Abstract
Purpose
To study the cellular responses to molecular and polymeric forms of plutonium using PC12 cells derived from rat adrenal glands.
Materials and methods
Serum starved PC12 cells were exposed to polymeric and molecular forms of plutonium for three hours. Cells were washed with 10 mM EGTA, 100 mM NaCl at pH 7.4 to remove surface sorbed plutonium. Localization of plutonium in individual cell was quantitatively analyzed by synchrotron X-ray fluorescence (XRF) microscopy.
Results
Molecular plutonium complexes introduced to cell growth media in the form of NTA, citrate, or transferrin complexes were taken up by PC12 cells, and mostly co-localized with iron within the cells. Polymeric plutonium prepared separately was not internalized by PC12 cells but it was always found on the cell surface as big agglomerates; however polymeric plutonium formed in situ was mostly found within the cells as agglomerates.
Conclusions
PC12 cells can differentiate molecular and polymeric forms of plutonium. Molecular plutonium is taken up by PC12 cells and mostly co-localized with iron but aged polymeric plutonium is not internalized by the cells.
Keywords: PC12 Cells, plutonium polymer, molecular plutonium, X-ray fluorescence microscopy
INTRODUCTION
Environmental contamination of radionuclides, such as plutonium, presents important potential health risks. Although significant amounts of plutonium do not occur in nature, environmental plutonium contamination is a concern because of historical events, (e.g., fallout from weapons testing, accidental release from nuclear reactors, and inadequate disposal practices), and also because of current concerns about nuclear terrorism and future plans for geological disposal of radioactive wastes (Ewing 1999; León Vintró et al. 2009). Plutonium is dangerous to health (tumorigenic) when it finds its way into the body. It is accessible from ingested food and water, inhaled particles from the contaminated environment or through cuts and broken skin. Up to 25% of the inhaled or ingested plutonium is deposited into the body and remains there for decades (Taylor 1995; Voelz 2000). Due to long term retention of plutonium isotopes and their energetic α-particle emissions, even small amounts of plutonium easily damage surrounding tissues.
Plutonium exists in nature in different chemical forms and as many as four different oxidation states (Pu(III), Pu(IV), Pu(V), Pu(VI)). The uptake and retention of plutonium will depend upon its physico-chemical properties (Fouillit et al. 2004) as determined by its oxidation state and aggregation state (i.e., mononuclear molecular complexes vs. polymeric or colloidal forms of plutonium). Pu(V) is believed to be important in the environment at trace concentrations (Nelson & Lovett 1978) but Pu(IV) is generally believed to be the most stable oxidation state at environmental pH values (Choppin 2003). The Pu4+ cation is the predominant species only at pH < 0.5 or when bound by strong ligands. At higher pH values, even under acidic condition and very low concentrations, Pu(IV) readily hydrolyses to form nanoparticles of PuO2, (Thiyagarajan et al. 1990) a colloidal or polymeric species, before eventually precipitating as a refractory hydrous plutonium dioxide (Choppin et al. 1997; Walther et al. 2007). Because of this tendency to form polymeric plutonium under neutral conditions, Pu polymer either as a pure species or sorbed to colloids is an important form of plutonium in nature even at very low Pu concentrations (Kersting et al. 1999).
Although the biological hazards of plutonium have been long appreciated, very little specific information is known about cellular uptake, transfer, and distribution of molecular and polymeric plutonium (Taylor et al. 1987). To better understand the interactions of plutonium with cells, we have used X-ray fluorescence (XRF) microscopy to examine the uptake and distribution of various forms of plutonium in mammalian cells. XRF microscopy is a powerful technique for quantitative detection of elements in a small area. It has higher spatial resolution than α-autoradiography and the penetrating power of X-rays allows one to study thick samples, or samples that have been encapsulated to meet radiation safety regulations. XRF microscopy has been very informative for the simultaneous quantitative detection of multiple elements within cells where it has been successfully used to track transition metals like Cu, Zn, Fe and other biologically relevant elements at the sub-cellular level (Kemner et al. 2004; Paunesku et al. 2006; Fahrni 2007). Moreover, while metal-specific fluorescent dyes have been developed for many biologically essential metals (Gee et al. 2002; Yang et al. 2005), none are known for transuranic elements. Therefore, XRF microprobe is becoming an essential technique for studying the sub-cellular distribution of elements such as uranium and plutonium.
Prior work has established that the liver and skeleton are the main deposition site for intravenously injected polymeric and monomeric plutonium respectively with lesser amounts depositing in spleen, lung, and lymph nodes (Rahman et al. 1964; Baxter et al. 1973; Mahlum & Sikov 1974). In addition to the liver and skeleton, the adrenal gland has also been reported as deposition site for plutonium (Ballou 1964). Therefore, we selected PC12 cell lines from rat adrenal gland for our study. Phagocytic cells are known to interact with colloidal plutonium but, very little information is known about the interaction of plutonium with cells outside the reticuloendothelial system. PC12 cells are not phagocytes but they are known for the internalization of Mn-40 nanoparticles (Hussain et al. 2006). This particularly attracted our attention to understand cellular responses to the polymeric and molecular plutonium. As most environmental plutonium is expected to be found in its polymeric form, it is important to know the cellular response for plutonium polymer. Using XRF we directly observe differences in the sub-cellular distribution of molecular and polymeric forms of plutonium in PC12 cells. Plutonium is internalized by PC12 cells when it is presented to the cells as molecular complexes of chelating agents that inhibit Pu polymer formation in the growth media, or fresh polymer formed in situ. In contrast, aged polymeric plutonium nanoparticles are not internalized by PC12 cells; instead, it stays on the surface of cells as large agglomerates. Co-localization of plutonium with iron also depends on type of plutonium complexes interacting with cells.
