Abstract
An increasing number of studies use functional MRI and blood oxygen level-dependent signal (BOLD) to investigate neurofunctional basis of acute alcohol effects on the brain. However, the BOLD signal reflects neural activity only indirectly as it depends on regional hemodynamic changes and is therefore sensitive to vasoactive substances such as alcohol. We used MRI-based pulsed arterial-spin labeling (PASL) method to quantify effects of acute intoxication on resting cerebral perfusion. Gender effects have not been previously examined and yet they are of particular interest given differences in hormonal dynamics, alcohol metabolism, and hemodynamic regulation. Nineteen young, healthy individuals (9 women) with no personal or familial alcohol- or drug-related problems served as their own controls by participating in both alcohol (0.6 g/kg ethanol for men, 0.55 g/kg for women) and placebo scanning sessions in a counterbalanced manner. Regionally specific effects of the moderate alcohol dose on gray matter perfusion were examined with voxel-wise and region-of-interest analyses suggesting an interaction between gender and alcohol beverage. Acute intoxication increased perfusion in bilateral frontal regions in men, but not in women. Under placebo, stronger cortical perfusion was observed in women compared to men primarily in the left hemisphere in frontal, parietal and temporal areas. These results emphasize gender differences and regional specificity of alcohol's effects of cerebral perfusion possibly due to interactive influences on hormonal, metabolic, and hemodynamic autoregulatory systems. Alcohol-induced perfusion increase correlated positively with impulsivity/antisocial tendencies, consistent with dopaminergic mediation of reward and its effects on cortical perfusion. Additional ASL studies are needed to investigate dose- and time-dependent effects of alcohol intoxication and gender on the hemodynamic factors that conjointly influence BOLD signal, in order to disambiguate the vascular/metabolic mechanisms from the neurally-based changes.
Keywords: Cerebral blood flow, CBF, perfusion, MRI, ASL, alcohol, gender, impulsivity
Introduction
In spite of its widespread use and vast costs resulting from its abuse, alcohol's effects on the functional neuroanatomy are still poorly understood. Better understanding of the neural basis of alcohol’s effects on cognition and behavioral regulatory functions could provide crucial insight into alcohol-induced cognitive impairments as well as dysregulation of self-control and inability to desist drinking. Studies of acute alcohol challenge are important as they can reveal the neural circuits underlying behavioral impairments due to intoxication and can inform and guide pharmacological research on possible agents aiming to diminish or reverse alcohol's effects. In concert with studies on chronic alcoholics and populations at risk, they help to parse out the effects of alcohol neurotoxicity, genetic susceptibility, and environmental factors, offering insight into neural systems that may be most susceptible to chronic alcohol abuse.
The most common method of choice in studies investigating functional neuroanatomy is T2*-weighted BOLD contrast due to its high sensitivity and excellent spatial resolution. Consequently, the prominence of BOLD fMRI studies examining effects of acute intoxication on cognitive functions has been rising with increased reliance on neuroimaging methods in the field of alcohol use and alcoholism. Working memory tasks resulted in BOLD signal changes in frontal and parietal areas when the average blood alcohol concentration (BAC) levels reached ~0.08 % (Gundersen et al., 2008; Paulus et al., 2006). Similar intoxication levels affected activity primarily in frontal circuitry during simulated driving (Calhoun et al., 2004; Meda et al., 2009). Intoxication levels at ~0.08 % also affected limbic activation to emotional faces (Gilman et al., 2008) and to alcoholic drink odors at ~0.05 % (Bragulat et al., 2008).
However, the BOLD signal reflects neural activity only indirectly as a result of neurovascular coupling and it depends on regional changes in cerebral blood flow (CBF), volume (CBV), and cerebral metabolic rate of oxygen use (CMRO2) (Buxton, 2002). Therefore, it is possible that these observed effects are partly due to alcohol's effects on factors other than the neural activation (Iannetti and Wise, 2007). Due to its complex dependence on hemodynamic regulation, the BOLD signal is sensitive to anything that can alter hemodynamics and the neurovascular coupling, including pharmacological agents, disease, etc. Under such circumstances, the neural activation is confounded with vascular changes and the BOLD signal cannot be interpreted unambiguously in isolation (Brown et al., 2007; Buxton et al., 2004; Liu and Brown, 2007). Alcohol is a vasoactive pharmacological agent which modulates regional cerebral perfusion in a dose-dependent manner (Mathew and Wilson, 1991). It may affect the baseline perfusion reflected in a change in BOLD signal, which would in turn affect task-induced BOLD changes (Brown et al., 2003). Indeed, studies using positron emission tomography (PET) or related single photon emission tomography (SPECT) methods have reported regionally specific changes in CBF during rest that were alcohol dose-dependent. Alcohol increased CBF in prefrontal and temporal areas (Mathew and Wilson, 1986; Sano et al., 1993; Tiihonen et al., 1994; Volkow et al., 1988), as well as the anterior cingulate (AC) cortex and brainstem (Ingvar et al., 1998). Alcohol-induced cerebral vasodilation and consequently increased CBF were confirmed with transcranial Doppler (Blaha et al., 2003; Stendel et al., 2006).
Arterial-spin labeling (ASL) is a noninvasive MRI-based technique that uses magnetically labeled arterial blood as an endogenous tracer in order to measure regional CBF (Buxton et al., 1998a; Detre et al., 2009; Golay et al., 2004; Wong et al., 1999). Diffusion of labeled blood into tissue alters the local magnetization revealing a component of the MRI signal that is dependent on the local rate of blood flow (Calamante et al., 1999; Detre and Alsop, 1999). It permits quantification of cerebral perfusion in physiological units expressed in ml of blood per 100 g of tissue per minute. The ASL method has been used to examine cerebral blood flow in abstinent alcohol dependent individuals and showed decreased fronto-parietal perfusion (Clark et al., 2007; Mon et al., 2009). In a study investigating relapse, a group of treatment-seeking alcohol-dependent individuals were scanned at baseline and after ~35 days of abstinence and followed for one year. Individuals who resumed drinking had lower frontal CBF both at baseline and after ~35 days of abstinence in comparison to those who remained abstinent during the follow up period (Durazzo et al., 2010). ASL-measured cortical perfusion was also found to be reduced by cocaine infusion (Gollub et al., 1998) and chronic cigarette smoking in alcohol-dependent individuals (Gazdzinski et al., 2006; Mon et al., 2009).
In the present study we used the ASL technique in order to measure resting gray matter perfusion in a group of healthy individuals who served as their own controls by participating in both placebo and moderate (0.6 g/kg ethanol for men, 0.55 g/kg for women) alcohol conditions in a counterbalanced manner. Previous studies confirmed that the ASL CBF measurements are highly stable across sessions (Hermes et al., 2007; Parkes et al., 2004; Pfefferbaum et al., 2010; Wang et al., 2011), making it appropriate for inter-session comparisons. Furthermore, the anatomical MR images were acquired in the same session with the ASL scans, facilitating precise co-registration (Brown et al., 2007; Tracey, 2001). Despite previous evidence of the gender differences in CBF (Hermes et al., 2007; Parkes et al., 2004), in hormonal balance (Baxter et al., 1987), alcohol metabolism (Kwo et al., 1998), and autonomic vascular regulation (Hart et al., 2009), gender differences in CBF under alcohol challenge have not been investigated. The goal of our study was to use ASL to examine regional effects of alcohol intoxication on gray matter perfusion in men and women and provide a preliminary insight into the hemodynamic changes underlying the BOLD signal effects in acute intoxication studies with moderate alcohol dose.
Methods
Participants
Nineteen individuals (9 women, age (M ± SD) = 24.9 ± 2.6 yrs, range = 22 – 33 yrs) served as their own controls as they participated in both alcohol and placebo sessions in a counterbalanced manner. Answers on the adapted Alcohol Use Questionnaire (Cahalan et al., 1969) indicated that the study participants were light-moderate drinkers who reported drinking occasionally (1.8 ± 0.9 times per week on average) and in low to moderate amounts (2.2 ± 0.7 drinks per occasion). All participants reported drinking in social settings on a regular basis. No gender differences were observed in the amount or frequency of drinking. All participants were young, healthy, right-handed non-smokers with no alcohol or drug-related problems. They reported experiencing no health issues, never suffered from seizures or concussions and were not taking any medications at the time of the study. None of the subjects were ever arrested or treated for alcohol or drug problems. Short Michigan Alcohol Screening Test (SMAST) questionnaire (Selzer et al., 1975) detected no alcoholism-related symptoms and the participants reported no family history of alcoholism or drug abuse for the first or second degree relatives. The participants' reported drinking habits were quite a bit lighter than the nation-wide average of 3.7 drinks per occasion for young adults (Chen et al., 2004/2005), indicating that it is unlikely that they had acquired high levels of tolerance to alcohol, or that they suffered from the long-term CNS effects observed in heavy social drinkers (Nichols and Martin, 1996; Parsons and Nixon, 1998). Data were collected from two other individuals but due to technical problems with one of their scans they were not included in the analyses.
Experimental Procedure
The participants were requested to abstain from food for at least 3 hours and from alcohol at least 48 hours before each experimental session and were asked about their compliance upon their arrival to the laboratory. Participants were screened with an electronic breathalyzer (Alcotest 7410, Draeger Safety, Inc.) for the presence of alcohol. They were also tested for other substances including marijuana, cocaine, methamphetamine, nicotine, opiates, and phencyclidine with a FDA approved multi-drug screen test kit (Medimpex Inc.). Female subjects were tested for pregnancy prior to each scanning session to ascertain that they were not pregnant. All participants tested negative on all tests. In addition to self-report of the phase of their menstrual cycle, we obtained quick-screen measures of luteinizing hormone (Medimpex, Inc.). For seven women both scans took place during low hormonal levels; two were in the early follicular phase (menstruation) for both sessions and five women were using birth control, providing a constant hormonal status. For one woman both scans fell during her luteal phase and for another one session took place in the early follicular and the other during the luteal phase. Caffeine intake was not quantified. However, participants were encouraged to refrain from drinking coffee for at least three hours prior to the beginning of each session. In addition, all scanning sessions took place in the early evening when caffeine intake is lowest (Smith, 2002).
