Summary
Intrinsic immunosuppression is a major obstacle for a successful cancer therapy. Mechanisms how immunosuppression is induced and regulated in humans are ill-defined. A micro-environmental component that might prevent anti-tumor immunity is the presence of dying tumor cells, which is abundant following conventional cancer ablation methods such as chemo- or radiotherapy. Shedding of apoptotic debris and/or secretion of factors to the tumor bed or draining lymph nodes thus might have a profound impact on professional phagocytes such as DC and subsequent priming of lymphocytes. In this study, we exposed human DC to supernatants of living, apoptotic or necrotic human breast cancer cells and co-cultured them with autologous T cells. Priming with apoptotic debris prevented DC from establishing cytotoxicity towards living human tumor cells by inducing a regulatory T cell population, defined by co-expression of CD39 and CD69. Immunosuppression via Treg was transferable and required the release of sphingosine-1-phosphate (S1P) from apoptotic cells, acting via S1P receptor 4 on DC to induce IL-27 secretion. We propose that CD69-expression on CD39+ Treg enables them to interact with CD73-expressing CD8+ T cells to generate adenosine, thereby suppressing cytotoxicity. These findings aid the understanding how dying tumor cells limit anti-tumor immunity.
Keywords: cell death, cancer, tumor immunology, regulatory T cells, dendritic cells
Introduction
A growing tumor activates the immune system in ways to ensure its own survival and further on, encourage the formation of metastases. Polarization to a tumor-supportive state can be observed for APC of the innate immunity such as macrophages or DC [1]. These phagocytes program adaptive immunity [2] by generating e.g. tumor-specific Treg, which are a major obstacle for anti-tumor immunity [3]. Treg can be primed and activated mainly in the tumor-adjacent draining lymph nodes (TDLN) by factors shed from the tumor [4]. Once primed, Treg travel to the tumor site, where they prevent effector T cells from eradicating the tumor and further stimulate metastasis of tumor cells [5]. Thus, TDLN-derived suppressive Treg can potentially curb the benefit of an adoptive immunotherapy by suppressing the function of cytotoxic T cells or preventing immune activation following conventional therapy, which is required to eradicate residual tumor masses [6]. Hence, mechanisms of Treg generation within the TDLN have to be defined in order to design effective therapeutic strategies.
Generation/priming of tumor-specific Treg, requires antigen uptake and presentation by professional APC, i.e., DC. Tumor-derived antigens are acquired by DC directly at the tumor site [7], from metastasing tumor cells [8], or through tumor-released exosomes/microvesicles [9], which are primarily drained to the TDLN. In addition to antigens, DC receive other tumor-derived signals that shape their phenotype and the subsequent profiling of T cells [1, 10]. Depending on the tumor microenvironment, DC may exist in different states of maturation and activation [8, 11].
A prominent microenvironmental niche in tumors are dying tumor or stromal cells that are continouosly produced during tumor growth [12] or by conventional cancer-ablation methods. Regarding the immunological outcome of tumor cell death, the two extremes are 1) antigen cross-presentation and induction of an inflammatory response by DC that prepares immune effectors to eradicate malfunctioning cells or 2) tolerance to dampen an over-activated immune reaction [13]. The decision towards inflammation versus tolerance depends on the surface protein/lipid signatures as well as immune-modulating factors released by dying cells, determined by the mode of cell death [14]. For instance, necrosis induces shedding of danger-associated molecular patterns, which activate TLRs on DC [14]. The immunological outcome of apoptosis is ambiguous due to varieties in the apoptotic cell (AC) surface proteome [15]. Apoptosis can be immunogenic as demonstrated by an increase in antigen-cross-presentation and the induction of cytotoxic T cells upon priming with AC in vivo [16]. On the other hand, triggering of multiple immunosuppressive pathways upon priming with AC has also been recognized [17]. In case of cancer-ablation treatments such as chemotherapy, the decision towards generating an anti-tumor response or tolerance might be determined by the drug being used, as recent evidence suggests that certain chemotherapeutic drugs such as oxaliplatin trigger immunogenic cancer cell death [18]. However, cross-presentation of AC-derived antigens after chemotherapy does not necessarily culminate in anti-tumor immunity [19]. Hence, the surface alterations on dying cells, signalling molecules secreted from dying cells that drain the adjacent lymph nodes together with tumor antigens may also be important for inducing tolerance and possibly favour relapse [4]. AC secrete immunomodulators in a regulated manner, among them lipids such as lysophosphatidylcholine (LPC) or sphingosine-1-phosphate (S1P), anti-inflammatory proteins such as transforming growth factor (TGF)-β as well as nucleotides, which have the capacity to modify DC-dependent immunity [14]. Understanding how priming by dying cells impacts anti-tumor immune responses might benefit cancer therapy.
Materials and methods
Primary human immune cell isolation and expansion
Primary human blood cells were obtained from Buffy Coats (DRK-Blutspendedienst Baden-Württemberg-Hessen, Institut für Transfusionsmedizin und Immunhämatologie, Frankfurt, Germany). For isolation of CD14+ human monocytes, PBMC were obtained using Ficoll-Isopaque (PAA, Cölbe, Germany) gradient centrifugation. CD14+ monocytes were isolated from PBMC by magnetic sorting using human CD14 microbeads and the autoMACS™ Separator (Miltenyi, Bergisch Gladbach, Germany). The negative fraction was used for T cell enrichment in T cell medium [20] containing IL-2 (100 U/ml) (Immunotools, Friesoythe, Germany) for 6 days.
Monocyte-derived DC generation
2 × 105 human primary monocytes were cultured in 12-well plates in RPMI 1640 containing 10% FCS, GM-CSF (100 ng/ml) (Miltenyi) and IL-4 (5 ng/ml) (Immunotools) for 6 days to generate DC.
Preparation of tumor cell supernatants
MCF-7 human breast carcinoma cells were grown in RPMI 1640 with 10% FCS. Supernatants of living (VCM), apoptotic (ACM) or necrotic (NCM) MCF-7 cells were prepared as follows. MCF-7 cells remained untreated (living), were exposed to 0.5 μg/ml staurosporine for 1 h (apoptosis) or 30 μM oxaliplatin for 16 h (immunogenic cell death) (both from Sigma, Steinheim, Germany) or were incubated at 56°C for 30 min (necrosis), followed by washing and incubation for another 5 h in full medium. Conditioned media were harvested by centrifugation (1.000 × g, 10 min) and filtration through 0.2-μm pore filters to remove residual cellular particles.
Reagents
S1P and the S1PR1/3 inhibitor VPC23019 (1 μM) (Avanti Polar Lipids, AL, USA) were dissolved following the manufacturer’s instructions. S1PR2/4 antagonist JTE-013 (15 μM) (Biomol, Hamburg Germany) and the S1PR4 antagonists CYM50358 and CYM50374 (each 200 nM) [21] were dissolved in DMSO. DC were pre-incubated with these reagents for 30 min prior to the addition of tumor cell supernatants. The indolamine-2,3-dioxygenase (IDO) inhibitors L-1MT or D-1MT (1 mM) (Sigma) [22] were added to DC 2 h prior to T cell addition. IL-27 neutralizing antibody isotype control (R&D Systems, Wiesbaden-Nordernstadt, Germany) were added at 1 μg/ml to DC 30 min before addition of T cells. The CD39 inhibitor ARL67156 (250 μM), the CD73 inhibitor 5′-[αβ-methylene] diphosphate (APCP) [23] (100 μM) in ddH2O and the adenosine receptor A2a antagonist 8 (3-chlorostyryl) caffeine (CSC) [24] (10 mM) in DMSO, as well as the CD69 antibody (BD Biosciences, Heidelberg, Germany), TGF-β neutralizing antibody (R&D Systems) [25] and the respective isotype controls, were added to DC-T cell cultures at day 2.
DC-T cell co-culture
Tumor cell supernatants were added to 2 × 105 DC at ratios of 1:1 for 16 h, followed by washing. Afterwards 2 × 106 T cell-enriched PBMC were added and co-cultures were maintained for 3 days.
Cytotoxicity assay
Cytotoxicity was quantified using a modified assay [26]. In brief, 5 × 104 human breast carcinoma cells (MCF-7, T47D), pre-stained with 100 μM CellTracker Blue (Invitrogen, CA, USA) for 45 min, were cultured for 4 h in FACS tubes with T cells from DC co-cultures (ratios as indicated). The reaction mix was stained with PI for 10 min. Directly before sample acquisition, Flow-Count Fluorospheres (Beckman-Coulter, Krefeld, Germany) were added as an internal cell counting standard and 2000 living (PI−, CellTracker Blue+, FSChigh) breast cancer cells were recorded for each sample compared to unstimulated cells. Cytotoxicity was calculated as described [26]. In some experiments, blocking of CD8 in T cells was triggered using CD8 antibody [27] or the respective isotype control (BD Biosciences) 1 h before performing cytotoxicity experiments.
Treg and CD39+ cell isolation
Treg were isolated from DC-T cell co-cultures using the CD4+CD25+ Regulatory T Cell isolation Kit (Miltenyi) by magnetic separation. Treg-depleted populations were added back to the respective co-cultures. Isolated Treg (controlled via FACS, Fig. S3) were either used for RNA isolation or were interchanged between ACM and VCM groups at ratios reconstituting mean FoxP3-expressing cells (0.5%) to monitor their specific suppressive potential. CD39-expressing T cells from IL-2 enriched T cell cultures were removed by staining with CD39-FITC antibody (Miltenyi) and magnetic separation using anti-FITC microbeads (Miltenyi). The CD39-depleted populations were then used for co-cultures.
