Abstract
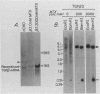
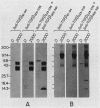
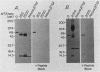
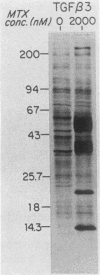
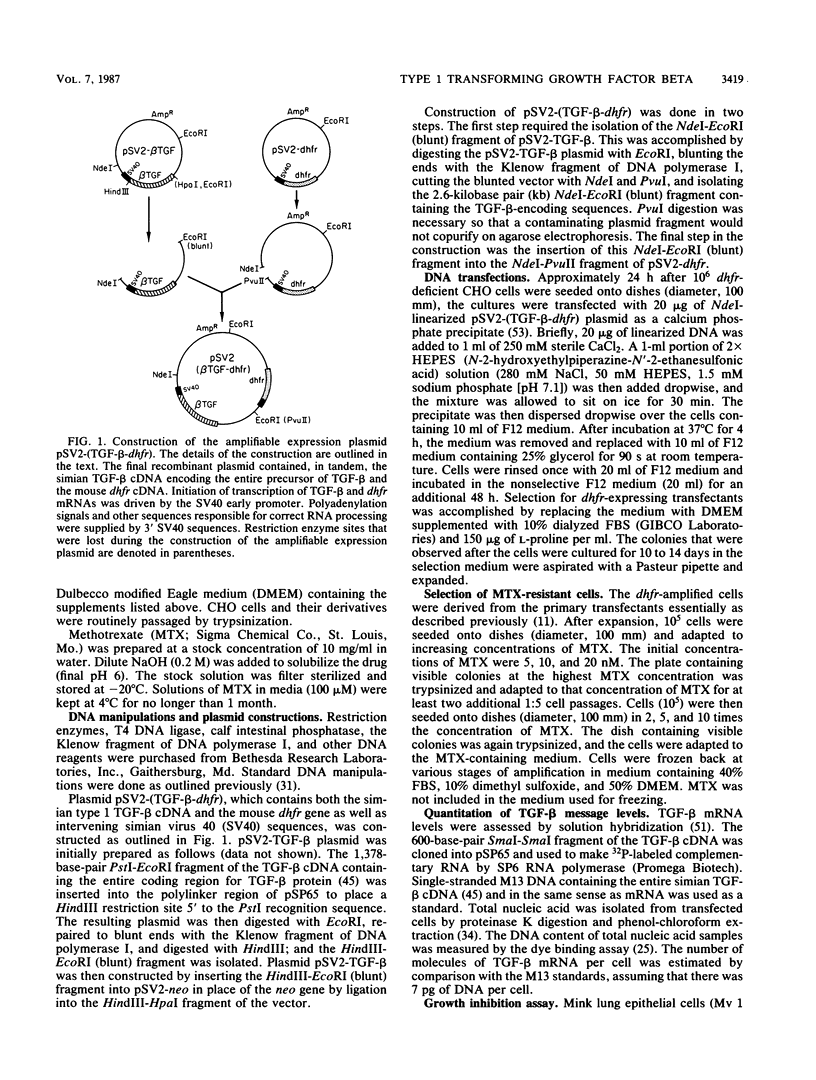
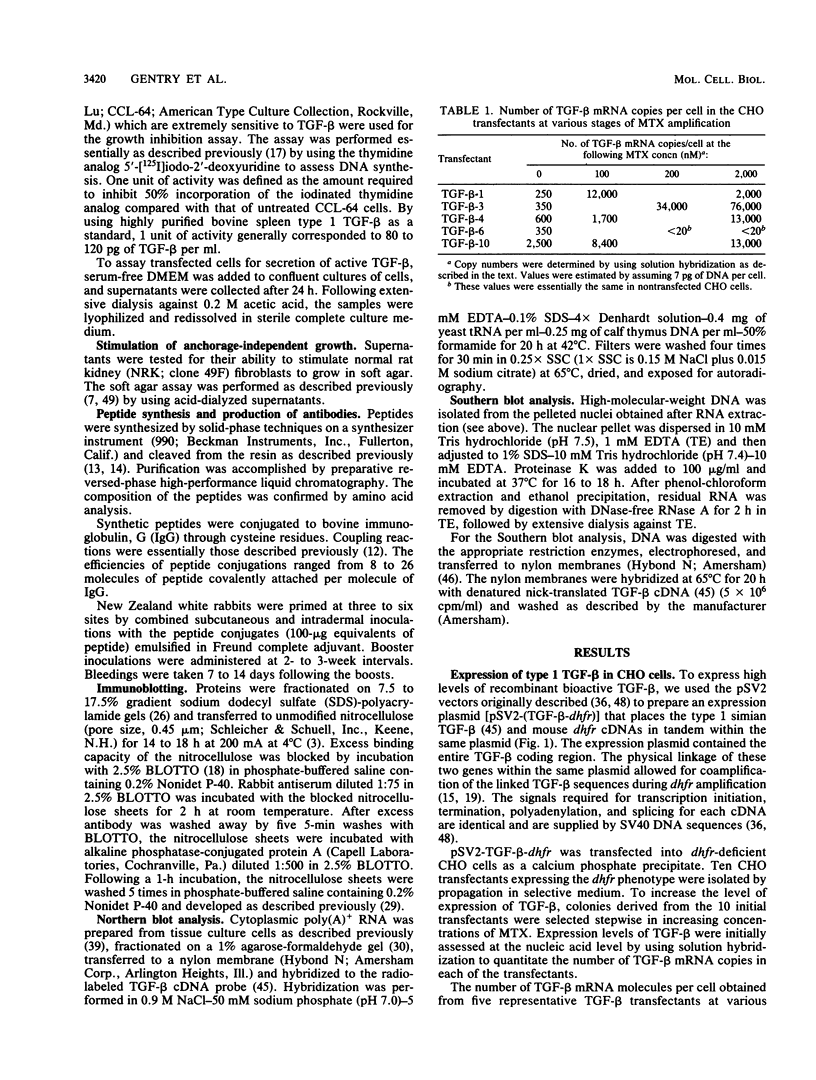
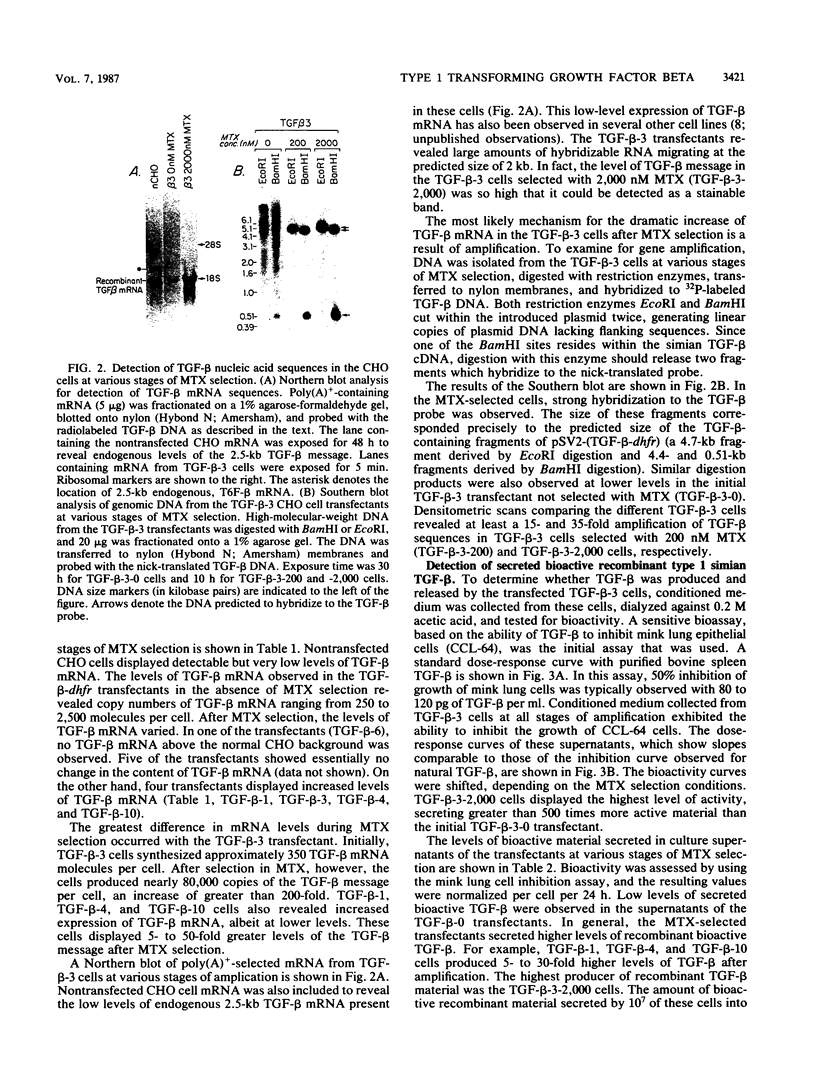
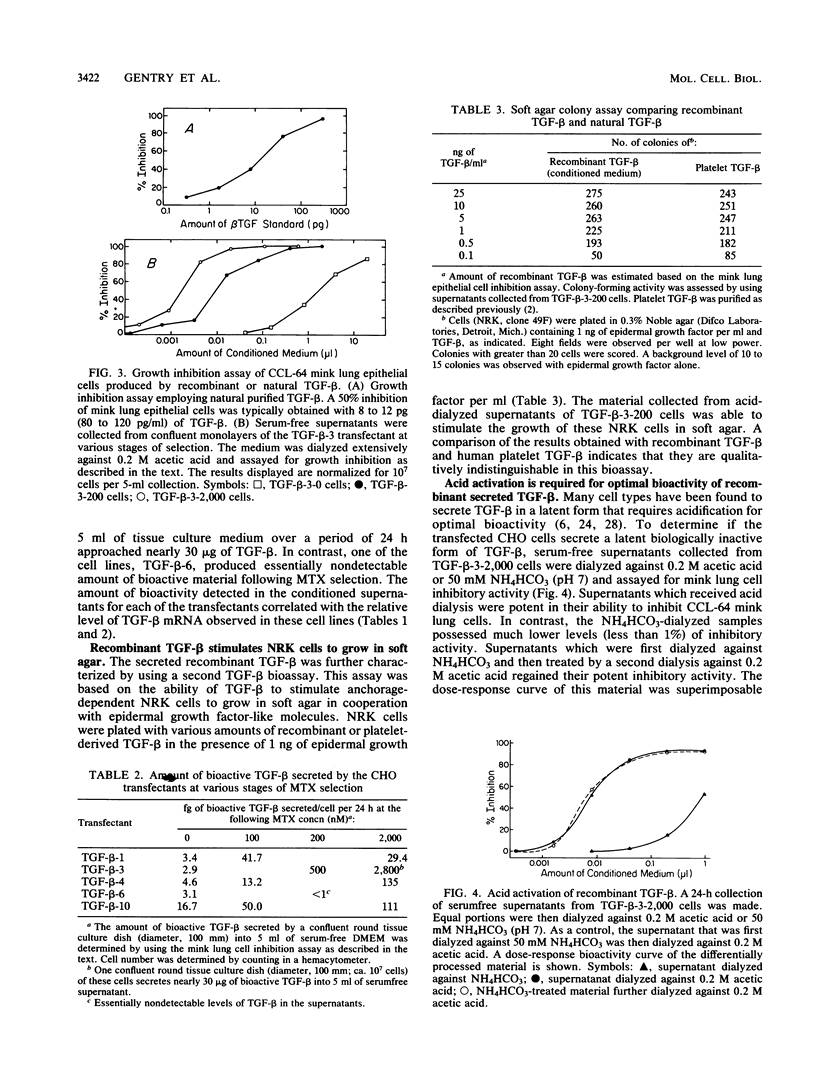
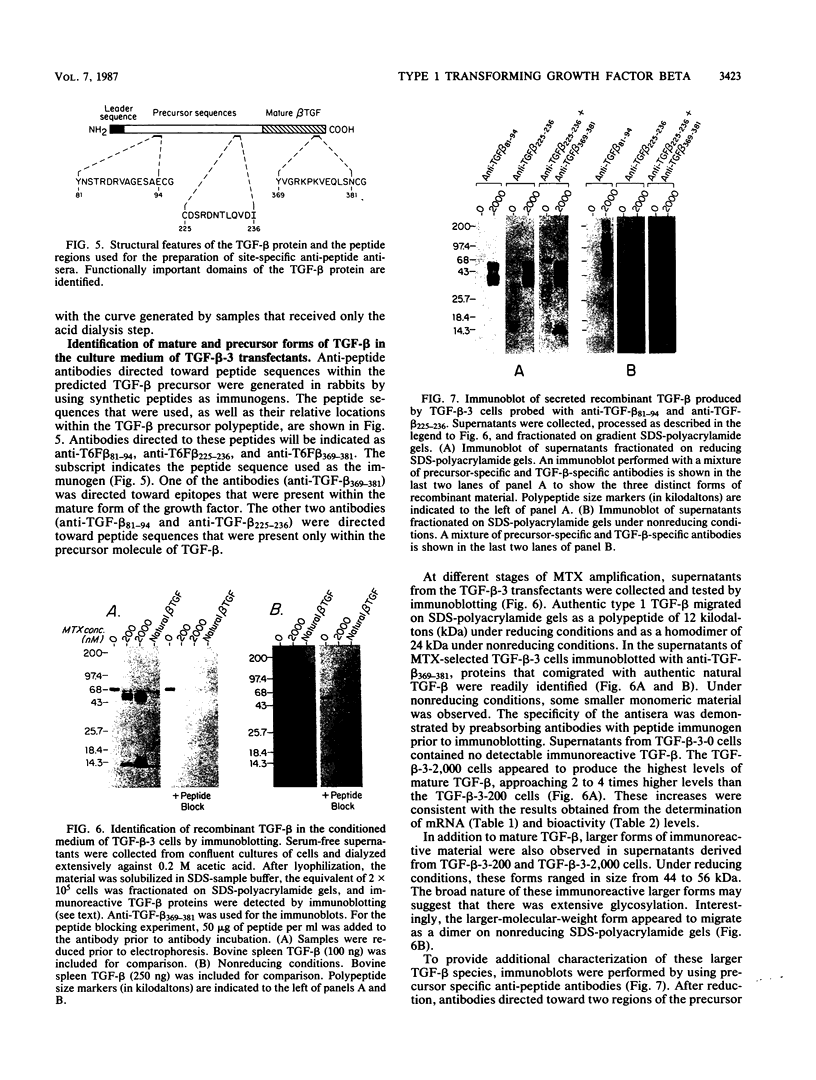
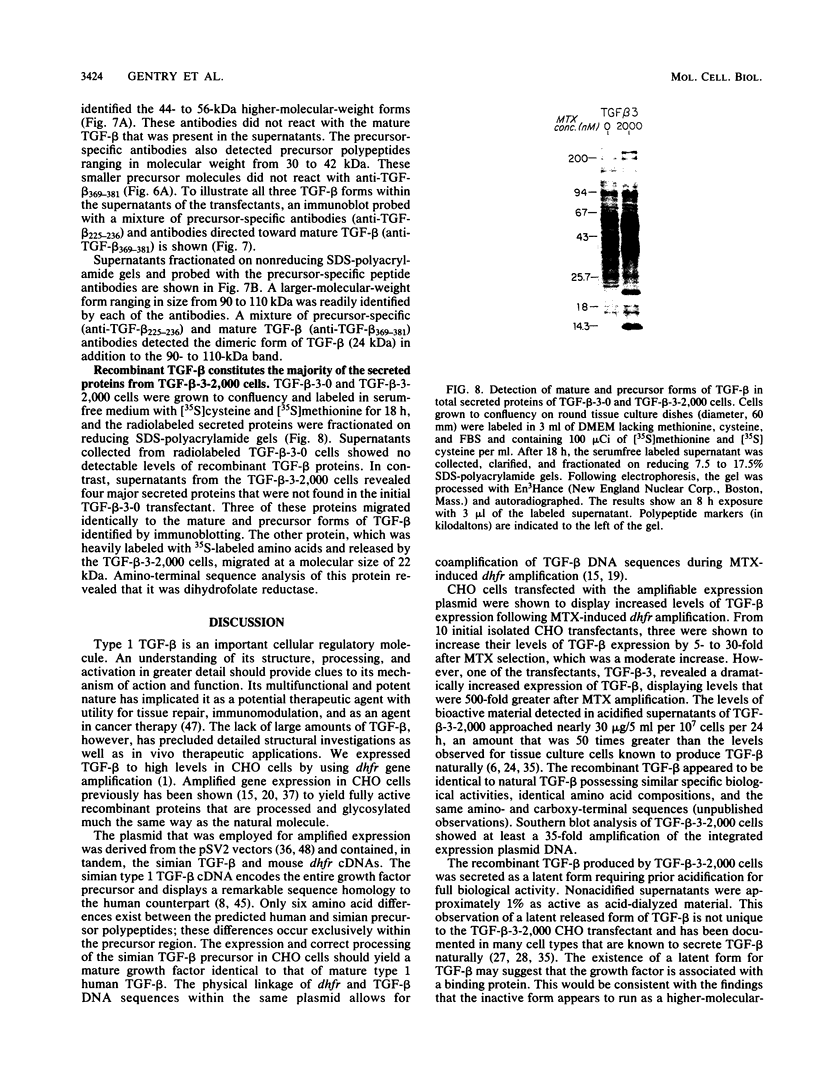
Recombinant type 1 transforming growth factor beta (TGF-beta) was expressed to high levels in CHO cells by using dihydrofolate reductase (dhfr) gene amplification. The expression plasmid was derived from the pSV2 vectors and contained, in tandem, the simian TGF-beta and mouse dhfr cDNAs. Transcription of both cDNAs was controlled by the simian virus 40 early promoter. Stepwise selection of transfected CHO cells in increasing concentrations of methotrexate yielded cell lines that expressed amplified TGF-beta nucleic acid sequences. The expression plasmid DNA was amplified greater than 35-fold in one of the methotrexate-selected transfectants. The major proteins secreted by these cells consisted of latent TGF-beta and TGF-beta precursor polypeptides, as judged by immunoblots by using site-specific anti-peptide antibodies derived from various regions of the TGF-beta precursor. Levels of recombinant TGF-beta protein secreted by these cells approached 30 micrograms/24 h per 10(7) cells and required prior acidification for optimal activity; nonacidified supernatants were approximately 1% as active as acidified material. Antibodies directed toward sequences present in the mature growth factor readily identified a proteolytically processed recombinant