Abstract

A novel gold(I)-catalyzed cycloisomerization of propargylic esters leading to unsymmetrically substituted naphthalenes has been developed. This cascade reaction involves an unprecedented tandem sequence of 1,3- and 1,2-migration of two different migrating groups. It is believed that this transformation likely proceeds via the formation of 1,3-diene intermediate or its precursor, which upon cyclization and aromatization steps transforms into the naphthalene core.
In recent years, transition-metal-catalyzed transformations of propargylic esters1 have received much attention. Particularly intriguing is reactivity of these easily accessible compounds in the context of gold catalysis,2 which has been reflected in the development of a variety of diverse and elegant transformations leading to an immense array of complex organic molecules. Remarkable propensity of propargylic esters 1 to undergo 1,3-acyl migration,1,2 through the formation of an activated allene equivalent, intermediate i,1 allowed for efficient and expeditious assembly of various acyclic unsaturated synthons3 and complex carbo-4 and heterocycles5 (eq 1). Herein, we wish to report a gold(I)-catalyzed double 1,3-/1,2-migration-benzannulation cascade of propargylic esters 1 into naphthalenes 2 (eq 2).
 |
(1) |
 |
(2) |
Considering the enhanced electrophilicity of the sp3 center1b in intermediate i, we envisioned that incorporation of a suitable 1,2-migrating group (MG) in ii would provoke a subsequent 1,2-shift6 into iii, which upon proton loss and protiodeauration would afford 1,3-diene7 3. It is reasonable to propose that the latter may undergo 6π-electrocyclization into naphthalene8,9 2, analogously to the known cyclization of acyloxy-1,3,5-trienes10 (eq 3).
 |
(3) |
To this end, a possible isomerization of propargyl phosphate 1a (MG = H) in the presence of different catalysts has been tested (Table 1). It was found that employment of Ag triflate gave corresponding allene 4a in good yield (entry 1). Remarkably, switching to cationic Au(I) triflate led to the formation of target 1,3-diene 3a in 86% yield (entry 2). Monitoring of the reaction course revealed that this transformation proceeded through allenic intermediate 3a.12 Employment of Au(III) complexes (entries 3 and 4), non-cationic Au(I) halides (entries 5 and 6), Cu(I) and Cu(II) triflates, as well as Brønsted or Lewis acids, resulted in no reaction.12
Table 1.
Optimization of Reaction Conditions

| |||
|---|---|---|---|
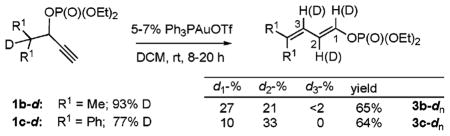
| entry | catalyst | yield 4a, %a | yield 3a, %a |
| 1 | 10% AgOTf | 73 | 0 |
| 2 | 5% Ph3PAuCl, 5% AgOTf | 0 | 0 |
| 3 | 5% AuCl3 | 0 | 0 |
| 4 | 5% AuCl3, 15% AgOTf | 0 | 86 |
| 5 | 5% AuI | 0 | 86 |
| 6 | 5% R3PAuCl (R = Et, Ph) | 0 | 86 |
Isolated yield of product for reaction performed on 0.1–0.2 mmol scale.
It deserves mentioning that the isomerization of acetates 1 into 1,3-dienes 5 in the presence of Ag-catalysts was reported (eq 4).11 However, the nature of the second step (1,2-H-shift or proton elimination) remained unclear. To address this issue, we performed mechanistic studies12 for MG = H (eq 3) employing Au(I) catalyst. Experiments revealed that the reaction proceeds exclusively via a 1,3-shift13–15 –elimination16 sequence.
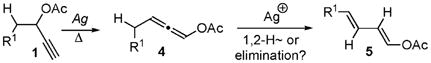 |
(4) |
Thus, we hypothesized that successful incorporation of a 1,2-migration into this cascade can only be achieved when a migrating group resides at a “proton-free” quaternary C-4 center. Therefore, isomerization of acetate 1d, possessing a strained cyclobutane ring, was examined. Indeed, a tandem 1,3-migration and ring expansion via a 1,2-shift occurred leading to 1,3-diene 3d in a moderate yield (eq 5). Moreover, isomerization of cyclopentyl homologs 1e and 1f afforded target naphthalenes 3e and 3f respectively (eq 5), thus providing a proof of concept for this cascade transformation.17
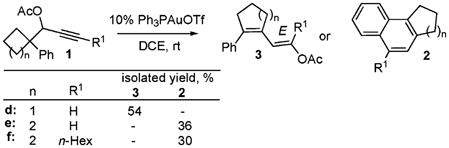 |
(5) |
Next, cycloisomerization of substrates possessing better 1,2-migrating groups was examined (Table 2). Thus, a tandem acyloxy- or phosphatyloxy- and Ph-group migration/benzannulation of propargylic esters 1g–n proceeded smoothly to provide naphthalenes 2g–n in good to excellent yields. Terminal, alkyl-, and aryl-substituted acetylenes were nearly equally efficient in this transformation. Unexpectedly, cy-clization of dimethylphenyl-substituted acetate 1k proceeded via exclusive 1,2-Me-group migration to give 3k in good yield (entry 5). Notably, a variety of substituents, such as methoxy (entry 6), trifluoromethyl (entry 7), and 2-furyl (entry 8), were perfectly tolerated under these reaction conditions.
Table 2.
Gold(I)-Catalyzed Synthesis of Naphthalenes