Materials and Methods
Preparation of Pu-NTA/Pu-citrate Complex
Plutonium-242 (99.96 atom% Pu-242, 0.035 atom% Pu-239, and 0.0014 atom% Pu-238) purified by anion exchange chromatography in 7.5 M HNO3, was oxidized to Pu(VI) in hot perchloric acid and diluted with water. This faint pink solution, containing 5 × 10−3 M Pu(VI), was subjected to electrochemical reduction at a Pt electrode in 1 M perchloric acid to form a blue solution of Pu(III). The oxidation state and purity of the resulting solution was confirmed by UV-vis spectroscopy, and the concentration of Pu(III) was determined by its sharp absorption peak at 600 nm (ε600 = 38 M−1 cm−1) and liquid scintillation counting (specific activity 242Pu = 3.93 × 10−3 Ci/g). The perchloric acid content of the Pu(III) solution was reduced to approximately 0.01 M by selective extraction of the HClO4 with 10% Amberlite LA2 in o-xylene. The Pu(III) solution was then treated with three equivalents of NTA solution and the remaining HClO4 was neutralized by addition of solid sodium carbonate. Solid sodium carbonate was added very cautiously until the effervescence ceased, indicating a final pH around 5.5 to 6.0. The resulting solution was left at room temperature for about 30 minutes to allow the Pu(III) to oxidize to Pu(IV), changing from a light blue to a faint pink solution. The resulting Pu(IV)-NTA solution was either syringe filtered through 0.22 μm filter or a 5k ultra centrifugal filter (Millipore Corporation). The completeness of oxidation was verified by UV-vis spectroscopy, and was always at least 99% Pu(IV). After oxidation color changes from light blue to faint pink. Mononuclear Pu(IV)-citrate complexes were prepared using similar protocol, substituting sodium citrate from NTA.
Preparation of Rat PuCFeNTf
Monoferric rat serum transferrin with iron in the N-lobe (FeNTf) was prepared by selectively depleting iron from the C-lobe of diferric rat transferrin (Fe2Tf) using a previously published protocol (Baldwin & Desousa 1981). Gel electrophoresis indicated a purity of 95 % FeNTf. The FeNTf thus prepared was treated with 1.2 equivalents of Pu(IV)-NTA and incubated at least 2 hours at 4°C, to produce PuCFeNTf. The excess Pu(IV)-NTA was removed using 10k Amicon ultra centrifugal filter. The Pu content of the transferrin was determined by liquid scintillation counting. This procedure produced transferrin containing greater than 95% plutonium in the C-lobe considering 100% removal of Fe from the C-lobe and 100 % iron in N-lobe. The protein was stored at 4°C and used within two to three days.
Plutonium Polymer
Plutonium colloids were generated by two methods. Polymeric plutonium was prepared in 0.3M HNO3 by refluxing under nitric oxide gas using the previously published protocol (Costanzo et al. 1973; Lloyd & Haire 1978). We refer to this as aged polymer. The resulting solutions were filtered though 0.22 μm filters before use. Alternately, solutions of electrochemically produced Pu(IV) in 1 M HClO4 were hydrolyzed by addition of a small aliquot to serum-free media followed by neutralization with small increments of NaOH and vigorous mixing in the presence of no more than one equivalent of ammonium citrate. We refer to this polymer as a fresh polymer in.
Incubation of PC12 Cells with Plutonium
PC12 cells (ATCC) were grown in F12K medium supplemented with 12.5 % horse serum, 2.5% fetal calf serum, and antibiotics in humidified 5% CO2 environment at 37°C. Cells were plated in 25 cm2 culture bottles in the appropriate density (ca. 70% confluency) the day before Pu exposure, and serum starved overnight before feeding with plutonium. The serum-free F12K media was replaced with 2 mL of complete media supplemented with 12.5 to 50 μM 242Pu(IV) in the desired chemical form and the cells were incubated at room temperature, or at 37 °C under 5% CO2 for up to 3 hours. At the end of the incubation, the cells were washed three times with a solution of 10 mM EGTA (ethyleneglycoltetraacetic acid) and 100 mM NaCl at pH 7.4 to remove surface sorbed molecular Pu and then suspended in 2 mL of the EGTA solution. The cells were then pelleted, the supernatant was discarded, and the cells were resuspended in a known volume of EGTA buffer. In select cases, the concentration and viability of the cells was checked by trypan blue exclusion. A fraction of the cells were lysed in cell lysis buffer (40 mM Tris-HCl pH 7.4, 120 mM NaCl, 0.5% Triton X-100, 0.3% SDS), and the lysate was used to measure the average plutonium uptake by liquid scintillation counting and for protein quantification quantify by BCA assay (Pierce Protein Research Products, Thermo Fisher Scientific Inc.). Depending upon the sample, 105 to 106 cells were used for liquid scintillation counting.
XRF Imaging
Cells for X-ray fluorescence microscopy were deposited from an aliquot of the final EGTA/NaCl suspension onto formvar coated copper electron microscopy grids. The cells were allowed to air dry overnight and were mounted in aluminum sample holders before being encapsulated between a pair of 500 nm SiN windows and epoxied underneath a layer of 6 μm polypropylene or 8 μm Kapton film.
