Abstract
Introduction
Two families of nucleoside analogs have been developed to treat viral infections and cancer, but these compounds can cause tissue and cell-specific toxicity related to their uptake and subcellular activity which are dictated by host enzymes and transporters. Cellular uptake of these compounds requires nucleoside transporters that share functional similarities but differ in substrate specificity. Tissue-specific cellular expression of these transporters enables nucleoside analogs to produce their tissue specific toxic effects, a limiting factor in the treatment of retroviruses and cancer.
Areas Covered
This review discusses the families of nucleoside transporters and how they mediate cellular uptake of nucleoside analogs. Specific focus is placed on examples of known cases of transporter-mediated cellular toxicity and classification of the toxicities resulting. Efflux transporters are also explored as a contributor to analog toxicity and cell-specific effects.
Expert Opinion
Efforts to modulate transporter uptake/clearance remain long-term goals of oncologists and virologists. Accordingly, subcellular approaches that either increase or decrease intracellular nucleoside analog concentrations are eagerly sought and include transporter inhibitors and targeting transporter expression. However, additional understanding of nucleoside transporter kinetics, tissue expression, and genetic polymorphisms are required to design better molecules and better therapies.
Keywords: anticancer, antiviral, antiretroviral, mitochondria, nucleoside analogs, nucleoside transporters
1. Introduction
Nucleoside analogs are either naturally occurring or chemically altered nucleosides that mimic the natural counterparts that compose DNA and RNA [1-3]. Humans utilize the same set of deoxynucleosides or nucleosides as the basis of forming DNA or RNA, respectively. Each nucleoside consists of a sugar moiety and a nitrogenous base. While each nucleoside analog is different, the process of becoming a component of DNA or RNA is the same.
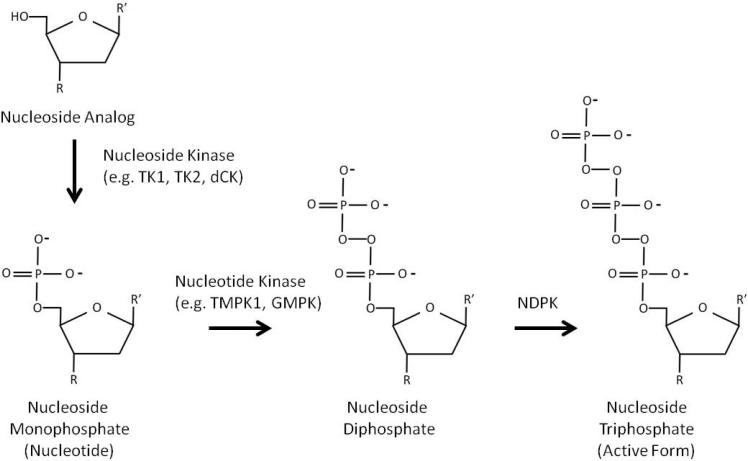
Each nucleoside requires phosphorylation to its triphosphate to become a substrate for target and/or off-target nucleic acid polymerases (Figure 1). This requires the nucleoside to be phosphorylated sequentially into its monophosphorylated, diphosphorylated, and triphosphorylated with the triphosphorylated nucleotide being the substrate of the polymerase that elongates and synthesizes the nucleic acid. (Note that in the case of tenofovir, it is a delivered as a nucleotide analog that only requires diphosphorylation and triphosphorylation to be incorporated. For simplicity, the term “nucleoside” will be used throughout the review to describe these analogs.) Chain elongation requires both triphosphate on the C5 position of the nucleotide and a hydroxyl group on the C3 position. When the C3 hydroxyl group is absent, as is the case for analogs, chain elongation is terminated until that nucleoside analog is removed and replaced with a correctly assembled nucleotide. In addition, if a bulky adduct (such as fluorine) is conjugated to the nucleoside, this can disrupt further nucleic acid replication by the incorporating polymerase. To that end, many nucleoside analogs are designed to chain terminate DNA or RNA elongation of a specific target and thereby eliminate replication, while other nucleoside analogs interfere with other crucial cell cycle-dependent DNA synthesis activities such as thymidine kinase or ribonucleotide reductase. Analogs are currently used in the treatment of cancer and viral infections (HIV-1, HCV, etc.), dividing nucleoside analogs into two pharmacological classes: anticancer (antineoplastics) and antivirals. Although nucleoside analogs share inhibition of nucleic acid replication as their central theme, it may be easy to view viral nucleoside analogs as those that inhibit viral reverse transcriptase (e.g. HIV-RT) while cancer nucleoside analogs inhibit cellular nuclear DNA polymerases in rapidly replicating cells in malignancies. Due to the difference in molecular targets (i.e. viral polymerase for antiviral analogs and nuclear DNA for anticancer analogs) and the type of cancer targeted, toxicities resulting from these compounds will vary (Tables 1 and 2). Nucleoside toxicity is based on a variety of factors (phosphorylation steps, polymerase specificity, etc.), but all toxicity is dependent initially on the uptake of nucleoside analogs into affected cells and cellular compartments by nucleoside transporters so the analog can be further processed.
Figure 1. Phosphorylation of nucleoside analogs.
Nucleoside analogs must be phosphorylated with intracellular kinases to activate the compound. A nucleoside analog is phosphorylated in a series of reactions from its nucleoside form to its monophosphorylated, diphosphorylated, and its final triphosphorylated form. The triphosphorylated form can be incorporated into DNA to perform chain termination. In this figure, R shows the location of hydroxyl groups required for chain extension; for antivirals, this hydroxyl group is missing. R’ denotes a generic base that is used for incorporation. Many structural differences exist between nucleoside analogs, including loss of the ribose ring and inclusion of bulky adducts used in many anticancer nucleoside analogs.
Table 1.
Antiviral Nucleoside Analogs*
| Nucleoside Analog | Abbreviation | Analogous to: | Used to Treat: | Toxicity |
|---|---|---|---|---|
| Abacavir | ABC | Guanosine | HIV-1 | Lactic acidosis, severe hepatomegaly with steatosis, hypersensitivity reaction, myocardial infarction |
| Didanosine | ddI | Adenosine | HIV-1 | Lactic acidosis, severe hepatomegaly with steatosis, pancreatitis, peripheral neuropathy |
| Emtricitabine | FTC | Cytidine | HIV-1 | Lactic acidosis, severe hepatomegaly with steatosis |
| Lamivudine | 3TC | Cytidine | HIV-1, HBV | Lactic acidosis, severe hepatomegaly with steatosis, pancreatitis |
| Stavudine | d4T | Thymidine | HIV-1 | Lactic acidosis, severe hepatomegaly with steatosis, pancreatitis, peripheral neuropathy |
| Tenofovir | TDF | Adenosine | HIV-1, HBV | Lactic acidosis, severe hepatomegaly with steatosis, nephrotoxicity, Fanconi's syndrome |
| Zidovudine | AZT | Thymidine | HIV-1 | Lactic acidosis, severe hepatomegaly with steatosis, bone marrow suppression, myopathy |
| Entecavir | ETV | Guanosine | HBV | Lactic acidosis and severe hepatomegaly with steatosis |
| Telbivudine | LdT | Thymidine | HBV | Lactic acidosis, severe hepatomegaly with steatosis, myopathy, peripheral neuropathy |
| Aciclovir | ACV | Guanosine | Herpes | Nephrotoxicity, thrombotic thrombocytopenic purpura, hemolytic anemia |
| Valaciclovir | VACV | Guanosine | Herpes | Nephrotoxicity, thrombotic thrombocytopenic purpura, hemolytic anemia, CNS adverse reactions |
| Ganciclovir | GCV | Guanosine | Herpes, CMV | Neutropenia, hemolytic anemia and thrombocytopenia |
| Famciclovir | FCV | Guanosine | Herpes | Acute renal failure, thrombocytopenia, hepatobiliary disorders |
| Adefovir | ADV | Adenosine | HBV | Lactic acidosis, severe hepatomegaly with steatosis, nephrotoxicity |
| Cidofovir | CDV | Cytidine | CMV | Acute renal failure, Fanconi's syndrome, neutropenia |
Information obtained from [88].