| |||
|---|---|---|---|
| entry | substrate | product | yield, %a,b |
| 1 |

|
 2g |
86c |
| 2 |

|
 2h |
94 |
| 3 |
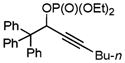
|
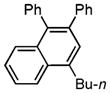 2h |
50 |
| 4 |

|
 2j |
75 |
| 5 |

|
 2k |
73 |
| 6 |

|
 2l |
90 |
| 7 |

|
 2m |
95 |
| 8 |

|
 2n |
94 |
Isolated yield.
Reactions were performed on a 0.5 mmol scale.
5% Au-catalyst was used.
We propose the following plausible mechanisms for this novel cascade transformation (Scheme 1). Au-catalyzed 1,3-migration transforms 1 via cyclic intermediate1b,3c,5b iv into allene 4.13 According to path A, 1,2-alkyl migration18 in iv produces benzylic cation v. The latter gives diene 3 upon protiodeauration or after proton transfer undergoes Friedel–Crafts alkylation to furnish naphthalene 2. Alternatively, a direct nucleophilic attack of vinyl-Au at the delocalized benzyl cation in v19 gives carbenoid intermediate vi, which upon 1,2-H-shift20 and aromatization produces naphthalene 2 (Path B).21 According to path C, Au-catalyzed 6π-electrocyclization14c,22 of 3 followed by elimination furnishes 2. In another scenario, direct intramolecular hydroarylation of 4 followed by 1,2-shift and proton loss in vii produces 2 (Path D). Unexpected exclusive Me- over Ph-migration in 1k is reasonably rationalized by stereoelectronic effect, according to which Ph group cannot accommodate requisite antiperiplanar orientation with the leaving group in iv.12,23,24 Successful cycloisomerization of 3e, obtained via 1,3-migration/elimination cascade, into naphthalene 2e provided an additional support for possible intermediacy of 1,3-dienes in this transformation (eq 6).
Scheme 1.