Flow cytometry
For analysis of DC maturation, DC were detached from the wells with accutase (PAA), and stained for 20 min with CD86-FITC (Immunotools), HLA-DR (MHC II)-PE-Cy7 (BD Biosciences) or HLA-ABC (MHC I)-FITC (Miltenyi) CD80-PE, CD83-APC, CD40-FITC (BioLegend). For polychromatic flow cytometry analysis of T cells from co-cultures, cells were harvested using a pipette. Non-specific antibody binding to FC-γ receptors was blocked using Human Fc Receptor Binding Inhibitor (eBioscience, San Diego, CA USA) for 20 min, cells were resuspended in FACS staining buffer (BD Biosciences) and incubated with a antibody cocktail consisting of CD3-V450, CD4-V500, CD8-APC-H7, CD25-PE-Cy7, CD69-AlexaFlour 700 and CD73-PE (BD Biosciences), CD39-FITC (Miltenyi), CD19-Qdot 655 (Invitrogen, Carlsbad, CA, USA) on ice for 30 min. Then, cells were fixed and permeabilized using the FoxP3 buffer set (BD Biosciences) and incubated with FoxP3-APC antibody (BD Biosciences) for 60 min at RT. For analysis of TGF-β expression, cells were pre-treated with 500 ng/ml Brefeldin A (Sigma) and TGF-β1-PE antibody (IQ products, Groningen, Netherlands) was used alongside the FoxP3 antibody. Samples were acquired using a LSRII/Fortessa flow cytometer (BD Biosciences) and analyzed using FlowJo software 7.6.1 (Treestar, Ashland, OR, USA). Antibodies were titrated to determine optimal concentrations. Antibody-capturing CompBeads (BD Biosciences) were used to create the multi-color panel compensation matrix. For gating, fluorescence minus one (FMO) controls and/or isotype controls were used. Instrument calibration was controlled and adjusted daily using Cytometer Setup and Tracking (CST) beads.
RNA isolation, cDNA synthesis and qPCR
RNA from DC was isolated using PeqGold (Peqlab, Erlangen, Germany) and quantitated using the NanoDrop spectrophotometer (NanoDrop, Wilmington, USA). 1 μg RNA was used for cDNA synthesis. RNA from < 105 Treg was isolated using the RNeasy micro kit (Qiagen, Hilden, Germany), quantitated using the Bioanalyzer from Agilent (Böblingen, Germany) and transcribed using sensiscript RT kits (Qiagen). Quantitative PCR was performed as described [28]. Human ebi3, actin and 18S rRNA were amplified using QuantiTect Primer Assays (Qiagen, Hilden, Germany). Additional primer sets were p35: sense 5′-AGATAAAACCAGCACAGTGGAGGC-3′, antisense: 5′-GCCAGGCAACTCCCATTAGTTAT-3′; p28 sense: 5′-AGGAGCTGCGGAGGGAGTT-3′, antisense: 5′-AGGGGCAGGAGGTACAGGTTC-3′; IL-10 sense: 5′-AAGCCTGACCACGCTTTCTA3-′, antisense: 5′-TAGCAGTTAGGAAGCCCCAA-3′. Results were analyzed using Gene Expression Macro (Bio-Rad, München, Germany). Actin and 18S were internal controls.
Cytokine Quantitation
TNF-α, IL-10, IL-6, IL-12 concentrations in DC supernatants and IFN-γ, IL-10, IL-4, IL-17, IL-2 from DC/T cell co-cultures were quantified using Human Inflammatory Cytokine or Human Th1/TH2/TH17 kits (BD Biosciences). Samples were acquired by FACS and processed with BD Biosciences FCAP software. IL-27 levels in DC supernatants were quantified using sandwich ELISA (Biolegend, San Diego, CA USA).
Statistical analysis
Data were analyzed using GraphPad Prism 5.0 (GraphPad Software, San Diego, CA, USA). p-values were calculated using ANOVA with Bonferroni’s correction. Differences were considered significant at p < 0.05.
Results
Priming with apoptotic cell supernatants suppresses DC-dependent tumor cell killing
We aimed to investigate how priming of DC by factors/exosomes shed by dying versus living tumor cells affects their ability to initiate tumor cell-specific cytotoxic T cell responses. Conditioned media of apoptotic, necrotic, or viable MCF7 cells (ACM, NCM, VCM) were incubated with primary monocyte-derived human DC at a ratio of one tumor cell/DC. Higher ratios of tumor cells/DC induced cell death in DC, especially when using NCM (Fig. S1A). This might be a mechanism of suppressing DC-dependent immunity by highly abundant dying tumor cells occurring e.g. after chemo/radiotherapy. Tumor cell supernatant-primed or unstimulated DC were co-cultured with IL-2-enriched autologous PBMC for 3 d (Fig. 1A). IL-2 enriched PBMCs were mainly T cells, lacking significant amounts of mononuclear phagocytes, B cells or NK cells (< 1%) (Fig. S2). Lymphocytes derived from these co-cultures were then added to living CellTracker Blue-stained MCF-7 cells for 4 h at different ratios and MCF-7/lymphocyte co-cultures were assessed for tumor cell death. Specific cytotoxicity was not observed in any experimental group up to a ratio of 1:2 (tumor cells to T cells) and reached a plateau at and above a ratio of 1:5 (Fig. 1B). At a ratio of 1:5, the VCM group unexpectedly showed significantly higher cytotoxicity toward living MCF-7 cells compared to the control group, whereas T cells from the NCM group were not cytotoxic (Fig. 1C). In contrast, cytotoxicity towards living MCF-7 cells was reduced below controls when T cells from the ACM group were used. Cytotoxicity in this group was comparable to the basal cytotoxicity exhibited by IL-2 activated T cells without DC co-culture (Fig. 1C). Importantly, VCM-induced cytotoxicity was cell-specific, since alterations in cytotoxicity were not observed when lymphocytes from MCF-7 supernatant-primed DC co-cultures where added to T47D cells (Fig. 1D).
Figure 1.
Viable cancer cell supernatants prime whereas supernatants of apoptotic cells suppress specific cytotoxicity. (A) Experimental outline. Human monocyte-derived DC were controls or incubated with supernatants of viable (VCM), apoptotic (ACM) or necrotic (NCM) MCF-7 cells at a ratio of 1:1 (Supernatants of 2 × 105 MCF-7 added to 2 × 105 DC) for 16 h. Subsequently, supernatants were removed by washing and autologous T cell-enriched PBMC were added at a ratio of 10:1 (Tcells/DC) and cultured for another 3 d. The resulting polarized T cells were then co-cultured with living tumor cells for 4 h to determine cytotoxicity as described under Materials and Methods. Experimental interventions as outlined in the manuscript are indicated as dotted arrows. (B) CellTracker Blue-stained MCF-7 cells were incubated with T cells from individual co-cultures at effector to target (E:T) ratios of 0.1:1, 0,5:1, 1:1, 1:2, 1:5, 1:10, 1:20 for 4h. Cytotoxicity calculated for the ACM, VCM, NCM groups was compared to the control group. Data are means ± SEM from five individual donors. (C) CellTracker Blue-stained MCF-7 cells were incubated with T cells from individual co-cultures as well as control T cells at ratios of 1:5 for 4 h. Cytotoxicity was calculated compared to non co-cultured MCF-7. Data are means ± SEM from five individual donors. (D) CellTracker Blue-stained MCF7 (black bars) or T47D (white bars) breast carcinoma cells were incubated with T cells from individual co-cultures at 1:5 ratio for 4 h prior to cytotoxicity measurements. Data are means ± SEM from four individual donors. Asterisks indicate significant differences between groups, * = p < 0.05. p-values were calculated using ANOVA with Bonferroni’s correction.
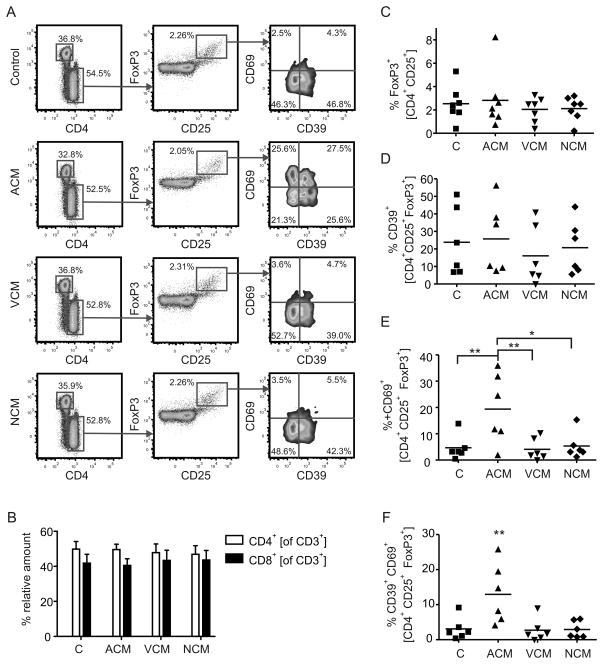
Accumulation of CD39/CD69-expressing FoxP3+ Treg in ACM-primed co-cultures
Since ACM-priming of DC suppressed cytotoxicity compared to the VCM- or NCM-primed groups, we determined whether alterations in the T cell populations occurred and if such alterations accounted for suppression of tumor killing. We analyzed the T cell profile in the co-culture using polychromatic flow cytometry (Fig. 2A). Regarding whole T cell numbers or basal T cell subsets, CD3+ T cell levels (not shown) and the ratio of CD4+ versus CD8+ T cells (Fig. 2B) remained unchanged throughout the experimental groups. Next, we checked for a possible expansion of Treg, which were detected by intracellular staining of FoxP3 in CD4+CD25+ T cells (Fig. 2A). The relative amount of FoxP3+ cells in the different experimental groups was unchanged (Fig. 2C). However, a different picture emerged when we investigated markers of Treg function. We stained Treg for expression of CD39 and CD69 (Fig. 2A). The ectonucleotidase CD39 is expressed on naturally occurring FoxP3+ Treg [29], while the lymphocyte activation marker CD69 was shown to be expressed by Treg of cervical cancer patients [30]. Whereas the expression of CD39 by Treg was not significantly different between the co-culture set-ups (Fig. 2D), there were significant differences with regard to CD69 expression. CD69 was upregulated on Treg selectively in the ACM group (Fig. 2E), which was most significant in the population co-expressing CD39 (Fig. 2F). This regulation pattern was Treg-specific, since neither CD39 nor CD69 were significantly upregulated in the total CD4+CD25+ population (Fig. S3A). CD39 was usually not expressed by CD8+ T cells. However, in approximately 20% of all donors, a small subpopulation of CD8+ T cells expressed CD39, which was selectively elevated in the ACM group, whereas CD69 expression was unaltered (Fig. S3B). Conclusively, ACM-primed, but not VCM or NCM-primed DC significantly induce surface CD69 expression in co-cultured CD39+ Treg.