TGF-beta which, on sodium dodecyl sulfate-polyacrylamide gels, comigrated with highly purified natural TGF-beta. In addition to mature recombinant TGF-beta, site-specific antibodies demonstrated the existence of larger TGF-beta precursor polypeptides. The availability of biologically active recombinant type 1 TGF-beta and precursor forms should provide a means to examine the structure, function, and potential in vivo therapeutic use of this growth factor.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Kellems R. E., Bertino J. R., Schimke R. T. Selective multiplication of dihydrofolate reductase genes in methotrexate-resistant variants of cultured murine cells. J Biol Chem. 1978 Mar 10;253(5):1357–1370. [PubMed] [Google Scholar]
- Assoian R. K., Komoriya A., Meyers C. A., Miller D. M., Sporn M. B. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem. 1983 Jun 10;258(11):7155–7160. [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cheifetz S., Weatherbee J. A., Tsang M. L., Anderson J. K., Mole J. E., Lucas R., Massagué J. The transforming growth factor-beta system, a complex pattern of cross-reactive ligands and receptors. Cell. 1987 Feb 13;48(3):409–415. doi: 10.1016/0092-8674(87)90192-9. [DOI] [PubMed] [Google Scholar]
- Childs C. B., Proper J. A., Tucker R. F., Moses H. L. Serum contains a platelet-derived transforming growth factor. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5312–5316. doi: 10.1073/pnas.79.17.5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey R. J., Jr, Shipley G. D., Moses H. L. Production of transforming growth factors by human colon cancer lines. Cancer Res. 1986 Mar;46(3):1164–1169. [PubMed] [Google Scholar]