Synchrotron X-ray fluorescence microscopy was performed at beamline 2-ID-D of the Advanced Photon Source at Argonne National Laboratory (Cai et al. 2003). Prior to the X-ray fluorescence microscopy, samples were placed in kinematic mounts developed to fit both a light microscope and the X-ray microprobe, and optical micrographs of selected intact cells were obtained. Then, the distributions of the elements from P to Pu in individual cells were mapped by raster scanning the incident 18.1 keV X-rays focused to a spot size of 0.2 μm × 0.5 μm or smaller across a cell of interest in 0.5 μm steps. The fluorescence signal for each element at each pixel was converted to elemental content in μg/cm2. The estimated absolute accuracy is better than 40%, and the relative accuracy is better than 10%. The resulting elemental maps were visualized and analyzed using the MAPS software (Vogt 2003).
RESULTS
Cellular Responses to Molecular Plutonium
Molecular plutonium in this study was added to the media in the form of NTA, citrate, or transferrin complexes. We used long-lived plutonium isotope, 242Pu, throughout our experiment to minimize radiation dose to the cells. To avoid the possible hydrolysis of Pu(IV), NTA and citrate complexes were prepared with Pu(III) at low pH, and allowed air oxidise to Pu(IV). It is known that both citrate and NTA form stable complexes with Pu(IV) (Kantar & Honeyman 2005; Bonin et al. 2009), however the kinetic stability of the Pu(IV)-citrate complex in solution seems to be lower than Pu(IV)-NTA complex. Slow hydrolysis of plutonium in plutonium citrate complex that depends on pH and citrate concentration has been reported by others (Francis et al. 2006). In contrast, the optical spectrum of the Pu(IV)-NTA complexes prepared using a 1:3 ratio is stable for more than a month and 100% of the Pu(IV)-NTA was recovered in the filtrate after passing through Ultra 5k centrifugal filter (Millipore Corporation) one month after preparation. In contrast, similar 1:3 Pu(IV)-citrate solutions start to hydrolyze within 15 days. To avoid the formation of polymeric plutonium, all Pu-complexes were filtered and used within a week of preparation. Light microscopic images show that there is no significant change in the cell morphology when PC12 cells were exposed to Pu-complexes. Cell viability (80–90% as determined by trypan blue exclusion) was similar for plutonium treated cells compared to plutonium free cells.
Pu (IV)-NTA
The determination of cell-associated 242Pu by liquid scintillation counting of lysed cells after incubation with 50 μM Pu(IV)-NTA shows that detectable amounts of plutonium were taken up by PC12 cells over 3 hours. An average of about 35 μg of plutonium per gram of protein was measured from the liquid scintillation counting experiment (figure 1). Plutonium uptake was slightly affected when cells were incubated with Pu(IV)-NTA in presence of diferric transferrin as reported previously (Planasbohne et al. 1983). Six Pu(IV)-NTA exposed cells were imaged using XRF microscopy. Values obtained from XRF analysis also show similar patterns (figure 1B) and results are comparable to the bulk uptake results which are well within the uncertainty of the measurements. The number of cells taken into consideration is vastly different for the two approaches, in XRF microscopy the specific Pu content of only a few particular cells are studied while liquid scintillation counting provides the average Pu content for 105 cells or more. However, the agreement between the Pu contents derived from the two methods, and the cell to cell consistency of the XRF measured Pu contents suggest that the amount of Pu associated with each cell is relatively constant.
Figure 1.
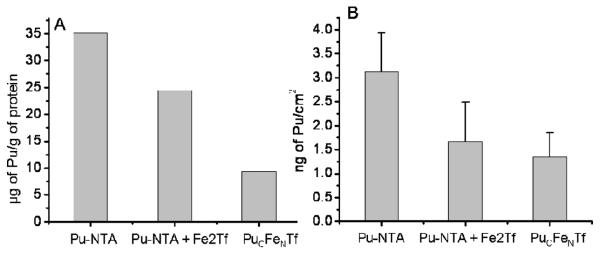
Uptake of plutonium by PC12 cells from Pu(IV)-NTA (50 μM) in presence and absence of diferric transferrin (25 μM), and from PuCFeNTf (25 μM). Corresponding values were calculated from XRF analysis (A) and liquid scintillation counting data (B). The amount of plutonium from XRF analysis was calculated as the mean values from three to five cells after subtraction of background fluorescence while values from liquid scintillation counting were for approximately 105 cells. The incubations were carried out for 3 hours at 37 °C.
Pu(IV)-transferrin
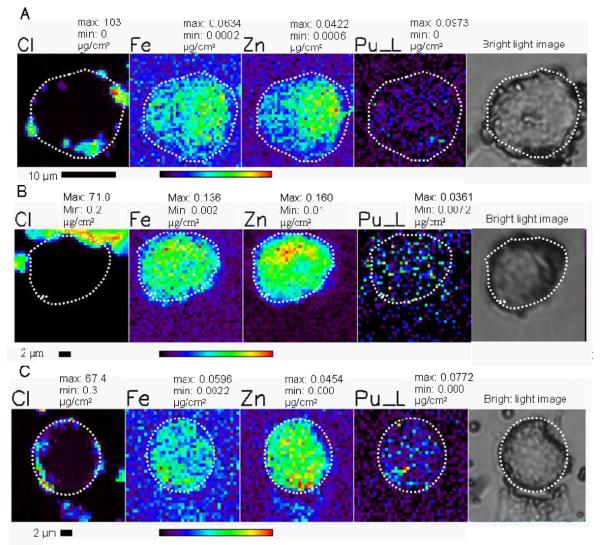
Transferrin is a bilobal iron-binding glycoprotein that binds and transfers iron into cells through receptor mediated endocytosis (Dautryvarsat et al. 1983). In addition to iron, transferrin also binds numerous other metals (Planasbohne et al. 1983; Planasbohne et al. 1985). Transferrin forms stable complexes with molecular Pu(IV), (Lehmann et al. 1983) however, both lobes behave differently with plutonium. We have recently shown that only one form of the plutonium-transferrin complex (PuCFeNTf) is taken up by PC12 cells. The total of 10 cells were imaged using XRF microscopy to evaluate the plutonium uptake. XRF images show that plutonium taken up by PC12 cells in the form of PuCFeNTf is mostly colocalized with iron within the cytoplasm of cells (figure 2C, 4A). Plutonium taken up by cells in the form of a transferrin complex is homogeneously distributed thoughout the cytoplasm of the cell without any sign of aggregation.