Figure 2.
Apoptotic cell supernatants induce CD69-expressing Treg. A–F, The profile of human T cell subpopulations from 3 d co-cultures with unprimed ACM-, VCM- or NCM-primed human monocyte-derived autologous DC were quantified using polychromatic flow cytometry. (A) Representative FACS traces for each co-culture setting are displayed. CD3+ T cells were sub-classed depending on expression of CD4 and CD8. CD4+ T cells were analyzed for expression CD25+ versus FoxP3+. CD4+CD25+FoxP3+ cells were further sub-divided based on expression of CD39 and CD69. (B) Graphs display statistical quantitation of relative CD4 versus CD8 expression by CD3+ T cells. Data displayed as mean ± SEM from five individual donors. (C) Statistical quantification of FoxP3-expressing CD4+CD25+ T cells (Treg). Individual data points and the mean of seven individual donors are shown. (D-F) Statistical quantification of CD39+ CD69− Treg (D) CD39− CD69+ Treg (E) CD39+ CD69+ Treg (F). Individual data points and the mean of six individual donors are shown. Asterisks indicate significant differences between groups, * = p < 0.05, ** =p < 0.01. p-values were calculated using ANOVA with Bonferroni’s correction.
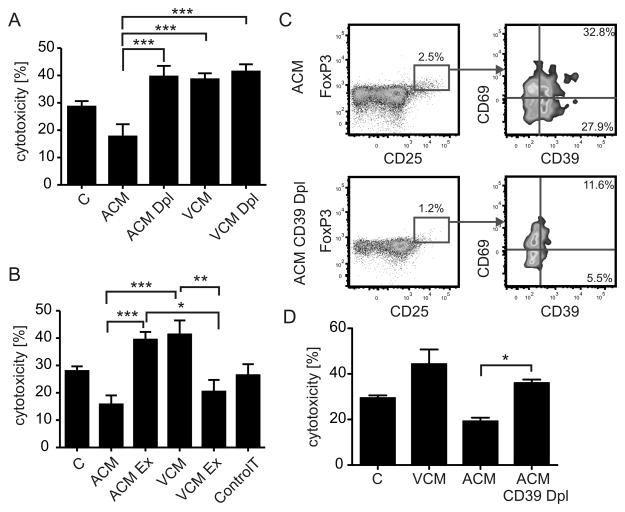
Next, we asked if the accumulation of CD69-expressing Treg in the ACM group (Fig. 2A,F) contributed to reduced MCF-7 cell killing (Fig. 1B). In a first approach, we depleted Treg from unprimed, ACM-primed or VCM-primed co-cultures using commercial kits, before subjecting the remaining cells to the cytotoxicity assay. Of the isolated CD4+ CD25+ T cells, 40% expressed intracellular FoxP3, largely co-expressed CD39, and thus likely represent functional Treg (Fig. S4). Interestingly, Treg-depleted lymphocytes from the ACM group were significantly more cytotoxic compared to the complete lymphocyte fraction, whereas Treg-depletion from the VCM-primed co-culture did not affect the enhanced cytotoxicity observed in the VCM group (Fig. 3A). Next, we asked whether the Treg-dependent suppression of cytotoxicity in the ACM group might be transferable. We isolated Treg from ACM-primed or VCM-primed co-cultures on day 2 and added the Treg from the ACM group to the Treg-depleted lymphocytes of the VCM group and vice versa. After another 24 h of co-incubation, these mixed lymphocyte populations were used in the cytotoxicity assay. Indeed, Treg from the ACM group significantly suppressed cytotoxicity of Treg-depleted lymphocytes from the VCM group. On the other hand Treg from the VCM group were unable to suppress cytotoxicity in the ACM group (Fig. 3B). Thus, ACM-priming provided FoxP3+ T cells with the ability to suppress cytotoxicity, which was correlated to expression of CD39 and CD69. Depletion of these cells restored cytotoxicity to a level comparable to the VCM-primed group. We explored the relationship of CD39 and CD69-expressing Treg with suppression of cytotoxicity by further depleting CD39+ cells from IL-2-enriched lymphocytes before adding them to DC co-cultures. T cells from these co-cultures displayed lower levels of Treg and completely lacked the CD39+ subpopulation, indicating that CD39+ Treg upregulate CD69 expression in ACM co-cultures, whereas upregulation of CD39 by CD69+ cells could be ruled out (Fig. 3C). Importantly, depletion of CD39+ T cells restored cytotoxicity in the ACM group as observed when depleting total CD25+ cells (Fig. 3D).
Figure 3.
Treg confer ACM-induced suppression of cytotoxicity. (A) Treg were isolated from 2 d co-cultures of ACM- or VCM-primed human monocyte-derived autologous DC using automated magnetic bead-sorting. Residual T cells were added back to co-cultures for 24 h. MCF7 cells were then incubated with T cells from the ACM or VCM groups with or without (Dpl) Treg as well as controls and cytotoxicity was analyzed. Data are means ± SEM from five individual donors. (B) Treg were isolated from 2 d co-cultures of ACM- or VCM-primed human monocyte-derived autologous DC using automated magnetic bead-sorting. Treg from ACM groups were then mixed with Treg-depleted T cells from VCM groups and vice versa and added back to the co-cultures for 24 h. MCF-7 cells were subsequently incubated with T cells from the Treg exchange groups (Ex) compared to non-exchanged groups and the control. T cells alone cultured without DC are indicated as control T. Data are means ± SEM from six individual donors. (C,D) CD39+ cells were depleted from IL-2 enriched PBMC using anti-FITC microbeads after labeling with CD39-FITC antibody by automated magnetic bead-sorting. CD39-depleted (CD39 Dpl) T cells were added to co-cultures in the same ratio as unmodified T cells. (C) Representative FACS traces displaying the relative amount of CD39+CD69+ cells in CD4+CD25+FoxP3+ T cells in ACM co-cultures of CD39-depleted and unmodified T cells. (D) MCF7 cells were incubated with T cells from the VCM group, the ACM group with or without CD39+ T cell depletion (Dpl) as well as controls and cytotoxicity was analyzed. Data are means ± SEM from four individual donors. Asterisks indicate significant differences between groups, * = p < 0.05, ** = p < 0.01, *** = p < 0.001. p-values were calculated using ANOVA with Bonferroni’s correction.
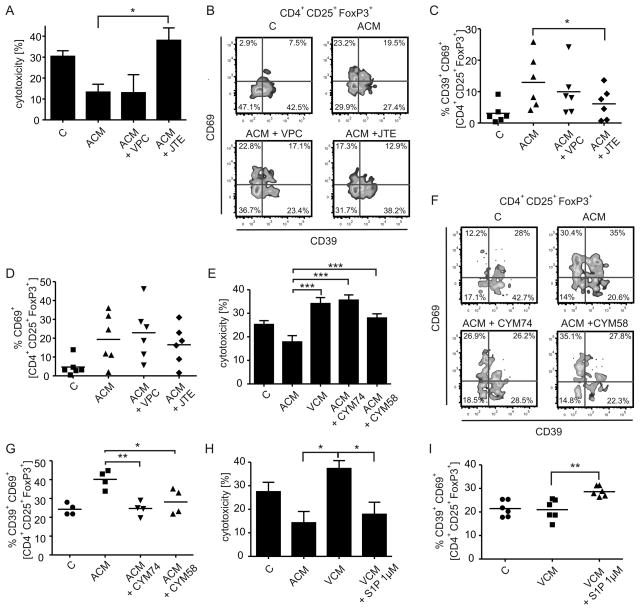
S1P in ACM confers suppression of cytotoxicity by activating S1PR4 on DC
Next, we performed experiments to interfere with the immunosuppressive properties of ACM in order to determine whether CD69 expression on FoxP3+ T cells accounted for reduced cytotoxicity. We asked for the factor(s) in ACM inducing DC-dependent suppression of cytotoxicity. Among the immunomodulatory factors secreted by AC is the lipid mediator S1P [14], which was present in ACM (~ 10 nM, determined routinely [11]). S1P couples to five specific receptors (S1PR), with human DC expressing S1PR1-4. Pharmacological inhibition of S1PRs during ACM-priming of human DC was used to test an impact of AC-derived S1P on DC-dependent T cell activation. JTE-013, a partial inhibitor for S1PR2 (IC50 1.5 μM) and a full inhibitor of S1PR4 (IC50 4.5 μM) [31], significantly prevented ACM-induced suppression of cytotoxicity when used at high concentrations (15 μM), whereas the S1PR1/3 inhibitor VPC23019 (1μM) did not (Fig. 4A). Diminishing cytotoxicity with the S1PR2/4 inhibitor JTE-013 correlated with a significant reduction of CD39+CD69+ Treg, whereas the relative proportion of CD69+CD39− Treg was unchanged (Fig. 4B–D). To substantiate these findings and to explicitly identify the S1PR subtype, we used the specific S1PR4 antagonists CYM50374 and CYM50358 (200 nM each) [21]. Both substances reversed suppression of cytotoxicity induced by ACM priming (Fig. 4E) and decreased the expansion of CD39+CD69+ Treg (Fig. 4F,G). Moreover, supplying S1P (1 μM) during VCM-priming of DC suppressed the VCM-induced cytotoxicity (Fig. 4H) and increased the amount of CD39+CD69+ Treg (Fig. 4I). These findings indicate that S1PR4 activation by S1P in ACM enabled DC to induce CD69 expression on Treg, correlating to suppressed cytotoxicity.