- Derynck R., Jarrett J. A., Chen E. Y., Eaton D. H., Bell J. R., Assoian R. K., Roberts A. B., Sporn M. B., Goeddel D. V. Human transforming growth factor-beta complementary DNA sequence and expression in normal and transformed cells. Nature. 1985 Aug 22;316(6030):701–705. doi: 10.1038/316701a0. [DOI] [PubMed] [Google Scholar]
- Derynck R., Jarrett J. A., Chen E. Y., Goeddel D. V. The murine transforming growth factor-beta precursor. J Biol Chem. 1986 Apr 5;261(10):4377–4379. [PubMed] [Google Scholar]
- Frolik C. A., Dart L. L., Meyers C. A., Smith D. M., Sporn M. B. Purification and initial characterization of a type beta transforming growth factor from human placenta. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3676–3680. doi: 10.1073/pnas.80.12.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser C. S., Schimke R. T. Cell cycle regulation of transfected murine dihydrofolate reductase genes. J Biol Chem. 1986 May 25;261(15):6938–6946. [PubMed] [Google Scholar]
- Gentry L. E., Lawton A. Characterization of site-specific antibodies to the erbB gene product and EGF receptor: inhibition of tyrosine kinase activity. Virology. 1986 Jul 30;152(2):421–431. doi: 10.1016/0042-6822(86)90144-3. [DOI] [PubMed] [Google Scholar]
- Gentry L. E., Rohrschneider L. R., Casnellie J. E., Krebs E. G. Antibodies to a defined region of pp60src neutralize the tyrosine-specific kinase activity. J Biol Chem. 1983 Sep 25;258(18):11219–11228. [PubMed] [Google Scholar]