Figure 2.
PC12 cells after treatment with 50 μM molecular Pu(IV)-NTA (A), 50 μM Pu(IV)-NTA + 25 μM Fe2Tf (B), and 12.5 μM PuCFeNTf (C) for three hours. The cell outline is indicated with a dotted line on the XRF images and the corresponding bright-light micrograph. The concentration of selected elements and the maximum and minimum values for the color scale are given above each image in micrograms per square centimeter. The rainbow colored scale bar represents the signal intensity; black pixels represents the low concentration and red pixels represents the highest concentration of corresponding elements. The numerical scale is unique for each element. A scale bar is shown below the elemental map.
Figure 4.
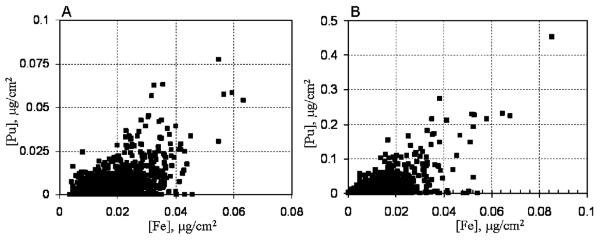
Scatter plot showing the co-localization of the highest iron and plutonium concentrations in PC12 cells treated with FeNPuCTf (A) or a mixture of Pu-citrate and iron citrate (B) for three hours. Values in the plots A and B correspond to the cell images 2C and 3A respectively.
Pu(IV)-citrate
Soluble Pu(IV)-citrate complexes were prepared in a similar way to the Pu(IV)-NTA complexes. XRF images of cells incubated with media supplemented with serum and this molecular form of Pu-citrate (50 μM) show that Pu is taken up by PC12 cells and it is mostly co-localized with iron (figures 3A and 4B). Plutonium added to the media in the form of molecular Pu(IV)-citrate complexes does not agglomerate inside the cells within three hours. A total of 15 cells were imaged for metal contents using XRF microscopy. Comparing XRF images with the light microscopic image of the corresponding cell (figure 3A), plutonium is mostly seen in the cytoplasm and it is absent in the nuclear region of cell. Within the cytoplasm plutonium is homogeneously distributed, suggesting that it exists as soluble complexes with small molecules like citrate, or biomolecules such as transferrin and ferritin..
Figure 3.
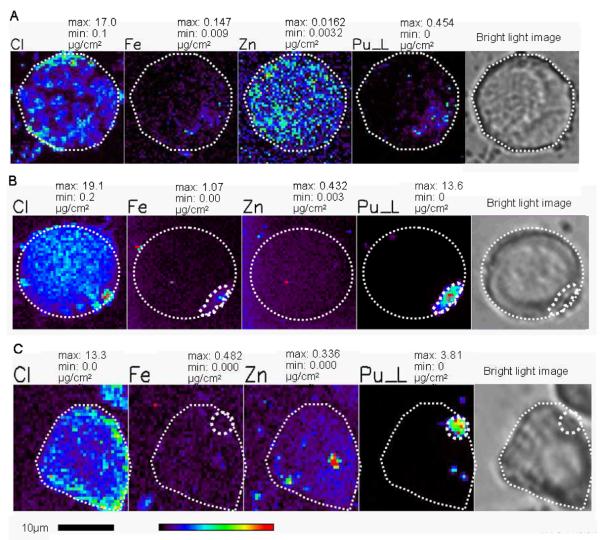
PC12 cells after treatment with the molecular form of Pu(IV)-citrate (50μM) for three hours (A) and treatment with fresh polymer from 50 μM of 1:1 Pu(IV)-citrate in 1 M perchloric acid and 50 μM iron(III)-citrate (B) or from 50 μM of 1:1 Pu(IV)-citrate in 1 M perchloric acid (C). pH was adjusted before adding serum to the media (for B and C). The Location of plutonium within a cell and corresponding position of highest iron concentrations is denoted by small white circle. The maximum and minimum threshold concentrations in micrograms per square centimeter for a given element are above each image. The rainbow colored scale bar represents the signal intensity; dark pixels represents the low concentration and red pixel represents the highest concentration of corresponding elements. A scale bar is shown below the elemental map.
