Abstract
Integrin-binding peptides increase cell adhesion to naive hydroxyapatite (HA), however, in the body, HA becomes rapidly modified by protein adsorption. Previously we reported that, when combined with an adsorbed protein layer, RGD peptides interfered with cell adhesion to HA. In the current study we evaluated mesenchymal stem cell (MSC) interactions with HA disks coated with the collagen-mimetic peptides, DGEA, P15 and GFOGER. MSCs adhered equally well to disks coated with DGEA, P15, or collagen I, and all three substrates, but not GFOGER, supported greater cell adhesion than uncoated HA. When peptide-coated disks were overcoated with proteins from serum or the tibial microenvironment, collagen mimetics did not inhibit MSC adhesion, as was observed with RGD, however neither did they enhance adhesion. Given that activation of collagen-selective integrins stimulates osteoblastic differentiation, we monitored osteocalcin secretion and alkaline phosphatase activity from MSCs adherent to DGEA or P15-coated disks. Both of these osteoblastic markers were upregulated by DGEA and P15, in the presence and absence of differentiation-inducing media. Finally, bone formation on HA tibial implants was increased by the collagen-mimetics. Collectively these results suggest that collagen-mimetic peptides improve osseointegration of HA, most probably by stimulating osteoblastic differentiation, rather than adhesion, of MSCs.
Keywords: Bioadsorption, Bone Tissue Engineering, Cell Adhesion, Hydroxyapatite, Collagen, Peptide
Introduction
In order for hard tissue implants to integrate into existing bone, osteoblast precursor cells must bind to the implant surface, differentiate, and form a new bone matrix to tether the implant in place. One common strategy for improving implant integration is to functionalize biomaterial surfaces with peptides that mimic the native extracellular matrix, with the goal of providing attachment sites for adhesion receptors present on osteogenic cells. One of the most widely-studied adhesion-promoting peptides is Arg-Gly-Asp (RGD), a tri-amino acid sequence found within matrix proteins such as fibronectin (FN) and vitronectin (VN). RGD is a principal ligand for the integrin family of adhesion receptors, although it is known that other domains within FN and VN act synergistically with RGD to more strongly activate integrins [1-8]. Many types of biomaterials have been modified with RGD, and in vitro studies consistently suggest that RGD-modified surfaces promote better cell attachment than unmodified surfaces [9-15].
However, some types of biomaterials, including hydroxyapatite (HA), are very efficient at adsorbing adhesive proteins present within body fluids at the surgical site, and therefore it isn’t clear that functionalizing hydroxyapatite (HA) with RGD would be beneficial in vivo. We and others have shown that FN, VN and fibrinogen (Fbg) from the surgical environment become adsorbed to the HA surface within minutes following implantation [16-19]. Thus, an RGD-modified HA surface would be presented to cells within the context of an adsorbed layer of endogenous integrin-binding proteins. To model the role of protein adsorption in influencing cell attachment to RGD-modified HA biomaterials, we previously studied mesenchymal stem cell (MSC) adhesion to RGD-modified HA disks that were either over-coated with fetal bovine serum (FBS) [15], or implanted for 30 minutes into rat tibiae to allow endogenous protein adsorption [19]. Surprisingly, these studies revealed that RGD was detrimental to MSC attachment; more cells adhered to the FBS-coated [15] or tibial-implanted [19] HA disks that lacked RGD pre-coatings. Moreover, the combined RGD/FBS-coated HA surfaces elicited greater activation of the cell apoptotic marker, caspase 3, than FBS-coated HA [19], suggesting that RGD had a negative effect on cell survival. Finally, a comparison of uncoated and RGD-coated HA disks implanted for 5 days into rat tibiae showed that the RGD coatings significantly inhibited the amount of new bone formed on the implant surface, as well as the degree of direct bone-implant contact [19]. Taken together, these results suggested that RGD inhibits the osseointegration of HA biomaterials, most probably through diminished attachment and survival of MSCs.
The mechanisms underlying the inhibitory effects of RGD on cytocompatibility and implant integration are not currently understood, however we hypothesize that synthetic RGD peptides on the HA surface compete with adsorbed proteins such as FN, VN or Fbg for binding to cell surface integrin receptors. FN, VN and Fbg are among the most abundant adhesion molecules in blood [20-22], and all of these proteins bind to integrins through an RGD-dependent mechanism [16, 23, 24]. It is possible that synthetic RGD peptides divert integrin receptors away from binding adsorbed FN, VN or Fbg proteins, and thereby elicit diminished integrin signaling, given that RGD peptides are weaker integrin ligands than native full-length adhesion proteins [7, 25]. We further speculate that RGD will be detrimental for biomaterials that have high affinity for adsorbing integrin ligands from the tissue environment, but alternately beneficial for biomaterials that lack any other integrin ligand (e.g., nonfouling types of materials).
Regardless of mechanism, our prior results clearly showed that RGD peptides were not beneficial for HA biomaterials, and we therefore questioned whether there were any other adhesive peptides that would improve cell and/or tissue responses to HA. As an alternative to RGD, many investigators have evaluated osteogenic cell attachment to materials functionalized with collagen-derived peptides. Collagen mimetic peptides are attractive for a number of reasons. First, collagen I binds to different integrin receptors than FN, VN and Fbg, and the integrin/collagen I interaction is thought to be RGD-independent. Hence, collagen mimetic peptides would not be expected to compete with adsorbed FN, VN or Fbg for binding to cell surface integrins. Secondly, it is unlikely that collagen I would adsorb to the HA surface in substantial amounts upon surgical placement, given that collagen I is not highly abundant within the blood. Finally, activation of the collagen-selective integrin, α2β1, induces osteoblastic differentiation [26-29], and therefore it is possible that collagen-derived peptides could serve as both cell attachment and differentiation factors. In light of these considerations, the focus of the current study was to monitor the cytocompatibility and osseointegration of HA biomaterials modified with collagen I-derived peptides. Three collagen I mimetics were evaluated; DGEA [30] and GTPGPQGIAGQRGVV (“P15”) [31], which are linear peptides derived from the α1 helix of collagen I, and the GFOGER peptide [32, 33], which spontaneously assumes a triple-helical structure due to the presence of GPP repeats engineered onto the ends of the peptide. All of these peptides have shown some degree of efficacy in directing osteogenic cell attachment, however, to our knowledge, a side-by-side comparison of the peptides, when adsorbed to calcium phosphate biomaterials, has never been performed.
Materials and Methods
Peptide preparation
The collagen I mimetic peptides DGEA (370.4 g/mol) and P15 (GTPGPQIAGQAGVV, 1393.5 g/mol) were obtained from American Peptide, whereas the GFOGER peptide [32, 33] was a generous gift from Dr. Richard Farndale (Cambridge University). The RGD and RGE peptides were also purchased from American Peptide. The RGD peptide used in this study, GPenGRGDSPCA (948.1g/mol), is a cyclized peptide that has high affinity for vitronectin receptors. The RGE peptide (RGES, 447.5g/mol) is a control for RGD, given that the RGE sequence does not bind integrin receptors. All peptides were reconstituted in ddH2O at 1mg/ml, aliquoted and stored at -20°C.
Disk preparation
For in vitro studies, clinical grade HA powder (Fisher Scientific) was pressed into disks using a 15.875mm die, under 3000 psi. For in vivo studies, clinical grade HA powder (Fisher Scientific) was pressed into disks using a 3mm die, under 1000 psi. Pressed disks were sintered at 1000°C for 3 hours and allowed to cool in the furnace at decreasing intervals. Disks were then stored under sterile conditions. Peptides (1mg/ml) were coated onto sintered HA disks as previously reported [15]. For peptide-only coatings, disks were incubated at 4°C overnight in peptide solution. For sequential coatings, disks were incubated in peptide solution at 37°C for 1 hour, and then overcoated with serum overnight at 4°C. The disks were washed with PBS to remove unbound peptide, and warmed to 37°C prior to cell seeding or in vivo implantation.
