEwen BS. Adrenal hormones suppress cell division in the adult rat dentate gyrus. J Neurosci. 1992;12(9):3642–3650. doi: 10.1523/JNEUROSCI.12-09-03642.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajszan T, Milner TA, Leranth C. Sex steroids and the dentate gyrus. Prog Brain Res. 2007;163:399–816. doi: 10.1016/S0079-6123(07)63023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harburg GC, Hall FS, Harrist AV, Sora I, Uhl GR, Eisch AJ. Knockout of the mu opioid receptor enhances the survival of adult-generated hippocampal granule cell neurons. Neuroscience. 2007;144(1):77–87. doi: 10.1016/j.neuroscience.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan AH, von Rosenstiel P, Patchev VK, Holsboer F, Almeida OF. Exacerbation of apoptosis in the dentate gyrus of the aged rat by dexamethasone and the protective role of corticosterone. Exp Neurol. 1996;140(1):43–52. doi: 10.1006/exnr.1996.0113. [DOI] [PubMed] [Google Scholar]
- Hastings NB, Gould E. Neurons inhibit neurogenesis. Nat Med. 2003;9(3):264–266. doi: 10.1038/nm0303-264. [DOI] [PubMed] [Google Scholar]
- Heine VM, Maslam S, Joels M, Lucassen PJ. Prominent decline of newborn cell proliferation, differentiation, and apoptosis in the aging dentate gyrus, in absence of an age-related hypothalamus-pituitary-adrenal axis activation. Neurobiol Aging. 2004;25(3):361–375. doi: 10.1016/S0197-4580(03)00090-3. [DOI] [PubMed] [Google Scholar]
- Holmes MC, Abrahamsen CT, French KL, Paterson JM, Mullins JJ, Seckl JR. The mother or the fetus? 11beta-hydroxysteroid dehydrogenase type 2 null mice provide evidence for direct fetal programming of behavior by endogenous glucocorticoids. J Neurosci. 2006;26(14):3840–3844. doi: 10.1523/JNEUROSCI.4464-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger S, Nakashima K, Clemenson GD, Jr, Mejia E, Mathews E, Ure K, Ogawa S, Sinton CM, Gage FH, Hsieh J. Epigenetic modulation of seizure-induced neurogenesis and cognitive decline. J Neurosci. 2007;27(22):5967–5975. doi: 10.1523/JNEUROSCI.0110-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger S, Romer B, Babu H, Kempermann G. Seizures induce proliferation and dispersion of doublecortin-positive hippocampal progenitor cells. Exp Neurol. 2005;196(2):342–351. doi: 10.1016/j.expneurol.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Jortner BS. The return of the dark neuron. A histological artifact complicating contemporary neurotoxicologic evaluation. Neurotoxicology. 2006;27(4):628–634. doi: 10.1016/j.neuro.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Kee N, Sivalingam S, Boonstra R, Wojtowicz JM. The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. J Neurosci Methods. 2002;115(1):97–105. doi: 10.1016/s0165-0270(02)00007-9. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gage FH. Experience-dependent regulation of adult hippocampal neurogenesis: effects of long-term stimulation and stimulus withdrawal. Hippocampus. 1999;9(3):321–332. doi: 10.1002/(SICI)1098-1063(1999)9:3<321::AID-HIPO11>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130(2):391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- Krasnoff A, Weston LM. Puberal status and sex differences: activity and maze behavior in rats. Dev Psychobiol. 1976;9(3):261–269. doi: 10.1002/dev.420090310. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Biebl M, Wilhelm D, Li M, Friedlander RM, Winkler J. Increased generation of granule cells in adult Bcl-2-overexpressing mice: a role for cell death during continued hippocampal neurogenesis. Eur J Neurosci. 2005;22(8):1907–1915. doi: 10.1111/j.1460-9568.2005.04377.x. [DOI] [PubMed] [Google Scholar]
- Lee SJ, McEwen BS. Neurotrophic and neuroprotective actions of estrogens and their therapeutic implications. Annu Rev Pharmacol Toxicol. 2001;41:569–591. doi: 10.1146/annurev.pharmtox.41.1.569. [DOI] [PubMed] [Google Scholar]
- Lemaire V, Koehl M, Le Moal M, Abrous DN. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci U S A. 2000;97(20):11032–11037. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire V, Lamarque S, Le Moal M, Piazza PV, Abrous DN. Postnatal stimulation of the pups counteracts prenatal stress-induced deficits in hippocampal neurogenesis. Biol Psychiatry. 2006;59(9):786–792. doi: 10.1016/j.biopsych.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Li H, Pin S, Zeng Z, Wang MM, Andreasson KA, McCullough LD. Sex differences in cell death. Ann Neurol. 2005;58(2):317–321. doi: 10.1002/ana.20538. [DOI] [PubMed] [Google Scholar]