All procedures were in accordance with the ethical standards of the Declaration of Helsinki. Written informed consent approved by the Human Research Committee at Massachusetts General Hospital and the Partners Healthcare Network was obtained from all subjects before participation. Prior to the first experimental session, the participants were familiarized with the setup and the scanner. No beverages were administered at that time but the participants filled out questionnaires probing their handedness (Oldfield, 1971), quantity and frequency of alcohol use (Cahalan et al., 1969), severity of alcoholism-related symptoms (Selzer et al., 1975), level of response to alcohol (Schuckit et al., 1997) and personality (Eysenck and Eysenck, 1975; Zuckerman, 1971), in addition to providing information about their medical history and family history of alcoholism. Subsequently, the subjects participated in placebo and alcohol sessions that were counterbalanced in order of presentation. The two sessions took place 31.6 ± 24.0 days apart on average.
Subjects were given a beverage that consisted of 0.6 g/kg ethanol for men and 0.55 g/kg for women to adjust for the body mass index difference (Friel et al., 1999). Alcohol (vodka Gray Goose®) was mixed with orange juice (20% v/v). In the placebo condition, orange juice of the same volume was administered (Marinkovic et al., 2001). The beverage was served in two glasses which the subjects were asked to consume in a ten minute period. The entire session including preparations, beverage consumption, and task lasted approximately two hours.
The blood alcohol concentration (BAC) of each participant was checked upon arrival to the laboratory at the start of each session and was estimated with the electronic breathalyzer during the times when the subject was outside the scanning chamber, approximately every 5 min, starting 15 min after drinking. Since no electronic devices could be used inside the scanner room, a Saliva Alcohol Test (Q.E.D., STC Technologies, Inc.) was used to estimate the BAC during scans. Participants performed an Antisaccade task (results reported in a separate manuscript) prior to resting ASL scan, which was administered at the end of the scanning session, on a descending limb of BAC. The average BAC measured immediately after the ASL scan was 0.043% ± 0.01%, at 98 min after the start of drinking. Although female participants tended to have a lower average BAC than males (0.038% vs 0.047%), the difference was not significant, F(1, 17) = 2.1, p > 0.17.
Image Acquisition and Analysis
Imaging data were acquired at the Martinos Center in Boston, MA with a 3T Siemens Trio Tim whole-body scanner system (Siemens Healthcare, Erlangen, Germany) fitted with the standard vendor’s 12-channel head coil. Special care was taken to minimize head motion with the use of a special pillow, foam padding, and head "clamps" that allowed participants to maintain a comfortable but stable position during scanning. Exposure to scanner noise was reduced with 29 db earplugs and pillow padding.
The resting-state scans were acquired with pulsed arterial spin labeling (PASL) (Kim, 1995; Kwong et al., 1995) for perfusion-weighted imaging using an echo planar imaging (EPI) readout. The protocol combined quantitative imaging of perfusion using a single subtraction - second version (QUIPSS-II) with the flow-sensitive alternating inversion recovery (FAIR), slice selective and non slice selective hyperbolic secant inversion pulse labeling scheme (Wong et al., 1998). The ASL sequence lasted 7 min and comprised axial-oblique AC-PC oriented images of 24 slices with 5-mm thickness that were acquired using identical sequence parameters in ascending slice order (inferior-superior): TR = 4000ms, TE = 13ms, tagging duration TI1 = 600ms after which QUIPSS-II saturation was done; starting time of EPI read-out of TI2 = 1600ms (first slice); voxel size 3.1 × 3.1 × 5.0 mm3; matrix size = 64 × 64, FOV = 200mm; FA = 90°, EPI readout bandwidth = 2298 Hz/Px. In order to minimize the impact of static tissue, two presaturation pulses were applied in the imaging planes immediately before the inversion pulse. QUIPSS-II was done with an inferior as well as a superior saturation slab outside the slices.
Structural data were acquired with two high resolution three-dimensional, Fourier-transformed magnetization-prepared rapid acquisition gradient echo (MPRAGE) T1-weighted sequences that optimize contrast for a range of tissue properties (TR = 2530ms, TE = 3.25ms, FA = 7°, FOV = 256, 128 sagittal slices, 1.33mm thickness, in-plane resolution 1 × 1 mm. The FreeSurfer (surfer.nmr.mgh.harvard.edu) analysis package was used to analyze structural images and ASL data from each subject (Dale et al., 1999; Fischl et al., 1999a). Each participant's cortical surface was reconstructed using an automatic gray/white segmentation, tessellation and inflation of the folded surface tessellation patterns. These surfaces were registered with a canonical brain surface created from an average of 40 brains (Fischl et al., 1999b) allowing for high-resolution group averaging based on surface alignment. The affine transform that mapped the anatomical for each individual to the MNI305 average brain was also computed (Collins et al., 1995).
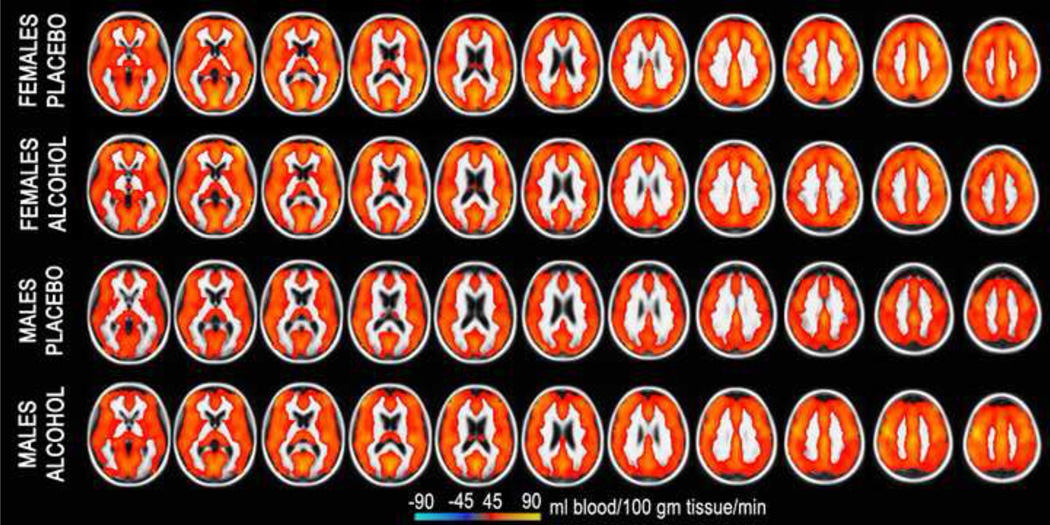
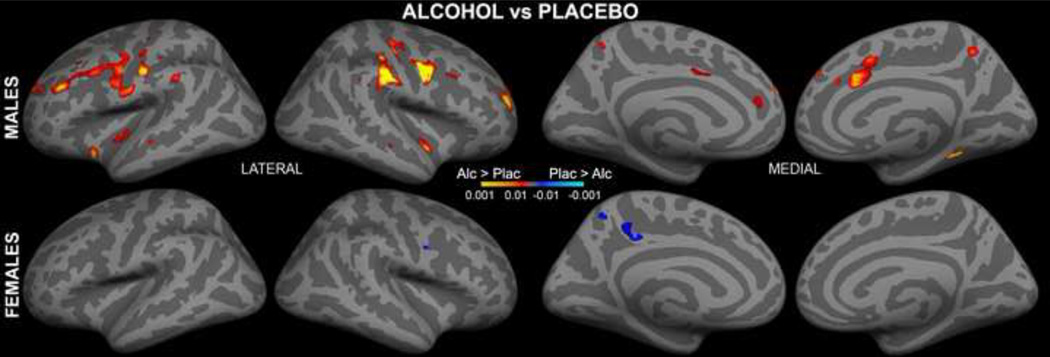
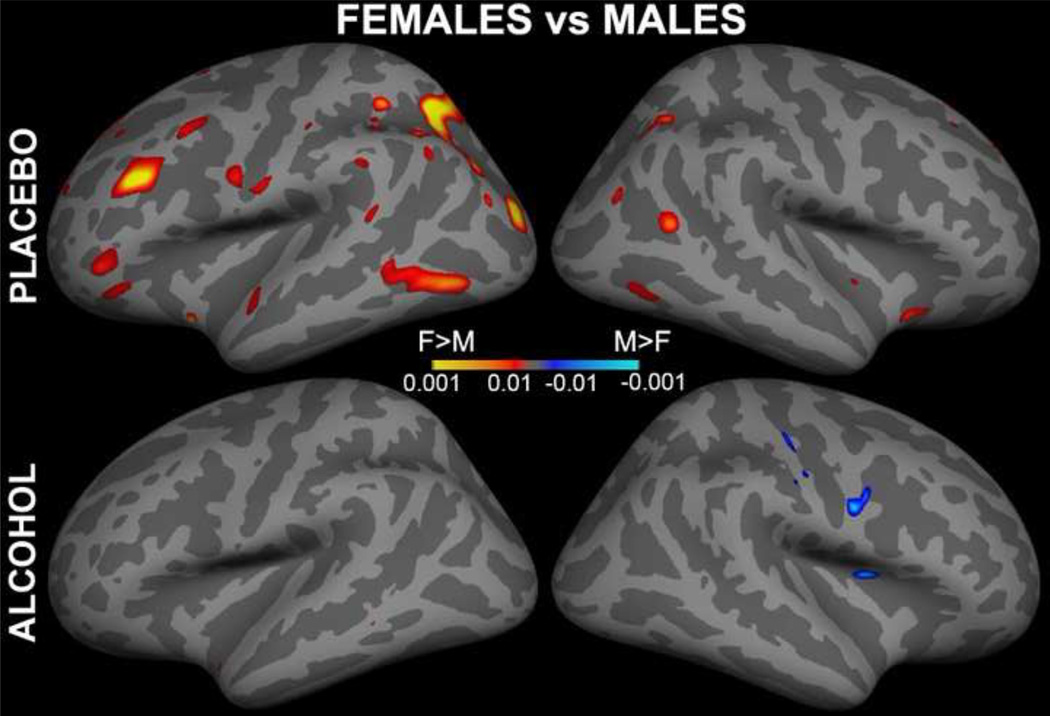
ASL data were motion corrected with AFNI algorithm (Cox, 1996). The amount of head motion did not differ between genders or sessions and did not exceed the maximum of 2 mm in any subject. The Siemens ASL sequence automatically computed a perfusion-weighted map and a relative CBF map with the formula described by Wang and colleagues (2003) using a fully relaxed M0 volume acquired at the beginning of each series (lambda = 0.9 mL/g, alpha = 95%, T1a_blood = 1500 ms). Each individual's map was aligned with the anatomical images using boundary-based registration (Greve and Fischl, 2009). The CBF maps were then resampled to the MNI305 space by concatenating the CBF-anatomical and anatomical-MNI305 transforms. Voxel-wise general linear model analysis was performed with random effects model using the FreeSurfer mri_glmfit program. Images of the overall group average ASL-CBF for each gender and beverage condition are shown in Fig 1. Beverage differences were computed as two-sample t-tests for males and females separately. Similarly, gender differences were computed for alcohol and placebo separately. These are shown in Fig 2 and Fig 3 respectively in the form of the statistical parametric maps in cortical surface space.