Cell Isolation and culture
As previously described [34], human bone marrow cells were subjected to low speed centrifugation, and resuspended in Dulbecco's modified Eagle's Medium (DMEM). The cell suspension was then applied to a histopaque-1077 column, and centrifuged to establish a density gradient. The MSC layer was extracted from the gradient, and the cells grown in DMEM supplemented with 10% FBS (standard growth media). Cells from passage 3-13 were used for our experiments. MSCs grown in standard growth media maintain a multipotent phenotype, with the ability to differentiate along the chondrogenic, osteogenic, and adipogenic lineages. Human bone marrow samples were obtained with prior approval from the University of Alabama Institutional Review Board.
For differentiation experiments, osteogenic media (OS media), consisting of DMEM supplemented with PenStrep, Amphotericin B, 10% FBS, 100nM dexamethasone, 10mM sodium β-glycerolphosphate, and 0.05mM L-ascorbic acid-2-phosphate [35], was used.
Cell adhesion to peptide-coated disks
MSC adhesion to peptide-coated disks was evaluated using a standard fluorescence-based assay [36]. As recommended by the vendor, cells were incubated in serum-free media containing 2 μM CMFDA, a fluorescent dye (“Cell Tracker Green”, Molecular Probes). After labeling, cells were detached from tissue culture flasks by trypsinization, followed by incubation in trypsin inhibitor (Sigma). 1×105 labeled cells were re-suspended in serum-free media, seeded onto HA substrates, and allowed to adhere for 1 hour. This time interval was selected because cells are well-spread at 1 hour, and because at this time point, differences in cell spreading can be attributed directly to the adhesion molecules that were pre-adsorbed onto the surfaces (RGD, DGEA, etc). At later time points, cells secrete their own adhesion molecules, which complicates analysis of the effect of surface treatments. After the 1-hr binding interval, loosely-bound cells were removed by washing with phosphate-buffered saline (PBS), and the remaining adherent cells were lysed (1% Triton-X-100 in 50mM Tris) to release the fluorescent marker into solution. Fluorescence was quantified by reading samples on a fluorometer.
Cell morphology
5×104 MSCs were seeded in serum-free media onto disks for 1 hour. Unbound cells were washed away with PBS, while the adherent cells were fixed in 3.7% formaldehyde, permeabilized with 0.2% Triton-X-100, and then stained with Alexa-488 phalloidin (Molecular Probes). The cells were then mounted with 4.7mM n-propyl-gallate mounting fluid, and visualized using a Nikon fluorescent microscope.
Western blot analysis of adsorbed serum proteins
Disks which had been coated with FBS or sequentially coated with peptide/FBS overnight were washed in PBS to remove loosely-bound proteins. Proteins remaining on the surface were solubilized in boiling sodium dodecyl sulfate (SDS)-buffer (50mM Tris, 2% SDS, 5% β-mercaptoethanol) for 30 minutes, with constant agitation. The supernatant was collected and stored at −80°C. Desorbed proteins were resolved on a 7% polyacrylamide gel. Proteins were transferred to a polyvinyldifluoride (PVDF) membrane, and exposed to antibodies for FN (Chemicon), or VN (Abcam); followed by an HRP-conjugated secondary antibody (Amersham Life Sciences). Proteins were detected using chemiluminescence reagents (Amersham Life Sciences or Millipore).
MSC differentiation
Disks which had been coated with FBS or sequentially coated with peptide/FBS were washed with PBS to remove loosely-bound proteins. 2×105 MSCs were seeded onto the disks in serum-free media and allowed to attach for 24 hours. The media was then switched to standard growth media, and the cells were allowed to grow for 72 hours in order to reach confluence. After this interval, the growth media was replaced by osteogenic media, and the cells were allowed to grow for an additional 2 weeks (the osteogenic media was replaced every 2 days). Following incubation in osteogenic media, samples were stained for alkaline phosphatase (ALP) activity, or alternately, the supernatants were collected for osteocalcin (OCN) ELISA.
ALP Activity
Samples exposed to osteogenic media were washed 3 times in PBS, and then a colorimetric ALP activity kit (Sigma) was used to detect ALP on each substrate. Images of the relative activity were taken.
OCN ELISA
Supernatants from the samples exposed to osteogenic media were collected and then tested for OCN secretion using a commercially available kit (Biomedical Technologies, Inc.). The supernatants were incubated on a capture antibody-coated plate in the presence of antiserum. The plates were washed, and detection reagent was then added. The colorimetric product was analyzed at 450nm, and readings were compared with a standard curve.
Tibial implantation
Male Sprague-Dawley rats were anesthetized, and an osteotomy was created in the right proximal tibia as previously reported by our group [19]. 3mm HA disks, either left uncoated or coated with collagen I mimetic peptides, were placed into the defects, and extracted at either 30 minutes or 5 days. All protocols were performed in accordance with guidelines established by the University of Alabama Institutional Animal Care and Use Committee.
Cell adhesion on disks retrieved from tibiae
HA disks were retrieved from rat tibial osteotomies after a 30 min-implantation and used for in vitro cell adhesion assays. As described [19], the retrieved disks were washed with PBS, and then human MSCs were seeded onto the disks and allowed to adhere for 1 hr. The disks were washed again to remove loosely-bound cells, and the adherent cells were stained with Alexa-488 phalloidin (Cell Signaling) and 4',6-diamidino-2-phenylindole (DAPI) (Cell Signaling) Cell adhesion was quantified by counting the number of cells in a microscopic field. Five implants were evaluated per treatment group, with multiple microscopic fields examined per implant.
Protein adsorption from retrieved tibial implants
HA disks were retrieved following a 30-min implantation, and washed with PBS. Disks were then incubated in boiling-SDS buffer as described above. Samples were resolved by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to PVDF membranes. Membranes were incubated with antibodies specific for FN (Chemicon), VN (Chemicon), or Fbg (Abcam). Secondary antibodies to each of the primaries were then added (Amersham), and signal was detected using chemiluminescence (Amersham Life Science or Millipore).
Bone formation on implanted HA disks
Following 5 days of implantation, tibiae, with disks in place, were retrieved, and embedded in poly(methyl methacrylate) (PMMA) for Goldner's trichrome staining, which stains mineralized tissue green [19]. 5 implants were analyzed for each of the three treatment groups (15 animals total), with at least two tissue sections per implant evaluated.
The amount of total new bone surrounding 5 day implants, as well as the amount of bone in direct contact with the implant perimeter, were quantified from Goldner's stained sections using Bioquant imaging software as previously described [19].
Statistics
In vitro assays were performed at least three independent times, with each experiment performed in triplicate. In vivo experiments were performed with five animals per treatment group, with at least two fields or sections analyzed for quantification. Data sets were assessed using students t-test parametric analysis. A confidence level of 95% (p<0.05) was considered significant.
Results
MSC adhesion to HA disks coated with collagen I mimetic peptides
To determine the efficacy of collagen-derived peptides in promoting cell adhesion, we monitored MSC attachment to HA disks coated with DGEA, P15, GFOGER or collagen I. These experiments revealed that DGEA and P15 were able to support a level of cell adhesion equivalent to that of collagen I, and all three of these integrin ligands stimulated greater binding than uncoated HA disks (Figure 1A). However, in contrast to DGEA and P15, the GFOGER peptide failed to stimulate cell adhesion above that of uncoated HA.
Figure 1. DGEA and P15 increase cell adhesion and cell spreading on HA.
A) MSCs labeled with Cell Tracker dye were allowed to adhere for 1 hour to HA disks pre-coated with collagen I (Col I), DGEA, P15 or GFOGER. The adherent cells were subsequently lysed and adhesion was quantified by measuring fluorescence. Values were folded to uncoated HA (dotted line) (*=p<0.05 to uncoated HA). B) Representative images of MSCs allowed to adhere for 1 hour to collagen I (Col I), DGEA, P15 or GFOGER-coated HA. Cells were labeled with Alexa-488 phalloidin (Cell Signaling Technologies).