Cellular Responses to Plutonium Polymer
PC12 cells behave differently if they are exposed to plutonium-citrate in slightly different way. Serum starved PC12 cells were treated with an equimolar amount of Pu(IV) and citrate (1:1) in 1M perchloric acid after adjusting the pH to 7.4 with NaOH but before adding serum to the medium. Because of the hydrolytic behavior of Pu(IV), Pu(IV)-citrate under this condition does not exist in pure molecular form, it is mostly hydrolyzed to fresh plutonium polymer, representing the formation of Pu colloids in situ. In separate serum-free tests approximately 90% of the Pu prepared by this method could not pass a 0.22 μm filter. After three hours incubation, large amounts of plutonium, up to 1000 times the amounts observed for in the uptake of molecular Pu complexes, appeared as agglomerates near the cell membranes (figures 3B and 3C) in 32 of the 38 plutonium-containing cells examined. (Two other cells were associated with Pu particles that maybe internal or associated with the cell membrane above or below the cells, and 4 other cells showed only low levels of plutonium). Moreover, when the media was supplemented with iron and plutonium, both elements colocalized as agglomerates, while in media supplemented with only plutonium, the plutonium:iron ratio in the cells was higher and plutonium and iron did not colocalize. In these cells plutonium is principally found just inside the cell embedded within the cell membrane (figures 3B and 3C).
PC-12 cells were also incubated with pre-prepared plutonium polymer (aged polymer) for three hours and plutonium uptake was determined by LSC and XRF microscopy. Among the 10 cells selected for XRF imaging, polymeric plutonium was not found distributed inside the cell as observed with the molecular complexes of plutonium. Instead it was always found as discrete deposits on the cell surfaces sticking on or in the cell membrane in micron-sized particles (figures 3B, 3C and 5). This is in contrast to our observations when the Pu polymer was freshly generated in the media.
Figure 5.
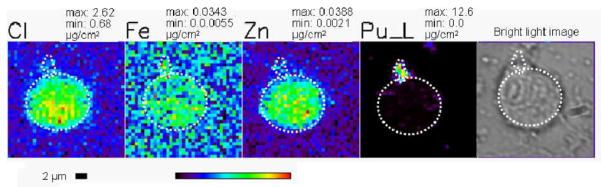
PC12 cells after treatment with aged Pu-polymer for three hours. The white circle indicates the cell outline and position of plutonium in the cell surface is indicated with a small dotted outline in XRF images and bright light microscopic image. The maximum and minimum threshold concentrations in micrograms per square centimeter for a given element are. The rainbow colored scale bar represents the signal intensity; black pixels represents the lowest concentration and red pixel represents the highest concentration of corresponding elements. A scale bar is shown below the elemental map.
DISCUSSION
Our study of the cellular uptake of Pu by PC12 cells using XRF imaging provides direct insights about the different uptake and distribution pattern of polymeric and molecular forms of plutonium at the cellular level in vitro. In our experiments, only molecular forms of plutonium added to the growth media (figures 2, 3A), or fresh polymer formed in situ (figures 3B and 3C) were taken into PC12 cells. After 3 hours of incubation, the plutonium taken up from molecular complexes is mostly co-localized with iron (figure 4) in the cytoplasm and is evenly distributed. In addition, the uptake and cellular distribution of molecular plutonium complexes is similar whether the Pu is added to the media as Pu-NTA, Pu-citrate, or pre-formed Pu-transferrin complexes. The molecular forms of plutonium taken up by cells are clearly resistant to aggregation in the media or within the cells over three hours, and the colocalization of intracellular Pu and Fe in the PC12 cells is consistent with previous reports that Pu is associated with the iron-containing proteins transferrin, ferritin, or hemosiderin (Taylor 1972; Taylor et al. 1987). Different uptake pathways for plutonium and iron have been suggested for human lymphoblast cells (Planasbohne et al. 1985), but those cells showed similar uptake behavior for molecular plutonium from citrate and transferrin complex. These observations are consistent with our results for molecular plutonium but none of these previous reports are based on high resolution imaging of individual cells.
Pu(IV) forms both 1:1 and 1:2 complexes with citric acid with reported stability constants of 1015.5 and 1030 respectively (Hummel 2005). At lower citrate concentrations, however, Pu(IV) will still be hydrolyzed to polymeric form at physiological pHs. When cells were exposed to fresh plutonium polymer generated from such solutions, the plutonium is still taken into cells and is typically retained as agglomerates at the interface between the cell membrane and the cytoplasm in less than three hours (Figure 3B, 3C). Significant amounts of plutonium from the polymeric plutonium does not appear to specifically bind with transferrin or other biomolecules to form soluble complexes, as it does not distribute through the cytoplasm as we observe for the molecular complexes. However, a similar type of aggregation was observed for the neighboring actinide element, neptunium, in the nuclei of hepatocytes and kidney proximal tubule cells when 237Np nitrate was intraperitoneally injected to rats. Clusters of dense granules with maximum diameter of 2 μm were found in the central part of the nucleus (Boulahdour et al. 1995).
These studies of fresh polymer are relevant to plutonium uptake because even molecular plutonium not complexed by transferrin or ferritin, starts aggregating over time in vivo. Previous studies have shown that plutonium citrate intravenously administered to rat showed 64% of the total liver plutonium in the soluble fraction within an hour of injection. After 8 days, fractions of plutonium significantly decreased to 5% in soluble fractions (Boocock et al. 1970) indicating the aggregation of plutonium over time. Within a few hours of injection, plutonium starts to aggregate and accumulated in lysosomes. Colloidal or aggregated forms of plutonium are mostly associated with sinusoidal (phagocytic) cells in the liver (Grube et al. 1978).