- Gentry L. E., Twardzik D. R., Lim G. J., Ranchalis J. E., Lee D. C. Expression and characterization of transforming growth factor alpha precursor protein in transfected mammalian cells. Mol Cell Biol. 1987 May;7(5):1585–1591. doi: 10.1128/mcb.7.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes J., Weissmann C. Constitutive, long-term production of human interferons by hamster cells containing multiple copies of a cloned interferon gene. Nucleic Acids Res. 1983 Feb 11;11(3):687–706. doi: 10.1093/nar/11.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Type beta transforming growth factor controls the adipogenic differentiation of 3T3 fibroblasts. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8530–8534. doi: 10.1073/pnas.82.24.8530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda T., Lioubin M. N., Marquardt H. Human transforming growth factor type beta 2: production by a prostatic adenocarcinoma cell line, purification, and initial characterization. Biochemistry. 1987 May 5;26(9):2406–2410. doi: 10.1021/bi00383a002. [DOI] [PubMed] [Google Scholar]
- Kaufman R. J., Sharp P. A. Amplification and expression of sequences cotransfected with a modular dihydrofolate reductase complementary dna gene. J Mol Biol. 1982 Aug 25;159(4):601–621. doi: 10.1016/0022-2836(82)90103-6. [DOI] [PubMed] [Google Scholar]
- Kaufman R. J., Wasley L. C., Spiliotes A. J., Gossels S. D., Latt S. A., Larsen G. R., Kay R. M. Coamplification and coexpression of human tissue-type plasminogen activator and murine dihydrofolate reductase sequences in Chinese hamster ovary cells. Mol Cell Biol. 1985 Jul;5(7):1750–1759. doi: 10.1128/mcb.5.7.1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehrl J. H., Roberts A. B., Wakefield L. M., Jakowlew S., Sporn M. B., Fauci A. S. Transforming growth factor beta is an important immunomodulatory protein for human B lymphocytes. J Immunol. 1986 Dec 15;137(12):3855–3860. [PubMed] [Google Scholar]