Cell spreading on HA disks coated with DGEA, P15, GFOGER or collagen I
To evaluate cell morphology, MSCs were seeded onto HA disks that had been pre-coated with DGEA, P15, GFOGER, or collagen I. Following a 1 hour attachment interval, cells were stained with phalloidin to visualize the actin cytoskeleton. As shown in Figure 1B, DGEA, P15 and collagen I were all able to induce some degree of cell spreading, whereas GFOGER was completely ineffective. These results suggest that DGEA and P15 engaged integrin receptors and elicited sufficient integrin activation to promote some restructuring of the actin cytoskeleton.
It was surprising that the GFOGER peptide was ineffective in promoting cell adhesion and spreading, given prior reports in the literature suggesting that this triple helical peptide promotes robust integrin activation [32, 33, 37-39]. This result could be due to a number of factors including; poor GFOGER adsorption to HA, a change in the GFOGER tertiary structure upon HA adsorption, or inactivity of the peptide preparation. To insure that there were no problems with our peptide preparation, we examined cell attachment to GFOGER-coated tissue culture plastic, the material substrate on which the peptide had originally been tested [38]. We found that, when adsorbed to tissue culture plastic, the GFOGER peptide induced cell adhesion and spreading equivalent to that stimulated by intact collagen I, indicating that the peptide was active (data not shown). However, because of the poor performance of the GFOGER peptide when adsorbed to HA, this peptide was not studied further in the current investigation.
MSC adhesion to HA disks coated sequentially with peptide and FBS
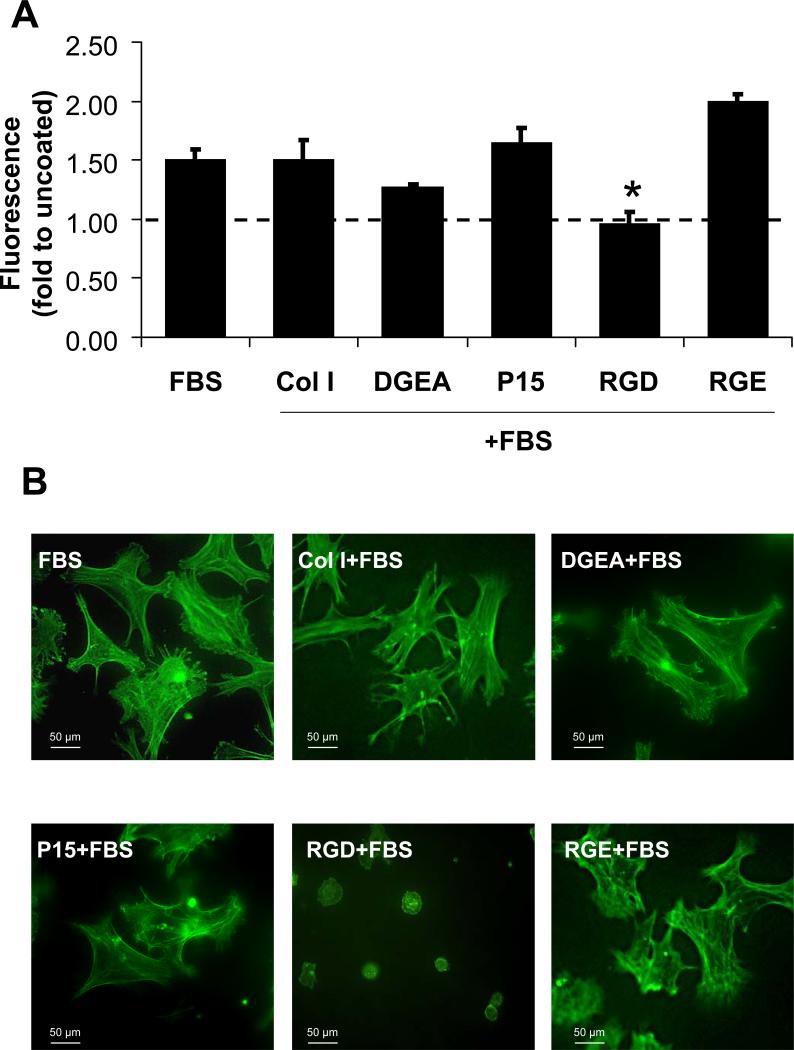
Because HA is known to rapidly adsorb significant quantities of proadhesive proteins such as FN, VN and Fbg from the in vivo environment [16-19], it is unlikely that cells within a patient would ever encounter peptide-coated HA in the absence of an adsorbed protein layer. In order to mimic the process of endogenous protein adsorption, peptide-coated HA disks were overcoated with FBS, which we have previously shown deposits abundant FN and VN onto HA surfaces [15]. MSCs were then seeded onto these sequentially-coated surfaces and evaluated for attachment. As shown in Figure 2A, MSC adhesion to the DGEA/FBS and P15/FBS-coated surfaces was equivalent to that of adhesion observed on HA coated with FBS alone, suggesting that adsorbed serum proteins are sufficient to induce maximal cell attachment. In contrast, RGD peptides significantly inhibited cell adhesion when presented in combination with serum proteins. The RGE control peptide, which has a similar charge and structure as RGD, but does not engage or activate integrin receptors, had no effect on cell adhesion. Thus, the binding of RGD, but not collagen-derived peptides, to cell surface integrins has a detrimental effect on cell adhesion.
Figure 2. DGEA and P15 do not inhibit cell adhesion when presented in the context of adsorbed serum proteins.
A) HA disks were coated with collagen I (Col I), DGEA, P15, RGD or RGE followed by an over-coating with serum (FBS) to allow protein adsorption. As a control, some disks were coated with FBS only. Fluorescently-labeled MSCs were then seeded onto the disks and monitored for cell adhesion as previously described. Values were folded to uncoated HA (dotted line) (*=p<0.05 to FBS). B) Representative images of Alexa-488 phalloidin stained MSCs adherent to HA disks coated with FBS alone or to disks coated first with either collagen I (Col I), DGEA, P15, RGD or RGE, followed by an FBS over-coating.
Cell spreading on HA disks coated sequentially with peptide and FBS
To determine whether DGEA or P15 peptides had any effect on cell spreading when presented in combination with adsorbed serum proteins, cells were seeded onto disks coated with DGEA, P15, collagen I, RGD or RGE, followed by an FBS-coating (Figure 2B). After a 1 hour incubation, cell morphology was evaluated by staining cells with phalloidin. These experiments revealed extensive cell spreading on disks coated with DGEA/FBS, P15/FBS, collagen I/FBS, RGE/FBS or FBS alone. In contrast, the RGD/FBS-coated samples did not support any degree of cell spreading, suggesting that the presence of RGD in combination with adsorbed serum proteins blocks integrin-mediated signaling.
MSC adhesion to HA disks adsorbed with proteins from the tibial microenvironment
To better model the process of protein adsorption on a biomaterial surface, we placed uncoated or peptide-coated disks into rat tibial osteotomies for 30 minutes to allow the adsorption of native proteins present within the bone microenvironment. The disks were then retrieved, washed extensively with PBS, and MSCs were subsequently seeded onto the disks and evaluated for degree of cell attachment (Figure 3). Similar to results generated from disks overcoated with FBS, neither DGEA nor P15 inhibited cell adhesion to disks coated with endogenous proteins. Notably, we previously reported that RGD inhibits at least 75% of cell adhesion when presented in the context of adsorbed tibial proteins [19].
Figure 3. DGEA and P15 do not inhibit cell adhesion when presented in the context of adsorbed proteins from the tibial microenvironment.
A) HA disks were either left uncoated (UNC, panels 1-2) or coated with DGEA (panels 3-4) or P15 (panels 5-6). The disks were then implanted into the rat tibiae for 30 minutes to allow protein adsorption, retrieved, and washed extensively with PBS. MSCs were seeded onto the surfaces, and allowed to adhere for 1 hour. Cells were then fixed and labeled with Alexa-488 phalloidin (1,3,5) and DAPI (2,4,6). Representative images are shown. B) Adherent cells were quantified by counting cells from multiple microscopic fields from 5 separate implants per group. Data are reported as fold to the uncoated HA implants. No significant difference was observed in the number of adherent cells for the 3 surface treatments.