Figure 4.
S1PR4 on DC conveys ACM-dependent suppression of cytotoxicity. (A–D) T cells were co-cultured with control or ACM-primed autologous DC with or without the S1PR2/4 antagonist JTE-013 or the S1PR1/3 antagonist VPC23019. (A) Cytotoxicity induced by T cells from individual co-cultures towards living MCF-7 cells. Data are means ± SEM from six individual donors. (B) Representative FACS traces of CD39 and CD69 expression by Treg from the individual co-cultures. C,D, Statistical evaluation of CD39+ CD69+ Treg (C)−or CD39− CD69+ Treg (D). Individual data points and the mean of six individual donors are shown. (E–G) T cells were co-cultured with control or ACM-primed autologous DC with or without the S1PR4 antagonists CYM74 or CYM58 (E) Cytotoxicity induced by T cells from individual co-cultures towards living MCF-7 cells. Data are means ± SEM from four individual donors. (F) Representative FACS traces of CD39 and CD69 expression by Treg from the individual co-cultures. (G) Statistical evaluation of CD39+ CD69+ Treg from individual co-cultures. Individual data points and the mean of four individual donors are shown. (H,I) T cells were co-cultured with control, ACM- or NCM-primed autologous DC with or without adding 1 μM S1P (H), Cytotoxicity induced by T cells from individual co-cultures towards living MCF-7 cells. Data are means ± SEM from six individual donors. (I), Statistical evaluation of CD39+ CD69+ Treg from individual co-cultures. Individual data points and the mean of four individual donors are shown. Asterisks indicate significant differences between groups, * = p < 0.05, **.= p < 0.01, *** = p < 0.001. p-values were calculated using ANOVA with Bonferroni’s correction.
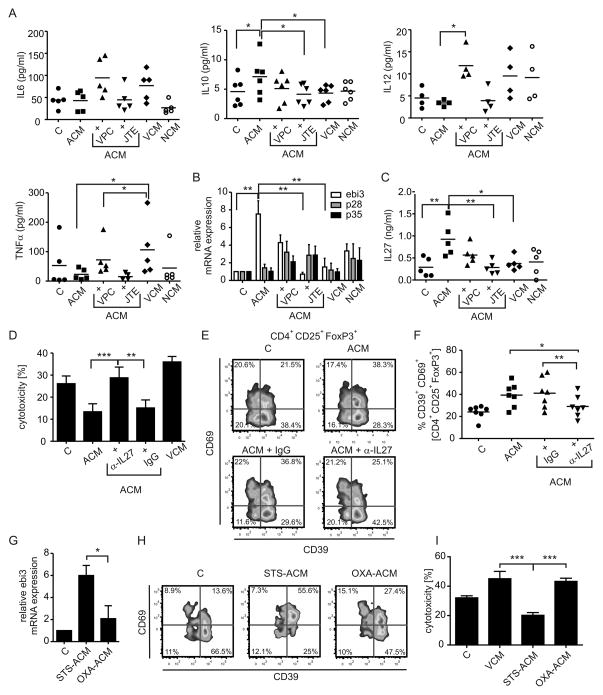
ACM-primed DC secrete IL-27 to activate suppressive Treg
Apparently, tumor-associated DC exist in different functional states depending on the microenvironment they are exposed to (i.e. tumor mass versus TDLN) [11]. We asked which parameters of DC functionality were altered by ACM. For maturation markers, each tumor supernatant slightly induced HLA-DR expression on DC as compared to the control group. CD80 expression remained unchanged. CD83 expression was induced slightly only in the ACM group. HLA-ABC expression remained largely unchanged, except for a reduction with NCM, whereas CD86 and CD40 expression were strongly induced with all conditioned media (Fig. S1B). Conclusively, immunosuppression by ACM-priming was not dependent on altered maturation.
Apart from maturation, functional markers of tolerogenic DC include proteins such as IDO. IDO is an intracellular enzyme involved in tryptophan metabolism along the kynurenine pathway. IDO has been implicated in tolerance induction mediated through depleting tryptophan, which halts T cell proliferation and/or by accumulation of 3-hydroxykynurenine or 3-hydoxyanthranilic acid, which is toxic to lymphocytes [32]. IDO is expressed by DC interacting with AC [33]. Thus, we asked whether this enzyme had any impact on the observed immunosuppression in the ACM co-cultures. We found that neither inhibition of IDO1 using D-1MT nor IDO2 with L-1MT was able to significantly restore cytotoxicity compared to the VCM group (Fig. S5). However, in some experiments L-1MT had minor efficiency in elevating cytotoxicity. Next we measured the release of inflammatory cytokines 16 h after stimulation of DC with tumor cell supernatants. ACM stimulation together with S1PR antagonists was included to detect meaningful regulation patterns. IL-12 was not secreted in relevant amounts, although there was a tendency towards enhanced IL-12 secretion by VCM-primed DC versus their ACM-primed counterparts (Fig. 5A). IL-6 showed a trend towards enhanced production in the VCM group, which was significant for TNF-α. However, compared to ACM, secretion of these cytokines was enhanced when using VPC23019, but not JTE-013, which did not correlate with changes in cytotoxicity. Release of the anti-inflammatory cytokine IL-10 followed a meaningful regulation pattern, being elevated only with ACM and ACM+VPC23019 (Fig. 5A). However, release of IL-10 was in the low pg/ml range, largely restricting its potential impact. Searching for other candidates, we next focused on IL-12 family cytokines. Despite having structurally similar subunits or sharing identical subunits, these cytokines may enhance generation of either Th1, Th17 or Treg [34]. For instance, the protein encoded by Epstein-Barr virus induced gene 3 (ebi3) is a common subunit of IL-35 and IL-27. Analyzing mRNA expression in human DC in analogy to secreted cytokines, we noticed that ebi3 mRNA was upregulated with ACM after 16 h in a S1PR2/4-dependent manner as determined by using JTE-013 versus VPC23019 (Fig. 5B). Since expression of p28 and p35, the complementary subunits for IL-27 or IL-35 respectively, were unchanged with ACM treatment (Fig. 5B), we analyzed secretion of IL-35 and IL-27 using commercial ELISA kits. While detecting IL-35 was unsuccessful, IL-27 was significantly upregulated in ACM-treated DC compared to controls or VCM-primed DC, which was abolished when inhibiting S1PR2/4 (Fig. 5C). To validate the role of DC-secreted IL-27 in generating suppressive Treg, we added a specific IL-27-neutralizing antibody versus a non-specific isotype control (each 1 μg/ml) to DC before adding autologous T cells. Blocking IL-27 potently reduced ACM-induced suppression of cytotoxicity as compared to the isotype control (Fig. 5D), which again correlated with attenuated ACM-induced CD69 expression in CD39+ Treg (Fig. 5E,F). We wondered whether induction of cell death using immunogenic drugs would deliver comparable results with regard to IL-27-dependent CD39+ CD69+ Treg expansion. When we compared effects of supernatants of oxaliplatin-treated MCF-7 cells (OXA-ACM) with staurosporine-treated cells (STS-ACM) on DC, we noticed significantly lower ebi-3 expression in OXA-ACM-treated DC (Fig. 5G). Correlating to that, we observed reduced CD69+CD39+ Treg in OXA-ACM co-cultures compared to STS-ACM co-cultures (Fig. 5H). This difference in the Treg population was again reflected by the cytotoxic potential of co-cultured T cells as T cells from the OXA-ACM group were significantly more cytotoxic compared to T cells from the STS-ACM group (Fig. 5I). Hence, also in our hands oxaliplatin induced immunogenic cell death, which correlated with reduced ebi-3 expression and thus, likely IL-27 production, as well as reduced CD69 expression on CD39+ Treg.
Figure 5.