Mechanistic Rationale for Au(I)-Catalyzed Cascade
 |
(6) |
In summary, we have developed a novel gold(I)-catalyzed approach toward polysubstituted naphthalenes, which features an unprecedented tandem sequence of 1,3- and 1,2-migration of different migrating groups in propargylic esters. A more detailed investigation on the mechanism, as well as the scope of this cascade, is ongoing and will be reported in due course.
Supplementary Material
Acknowledgments
The support of the National Institutes of Health (Grant GM-64444) is gratefully acknowledged.
Footnotes
Supporting Information Available: Preparative procedures and analytical and spectral data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.For recent reviews, see: Marco-Contelles J, Soriano E. Chem Eur J. 2007;13:1350. doi: 10.1002/chem.200601522.Marion N, Nolan SP. Angew Chem, Int Ed. 2007;46:2750. doi: 10.1002/anie.200604773.
- 2.For recent reviews, see: Ma S, Yu S, Gu Z. Angew Chem, Int Ed. 2006;45:200. doi: 10.1002/anie.200502999.Hashmi ASK. Angew Chem, Int Ed. 2005;44:6990. doi: 10.1002/anie.200502735.Hoffmann-Röder A, Krause N. Org Biomol Chem. 2005;3:387. doi: 10.1039/b416516k.Echavarren AM, Nevado C. Chem Soc Rev. 2004;33:431. doi: 10.1039/b308768a.Dyker G. Angew Chem, Int Ed. 2000;39:4237. doi: 10.1002/1521-3773(20001201)39:23<4237::AID-ANIE4237>3.0.CO;2-A.Hashmi ASK. Chem Rev. 2007;107:3180. doi: 10.1021/cr000436x.Hashmi ASK, Hutchings GJ. Angew Chem, Int Ed. 2006;45:7896. doi: 10.1002/anie.200602454.Fürstner A, Davies PW. Angew Chem, Int Ed. 2007;46:3410. doi: 10.1002/anie.200604335.Yamamoto Y. J Org Chem. 2007;72:7817. doi: 10.1021/jo070579k.Zhang L, Sun J, Kozmin SA. Adv Synth Catal. 2006;348:2271.Widenhoefer RA, Han X. Eur J Org Chem. 2006:4555.Ishida T, Haruta M. Angew Chem, Int Ed. 2007;46:7154. doi: 10.1002/anie.200701622.Gorin DJ, Toste FD. Nature. 2007;446:395. doi: 10.1038/nature05592.
- 3.(a) Amijs CHM, López-Carrillo V, Echavarren AM. Org Lett. 2007;9:4021. doi: 10.1021/ol701706d. [DOI] [PubMed] [Google Scholar]; (b) Yu M, Zhang G, Zhang L. Org Lett. 2007;9:2147. doi: 10.1021/ol070637o. [DOI] [PubMed] [Google Scholar]; (c) Wang S, Zhang L. J Am Chem Soc. 2006;128:8414. doi: 10.1021/ja062777j. [DOI] [PubMed] [Google Scholar]; (d) Wang S, Zhang L. Org Lett. 2006;8:4585. doi: 10.1021/ol0618151. [DOI] [PubMed] [Google Scholar]
- 4.(a) Lemière G, Gandon V, Cariou K, Fukuyama T, Dhimane AL, Fensterbank L, Malacria M. Org Lett. 2007;9:2207. doi: 10.1021/ol070788r. [DOI] [PubMed] [Google Scholar]; (b) Buzas A, Gagosz F. J Am Chem Soc. 2006;128:12614. doi: 10.1021/ja064223m. [DOI] [PubMed] [Google Scholar]; (c) Marion N, Díez-González S, Frémont P, Noble AR, Nolan SP. Angew Chem, Int Ed. 2006;45:3647. doi: 10.1002/anie.200600571. [DOI] [PubMed] [Google Scholar]; (d) Zhang L, Wang S. J Am Chem Soc. 2006;128:1442. doi: 10.1021/ja057327q. [DOI] [PubMed] [Google Scholar]
- 5.(a) Buzas A, Istrate F, Gagosz F. Org Lett. 2006;8:1957. doi: 10.1021/ol0606839. [DOI] [PubMed] [Google Scholar]; (b) Zhang L. J Am Chem Soc. 2005;127:16804. doi: 10.1021/ja056419c. [DOI] [PubMed] [Google Scholar]; (c) Luo T, Schreiber SL. Angew Chem, Int Ed. 2007;46:8250. doi: 10.1002/anie.200703276. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Schwier T, Sromek AW, Yap DML, Chernyak D, Gevorgyan V. J Am Chem Soc. 2007;129:9868. doi: 10.1021/ja072446m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.For general review, see: Ducrot PH. One or More CH and/or CC Bond(s) Formed by Rearrangement. In: Katritzky AR, Taylor RJK, editors. Comprehensive Organic Functional Group Transformations II. Vol. 1. Elsevier; Oxford, UK: 2005. pp. 375–426.
- 7.For syntheses of 1,3-dienes employing Au catalysis, see: Buzas A, Istrate F, Gagosz F. Org Lett. 2007;9:985. doi: 10.1021/ol063031t.(b) See also ref 3d.
- 8.For a recent review on naphthalene syntheses, see: de Koning CB, Rousseau AL, van Otterlo WAL. Tetrahedron. 2003;59:7.
- 9.For selected examples, see: Dyker G, Hilderbrandt D, Liu J, Merz K. Angew Chem, Int Ed. 2003;42:4399. doi: 10.1002/anie.200352160.Zhao J, Hughes CO, Toste FD. J Am Chem Soc. 2006;128:7436. doi: 10.1021/ja061942s.Asao N, Takahashi K, Lee S, Kasahara T, Yamamoto Y. J Am Chem Soc. 2002;124:12650. doi: 10.1021/ja028128z.See also: Grisé CM, Barriault L. Org Lett. 2006;8:5905. doi: 10.1021/ol062582g.Asao N, Sato K. Org Lett. 2006;8:5361. doi: 10.1021/ol062268m.Wang S, Zhang L. J Am Chem Soc. 2006;128:14274. doi: 10.1021/ja066220f.
- 10.Hamura T, Morita M, Matsumoto T, Suzuki K. Tetrahedron Lett. 2003;44:167. and references therein. [Google Scholar]
- 11.(a) Saucy G, Marbet R, Lindlar H, Isler O. Helv Chim Acta. 1959;42:1945. [Google Scholar]; (b) Schlossarczyk H, Sieber W, Hesse M, Hansen HJ, Schmid H. Helv Chim Acta. 1973;56:875. [Google Scholar]; (c) Cookson RC, Cramp MC, Parsons PJ. J Chem Soc, Chem Comm. 1980:197. [Google Scholar]
- 12.See Supporting Information for details.
- 13.Direct observation of the allenes 4 supported 1,3-migration path.12
- 14.For reviews, see: Allin SM, Baird RD. Curr Org Chem. 2001;5:395.Nubbemeyer U. Synthesis. 2003;7:961.Fanning KN, Jamieson AG, Sutherland A. Curr Org Chem. 2006;10:1007.
-
15.D-Labeling studies on isomerization of 1a-d ruled out possible involvement of alkyne-vinylidene isomerization path.