The effect of collagen I mimetic peptides on protein adsorption from serum and the tibial microenvironment
We next tested whether DGEA or P15 had any effect on the adsorption of proadhesive proteins. To this end, disks were coated with DGEA/FBS, P15/FBS, or FBS alone. The disks were then washed to remove loosely-bound proteins, and the adsorbed proteins were subsequently desorbed by incubation in boiling SDS buffer. Desorbed samples were immunoblotted for FN and VN. As shown in Figure 4A, all of the surfaces adsorbed similar amounts of FN and VN.
Figure 4. DGEA and P15 pre-coatings do not affect protein adsorption from serum or the tibial microenvironment.
A) Representative western blots of FN and VN desorbed from disks coated with either FBS alone, or with DGEA or P15, followed by FBS. B) Western blots of FN, VN and Fbg desorbed from uncoated, DGEA-coated or P15-coated, HA disks implanted for 30 minutes into rat tibiae.
We also evaluated protein adsorption to peptide-coated disks placed into tibial osteotomies. Specifically, uncoated, DGEA-coated, or P15-coated HA disks were implanted for 30 minutes; the disks were then retrieved, proteins desorbed and analyzed by immunoblotting. Similar to results obtained with serum overcoatings (Figure 4A), peptide-coated and uncoated disks adsorbed similar amounts of FN, VN and Fbg from the tibial microenvironment (Figure 4B).
The influence of collagen-mimetic peptides on alkaline phosphatase activity
Results presented in Figures 2 and 3 indicated that, unlike RGD, the DGEA and P15 peptides did not inhibit cell attachment or spreading on protein-coated HA. However, DGEA and P15 did not enhance cell adhesion either, suggesting that there is little benefit to using these peptides as cell attachment factors. However, there is a growing literature suggesting that activation of a collagen-selective integrin, the α2β1 species, induces MSC differentiation along the osteoblastic lineage [26-29]. Accordingly, we were interested in whether collagen-derived peptides might serve as differentiation factors for MSCs. To test this hypothesis, cells were seeded onto peptide-coated disks overcoated with FBS, or disks coated with FBS alone as a control. The cells were then grown for 2 weeks in osteogenic media, and stained for ALP activity. As shown in Figure 5A, the disks coated with DGEA/FBS and P15/FBS supported greater levels of ALP activation than disks coated with FBS alone, indicating a greater degree of osteoblastic differentiation.
Figure 5. DGEA and P15 increase osteoblastic markers in the presence of osteogenic media.
A) MSCs were seeded onto disks coated with FBS alone, or on disks sequentially coated with either DGEA/FBS or P15/FBS. Cells were allowed to grow in osteogenic media (OS media) for 2 weeks. A representative image of an alkaline phosphatase (ALP) activity assay is shown. B) MSCs adherent to peptide/protein-coated disks were grown in OS media for 2 weeks as previously described. As a control, some cells were grown on FBS-coated disks in growth media rather than OS media. Following a 2-week incubation in either OS or growth media, culture supernatants were collected and evaluated for osteocalcin (OCN) secretion by ELISA. Values are reported as fold to the samples incubated in growth media (*=p<0.05 relative to FBS samples in growth media and †=p<0.05 relative to FBS in OS media).
The influence of collagen-mimetic peptides on osteocalcin secretion
To confirm that DGEA and P15 stimulated osteoblastic differentiation of MSCs, we examined secretion of OCN using an ELISA assay (Figure 5B). As a negative control, we allowed cells seeded onto FBS-coated disks to grow for two weeks in standard growth media, which maintains cells in an undifferentiated state. A comparison of the FBS-coated samples incubated in either growth media or osteogenic media revealed a 12-fold increase in OCN secretion from the cells exposed to osteogenic media, reflecting osteoblastic differentiation. Importantly, the cells adherent to DGEA/FBS and P15/FBS-coated surfaces secreted more OCN than cells adherent to FBS coatings alone when incubated in the presence of osteogenic media. These results, combined with the measurements of ALP activity, suggest that DGEA and P15 are able to enhance MSC differentiation along the osteoblast lineage.
The influence of collagen-mimetic peptides on ALP activity and OCN secretion in the absence of osteogenic media
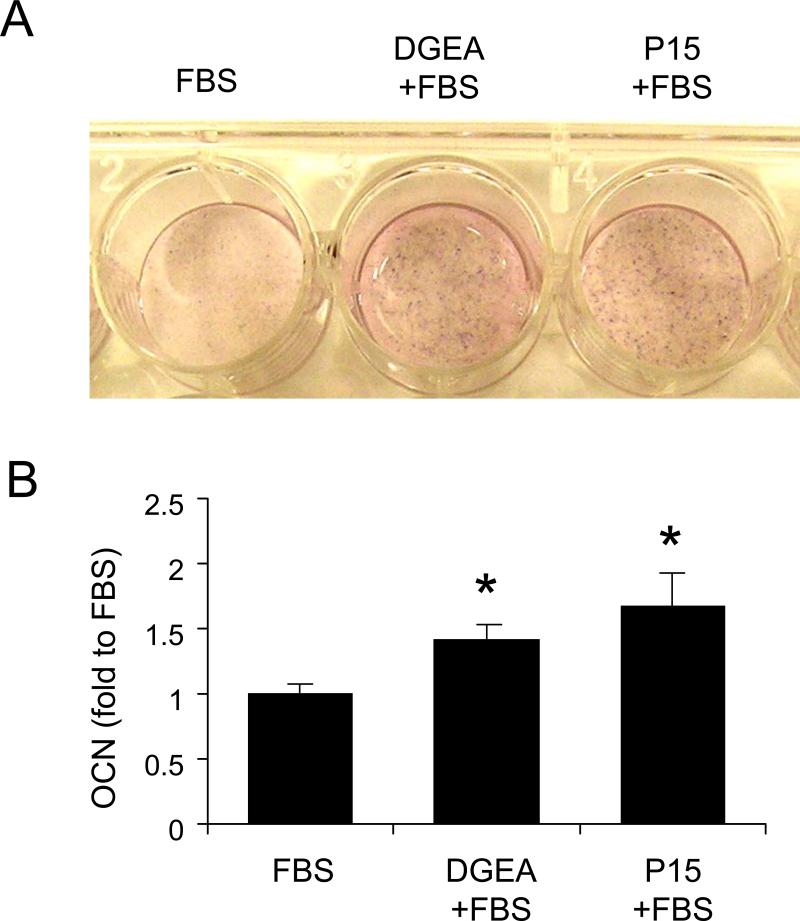
Results shown in Figure 5 indicated that DGEA and P15 were able to upregulate the expression of osteoblastic markers when presented to cells in the presence of osteogenic media. However, some studies have suggested that activation of α2β1 integrins can stimulate osteoblastic differentiation even in the absence of other differentiation-inducers [26-29]. Hence, we tested whether DGEA and P15 were able to stimulate osteoblastic differentiation in the absence of osteogenic media. To this end, cells adherent to peptide-coated disks were incubated in standard growth media for two weeks and then ALP activity and OCN levels were measured. We found that cells grown on HA disks coated with DGEA/FBS or P15/FBS exhibited greater ALP activity and OCN secretion than cells grown on disks coated with FBS alone (Figure 6A and B). These results suggest that DGEA and P15 are able to activate collagen-binding integrins enough to induce some degree of osteoblastic differentiation in the absence of standard differentiation factors.
Figure 6. DGEA and P15 increase osteogenic markers in the absence of osteogenic media.
A) Representative image of an alkaline phosphatase activity assay for MSCs incubated in growth media on HA disks pre-coated with FBS, DGEA/FBS, or P15/FBS. B) Quantification of osteocalcin (OCN) secretion from samples incubated in standard growth media for two weeks. (*=p<0.05 to FBS).