Figure 1.
Group average CBF for each gender and beverage condition. Inter-slice distance is 5 mm and the CBF is quantified in ml of blood per 100g of tissue per minute.
Figure 2.
Voxel-wise analysis of beverage differences for each gender displayed on the inflated cortical surface of both hemispheres. Acute intoxication increased CBF in men but not in women.
Figure 3.
Voxel-wise analysis of gender differences for each beverage displayed on the inflated lateral cortical surface of both hemispheres. Under placebo, stronger CBF was observed in women compared to men primarily in the left hemisphere.
In an effort to further quantify and examine potential regional differences due to alcohol intoxication and gender, a region-of-interest (ROI) analysis was conducted on the perfusion measured in the cortical ribbon. Each subject's cortical surface was parcellated into neuroanatomical areas based on a probabilistic atlas (Desikan et al., 2006; Fischl et al., 2004). Within these anatomical boundaries, measures of perfusion (ml/100g/min) were calculated for each ROI, each participant and for each session. Since the slice prescription did not cover the entire brain reliably across all subjects, the areas in the inferoventral temporal (i.e. fusiform, parahippocampal and entorhinal cortices), orbitofrontal and frontopolar regions were excluded from the ROI analyses. Forty ROIs were analyzed in this manner and they included the following cortical areas in both hemispheres: 1) superior frontal gyrus, 2) rostral middle frontal gyrus, 3) pars triangularis of the inferior frontal gyrus, 4) pars opercularis of the inferior frontal gyrus, 5) caudal middle frontal gyrus, 6) rostral anterior cingulate cortex, 7) caudal anterior cingulate cortex, 8) precentral gyrus, 9) superior temporal gyrus, 10) middle temporal gyrus, 11) banks of the superior temporal sulcus (i.e. the posterior aspect of the superior temporal sulcus), 12) postcentral gyrus, 13) superior parietal cortex, 14) supramarginal gyrus, 15) inferior parietal cortex, 16) precuneus cortex, 17) lateral occipital cortex, 18) lingual gyrus, 19) cuneus cortex, 20) pericalcarine cortex. Detailed description of the anatomical ROI delineation can be found in (Desikan et al., 2006).
Mixed factorial design ANOVA with gender as a between-group factor and beverage and hemisphere as within-subject factors was carried out on the average perfusion values for each gender, beverage, and left and right hemisphere (Woodward et al., 1990). In order to examine potential regional sensitivity of cerebral perfusion to the effects of alcohol, gender, and hemispheric laterality, the ROIs were grouped into frontal, temporal, parietal, and occipital regions for each hemisphere and submitted to a mixed design ANOVA with gender as a between-group factor and beverage, hemispheric laterality and cortical regions as within-subject factors. Finally, in order to examine these effects across all ROIs simultaneously while controlling for their mutual dependence, we employed a multivariate analysis of variance (MANOVA). With the goal of exploring potential trends in the data, a series of univariate ANOVAs were additionally carried out across the ROIs. With the overall α level maintained at p < 0.05, the Sidak's correction of the Bonferroni method for protection against inflated type one error adjusted alpha level for each ROI to p < 0.001.
Results
Images of the overall group average CBF for both genders and beverage conditions are shown in Fig 1. Voxel-wise analyses were performed using random-effects analysis model of the group data for each gender in surface space. Differential images contrasting alcohol and placebo for each gender separately showed significantly stronger perfusion under alcohol in men, but not women, in fronto-parietal regions (Fig 2), suggesting an interaction between gender and beverage. Fig. 3 presents voxel-wise statistical parametric maps of gender differences for each beverage displayed on the inflated cortical surfaces of both hemispheres. Women had stronger perfusion than men under placebo especially in the left hemisphere in the frontal, parietal, and temporal areas.
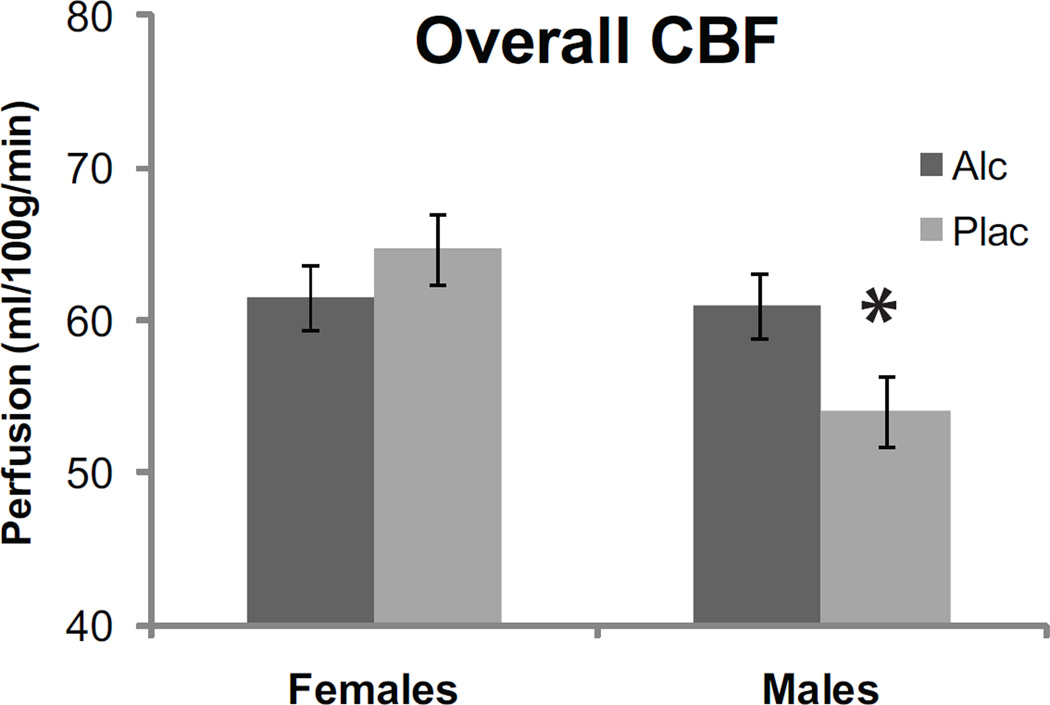
ROI analysis of the cortical CBF with respect to gender, intoxication, and regional specificity confirmed these observations as described here below. This analysis was performed with graded degrees of anatomical precision comprising the overall hemispheric (Fig 4), lobar (Fig 5), and anatomically parcellated CBF measures (Fig 6). Effects of gender, alcohol intoxication, and hemispheric laterality on the overall cerebral perfusion were analyzed with a 2 × 2 × 2 mixed design ANOVA (Woodward et al., 1990). The dependent variable was CBF averaged across all the ROIs for each hemisphere. The analysis indicated a significant Gender and Beverage interaction, F(1,17) = 5.1, p < 0.05 and a significant Gender x Hemisphere interaction, F(1,17) = 5.6, p < 0.05. These interactions were due to alcohol-induced CBF increase in men, F(1,17) = 5.1, p < 0.05, but not in women, F(1,17) = 1.0, p > 0.30. Perfusion was stronger in women than in men under placebo only, F(1,17) = 6.0, p < 0.05, with no gender differences observed under alcohol, F(1,17) = 0.0, p > 0.5 (Fig 4). Gender differences tended to be stronger in the left hemisphere overall, F(1,17) = 4.1, p < 0.06, and were not significant on the right, F(1,17) = 0.7, p > 0.4, (Fig 3).
Figure 4.
Effects of gender and alcohol intoxication on the gray matter perfusion. Alcohol-induced CBF increase was observed in men only. Women show stronger CBF than men under placebo.