- Kehrl J. H., Wakefield L. M., Roberts A. B., Jakowlew S., Alvarez-Mon M., Derynck R., Sporn M. B., Fauci A. S. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986 May 1;163(5):1037–1050. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keski-Oja J., Leof E. B., Lyons R. M., Coffey R. J., Jr, Moses H. L. Transforming growth factors and control of neoplastic cell growth. J Cell Biochem. 1987 Feb;33(2):95–107. doi: 10.1002/jcb.240330204. [DOI] [PubMed] [Google Scholar]
- Knabbe C., Lippman M. E., Wakefield L. M., Flanders K. C., Kasid A., Derynck R., Dickson R. B. Evidence that transforming growth factor-beta is a hormonally regulated negative growth factor in human breast cancer cells. Cell. 1987 Feb 13;48(3):417–428. doi: 10.1016/0092-8674(87)90193-0. [DOI] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawrence D. A., Pircher R., Jullien P. Conversion of a high molecular weight latent beta-TGF from chicken embryo fibroblasts into a low molecular weight active beta-TGF under acidic conditions. Biochem Biophys Res Commun. 1985 Dec 31;133(3):1026–1034. doi: 10.1016/0006-291x(85)91239-2. [DOI] [PubMed] [Google Scholar]
- Lawrence D. A., Pircher R., Krycève-Martinerie C., Jullien P. Normal embryo fibroblasts release transforming growth factors in a latent form. J Cell Physiol. 1984 Oct;121(1):184–188. doi: 10.1002/jcp.1041210123. [DOI] [PubMed] [Google Scholar]