ACM induces IL-27 in DC in a S1PR4-dependent manner to generate Treg. (A–C) Human monocyte-derived DC were controls or incubated with VCM, NCM or ACM with or without the addition of JTE-013 (15 μM) or VPC23019 (1μM) for 16 h. (A) Secreted cytokines were quantified using Human inflammatory cytokine Cytometric Bead Arrays. Quantification of IL-6, TNF-α, IL-12 and IL-10 in DC supernatants is shown. Individual data points and the mean of five individual donors are shown. (B) Relative mRNA expression of ebi3, p28 and p35 quantitated via qPCR is displayed. Data are means ± SEM from four individual donors. (C) ELISA quantification of IL-27 protein secretion by DC from the individual groups is shown. Individual data points and the mean of five individual donors are shown. (D–F) T cells were co-cultured with control, VCM- or ACM-primed autologous DC with or without the addition of an IL-27-neutralizing antibody (α-IL27) or the isotype control (IgG). (D) Cytotoxicity induced by T cells from individual co-cultures towards living MCF-7 cells. Data are means ± SEM from seven individual donors. (E) Representative FACS traces of CD39 and CD69 expression by Treg from the individual co-cultures. (F) Statistical quantification of CD39+ CD69+ Treg. Individual data points and the mean of seven individual donors are shown, n = 7. (G–I) MCF-7 cells were subjected to 0.5 μg/ml staurosporine (STS) or 30 μM oxaliplatin (OXA) to induce cell death and supernatants were harvested. (G) Expression of ebi3 mRNA in DC treated with STS-ACM or OXA-ACM compared to control DC is displayed. Data are means ± SEM from four individual donors. (H) T cells were co-cultured with control, VCM-, or STS-ACM- or OXA-ACM-primed autologous DC. Representative FACS traces of CD39 and CD69 expression by Treg from the individual co-cultures are shown. (I) Cytotoxicity induced by T cells from individual co-cultures towards living MCF-7 cells. Data are means ± SEM from five individual donors. Asterisks indicate significant differences between groups, * = p < 0.05, ** = p < 0.01, *** = p < 0.001. p-values were calculated using ANOVA with Bonferroni’s correction.
Suppression of cytotoxicity is reduced by interference with adenosine generation
We were interested in the mechanism how Treg suppressed cytotoxicity. Analyzing the contents of the T cell-derived cytokines IFN-γ, IL-10, IL-4, IL-17, IL-2 from total co-cultures after day 3, did not reveal any meaningful regulation patterns (Fig. S6). However, Treg can suppress cytotoxic T cells by various mechanisms including production of effector cytokines such as TGF-β and IL-10 [35]. We analyzed the expression of these cytokines specifically in Treg. IL-10 expression was determined on mRNA level after isolation of Treg, whereas TGF-β was quantified by intracellular staining using flow cytometry. Unexpectedly, neither IL-10 (Fig. 6A) nor TGF-β expression (Fig. 6B) was significantly altered in Treg upon ACM-stimulation. In addition, neutralizing TGF-β in ACM co-cultures with a specific antibody did not restore cytotoxicity (Fig. 6C), thus ruling out TGF-β as a candidate for immunosuppression in this set-up. Another molecule known for its immunosuppressive function is the purine nucleotide adenosine. Extracellular adenosine inhibits, among others, proliferation and/or priming of CD8+ T cells [36]. Importantly, CD8 depletion experiments suggested that CD8+ T cells were required for VCM-induced cytotoxicity in our system (Fig. 6D). Adenosine is produced by the sequential breakdown of ATP by e.g. the ectonucleotidases CD39 and CD73. Previous reports indicated CD39 is expression by human Treg, whereas CD73 might not be co-expressed by these cells [37]. Indeed, whereas CD39 was mainly expressed by CD4+ Treg, which did not express CD73 (Fig. 6C), CD73 was expressed only by CD8+ cells (Fig. 6D), which was unaltered upon priming with either ACM or VCM (Fig. 6E). However adenosine was involved in ACM-induced suppression of cytotoxicity in our system. Addition of the CD39 inhibitor ARL67156, the CD73 inhibitor APCP [23] as well as the adenosine receptor A2a inhibitor CSC [24] to co-cultures restored cytotoxicity brought about by ACM-priming (Fig. 6F). These suggested a role for adenosine generation and function in our system. None of the compounds altered expression of CD39 by Treg or of CD73 by CD8+ T cells except for the CD73 inhibitor APCP, which diminished surface expression of CD73 on CD8+ T cells, an unrecognized mechanism of its action (Fig. 6G).
Figure 6.
Interfering with adenosine generation and signaling restores cytotoxicity. (A) Magnetic bead-isolated Treg from the individual co-cultures at day 3 were analyzed for relative IL-10 mRNA expression via qPCR. Data are means ± SEM from five individual donors. (B) TGF-β expression in CD4+CD25+FoxP3+ Treg from whole individual co-cultures was determined via intracellular staining and polychromatic flow cytometry. Statistical evaluation TGF-β-dependent mean fluorescence intensity (MFI) is displayed. Data are means ± SEM from four individual donors. (C) T cells were co-cultured with control, VCM- or ACM-primed autologous DC with or without the addition of a TGF-β-neutralizing antibody (α-TGF-β) or the isotype control (IgG). Cytotoxicity induced by T cells from individual co-cultures towards living MCF-7 cells was determined. Data are means ± SEM from five individual donors. (D) Relative FACS traces indicating CD39-expression by CD8− T cells from control co-cultures. Gating on these cells and evaluation of FoxP3 versus CD73 expression reveals that CD39 is selectively expressed by CD4+ Treg. (E) Relative FACS traces indicating CD73-expression by CD8+ T cells from control co-cultures. Gating on these cells and evaluation of FoxP3 versus CD39 expression reveals that CD73 is selectively expressed by CD8+ FoxP3− T cells. (F) Quantification of CD73 expression by CD8+ T cells from individual co-cultures as indicated analyzed by flow cytometry is displayed. Individual data points and the mean of seven individual donors are shown. (G) T cells derived from VCM co-cultures were pre-incubated for 1h with anti-CD8 or the respective isotype control. These T cell were used to determine cytotoxicity against living MCF-7 cells compared to T cells from control co-cultures. (H) T cells were co-cultured with control, VCM- or ACM-primed autologous DC with or without addition of the CD39 inhibitor (ARL) (250 μM), the CD73 inhibitor (APCP) (100 μM) or the adenosine receptor A2a (CSC) (10 mM). Cytotoxicity induced by T cells from individual co-cultures towards living MCF-7 cells is shown. Data are means ± SEM from six individual donors. (F) Quantification of CD73 expression by CD8+ T cells from individual co-cultures as indicated, analyzed using flow cytometry, is displayed. Individual data points and the mean of four individual donors are shown. Asterisks indicate significant differences between groups, * = p < 0.05, ** = p < 0.01, *** = p < 0.001. p-values were calculated using ANOVA with Bonferroni’s correction.
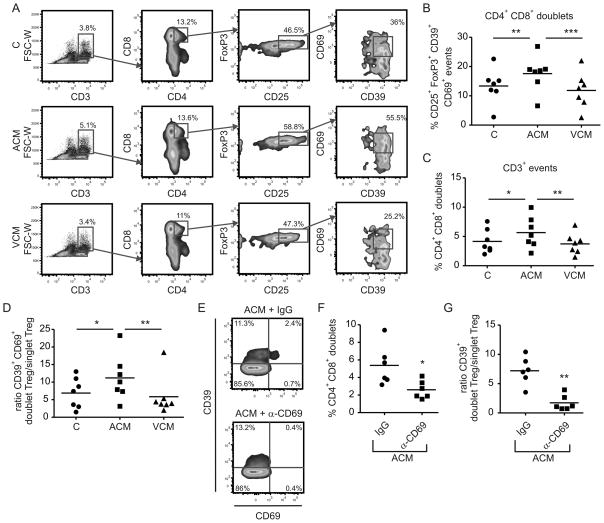
So far, we observed accumulation of CD69-expressing CD39+ Treg in ACM-primed co-cultures, which directly correlated to suppression of cytotoxicity. We wondered whether CD69 expression on Treg might be directly involved in suppressing CD8+ T cell function. The concerted action of CD39 and CD73 seemed to be important for suppression of cytotoxicity induced by ACM-priming although these molecules were expressed by different cells. CD69 is a member of the c-type lectin family, proteins which regulate cell-cell contact. We hypothesize that CD69-bearing CD39+ Treg might establish direct contact with CD73-expressing CD8+ T cells to ensure efficient adenosine generation and subsequent immunosuppression. To approach this question, we analyzed CD4+CD8+ events within CD3+ doublets from control, ACM and VCM co-cultures using polychromatic flow cytometry (Fig. S7). These CD4+CD8+ doublets were generally enriched in CD25+FoxP3+ cells expressing CD69 (Fig. S7). However this enrichment was strongly pronounced in the ACM group compared to the control or VCM groups (Fig. 7A,B). Additionally, there was a significant reduction in enrichment of CD4+CD8+ events within whole CD3+ doublets in the ACM group as compared to the control or VCM co-cultures (Fig. 7C). This pattern was also observed when analyzing whether CD25+CD39+CD69+ events were specifically enriched in doublets as compared to the singlet population (Fig. 7D). Interestingly, though Treg numbers were generally lower in doublets compared to singlets, the percentages of CD25+CD39+CD69+ events was higher in doublets compared to singlets. As a next step, we wondered whether antibody-mediated CD69 depletion would reduce CD4+CD8+ doublet formation as well as enrichment of CD25+CD39+CD69+ events in doublets of the ACM group. CD69 depletion was efficiently induced in ACM co-cultures using CD69 antibody compared to the isotype, without altering the total amount of CD39+ cells (Fig. 7E). Strikingly, CD4+CD8+ doublets (Fig. 7F) as well as CD25+CD39+ events in the doublet population (Fig. 7G) of the ACM group were decreased upon CD69 neutralization. These findings provide a first hint that CD69-expressing Treg may bind to an unidentified ligand on CD8+ T cells, which might aid efficient adenosine production and subsequent suppression of cytotoxicity.