Cellular uptake of larger particles in the nanometer to micrometer range involves several mechanisms including receptor mediated endocytosis, non-specific endocytosis, pinocytosis, and phagocytosis (Mailander & Landfester 2009) and depends on the size, morphology and surface charge. We observe that pre-formed, aged Pu(IV) polymer behaves differently than either the molecular forms of plutonium we studied or fresh plutonium polymer formed in situ. When PC12 cells are exposed to aged plutonium polymer, polymeric particles are not internalized by cells; instead, we observe them only as approximately micron-sized deposits on the cell surface. In vivo, the largest fraction of plutonium (more than 30%) retained from intravenously injected polymeric plutonium was found in the lysosomes in liver (Rahman et al. 1964). We find that in the absence of phagocytic activity aged polymeric plutonium is not internalized within the adrenal gland cells studied.
The micron-sized plutonium particles on the surfaces of the cells treated with aged polymer are larger than expected. The stock solution of aged polymer had passed a 0.22 μm filter immediately prior to incubation. In addition, typical plutonium polymers in aqueous solution prepared from plutonium nitrate are reported to be ellipsoidal in shape with about 4.7 nm diameter and 190 nm long (Thiyagarajan et al. 1990), which is also considerably smaller than the Pu deposits we observe (figure 5). Consequently, the micron-sized particles of aged polymer present on cell surface would appear to be due to the accumulation of multiple polymeric plutonium particles.
Such bioaggregation would not be unique to eukaryotic cells. Actinide bioaccumulation or bioprecipitation using redox chemistry or the secretion of precipitating agents is well known in microorganisms. Some bacteria are known to reduce more toxic U(VI) to insoluble less toxic U(IV) (Suzuki & Banfield 2004; Liu et al. 2009). Intracellular bioaccumulation of clusters of uranium granules is considered as detoxification process in bacteria. Biotransformation of Pu(VI) or Pu(V) to insoluble Pu(IV) species by some bacteria also has been reported (Francis 2007), but the specific mechanisms that could spur bioaccumulation in mammalian cells is not understood.
Interestingly, PC12 cells behave differently with aged polymeric plutonium (hydrous plutonium oxide) particles than transition metal nanoparticles. The observation that aged Pu polymer is not internalized by PC12 cells contrasts recent reports on the uptake of Mn-40 (manganese oxide) nanoparticles by PC12 cells. Mn-40 nanoparticles were observed both inside and outside the PC12 cells as agglomerates 1 to 10 μm in size (Hussain et al. 2006). It is well known that nanoparticles interacting on the cell surface receptors do not cross the plasma membrane under normal conditions. Instead, they are enveloped in a vesicle as a part of the cytoplasmic membrane (Qualmann & Kessels 2002). However, nanoparticles producing reactive oxygen species (ROS) can damage the vesicle and enter into the cells. ROS induced cell permeability by Fe2O3 nanoparticles have been shown in human microvascular endothelial cells (Apopa et al. 2009). Mn-40 nanoparticles are also known to produce ROS which may have facilitated nanoparticle uptake for PC12 cells. Despite the production of ROS by most toxic metal nanoparticles, the surface chemistry and potential for ROS mediated toxic effects by plutonium particles is not understood.
By using low concentrations of long-lived 242Pu we have minimized the potential for radiation-induced cell damage that could facilitate particle transport. The rate of α-particle emission by cell-associated 242Pu is small, ranging from an average of 1–2 disintegrations per day for cells with high concentrations of Pu to 1 disintegration every 60 days for cells with the lowest Pu contents currently measurable by synchrotron XRF microscopy. One might hypothesize that unlike transition metal oxide nanoparticles, the our aged Pu particles do not generate significant concentrations of ROS either chemically or from radioactive decay. It is not clear, however, why plutonium polymer generated in situ, is able to penetrate the PC12 cells while aged plutonium polymer aggregates on the cell surface. The amounts of cell associated plutonium are similar for both forms of the polymer so any radiation-induced effects should be similar. One might speculate that the surface chemistry and reactivity of the fresh and aged polymer differ, but this is an idea that requires further study.
In summary, plutonium is taken up by PC12 cells when they are exposed to soluble molecular plutonium complexes, or freshly formed polymers in situ. The aged plutonium polymers, which would represent the plutonium aggregates found in the environment, are not internalized by PC12 cells. The amount of plutonium deposited per cell (inside, or on the cell surface) from aged or freshly formed polymeric plutonium is significantly higher than the plutonium deposition from monomeric form which is more likely to cause radiation damage or cell death. Further study is required to understand why PC12 cells prefer to internalized larger fresh plutonium polymer but not the aged polymer.
