Abstract
The acute response of human skin to ultraviolet B (UVB) radiation has not been fully characterized. We sought to define the cutaneous response at 24 hours following narrow-band UVB (NB-UVB, 312 nm peak), a therapeutically relevant source of UVB, using transcriptional profiling, immunohistochemistry, and immunofluorescence. There were 1,522 unique differentially-regulated genes, including upregulation of antimicrobial peptides (AMPs) (S100A7, S100A12, human beta-defensin 2, and elafin), neutrophil and monocyte/dendritic cell (DC) chemoattractants (IL-8, CXCL1, CCL20, CCL2). Ingenuity Pathway Analysis demonstrated activation of innate defense and early adaptive immune pathways. Immunohistochemistry confirmed increased epidermal staining for AMPs (S100A7, S100A12, human beta-defensin 2, and elafin). Inflammatory myeloid CD11c+BDCA1− DCs were increased in irradiated skin, which were immature as shown by minimal co-localization with DC-LAMP, and co-expressed inflammatory markers TNF and TRAIL in irradiated skin. There were increased BDCA3+ DCs, a cross-presenting DC subtype with immunosuppressive functions, and these cells have not been previously characterized as part of the response to UVB. These results show that the acute response of human skin to erythemogenic doses of NB-UVB includes activation of innate defense mechanisms, as well as early infiltration of multiple subtypes of inflammatory DCs, which could serve as a link between innate and adaptive immunity.
Keywords: acute skin inflammation, inflammatory dendritic cells, macrophages, antimicrobial peptides, BDCA-3
INTRODUCTION
The acute whole-tissue response of human skin to UVB has not been well characterized. Past studies have examined only the epidermal response to broad-band UVB (Enk et al., 2004), or the whole-tissue response to irradiation performed with solar-simulated radiation (which combines UVA and UVB) (Enk et al., 2004; Mouchet et al., 2010). We sought to define the acute response of human skin to erythemogenic doses of narrowband UVB (NB-UVB; 312 nm peak), a source of UVB used in phototherapy, at 24 hours following two minimal erythema doses (2MED) exposure using transcriptional profiling and immune cell characterization.
Depending on the level of exposure, the response of human skin to UVB irradiation ranges from mild erythema and pain to more extensive damage that includes apoptosis of keratinocytes (KCs) (“sunburn cells”) or extensive epidermal necrosis and blistering. Larger exposures to UV light, therefore, represent a form of epidermal wounding, which must engage an appropriate wound repair response in order to restore the epidermal barrier and protect against infection. Studies have shown that UVB irradiation of human skin can upregulate protective innate immune mechanisms, including antimicrobial peptides (AMPs) (S100A7, S100A8, RNase7, and human beta-defensin) (Glaser et al., 2009; Lee et al., 2009) which are chemotactic for neutrophils, dendritic cells (DCs) and macrophages, and provide direct protection against pathogens. KCs are known to generate AMPs in response to mechanical wounding (Kesting et al., 2010; Sorensen et al., 2006).
Prior murine studies have shown that acute UVB exposure creates an immunosuppressive environment in irradiated skin (Fisher and Kripke, 1977; Phan et al., 2006). Langerhans cells (LC) are reduced by 50% after a single dose of 1.5 MEDs of UVB (Murphy et al., 1993), while at the same time there is an accumulation of a poorly characterized “UV-DC” population, that is HLA-DR+CD45+ (subset CD36+) (Meunier et al., 1995). However, at the time of that work, CD11c, which stained some cells in the UVB-induced infiltrate, was considered to be an extended marker of macrophages, and clear distinctions between dermal macrophages and DC populations were lacking. It is now recognized that CD11c is the most general marker of myeloid DCs in human skin, with further sub-classification of myeloid DCs into specific subtypes (such as BDCA1+/CD1c+ and BDCA3/CD141+), with different states of maturity (e.g. having maturation markers DC-LAMP or DEC-205), as well as inflammatory subsets (Zaba et al., 2007; Zaba et al., 2009b). We have previously reported that there is markedly decreased epidermal staining for Langerin, a specific marker for Langerhans cells, in irradiated versus non-irradiated samples (Kennedy-Crispin et al., 2012). In this study, we further characterized the effect of NB-UVB on cutaneous DC populations by a combination of IHC and IF analysis for specific subsets.
Overall, we provide the first transcriptional profiling of full thickness human skin during the acute response to erythemogenic doses of narrowband UVB. We identified 1,522 unique differentially regulated genes between irradiated and non-irradiated skin including many immune-modulating cytokines, chemokines, AMPs, and leukocyte surface markers. Using protein staining, we provided further evidence of the early upregulation of AMPs in the epidermis. We demonstrated that following NB-UVB irradiation, the mononuclear cell infiltrate included macrophages and multiple subtypes of DCs including CD11c+ BDCA3+ cells, a DC subtype that has not been previously identified in UVB-irradiated skin and that has recently been shown to cause immunosuppression (Chu et al., 2012). Our study shows that the acute response of human skin to NB-UVB includes upregulation of innate defense pathways and mobilization of distinct DC subsets that may provide defense against pathogens and ultimately restore cutaneous immunity.
RESULTS
Microarray profiling identifies UVB-responsive genes
Ten subjects with skin type II-III were irradiated with 2MED of 312 nm narrow-band ultraviolet B (NB-UVB) radiation (Judson et al., 2010), under a Rockefeller University IRB-approved protocol. Informed consent was obtained and the study was performed in adherence with the Principles of the Declaration of Helsinki. NB-UVB was chosen as it is used therapeutically to treat chronic skin diseases, and 2MEDs was chosen as this level of NB-UVB is known to induce “sunburn cells” in the epidermis (Coven et al., 1997). A 24-hour time-point was evaluated as cellular changes can be measured at this time after cutaneous injury (Johnson-Huang et al., 2012). Punch biopsies (6mm) of irradiated and non-irradiated (protected from UVB) skin were taken from each patient after 24 hours of NB-UVB exposure, and RNA was extracted and hybridized to HG U133 plus 2.0 chips. In this analysis, 2,019 probe sets were identified, encoding 1,522 unique differentially expressed genes (DEG) between irradiated and non-irradiated samples (FCH>2, FDR<0.01) (Supplemental Table S1). A selected list of inflammatory and immune-related genes from 818 upregulated and 704 downregulated DEG, is presented in Table 1. The list of upregulated genes included a large number of AMPs, cytokines, chemokines, and immune cell markers, discussed further below.
Table 1.