Figure 7.
CD39/CD69-expressing Treg are enriched in CD4+CD8+ doublet events. (A) Representative FACS traces show analysis of the FSC-W gated CD3+ doublet population of cells from control, ACM- or VCM-primed co-cultures. CD4+CD8+ events in the doublets, most likely aggregates of CD4+ and CD8+ T cells were analyzed for expression of CD25, FoxP3, CD39 and CD69. (B) Quantification of CD25+FoxP3+CD39+CD69+ events in the doublet CD4+CD8+ population is shown. Individual data points and the mean of seven individual donors are shown. (C) The amount of CD4+ and CD8+ positive events in the CD3+ doublets was quantified. Individual data points and the mean of seven individual donors are shown. (D) The ratio of CD25+FoxP3+CD39+CD69+ events in doublets versus singlets in ACM-, VCM- or control co-cultures is shown. Individual data points and the mean of seven individual donors are shown. (E) ACM co-cultures were treated with either anti-CD69 or the isotype control on day 2. Representative FACS traces show CD39 and CD69 expression in CD4+ T cells on day 3. (F) The amount of CD4+ and CD8+ positive events in CD3+ doublets in ACM-co-cultures with anti-CD69 (α-CD69) or the isotype control (IgG) on day 3 is displayed. Individual data points and the mean of six individual donors are shown, n = 6. (G) The ratio of CD25+FoxP3+CD39+ events in doublets versus singlets of ACM-co-cultures with anti-CD69 (α-CD69) or the isotype control (IgG) on day 3 is shown. Individual data points and the mean of six individual donors are shown. Asterisks indicate significant differences between groups, * = p < 0.05, ** = p < 0.01, *** = p < 0.001. p-values were calculated using ANOVA with Bonferroni’s correction or paired Student’s t-test.
Discussion
A growing tumor or a tumor subjected to conventional therapy sheds tumor-derived factors/exosomes [38]. We analyzed the impact of factors released from dying tumor cells on T cell-dependent cytotoxicity. Factors shed from apoptotic tumor cells reduced cytotoxicity, whereas priming with VCM increased cytotoxicity against living tumor cells. We propose that antigens contained in exosomes in VCM might be responsible for inducing cytotoxicity via CD8+ T cells, whereas Treg induced by immunosuppressive factors contained in ACM prevent cytotoxicity. However, tumor cell-specific CTL might still be generated by ACM as indicated by the Treg depletion experiments, which restored cytotoxicity. Furthermore, suppressive potential of ACM-induced Treg was transferable to VCM-primed lymphocytes. Hence apoptotic debris resulting from cytotoxic cancer therapy, which is known to induce immune paralysis [6], when shed to the TDLN might induce generation of Treg that block potent endogenous CTL or exogenous CTL activity. Along this line, depletion of Treg using anti-CD25 or anti-CTLA4 along with T cell or DC immunotherapy restored anti-tumor immunity [39].
Generation of suppressive Treg required activation of S1PR4 on DC, likely due to S1P that is secreted by AC [11]. S1P is a potent lipid mediator that signals via specific G protein coupled receptors to control aspects of phagocyte biology [40]. We previously demonstrated that S1P is involved in activating macrophages to a potentially tumor-supportive phenotype [41]. Furthermore, during LPS-induced maturation of DC S1P inhibits Th1 responses by suppressing IL-12 release and instead promotes Th2 responses by increasing IL-4 and IL-10 production [40]. We observed that inhibition of S1PR1/3 upon treatment with ACM indeed marginally increased secretion of the pro-inflammatory cytokines IL-12, TNF-α and IL-6, while moderately increasing IL-10 (Fig. 5A). This suggests that S1P acting on S1PR1/3 might be an intrinsic attenuating signal in AC-induced inflammation. However, for suppression of cytotoxicity these changes seemed irrelevant. Rather, S1P in ACM induced expression of ebi3 and the release of IL-27 through S1PR4 (Fig. 5B,C). Interestingly, expression of IL-27 by APCs in the lymphatic system upon interaction with tumor cells has been demonstrated before [42].
Generally, the function of IL-27 in T cell biology is ambiguous, varying between pro-inflammatory (induction of Th1) and anti-inflammatory (immunosuppression) [43]. IL-27 induces expression of the Th1 transcription factor T-bet [44] and was recently shown to promote CD8+ T cell proliferation by activating STAT1 [45], which argues for an inflammatory function. However, also Treg can express T-bet. These specialized Treg migrate to areas of Th1 inflammation and contribute to immunosuppression [46], thereby dampening over-activation of immunity. A similar mechanism may be employed by ACM-primed DC expressing IL-27 to induce Treg that in turn suppress cytotoxicity even in a milieu that is not strictly anti-inflammatory. Besides inducing T bet, IL-27 potently reduces expression of the Th17 cell-determining transcription factor (TF) RORC as well as the Th2-detemining TF GATA3, but not Foxp3 [47]. In our hands, interfering with IL-27 had no effect on FoxP3 expression (data not shown). Instead, IL-27 inhibition in the ACM group decreased the CD39+CD69+ Treg population (Fig. 5E). A mechanism, how IL-27 induces CD69 expression, remains to be discovered. Interestingly, IL-35, which shares the subunit ebi3 with IL-27, is known to induce CD39 expression on CD4+Foxp3+ Treg [48].
The exact mechanism employed by Treg to suppress cytotoxicity in our system has yet to be defined. CD4+ Treg can be divided among others into CD4+CD25+Foxp3+ Treg and T regulatory type 1 (Tr1) FoxP3− cells. IL-27 was shown to enhance the generation of IL-10 expressing Tr1 cells in mice [49]. In our system, using human cells, IL-27 did not upregulate IL-10 expression in the ACM group (Fig. 6A). The defining characteristic of the suppressive Treg subpopulation expanded by ACM-primed DC was expression of the ectonucleotidase CD39 and the activation marker CD69. As suggested previously, CD39 was mainly expressed by FoxP3+ cells [29]. CD39 metabolizes ATP and ADP to AMP, the former being produced by activated T cells that have undergone TCR engagement and calcium influx [50]. Since DC treated with ACM do not show deficiencies in maturation or activation, they might well engage the TCR of T effector cells to stimulate ATP release, which can be metabolized to AMP by CD39 present on the surface of Treg. Further on, AMP can be degraded to adenosine by CD73, present exclusively on CD8+ T cells in our system. Extracellular adenosine was shown to inhibit many aspects of T cell function such as effector differentiation, activation (by increasing cAMP levels), cytokine production, metabolic activity and proliferation [36]. Interestingly, ectonucleotidase expression and activity was increased in Treg of head and neck cancer patients, presumably contributing to their immunosuppressive function [23]. A similar mechanism of immunosuppression is employed by ectonucleotidase-expressing ovarian carcinoma cells, which generate adenosine to inhibit CD4+ T cell proliferation as well as NK cell cytotoxicity through activation of AdorA2a on these cells [51]. Hence adenosine generated through CD39 and CD73 expressed by Treg and CD8+ T cells respectively might suppress the function of CTL in our system acting via AdorA2a, whose inhibition indeed restored cytotoxicity in the ACM group.
The most defining aspect of Treg from the ACM group was expression of CD69. CD69 expression on T cells inhibits S1P1 expression [52], which may act to maintain Treg in a suppressive state, since S1P has been shown to overcome FoxP3+ Treg-mediated suppression [53]. Besides, we hypothesize that CD69 on Treg might directly suppress the activity of effector T cells. CD69 is a C-type lectin, which can trigger e.g. TGF-β production [54]. Although TGF-β was connected with suppression of cytotoxic CD8+ T cells before [55], we did not observe significantly up-regulation of TGF-β in Treg of the ACM group and TGF-β neutralizing did not restore cytotoxicity in our set-up. An alternative option might be binding of CD69 to a putative ‘ligand/receptor’ on the surface of CD8+ T cells, which is a common pattern for C-type lectins. This is true e.g. for interactions between cytotoxic lymphocytes and their targets [56]. However, a binding partner for CD69 is not known. If this putative molecule is expressed on CD8+ T cells, CD69-expressing Treg might bind to these cells to create a functional platform for adenosine production by bringing CD39 and CD73 in close proximity. Our analysis of CD4+ CD8+ doublets is a first hint to support this hypothesis. Future experiments addressing the function and the putative ligand for CD69 are needed.
A fully functional immune system is essential for mounting an inflammatory response in the presence of a tumor. This is exemplified by the prevention of breast tumor destruction in individuals with a mutation in TLR4 [57]. Therefore, the efficacy of chemotherapeutics administered to cancer patients may also depend on their impact on the immune system. Chemotherapeutics may either kill tumor cells without immune involvement, which may or may not be immunogenic, may cause tumor cell death by activating immune cells or cause immunosuppression by also killing immune cells [58]. The immunological outcome depends on surface alterations of dying cells or, as seen in our system, on the apoptotic cell secretome, which depends on the cell death-inducing agent. In our studies, the immunogenic cell death inducer oxaliplatin did not induce immune suppression as opposed to staurosporine. One might speculate that this was due to the absence of S1P production by oxaliplatin-treated MCF-7 cells.