-
16.Significant loss of D-label, as well as scrambling of the latter between C-1 and C-2, was observed for labeled phosphates 1b-d and 1c-d. Reversible protonation at C-1 under the prolonged reaction times is most likely the reason for the observed notable incorporation of D at C-1.
- 17.For reasons, which are not clearly understood, 3d did not cyclize into 2 even under forcing reaction conditions.
- 18.For selected examples, see: Dudnik AS, Gevorgyan V. Angew Chem, Int Ed. 2007;46:5195. doi: 10.1002/anie.200701128.Kirsch SF, Binder JT, Liébert C, Menz H. Angew Chem, Int Ed. 2006;45:5878. doi: 10.1002/anie.200601836.
- 19.See, for example: Luzung MR, Mauleón P, Toste FD. J Am Chem Soc. 2007;129:12402. doi: 10.1021/ja075412n.(b) See also ref 4a,b.
- 20.For selected examples, see: Markham JP, Staben ST, Toste FD. J Am Chem Soc. 2005;127:9708. doi: 10.1021/ja052831g.Gorin DJ, Davis NR, Toste FD. J Am Chem Soc. 2005;127:11260. doi: 10.1021/ja053804t.Sromek AW, Rubina M, Gevorgyan V. J Am Chem Soc. 2005;127:10500. doi: 10.1021/ja053290y.
-
21.Path B involving clean 1,2-H-shift to Au-carbenoid is not supported by the observed significant loss of D-label in cycloisomerization of 1j-d.

- 22.See also: Menz H, Kirsch SF. Org Lett. 2006;8:4795. doi: 10.1021/ol061856x.
- 23.For similar considerations, see: Aggarwal VK, Sheldon CG, Macdonald GJ, Martin WP. J Am Chem Soc. 2002;124:10300. doi: 10.1021/ja027061c.
- 24.Obviously, exclusive Me- vs Ph-migration can also be explained via Path D, according to which 1,2-migration occurs after cyclization step. Alternatively, as proposed by the reviewer, migration of the Me group leading to the more stable intermediate benzylic carbocation under thermodynamic control can also saccount for the observed chemoselectivity.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