Selected DEG for paired irradiated versus non-irradiated skin
| Gene symbol/protein |
Description | Gene chip FCH a, b |
PCR FCH |
PCR p value |
|---|---|---|---|---|
| MMP1 | matrix metallopeptidase 1 (interstitial collagenase) |
149.3 | ||
| DEFB4A/HBD2 | Defensin, beta 4A | 73.0 | ||
| CXCL1 | chemokine (C-X-C motif) ligand 1 (melanoma growth stimulating activity, alpha) |
60.7 | 5.8 | 5.3 × 10−4 |
| CCL20 | chemokine (C-C motif) ligand 20 | 52.2 | 8.3c | 2.6 × 10−3 |
| PI3/Elafin | peptidase inhibitor 3, skin-derived | 45.1 | ||
| MMP3 | matrix metallopeptidase 3 (stromelysin 1, progelatinase) |
44.6 | ||
| CXCL2 | chemokine (C-X-C motif) ligand 2 | 35.9 | ||
| IL6 | Interleukin 6 | 33.5 | ||
| S100A9 | S100 calcium binding protein A9 | 28.7 | ||
| CXCL3 | chemokine (C-X-C motif) ligand 3 | 26.5 | ||
| IL8 | Interleukin 8 | 24.4 | 10.5 | 2.7 × 10−4 |
| S100A7 | S100 calcium binding protein A7 | 14.4 | ||
| CCL8 | chemokine (C-C motif) ligand 8 | 14.1 | ||
| PTGS2 | Prostaglandin-endoperoxide synthase 2 (prostaglandin G/H synthase and cyclooxygenase) |
11.1 | ||
| CCR1 | Chemokine (C-C motif) receptor 1 | 10.9 | ||
| CCL18 | Chemokine (C-C motif) ligand 18 | 9.9 | ||
| S100A8 | S100 calcium binding protein A8 | 9.0 | ||
| S100A12 | S100 calcium binding protein A12 | 8.5 | ||
| ICAM1 | Intercellular adhesion molecule 1 | 6.7 | ||
| KRT16 | Keratin 16 | 6.0 | ||
| CCL2 | Chemokine (C-C motif) ligand 2 | 5.6 | 3.4 | 7.9× 10−3 |
| IL1B | Interleukin 1, beta | 5.5 | ||
| CD209 | CD209 molecule | 5.03 | ||
| CD163 | CD163 molecule | 4.8 | ||
| IL4R | Interleukin 4 receptor | 4.2 | ||
| CCR7 | Chemokine (C-C motif) receptor 7 | 3.7 | ||
| IL24 | Interleukin 24 | 3.6 | ||
| CCR2 | Chemokine (C-C) receptor 2 | 3.4 | ||
| CD14 | CD14 molecule | 3.4 | ||
| CCL22 | Chemokine (C-C) ligand 22 | 3.3 | ||
| TLR2 | Toll-like receptor 2 | 2.9 | ||
| CD83 | CD83 molecule | 2.8 | ||
| S100A2 | S100 calcium binding protein A2 | 2.6 | ||
| IL15 | Interleukin 15 | 2.1 | ||
| CD207 | CD207 molecule, langerin | −9.8 | ||
| CD1A | CD1A molecule | −7.7 | ||
| IL-10 | 2.5 | 3.6 × 10−3 |
FCH = Fold-change in gene expression from microarray data
FDR < 0.01 for all genes
Previously published (Kennedy-Crispin et al., 2012)
Immunohistochemistry confirms upregulation of AMPs in the epidermis and dermis
According to our microarray analysis, NB-UVB induced increased expression of many AMPs in human skin. Prior in vivo studies of human skin have shown that broad-band UVB upregulates protein expression of some AMPs such as S100A7 and HBD-2 several days following initial UVB exposure (Glaser et al., 2009), but their expression during the acute stage of inflammation following irradiation (<24 hours) has not been defined. We performed IHC on skin biopsies (n=7-10) for differentially upregulated AMPs, which included S100A7, S100A12, S100A8/9 (encodes calprotectin), DEFB4 (encodes human beta-defensin 2 protein, HBD-2), and PI3 (encodes elafin protein).
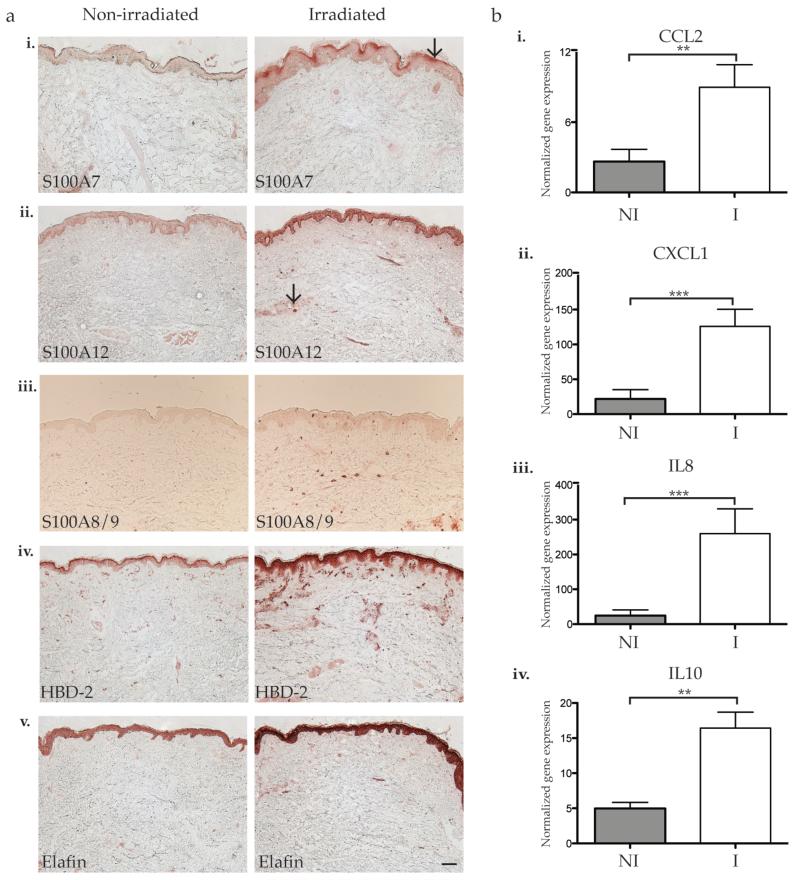
Epidermal staining of S100A7 was increased following irradiation compared to the non-irradiated skin (Figure 1a, i). Low level expression of S100A7 was noted in the epidermis of non-irradiated skin while an increased focal expression in the granular layer was observed in irradiated skin. S100A12 was detectable in the epidermis in non-irradiated skin, but an increased staining throughout the entire epidermis, with a few dermal cells was noted in irradiated skin (Figure 1a, ii). Most patients demonstrated faint expression of S100A8/9 in the epidermis of non-irradiated skin (Figure 1a, iii). Following irradiation, there was increased dermal staining of S100A8/9, which may be due to neutrophilic infiltration, as both proteins are produced by neutrophils during acute inflammation (Ryckman et al., 2003). In non-irradiated skin, HBD-2 was expressed in the epidermis and concentrated more heavily in the upper granular layer. Following irradiation, there was increased epidermal staining with the intensity more concentrated in the granular layer (Figure 1a, iv). Similarly, elafin staining was increased throughout the epidermis and dermis of irradiated skin compared to non-irradiated skin (Figure 1a, v). Therefore, in the acute inflammatory phase following NB-UVB irradiation, there was increased expression of S100A7, S100A12, HBD-2, and elafin in the epidermis, and S100A8/9 also appeared to increase in dermal cells.
Figure 1. Differentially expressed antimicrobial peptides and chemokines were validated by immunohistochemistry or qRT-PCR.