- Li H, Zhu ZL, Jia N, Bai ZL, Cai Q, Chen R, Song TB, Liu JK. Gender-dependent difference of NF-kappaB expression in the hippocampus of prenatally stressed offspring rats. Sheng Li Xue Bao. 2006;58(6):577–583. [PubMed] [Google Scholar]
- Liu L, Li A, Matthews SG. Maternal glucocorticoid treatment programs HPA regulation in adult offspring: sex-specific effects. Am J Physiol Endocrinol Metab. 2001;280(5):E729–739. doi: 10.1152/ajpendo.2001.280.5.E729. [DOI] [PubMed] [Google Scholar]
- Lucassen PJ, Heine VM, Muller MB, van der Beek EM, Wiegant VM, De Kloet ER, Joels M, Fuchs E, Swaab DF, Czeh B. Stress, depression and hippocampal apoptosis. CNS Neurol Disord Drug Targets. 2006;5(5):531–546. doi: 10.2174/187152706778559273. [DOI] [PubMed] [Google Scholar]
- Maccari S, Piazza PV, Kabbaj M, Barbazanges A, Simon H, Le Moal M. Adoption reverses the long-term impairment in glucocorticoid feedback induced by prenatal stress. J Neurosci. 1995;15(1 Pt 1):110–116. doi: 10.1523/JNEUROSCI.15-01-00110.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson A, Dinkel K, Sapolsky R. Glucocorticoids worsen excitotoxin-induced expression of pro-inflammatory cytokines in hippocampal cultures. Exp Neurol. 2005;194(2):376–383. doi: 10.1016/j.expneurol.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Mandyam CD, Harburg GC, Eisch AJ. Determination of key aspects of precursor cell proliferation, cell cycle length and kinetics in the adult mouse subgranular zone. Neuroscience. 2007;146(1):108–122. doi: 10.1016/j.neuroscience.2006.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandyam CD, Norris RD, Eisch AJ. Chronic morphine induces premature mitosis of proliferating cells in the adult mouse subgranular zone. Journal of Neuroscience Research. 2004;76(6):783–794. doi: 10.1002/jnr.20090. [DOI] [PubMed] [Google Scholar]
- Mandyam CD, Wee S, Eisch AJ, Richardson HN, Koob GF. Methamphetamine self-administration and voluntary exercise have opposing effects on medial prefrontal cortex gliogenesis. J Neurosci. doi: 10.1523/JNEUROSCI.2505-07.2007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markakis EA, Palmer TD, Randolph-Moore L, Rakic P, Gage FH. Novel neuronal phenotypes from neural progenitor cells. J Neurosci. 2004;24(12):2886–2897. doi: 10.1523/JNEUROSCI.4161-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick CM, Smythe JW, Sharma S, Meaney MJ. Sex-specific effects of prenatal stress on hypothalamic-pituitary-adrenal responses to stress and brain glucocorticoid receptor density in adult rats. Brain Res Dev Brain Res. 1995;84(1):55–61. doi: 10.1016/0165-3806(94)00153-q. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Sex, stress and the hippocampus: allostasis, allostatic load and the aging process. Neurobiol Aging. 2002;23(5):921–939. doi: 10.1016/s0197-4580(02)00027-1. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Lieberburg I, Chaptal C, Krey LC. Aromatization: important for sexual differentiation of the neonatal rat brain. Horm Behav. 1977;9(3):249–263. doi: 10.1016/0018-506x(77)90060-5. [DOI] [PubMed] [Google Scholar]
- Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8(9):955–962. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- Montano MM, Wang MH, Even MD, vom Saal FS. Serum corticosterone in fetal mice: sex differences, circadian changes, and effect of maternal stress. Physiol Behav. 1991;50(2):323–329. doi: 10.1016/0031-9384(91)90073-w. [DOI] [PubMed] [Google Scholar]
- Montaron MF, Piazza PV, Aurousseau C, Urani A, Le Moal M, Abrous DN. Implication of corticosteroid receptors in the regulation of hippocampal structural plasticity. Eur J Neurosci. 2003;18(11):3105–3111. doi: 10.1111/j.1460-9568.2003.03048.x. [DOI] [PubMed] [Google Scholar]
- Moore CL, Power KL. Prenatal stress affects mother-infant interaction in Norway rats. Dev Psychobiol. 1986;19(3):235–245. doi: 10.1002/dev.420190309. [DOI] [PubMed] [Google Scholar]