Table 2.
Anticancer Nucleoside Analogs**
| Nucleoside Analog | Abbreviation | Analogous to: | Used to Treat: | Toxicity |
|---|---|---|---|---|
| Gemcitabine | dFdC | Cytidine | ovarian cancer, breast cancer, pancreatic cancer, non-small cell lung cancer | Anemia, neutropenia, thrombocytopenia, pulmonary toxicity, hepatic impairment, renal impairment |
| Cytarabine | ARA-C | Cytidine | acute nonlymphocytic leukemia, acute lymphocytic leukemia | Anemia, neutropenia, thrombocytopenia, renal impairment, stomatitis, peripheral neuropathy |
| Clofarabine | Cl-F-ARA-A | Adenosine | acute lymphoblastic leukemia | Anemia, neutropenia, thrombocytopenia, hepatic impairment, renal failure, systemic inflammatory response syndrome |
| Cladribine | CdA | Adenosine | Hairy cell leukemia | Anemia, neutropenia, thrombocytopenia, peripheral neuropathy, renal failure |
| Fludarabine | F-ARA-A | Adenosine | chronic B-cell lymphocytic leukemia | Anemia, neutropenia, thrombocytopenia, pulmonary toxicity, autoimmune reaction, neurological toxicity |
| Azacitidine | 5-AZC, 5-AC | Cytidine | myelodysplastic syndromes, chronic myelomonocytic leukemia | Anemia, neutropenia, thrombocytopenia, hepatic impairment, renal impairment and failure |
| Decitabine | DAC, 5-AZA | Cytidine | myelodysplastic syndromes, chronic myelomonocytic leukemia | Anemia, neutropenia, thrombocytopenia, neurological reactions |
| Nelarabine | ARA-G | Guanosine | T-cell acute lymphoblastic leukemia, T-cell lymphoblastic lymphoma | Anemia, neutropenia, thrombocytopenia, neurological reactions |
| Pentostatin | dCF | Adenosine | Hairy cell leukemia | Anemia, neutropenia, thrombocytopenia, peripheral neuropathy, hepatic impairment |
Each cell can express multiple types of nucleoside transporters, and each nucleoside transporters demonstrate broad substrate specificity so that redundancy can be an adaptive advantage. Reviews that focus on classification, cell expression, and function of nucleoside transporters are available [4-8]; however, the present review will address how mechanisms of nucleoside uptake promote cellular toxicity, utilizing specific examples of transporter-mediated toxicity.
2. Classification of Nucleoside Transporters
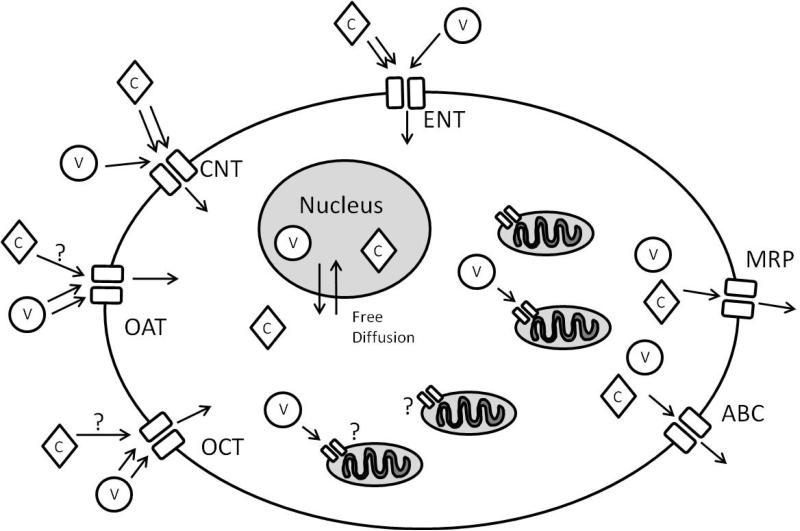
Specificity of nucleoside analog uptake by nucleoside transporters determines their access to selected intracellular compartments. It follows that the toxic effects of nucleoside analogs are mediated by the availability of uptake-compliant transporters (Figure 2). In addition, other transporters have been found to promote uptake of nucleoside analogs into specific cell types. There are also families of transporters that regulate the export of nucleoside analogs, playing an important role in tissue toxicity.
Figure 2. Nucleoside analog transporters.
This simplified cell view demonstrates the transporters involved in nucleoside uptake and clearance. Most nucleoside transporters are located on the plasma membrane of cell to regulate the influx of nucleosides and nucleoside analogs. Anticancer compounds (diamonds, C) and antiviral compounds (circles, V) display different uptake kinetics with each transporter, with anticancer compounds more readily transporter by ENTs and CNTs while antiviral compounds demonstrating uptake through OATs and OCTs. It is currently unknown to what extent OATs and OCTs play in anticancer nucleoside analog uptake. It is also unknown what transporter(s) mediate uptake of antiretroviral analogs into the mitochondrial matrix. Export of the nucleoside analogs is mediated by ABC/MRP transporters. Please note that not all cells express every transporter.
2.1 Equilibrium Nucleoside Transporters
Equilibrium transporters (ENT) facilitate the transport of nucleoside analogs down the concentration gradient formed across the plasma membrane of the cell [8]. Delivery of nucleoside a given analog to a patient leads to its detection in blood. This concentration gradient enables transport of nucleoside analogs across sodium-independent transporters to promote concentration equilibration across the membrane [9, 10]. It is worth noting that passive diffusive of nucleoside analogs across the plasma membrane is possible but operates at a much lower efficiency than ENT-mediated uptake.
At present, there are four known human equilibrative nucleoside transporters, termed hENT 1-4. The substrate specificity of nucleosides and analogs are different for each transporter. For example, hENT 1 and 2 will transport anticancer drug fludarabine effectively while hENT 3 will transport fludarabine less efficiently [11-13]. There are differences in tissue expression of these transporters, with some ENTs expressing ubiquitously and others relatively tissue specific [9, 13-15]. In general, hENTs are found on the plasma membrane to facilitate nucleoside uptake into the cell; however, some hENTs can localize to other subcellular membranes where nucleoside transport across a membrane is important (i.e. mitochondrial membrane, ER membrane, etc.) [16, 17].
The hENT transporter family is capable of transporting antiviral nucleoside analogs in addition to the anticancer analogs. However, transport is limited due to the lack of a 3’-OH group on antiviral nucleoside analogs [18, 19]. The 3’OH group appears to be a necessary component for efficient uptake, but less efficient uptake of antiviral nucleoside analogs have been documented through hENT 2, hENT 3, and, to a lesser extent, hENT 1 [13, 18-20].
2.2 Concentrating Nucleoside Transporters
Concentrating transporters (CNT) transport nucleoside analogs against a concentration gradient [21]. These transporter are sodium-dependent, which defines their separate classification from equilibrium transporters, but they share many of the same characteristics of substrate specificity and cellular localization. There are five human concentrative nucleoside transporters (hCNT 1-5), of which hCNT 1-3 have been characterized for nucleoside analog transport efficiency [22-24]. They localize primarily to the plasma membrane to promote cellular uptake of nucleosides, but studies have shown they can have subcellular localization as well [21]. The two additional CNTs (hCNT 4 and hCNT 5) have been identified but have not been as extensively characterized as hCNT 1-3 [25-27]. The ability of these two additional CNTs to contribute to nucleoside analog uptake have yet to be determined, as well as tissue specificity and cellular/subcellular localization in human tissues.