(a) Representative IHC for (i) S100A7, (ii) S100A8/9, (iii) S100A12, (iv) HBD-2, and (v) elafin showed increased S100A7, S100A12, HBD-2, and elafin staining in the epidermis and increased S100A8/9 and HBD-2 in the dermis of irradiated samples. Arrow indicates (i) focal epidermal expression of S100A7, and (ii) S100A12+ dermal cell. Size bars = 100 μm. (b) qRT-PCR using mRNA from paired non-irradiated and irradiated skin (n=9) showed increased expression of (i) CCL2, (ii) CXCL1, (iii) IL-8, and (iv) IL-10 in irradiated skin. All data were normalized to hARP (human acidic ribosomal protein) housekeeping gene. Error bars indicate SEM, NI = non-irradiated skin, I = irradiated skin. * p<0.05, ** p<0.01, *** p<0.001.
RT-PCR confirms differentially expressed genes in microarray profiling
To confirm microarray findings, quantitative reverse transcriptase-PCR (qRT-PCR) was performed on irradiated and non-irradiated skin biopsies for several key chemokines. (Figure 1b, Table 1 for FCH and p value). We have previously published that NB-UVB increased CCL20 mRNA and protein in this group of samples (Kennedy-Crispin et al., 2012). In addition, CCL2, CXCL1 and IL8 were significantly upregulated in irradiated versus non-irradiated skin biopsies (p < 0.05 for all; Figure 1bi, ii, iii; Table 1). The expression of IL-10 was also of interest, as IL-10 is known to be upregulated acutely in response to UVB (Kang et al., 1994). Although microarray analysis did not detect differential expression of this cytokine, which is often the case for low expression transcripts (Suarez-Farinas et al., 2010), IL-10 was upregulated in irradiated skin by qRT-PCR (p=0.01).
As we have previously reported, there was no increase in T cells in irradiated versus non-irradiated skin (Kennedy-Crispin et al., 2012), and prior studies have shown that T cell infiltration/expansion does not occur until approximately 48 hours after UVB irradiation (Di Nuzzo et al., 1998). Common T cell cytokines were measured, including IL-4, IL-5, IL-17, IL-22, TGF-β, and IFN-γ, and were not differentially expressed 24 hours after NB-UVB irradiation (data not shown).
Ingenuity Pathway Analysis identified multiple significant innate defense and early adaptive immune pathways
As microarray analysis identified over 1,500 DEG, Ingenuity Pathway Analysis (IPA) was conducted to elucidate the immune pathways that were significant during acute NB-UVB injury, shown in Supplemental Table S2 (p < 0.05). As UVB causes cell death and DNA damage (including the formation of pyrimidine dimers), it was not surprising that Cell Cycle and DNA Repair, Pyrimidine Metabolism, p53, and Apoptosis Signaling were activated. Additionally, melanocytes are known to upregulate their production of melanin as a protective response to UVB exposure and accordingly, Phenylalanine, Tyrosine and Tryptophan Biosynthesis pathway was also significant. Innate immune protective pathways identified included Acute Phase Response Signaling, Toll-like Receptor Signaling, Role of Pattern Recognition of Bacteria and Viruses, and IL-8 Signaling. IL-10 Signaling was upregulated, and surprisingly, multiple IL-17 pathways were activated even though IL-17 was not found to be differentially expressed between irradiated and non-irradiated samples as measured by microarray or RT-PCR. Pathways of early activation of adaptive immunity included DC Maturation, Communication between Innate and Adaptive Immune Cells, and IL-2 Signaling. Overall, IPA identified pathways known to be associated with UV skin damage, as well as multiple innate and early adaptive immune pathways.
Acute response to UVB includes distinct populations of DCs and macrophages
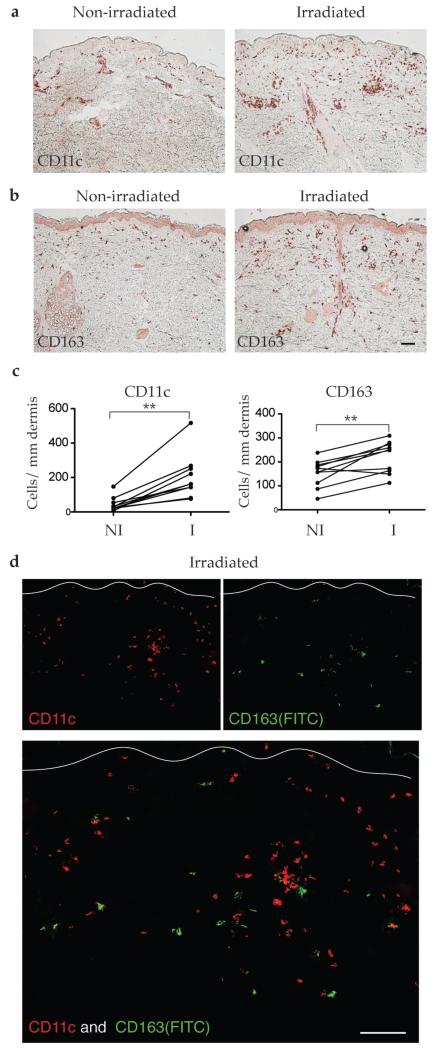
Genomic markers of leukocyte lineage were increased (CD209, CD163, CD14, and CD83) or decreased (CD207 and CD1A) by irradiation (Table 1; Supplemental Table S1). We have previously shown a decrease in Langerin+ Langerhans cells in irradiated skin (Kennedy-Crispin et al., 2012). The acute response to UVB was thought to include a monocytic infiltrate composed largely of macrophages. It is now known that some of these cells also express CD11c (Meunier et al., 1995), a specific marker for dermal DCs in human skin. There was a five-fold increase in CD11c+ dendritic cells in the dermis of irradiated skin (Figure 2a, c, p=0.002), and approximately a two-fold increase in CD163+ macrophages in the dermis (Figure 2b, d, p=0.006). As previously shown in normal skin, CD11c and CD163 represent distinct dermal cell populations (Zaba et al., 2007), which was confirmed by two-color immunofluorescence (IF) with these markers in irradiated skin (Figure 2d).
Figure 2. Irradiated skin contained increased CD11c+ dendritic cells and CD163+ macrophages.
Representative immunohistochemistry and cell counts showed (a, c) increased CD11c+ dendritic cells, and (b, c) increased CD163+ macrophages in the dermis of irradiated skin compared to paired non-irradiated skin. (d) There was no co-expression between CD11c+ myeloid DCs (red) and CD163+ macrophages (green) in irradiated skin.