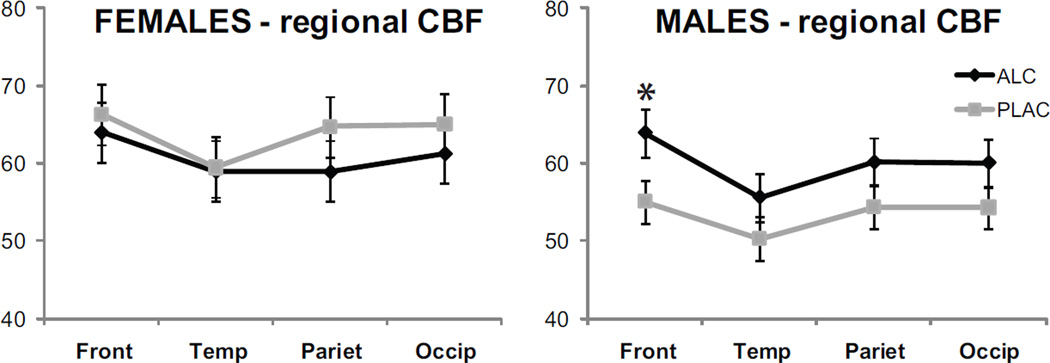
Figure 5.
Regional CBF sensitivity to gender and alcohol intoxication. Alcohol increased perfusion in prefrontal regions bilaterally in men. Furthermore, the statistical analysis indicated that stronger CBF was observed in women as compared to men under placebo in the frontal, temporal, and parietal regions.
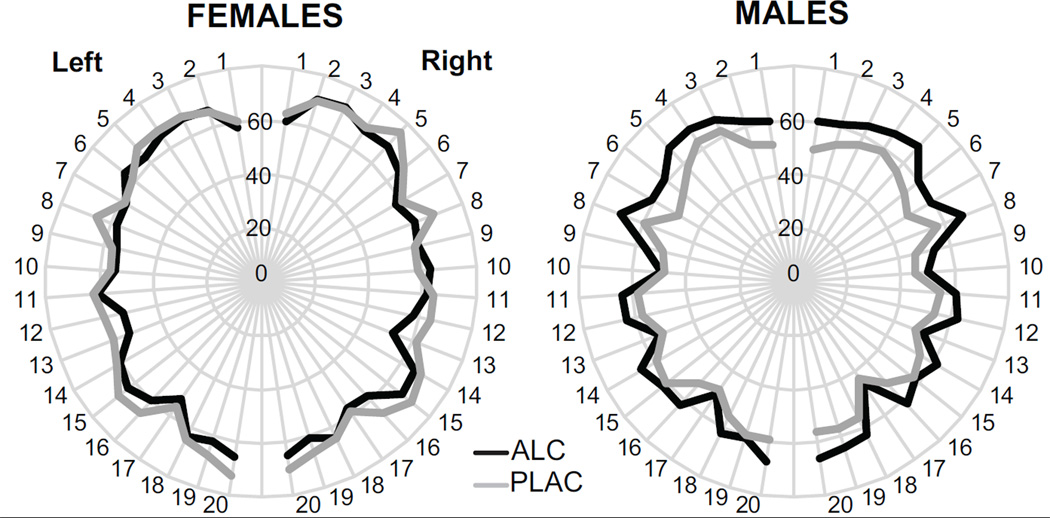
Figure 6.
Group average CBF for forty ROIs for the left and right hemispheres (Desikan et al., 2006; Fischl et al., 2004). Even though the overall MANOVA did not show significant results when corrected for multiple comparisons, alcohol tended to increase perfusion in men in the caudal middle frontal and supramarginal gyri bilaterally. Women tended to show stronger CBF in the left caudal middle frontal, inferior parietal, supramarginal and superior temporal areas. The list of the ROIs is included in the text.
Regional CBF sensitivity to the effects of gender, alcohol intoxication, and hemispheric laterality was analyzed with ROIs grouped into frontal, temporal, parietal, and occipital regions for each hemisphere in a 2 × 2 × 2 × 4 mixed design ANOVA (Fig 5), (Hermes et al., 2007). Significant interaction was observed for the factors of Gender x Beverage, F(1,17) = 4.5, p < 0.05, with stronger CBF observed in women than in men under placebo in the left hemisphere in the frontal, F(1,17) = 7.9, p < 0.01, temporal, F(1,17) = 8.2, p < 0.01, and parietal areas, F(1,17) = 8.4, p < 0.01. There were no gender differences under alcohol. Alcohol increased perfusion in men particularly in frontal regions both on the left, F(1,17) = 5.9, p < 0.05, and on the right, F(1,17) = 6.2, p < 0.05 (Figs 2 and 5). Gender x Hemisphere interaction, F(1,17) = 5.6, p < 0.05, was due to stronger gender differences in the left hemisphere (Fig 3). Main effect of Region, F(3,51) = 7.5, p < 0.001, indicated that, when summed across the factors of Beverage and Gender, the overall perfusion was strongest in prefrontal, compared to all other areas, F(1,17) = 11.2, p < 0.01, and weakest in temporal cortical areas, F(1,17) = 18.8, p < 0.001.
Finally, ROI-based MANOVA was carried out across all forty ROIs as dependent variables with the factors of Gender, Beverage, and Hemisphere in an effort to increase spatial precision of potential gender- or beverage-based regional differences (Fig 6). The overall multivariate analysis across all subjects did not show any significant effects. Similarly, none of the univariate comparisons carried out for each ROI reached Sidak's correction of Bonferroni critical value of p < 0.001 for multiple comparisons that would maintain the overall α at < 0.05 (all p's > 0.005). However, the observed trends further refined the spatial foci of the regional differences reported above. Here presented are p-values that were not corrected for the inflated probability of type I error due to multiple comparisons (Woodward et al., 1990) but can be considered to represent trends in the data. Cortical perfusion showed a trend towards higher values in women than men under placebo in the left hemisphere in caudal middle frontal gyrus, F(1,17) = 10.4, p < 0.005, inferior parietal cortex, F(1,17) = 10.3, p < 0.005, supramarginal gyrus, F(1,17) = 8.0, p < 0.01, and superior temporal gyrus, F(1,17) = 9.5, p < 0.01. Alcohol increased perfusion in men bilaterally in the caudal middle frontal gyrus, F(1,17) = 8.0, p < 0.01, caudal anterior cingulate, F(1,17) = 8.0, p < 0.01, supramarginal gyrus, F(1,17) = 8.6, p < 0.01, and superior temporal cortex, F(1,17) = 4.6, p < 0.05.
Correlational analysis
Correlations between scores on personality questionnaires and perfusion measures were calculated as a function of beverage, gender, and hemispheric laterality. The Psychoticism/Socialization scale of the EPQ (Eysenck and Eysenck, 1975) correlated with perfusion under alcohol in the right hemisphere (r = 0.72, p < 0.001), and marginally on the left (r = 0.43, p < 0.1). This correlation was significant for both genders (females: 0.77, p < 0.05 and males: 0.70, p < 0.05). In contrast, there was no correlation under placebo for either right (0.03, p > 0.5) or left hemisphere (−0.18, p > 0.5).
Discussion
In this experiment we sought to investigate effects of alcohol intoxication on cortical perfusion in a cohort of young and healthy men and women during rest. CBF was measured from the same participants after ingesting a moderate alcohol dose or placebo in a counterbalanced manner on a descending limb of the BAC curve. The main finding was that acute intoxication increased cortical perfusion in bilateral frontal regions in men, but not in women. Under placebo, stronger perfusion was observed in women as compared to men primarily in the left hemisphere. Results of the ROI-based analyses were consistent across levels of regional specificity and indicated analogous conclusions while adding refinement of spatial precision to the observed effects.
The present results are in overall agreement with previous PET or SPECT studies of resting CBF that used a range of alcohol doses. In most previous studies, no significant CBF changes were observed at the lowest administered alcohol dose (0.5 g/kg) (Mathew and Wilson, 1986; Volkow et al., 1988), although in one study a bilateral global increase was seen after the measured CBF values were corrected for CO2 level (Mathew and Wilson, 1986). In a SPECT study, Schwartz and colleagues (Schwartz et al., 1993) administered 0.6 g/kg to male subjects and observed a 4% CBF increase more than two hours after drinking. Tiihonen and colleagues (1994) reported 8% CBF increase in the right prefrontal area after administering 0.7 g/kg to male subjects. Similarly, the same dose increased CBF by 12% especially in prefrontal areas in a group of male subjects (Sano et al., 1993). Newlin and colleagues (1982) administered 0.75 g/kg to a group consisting of male and female participants and observed a global gray matter CBF increase of ~20%. Measured one hour after drinking in males only, a dose of 1 g/kg increased blood flow to the prefrontal and temporal cortices by ~8%, but decreased CBF in cerebellum (Volkow et al., 1988). While most of these studies employed male participants only, those with mixed subject groups (Mathew and Wilson, 1986; Newlin et al., 1982) did not report effects of gender. Our results extend previous findings by indicating that gender modulates effects of alcohol intoxication on cortical perfusion.
Gender exerts powerful effects on resting CBF with greater perfusion observed in women than men under placebo in the left hemisphere, particularly in the frontal (caudal middle frontal gyrus), temporal (superior temporal gyrus), and parietal regions (supramarginal gyrus and inferior parietal cortex). The observed overall gender-based difference replicates previous reports of greater resting cortical perfusion in women compared to men with both MRI-based (Hermes et al., 2007; Parkes et al., 2004; Shin et al., 2007), as well as PET-based methods (Daniel et al., 1989; Mathew et al., 1986; Rodriguez et al., 1988; Shaw et al., 1979). The prevalent supposition for this robust finding rests on hormonal differences between men and women (Baxter et al., 1987; Goldman et al., 1976). This hypothesis is well supported by the evidence of CBF sensitivity to the manipulation of hormonal balance. Pharmacological suppression of gonadal hormones in women results in decreased CBF prefrontally during a cognitive task (Berman et al., 1997). Furthermore, estrogen is correlated with CBF velocity as measured with transcranial Doppler during ovulation induction and after pituitary suppression (Shamma et al., 1992). When measured across a wide age range, the CBF gender difference is the strongest in the decades prior to the onset of menopause (Shaw et al., 1979).