Specificity for nucleoside analogs is different for each CNT. Generally, anticancer nucleoside analogs are transported well by all CNTs, though there are varying levels of efficiency for each anticancer compound. This is also the case with antiviral compounds that have different uptake specificity. For example, AZT can be transported by hCNT1 but lamivudine is poorly transported [28, 29]. Differential uptake of nucleoside analogs into tissues may pharmacologically relate to tissue-specific expression of CNTs and thus may account for tissue-specific toxicity.
2.3 Organic Cationic Transporters and Organic Anionic Transporters
Organic cationic transporters (OCT) and organic anionic transporters (OAT) utilize facilitated transport to promote the uptake of nucleoside analogs (and other charged compounds) into a variety of cells. Interestingly, these transporters do not transport naturally occurring nucleosides, but modifications to the nucleoside structure can promote uptake by these transporters [7]. OCTs and OATs are plasma membrane proteins that mediate the uptake of nucleoside analogs into the cell; unlike ENTs, no intracellular populations of these transporters have been documented. OCT 1 and OCT 2 are expressed primarily in the liver and kidney, respectively, while OCT 3 is expressed more ubiquitously [30-32]. The OAT family consists of 10 different transporters, with each transporter expressed differently in each tissue [33]. Uptake studies of nucleoside analogs for specific transporters have revealed that each transporter exhibits compound-specific uptake. For example, not all antiretroviral nucleoside analogs have a high specificity for OCT1 and OCT2; lamivudine and zalcitabine show uptake by OCT1 and OCT2 while other NRTIs show little or no uptake [34]. Similarly, the uptake of nucleoside analogs by OATs is dependent on the nucleoside analog structure as not all nucleoside analogs are transported as efficiently by OATs.
2.4 ATP-binding Cassette Transporters and Multidrug Resistance Proteins
ATP-binding cassette (ABC) transporters are a protein superfamily that mediates the ATP-dependent transport of compounds [35, 36]. While both import and export ABC transporters exist, this section will focus on the export transporters that mediate the clearance of compounds, specifically nucleoside analogs, from the cell. These transporters utilize ATP to transport the compound against a concentration gradient and promote intracellular clearance of compounds. They are important in the resistance of cells to a variety of chemotherapeutic agents and the development of an effective clinical treatment strategy. With viral nucleoside analogs, they can eliminate tissue toxicity by clearing the analogs from unwanted tissues. These efflux transporters do not transport the non-phosphorylated nucleoside analog. The nucleoside analog must be phosphorylated into its monophosphorylated nucleotide form, and the nucleotide can be transported by one of these transporters [37]. These transporters include proteins such as P-glycoprotein (ABCB1) and the breast cancer resistance protein (BCRP or ABCG2) [38-42]. This superfamily includes the drug-exporting multidrug resistance proteins (MRP), and they are regulators of the efflux of many compounds, including nucleoside analogs. While not of the same family of transporters required for the successful uptake of nucleoside analogs, MRPs are important in the clearance of these compounds by an ATP-dependent mechanism [43]. There are nine characterized members of the MRP family of transporters with broad tissue specificity and broad substrate specificity [44]. The efflux of nucleoside analogs from the kidney is a key aspect of nucleoside analog toxicity in the kidney, a point discussed in more detail below (see Section 5.1- Tenofovir and Renal Toxicity). The expression of clearance transporters can reduce the effectiveness of anticancer nucleoside analogs by promoting the export of these compounds from neoplastic tissue (see Section 5.2- Nelarabine (Ara-G), Cytarabine (Ara-C), and Neurotoxicity).
3. Antiviral Nucleoside Toxicity
As shown in Table 1, antiviral nucleoside analogs have both unique and shared characteristic toxicities. The observed toxicities probably reflect activity of a combination of factors including transporters utilized for cell entry, metabolism of the compound to its active form, specificity of the compound for its desired target, and the clearance of the compound following biological inactivation. The combination of these effects can promote the cell and tissue dysfunction that is characteristic to a family of compounds. For antiviral compounds, one acknowledged toxic effect is mediated by mitochondrial dysfunction relating to inhibition of the mitochondrial polymerase, pol γ [45-51]. The inhibition of mtDNA replication leads to a decrease in energy production and a subsequent increase in reactive oxygen species and tissue dysfunction [47, 49, 52].
As is the case with the entire class of such compounds, the toxicity of antivirals is dependent on the entry of the nucleoside analog into the cell and its uptake into the mitochondrial compartment. As discussed previously, antiviral nucleoside analogs can be transported across the cellular membrane through uptake by ENTs, CNTs, OCTs, and OATs. This allows the compound to gain access to the cytoplasm of the cell. The mechanism by which nucleoside analogs are transported into the mitochondrial compartment is still debated. Research originally suggested that the deoxynucleoside carrier (DNC) transported antiretroviral compounds across the mitochondrial membrane [53, 54]. This finding was questioned when DNC was shown not contribute to mtDNA depletion induced by NRTIs [55]. While the mechanism of nucleoside analog uptake into the mitochondrial is not definitive, the presence of ENTs and CNTs in the inner mitochondrial membrane does suggest one possible pathway of analog access to the mitochondrial compartment. Further experiments are required to define the precise mechanism of nucleoside analog entry into mitochondria.
4. Anticancer Nucleoside Toxicity
Similar to antiviral nucleoside analogs, anticancer nucleoside analogs exhibit toxicities shared with related compounds and those that are unique (Table 2). As with antiviral nucleoside analogs, the toxicity of these analogs is multifactorial with transporter uptake contributing to the cellular toxicity. These compounds are designed to inhibit cellular replication of cancer cells and promote cell death; the toxicities arising from these compounds is due to the undesired inhibition of mammalian host cells that require replication to maintain proper homeostasis. Although all rapidly replicating cells could potentially serve as targets, one important group includes hematopoietic stem cells and the pluripotent cells derived from them [56-59]. Resultant toxicity leads to a decrease in blood cell lineages from bone marrow suppression, an effect that fortunately is reversible following cessation of anticancer therapy.
Toxicity of anticancer nucleoside analogs requires uptake into the cell and inhibition of cellular replication by inhibiting nuclear DNA replication. Cellular uptake is mediated primarily by ENTs and CNTs [9, 11]. Uptake into the nuclear compartment does not appear to be mediated by nucleoside transporters as no concentration gradient is observed across the porous nuclear membrane [60]. Since all replicating cells use similar DNA replicating machinery, the toxicity of anticancer nucleoside analogs is dependent on the targeting of replicating cells and the expression of nucleoside transporters that mediate the uptake of the nucleoside analog.
5. Nucleoside Transporters Involved in Nucleoside Analog Toxicity
Due to the differential tissue expression and cellular localization of the nucleoside transporters, the tissue-specific toxic effects of analogs can vary. Toxicity is mediated by many cellular processes that work to activate and localize the analogs subcellularly in an undesired manner. While tissue-specific toxicity is dependent on nucleoside phosphorylating enzymes that activate the anticancer and antiviral nucleoside analogs to their triphosphate form, the uptake of nucleoside analogs is the first step of tissue-specific analog-induced toxicity [47, 49, 50, 52, 61, 62]. There are certain cases where nucleoside transporters were found to be directly involved in tissue-specific nucleoside analog toxicity. The following sections will highlight examples of nucleoside transporter-mediated toxicity.
5.1 Tenofovir and Renal Toxicity
Among primary and most serious toxicities associated with antiretroviral nucleoside analogs are lactic acidosis and hepatic steatosis. Tenofovir, however, has a unique toxicity that includes kidney failure in the most extreme cases [63-65]. The renal toxicity of tenofovir has been linked to its uptake by two nucleoside transporters that concentrate it in the kidney and promote proximal tubule cell toxicity. (It is worth mentioning again that tenofovir is not a nucleoside analog but is a nucleotide analog. However, the term “nucleoside analog” will be used as a general term that will include tenofovir.)