UVB-irradiated skin includes multiple myeloid DC subtypes, including BDCA3+ DCs
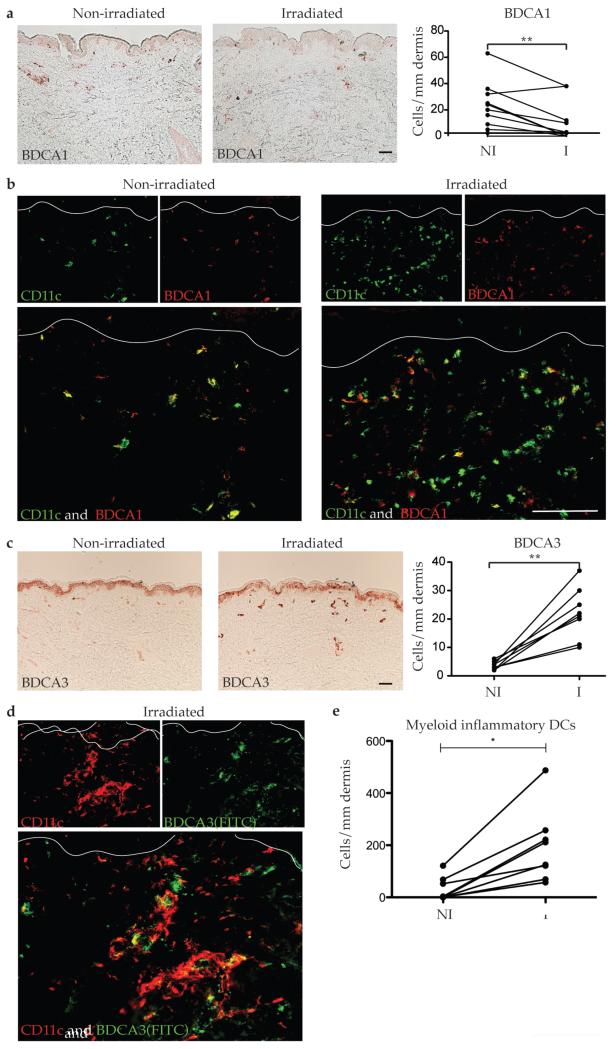
As DCs elaborate inflammatory molecules that recruit T cells to sites of inflammation and modulate T cell responses through direct communication, we next sought to further define the myeloid DC subtypes that infiltrate the skin following acute UVB exposure. There was an overall decrease in dermal BDCA1+ cells (Figure 3a, p=0.018), which is consistent with the prior observation that resident dermal DCs (CD11c+ BDCA1+) are depleted by UVB (Meunier et al., 1995). We have previously defined inflammatory DCs as CD11c+ BDCA1− cells based on our studies in psoriasis (Zaba et al., 2009b). One prior study showed that the infiltrating CD11c+ cells in UVB-irradiated skin were BDCA1− (Meunier et al., 1995). By CD11c and BDCA1 two color IF, almost all CD11c+ in non-irradiated skin cells were BDCA1+, while in irradiated skin, most of the CD11c+ cells were BDCA1− (Figure 3b). This demonstrated that the majority of dermal cells in irradiated skin were CD11c+ BDCA1−, most likely inflammatory DCs.
Figure 3. CD11c+ BDCA1− and CD11c+ BDCA3+ DCs were increased in NB-UVB-irradiated skin.
Representative immunohistochemistry, cell counts, and IF for DC surface markers CD11c, BDCA1, and BDCA3 in non-irradiated and irradiated skin (n=8-10) showed (a) BDCA1+ cells decreased two-fold in irradiated skin, with (b) decreased co-localization of CD11c and BDCA1 in irradiated skin compared to non-irradiated skin. (c) BDCA3+ cells increased 4.5-fold. (d) Most BDCA-3+ cells co-localizated with CD11c in irradiated skin (yellow cells), but there were many CD11c+ cells that did not co-localize with BDCA-3 (red cells). (e) Inflammatory DCs (CD11c+ BDCA1− BDCA3−) were elevated in irradiated vs non-irradiated skin, as calculated by subtracting BDCA1+ and BDCA3+ cells from CD11c+ cells. Size bar = 100μm. NI = non-irradiated skin, I = irradiated skin. * p<0.05.
Since the identification of CD11c+BDCA1− DCs that infiltrate the skin following UVB irradiation, additional DC subtypes have been identified as BDCA3+, but whether they are involved in the response to UVB irradiation has not been investigated. There was a five-fold increase in BDCA3+ cells in the dermis of irradiated skin (Figure 3c, p=0.0005), similar to the magnitude of increase in CD11c+ cells, but still lower in number. To confirm that the BDCA3+ cells were DCs, we co-stained CD11c with BDCA3 (Figure 3d) and most BDCA3+ cells co-localized with CD11c in irradiated skin. However, there were many CD11c+ cells that did not co-localize with BDCA-3 (Figure 3d). An estimate of cells that may be more correctly considered inflammatory myeloid DCs can be calculated by subtraction of both BDCA-1+ and BDCA-3+ cells from CD11c+ cells, as we have calculated for psoriasis skin lesions (Zaba et al., 2009a). Using this approach, there were significantly elevated inflammatory myeloid DCs in irradiated skin (p=0.014) (Figure 3e). Hence, we show that irradiation increased BDCA3+ DCs, a subset of CD11c+ myeloid DCs, as well as inflammatory DCs, defined as CD11c+ BDCA1− BDCA3−.
Characterization of UVB-induced inflammatory DCs
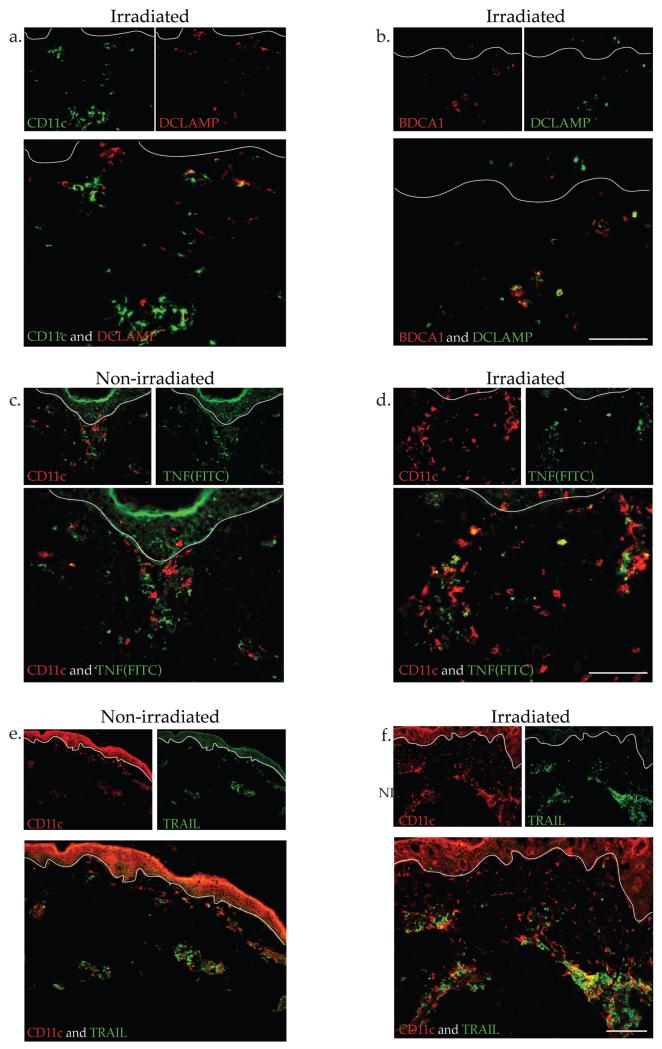
As DCs in normal skin are generally immature DCs (Zaba et al., 2009b), we were interested in evaluating the maturation state of DCs after irradiation. Few CD11c+ cells co-expressed the maturation marker DC-LAMP while most BDCA1+ cells co-expressed this marker (Figure 4a, b). To further determine if inflammatory DCs were present (Zaba et al., 2010), CD11c was co-stained with inflammatory DC markers TNF-α (Figure 4c, d) and TRAIL (Figure 4e, f). In irradiated skin, there were many CD11c+ cells that co-localized with TNF-α, and some that co-localized with TRAIL compared to non-irradiated skin. These results suggests that it is the BDCA1+ DCs that mature with irradiation, and the CD11c+ inflammatory myeloid DCs induced by irradiation are expressing markers seen in other states of inflammation such as psoriasis.