- Leary J. J., Brigati D. J., Ward D. C. Rapid and sensitive colorimetric method for visualizing biotin-labeled DNA probes hybridized to DNA or RNA immobilized on nitrocellulose: Bio-blots. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4045–4049. doi: 10.1073/pnas.80.13.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Massagué J., Cheifetz S., Endo T., Nadal-Ginard B. Type beta transforming growth factor is an inhibitor of myogenic differentiation. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8206–8210. doi: 10.1073/pnas.83.21.8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masui T., Wakefield L. M., Lechner J. F., LaVeck M. A., Sporn M. B., Harris C. C. Type beta transforming growth factor is the primary differentiation-inducing serum factor for normal human bronchial epithelial cells. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2438–2442. doi: 10.1073/pnas.83.8.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight G. S., Lee D. C., Hemmaplardh D., Finch C. A., Palmiter R. D. Transferrin gene expression. Effects of nutritional iron deficiency. J Biol Chem. 1980 Jan 10;255(1):144–147. [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Factors governing the expression of a bacterial gene in mammalian cells. Mol Cell Biol. 1981 May;1(5):449–459. doi: 10.1128/mcb.1.5.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachl C., Burke R. L., Stuve L. L., Sanchez-Pescador L., Van Nest G., Masiarz F., Dina D. Expression of cell-associated and secreted forms of herpes simplex virus type 1 glycoprotein gB in mammalian cells. J Virol. 1987 Feb;61(2):315–325. doi: 10.1128/jvi.61.2.315-325.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E. Variants of the cell recognition site of fibronectin that retain attachment-promoting activity. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5985–5988. doi: 10.1073/pnas.81.19.5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purchio A. F., Fareed G. C. Transformation of human embryonic kidney cells by human papovarirus BK. J Virol. 