Ex-vivo priming of DC with tumor lysates and in vivo DC activation strategies have been employed in cancer immunotherapy [59]. Therefore, our data might add to the understanding how priming with viable or killed tumor cells affects DC anti-tumor activity and thus might aid in improving strategies for ex vivo DC activation. Also, conventional therapy will probably benefit from inhibition of intrinsic immunosuppressive pathways. Our results provide evidence that interfering with S1PR4 and/or IL-27 might restrict tumor-induced immune suppression.
Supplementary Material
Acknowledgments
The authors are grateful to Miguel Guerrero and Mariangela Urbano for the S1P4R antagonists CYM74 or CYM58. We further thank Franz-Josef Streb and Margarethe Wiebe for excellent technical existence. The work was supported by a grant from Medical Faculty, Goethe-University Frankfurt to A.W. B.B. is supported by Sander Foundation (2007.070.2) and DFG (Br999, FOG784, ECCPS). E.R. is supported by a National Institute of Health Molecular Library Screen Center Network grant (U54 MH084512A).
Abbreviations
- TDLN
tumor-adjacent draining lymph nodes
- S1P
sphingosine-1-phosphate
- S1PR
S1P receptor
- ACM
apoptotic tumor cell condition medium
- VCM
viable tumor cell condition medium
- NCM
necrotic tumor cell condition medium
- AdorA2a
adenosine receptor A2a
- IDO
indoleamine-2,3 dioxygenase
Footnotes
Conflict-of-interest disclosure
The authors declare no competing financial interests.
References
- 1.Dhodapkar MV, Dhodapkar KM, Palucka AK. Interactions of tumor cells with dendritic cells: balancing immunity and tolerance. Cell Death Differ. 2008;15:39–50. doi: 10.1038/sj.cdd.4402247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berzofsky JA, Terabe M. NKT cells in tumor immunity: opposing subsets define a new immunoregulatory axis. J Immunol. 2008;180:3627–3635. doi: 10.4049/jimmunol.180.6.3627. [DOI] [PubMed] [Google Scholar]
- 3.Piersma SJ, Welters MJ, van der Burg SH. Tumor-specific regulatory T cells in cancer patients. Hum Immunol. 2008;69:241–249. doi: 10.1016/j.humimm.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Munn DH, Mellor AL. The tumor-draining lymph node as an immune-privileged site. Immunol Rev. 2006;213:146–158. doi: 10.1111/j.1600-065X.2006.00444.x. [DOI] [PubMed] [Google Scholar]
- 5.Tan W, Zhang W, Strasner A, Grivennikov S, Cheng JQ, Hoffman RM, Karin M. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature. 2011;470:548–553. doi: 10.1038/nature09707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- 7.Bell D, Chomarat P, Broyles D, Netto G, Harb GM, Lebecque S, Valladeau J, Davoust J, Palucka KA, Banchereau J. In breast carcinoma tissue, immature dendritic cells reside within the tumor, whereas mature dendritic cells are located in peritumoral areas. J Exp Med. 1999;190:1417–1426. doi: 10.1084/jem.190.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDonnell AM, Robinson BW, Currie AJ. Tumor antigen cross-presentation and the dendritic cell: where it all begins? Clin Dev Immunol. 2010;2010:539519. doi: 10.1155/2010/539519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iero M, Valenti R, Huber V, Filipazzi P, Parmiani G, Fais S, Rivoltini L. Tumour-released exosomes and their implications in cancer immunity. Cell Death Differ. 2008;15:80–88. doi: 10.1038/sj.cdd.4402237. [DOI] [PubMed] [Google Scholar]
- 10.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 11.Weigert A, Cremer S, Schmidt MV, von Knethen A, Angioni C, Geisslinger G, Brune B. Cleavage of sphingosine kinase 2 by caspase-1 provokes its release from apoptotic cells. Blood. 2010;115:3531–3540. doi: 10.1182/blood-2009-10-243444. [DOI] [PubMed] [Google Scholar]
- 12.Gregory CD, Pound JD. Cell death in the neighbourhood: direct microenvironmental effects of apoptosis in normal and neoplastic tissues. J Pathol. 2010;223:177–194. doi: 10.1002/path.2792. [DOI] [PubMed] [Google Scholar]
- 13.Albert ML. Death-defying immunity: do apoptotic cells influence antigen processing and presentation? Nat Rev Immunol. 2004;4:223–231. doi: 10.1038/nri11308. [DOI] [PubMed] [Google Scholar]
- 14.Zitvogel L, Kepp O, Kroemer G. Decoding cell death signals in inflammation and immunity. Cell. 2010;140:798–804. doi: 10.1016/j.cell.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 15.Tesniere A, Apetoh L, Ghiringhelli F, Joza N, Panaretakis T, Kepp O, Schlemmer F, Zitvogel L, Kroemer G. Immunogenic cancer cell death: a key-lock paradigm. Curr Opin Immunol. 2008;20:504–511. doi: 10.1016/j.coi.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 16.Nowak AK, Lake RA, Marzo AL, Scott B, Heath WR, Collins EJ, Frelinger JA, Robinson BW. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J Immunol. 2003;170:4905–4913. doi: 10.4049/jimmunol.170.10.4905. [DOI] [PubMed] [Google Scholar]
- 17.Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007;7:610–621. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- 18.Panaretakis T, Kepp O, Brockmeier U, Tesniere A, Bjorklund AC, Chapman DC, Durchschlag M, Joza N, Pierron G, van Endert P, Yuan J, Zitvogel L, Madeo F, Williams DB, Kroemer G. Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. Embo J. 2009;28:578–590. doi: 10.1038/emboj.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Most RG, Currie AJ, Robinson BW, Lake RA. Decoding dangerous death: how cytotoxic chemotherapy invokes inflammation, immunity or nothing at all. Cell Death Differ. 2008;15:13–20. doi: 10.1038/sj.cdd.4402255. [DOI] [PubMed] [Google Scholar]
- 20.Exley MA, Wilson B, Balk SP. Isolation and functional use of human NKT cells. Curr Protoc Immunol. Chapter 14(Unit 14):11. doi: 10.1002/0471142735.im1411s90. [DOI] [PubMed] [Google Scholar]
- 21.Guerrero M, Urbano M, Velaparthi S, Zhao J, Schaeffer MT, Brown S, Rosen H, Roberts E. Discovery, design and synthesis of the first reported potent and selective sphingosine-1-phosphate 4 (S1P(4)) receptor antagonists. Bioorg Med Chem Lett. 2011;21:3632–3636. doi: 10.1016/j.bmcl.2011.04.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qian F, Villella J, Wallace PK, Mhawech-Fauceglia P, Tario JD, Jr, Andrews C, Matsuzaki J, Valmori D, Ayyoub M, Frederick PJ, Beck A, Liao J, Cheney R, Moysich K, Lele S, Shrikant P, Old LJ, Odunsi K. Efficacy of levo-1-methyl tryptophan and dextro-1-methyl tryptophan in reversing indoleamine-2, 3-dioxygenase-mediated arrest of T-cell proliferation in human epithelial ovarian cancer. Cancer Res. 2009;69:5498–5504. doi: 10.1158/0008-5472.CAN-08-2106. [DOI] [PubMed] [Google Scholar]
- 23.Mandapathil M, Szczepanski MJ, Szajnik M, Ren J, Lenzner DE, Jackson EK, Gorelik E, Lang S, Johnson JT, Whiteside TL. Increased ectonucleotidase expression and activity in regulatory T cells of patients with head and neck cancer. Clin Cancer Res. 2009;15:6348–6357. doi: 10.1158/1078-0432.CCR-09-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eltzschig HK, Thompson LF, Karhausen J, Cotta RJ, Ibla JC, Robson SC, Colgan SP. Endogenous adenosine produced during hypoxia attenuates neutrophil accumulation: coordination by extracellular nucleotide metabolism. Blood. 2004;104:3986–3992. doi: 10.1182/blood-2004-06-2066. [DOI] [PubMed] [Google Scholar]
- 25.Herr B, Zhou J, Werno C, Menrad H, Namgaladze D, Weigert A, Dehne N, Brune B. The supernatant of apoptotic cells causes transcriptional activation of hypoxia-inducible factor-1alpha in macrophages via sphingosine-1-phosphate and transforming growth factor-beta. Blood. 2009;114:2140–2148. doi: 10.1182/blood-2009-01-201889. [DOI] [PubMed] [Google Scholar]
- 26.Zimmermann SY, Esser R, Rohrbach E, Klingebiel T, Koehl U. A novel four-colour flow cytometric assay to determine natural killer cell or T-cell-mediated cellular cytotoxicity against leukaemic cells in peripheral or bone marrow specimens containing greater than 20% of normal cells. J Immunol Methods. 2005;296:63–76. doi: 10.1016/j.jim.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 27.Campanelli R, Palermo B, Garbelli S, Mantovani S, Lucchi P, Necker A, Lantelme E, Giachino C. Human CD8 co-receptor is strictly involved in MHC-peptide tetramer-TCR binding and T cell activation. Int Immunol. 2002;14:39–44. doi: 10.1093/intimm/14.1.39. [DOI] [PubMed] [Google Scholar]