Figure 4. CD11c+ cells were immature and expressed inflammatory markers TNF and TRAIL.
(a) Representative IF showed occasional co-localization of CD11c+ cells with maturation marker DC-LAMP in non-irradiated skin but (b) many BDCA-1+ cells expressed DC-LAMP after irradiation. UVB-irradiated skin had increased co-localization of CD11c and (c, d) TNF and (e, f) TRAIL, compared to non-irradiated skin.
DISCUSSION
The acute response to erythemal doses of UVB is epidermal damage, leading to apoptosis of keratinocytes (“sunburn cells”) and epidermal loss (blistering). There are molecular changes associated with acute UV exposure, such as mutations in p53 and ras, setting up the need for a protective response to this histological and molecular cutaneous damage. Our view is that the response of skin to high levels of UVB must accomplish three goals: (1) repair damaged keratinocytes, (2) increase innate immune defenses, and (3) restore cutaneous homeostasis. Our study provides support for the acute inflammatory effects of high dose narrowband UVB exposure with expression of abundant inflammatory molecules, and alterations in populations of dendritic cells as the tissue tries to restore homeostasis.
To our knowledge, there is only one other microarray study that examines the acute effect of UVB on human skin in vivo (Enk et al., 2004). Using the Affymetrix Human Focus oligonucleotide array, that study found differential regulation of approximately 800 genes after epidermal suction blisters were obtained from normal human volunteers (n=3), 24 hours after irradiation with 4MED of broadband UVB. Our study differs from the Enk et al. report in several ways: a lower dose (2MED) of narrowband UVB was given, full-thickness skin was examined, and Affymetrix HG U133 plus 2.0 chips were studied. Almost twice as many gene transcripts were detected in our study, most likely due to the modern microarray chip, the inclusion of dermal tissue, as well as increased statistical power from study of more patients (n=10 vs. 3).
As keratinocytes have been shown to upregulate innate immune molecules during the early stages of wound healing (Barrientos et al., 2008), UVB irradiation similarly upregulates protective immune mechanisms. For instance, sunburn patients rarely suffer from bacterial superinfections or secondary impetiginization (Termorshuizen et al., 2002), and patients with impetiginized atopic dermatitis can improve from UVB without additional antibiotic treatment (Silva et al., 2006).
Our study provides evidence that NB-UVB provokes early induction of innate immune mediators, such as the production of cytokines and chemokines that serve as neutrophil chemoattractants (IL-8, CXCL1, CXCL2, CXCL3) (Devalaraja et al., 2000; Gillitzer and Goebeler, 2001) and monocyte/DC chemoattractants (CCL20, CCL2) (Dieu-Nosjean et al., 1999; Gillitzer et al., 1993) (Kennedy-Crispin et al., 2012). This is consistent with other studies. Microarray profiling of a number of cytokines/chemokines that are known to activate KCs and recruit neutrophils following acute epidermal injury were increased in response to UVB, including IL-8, CXCL1, CXCL2, and CXCL3 (Barrientos et al., 2008). Chemokines known to recruit immature DCs and T cells into the skin included CCL2 and CCL20 (Caux et al., 2000; Dieu-Nosjean et al., 1999) were also increased. Cytokines known to be upregulated during acute epidermal injury and in response to UVB irradiation included IL-6 and IL-1β (Barrientos et al., 2008). Hence UVB induces many chemokines and cytokines that play a role activating the innate immune system and recruiting leukocytes to respond to the cutaneous injury.
AMPs are a conserved component of the innate immune response that have potent antibiotic activity as well as immunomodulatory capabilities by altering cytokine/chemokine production and modulating DC and T cell responses, thereby playing a role in bridging innate and adaptive immunity (Braff and Gallo, 2006; Gallo and Nakatsuji, 2011). Our studies showed that different AMPs appear to be rapidly regulated, including AMPs that share chromosomal localization at 1q21 as part of the epidermal differentiation complex (EDC) (Marenholz et al., 2001). For example, S100A7, S100A12, HBD-2 and elafin showed an increase in mRNA expression and epidermal protein by 24 hours. In contrast, neutrophilic S100A8/9 mRNA was upregulated after 24 hours but protein was only expressed in dermal cells. Other studies have shown that UVB upregulated AMPs (S100A7, S100A8/9, S100A12, human beta-defensin 2, and elafin), which can serve as both neutrophil and monocyte/DC chemoattractants (Eckert et al., 2004; Schroder and Harder, 2006). AMPs provide direct protection against gram positive and gram negative bacteria, mycobacteria, fungi, enveloped viruses, and even actinically-damaged cells (Conner et al., 2002; Gallo and Nakatsuji, 2011; Glaser et al., 2005; Lopez-Garcia et al., 2005). They also have immunomodulatory activities such as chemotaxis of innate and adaptive immune cells (Kerkhoff et al., 1999; Miranda et al., 2001; Wolf et al., 2008; Yan et al., 2008; Yang et al., 1999). Prior in vivo studies in humans have shown that UVB increases epidermal mRNA and/or protein expression of S100A7, S100A8, and hBD-2 two or more days following UVB (Glaser et al., 2009; Lee et al., 2009). Overall, these results show that S100A7, S100A12 and HBD-2 were prominent components of the acute epidermal inflammatory response to UVB, but that S100A8/9 protein expression by epidermal keratinocytes is likely upregulated in later stages of the UVB response, similar to chronic inflammatory skin diseases such as psoriasis (Broome et al., 2003; Lee et al., 2009; Madsen et al., 1991).
There was also a differential response of antigen presenting cells to acute UVB. Prior studies have shown that in response to UVB, LCs and classic CD11c+ BDCA1+ dermal DCs are depleted from the epidermis and dermis while there was concomitant expansion of HLA-DR+ CD11c+ BDCA1− CD1a− cells in the dermis (Kennedy-Crispin et al., 2012; Meunier et al., 1995). We confirmed these findings using in situ methods. Furthermore, after acute NB-UVB irradiation, dermal CD11c+ cells were indeed consistent with inflammatory DCs as they co-expressed TNF and TRAIL, and lacked the maturity marker DC-LAMP. In addition, there was an increased population of BDCA3+ DCs, which have not previously been identified in UV-irradiated skin. These BDCA3+ DCs are considered to be analogous to mouse CD8+ DCs, which have the ability to cross-present antigens to CD8+ T cells (Guilliams et al., 2010). In a recent publication by Chu et al, cutaneous human BDCA3+ cells were able to suppress skin inflammation. This immunosuppressive capacity may be an important immune mechanism for identifying and eliminating UV-damaged cells, thus maintaining self-tolerance during this process. It has been shown that the mononuclear cell infiltrate in UVB-irradiated murine skin is necessary for local immunosuppression to occur (Hammerberg et al., 1994, 1996), and that this immunosuppression is also tightly linked to the activity of regulatory T cells 48-72 hours following exposure (Schwarz, 2008). It will be important to determine the role of BDCA3+ cells during chronic UV exposure, to see if they fulfill this immunoprotective role.