1979 Feb;29(2):763–769. doi: 10.1128/jvi.29.2.763-769.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp U. R., Gunnell M., Marquardt H. Normal mouse serum contains peptides which induce fibroblasts to grow in soft agar. J Cell Biochem. 1983;21(1):29–38. doi: 10.1002/jcb.240210105. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Lamb L. C., Smith J. M., Sporn M. B. New class of transforming growth factors potentiated by epidermal growth factor: isolation from non-neoplastic tissues. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5339–5343. doi: 10.1073/pnas.78.9.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Meyers C. A., Wideman J., Blacher R., Pan Y. C., Stein S., Lehrman S. R., Smith J. M., Lamb L. C. Purification and properties of a type beta transforming growth factor from bovine kidney. Biochemistry. 1983 Dec 6;22(25):5692–5698. doi: 10.1021/bi00294a002. [DOI] [PubMed] [Google Scholar]
- Seyedin S. M., Segarini P. R., Rosen D. M., Thompson A. Y., Bentz H., Graycar J. Cartilage-inducing factor-B is a unique protein structurally and functionally related to transforming growth factor-beta. J Biol Chem. 1987 Feb 15;262(5):1946–1949. [PubMed] [Google Scholar]
- Seyedin S. M., Thompson A. Y., Bentz H., Rosen D. M., McPherson J. M., Conti A., Siegel N. R., Galluppi G. R., Piez K. A. Cartilage-inducing factor-A. Apparent identity to transforming growth factor-beta. J Biol Chem. 1986 May 5;261(13):5693–5695. [PubMed] [Google Scholar]