- Olney JW, Tenkova T, Dikranian K, Muglia LJ, Jermakowicz WJ, D’Sa C, Roth KA. Ethanol-induced caspase-3 activation in the in vivo developing mouse brain. Neurobiol Dis. 2002;9(2):205–219. doi: 10.1006/nbdi.2001.0475. [DOI] [PubMed] [Google Scholar]
- Palanza P. Animal models of anxiety and depression: how are females different? Neurosci Biobehav Rev. 2001;25(3):219–233. doi: 10.1016/s0149-7634(01)00010-0. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- Peissner W, Kocher M, Treuer H, Gillardon F. Ionizing radiation-induced apoptosis of proliferating stem cells in the dentate gyrus of the adult rat hippocampus. Brain Res Mol Brain Res. 1999;71(1):61–68. doi: 10.1016/s0169-328x(99)00170-9. [DOI] [PubMed] [Google Scholar]
- Perfilieva E, Risedal A, Nyberg J, Johansson BB, Eriksson PS. Gender and strain influence on neurogenesis in dentate gyrus of young rats. J Cereb Blood Flow Metab. 2001;21(3):211–217. doi: 10.1097/00004647-200103000-00004. [DOI] [PubMed] [Google Scholar]
- Rao MS, Shetty AK. Efficacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur J Neurosci. 2004;19(2):234–246. doi: 10.1111/j.0953-816x.2003.03123.x. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Zorrilla EP, Mandyam CD, Rivier CL. Exposure to repetitive versus varied stress during prenatal development generates two distinct anxiogenic and neuroendocrine profiles in adulthood. Endocrinology. 2006;147(5):2506–2517. doi: 10.1210/en.2005-1054. [DOI] [PubMed] [Google Scholar]
- Rivier C, Vale W. Cytokines act within the brain to inhibit luteinizing hormone secretion and ovulation in the rat. Endocrinology. 1990;127(2):849–856. doi: 10.1210/endo-127-2-849. [DOI] [PubMed] [Google Scholar]
- Rivier J, Amoss M, Rivier C, Vale W. Synthetic luteinizing hormone releasing factor. Short chain analogs. J Med Chem. 1974;17(2):230–233. doi: 10.1021/jm00248a019. [DOI] [PubMed] [Google Scholar]
- Rodriguez JJ, Montaron MF, Petry KG, Aurousseau C, Marinelli M, Premier S, Rougon G, Le Moal M, Abrous DN. Complex regulation of the expression of the polysialylated form of the neuronal cell adhesion molecule by glucocorticoids in the rat hippocampus. Eur J Neurosci. 1998;10(9):2994–3006. doi: 10.1046/j.1460-9568.1998.00316.x. [DOI] [PubMed] [Google Scholar]
- Roof RL, Stein DG. Gender differences in Morris water maze performance depend on task parameters. Physiol Behav. 1999;68(1-2):81–86. doi: 10.1016/s0031-9384(99)00162-6. [DOI] [PubMed] [Google Scholar]
- Roussel S, Boissy A, Montigny D, Hemsworth PH, Duvaux-Ponter C. Gender-specific effects of prenatal stress on emotional reactivity and stress physiology of goat kids. Horm Behav. 2005;47(3):256–266. doi: 10.1016/j.yhbeh.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Pauels HG, Lugering N, Lugering A, Domschke W, Kucharzik T. Glucocorticoids induce apoptosis in human monocytes: potential role of IL-1 beta. J Immunol. 1999;163(6):3484–3490. [PubMed] [Google Scholar]
- Schmitz C, Rhodes ME, Bludau M, Kaplan S, Ong P, Ueffing I, Vehoff J, Korr H, Frye CA. Depression: reduced number of granule cells in the hippocampus of female, but not male, rats due to prenatal restraint stress. Mol Psychiatry. 2002;7(7):810–813. doi: 10.1038/sj.mp.4001118. [DOI] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, Collado-Morente L, McEwen BS, Alvarez-Buylla A. Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J Comp Neurol. 2004;478(4):359–378. doi: 10.1002/cne.20288. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Richardson HN, Chappell PE, Levine JE. In vivo gonadotropin-releasing hormone secretion in female rats during peripubertal development and on proestrus. Endocrinology. 2001;142(7):2929–2936. doi: 10.1210/endo.142.7.8239. [DOI] [PubMed] [Google Scholar]
- Sutcliffe JS, Marshall KM, Neill JC. Influence of gender on working and spatial memory in the novel object recognition task in the rat. Behav Brain Res. 2007;177(1):117–125. doi: 10.1016/j.bbr.2006.10.029. [DOI] [PubMed] [Google Scholar]