ACKNOWLEDGMENTS
This work at Argonne National Laboratory and its Advanced Photon Source was supported by the Office of Basic Energy Sciences, U. S. Department of Energy, while B.P.A. was supported by the University of Chicago and the Department of Energy under section H.35 of U.S. Department of Energy Contract No. DE.AC02-06CHl1357 awarded to UChicago Argonne, LLC, operator of Argonne National Laboratory
REFERENCES
- Apopa PL, Qian Y, Shao R, Guo NL, Schwegler-Berry D, Pacurari M, Porter D, Shi XL, Vallyathan V, Castranova V, et al. Iron oxide nanoparticles induce human microvascular endothelial cell permeability through reactive oxygen species production and microtubule remodeling. Particle and Fibre Toxicology. 2009;6 doi: 10.1186/1743-8977-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin DA, Desousa DMR. The effect of salts on the kinetics of iron release from n-terminal and c-terminal monoferric-transferrins. Biochemical and Biophysical Research Communications. 1981;99(4):1101–1107. doi: 10.1016/0006-291x(81)90732-4. [DOI] [PubMed] [Google Scholar]
- Ballou JE. Distribution and Retention of Plutonium-239 and Neptunium-237 in the Rat Adrenal. Radiation Research. 1964;22(1):81–94. [PubMed] [Google Scholar]
- Baxter DW, Rosenthal MW, Russell JJ, Moretti E, Chladek D, Lindenbaum A. Comparison of Monomeric and Polymeric Plutonium in the Dog and Mouse. Radiation Research. 1973;54(3):556–565. [PubMed] [Google Scholar]
- Bonin L, Guillaumont D, Jeanson A, Den Auwer C, Grigoriev M, Berthet JC, Hennig C, Scheinost A, Moisy P. Thermodynamics and Structure of Actinide(IV) Complexes with Nitrilotriacetic Acid. Inorganic Chemistry. 2009;48(9):3943–3953. doi: 10.1021/ic801453w. [DOI] [PubMed] [Google Scholar]
- Boocock G, Danpure CJ, Popplewell DS, Taylor DM. The Subcellular Distribution of Plutonium in Rat Liver. Radiation Research. 1970;42(2):381–396. [PubMed] [Google Scholar]
- Boulahdour H, Poncy JL, Berry JP, Galle P. Intranuclear sites of np-237 in mammalian-cells - a study using electron-microscopy and electron-probe microanalysis. International Journal of Radiation Biology. 1995;68(1):55–61. doi: 10.1080/09553009514550921. [DOI] [PubMed] [Google Scholar]
- Cai Z, Lai B, Xiao Y, Xu S. An X-ray diffraction microscope at the Advanced Photon Source. Journal De Physique Iv. 2003;104:17–20. [Google Scholar]
- Choppin GR. Actinide speciation in the environment. Radiochimica Acta. 2003;91(11):645–649. [Google Scholar]
- Choppin GR, Bond AH, Hromadka PM. Redox speciation of plutonium. Journal of Radioanalytical and Nuclear Chemistry. 1997;219(2):203–210. [Google Scholar]
- Costanzo DA, Biggers RE, Bell JT. Plutonium polymerization--I A spectrophotometric study of the polymerization of plutonium(IV) Journal of Inorganic and Nuclear Chemistry. 1973;35(2):609–622. [Google Scholar]
- Dautryvarsat A, Ciechanover A, Lodish HF. PH AND THE RECYCLING OF TRANSFERRIN DURING RECEPTOR-MEDIATED ENDOCYTOSIS. Proceedings of the National Academy of Sciences of the United States of America-Biological Sciences. 1983;80(8):2258–2262. doi: 10.1073/pnas.80.8.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing RC. Nuclear waste forms for actinides. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(7):3432–3439. doi: 10.1073/pnas.96.7.3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrni CJ. Biological applications of X-ray fluorescence microscopy: exploring the subcellular topography and speciation of transition metals. Current Opinion in Chemical Biology. 2007;11(2):121–127. doi: 10.1016/j.cbpa.2007.02.039. [DOI] [PubMed] [Google Scholar]
- Fouillit M, Grillon G, Fritsch P, Rateau G, Pave D, Delforge J, Le Gall B. Comparative tissue uptake and cellular deposition of three different plutonium chemical forms in rats. International Journal of Radiation Biology. 2004;80(9):683–689. doi: 10.1080/09553000400005486. [DOI] [PubMed] [Google Scholar]
- Francis AJ. Microbial mobilization and immobilization of plutonium. Journal of Alloys and Compounds. 2007;444–445:500–505. [Google Scholar]
- Francis AJ, Dodge CJ, Gillow JB. Biotransformation of plutonium complexed with citric acid. Radiochimica Acta. 2006;94(9–11):731–737. [Google Scholar]
- Gee KR, Zhou Z-L, Qian W-J, Kennedy R. Detection and Imaging of Zinc Secretion from Pancreatic β-Cells Using a New Fluorescent Zinc Indicator. Journal of the American Chemical Society. 2002;124(5):776–778. doi: 10.1021/ja011774y. [DOI] [PubMed] [Google Scholar]
- Grube BJ, Stevens W, Atherton DR. Retention of plutonium in hepatocytes and sinusoidal lining cells isolated from rat-liver. Radiation Research. 1978;73(1):168–179. [PubMed] [Google Scholar]
- Hummel W, Anderegg G, Rao L, Puigdomenech I, Tochiyama O. Chemical Thermodynamics of Compounds and Complexes of U, Np, Pu, Am, Se, Ni, and Zr with selected organic ligands. Elsevier; Boston: 2005. [Google Scholar]
- Hussain SM, Javorina AK, Schrand AM, Duhart HM, Ali SF, Schlager JJ. The interaction of manganese nanoparticles with PC-12 cells induces dopamine depletion. Toxicological Sciences. 2006;92(2):456–463. doi: 10.1093/toxsci/kfl020. [DOI] [PubMed] [Google Scholar]