In the present study we endeavored to scan our female subjects during the low hormone phase windows as none was scanned during the periovulatory hormonal surge. Nevertheless, it is likely that differences in the chronic hormonal state between men and women contributed to the greater perfusion in women under placebo (Baxter et al., 1987). However, interaction with alcohol is not as easily explained. If higher estrogen levels in women are primarily responsible for the observed gender differences in CBF, one would expect this difference to be even higher under alcohol given that acute alcohol intoxication increases plasma estradiol in healthy women (Mendelson et al., 1988) which, in turn, increases perfusion (Goldman et al., 1976). Instead, a complex interaction of hormonal balance and alcohol metabolism seems to contribute to CBF differences between men and women. Due to their relatively larger liver volume, women metabolize alcohol faster than men (Kwo et al., 1998). Women's faster elimination rate can explain the slightly, though nonsignificantly, lower BAC in women on the descending BAC limb observed in this study. However, the BAC did not correlate with CBF for either gender, r = 0.33 for women and r = −0.09 for men. Given that the ASL scan took place on the descending BAC limb, it is possible that metabolic products of alcohol's breakdown such as acetate contributed to interindividual and gender differences in blood flow by affecting microcirculatory blood vessels. Acetate causes sedation and decrease in motor activity (Correa et al., 2003) and is present in the bloodstream for much longer than alcohol (Hannak et al., 1985), suggesting that it may underlie the sedative effects observed on the descending limb of BAC. By causing vasodilation via adenosine receptors, acetate exerts potent effects on CBF. In a SPECT study (Schwartz et al., 1993), a moderate dose of alcohol (0.6 g/kg), equivalent to the dose used in the present experiment, was administered to healthy men. Whereas the BAC correlated negatively with CBF which increased by 4%, a significant positive correlation was observed between CBF and blood acetate. In that study, the measurements were taken 134 minutes after drinks were consumed, on the descending BAC limb when the effects of acetate were dominant. As a result of the same alcohol dose, we have also observed a significant perfusion increase in young, healthy men by 12.9 % overall (expressed as ((alc-plac)/plac)*100), which was most prevalent in prefrontal areas bilaterally. At the same time, a non-significant alcohol-induced CBF decrease of 4.9 % was observed in women. No gender differences in CBF were observed under intoxication. Thus, our results confirm previous observations using a different method and extend them by reporting a robust interaction between the factors of alcohol and gender.
Given the complexity of the multifactorially determined hemodynamic mechanisms, some other possible routes of affecting perfusion could be considered as possibly contributing to the observed gender and beverage interactive effects. One such possible influence is through respiratory changes. By altering respiration, alcohol could potentially affect CO2, which is known to be an effective vasodilator exerting strong effects on CBF (Birn et al., 2006). However, even rather high alcohol levels do not affect arterial CO2 (Murray et al., 1986), nor the respiratory motor network (Vecchio et al.) as measured in rodents. Since only a moderate alcohol dose was administered in our study, it is unlikely that respiration was affected sufficiently differently in men and women to cause changes in CO2. Another previously suggested factor is the gender-based difference in viscosity of blood (Shaw et al., 1979). Contrary to this hypothesis, however, studies indicate that the CBF is not related to blood viscosity but to arterial oxygen content (Brown and Marshall, 1985). Furthermore, blood viscosity does not appear to be affected even by rather high alcohol dose (1.5 g/kg) (Hillbom et al., 1983). However, changes in cerebral perfusion could derive from sympathetic effects on the cerebral vasculature (Jordan et al., 2000). Recent studies indicate that autonomic vascular autoregulation differs fundamentally between men and women (Hart et al., 2009), which is again attributed to hormonal differences between genders (Maki and Resnick, 2001). Thus, it is clear that regulatory configuration of the hormonal and vascular systems comprise complicated feedback loops. Consequently, the nature of the alcohol's interactions with gonadal hormones on one hand, and the physiological basis of the gender-based differences in CBF on the other, will need to be disentangled in a series of future studies. Finally, even though the CBF was measured during resting, it is possible that different participants engaged in somewhat different types of cognitive or emotional states, increasing the variability in CBF. Esposito and colleagues (Esposito et al., 1996) reported that gender differences in PET-measured perfusion depend on the cognitive task as the largest differences were observed during the most challenging tasks probing frontal functions.
The present results extend and augment the existing evidence indicating associations between personality traits and cerebral blood flow (Ebmeier et al., 1994; O'Gorman et al., 2006). In our study, perfusion under alcohol condition correlated with scores on Psychoticism/Antisocial (P) scale of the EPQ in both genders. The P-scale is taken to represent impulsivity and antisocial tendencies (Hare, 1982). It has been clearly established that personality aspects such as impulsivity and antisocial behavior are strongly related to vulnerability to alcohol addiction (Begleiter and Porjesz, 1999; Muller et al., 2008; Schuckit et al., 2004), with P-scale being a strong prospective predictor of a substance use disorder diagnosis (Sher et al., 2000). By the same token, increased mesolimbic dopaminergic activation may underlie vulnerability to drug abuse (Everitt et al., 2008) and is elicited by acute alcohol intoxication (Gessa et al., 1985; Yoder et al., 2009). A recent study in humans demonstrated that impulsive/antisocial tendencies correlated with amphetamine-induced dopamine release in nucleus accumbens, particularly on the right (Buckholtz et al., 2010). Furthermore, administration of a dopamine agonist increased blood flow in prefrontal areas in a right-dominant fashion (Grasby et al., 1993). Thus, our observation that alcohol-induced increase in blood flow correlates with baseline impulsivity is consistent with dopaminergic mediation of the rewarding aspects of alcohol and concomitant with its effects on cerebral perfusion.
Overall, the greatest overall CBF was observed in the frontal regions bilaterally, in agreement with previous reports (Ingvar, 1976; Prohovnik et al., 1980; Rodriguez et al., 1988; Wilkinson et al., 1969), but see (Hermes et al., 2007; Pfefferbaum et al., 2010). Studies show that this pattern of regional specificity is maintained under normocapnic anaesthesia, but is abolished by hypocapnic anaesthesia (Wilkinson, 1971). Given the vulnerability of frontal lobes to alcohol effects (Oscar-Berman and Marinkovic, 2007) and their fundamental importance in subserving cognitive functions (Miller and Cohen, 2001), it is important to gain better insight into alcohol's effects on the regional CBF differences during resting and cognitive activity. Regionally-specific vascular and metabolic changes exerted by alcohol may be important as markers of cerebral specificity of alcohol-induced vascular changes and could potentially illuminate the physiological basis of strokes and sudden death syndrome in binge drinkers (Altura et al., 1983).
Taken together, evidence suggests that CBF changes may be observed starting at 0.5 g/kg, depending on the time after drinking, gender, type of experimental design and sample size. This also means that, due to alcohol's vasoactive effects, it may not be possible to interpret results of the fMRI-BOLD studies using higher-level acute alcohol intoxication unambiguously. Whereas the fMRI-BOLD method is an excellent mapping tool, its relative magnitude difference may not accurately reflect neural changes due to its sensitivity to vasoactive influences. This issue is particularly important given the increasing prominence of fMRI-BOLD studies in alcohol research on one hand, and a limited understanding of alcohol-induced changes in the physiology underlying BOLD on the other (Brown et al., 2003; Iannetti and Wise, 2007; Tracey, 2001). Furthermore, our results indicate that acute intoxication affects resting cortical perfusion in men but not women on the descending BAC limb. Additional ASL studies with larger samples, different alcohol doses and measurements at different points after drinking are needed in order to sort out the effects of alcohol intoxication and gender as they pertain to effects of alcohol and its metabolites, hormonal dynamics, and differences in hemodynamic autoregulation. Future studies will also need to examine whether these results can be generalized across different age groups given significant age-related decrease in cortical perfusion (Bangen et al., 2009; Parkes et al., 2004), as well as age-dependent effects of alcohol on brain function (Oscar-Berman and Marinkovic, 2007).
Furthermore, additional studies are needed to provide a more exact assessment of the relationship between the essential hemodynamic factors and the BOLD signal under intoxication, disambiguating the vascular/metabolic mechanisms underlying the BOLD signal from the neurally-based changes. Such studies would mitigate interpretational confounds of non-neural origin and would insure interpretability of the alcohol-induced effects on the BOLD signal (Ances et al., 2008; Hyder, 2004; Liu and Brown, 2007; Perthen et al., 2008). Due to its superior signal-to-noise ratio, higher spatial and temporal resolution and better brain coverage, the BOLD signal has been the method of choice in neuroimaging studies (Brown et al., 2007; Buxton, 2002). Although the BOLD method is an excellent mapping tool, interpreting the magnitude changes as proportionally reflecting neural events is inherently ambiguous (Leontiev et al., 2007). In contrast, the ASL provides quantification of the blood flow and it may be a more faithful index of the functional activity than BOLD due to its sensitivity to changes in capillary bed (Lee et al., 2001). It is sensitive, however, to the factors influencing cerebrovascular structure such as aging or disease (D'Esposito et al., 2003) that result in increased variation in transit times (Buxton et al., 1998a). Employing these two complementary methods synergistically provides an opportunity to discern vascular from neurally-based changes underlying BOLD. More specifically, dual echo acquisition allows simultaneous measurement of the CBF and BOLD signals (Wong et al., 1997). Cerebral metabolic rate of oxygen use (CMRO2), which is coupled with neural activity (Hyder, 2004), can further be derived with the addition of simultaneous measures of CBF and BOLD during mild hypercapnic (increased arterial CO2) manipulation. This "calibrated BOLD" method (Davis et al., 1998) relies on the observation that ASL reflects changes in CBF while the BOLD is sensitive to changes in both CBF and CMRO2. Mild hypercapnia increases CBF but not CMRO2, effectively providing scaling (calibration) for the BOLD. Estimates of local CMRO2 reflecting neural activity are based on simultaneous measurements of the BOLD and CBF responses within the Davis' model. Consequently, these measures provide excellent insight into the physiological factors underlying BOLD and a way to deconvolve vascular confounds from neural activity (Buxton et al., 2004; Buxton et al., 1998b; Liu and Brown, 2007).