- Szuran TF, Pliska V, Pokorny J, Welzl H. Prenatal stress in rats: effects on plasma corticosterone, hippocampal glucocorticoid receptors, and maze performance. Physiol Behav. 2000;71(3-4):353–362. doi: 10.1016/s0031-9384(00)00351-6. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Galea LA, Gould E. Stress inhibits the proliferation of granule cell precursors in the developing dentate gyrus. Int J Dev Neurosci. 1998;16(3-4):235–239. doi: 10.1016/s0736-5748(98)00029-x. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;19(14):5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Hove DL, Blanco CE, Aendekerk B, Desbonnet L, Bruschettini M, Steinbusch HP, Prickaerts J, Steinbusch HW. Prenatal restraint stress and long-term affective consequences. Dev Neurosci. 2005;27(5):313–320. doi: 10.1159/000086711. [DOI] [PubMed] [Google Scholar]
- Van den Hove DL, Steinbusch HW, Scheepens A, Van de Berg WD, Kooiman LA, Boosten BJ, Prickaerts J, Blanco CE. Prenatal stress and neonatal rat brain development. Neuroscience. 2006;137(1):145–155. doi: 10.1016/j.neuroscience.2005.08.060. [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415(6875):1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Garabedian MJ. Modulation of glucocorticoid receptor transcriptional activation, phosphorylation, and growth inhibition by p27Kip1. J Biol Chem. 2003;278(51):50897–50901. doi: 10.1074/jbc.M310297200. [DOI] [PubMed] [Google Scholar]
- Ward IL, Weisz J. Maternal stress alters plasma testosterone in fetal males. Science. 1980;207(4428):328–329. doi: 10.1126/science.7188648. [DOI] [PubMed] [Google Scholar]
- Ward IL, Weisz J. Differential effects of maternal stress on circulating levels of corticosterone, progesterone, and testosterone in male and female rat fetuses and their mothers. Endocrinology. 1984;114(5):1635–1644. doi: 10.1210/endo-114-5-1635. [DOI] [PubMed] [Google Scholar]
- Weinstock M. Gender differences in the effects of prenatal stress on brain development and behaviour. Neurochem Res. 2007;32(10):1730–1740. doi: 10.1007/s11064-007-9339-4. [DOI] [PubMed] [Google Scholar]
- Weinstock M, Matlina E, Maor GI, Rosen H, McEwen BS. Prenatal stress selectively alters the reactivity of the hypothalamic-pituitary adrenal system in the female rat. Brain Res. 1992;595(2):195–200. doi: 10.1016/0006-8993(92)91049-k. [DOI] [PubMed] [Google Scholar]
- Welberg LA, Seckl JR, Holmes MC. Inhibition of 11beta-hydroxysteroid dehydrogenase, the foeto-placental barrier to maternal glucocorticoids, permanently programs amygdala GR mRNA expression and anxiety-like behaviour in the offspring. Eur J Neurosci. 2000;12(3):1047–1054. doi: 10.1046/j.1460-9568.2000.00958.x. [DOI] [PubMed] [Google Scholar]
- West MJ, Andersen AH. An allometric study of the area dentata in the rat and mouse. Brain Res. 1980;2(3):317–348. doi: 10.1016/0165-0173(80)90012-0. [DOI] [PubMed] [Google Scholar]
- West MJ, Gundersen HJ. Unbiased stereological estimation of the number of neurons in the human hippocampus. J Comp Neurol. 1990;296(1):1–22. doi: 10.1002/cne.902960102. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231(4):482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Westenbroek C, Den Boer JA, Veenhuis M, Ter Horst GJ. Chronic stress and social housing differentially affect neurogenesis in male and female rats. Brain Res Bull. 2004;64(4):303–308. doi: 10.1016/j.brainresbull.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Williams CL, Barnett AM, Meck WH. Organizational effects of early gonadal secretions on sexual differentiation in spatial memory. Behav Neurosci. 1990;104(1):84–97. doi: 10.1037//0735-7044.104.1.84. [DOI] [PubMed] [Google Scholar]
- Yu IT, Lee SH, Lee YS, Son H. Differential effects of corticosterone and dexamethasone on hippocampal neurogenesis in vitro. Biochem Biophys Res Commun. 2004;317(2):484–490. doi: 10.1016/j.bbrc.2004.03.071. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Li X, Chen W, Zhao Y, Li H, Qing C, Jia N, Bai Z, Liu J. Prenatal stress causes gender-dependent neuronal loss and oxidative stress in rat hippocampus. J Neurosci Res. 2004;78(6):837–844. doi: 10.1002/jnr.20338. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP. Multiparous species present problems (and possibilities) to developmentalists. Dev Psychobiol. 1997;30(2):141–150. doi: 10.1002/(sici)1098-2302(199703)30:2<141::aid-dev5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]