Tenofovir is transported from the blood into the kidneys via OAT1 and OAT3 [66, 67]. While OAT 3 exhibits localization throughout the cortical tubule, OAT 1 is co-localized with OAT3 in proximal tubule cells [67]. The uptake of antiretroviral nucleoside analogs is not limited to tenofovir in the proximal tubules: zidovudine and lamivudine were shown to be taken up through OAT1 in the proximal tubules. However, the clearance of these analogs from the proximal tubules is performed by the multidrug-resistance protein 4 (MRP4). MRP4 knockout mice had a decreased efflux of organic anions in the proximal tubules, an effect that could enable concentration of tenofovir in proximal tubule cells [68]. Pathological and biochemical studies of tenofovir-induced mitochondrial toxicity revealed that OAT1 is a major transporter of tenofovir into the proximal tubule cells [45, 69]. Additionally, MRP4 was found to be necessary for elimination of tenofovir from renal proximal tubule cells [69, 70]. A decrease in MRP4 was associated with an increase in tenofovir-induced mitochondrial toxicity. Due to broad substrate specificity of MRP4, enhanced clearance of other biological compounds can decrease clearance of tenofovir and increase its cellular concentration [44]. In this situation, tenofovir appears to cause renal toxicity as a result of the colocalization of OAT1, OAT3, and MRP4 in renal proximal tubule cells, with tenofovir toxicity likely resulting from decreased elimination and increased intracellular concentration.
5.2 Nelarabine (Ara-G), Cytarabine (Ara-C), and Neurotoxicity
Anticancer nucleoside analogs exhibit myelosuppression as a common side effect. However, within this class of analogs, a few compounds display unique toxicities that can limit dosage. As an example, neurotoxicity and peripheral neuropathy are seen with high concentrations within a subset of anticancer compounds including nelarabine and cytarabine [71].
Nelarabine was observed in Phase I clinical trials to cause reversible neurotoxicity in 40% of adult patients at a dose of 40mg/kg/dose [72]. The neurological conditions observed included drowsiness, headaches, malaise and fatigue, confusion, motor dysfunction, peripheral neuropathy. The ability of nelarabine to enter the neurological cells and cause this toxicity has not been studied extensively. Research has demonstrated that the effectiveness of nelarabine is diminished in leukemia cells that have a lower expression of hENT1 and hENT2, but this correlation only suggests the primary method of uptake in leukemia cell [73]. Microarray studies have implicated overexpression of ABCB1, a member of the family of clearance transporters including the MRP family, in reducing nelarabine effectiveness against leukemia cells [74]. Further studies would be required to determine the transporters that are mediating nelarabine-induced neurotoxicity, but research has shown modulation of plasma membrane transporters can modulate nelarabine efficacy and toxicity.
The neurotoxicity of cytarabine is primarily a concern related to high-dose regimens (3g/m2 every 12 hours in adults) [75]. The mechanism of cellular uptake into the affected neurological cells has not been extensively studied, but evidence from other cell models provide some clues as to the mechanism. Peripheral blood mononuclear cells exhibited decreased cytarabine-associated toxicity when treated with a hENT1 inhibitor, demonstrating hENT1 is an important transporter for cytarabine uptake [76]. Experiments in transfected cells showed that hCNT1 can transport cytarabine, but cytarabine is a poor permeant [29]. The clearance of cytarabine from the cell appears to be mediated, at least in part, by MRP7, as knockout mouse cells for MRP7 were hypersensitive to cytarabine [77]. Other studies have implicated the expression of ABCG2 (also known as Breast Cancer Resistance Protein-BCRP) in cellular resistance to cytarabine toxicity [78-80]. As with nelarabine, cytarabine-induced toxic effects appear to be mediated by the availability of uptake transporters and clearance transporters that facility the intracellular toxic effects. The presence of these transporters in the affected neurological tissues will need to be further studied to determine which transporters contribute to additional neurotoxicity.
6. Expert Opinion
The toxicity of nucleoside analogs is dependent on a broad range of factors that contribute to pathophysiological manifestations in affected tissues. Nucleoside transporters (both uptake and clearance) represent one component that is important in the generation of toxic effects of nucleoside analogs, both antiviral and anticancer. Limiting the toxic effects of both antiviral and anticancer nucleoside analogs is paramount to better treatment regimens and better patient adherence. To minimize toxic effects, the development of better nucleoside analogs with reduced toxicity is the first goal. In addition, the reduction of unwanted cell uptake or enhanced clearance of nucleoside analogs from non-target cells is another therapeutic goal. The reduction of unwanted cell uptake is one therapeutic method that has some potential. There are current inhibitors of hENT that can limit the uptake of nucleoside analogs through specific hENTs; for example, NBMPR (nitrobenzylmercaptopurine ribonucleoside) inhibit hENT1 better than hENT2 while hENT3 is insensitive to NBMPR inhibition [13, 81]. While NBMPR is not a good compound for clinical application, this differential inhibition can be utilized in cases where a nucleoside analog preferentially enters through one transporter over another. This presents the obvious problem of inhibiting normal nucleoside uptake, but as multiple methods of nucleoside entry are possible and the selective uptake of nucleoside analogs through specific transporters is determined, the development of nucleoside transporter inhibitors does remain a possible therapeutic intervention to reduce unwanted cell toxicity. And as antiviral nucleoside analogs are more likely to enter through OATs and OCTs, inhibitors of these transporters have a decreased likelihood of reducing normal nucleoside uptake into the cell. The ability to inhibit antiviral uptake to reduce tissue toxicity has been documented and does suggest intervention may be possible [82, 83].
A second possible avenue of therapeutic intervention is the enhanced clearance of nucleoside analogs through ABC/MRP transporters. As discussed above, many of the antiviral and anticancer compounds are cleared from cells via these transporters. Through the development of gene expression vectors, promoting the clearance of nucleoside compounds through enhanced expression of pertinent transporters may be possible. As is the case with tenofovir, enhanced expression of MRP4 could reduce tenofovir-induced kidney toxicity and enable higher doses of tenofovir [69]. However, this option is still in a non-clinical test phase due to the successful delivery of these vectors without disrupting normal cell function.
In addition to enhanced clearance of nucleoside analogs, the reduced expression of nucleoside transporters on the tissues can reduce the toxicity of these compounds. From an expression/vector approach, this method is still in development also. However, in clinical settings, the reduction of nucleoside transporter expression has been documented and can be used as a predictor of nucleoside analog effectiveness in cancer treatment. This was shown in a study of Waldenström's Macroglobulinemia and small lymphocytic lymphoma [84]. This study revealed that patients with reduced hCNT1 had a lower clinical success rate following nucleoside analog therapy than those patients with higher hCNT1 levels. This was attributed to a higher uptake of cladribine. Another study showed that enhanced levels of ABCG2 could enhance clearance of cladribine and other nucleoside analogs and reduce the effectiveness of these compounds in breast cancer [78-80]. One study revealed that for clofarabine to be effective, ABCG2 expression had to be reduced or inhibited to permit the cellular accumulation of the analog; the mechanism of altered ABCG2 expression was linked to intracellular deoxycytidine kinase activity [85]. Inhibition of ABCG2 would have the benefit of increasing clofarabine in targeted tissue but could also enhance cellular toxicity in other affected tissues. Future combination therapy of clearance inhibitors or genetic modulation of surface transporters may help enhance the targeting of anticancer nucleoside analogs to cancer and reduce unwanted cell toxicity.