We have defined the acute transcriptional response of human skin to erythemal doses of UVB radiation, which included a vast array of inflammatory molecules and immune cell markers. Chemokines were elevated, and keratinocytic AMPs were induced, including S100A7, S100A12, HBD-2, and elafin, suggesting a high-level activation of innate immune mechanisms. We also showed that, while Langerhans cells and BDCA-1+ resident dendritic cells (DCs) were depleted by high levels of UVB irradiation, several DC subtypes, including inflammatory myeloid DCs and BDCA3+ DCs, infiltrated the dermis within 24 hours of an erythemal dose of UVB. These inflammatory DCs are likely to contribute to inflammation, while the BDCA-3+ DC responses may be involved in the longer phase homeostatic cutaneous response to UVB.
MATERIALS AND METHODS
Ultraviolet B radiation
NB-UVB-treated skin and paired non-irradiated samples from a previous study were used (Judson et al., 2010). Archived materials included whole tissue RNA extracts and cryostat sections of skin biopsies.
RNA extraction, quantification, and microarray
Total RNA was extracted using RNeasy Micro Kit (Qiagen Valencia, CA) according to manufacturer’s protocol with on-column Dnase digestion. The amount of RNA was assessed by quantitative RT-PCR for human acidic ribosomal protein (hARP) using the 7900HT Fast Real-Time PCR System (Applied Biosystems). Quality of extracted RNA was examined using Agilent Bioanalyzer 2100 (Agilent Technologies, Palo Alto, CA). RNA was hybridized to HG U133 plus 2.0 chips to measure relative gene expression.
Quantitative RT-PCR
Quantitative RT-PCR was performed using Taqman gene expression assays as previously described (Chamian et al., 2005). A list of primers is included in Supplemental Table S3. All data were analyzed by the Applied Biosystems PRISM 7700 software (Sequence Detection Systems, ver. 1.7) and normalized to hARP housekeeping gene.
Immunohistochemistry and Immunofluorescence
Standard procedures were used for IHC and IF as previously described (Zaba et al., 2009a). Frozen tissue sections of normal and irradiated skin sections (n=7-10) were stained with CD11c, BDCA1, BDCA3, DCLAMP, CD163, S100A7, S100A8/9, S100A12, HBD-2 (Supplemental Table S4) and the signal was amplified with avidin-biotin complex (Vector Laboratories) and developed using chromogen 3-amino-9-ethylcarbazole (Sigma-Aldrich). The number of positive cells per mm was counted manually per field using computer-assisted image analysis (NIH Image 6.1;http://rsb.info.nih.gov/ nih-image) as previously described (Fuentes-Duculan et al., 2010).
IF images were acquired using the appropriate filters of a Zeiss Axioplan 2 widefield fluorescence microscope with a Plan Neofluar 20 × 0.7 numerical aperture lens and a Hamamatsu Orca Ercooled charge-coupled device camera, controlled by METAVUE software (MDS Analytical Technologies, Downington, PA). Images in each figure are presented both as single color stains (green and red) located above the merged image, so that localization of two markers on similar or different cells can be appreciated. Cells that coexpress the two markers in a similar location are yellow in color. A white line denotes the dermoepidermal junction. Dermal collagen fibers gave green autofluorescence, and antibodies conjugated with a fluorochrome often gave background epidermal fluorescence.
Statistical analysis
Affymetrix CEL files were scanned using software packages Harshlight (Suarez-Farinas et al., 2005) and arrayQualityMetrics from R/Bioconductor (www.bioconductor.org). Expression values (in log2-scale) were obtained using the GCRMA algorithm. Genes with expression higher than three in at least two samples and standard deviation > 0.05 were included in the statistical analysis. To identify differentially expressed genes (DEG), a moderated t-test was used in the limma package framework. Resultant P-values were adjusted for multiple hypotheses using the Benjamini-Hochberg procedure, which controls for the False Discovery Rate (FDR). The data discussed in this publication have been deposited in the National Center for Biotechnology Information’s Gene Expression Omnibus (GSEXXXX).
Differential gene expression as measured by PCR was normalized by hARP. Log-transformed PCR data and cell counts were analyzed by two-tailed non-parametric paired test (Wilcoxon signed rank test) using GraphPad Prism software, and significance accepted as p<0.05. Ingenuity pathway analysis (Ingenuity Systems, Redwood City, CA) was used to identify the Pathways enriched in the DEG lists.
Supplementary Material
ACKNOWLEGEMENTS
Research supported by National Institutes of Health (NIH) grant UL1 RR024143 from the National Center for Research Resources (NCRR) and the Milstein Medical Program. NG was supported by NIH MSTP grant GM07739. TL is supported by 3K23AR052404-04S1; MAL is supported by NIH 1R01AR060222; LMJ-H is supported by the Linda and Leonard Berkowitz Postdoctoral Fellowship.
Abbreviations
- AMP
antimicrobial peptide
- DCs
dendritic cells
- DEG
differentially expressed genes
- FCH
fold change
- FDR
false discovery rate
- hARP
human acidic ribosomal protein
- IF
immunofluorescence
- IHC
immunohistochemistry
- IPA
Ingenuity Pathway Analysis
- KC
keratinocyte
- LC
Langerhans cell
- MED
minimal erythemal dose
- qRT-PCR
quantitative reverse transcriptase-PCR
- NB-UVB
narrow-band ultraviolet B
REFERENCES
- Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- Braff MH, Gallo RL. Antimicrobial peptides: an essential component of the skin defensive barrier. Current topics in microbiology and immunology. 2006;306:91–110. doi: 10.1007/3-540-29916-5_4. [DOI] [PubMed] [Google Scholar]
- Broome AM, Ryan D, Eckert RL. S100 protein subcellular localization during epidermal differentiation and psoriasis. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2003;51:675–85. doi: 10.1177/002215540305100513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caux C, Ait-Yahia S, Chemin K, de Bouteiller O, Dieu-Nosjean MC, Homey B, et al. Dendritic cell biology and regulation of dendritic cell trafficking by chemokines. Springer seminars in immunopathology. 2000;22:345–69. doi: 10.1007/s002810000053. [DOI] [PubMed] [Google Scholar]
- Chamian F, Lowes MA, Lin SL, Lee E, Kikuchi T, Gilleaudeau P, et al. Alefacept reduces infiltrating T cells, activated dendritic cells, and inflammatory genes in psoriasis vulgaris. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:2075–80. doi: 10.1073/pnas.0409569102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu CC, Ali N, Karagiannis P, Di Meglio P, Skowera A, Napolitano L, et al. Resident CD141 (BDCA3)+ dendritic cells in human skin produce IL-10 and induce regulatory T cells that suppress skin inflammation. The Journal of experimental medicine. 2012;209:935–45. doi: 10.1084/jem.20112583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner K, Nern K, Rudisill J, O’Grady T, Gallo RL. The antimicrobial peptide LL-37 is expressed by keratinocytes in condyloma acuminatum and verruca vulgaris. Journal of the American Academy of Dermatology. 2002;47:347–50. doi: 10.1067/mjd.2002.122190. [DOI] [PubMed] [Google Scholar]