The effect of collagen-mimetic peptides on bone/implant contact and new bone synthesis surrounding tibial implants
In order to examine if the differentiation-inducing features of collagen-mimetic peptides had any effect on implant osseointegration, uncoated, DGEA-coated, or P15-coated HA disks were placed into rat tibial osteotomies and monitored for bone formation. Specifically, implants were left in place for 5 days; the tibiae were then removed (with implants in place), and embedded in PMMA. Sections of the embedded tibiae were stained with Goldner's Trichrome, a stain which labels mineralized tissue green (Figure 7A). When the amount of new bone was quantified (Figure 7B), we found that both DGEA and P15 stimulated greater bone formation around the HA implants. In addition, DGEA was able to increase the amount of bone directly contacting the perimeter of the implants. P15 showed a trend toward increased bone-implant contact, but this was not statistically significant. Taken together these results suggest that collagen-mimetic peptides improve bone tissue responses to HA biomaterials.
Figure 7. DGEA and P15 increase bone formation around HA implants.
A) Representative images of Goldner's Trichrome-stained sections from tibiae implanted with uncoated (UNC), DGEA-coated, or P15-coated, HA disks. B) Quantification of the amount of new bone formed (black bars) and the amount of bone directly contacting the implant perimeter (white bars) around uncoated (UNC), DGEA-coated or P15-coated implants (*=p<0.05 to UNC)
Discussion
Previous studies have shown that the addition of adhesive proteins, such as FN [40-43], VN [41], and Fbg [44], to the surface of HA biomaterials increases cell adhesion. Furthermore, we and others have reported that HA adsorbs abundant adhesive proteins, including FN, and VN, from serum [15, 34, 45] as well as from the tibial microenvironment [19]. We also found that HA adsorbs significantly more VN and FN from serum than titanium or stainless steel, and these adsorbed proteins are present on the HA surface in conformations appropriate for binding purified integrin receptors and MSCs [34]. The importance of proadhesive blood proteins in regulating cell attachment to HA has been confirmed by other studies. For example, Zreiqat et al. [46], showed that human bone-derived (HBD) cells do not adhere to hydroxyapatite if vitronectin is depleted from serum.
In light of these results, we questioned the utility of synthetic adhesive peptides, and proposed instead that adsorbed endogenous integrin-binding proteins may be sufficient for promoting optimal osteogenic cell attachment and survival. Indeed we previously reported that cell adhesion to serum-coated HA is significantly better than cell adhesion to RGD-coated HA [15], and this result was observed with 5 different species of serum [1] and both linear and cyclic RGD peptides [15]. Moreover, when RGD was combined with adsorbed serum [15] or tibial proteins [19], it was unexpectedly found that RGD peptides were strongly detrimental to cell attachment and survival. RGD also significantly inhibited the osseointegration of HA disks implanted into rat tibiae [19].
The unfavorable cell and tissue responses to RGD-coated HA biomaterials prompted an evaluation of whether other adhesive peptides would be beneficial for HA. In the current study, three peptides derived from collagen I were examined; DGEA, P15, and GFOGER. These peptides are α2β1 integrin-binding motifs, [30-33, 47], and all have been shown to increase MSC adhesion to a variety of biomaterials [31-33, 48, 49]. Interestingly, we found that GFOGER was unable to promote cell adhesion or spreading on HA surfaces, although the mechanism underlying this negative result is not currently understood. In contrast, DGEA and P15 coatings supported a level of cell adhesion equivalent to that of HA coated with full-length collagen I. In addition, the DGEA and P15 peptides did not inhibit cell adhesion when presented in combination with adsorbed adhesive proteins from either serum or the tibial microenvironment. This striking disparity between the effects of RGD vs. collagen-derived peptides on MSC adhesion suggests that collagen-derived peptides interact with MSCs through different mechanistic pathways. We hypothesize that the binding of DGEA and P15 to collagen-specific integrins, including the α2β1 receptor, would not compete with adsorbed blood proteins such as FN, VN and Fbg for cell surface integrins, given that these latter proteins bind distinct integrin species including α5β1, αvβ3 and αIIbβ3. As well, FN, VN and Fbg all bind integrins through RGD-dependent mechanisms, whereas the interaction between α2β1 and collagen I is RGD-independent.
While it was encouraging that DGEA and P15 did not inhibit cell adhesion when presented within the context of an adsorbed protein layer, it was noted that these peptides did not enhance cell adhesion either. Adsorbed serum or tibial proteins (including FN, VN and Fbg) appeared to promote maximal cell adhesion and spreading, suggesting that the use of collagen-derived peptides as attachment factors is of limited value. However, our results alternately suggested that DGEA and P15 may serve as effective differentiation factors. Activation of the α2β1 integrin receptor has been reported to upregulate many markers of osteoblastic differentiation including osteopontin mRNA [29] and protein [26, 50], ALP activity [26, 50], OCN protein [26, 50] and matrix mineralization [26, 27, 50]. In our studies, MSCs grown on HA disks coated with DGEA or P15 exhibited higher ALP activity and greater secretion of OCN, as compared MSCs grown on serum-coated HA. This enhanced osteoblastic activity was observed both in the presence and absence of osteogenic media, indicating that collagen-derived peptides can stimulate some degree of osteoblastic differentiation even in the absence of other differentiation-inducing agents. Consistent with these results, DGEA and P15-coated HA disks implanted into rat tibiae promoted better bone in-growth and DGEA stimulated greater bone-implant direct contact than unmodified HA. Collectively these results are in excellent agreement with many other studies implicating collagen and collagen-derived peptides in osteogenesis. In particular, the P15 peptide has been extensively studied, and has been used for many clinical applications. P15-coated anorganic bovine mineral (ABM), used for periodontal defects, has been shown to increase bone regeneration at dental implant sites in humans [51-58]. In addition, in a side-by-side comparison study, P15-coated ABM performed better than open flab debridement [56, 57], Puros, a form of allograft, [58] and C-Graft 228, a calcified biomaterial derived from algae [58]. Finally, P15-coated ABM has been successfully used in maxillary sinus augmentation to induce bone growth [59].
Collagen I and HA are the two principal components of native bone, and therefore HA biomaterials modified with collagen-derived peptides represent a matrix that mimics the endogenous surface that MSCs would likely encounter in vivo. Both HA [60-62] and collagen I [26, 29, 50] have been reported to enhance osteoblastic differentiation of MSCs, and composite biomaterials encompassing collagen I and calcium phosphates including HA and tri-calcium phosphate have been shown to significantly increase osteoblastic differentiation [63, 64] and bony ingrowth [63, 65]. In the aggregate, these studies indicate a promising role for collagen-related biomaterials in bone regeneration. However there are some concerns regarding the use of collagen I. Collagen I can be immunogenic in some instances, and has the potential to transmit pathogens when xenografted. These obstacles could theoretically be circumvented by using collagen-derived peptides such as DGEA and P15. As well, synthetic peptides are significantly less expensive to produce than native collagen I, providing a more cost-effective strategy for optimizing biomaterials used for bone repair.