In sum, our results indicate that cortical perfusion is affected differently in men versus women by moderate alcohol intoxication on a descending BAC limb as alcohol-induced CBF increase was observed bilaterally in frontal regions in men only. Under placebo, greater perfusion was observed in women compared to men, confirming previous robust evidence of this effect. These gender-based differences may be due to a complex and possibly interactive set of factors influencing hormonal, metabolic, and hemodynamic autoregulatory systems in the context of alcohol intoxication. Additional ASL studies are needed to investigate the interactive dose-dependent effects of alcohol intoxication and gender and to disambiguate the vascular/metabolic mechanisms underlying the BOLD signal from the neurally-based changes. Taken together with previous PET and SPECT studies, our results support the feasibility of fMRI-BOLD at low levels of alcohol intoxication.
Acknowledgments
This work was supported by funds from the National Institutes of Health (R01-AA016624 and P41RR14075) and Medical Investigation of Neurodevelopmental Disorders (MIND) Institute. The study was carried out at Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Boston. We thank Elinor Artsy for help with data collection.
References
- Altura BM, Altura BT, Carella A. Ethanol produces coronary vasospasm: evidence for a direct action of ethanol on vascular muscle. Br J Pharmacol. 1983;78:260–262. doi: 10.1111/j.1476-5381.1983.tb09389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ances BM, Leontiev O, Perthen JE, Liang C, Lansing AE, Buxton RB. Regional differences in the coupling of cerebral blood flow and oxygen metabolism changes in response to activation: implications for BOLD-fMRI. Neuroimage. 2008;39:1510–1521. doi: 10.1016/j.neuroimage.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangen KJ, Restom K, Liu TT, Jak AJ, Wierenga CE, Salmon DP, Bondi MW. Differential age effects on cerebral blood flow and BOLD response to encoding: associations with cognition and stroke risk. Neurobiol Aging. 2009;30:1276–1287. doi: 10.1016/j.neurobiolaging.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter LR, Jr., Mazziotta JC, Phelps ME, Selin CE, Guze BH, Fairbanks L. Cerebral glucose metabolic rates in normal human females versus normal males. Psychiatry Res. 1987;21:237–245. doi: 10.1016/0165-1781(87)90028-x. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B. What is inherited in the predisposition toward alcoholism? A proposed model [see comments] Alcohol Clin Exp Res. 1999;23:1125–1135. doi: 10.1111/j.1530-0277.1999.tb04269.x. [DOI] [PubMed] [Google Scholar]
- Berman KF, Schmidt PJ, Rubinow DR, Danaceau MA, Van Horn JD, Esposito G, Ostrem JL, Weinberger DR. Modulation of cognition-specific cortical activity by gonadal steroids: a positron-emission tomography study in women. Proc Natl Acad Sci U S A. 1997;94:8836–8841. doi: 10.1073/pnas.94.16.8836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Diamond JB, Smith MA, Bandettini PA. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. Neuroimage. 2006;31:1536–1548. doi: 10.1016/j.neuroimage.2006.02.048. [DOI] [PubMed] [Google Scholar]
- Blaha M, Aaslid R, Douville CM, Correra R, Newell DW. Cerebral blood flow and dynamic cerebral autoregulation during ethanol intoxication and hypercapnia. J Clin Neurosci. 2003;10:195–198. doi: 10.1016/s0967-5868(02)00126-1. [DOI] [PubMed] [Google Scholar]
- Bragulat V, Dzemidzic M, Talavage T, Davidson D, O'Connor SJ, Kareken DA. Alcohol sensitizes cerebral responses to the odors of alcoholic drinks: an fMRI study. Alcohol Clin Exp Res. 2008;32:1124–1134. doi: 10.1111/j.1530-0277.2008.00693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GG, Eyler Zorrilla LT, Georgy B, Kindermann SS, Wong EC, Buxton RB. BOLD and perfusion response to finger-thumb apposition after acetazolamide administration: differential relationship to global perfusion. J Cereb Blood Flow Metab. 2003;23:829–837. doi: 10.1097/01.WCB.0000071887.63724.B2. [DOI] [PubMed] [Google Scholar]
- Brown GG, Perthen JE, Liu TT, Buxton RB. A primer on functional magnetic resonance imaging. Neuropsychol Rev. 2007;17:107–125. doi: 10.1007/s11065-007-9028-8. [DOI] [PubMed] [Google Scholar]
- Brown MM, Marshall J. Regulation of cerebral blood flow in response to changes in blood viscosity. Lancet. 1985;1:604–609. doi: 10.1016/s0140-6736(85)92145-2. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Benning SD, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Shelby ES, et al. Mesolimbic dopamine reward system hypersensitivity in individuals with psychopathic traits. Nat Neurosci. 2010;13:419–421. doi: 10.1038/nn.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton RB. Introduction to Functional Magnetic Resonance Imaging. New York, NY: Cambridge University Press; 2002. [Google Scholar]
- Buxton RB, Frank LR, Wong EC, Siewert B, Warach S, Edelman RR. A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn Reson Med. 1998a;40:383–396. doi: 10.1002/mrm.1910400308. [DOI] [PubMed] [Google Scholar]
- Buxton RB, Uludag K, Dubowitz DJ, Liu TT. Modeling the hemodynamic response to brain activation. Neuroimage. 2004;1(23 Suppl):S220–S233. doi: 10.1016/j.neuroimage.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Buxton RB, Wong EC, Frank LR. Dynamics of blood flow and oxygenation changes during brain activation: the balloon model. Magn Reson Med. 1998b;39:855–864. doi: 10.1002/mrm.1910390602. [DOI] [PubMed] [Google Scholar]
- Cahalan D, Cisin IH, Crossley HM. Monograph #6. Rutgers Center of Alcohol Studies. New Brunswick: NJ; 1969. American drinking practices: A national study of drinking behavior and attitudes. [Google Scholar]
- Calamante F, Thomas DL, Pell GS, Wiersma J, Turner R. Measuring cerebral blood flow using magnetic resonance imaging techniques. J Cereb Blood Flow Metab. 1999;19:701–735. doi: 10.1097/00004647-199907000-00001. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Pekar JJ, Pearlson GD. Alcohol intoxication effects on simulated driving: exploring alcohol-dose effects on brain activation using functional MRI. Neuropsychopharmacology. 2004;29:2097–2017. doi: 10.1038/sj.npp.1300543. [DOI] [PubMed] [Google Scholar]
- Chen CM, Dufour MC, Yi H. Alcohol consumption among young adults ages 18–24 in the United States: Results from the 2001–2002 NESARC survey. Alcohol Research and Health. 2004/2005;28:269–280. [Google Scholar]
- Clark CP, Brown GG, Eyler LT, Drummond SP, Braun DR, Tapert SF. Decreased perfusion in young alcohol-dependent women as compared with age-matched controls. Am J Drug Alcohol Abuse. 2007;33:13–19. doi: 10.1080/00952990601082605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DL, Holmes CJ, Peters TM, Evans AC. Automatic 3-D model-based neuroanatomical segmentation. Hum Brain Mapp. 1995;3:190–208. [Google Scholar]
- Correa M, Arizzi MN, Betz A, Mingote S, Salamone JD. Open field locomotor effects in rats after intraventricular injections of ethanol and the ethanol metabolites acetaldehyde and acetate. Brain Res Bull. 2003;62:197–202. doi: 10.1016/j.brainresbull.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Deouell LY, Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nat Rev Neurosci. 2003;4:863–872. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Daniel DG, Mathew RJ, Wilson WH. Sex roles and regional cerebral blood flow. Psychiatry Res. 1989;27:55–64. doi: 10.1016/0165-1781(89)90009-7. [DOI] [PubMed] [Google Scholar]
- Davis TL, Kwong KK, Weisskoff RM, Rosen BR. Calibrated functional MRI: mapping the dynamics of oxidative metabolism. Proc Natl Acad Sci U S A. 1998;95:1834–1839. doi: 10.1073/pnas.95.4.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Detre JA, Alsop DC. Perfusion magnetic resonance imaging with continuous arterial spin labeling: methods and clinical applications in the central nervous system. Eur J Radiol. 1999;30:115–124. doi: 10.1016/s0720-048x(99)00050-9. [DOI] [PubMed] [Google Scholar]
- Detre JA, Wang J, Wang Z, Rao H. Arterial spin-labeled perfusion MRI in basic and clinical neuroscience. Curr Opin Neurol. 2009;22:348–355. doi: 10.1097/WCO.0b013e32832d9505. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Mon A, Meyerhoff DJ. Cortical perfusion in alcohol-dependent individuals during short-term abstinence: relationships to resumption of hazardous drinking after treatment. Alcohol. 2010;44:201–210. doi: 10.1016/j.alcohol.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebmeier KP, Deary IJ, Ocarroll RE, Prentice N, Moffoot APR, Goodwin GM. Personality Associations with the Uptake of the Cerebral Blood-Flow Marker (99m)Tc-Exametazime Estimated with Single-Photon Emission Tomography. Pers Indiv Differ. 1994;17:587–595. [Google Scholar]