- Coven TR, Burack LH, Gilleaudeau R, Keogh M, Ozawa M, Krueger JG. Narrowband UV-B produces superior clinical and histopathological resolution of moderate-to-severe psoriasis in patients compared with broadband UV-B. Archives of dermatology. 1997;133:1514–22. [PubMed] [Google Scholar]
- Devalaraja RM, Nanney LB, Du J, Qian Q, Yu Y, Devalaraja MN, et al. Delayed wound healing in CXCR2 knockout mice. The Journal of investigative dermatology. 2000;115:234–44. doi: 10.1046/j.1523-1747.2000.00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Nuzzo S, Sylva-Steenland RM, de Rie MA, Das PK, Bos JD, Teunissen MB. UVB radiation preferentially induces recruitment of memory CD4+ T cells in normal human skin: long-term effect after a single exposure. The Journal of investigative dermatology. 1998;110:978–81. doi: 10.1046/j.1523-1747.1998.00220.x. [DOI] [PubMed] [Google Scholar]
- Dieu-Nosjean MC, Vicari A, Lebecque S, Caux C. Regulation of dendritic cell trafficking: a process that involves the participation of selective chemokines. Journal of leukocyte biology. 1999;66:252–62. doi: 10.1002/jlb.66.2.252. [DOI] [PubMed] [Google Scholar]
- Eckert RL, Broome AM, Ruse M, Robinson N, Ryan D, Lee K. S100 proteins in the epidermis. The Journal of investigative dermatology. 2004;123:23–33. doi: 10.1111/j.0022-202X.2004.22719.x. [DOI] [PubMed] [Google Scholar]
- Enk CD, Shahar I, Amariglio N, Rechavi G, Kaminski N, Hochberg M. Gene expression profiling of in vivo UVB-irradiated human epidermis. Photodermatology, photoimmunology & photomedicine. 2004;20:129–37. doi: 10.1111/j.1600-0781.2004.00097.x. [DOI] [PubMed] [Google Scholar]
- Fisher MS, Kripke ML. Systemic alteration induced in mice by ultraviolet light irradiation and its relationship to ultraviolet carcinogenesis. Proceedings of the National Academy of Sciences of the United States of America. 1977;74:1688–92. doi: 10.1073/pnas.74.4.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes-Duculan J, Suárez-Fariñas M, Zaba LC, Nograles KE, Pierson KC, Mitsui H, et al. A subpopulation of CD163-positive macrophages is classically activated in psoriasis. The Journal of investigative dermatology. 2010;130:2412–22. doi: 10.1038/jid.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo RL, Nakatsuji T. Microbial symbiosis with the innate immune defense system of the skin. The Journal of investigative dermatology. 2011;131:1974–80. doi: 10.1038/jid.2011.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillitzer R, Goebeler M. Chemokines in cutaneous wound healing. Journal of leukocyte biology. 2001;69:513–21. [PubMed] [Google Scholar]
- Gillitzer R, Wolff K, Tong D, Muller C, Yoshimura T, Hartmann AA, et al. MCP-1 mRNA expression in basal keratinocytes of psoriatic lesions. The Journal of investigative dermatology. 1993;101:127–31. doi: 10.1111/1523-1747.ep12363613. [DOI] [PubMed] [Google Scholar]
- Glaser R, Harder J, Lange H, Bartels J, Christophers E, Schroder JM. Antimicrobial psoriasin (S100A7) protects human skin from Escherichia coli infection. Nature immunology. 2005;6:57–64. doi: 10.1038/ni1142. [DOI] [PubMed] [Google Scholar]
- Glaser R, Navid F, Schuller W, Jantschitsch C, Harder J, Schroder JM, et al. UV-B radiation induces the expression of antimicrobial peptides in human keratinocytes in vitro and in vivo. The Journal of allergy and clinical immunology. 2009;123:1117–23. doi: 10.1016/j.jaci.2009.01.043. [DOI] [PubMed] [Google Scholar]
- Guilliams M, Henri S, Tamoutounour S, Ardouin L, Schwartz-Cornil I, Dalod M, et al. From skin dendritic cells to a simplified classification of human and mouse dendritic cell subsets. European journal of immunology. 2010;40:2089–94. doi: 10.1002/eji.201040498. [DOI] [PubMed] [Google Scholar]
- Hammerberg C, Duraiswamy N, Cooper KD. Active induction of unresponsiveness (tolerance) to DNFB by in vivo ultraviolet-exposed epidermal cells is dependent upon infiltrating class II MHC+ CD11bbright monocytic/macrophagic cells. J Immunol. 1994;153:4915–24. [PubMed] [Google Scholar]
- Hammerberg C, Duraiswamy N, Cooper KD. Reversal of immunosuppression inducible through ultraviolet-exposed skin by in vivo anti-CD11b treatment. J Immunol. 1996;157:5254–61. [PubMed] [Google Scholar]
- Johnson-Huang LM, Suarez-Farinas M, Pierson KC, Fuentes-Duculan J, Cueto I, Lentini T, et al. A single intradermal injection of IFN-gamma induces an inflammatory state in both non-lesional psoriatic and healthy skin. The Journal of investigative dermatology. 2012;132:1177–87. doi: 10.1038/jid.2011.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judson BL, Miyaki A, Kekatpure VD, Du B, Gilleaudeau P, Sullivan-Whalen M, et al. UV radiation inhibits 15-hydroxyprostaglandin dehydrogenase levels in human skin: evidence of transcriptional suppression. Cancer Prev Res (Phila) 2010;3:1104–11. doi: 10.1158/1940-6207.CAPR-10-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang K, Hammerberg C, Meunier L, Cooper KD. CD11b+ macrophages that infiltrate human epidermis after in vivo ultraviolet exposure potently produce IL-10 and represent the major secretory source of epidermal IL-10 protein. J Immunol. 1994;153:5256–64. [PubMed] [Google Scholar]
- Kennedy-Crispin M, Billick E, Mitsui H, Gulati N, Fujita H, Gilleaudeau P, et al. Human keratinocytes’ response to injury upregulates CCL20 and other genes linking innate and adaptive immunity. The Journal of investigative dermatology. 2012;132:105–13. doi: 10.1038/jid.2011.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkhoff C, Klempt M, Kaever V, Sorg C. The two calcium-binding proteins, S100A8 and S100A9, are involved in the metabolism of arachidonic acid in human neutrophils. The Journal of biological chemistry. 1999;274:32672–9. doi: 10.1074/jbc.274.46.32672. [DOI] [PubMed] [Google Scholar]
- Kesting MR, Stoeckelhuber M, Holzle F, Mucke T, Neumann K, Woermann K, et al. Expression of antimicrobial peptides in cutaneous infections after skin surgery. The British journal of dermatology. 2010;163:121–7. doi: 10.1111/j.1365-2133.2010.09781.x. [DOI] [PubMed] [Google Scholar]
- Lee YM, Kim YK, Eun HC, Chung JH. Changes in S100A8 expression in UV-irradiated and aged human skin in vivo. Archives of dermatological research. 2009;301:523–9. doi: 10.1007/s00403-009-0960-8. [DOI] [PubMed] [Google Scholar]