On the opposite spectrum is the desire by oncologists to increase tissue toxicity in cancer cells to reduce the treatment duration or exposure and increase the effectiveness of these nucleoside analogs in a greater variety of cancers. This is conceptually intertwined with many of the same mechanisms discussed to reduce tissue toxicity. To increase the toxicity, an increase in nucleoside analog uptake or a reduction in export would be desired. Inhibitors of export transporters or the use of RNAi to reduce tissue-specific expression of clearance transporters show promise. Increased expression of importing nucleoside transporters is still in a non-clinical test phase, with its usage limited or non-existent in clinical applications. It is hopeful that a mechanism may be found to exploit these transporter systems and modulate the balance of intracellular nucleoside analog concentrations.
Modulation of the expression of transporters is crucial to understanding the mechanisms of nucleoside analog toxicity. Very little is known about the factors that can induce these changes, though the changes are important (as discussed for anticancer analog success above). There have been some insightful studies that have provided a glimpse into possible mechanisms of expression regulation. In one study, transporters hCNT1, hCNT3, and hENT2 were found to be expressed differently between HIV-1 infected and non-infected individuals [86]. This work found that the infection process, likely mediated in part by TNF-α, was able to stimulate the expression of these transporters in adipocytes. This effect could lead to changes in the effectiveness of nucleoside analogs that require these transporters while also providing analogs access to tissues that may not normally be as convivial to nucleoside analogs. Additional studies will be required to determine how modulating tissue expression alters analog effectiveness and toxicity.
Finally, it is worth noting that polymorphisms in nucleoside transporters represent another mechanism of reduced or enhanced nucleoside uptake. Single nucleotide polymorphisms in nucleoside transporters have been documented in patients that are either more receptive or less receptive to nucleoside analogs. In a review of these effects on gemcitabine, over fourteen polymorphisms were detected in both coding and promoter regions of three nucleoside transporters [87]. These polymorphisms can either promote or hinder the efficacy of gemcitabine-inclusive therapies, an important concern for the application of these compounds. In this case, additional genetic profiling would enable the development of personalized therapies for both cancer and viral infections based on the polymorphisms present in a patient's nucleoside transporter.
The description of nucleoside analog transporters has been increasing in recent years due to additional identification and classification of how these transporters promote differential cellular toxicity. Further research, from substrate specificity to pharmacological inhibitor identification, will be necessary for additional advances in the clinical setting of nucleoside analogs. As our understanding of nucleoside analog uptake and clearance is expanded, the applicability of nucleoside analogs to other cancers or the benefits to current treatment regimens will increase as will the effectiveness of this drug class, thereby reducing patient discomfort and the need for additional therapies.
Article Highlights.
Nucleoside transporters mediate the uptake and clearance of antiviral and anticancer nucleoside analogs, a growing class of therapeutic compounds with increasing potential.
The current knowledge of nucleoside analog transporters, including ENTs, CNTS, OATs, and OCTs, demonstrates the importance of these transporters in analog-mediated toxicity but also highlights the limits of current understanding.
ABC/MRP transporters promote clearance of nucleoside analogs from tissues, and the resulting toxicities from nucleoside analogs are can be directly linked their reduced clearance by these efflux transporters.
Nucleoside analog toxicity can be linked to tissue-specific expression of of uptake and efflux transporters that mediate intracellular accumulation.
Clinical and experimental attempts to modulate transporter activity require further studies on substrate specificity and tissue expression to develop viable therapeutic options.
Acknowledgments
Declaration of Interest
The authors are supported by grant support from the National Institute of Drug Abuse /National Institutes of Health/United States Department of Health and Human Services (grant number -1R01DA030996)
Contributor Information
Christopher A Koczor, Emory University - Pathology 101 Woodruff Circle WMRB 7207-SOM:Pathology , Atlanta, Georgia 30322 United States.
Rebecca A Torres, Emory University - Pathology 101 Woodruff Circle, Atlanta, Georgia 30322 United States.
W Lewis, Department of Pathology, Emory University, Atlanta, GA, USA.
REFERENCES
- 1.Koczor CA, Lewis W. Nucleoside reverse transcriptase inhibitor toxicity and mitochondrial DNA. Expert Opin Drug Metab Toxicol. 2010;6(12):1493–1504. doi: 10.1517/17425255.2010.526602. [DOI] [PubMed] [Google Scholar]
- 2.Razonable RR. Antiviral drugs for viruses other than human immunodeficiency virus. Mayo Clin Proc. 2011;86(10):1009–1026. doi: 10.4065/mcp.2011.0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galmarini CM, Jordheim L, Dumontet C. Pyrimidine nucleoside analogs in cancer treatment. Expert Rev Anticancer Ther. 2003;3(5):717–728. doi: 10.1586/14737140.3.5.717. [DOI] [PubMed] [Google Scholar]
- 4*.Leung GP, Tse CM. The role of mitochondrial and plasma membrane nucleoside transporters in drug toxicity. Expert Opin Drug Metab Toxicol. 2007;3(5):705–718. doi: 10.1517/17425255.3.5.705. [A excellent review on equilibrative and concentrative nucleoside transporters including the analogs that they transport.] [DOI] [PubMed] [Google Scholar]
- 5.Burckhardt G, Burckhardt BC. In vitro and in vivo evidence of the importance of organic anion transporters (OATs) in drug therapy. Handb Exp Pharmacol. 2011;(201):29–104. doi: 10.1007/978-3-642-14541-4_2. [DOI] [PubMed] [Google Scholar]
- 6.Ciarimboli G. Role of organic cation transporters in drug-induced toxicity. Expert Opin Drug Metab Toxicol. 2011;7(2):159–174. doi: 10.1517/17425255.2011.547474. [DOI] [PubMed] [Google Scholar]
- 7.Huber-Ruano I, Pastor-Anglada M. Transport of nucleoside analogs across the plasma membrane: a clue to understanding drug-induced cytotoxicity. Curr Drug Metab. 2009;10(4):347–358. doi: 10.2174/138920009788499030. [DOI] [PubMed] [Google Scholar]
- 8*.Young JD, Yao SY, Sun L, et al. Human equilibrative nucleoside transporter (ENT) family of nucleoside and nucleobase transporter proteins. Xenobiotica. 2008;38(7-8):995–1021. doi: 10.1080/00498250801927427. [An excellent review of equilibrative transporters.] [DOI] [PubMed] [Google Scholar]