Beyond identifying collagen-derived peptides as a promising substrate for enhancing HA bioactivity, our study is noteworthy because it highlights the importance of considering endogenous processes such as protein adsorption when evaluating cell responses to biomaterials. In our studies, we modeled protein adsorption by coating HA with either serum, or implanting disks for 30 minutes into tibial osteotomies to allow protein adsorption from the bone microenvironment. We recognize that this is an approximation of initial events at the implant site, and that other factors undoubtedly influence the characteristics of material surfaces presented to endogenous MSCs. For example, it is possible that protein-adsorbed material surfaces might be remodeled by other cells within the wound site (e.g., blood cells) prior to the arrival of MSCs. Nonetheless, it is important to note that our in vitro studies incorporating a protein adsorption modeling step are far more predictive of in vivo biomaterial performance than studies comparing peptide-modified HA with uncoated HA (the latter being the standard approach for evaluating adhesive peptides in vitro). For instance, when compared with unmodified HA (i.e., no protein adsorption), HA substrates coated with RGD, DGEA or P15 significantly increase MSC adhesion [14, 31, 49, 66, 67], suggesting that all of these integrin-binding peptides would enhance implant integration. However, our studies of HA disks implanted into rat tibiae clearly show that RGD inhibits, whereas DGEA and P15 stimulate, the amount of new bone deposited around HA implants. These in vivo results are, in fact, very consistent with in vitro studies incorporating a protein adsorption step. Our current working model (Figure 8) is that RGD competes with adsorbed proteins for integrin receptors, and thereby elicits diminished overall integrin signaling, whereas DGEA and P15 enhance integrin signaling from the cell surface by binding integrin receptors that are distinct from those that would be bound by endogenous adsorbed proteins.
Figure 8. Model describing the effects of integrin-binding peptides on osseointegration of HA implants.
A) Integrin activation by adsorbed proteins, such as FN and VN, plays a key role in MSC adhesion, survival and osteoblastic differentiation. When RGD is present on the HA surface, we hypothesize that integrins such as αvβ3 bind the RGD rather than full-length FN or VN, leading to poor cell adhesion and survival. Collagen-binding integrins such as α2β1 would not likely be engaged with ligand, given that minimal amounts of collagen I would adsorb to the HA surface from blood (given that fibrillar collagen I is not abundant in blood). The combination of weak signaling from RGD-dependent integrins (e.g. αvβ3, α5β1, αIIbβ3), and a lack of signaling from collagen-selective integrins, is proposed to contribute to poor implant integration. B) Conversely, the presence of either DGEA or P15 on the HA surface provides a ligand for collagen-selective integrins that, upon activation, initiate signaling mechanisms promoting osteoblastic differentiation. As well, RGD-dependent integrins would engage the native FN, VN or Fbg adsorbed from blood, resulting in strong adhesive and survival signaling. Collectively, signaling from these multiple integrin species is hypothesized to enhance osseointegration of HA biomaterials.
Conclusions
When compared with unmodified HA substrates, MSCs adhere significantly better to HA surfaces coated with RGD, DGEA and P15, consistent with the known role of these peptides as integrin-binding attachment factors. However, as HA is a highly adsorptive biomaterial, it is unlikely that cells would ever encounter an HA surface in the absence of an adsorbed protein layer. Our prior and current studies show that, when presented within the context of adsorbed adhesion proteins, the collagen-derived peptides, DGEA and P15, enhance the osseointegration of HA implants, whereas RGD is strongly detrimental. Our results further suggest that the beneficial effects of DGEA and P15 are due to the role of these peptides as differentiation, rather than adhesive, factors.
Acknowledgements
This research was supported by NIH/NIAMS grant R01AR51539 (SLB) and a Predoctoral NRSA from NIBIB (KMH). The authors gratefully acknowledge the Bone Histomorphometry Core Facility for their assistance with tissue processing and staining.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sawyer AA, Hennessy KM, Bellis SL. The effect of adsorbed serum proteins, RGD and proteoglycan-binding peptides on the adhesion of mesenchymal stem cells to hydroxyapatite. Biomaterials. 2007;28(3):383–92. doi: 10.1016/j.biomaterials.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 2.Dalton BA, McFarland CD, Underwood PA, Steele JG. Role of the heparin binding domain of fibronectin in attachment and spreading of human bone-derived cells. J Cell Sci. 1995;108(Pt 5):2083–92. doi: 10.1242/jcs.108.5.2083. [DOI] [PubMed] [Google Scholar]
- 3.Woods A, McCarthy JB, Furcht LT, Couchman JR. A synthetic peptide from the COOH-terminal heparin-binding domain of fibronectin promotes focal adhesion formation. Mol Biol Cell. 1993;4(6):605–13. doi: 10.1091/mbc.4.6.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aota S, Nagai T, Yamada KM. Characterization of regions of fibronectin besides the arginine-glycine-aspartic acid sequence required for adhesive function of the cell-binding domain using site-directed mutagenesis. J Biol Chem. 1991;266(24):15938–43. [PubMed] [Google Scholar]
- 5.Woods A, Couchman JR, Johansson S, Hook M. Adhesion and cytoskeletal organization of fibroblasts in response to fibronectin fragments. Embo J. 1986;5(4):665–70. doi: 10.1002/j.1460-2075.1986.tb04265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grant RP, Spitzfaden C, Altroff H, Campbell ID, Mardon HJ. Structural requirements for biological activity of the ninth and tenth FIII domains of human fibronectin. J Biol Chem. 1997;272(10):6159–66. doi: 10.1074/jbc.272.10.6159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hautanen A, Gailit J, Mann DM, Ruoslahti E. Effects of modifications of the RGD sequence and its context on recognition by the fibronectin receptor. J Biol Chem. 1989;264(3):1437–42. [PubMed] [Google Scholar]
- 8.Garcia AJ, Reyes CD. Bio-adhesive surfaces to promote osteoblast differentiation and bone formation. J Dent Res. 2005;84(5):407–13. doi: 10.1177/154405910508400502. [DOI] [PubMed] [Google Scholar]
- 9.Garcia AJ, Keselowsky BG. Biomimetic surfaces for control of cell adhesion to facilitate bone formation. Crit Rev Eukaryot Gene Expr. 2002;12(2):151–62. doi: 10.1615/critreveukaryotgeneexpr.v12.i2.50. [DOI] [PubMed] [Google Scholar]
- 10.Yang XB, Roach HI, Clarke NM, Howdle SM, Quirk R, Shakesheff KM, et al. Human osteoprogenitor growth and differentiation on synthetic biodegradable structures after surface modification. Bone. 2001;29(6):523–31. doi: 10.1016/s8756-3282(01)00617-2. [DOI] [PubMed] [Google Scholar]
- 11.Behravesh E, Mikos AG. Three-dimensional culture of differentiating marrow stromal osteoblasts in biomimetic poly(propylene fumarate-co-ethylene glycol)-based macroporous hydrogels. J Biomed Mater Res A. 2003;66(3):698–706. doi: 10.1002/jbm.a.10003. [DOI] [PubMed] [Google Scholar]