- Lopez-Garcia B, Lee PH, Yamasaki K, Gallo RL. Anti-fungal activity of cathelicidins and their potential role in Candida albicans skin infection. The Journal of investigative dermatology. 2005;125:108–15. doi: 10.1111/j.0022-202X.2005.23713.x. [DOI] [PubMed] [Google Scholar]
- Madsen P, Rasmussen HH, Leffers H, Honore B, Dejgaard K, Olsen E, et al. Molecular cloning, occurrence, and expression of a novel partially secreted protein “psoriasin” that is highly up-regulated in psoriatic skin. The Journal of investigative dermatology. 1991;97:701–12. doi: 10.1111/1523-1747.ep12484041. [DOI] [PubMed] [Google Scholar]
- Marenholz I, Zirra M, Fischer DF, Backendorf C, Ziegler A, Mischke D. Identification of human epidermal differentiation complex (EDC)-encoded genes by subtractive hybridization of entire YACs to a gridded keratinocyte cDNA library. Genome research. 2001;11:341–55. doi: 10.1101/gr.114801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier L, Bata-Csorgo Z, Cooper KD. In human dermis, ultraviolet radiation induces expansion of a CD36+ CD11b+ CD1− macrophage subset by infiltration and proliferation; CD1+ Langerhans-like dendritic antigen-presenting cells are concomitantly depleted. The Journal of investigative dermatology. 1995;105:782–8. doi: 10.1111/1523-1747.ep12326032. [DOI] [PubMed] [Google Scholar]
- Miranda LP, Tao T, Jones A, Chernushevich I, Standing KG, Geczy CL, et al. Total chemical synthesis and chemotactic activity of human S100A12 (EN-RAGE) FEBS letters. 2001;488:85–90. doi: 10.1016/s0014-5793(00)02392-9. [DOI] [PubMed] [Google Scholar]
- Mouchet N, Adamski H, Bouvet R, Corre S, Courbebaisse Y, Watier E, et al. In vivo identification of solar radiation-responsive gene network: role of the p38 stress-dependent kinase. PloS one. 2010;5:e10776. doi: 10.1371/journal.pone.0010776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy GM, Norris PG, Young AR, Corbett MF, Hawk JL. Low-dose ultraviolet-B irradiation depletes human epidermal Langerhans cells. The British journal of dermatology. 1993;129:674–7. doi: 10.1111/j.1365-2133.1993.tb03330.x. [DOI] [PubMed] [Google Scholar]
- Phan TA, Halliday GM, Barnetson RS, Damian DL. Spectral and dose dependence of ultraviolet radiation-induced immunosuppression. Frontiers in bioscience : a journal and virtual library. 2006;11:394–411. doi: 10.2741/1807. [DOI] [PubMed] [Google Scholar]
- Ryckman C, Vandal K, Rouleau P, Talbot M, Tessier PA. Proinflammatory activities of S100: proteins S100A8, S100A9, and S100A8/A9 induce neutrophil chemotaxis and adhesion. J Immunol. 2003;170:3233–42. doi: 10.4049/jimmunol.170.6.3233. [DOI] [PubMed] [Google Scholar]
- Schroder JM, Harder J. Antimicrobial skin peptides and proteins. Cellular and molecular life sciences : CMLS. 2006;63:469–86. doi: 10.1007/s00018-005-5364-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz T. 25 years of UV-induced immunosuppression mediated by T cells-from disregarded T suppressor cells to highly respected regulatory T cells. Photochemistry and photobiology. 2008;84:10–8. doi: 10.1111/j.1751-1097.2007.00223.x. [DOI] [PubMed] [Google Scholar]
- Silva SH, Guedes AC, Gontijo B, Ramos AM, Carmo LS, Farias LM, et al. Influence of narrow-band UVB phototherapy on cutaneous microbiota of children with atopic dermatitis. Journal of the European Academy of Dermatology and Venereology : JEADV. 2006;20:1114–20. doi: 10.1111/j.1468-3083.2006.01748.x. [DOI] [PubMed] [Google Scholar]
- Sorensen OE, Thapa DR, Roupe KM, Valore EV, Sjobring U, Roberts AA, et al. Injury-induced innate immune response in human skin mediated by transactivation of the epidermal growth factor receptor. The Journal of clinical investigation. 2006;116:1878–85. doi: 10.1172/JCI28422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Farinas M, Lowes MA, Zaba LC, Krueger JG. Evaluation of the psoriasis transcriptome across different studies by gene set enrichment analysis (GSEA) PloS one. 2010;5:e10247. doi: 10.1371/journal.pone.0010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Farinas M, Pellegrino M, Wittkowski KM, Magnasco MO. Harshlight: a "corrective make-up" program for microarray chips. BMC Bioinformatics. 2005;6:294. doi: 10.1186/1471-2105-6-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Termorshuizen F, Garssen J, Norval M, Koulu L, Laihia J, Leino L, et al. A review of studies on the effects of ultraviolet irradiation on the resistance to infections: evidence from rodent infection models and verification by experimental and observational human studies. International immunopharmacology. 2002;2:263–75. doi: 10.1016/s1567-5769(01)00178-3. [DOI] [PubMed] [Google Scholar]
- Wolf R, Howard OM, Dong HF, Voscopoulos C, Boeshans K, Winston J, et al. Chemotactic activity of S100A7 (Psoriasin) is mediated by the receptor for advanced glycation end products and potentiates inflammation with highly homologous but functionally distinct S100A15. J Immunol. 2008;181:1499–506. doi: 10.4049/jimmunol.181.2.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan WX, Armishaw C, Goyette J, Yang Z, Cai H, Alewood P, et al. Mast cell and monocyte recruitment by S100A12 and its hinge domain. The Journal of biological chemistry. 2008;283:13035–43. doi: 10.1074/jbc.M710388200. [DOI] [PubMed] [Google Scholar]
- Yang D, Chertov O, Bykovskaia SN, Chen Q, Buffo MJ, Shogan J, et al. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–8. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- Zaba LC, Fuentes-Duculan J, Eungdamrong NJ, Abello MV, Novitskaya I, Pierson KC, et al. Psoriasis Is Characterized by Accumulation of Immunostimulatory and Th1/Th17 Cell-Polarizing Myeloid Dendritic Cells. The Journal of investigative dermatology. 2009a;129:79–88. doi: 10.1038/jid.2008.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaba LC, Fuentes-Duculan J, Eungdamrong NJ, Johnson-Huang LM, Nograles KE, White TR, et al. Identification of TNF-related apoptosis-inducing ligand and other molecules that distinguish inflammatory from resident dendritic cells in patients with psoriasis. The Journal of allergy and clinical immunology. 2010;125:1261–8. e9. doi: 10.1016/j.jaci.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaba LC, Fuentes-Duculan J, Steinman RM, Krueger JG, Lowes MA. Normal human dermis contains distinct populations of CD11c+BDCA-1+ dendritic cells and CD163+FXIIIA+ macrophages. The Journal of clinical investigation. 2007;117:2517–25. doi: 10.1172/JCI32282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaba LC, Krueger JG, Lowes MA. Resident and “inflammatory” dendritic cells in human skin. The Journal of investigative dermatology. 2009b;129:302–8. doi: 10.1038/jid.2008.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.