- 9.Griffiths M, Beaumont N, Yao SY, et al. Cloning of a human nucleoside transporter implicated in the cellular uptake of adenosine and chemotherapeutic drugs. Nat Med. 1997;3(1):89–93. doi: 10.1038/nm0197-89. [DOI] [PubMed] [Google Scholar]
- 10.Griffiths M, Yao SY, Abidi F, et al. Molecular cloning and characterization of a nitrobenzylthioinosine-insensitive (ei) equilibrative nucleoside transporter from human placenta. Biochem J. 1997;328(Pt 3):739–743. doi: 10.1042/bj3280739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.King KM, Damaraju VL, Vickers MF, et al. A comparison of the transportability, and its role in cytotoxicity, of clofarabine, cladribine, and fludarabine by recombinant human nucleoside transporters produced in three model expression systems. Mol Pharmacol. 2006;69(1):346–353. doi: 10.1124/mol.105.015768. [DOI] [PubMed] [Google Scholar]
- 12.Mackey JR, Mani RS, Selner M, et al. Functional nucleoside transporters are required for gemcitabine influx and manifestation of toxicity in cancer cell lines. Cancer Res. 1998;58(19):4349–4357. [PubMed] [Google Scholar]
- 13.Baldwin SA, Yao SY, Hyde RJ, et al. Functional characterization of novel human and mouse equilibrative nucleoside transporters (hENT3 and mENT3) located in intracellular membranes. J Biol Chem. 2005;280(16):15880–15887. doi: 10.1074/jbc.M414337200. [DOI] [PubMed] [Google Scholar]
- 14.Crawford CR, Patel DH, Naeve C, et al. Cloning of the human equilibrative, nitrobenzylmercaptopurine riboside (NBMPR)-insensitive nucleoside transporter ei by functional expression in a transport-deficient cell line. J Biol Chem. 1998;273(9):5288–5293. doi: 10.1074/jbc.273.9.5288. [DOI] [PubMed] [Google Scholar]
- 15.Barnes K, Dobrzynski H, Foppolo S, et al. Distribution and functional characterization of equilibrative nucleoside transporter-4, a novel cardiac adenosine transporter activated at acidic pH. Circ Res. 2006;99(5):510–519. doi: 10.1161/01.RES.0000238359.18495.42. [DOI] [PubMed] [Google Scholar]
- 16.Lai Y, Tse CM, Unadkat JD. Mitochondrial expression of the human equilibrative nucleoside transporter 1 (hENT1) results in enhanced mitochondrial toxicity of antiviral drugs. J Biol Chem. 2004;279(6):4490–4497. doi: 10.1074/jbc.M307938200. [DOI] [PubMed] [Google Scholar]
- 17.Mani RS, Hammond JR, Marjan JM, et al. Demonstration of equilibrative nucleoside transporters (hENT1 and hENT2) in nuclear envelopes of cultured human choriocarcinoma (BeWo) cells by functional reconstitution in proteoliposomes. J Biol Chem. 1998;273(46):30818–30825. doi: 10.1074/jbc.273.46.30818. [DOI] [PubMed] [Google Scholar]
- 18.Yao SY, Ng AM, Sundaram M, et al. Transport of antiviral 3′-deoxy-nucleoside drugs by recombinant human and rat equilibrative, nitrobenzylthioinosine (NBMPR)-insensitive (ENT2) nucleoside transporter proteins produced in Xenopus oocytes. Mol Membr Biol. 2001;18(2):161–167. doi: 10.1080/09687680110048318. [DOI] [PubMed] [Google Scholar]
- 19.Vickers MF, Zhang J, Visser F, et al. Uridine recognition motifs of human equilibrative nucleoside transporters 1 and 2 produced in Saccharomyces cerevisiae. Nucleosides Nucleotides Nucleic Acids. 2004;23(1-2):361–373. doi: 10.1081/ncn-120028333. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto T, Kuniki K, Takekuma Y, et al. Ribavirin uptake by cultured human choriocarcinoma (BeWo) cells and Xenopus laevis oocytes expressing recombinant plasma membrane human nucleoside transporters. Eur J Pharmacol. 2007;557(1):1–8. doi: 10.1016/j.ejphar.2006.10.062. [DOI] [PubMed] [Google Scholar]
- 21*.Pastor-Anglada M, Cano-Soldado P, Errasti-Murugarren E, et al. SLC28 genes and concentrative nucleoside transporter (CNT) proteins. Xenobiotica. 2008;38(7-8):972–994. doi: 10.1080/00498250802069096. [An excellent review on concentrative nucleoside transporters, including function and drug kinetics.] [DOI] [PubMed] [Google Scholar]
- 22.Ritzel MW, Yao SY, Huang MY, et al. Molecular cloning and functional expression of cDNAs encoding a human Na+-nucleoside cotransporter (hCNT1). Am J Physiol. 1997;272(2 Pt 1):C707–714. doi: 10.1152/ajpcell.1997.272.2.C707. [DOI] [PubMed] [Google Scholar]
- 23.Ritzel MW, Yao SY, Ng AM, et al. Molecular cloning, functional expression and chromosomal localization of a cDNA encoding a human Na+/nucleoside cotransporter (hCNT2) selective for purine nucleosides and uridine. Mol Membr Biol. 1998;15(4):203–211. doi: 10.3109/09687689709044322. [DOI] [PubMed] [Google Scholar]
- 24.Ritzel MW, Ng AM, Yao SY, et al. Molecular identification and characterization of novel human and mouse concentrative Na+-nucleoside cotransporter proteins (hCNT3 and mCNT3) broadly selective for purine and pyrimidine nucleosides (system cib). J Biol Chem. 2001;276(4):2914–2927. doi: 10.1074/jbc.M007746200. [DOI] [PubMed] [Google Scholar]
- 25.Gutierrez MM, Giacomini KM. Substrate selectivity, potential sensitivity and stoichiometry of Na(+)-nucleoside transport in brush border membrane vesicles from human kidney. Biochim Biophys Acta. 1993;1149(2):202–208. doi: 10.1016/0005-2736(93)90202-b. [DOI] [PubMed] [Google Scholar]
- 26.Flanagan SA, Meckling-Gill KA. Characterization of a novel Na+-dependent, guanosine-specific, nitrobenzylthioinosine-sensitive transporter in acute promyelocytic leukemia cells. J Biol Chem. 1997;272(29):18026–18032. doi: 10.1074/jbc.272.29.18026. [DOI] [PubMed] [Google Scholar]
- 27.Flanagan SA, Gandhi V, Secrist JA, 3rd, et al. The novel nucleoside transport system exhibited by NB4 cells, csg, transports deoxyguanosine analogues, including ara-G. Biochem Pharmacol. 2003;66(5):733–737. doi: 10.1016/s0006-2952(03)00393-9. [DOI] [PubMed] [Google Scholar]
- 28.Cano-Soldado P, Lorrayoz IM, Molina-Arcas M, et al. Interaction of nucleoside inhibitors of HIV-1 reverse transcriptase with the concentrative nucleoside transporter-1 (SLC28A1). Antivir Ther. 2004;9(6):993–1002. [PubMed] [Google Scholar]
- 29.Graham KA, Leithoff J, Coe IR, et al. Differential transport of cytosine-containing nucleosides by recombinant human concentrative nucleoside transporter protein hCNT1. Nucleosides Nucleotides Nucleic Acids. 2000;19(1-2):415–434. doi: 10.1080/15257770008033018. [DOI] [PubMed] [Google Scholar]
- 30.Zhang L, Dresser MJ, Gray AT, et al. Cloning and functional expression of a human liver organic cation transporter. Mol Pharmacol. 1997;51(6):913–921. doi: 10.1124/mol.51.6.913. [DOI] [PubMed] [Google Scholar]
- 31.Gorboulev V, Ulzheimer JC, Akhoundova A, et al. Cloning and characterization of two human polyspecific organic cation transporters. DNA Cell Biol. 1997;16(7):871–881. doi: 10.1089/dna.1997.16.871. [DOI] [PubMed] [Google Scholar]
- 32.Nies AT, Koepsell H, Winter S, et al. Expression of organic cation transporters OCT1 (SLC22A1) and OCT3 (SLC22A3) is affected by genetic factors and cholestasis in human liver. Hepatology. 2009;50(4):1227–1240. doi: 10.1002/hep.23103. [DOI] [PubMed] [Google Scholar]
- 33*.VanWert AL, Gionfriddo MR, Sweet DH. Organic anion transporters: discovery, pharmacology, regulation and roles in pathophysiology. Biopharm Drug Dispos. 2010;31(1):1–71. doi: 10.1002/bdd.693. [This review provides a good overview of OAT function and localization.] [DOI] [PubMed] [Google Scholar]