- 12.Kantlehner M, Schaffner P, Finsinger D, Meyer J, Jonczyk A, Diefenbach B, et al. Surface coating with cyclic RGD peptides stimulates osteoblast adhesion and proliferation as well as bone formation. Chembiochem. 2000;1(2):107–14. doi: 10.1002/1439-7633(20000818)1:2<107::AID-CBIC107>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 13.Guillou-Buffello DL, Bareille R, Gindre M, Sewing A, Laugier P, Amedee J. Additive Effect of RGD Coating to Functionalized Titanium Surfaces on Human Osteoprogenitor Cell Adhesion and Spreading. Tissue Eng Part A. 2008;14(8):1445–55. doi: 10.1089/ten.tea.2007.0292. [DOI] [PubMed] [Google Scholar]
- 14.Durrieu MC, Pallu S, Guillemot F, Bareille R, Amedee J, Baquey CH, et al. Grafting RGD containing peptides onto hydroxyapatite to promote osteoblastic cells adhesion. J Mater Sci Mater Med. 2004;15(7):779–86. doi: 10.1023/b:jmsm.0000032818.09569.d9. [DOI] [PubMed] [Google Scholar]
- 15.Sawyer AA, Hennessy KM, Bellis SL. Regulation of mesenchymal stem cell attachment and spreading on hydroxyapatite by RGD peptides and adsorbed serum proteins. Biomaterials. 2005;26(13):1467–75. doi: 10.1016/j.biomaterials.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Wilson CJ, Clegg RE, Leavesley DI, Pearcy MJ. Mediation of biomaterial-cell interactions by adsorbed proteins: a review. Tissue Eng. 2005;11(1-2):1–18. doi: 10.1089/ten.2005.11.1. [DOI] [PubMed] [Google Scholar]
- 17.Weathersby PK, Horbett TA, Hoffman AS. A new method for analysis of the adsorbed plasma protein layer on biomaterial surfaces. Trans Am Soc Artif Intern Organs. 1976;22:242–52. [PubMed] [Google Scholar]
- 18.Nygren H, Broberg M. Specific activation of platelets by surface-adsorbed plasma proteins. J Biomater Sci Polym Ed. 1998;9(8):817–31. doi: 10.1163/156856298x00172. [DOI] [PubMed] [Google Scholar]
- 19.Hennessy KM, Clem WC, Phipps MC, Sawyer AA, Shaikh FM, Bellis SL. The effect of RGD peptides on osseointegration of hydroxyapatite biomaterials. Biomaterials. 2008;29(21):3075–83. doi: 10.1016/j.biomaterials.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Preissner KT. Structure and biological role of vitronectin. Annu Rev Cell Biol. 1991;7:275–310. doi: 10.1146/annurev.cb.07.110191.001423. [DOI] [PubMed] [Google Scholar]
- 21.Dahlback B. Blood coagulation. Lancet. 2000;355(9215):1627–32. doi: 10.1016/S0140-6736(00)02225-X. [DOI] [PubMed] [Google Scholar]
- 22.Mosher DF. Physiology of fibronectin. Annu Rev Med. 1984;35:561–75. doi: 10.1146/annurev.me.35.020184.003021. [DOI] [PubMed] [Google Scholar]
- 23.Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 24.Ruoslahti E. The RGD story: a personal account. Matrix Biol. 2003;22(6):459–65. doi: 10.1016/s0945-053x(03)00083-0. [DOI] [PubMed] [Google Scholar]
- 25.Pierschbacher M, Hayman EG, Ruoslahti E. Synthetic peptide with cell attachment activity of fibronectin. Proc Natl Acad Sci U S A. 1983;80(5):1224–7. doi: 10.1073/pnas.80.5.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mizuno M, Fujisawa R, Kuboki Y. Type I collagen-induced osteoblastic differentiation of bone-marrow cells mediated by collagen-alpha2beta1 integrin interaction. J Cell Physiol. 2000;184(2):207–13. doi: 10.1002/1097-4652(200008)184:2<207::AID-JCP8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 27.Salasznyk RM, Williams WA, Boskey A, Batorsky A, Plopper GE. Adhesion to Vitronectin and Collagen I Promotes Osteogenic Differentiation of Human Mesenchymal Stem Cells. J Biomed Biotechnol. 2004;2004(1):24–34. doi: 10.1155/S1110724304306017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takeuchi Y, Suzawa M, Kikuchi T, Nishida E, Fujita T, Matsumoto T. Differentiation and transforming growth factor-beta receptor down-regulation by collagen-alpha2beta1 integrin interaction is mediated by focal adhesion kinase and its downstream signals in murine osteoblastic cells. J Biol Chem. 1997;272(46):29309–16. doi: 10.1074/jbc.272.46.29309. [DOI] [PubMed] [Google Scholar]
- 29.Carvalho RS, Kostenuik PJ, Salih E, Bumann A, Gerstenfeld LC. Selective adhesion of osteoblastic cells to different integrin ligands induces osteopontin gene expression. Matrix Biol. 2003;22(3):241–9. doi: 10.1016/s0945-053x(03)00038-6. [DOI] [PubMed] [Google Scholar]
- 30.Xiao G, Wang D, Benson MD, Karsenty G, Franceschi RT. Role of the alpha2-integrin in osteoblast-specific gene expression and activation of the Osf2 transcription factor. J Biol Chem. 1998;273(49):32988–94. doi: 10.1074/jbc.273.49.32988. [DOI] [PubMed] [Google Scholar]
- 31.Bhatnagar RS, Qian JJ, Gough CA. The role in cell binding of a beta-bend within the triple helical region in collagen alpha 1 (I) chain: structural and biological evidence for conformational tautomerism on fiber surface. J Biomol Struct Dyn. 1997;14(5):547–60. doi: 10.1080/07391102.1997.10508155. [DOI] [PubMed] [Google Scholar]
- 32.Knight CG, Morton LF, Onley DJ, Peachey AR, Messent AJ, Smethurst PA, et al. Identification in collagen type I of an integrin alpha2 beta1-binding site containing an essential GER sequence. J Biol Chem. 1998;273(50):33287–94. doi: 10.1074/jbc.273.50.33287. [DOI] [PubMed] [Google Scholar]
- 33.Knight CG, Morton LF, Peachey AR, Tuckwell DS, Farndale RW, Barnes MJ. The collagen-binding A-domains of integrins alpha(1)beta(1) and alpha(2)beta(1) recognize the same specific amino acid sequence, GFOGER, in native (triple-helical) collagens. J Biol Chem. 2000;275(1):35–40. doi: 10.1074/jbc.275.1.35. [DOI] [PubMed] [Google Scholar]
- 34.Kilpadi KL, Chang PL, Bellis SL. Hydroxylapatite binds more serum proteins, purified integrins, and osteoblast precursor cells than titanium or steel. J Biomed Mater Res. 2001;57(2):258–67. doi: 10.1002/1097-4636(200111)57:2<258::aid-jbm1166>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 35.Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64(2):295–312. [PubMed] [Google Scholar]
- 36.Kilpadi KL, Sawyer AA, Prince CW, Chang PL, Bellis SL. Primary human marrow stromal cells and Saos-2 osteosarcoma cells use different mechanisms to adhere to hydroxylapatite. J Biomed Mater Res A. 2004;68(2):273–85. doi: 10.1002/jbm.a.20043. [DOI] [PubMed] [Google Scholar]
- 37.Reyes CD, Petrie TA, Burns KL, Schwartz Z, Garcia AJ. Biomolecular surface coating to enhance orthopaedic tissue healing and integration. Biomaterials. 2007;28(21):3228–35. doi: 10.1016/j.biomaterials.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reyes CD, Garcia AJ. Alpha2beta1 integrin-specific collagen-mimetic surfaces supporting osteoblastic differentiation. J Biomed Mater Res A. 2004;69(4):591–600. doi: 10.1002/jbm.a.30034. [DOI] [PubMed] [Google Scholar]
- 39.Emsley J, Knight CG, Farndale RW, Barnes MJ. Structure of the integrin alpha2beta1-binding collagen peptide. J Mol Biol. 2004;335(4):1019–28. doi: 10.1016/j.jmb.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 40.Schonmeyr BH, Wong AK, Li S, Gewalli F, Cordiero PG, Mehrara BJ. Treatment of hydroxyapatite scaffolds with fibronectin and fetal calf serum increases osteoblast adhesion and proliferation in vitro. Plast Reconstr Surg. 2008;121(3):751–62. doi: 10.1097/01.prs.0000299312.02227.81. [DOI] [PubMed] [Google Scholar]