- 28.Weis N, Weigert A, von Knethen A, Brune B. Heme oxygenase-1 contributes to an alternative macrophage activation profile induced by apoptotic cell supernatants. Mol Biol Cell. 2009;20:1280–1288. doi: 10.1091/mbc.E08-10-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, Hopner S, Centonze D, Bernardi G, Dell’Acqua ML, Rossini PM, Battistini L, Rotzschke O, Falk K. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 30.Battaglia A, Buzzonetti A, Baranello C, Ferrandina G, Martinelli E, Fanfani F, Scambia G, Fattorossi A. Metastatic tumour cells favour the generation of a tolerogenic milieu in tumour draining lymph node in patients with early cervical cancer. Cancer Immunol Immunother. 2009;58:1363–1373. doi: 10.1007/s00262-008-0646-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wetter JA, Revankar C, Hanson BJ. Utilization of the Tango beta-arrestin recruitment technology for cell-based EDG receptor assay development and interrogation. J Biomol Screen. 2009;14:1134–1141. doi: 10.1177/1087057109343809. [DOI] [PubMed] [Google Scholar]
- 32.Lob S, Konigsrainer A, Rammensee HG, Opelz G, Terness P. Inhibitors of indoleamine-2,3-dioxygenase for cancer therapy: can we see the wood for the trees? Nat Rev Cancer. 2009;9:445–452. doi: 10.1038/nrc2639. [DOI] [PubMed] [Google Scholar]
- 33.Williams CA, Harry RA, McLeod JD. Apoptotic cells induce dendritic cell-mediated suppression via interferon-gamma-induced IDO. Immunology. 2008;124:89–101. doi: 10.1111/j.1365-2567.2007.02743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gee K, Guzzo C, Che Mat NF, Ma W, Kumar A. The IL-12 family of cytokines in infection, inflammation and autoimmune disorders. Inflamm Allergy Drug Targets. 2009;8:40–52. doi: 10.2174/187152809787582507. [DOI] [PubMed] [Google Scholar]
- 35.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 36.Linnemann C, Schildberg FA, Schurich A, Diehl L, Hegenbarth SI, Endl E, Lacher S, Muller CE, Frey J, Simeoni L, Schraven B, Stabenow D, Knolle PA. Adenosine regulates CD8 T-cell priming by inhibition of membrane-proximal T-cell receptor signalling. Immunology. 2009;128:e728–737. doi: 10.1111/j.1365-2567.2009.03075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kisley LR, Barrett BS, Bauer AK, Dwyer-Nield LD, Barthel B, Meyer AM, Thompson DC, Malkinson AM. Genetic ablation of inducible nitric oxide synthase decreases mouse lung tumorigenesis. Cancer Res. 2002;62:6850–6856. [PubMed] [Google Scholar]
- 38.Zeelenberg IS, van Maren WW, Boissonnas A, Van Hout-Kuijer MA, Den Brok MH, Wagenaars JA, van der Schaaf A, Jansen EJ, Amigorena S, Thery C, Figdor CG, Adema GJ. Antigen localization controls T cell-mediated tumor immunity. J Immunol. 2011;187:1281–1288. doi: 10.4049/jimmunol.1003905. [DOI] [PubMed] [Google Scholar]
- 39.von Bergwelt-Baildon MS, Popov A, Saric T, Chemnitz J, Classen S, Stoffel MS, Fiore F, Roth U, Beyer M, Debey S, Wickenhauser C, Hanisch FG, Schultze JL. CD25 and indoleamine 2,3-dioxygenase are up-regulated by prostaglandin E2 and expressed by tumor-associated dendritic cells in vivo: additional mechanisms of T-cell inhibition. Blood. 2006;108:228–237. doi: 10.1182/blood-2005-08-3507. [DOI] [PubMed] [Google Scholar]
- 40.Rivera J, Proia RL, Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat Rev Immunol. 2008;8:753–763. doi: 10.1038/nri2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weigert A, Schiffmann S, Sekar D, Ley S, Menrad H, Werno C, Grosch S, Geisslinger G, Brune B. Sphingosine kinase 2 deficient tumor xenografts show impaired growth and fail to polarize macrophages towards an anti-inflammatory phenotype. Int J Cancer. 2009;125:2114–2121. doi: 10.1002/ijc.24594. [DOI] [PubMed] [Google Scholar]
- 42.Larousserie F, Bardel E, Pflanz S, Arnulf B, Lome-Maldonado C, Hermine O, Bregeaud L, Perennec M, Brousse N, Kastelein R, Devergne O. Analysis of interleukin-27 (EBI3/p28) expression in Epstein-Barr virus- and human T-cell leukemia virus type 1-associated lymphomas: heterogeneous expression of EBI3 subunit by tumoral cells. Am J Pathol. 2005;166:1217–1228. doi: 10.1016/S0002-9440(10)62340-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoshida H, Nakaya M, Miyazaki Y. Interleukin 27: a double-edged sword for offense and defense. J Leukoc Biol. 2009;86:1295–1303. doi: 10.1189/jlb.0609445. [DOI] [PubMed] [Google Scholar]
- 44.Kamiya S, Owaki T, Morishima N, Fukai F, Mizuguchi J, Yoshimoto T. An indispensable role for STAT1 in IL-27-induced T-bet expression but not proliferation of naive CD4+ T cells. J Immunol. 2004;173:3871–3877. doi: 10.4049/jimmunol.173.6.3871. [DOI] [PubMed] [Google Scholar]
- 45.Schneider R, Yaneva T, Beauseigle D, El-Khoury L, Arbour N. IL-27 increases the proliferation and effector functions of human naive CD8+ T lymphocytes and promotes their development into Tc1 cells. Eur J Immunol. 2011;41:47–59. doi: 10.1002/eji.201040804. [DOI] [PubMed] [Google Scholar]
- 46.Sindrilaru A, Peters T, Schymeinsky J, Oreshkova T, Wang H, Gompf A, Mannella F, Wlaschek M, Sunderkotter C, Rudolph KL, Walzog B, Bustelo XR, Fischer KD, Scharffetter-Kochanek K. Wound healing defect of Vav3−/− mice due to impaired {beta}2-integrin-dependent macrophage phagocytosis of apoptotic neutrophils. Blood. 2009;113:5266–5276. doi: 10.1182/blood-2008-07-166702. [DOI] [PubMed] [Google Scholar]
- 47.Murugaiyan G, Mittal A, Lopez-Diego R, Maier LM, Anderson DE, Weiner HL. IL-27 is a key regulator of IL-10 and IL-17 production by human CD4+ T cells. J Immunol. 2009;183:2435–2443. doi: 10.4049/jimmunol.0900568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kochetkova I, Golden S, Holderness K, Callis G, Pascual DW. IL-35 stimulation of CD39+ regulatory T cells confers protection against collagen II-induced arthritis via the production of IL-10. J Immunol. 184:7144–7153. doi: 10.4049/jimmunol.0902739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Batten M, Kljavin NM, Li J, Walter MJ, de Sauvage FJ, Ghilardi N. Cutting edge: IL-27 is a potent inducer of IL-10 but not FoxP3 in murine T cells. J Immunol. 2008;180:2752–2756. doi: 10.4049/jimmunol.180.5.2752. [DOI] [PubMed] [Google Scholar]
- 50.Schenk U, Westendorf AM, Radaelli E, Casati A, Ferro M, Fumagalli M, Verderio C, Buer J, Scanziani E, Grassi F. Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Sci Signal. 2008;1:ra6. doi: 10.1126/scisignal.1160583. [DOI] [PubMed] [Google Scholar]
- 51.Hausler SF, Montalban Del Barrio I, Strohschein J, Anoop Chandran P, Engel JB, Honig A, Ossadnik M, Horn E, Fischer B, Krockenberger M, Heuer S, Seida AA, Junker M, Kneitz H, Kloor D, Klotz KN, Dietl J, Wischhusen J. Ectonucleotidases CD39 and CD73 on OvCA cells are potent adenosine-generating enzymes responsible for adenosine receptor 2A-dependent suppression of T cell function and NK cell cytotoxicity. Cancer Immunol Immunother. 2011;60:1405–1418. doi: 10.1007/s00262-011-1040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL, Cyster JG, Matloubian M. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 53.Liu G, Burns S, Huang G, Boyd K, Proia RL, Flavell RA, Chi H. The receptor S1P1 overrides regulatory T cell-mediated immune suppression through Akt-mTOR. Nat Immunol. 2009;10:769–777. doi: 10.1038/ni.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sancho D, Gomez M, Sanchez-Madrid F. CD69 is an immunoregulatory molecule induced following activation. Trends Immunol. 2005;26:136–140. doi: 10.1016/j.it.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 55.Chen ML, Pittet MJ, Gorelik L, Flavell RA, Weissleder R, von Boehmer H, Khazaie K. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-beta signals in vivo. Proc Natl Acad Sci U S A. 2005;102:419–424. doi: 10.1073/pnas.0408197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vogler I, Steinle A. Vis-a-vis in the NKC: genetically linked natural killer cell receptor/ligand pairs in the natural killer gene complex (NKC) J Innate Immun. 2011;3:227–235. doi: 10.1159/000324112. [DOI] [PubMed] [Google Scholar]
- 57.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, Yang H, Amigorena S, Ryffel B, Barrat FJ, Saftig P, Levi F, Lidereau R, Nogues C, Mira JP, Chompret A, Joulin V, Clavel-Chapelon F, Bourhis J, Andre F, Delaloge S, Tursz T, Kroemer G, Zitvogel L. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 58.Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol. 8:151–160. doi: 10.1038/nrclinonc.2010.223. [DOI] [PubMed] [Google Scholar]
- 59.Le DT, Pardoll DM, Jaffee EM. Cellular vaccine approaches. Cancer J. 2010;16:304–310. doi: 10.1097/PPO.0b013e3181eb33d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.