- 34.Jung N, Lehmann C, Rubbert A, et al. Relevance of the organic cation transporters 1 and 2 for antiretroviral drug therapy in human immunodeficiency virus infection. Drug Metab Dispos. 2008;36(8):1616–1623. doi: 10.1124/dmd.108.020826. [DOI] [PubMed] [Google Scholar]
- 35.Borst P, Balzarini J, Ono N, et al. The potential impact of drug transporters on nucleoside-analog-based antiviral chemotherapy. Antiviral Res. 2004;62(1):1–7. doi: 10.1016/j.antiviral.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 36.Schinkel AH, Jonker JW. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview. Adv Drug Deliv Rev. 2003;55(1):3–29. doi: 10.1016/s0169-409x(02)00169-2. [DOI] [PubMed] [Google Scholar]
- 37.Schuetz JD, Connelly MC, Sun D, et al. MRP4: A previously unidentified factor in resistance to nucleoside-based antiviral drugs. Nat Med. 1999;5(9):1048–1051. doi: 10.1038/12487. [DOI] [PubMed] [Google Scholar]
- 38.Cascorbi I. P-glycoprotein: tissue distribution, substrates, and functional consequences of genetic variations. Handb Exp Pharmacol. 2011;(201):261–283. doi: 10.1007/978-3-642-14541-4_6. [DOI] [PubMed] [Google Scholar]
- 39.Miller DS. Regulation of P-glycoprotein and other ABC drug transporters at the blood-brain barrier. Trends Pharmacol Sci. 2010;31(6):246–254. doi: 10.1016/j.tips.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marquez B, Van Bambeke F. ABC multidrug transporters: target for modulation of drug pharmacokinetics and drug-drug interactions. Curr Drug Targets. 2011;12(5):600–620. doi: 10.2174/138945011795378504. [DOI] [PubMed] [Google Scholar]
- 41.Ishikawa T, Nakagawa H. Human ABC transporter ABCG2 in cancer chemotherapy and pharmacogenomics. J Exp Ther Oncol. 2009;8(1):5–24. [PubMed] [Google Scholar]
- 42.Robey RW, Ierano C, Zhan Z, et al. The challenge of exploiting ABCG2 in the clinic. Curr Pharm Biotechnol. 2011;12(4):595–608. doi: 10.2174/138920111795163913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keppler D. Multidrug resistance proteins (MRPs, ABCCs): importance for pathophysiology and drug therapy. Handb Exp Pharmacol. 2011;(201):299–323. doi: 10.1007/978-3-642-14541-4_8. [DOI] [PubMed] [Google Scholar]
- 44*.Russel FG, Koenderink JB, Masereeuw R. Multidrug resistance protein 4 (MRP4/ABCC4): a versatile efflux transporter for drugs and signalling molecules. Trends Pharmacol Sci. 2008;29(4):200–207. doi: 10.1016/j.tips.2008.01.006. [An excellent review of MRP4 expression and function.] [DOI] [PubMed] [Google Scholar]
- 45.Kohler JJ, Hosseini SH, Hoying-Brandt A, et al. Tenofovir renal toxicity targets mitochondria of renal proximal tubules. Lab Invest. 2009;89(5):513–519. doi: 10.1038/labinvest.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kohler JJ, Hosseini SH, Cucoranu I, et al. Murine cardiac mtDNA: effects of transgenic manipulation of nucleoside phosphorylation. Lab Invest. 2009;89(2):122–130. doi: 10.1038/labinvest.2008.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kohler JJ, Hosseini SH, Green E, et al. Cardiac-targeted transgenic mutant mitochondrial enzymes: mtDNA defects, antiretroviral toxicity and cardiomyopathy. Cardiovasc Toxicol. 2008;8(2):57–69. doi: 10.1007/s12012-008-9015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kohler JJ, Hosseini SH, Lewis W. Mitochondrial DNA impairment in nucleoside reverse transcriptase inhibitor-associated cardiomyopathy. Chem Res Toxicol. 2008;21(5):990–996. doi: 10.1021/tx8000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lewis W, Day BJ, Kohler JJ, et al. Decreased mtDNA, oxidative stress, cardiomyopathy, and death from transgenic cardiac targeted human mutant polymerase gamma. Lab Invest. 2007;87(4):326–335. doi: 10.1038/labinvest.3700523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50*.Lewis W, Day BJ, Copeland WC. Mitochondrial toxicity of NRTI antiviral drugs: an integrated cellular perspective. Nat Rev Drug Discov. 2003;2(10):812–822. doi: 10.1038/nrd1201. [This review covers the “DNA pol γ hypothesis” and mechanisms of antiretroviral therapies.] [DOI] [PubMed] [Google Scholar]
- 51.Lewis W, Dalakas MC. Mitochondrial toxicity of antiviral drugs. Nat Med. 1995;1(5):417–422. doi: 10.1038/nm0595-417. [DOI] [PubMed] [Google Scholar]
- 52.Kohler JJ, Cucoranu I, Fields E, et al. Transgenic mitochondrial superoxide dismutase and mitochondrially targeted catalase prevent antiretroviral-induced oxidative stress and cardiomyopathy. Lab Invest. 2009;89(7):782–790. doi: 10.1038/labinvest.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lewis W, Haase CP, Miller YK, et al. Transgenic expression of the deoxynucleotide carrier causes mitochondrial damage that is enhanced by NRTIs for AIDS. Lab Invest. 2005;85(8):972–981. doi: 10.1038/labinvest.3700301. [DOI] [PubMed] [Google Scholar]
- 54.Lewis W, Kohler JJ, Hosseini SH, et al. Antiretroviral nucleosides, deoxynucleotide carrier and mitochondrial DNA: evidence supporting the DNA pol gamma hypothesis. AIDS. 2006;20(5):675–684. doi: 10.1097/01.aids.0000216367.23325.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lam W, Chen C, Ruan S, et al. Expression of deoxynucleotide carrier is not associated with the mitochondrial DNA depletion caused by anti-HIV dideoxynucleoside analogs and mitochondrial dNTP uptake. Mol Pharmacol. 2005;67(2):408–416. doi: 10.1124/mol.104.007120. [DOI] [PubMed] [Google Scholar]
- 56.Robak T, Blonski JZ, Kasznicki M, et al. Cladribine with prednisone versus chlorambucil with prednisone as first-line therapy in chronic lymphocytic leukemia: report of a prospective, randomized, multicenter trial. Blood. 2000;96(8):2723–2729. [PubMed] [Google Scholar]
- 57.Rai KR, Peterson BL, Appelbaum FR, et al. Fludarabine compared with chlorambucil as primary therapy for chronic lymphocytic leukemia. N Engl J Med. 2000;343(24):1750–1757. doi: 10.1056/NEJM200012143432402. [DOI] [PubMed] [Google Scholar]
- 58.Begleiter A, Verburg L, Ashique A, et al. Comparison of antitumor activities of 2-chlorodeoxyadenosine and 9-beta-arabinosyl-2-fluoroadenine in chronic lymphocytic leukemia and marrow cells in vitro. Leukemia. 1995;9(11):1875–1881. [PubMed] [Google Scholar]
- 59.Robak T. Cladribine in the treatment of chronic lymphocytic leukemia. Leuk Lymphoma. 2001;40(5-6):551–564. doi: 10.3109/10428190109097654. [DOI] [PubMed] [Google Scholar]
- 60.Leeds JM, Slabaugh MB, Mathews CK. DNA precursor pools and ribonucleotide reductase activity: distribution between the nucleus and cytoplasm of mammalian cells. Mol Cell Biol. 1985;5(12):3443–3450. doi: 10.1128/mcb.5.12.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shewach DS, Daddona PE, Ashcraft E, et al. Metabolism and selective cytotoxicity of 9-beta-D-arabinofuranosylguanine in human lymphoblasts. Cancer Res. 1985;45(3):1008–1014. [PubMed] [Google Scholar]
- 62.Verhoef V, Fridland A. Metabolic basis of arabinonucleoside selectivity for human leukemic T- and B-lymphoblasts. Cancer Res. 1985;45(8):3646–3650. [PubMed] [Google Scholar]