- Esposito G, Van Horn JD, Weinberger DR, Berman KF. Gender differences in cerebral blood flow as a function of cognitive state with PET. J Nucl Med. 1996;37:559–564. [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck HJ, Eysenck SBG. Manual of the Eysenck Personality Questionnaire. London: Hodder & Staughton; 1975. [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999a;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999b;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Friel PN, Logan BK, O'Malley D, Baer JS. Development of dosing guidelines for reaching selected target breath alcohol concentrations. J Stud Alcohol. 1999;60:555–565. doi: 10.15288/jsa.1999.60.555. [DOI] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo T, Jahng GH, Ezekiel F, Banys P, Meyerhoff D. Effects of chronic alcohol dependence and chronic cigarette smoking on cerebral perfusion: a preliminary magnetic resonance study. Alcohol Clin Exp Res. 2006;30:947–958. doi: 10.1111/j.1530-0277.2006.00108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessa GL, Muntoni F, Collu M, Vargiu L, Mereu G. Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res. 1985;348:201–203. doi: 10.1016/0006-8993(85)90381-6. [DOI] [PubMed] [Google Scholar]
- Gilman JM, Ramchandani VA, Davis MB, Bjork JM, Hommer DW. Why we like to drink: a functional magnetic resonance imaging study of the rewarding and anxiolytic effects of alcohol. J Neurosci. 2008;28:4583–4591. doi: 10.1523/JNEUROSCI.0086-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golay X, Hendrikse J, Lim TC. Perfusion imaging using arterial spin labeling. Top Magn Reson Imaging. 2004;15:10–27. doi: 10.1097/00002142-200402000-00003. [DOI] [PubMed] [Google Scholar]
- Goldman H, Skelley EB, Sandman CA, Kastin AJ, Murphy S. Hormones and regional brain blood flow. Pharmacol Biochem Behav. 1976;5:165–169. doi: 10.1016/0091-3057(76)90347-6. [DOI] [PubMed] [Google Scholar]
- Gollub RL, Breiter HC, Kantor H, Kennedy D, Gastfriend D, Mathew RT, Makris N, Guimaraes A, Riorden J, Campbell T, et al. Cocaine decreases cortical cerebral blood flow but does not obscure regional activation in functional magnetic resonance imaging in human subjects. J Cereb Blood Flow Metab. 1998;18:724–734. doi: 10.1097/00004647-199807000-00003. [DOI] [PubMed] [Google Scholar]
- Grasby PM, Friston KJ, Bench CJ, Cowen PJ, Frith CD, Liddle PF, Frackowiak RS, Dolan RJ. The effect of the dopamine agonist, apomorphine, on regional cerebral blood flow in normal volunteers. Psychol Med. 1993;23:605–612. doi: 10.1017/s0033291700025381. [DOI] [PubMed] [Google Scholar]
- Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage. 2009;48:63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen H, Gruner R, Specht K, Hugdahl K. The effects of alcohol intoxication on neuronal activation at different levels of cognitive load. Open Neuroimag J. 2008;2:65–72. doi: 10.2174/1874440000802010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannak D, Bartelt U, Kattermann R. Acetate formation after short-term ethanol administration in man. Biol Chem Hoppe Seyler. 1985;366:749–753. doi: 10.1515/bchm3.1985.366.2.749. [DOI] [PubMed] [Google Scholar]
- Hare RD. Psychopathy and the Personality Dimensions of Psychoticism, Extraversion and Neuroticism. Pers Indiv Differ. 1982;3:35–42. [Google Scholar]
- Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach JH, Joyner MJ. Sex differences in sympathetic neural-hemodynamic balance: implications for human blood pressure regulation. Hypertension. 2009;53:571–576. doi: 10.1161/HYPERTENSIONAHA.108.126391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermes M, Hagemann D, Britz P, Lieser S, Rock J, Naumann E, Walter C. Reproducibility of continuous arterial spin labeling perfusion MRI after 7 weeks. MAGMA. 2007;20:103–115. doi: 10.1007/s10334-007-0073-3. [DOI] [PubMed] [Google Scholar]
- Hillbom ME, Kaste M, Tarssanen L, Johnsson R. Effect of ethanol on blood viscosity and erythrocyte flexibility in healthy men. Eur J Clin Invest. 1983;13:45–48. doi: 10.1111/j.1365-2362.1983.tb00063.x. [DOI] [PubMed] [Google Scholar]
- Hyder F. Neuroimaging with calibrated FMRI. Stroke. 2004;35:2635–2641. doi: 10.1161/01.STR.0000143324.31408.db. [DOI] [PubMed] [Google Scholar]
- Iannetti GD, Wise RG. BOLD functional MRI in disease and pharmacological studies: room for improvement? Magn Reson Imaging. 2007;25:978–988. doi: 10.1016/j.mri.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Ingvar DH. Functional landscapes of the dominant hemisphere. Brain Res. 1976;107:181–197. doi: 10.1016/0006-8993(76)90109-8. [DOI] [PubMed] [Google Scholar]
- Ingvar M, Ghatan PH, Wirsen-Meurling A, Risberg J, Von Heijne G, Stone-Elander S, Ingvar DH. Alcohol activates the cerebral reward system in man. J Stud Alcohol. 1998;59:258–269. doi: 10.15288/jsa.1998.59.258. [DOI] [PubMed] [Google Scholar]
- Jordan J, Shannon JR, Diedrich A, Black B, Costa F, Robertson D, Biaggioni I. Interaction of carbon dioxide and sympathetic nervous system activity in the regulation of cerebral perfusion in humans. Hypertension. 2000;36:383–388. doi: 10.1161/01.hyp.36.3.383. [DOI] [PubMed] [Google Scholar]
- Kim SG. Quantification of relative cerebral blood flow change by flow-sensitive alternating inversion recovery (FAIR) technique: application to functional mapping. Magn Reson Med. 1995;34:293–301. doi: 10.1002/mrm.1910340303. [DOI] [PubMed] [Google Scholar]
- Kwo PY, Ramchandani VA, O'Connor S, Amann D, Carr LG, Sandrasegaran K, Kopecky KK, Li TK. Gender differences in alcohol metabolism: relationship to liver volume and effect of adjusting for body mass. Gastroenterology. 1998;115:1552–1557. doi: 10.1016/s0016-5085(98)70035-6. [DOI] [PubMed] [Google Scholar]
- Kwong KK, Chesler DA, Weisskoff RM, Donahue KM, Davis TL, Ostergaard L, Campbell TA, Rosen BR. MR perfusion studies with T1-weighted echo planar imaging. Magn Reson Med. 1995;34:878–887. doi: 10.1002/mrm.1910340613. [DOI] [PubMed] [Google Scholar]
- Lee SP, Duong TQ, Yang G, Iadecola C, Kim SG. Relative changes of cerebral arterial and venous blood volumes during increased cerebral blood flow: implications for BOLD fMRI. Magn Reson Med. 2001;45:791–800. doi: 10.1002/mrm.1107. [DOI] [PubMed] [Google Scholar]
- Leontiev O, Dubowitz DJ, Buxton RB. CBF/CMRO2 coupling measured with calibrated BOLD fMRI: sources of bias. Neuroimage. 2007;36:1110–1122. doi: 10.1016/j.neuroimage.2006.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TT, Brown GG. Measurement of cerebral perfusion with arterial spin labeling: Part 1. Methods. J Int Neuropsychol Soc. 2007;13:517–525. doi: 10.1017/S1355617707070646. [DOI] [PubMed] [Google Scholar]
- Maki PM, Resnick SM. Effects of estrogen on patterns of brain activity at rest and during cognitive activity: a review of neuroimaging studies. Neuroimage. 2001;14:789–801. doi: 10.1006/nimg.2001.0887. [DOI] [PubMed] [Google Scholar]
- Marinkovic K, Halgren E, Maltzman I. Arousal-related P3a to novel auditory stimuli is abolished by moderately low alcohol dose. Alcohol and Alcoholism. 2001;36:529–539. doi: 10.1093/alcalc/36.6.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew RJ, Wilson WH. Regional cerebral blood flow changes associated with ethanol intoxication. Stroke. 1986;17:1156–1159. doi: 10.1161/01.str.17.6.1156. [DOI] [PubMed] [Google Scholar]
- Mathew RJ, Wilson WH. Substance abuse and cerebral blood flow. Am J Psychiatry. 1991;148:292–305. doi: 10.1176/ajp.148.3.292. [DOI] [PubMed] [Google Scholar]
- Mathew RJ, Wilson WH, Tant SR. Determinants of resting regional cerebral blood flow in normal subjects. Biol Psychiatry. 1986;21:907–914. doi: 10.1016/0006-3223(86)90264-7. [DOI] [PubMed] [Google Scholar]
- Meda SA, Calhoun VD, Astur RS, Turner BM, Ruopp K, Pearlson GD. Alcohol dose effects on brain circuits during simulated driving: an fMRI study. Hum Brain Mapp. 2009;30:1257–1270. doi: 10.1002/hbm.20591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson JH, Lukas SE, Mello NK, Amass L, Ellingboe J, Skupny A. Acute alcohol effects on plasma estradiol levels in women. Psychopharmacology (Berl) 1988;94:464–467. doi: 10.1007/BF00212838. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mon A, Durazzo TC, Gazdzinski S, Meyerhoff DJ. The impact of chronic cigarette smoking on recovery from cortical gray matter perfusion deficits in alcohol dependence: longitudinal arterial spin labeling MRI. Alcohol Clin Exp Res. 2009;33:1314–1321. doi: 10.1111/j.1530-0277.2009.00960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller SE, Weijers HG, Boning J, Wiesbeck GA. Personality traits predict treatment outcome in alcohol-dependent patients. Neuropsychobiology. 2008;57:159–164. doi: 10.1159/000147469. [DOI] [PubMed] [Google Scholar]