- Kantar C, Honeyman BD. Plutonium (IV) complexation with citric and alginic acids at low Pu-T concentrations. Radiochimica Acta. 2005;93(12):757–766. [Google Scholar]
- Kemner KM, Kelly SD, Lai B, Maser J, O'Loughlin EJ, Sholto-Douglas D, Cai ZH, Schneegurt MA, Kulpa CF, Nealson KH. Elemental and redox analysis of single bacterial cells by X-ray microbeam analysis. Science. 2004;306(5696):686–687. doi: 10.1126/science.1103524. [DOI] [PubMed] [Google Scholar]
- Kersting AB, Efurd DW, Finnegan DL, Rokop DJ, Smith DK, Thompson JL. Migration of plutonium in ground water at the Nevada Test Site. Nature. 1999;397(6714):56–59. [Google Scholar]
- Lehmann M, Culig H, Taylor DM. Identification of Transferrin as the Principal Plutonium-binding Protein in the Blood Serum and Liver Cytosol of Rats: Immunological and Chromatographic Studies. International Journal of Radiation Biology. 1983;44(1):65–74. doi: 10.1080/09553008314550871. [DOI] [PubMed] [Google Scholar]
- León Vintró L, Mitchell PI, Omarova A, Burkitbayev M, Jiménez Nápoles H, Priest ND. Americium, plutonium and uranium contamination and speciation in well waters, streams and atomic lakes in the Sarzhal region of the Semipalatinsk Nuclear Test Site, Kazakhstan. Journal of Environmental Radioactivity. 2009;100(4):308–314. doi: 10.1016/j.jenvrad.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Liu C, Zachara JM, Zhong L, Heald SM, Wang Z, Jeon B-H, Fredrickson JK. Microbial Reduction of Intragrain U(VI) in Contaminated Sediment. Environmental Science & Technology. 2009;43(13):4928–4933. doi: 10.1021/es8029208. [DOI] [PubMed] [Google Scholar]
- Lloyd MH, Haire RG. Chemistry of plutonium in sol-gel processes. Radiochimica Acta. 1978;25(3–4):139–148. [Google Scholar]
- Mahlum DD, Sikov MR. Distribution and Toxicity of Monomeric and Polymeric239 Pu in Immature and Adult Rats. Radiation Research. 1974;60(1):75–88. [PubMed] [Google Scholar]
- Mailander V, Landfester K. Interaction of Nanoparticles with Cells. Biomacromolecules. 2009;10(9):2379–2400. doi: 10.1021/bm900266r. [DOI] [PubMed] [Google Scholar]
- Nelson DM, Lovett MB. Oxidation-state of plutonium in irish sea. Nature. 1978;276(5688):599–601. [Google Scholar]
- Paunesku T, Vogt S, Maser J, Lai B, Woloschak G. X-ray fluorescence microprobe imaging in biology and medicine. Journal of Cellular Biochemistry. 2006;99(6):1489–1502. doi: 10.1002/jcb.21047. [DOI] [PubMed] [Google Scholar]
- Planasbohne F, Taylor DM, Duffield JR. Role of transferrin in metal uptake by human-lymphoblasts invitro. Cell Biochemistry and Function. 1985;3(3):217–222. doi: 10.1002/cbf.290030309. [DOI] [PubMed] [Google Scholar]
- Planasbohne F, Taylor DM, Duffield JR, Darai G. Role of transferrin in uptake of non-physiological metals into cells. Cell Biochemistry and Function. 1983;1(3):141–142. doi: 10.1002/cbf.290010303. [DOI] [PubMed] [Google Scholar]
- Qualmann B, Kessels MM. International Review of Cytology - a Survey of Cell Biology. Vol 220. Academic Press Inc.; San Diego: 2002. Endocytosis and the cytoskeleton; pp. 93–144. [DOI] [PubMed] [Google Scholar]
- Rahman YE, Lindenbaum A, Westfall WM. Lysosome Particles and Subcellular Distributions of Polymeric Tetravalent Plutonium-239. Radiation Research. 1964;21(4):575–583. [PubMed] [Google Scholar]
- Suzuki Y, Banfield JF. Resistance to, and accumulation of, uranium by bacteria from a uranium-contaminated site. Geomicrobiology Journal. 2004;21(2):113–121. [Google Scholar]
- Taylor DM. Interactions between transuranium elements and the components of cells and tissues. Health Phys. 1972;22(6):575–81. doi: 10.1097/00004032-197206000-00007. [DOI] [PubMed] [Google Scholar]
- Taylor DM. Environmental plutonium in humans. Applied Radiation and Isotopes. 1995;46(11):1245–1252. doi: 10.1016/0969-8043(95)00167-c. [DOI] [PubMed] [Google Scholar]
- Taylor DM, Seidel A, Planasbohne F, Schuppler U, Neumuller M, Wirth RE. Biochemical-studies of the interactions of plutonium, neptunium and protoactinium with blood and liver-cell proteins. Inorganica Chimica Acta. 1987;140(1–2):361–363. [Google Scholar]
- Thiyagarajan P, Diamond H, Soderholm L, Horwitz EP, Toth LM, Felker LK. Plutonium(iv) polymers in aqueous and organic media. Inorganic Chemistry. 1990;29(10):1902–1907. [Google Scholar]
- Voelz GL. Plutonium and Health: How Great is the Risk. Los Alamos Science. 2000;(26):74–89. [Google Scholar]
- Vogt S. MAPS: A set of software tools for analsis and visualization of 3D X-ray fluorescence data sets. Journal de physique IV France. 2003;104:635–638. [Google Scholar]
- Walther C, Cho HR, Marquardt CM, Neck V, Seibert A, Yun JI, Fanghanel T. Hydrolysis of plutonium(IV) in acidic solutions: no effect of hydrolysis on absorption-spectra of mononuclear hydroxide complexes. Radiochimica Acta. 2007;95(1):7–16. [Google Scholar]
- Yang LC, McRae R, Henary MM, Patel R, Lai B, Vogt S, Fahrni CJ. Imaging of the intracellular topography of copper with a fluorescent sensor and by synchrotron x-ray fluorescence microscopy. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(32):11179–11184. doi: 10.1073/pnas.0406547102. [DOI] [PMC free article] [PubMed] [Google Scholar]