- 63.Cote HC, Magil AB, Harris M, et al. Exploring mitochondrial nephrotoxicity as a potential mechanism of kidney dysfunction among HIV-infected patients on highly active antiretroviral therapy. Antivir Ther. 2006;11(1):79–86. [PubMed] [Google Scholar]
- 64.Vidal F, Domingo JC, Guallar J, et al. In vitro cytotoxicity and mitochondrial toxicity of tenofovir alone and in combination with other antiretrovirals in human renal proximal tubule cells. Antimicrob Agents Chemother. 2006;50(11):3824–3832. doi: 10.1128/AAC.00437-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kohler JJ, Hosseini SH. Subcellular renal proximal tubular mitochondrial toxicity with tenofovir treatment. Methods Mol Biol. 2011;755:267–277. doi: 10.1007/978-1-61779-163-5_22. [DOI] [PubMed] [Google Scholar]
- 66.Cihlar T, Ho ES, Lin DC, et al. Human renal organic anion transporter 1 (hOAT1) and its role in the nephrotoxicity of antiviral nucleotide analogs. Nucleosides Nucleotides Nucleic Acids. 2001;20(4-7):641–648. doi: 10.1081/NCN-100002341. [DOI] [PubMed] [Google Scholar]
- 67.Nagle MA, Truong DM, Dnyanmote AV, et al. Analysis of three-dimensional systems for developing and mature kidneys clarifies the role of OAT1 and OAT3 in antiviral handling. J Biol Chem. 2011;286(1):243–251. doi: 10.1074/jbc.M110.139949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leggas M, Adachi M, Scheffer GL, et al. Mrp4 confers resistance to topotecan and protects the brain from chemotherapy. Mol Cell Biol. 2004;24(17):7612–7621. doi: 10.1128/MCB.24.17.7612-7621.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kohler JJ, Hosseini SH, Green E, et al. Tenofovir renal proximal tubular toxicity is regulated by OAT1 and MRP4 transporters. Lab Invest. 2011;91(6):852–858. doi: 10.1038/labinvest.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ray AS, Cihlar T, Robinson KL, et al. Mechanism of active renal tubular efflux of tenofovir. Antimicrob Agents Chemother. 2006;50(10):3297–3304. doi: 10.1128/AAC.00251-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reilly KM, Kisor DF. Profile of nelarabine: use in the treatment of T-cell acute lymphoblastic leukemia. Onco Targets Ther. 2009;2:219–228. doi: 10.2147/ott.s4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kurtzberg J, Ernst TJ, Keating MJ, et al. Phase I study of 506U78 administered on a consecutive 5-day schedule in children and adults with refractory hematologic malignancies. J Clin Oncol. 2005;23(15):3396–3403. doi: 10.1200/JCO.2005.03.199. [DOI] [PubMed] [Google Scholar]
- 73.Homminga I, Zwaan CM, Manz CY, et al. In vitro efficacy of forodesine and nelarabine (ara-G) in pediatric leukemia. Blood. 2011;118(8):2184–2190. doi: 10.1182/blood-2011-02-337840. [DOI] [PubMed] [Google Scholar]
- 74.Fyrberg A, Peterson C, Kagedal B, et al. Induction of fetal hemoglobin and ABCB1 gene expression in 9-beta-D-arabinofuranosylguanine-resistant MOLT-4 cells. Cancer Chemother Pharmacol. 2011;68(3):583–591. doi: 10.1007/s00280-010-1524-5. [DOI] [PubMed] [Google Scholar]
- 75.Lowenberg B, Pabst T, Vellenga E, et al. Cytarabine dose for acute myeloid leukemia. N Engl J Med. 2011;364(11):1027–1036. doi: 10.1056/NEJMoa1010222. [DOI] [PubMed] [Google Scholar]
- 76.Parmar S, Seeringer A, Denich D, et al. Variability in transport and biotransformation of cytarabine is associated with its toxicity in peripheral blood mononuclear cells. Pharmacogenomics. 2011;12(4):503–514. doi: 10.2217/pgs.10.200. [DOI] [PubMed] [Google Scholar]
- 77.Hopper-Borge EA, Churchill T, Paulose C, et al. Contribution of Abcc10 (Mrp7) to in vivo paclitaxel resistance as assessed in Abcc10(-/-) mice. Cancer Res. 2011;71(10):3649–3657. doi: 10.1158/0008-5472.CAN-10-3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.van den Heuvel-Eibrink MM, Wiemer EA, Prins A, et al. Increased expression of the breast cancer resistance protein (BCRP) in relapsed or refractory acute myeloid leukemia (AML). Leukemia. 2002;16(5):833–839. doi: 10.1038/sj.leu.2402496. [DOI] [PubMed] [Google Scholar]
- 79.Hampras SS, Sucheston L, Weiss J, et al. Genetic polymorphisms of ATP-binding cassette (ABC) proteins, overall survival and drug toxicity in patients with Acute Myeloid Leukemia. Int J Mol Epidemiol Genet. 2010;1(3):201–207. [PMC free article] [PubMed] [Google Scholar]
- 80.de Wolf C, Jansen R, Yamaguchi H, et al. Contribution of the drug transporter ABCG2 (breast cancer resistance protein) to resistance against anticancer nucleosides. Mol Cancer Ther. 2008;7(9):3092–3102. doi: 10.1158/1535-7163.MCT-08-0427. [DOI] [PubMed] [Google Scholar]
- 81.Ward JL, Sherali A, Mo ZP, et al. Kinetic and pharmacological properties of cloned human equilibrative nucleoside transporters, ENT1 and ENT2, stably expressed in nucleoside transporter-deficient PK15 cells. Ent2 exhibits a low affinity for guanosine and cytidine but a high affinity for inosine. J Biol Chem. 2000;275(12):8375–8381. doi: 10.1074/jbc.275.12.8375. [DOI] [PubMed] [Google Scholar]
- 82.Cundy KC, Li ZH, Lee WA. Effect of probenecid on the distribution, metabolism, and excretion of cidofovir in rabbits. Drug Metab Dispos. 1996;24(3):315–321. [PubMed] [Google Scholar]
- 83.Lacy SA, Hitchcock MJ, Lee WA, et al. Effect of oral probenecid coadministration on the chronic toxicity and pharmacokinetics of intravenous cidofovir in cynomolgus monkeys. Toxicol Sci. 1998;44(2):97–106. doi: 10.1006/toxs.1998.2481. [DOI] [PubMed] [Google Scholar]
- 84.Rabascio C, Laszlo D, Andreola G, et al. Expression of the human concentrative nucleotide transporter 1 (hCNT1) gene correlates with clinical response in patients affected by Waldenstrom's Macroglobulinemia (WM) and small lymphocytic lymphoma (SLL) undergoing a combination treatment with 2-chloro-2′-deoxyadenosine (2-CdA) and Rituximab. Leuk Res. 2010;34(4):454–457. doi: 10.1016/j.leukres.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 85.Nagai S, Takenaka K, Nachagari D, et al. Deoxycytidine kinase modulates the impact of the ABC transporter ABCG2 on clofarabine cytotoxicity. Cancer Res. 2011;71(5):1781–1791. doi: 10.1158/0008-5472.CAN-10-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guallar JP, Cano-Soldado P, Aymerich I, et al. Altered expression of nucleoside transporter genes (SLC28 and SLC29) in adipose tissue from HIV-1-infected patients. Antivir Ther. 2007;12(6):853–863. [PubMed] [Google Scholar]
- 87*.Wong A, Soo RA, Yong WP, et al. Clinical pharmacology and pharmacogenetics of gemcitabine. Drug Metab Rev. 2009;41(2):77–88. doi: 10.1080/03602530902741828. [An excellent review of the effects of nucleoside transporter polymorphisms on gemcitabine efficacy.] [DOI] [PubMed] [Google Scholar]
- 88.Drugs@FDA.gov [January, 2012];U.S. Food and Drug Administration Website. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/.
- 89.NCI Drug Dictionary [January, 2012];National Cancer Institute at the National Institutes of Health Website. http://www.cancer.gov/drugdictionary/.