- 41.Woo KM, Seo J, Zhang R, Ma PX. Suppression of apoptosis by enhanced protein adsorption on polymer/hydroxyapatite composite scaffolds. Biomaterials. 2007;28(16):2622–30. doi: 10.1016/j.biomaterials.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pecheva E, Pramatarova L, Altankov G. Hydroxyapatite grown on a native extracellular matrix: initial interactions with human fibroblasts. Langmuir. 2007;23(18):9386–92. doi: 10.1021/la700435c. [DOI] [PubMed] [Google Scholar]
- 43.Deligianni D, Korovessis P, Porte-Derrieu MC, Amedee J. Fibronectin preadsorbed on hydroxyapatite together with rough surface structure increases osteoblasts’ adhesion “in vitro”: the theoretical usefulness of fibronectin preadsorption on hydroxyapatite to increase permanent stability and longevity in spine implants. J Spinal Disord Tech. 2005;18(3):257–62. [PubMed] [Google Scholar]
- 44.Phang MY, Ng MH, Tan KK, Aminuddin BS, Ruszymah BH, Fauziah O. Evaluation of suitable biodegradable scaffolds for engineered bone tissue. Med J Malaysia. 2004;59(Suppl B):198–9. [PubMed] [Google Scholar]
- 45.Matsuura T, Hosokawa R, Okamoto K, Kimoto T, Akagawa Y. Diverse mechanisms of osteoblast spreading on hydroxyapatite and titanium. Biomaterials. 2000;21(11):1121–7. doi: 10.1016/s0142-9612(99)00264-1. [DOI] [PubMed] [Google Scholar]
- 46.Zreiqat HS, O. C., Gengenbach T, Steele JG, Howlett CR. The Role of Surface Characteristics in the Initial Adhesion of Human Bone-Derived Cells on Ceramics. Cells Mater. 1996;6(1):45–56. [Google Scholar]
- 47.Staatz WD, Fok KF, Zutter MM, Adams SP, Rodriguez BA, Santoro SA. Identification of a tetrapeptide recognition sequence for the alpha 2 beta 1 integrin in collagen. J Biol Chem. 1991;266(12):7363–7. [PubMed] [Google Scholar]
- 48.Bhatnagar RS, Qian JJ, Wedrychowska A, Sadeghi M, Wu YM, Smith N. Design of biomimetic habitats for tissue engineering with P-15, a synthetic peptide analogue of collagen. Tissue Eng. 1999;5(1):53–65. doi: 10.1089/ten.1999.5.53. [DOI] [PubMed] [Google Scholar]
- 49.Gilbert M, Giachelli CM, Stayton PS. Biomimetic peptides that engage specific integrin-dependent signaling pathways and bind to calcium phosphate surfaces. J Biomed Mater Res A. 2003;67(1):69–77. doi: 10.1002/jbm.a.10053. [DOI] [PubMed] [Google Scholar]
- 50.Mizuno M, Kuboki Y. Osteoblast-related gene expression of bone marrow cells during the osteoblastic differentiation induced by type I collagen. J Biochem. 2001;129(1):133–8. doi: 10.1093/oxfordjournals.jbchem.a002824. [DOI] [PubMed] [Google Scholar]
- 51.Yukna RA, Krauser JT, Callan DP, Evans GH, Cruz R, Martin M. Multi-center clinical comparison of combination anorganic bovine-derived hydroxyapatite matrix (ABM)/cell binding peptide (P-15) and ABM in human periodontal osseous defects. 6-month results. J Periodontol. 2000;71(11):1671–9. doi: 10.1902/jop.2000.71.11.1671. [DOI] [PubMed] [Google Scholar]
- 52.Yukna RA, Callan DP, Krauser JT, Evans GH, Aichelmann-Reidy ME, Moore K, et al. Multi-center clinical evaluation of combination anorganic bovine-derived hydroxyapatite matrix (ABM)/cell binding peptide (P-15) as a bone replacement graft material in human periodontal osseous defects. 6-month results. J Periodontol. 1998;69(6):655–63. doi: 10.1902/jop.1998.69.6.655. [DOI] [PubMed] [Google Scholar]
- 53.Barros RR, Novaes AB, Jr., Roriz VM, Oliveira RR, Grisi MF, Souza SL, et al. Anorganic bovine matrix/p-15 “flow” in the treatment of periodontal defects: case series with 12 months of follow-up. J Periodontol. 2006;77(7):1280–7. doi: 10.1902/jop.2006.050161. [DOI] [PubMed] [Google Scholar]
- 54.Yukna R, Salinas TJ, Carr RF. Periodontal regeneration following use of ABM/P-1 5: a case report. Int J Periodontics Restorative Dent. 2002;22(2):146–55. [PubMed] [Google Scholar]
- 55.Hahn J, Rohrer MD, Tofe AJ. Clinical, radiographic, histologic, and histomorphometric comparison of PepGen P-15 particulate and PepGen P-15 flow in extraction sockets: a same-mouth case study. Implant Dent. 2003;12(2):170–4. doi: 10.1097/01.id.0000064812.39660.ff. [DOI] [PubMed] [Google Scholar]
- 56.Radhakrishnan S, Anusuya CN. Comparative clinical evaluation of combination anorganic bovine-derived hydroxyapatite matrix (ABM)/cell binding peptide (P-15) and open flap debridement (DEBR) in human periodontal osseous defects: a 6 month pilot study. J Int Acad Periodontol. 2004;6(3):101–7. [PubMed] [Google Scholar]
- 57.Bhongade ML, Tiwari IR. A comparative evaluation of the effectiveness of an anorganic bone matrix/cell binding peptide with an open flap debridement in human infrabony defects: a clinical and radiographic study. J Contemp Dent Pract. 2007;8(6):25–34. [PubMed] [Google Scholar]
- 58.Thompson DM, Rohrer MD, Prasad HS. Comparison of bone grafting materials in human extraction sockets: clinical, histologic, and histomorphometric evaluations. Implant Dent. 2006;15(1):89–96. doi: 10.1097/01.id.0000202426.62007.60. [DOI] [PubMed] [Google Scholar]
- 59.Degidi M, Scarano A, Iezzi G, Orsini G, Perrotti V, Strocchi R, et al. Maxillary sinus augmentation using a synthetic cell-binding peptide: a histologic and transmission electron microscopy case study in man. Implant Dent. 2005;14(4):371–5. doi: 10.1097/01.id.0000188471.49933.f2. [DOI] [PubMed] [Google Scholar]
- 60.Rouahi M, Champion E, Hardouin P, Anselme K. Quantitative kinetic analysis of gene expression during human osteoblastic adhesion on orthopaedic materials. Biomaterials. 2006;27(14):2829–44. doi: 10.1016/j.biomaterials.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 61.Wang C, Duan Y, Markovic B, Barbara J, Howlett CR, Zhang X, et al. Phenotypic expression of bone-related genes in osteoblasts grown on calcium phosphate ceramics with different phase compositions. Biomaterials. 2004;25(13):2507–14. doi: 10.1016/j.biomaterials.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 62.Shu R, McMullen R, Baumann MJ, McCabe LR. Hydroxyapatite accelerates differentiation and suppresses growth of MC3T3-E1 osteoblasts. J Biomed Mater Res A. 2003;67(4):1196–204. doi: 10.1002/jbm.a.20021. [DOI] [PubMed] [Google Scholar]
- 63.Dawson JI, Wahl DA, Lanham SA, Kanczler JM, Czernuszka JT, Oreffo RO. Development of specific collagen scaffolds to support the osteogenic and chondrogenic differentiation of human bone marrow stromal cells. Biomaterials. 2008;29(21):3105–16. doi: 10.1016/j.biomaterials.2008.03.040. [DOI] [PubMed] [Google Scholar]
- 64.Song JH, Kim HE, Kim HW. Electrospun fibrous web of collagen-apatite precipitated nanocomposite for bone regeneration. J Mater Sci Mater Med. 2008;19(8):2925–32. doi: 10.1007/s10856-008-3420-7. [DOI] [PubMed] [Google Scholar]
- 65.Leupold B, An, Hartsock A comparison of ProOsteon, DBX, and collagraft in a rabbit model. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2006;79(2):292–7. doi: 10.1002/jbm.b.30541. [DOI] [PubMed] [Google Scholar]
- 66.Le Guillou-Buffello D, Bareille R, Gindre M, Sewing A, Laugier P, Amedee J. Additive effect of RGD coating to functionalized titanium surfaces on human osteoprogenitor cell adhesion and spreading. Tissue Eng Part A. 2008;14(8):1445–55. doi: 10.1089/ten.tea.2007.0292. [DOI] [PubMed] [Google Scholar]
- 67.Itoh D, Yoneda S, Kuroda S, Kondo H, Umezawa A, Ohya K, et al. Enhancement of osteogenesis on hydroxyapatite surface coated with synthetic peptide (EEEEEEEPRGDT) in vitro. J Biomed Mater Res. 2002;62(2):292–8. doi: 10.1002/jbm.10338. [DOI] [PubMed] [Google Scholar]