- Sharples K., Plowman G. D., Rose T. M., Twardzik D. R., Purchio A. F. Cloning and sequence analysis of simian transforming growth factor-beta cDNA. DNA. 1987 Jun;6(3):239–244. doi: 10.1089/dna.1987.6.239. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B., Wakefield L. M., Assoian R. K. Transforming growth factor-beta: biological function and chemical structure. Science. 1986 Aug 1;233(4763):532–534. doi: 10.1126/science.3487831. [DOI] [PubMed] [Google Scholar]
- Subramani S., Mulligan R., Berg P. Expression of the mouse dihydrofolate reductase complementary deoxyribonucleic acid in simian virus 40 vectors. Mol Cell Biol. 1981 Sep;1(9):854–864. doi: 10.1128/mcb.1.9.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twardzik D. R., Ranchalis J. E., Todaro G. J. Mouse embryonic transforming growth factors related to those isolated from tumor cells. Cancer Res. 1982 Feb;42(2):590–593. [PubMed] [Google Scholar]
- Twardzik D. R., Sherwin S. A. Transforming growth factor (TGF) activity in human urine: synergism between TGF-beta and urogastrone. J Cell Biochem. 1985;28(4):289–297. doi: 10.1002/jcb.240280407. [DOI] [PubMed] [Google Scholar]
- Uhler M. D., Carmichael D. F., Lee D. C., Chrivia J. C., Krebs E. G., McKnight G. S. Isolation of cDNA clones coding for the catalytic subunit of mouse cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1300–1304. doi: 10.1073/pnas.83.5.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urlaub G., Chasin L. A. Isolation of Chinese hamster cell mutants deficient in dihydrofolate reductase activity. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4216–4220. doi: 10.1073/pnas.77.7.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R., Urlaub G., Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]