- Murray KA, White WJ, Zagon IS. Ethanol exposure in rats: studies on blood gas concentrations, pH and temperature. Alcohol. 1986;3:5–10. doi: 10.1016/0741-8329(86)90063-7. [DOI] [PubMed] [Google Scholar]
- Newlin DB, Golden CJ, Quaife M, Graber B. Effect of alcohol ingestion on regional cerebral blood flow. Int J Neurosci. 1982;17:145–150. doi: 10.3109/00207458208985916. [DOI] [PubMed] [Google Scholar]
- Nichols JM, Martin F. The effect of heavy social drinking on recall and event-related potentials. J Stud Alcohol. 1996;57:125–135. doi: 10.15288/jsa.1996.57.125. [DOI] [PubMed] [Google Scholar]
- O'Gorman RL, Kumari V, Williams SC, Zelaya FO, Connor SE, Alsop DC, Gray JA. Personality factors correlate with regional cerebral perfusion. Neuroimage. 2006;31:489–495. doi: 10.1016/j.neuroimage.2005.12.048. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M, Marinkovic K. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol Rev. 2007;17:239–257. doi: 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes LM, Rashid W, Chard DT, Tofts PS. Normal cerebral perfusion measurements using arterial spin labeling: reproducibility, stability, and age and gender effects. Magn Reson Med. 2004;51:736–743. doi: 10.1002/mrm.20023. [DOI] [PubMed] [Google Scholar]
- Parsons OA, Nixon SJ. Cognitive functioning in sober social drinkers: a review of the research since 1986. J Stud Alcohol. 1998;59:180–190. doi: 10.15288/jsa.1998.59.180. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Pulido C, Schuckit MA. Alcohol attenuates load-related activation during a working memory task: relation to level of response to alcohol. Alcohol Clin Exp Res. 2006;30:1363–1371. doi: 10.1111/j.1530-0277.2006.00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perthen JE, Lansing AE, Liau J, Liu TT, Buxton RB. Caffeine-induced uncoupling of cerebral blood flow and oxygen metabolism: a calibrated BOLD fMRI study. Neuroimage. 2008;40:237–247. doi: 10.1016/j.neuroimage.2007.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Chanraud S, Pitel AL, Shankaranarayanan A, Alsop DC, Rohlfing T, Sullivan EV. Volumetric cerebral perfusion imaging in healthy adults: regional distribution, laterality, and repeatability of pulsed continuous arterial spin labeling (PCASL) Psychiatry Res. 2010;182:266–273. doi: 10.1016/j.pscychresns.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prohovnik I, Hakansson K, Risberg J. Observations on the functional significance of regional cerebral blood flow in "resting" normal subjects. Neuropsychologia. 1980;18:203–217. doi: 10.1016/0028-3932(80)90066-4. [DOI] [PubMed] [Google Scholar]
- Rodriguez G, Warkentin S, Risberg J, Rosadini G. Sex differences in regional cerebral blood flow. J Cereb Blood Flow Metab. 1988;8:783–789. doi: 10.1038/jcbfm.1988.133. [DOI] [PubMed] [Google Scholar]
- Sano M, Wendt PE, Wirsen A, Stenberg G, Risberg J, Ingvar DH. Acute effects of alcohol on regional cerebral blood flow in man. J Stud Alcohol. 1993;54:369–376. doi: 10.15288/jsa.1993.54.369. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Kalmijn J. The search for genes contributing to the low level of response to alcohol: patterns of findings across studies. Alcohol Clin Exp Res. 2004;28:1449–1458. doi: 10.1097/01.alc.0000141637.01925.f6. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Tipp JE. The Self-Rating of the Effects of alcohol (SRE) form as a retrospective measure of the risk for alcoholism. Addiction. 1997;92:979–988. [PubMed] [Google Scholar]
- Schwartz JA, Speed NM, Gross MD, Lucey MR, Bazakis AM, Hariharan M, Beresford TP. Acute effects of alcohol administration on regional cerebral blood flow: the role of acetate. Alcohol Clin Exp Res. 1993;17:1119–1123. doi: 10.1111/j.1530-0277.1993.tb05217.x. [DOI] [PubMed] [Google Scholar]
- Selzer ML, Vinokur A, Van Rooijen L. A self-administered Short Michigan Alcoholism Screening Test (SMAST) Journal of Studies on Alcohol. 1975;36:117–126. doi: 10.15288/jsa.1975.36.117. [DOI] [PubMed] [Google Scholar]
- Shamma FN, Fayad P, Brass L, Sarrel P. Middle cerebral artery blood velocity during controlled ovarian hyperstimulation. Fertil Steril. 1992;57:1022–1025. [PubMed] [Google Scholar]
- Shaw T, Meyer JS, Mortel K, Cutaia M, Sakai F, Yamaguchi F, Yamamoto M. Effects of normal aging, sex and risk factors for stroke on regional cerebral blood flow (rCBF) in normal volunteers. Acta Neurologica Scandinavica. 1979;60:462–463. [Google Scholar]
- Sher KJ, Bartholow BD, Wood MD. Personality and substance use disorders: a prospective study. J Consult Clin Psychol. 2000;68:818–829. [PubMed] [Google Scholar]
- Shin W, Horowitz S, Ragin A, Chen Y, Walker M, Carroll TJ. Quantitative cerebral perfusion using dynamic susceptibility contrast MRI: evaluation of reproducibility and age- and gender-dependence with fully automatic image postprocessing algorithm. Magn Reson Med. 2007;58:1232–1241. doi: 10.1002/mrm.21420. [DOI] [PubMed] [Google Scholar]
- Smith A. Effects of caffeine on human behavior. Food Chem Toxicol. 2002;40:1243–1255. doi: 10.1016/s0278-6915(02)00096-0. [DOI] [PubMed] [Google Scholar]
- Stendel R, Irnich B, al Hassan AA, Heidenreich J, Pietilae T. The influence of ethanol on blood flow velocity in major cerebral vessels. A prospective and controlled study. Alcohol. 2006;38:139–146. doi: 10.1016/j.alcohol.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Kuikka J, Hakola P, Paanila J, Airaksinen J, Eronen M, Hallikainen T. Acute ethanol-induced changes in cerebral blood flow. Am J Psychiatry. 1994;151:1505–1508. doi: 10.1176/ajp.151.10.1505. [DOI] [PubMed] [Google Scholar]
- Tracey I. Prospects for human pharmacological functional magnetic resonance imaging (phMRI) J Clin Pharmacol. 2001;41:21S–28S. [PubMed] [Google Scholar]
- Vecchio LM, Grace KP, Liu H, Harding S, Le AD, Horner RL. State-dependent vs. central motor effects of ethanol on breathing. J Appl Physiol. 2010;108:387–400. doi: 10.1152/japplphysiol.00797.2009. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Mullani N, Gould L, Adler SS, Guynn RW, Overall JE, Dewey S. Effects of acute alcohol intoxication on cerebral blood flow measured with PET. Psychiatry Res. 1988;24:201–209. doi: 10.1016/0165-1781(88)90063-7. [DOI] [PubMed] [Google Scholar]
- Wang J, Licht DJ, Jahng GH, Liu CS, Rubin JT, Haselgrove J, Zimmerman RA, Detre JA. Pediatric perfusion imaging using pulsed arterial spin labeling. J Magn Reson Imaging. 2003;18:404–413. doi: 10.1002/jmri.10372. [DOI] [PubMed] [Google Scholar]
- Wang Y, Saykin AJ, Pfeuffer J, Lin C, Mosier KM, Shen L, Kim S, Hutchins GD. Regional reproducibility of pulsed arterial spin labeling perfusion imaging at 3T. Neuroimage. 2011;54:1188–1195. doi: 10.1016/j.neuroimage.2010.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson IM. Regional blood flow in the human cerebral hemisphere during general anaesthesia, studied at normal and at reduced levels of arterial PCO2. Proc R Soc Med. 1971;64:80–82. doi: 10.1177/003591577106400148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson IM, Bull JW, Duboulay GH, Marshall J, Russell RW, Symon L. Regional blood flow in the normal cerebral hemisphere. J Neurol Neurosurg Psychiatry. 1969;32:367–378. doi: 10.1136/jnnp.32.5.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EC, Buxton RB, Frank LR. Implementation of quantitative perfusion imaging techniques for functional brain mapping using pulsed arterial spin labeling. NMR Biomed. 1997;10:237–249. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<237::aid-nbm475>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Wong EC, Buxton RB, Frank LR. Quantitative imaging of perfusion using a single subtraction (QUIPSS and QUIPSS II) Magn Reson Med. 1998;39:702–708. doi: 10.1002/mrm.1910390506. [DOI] [PubMed] [Google Scholar]
- Wong EC, Buxton RB, Frank LR. Quantitative perfusion imaging using arterial spin labeling. Neuroimaging Clin N Am. 1999;9:333–342. [PubMed] [Google Scholar]
- Woodward JA, Bonett DG, Brecht ML. Introduction to linear models and experimental design. San Diego: Harcourt Brace Jovanovich; 1990. [Google Scholar]
- Yoder KK, Morris ED, Constantinescu CC, Cheng TE, Normandin MD, O'Connor SJ, Kareken DA. When what you see isn't what you get: alcohol cues, alcohol administration, prediction error, and human striatal dopamine. Alcohol Clin Exp Res. 2009;33:139–149. doi: 10.1111/j.1530-0277.2008.00821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M. Dimensions of sensation seeking. Journal of Consulting and Clinical Psychology. 1971;36:45–52. [Google Scholar]