Abstract
Purpose.
While the role of the macular pigment carotenoids in the prevention of age-related macular degeneration has been extensively studied in adults, comparatively little is known about the physiology and function of lutein and zeaxanthin in the developing eye. We therefore developed a protocol using a digital video fundus camera (RetCam) to measure macular pigment optical density (MPOD) and distributions in premature infants and in children.
Methods.
We used blue light reflectance to image the macular pigment in premature babies at the time of retinopathy of prematurity (ROP) screening and in children aged under 7 years who were undergoing examinations under anesthesia for other reasons. We correlated the MPOD with skin carotenoid levels measured by resonance Raman spectroscopy, serum carotenoids measured by HPLC, and dietary carotenoid intake.
Results.
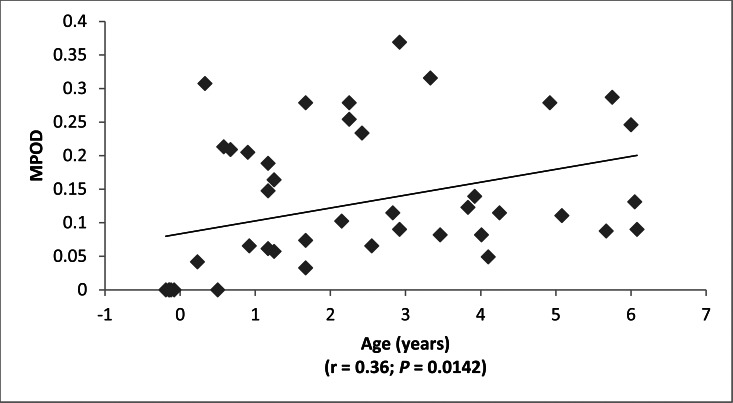
We enrolled 51 infants and children ranging from preterm to age 7 years. MPOD correlated significantly with age (r = 0.36; P = 0.0142), with serum lutein + zeaxanthin (r = 0.44; P = 0.0049) and with skin carotenoid levels (r = 0.42; P = 0.0106), but not with dietary lutein + zeaxanthin intake (r = 0.13; P = 0.50). All premature infants had undetectable macular pigment, and most had unusually low serum and skin carotenoid concentrations.
Conclusions.
Our most remarkable finding is the undetectable MPOD in premature infants. This may be due in part to foveal immaturity, but the very low levels of serum and skin carotenoids suggest that these infants are carotenoid insufficient as a consequence of low dietary intake and/or severe oxidative stress. The potential value of carotenoid supplementation in the prevention of ROP and other disorders of prematurity should be a fruitful direction for further investigation.
Keywords: macular pigment, carotenoid, imaging, lutein, zeaxanthin
Little is known about the physiology and function of lutein and zeaxanthin in the developing eye. We therefore developed a protocol using a digital fundus camera (RetCam) to measure macular pigment optical density (MPOD) and distributions in premature infants and in children.
Introduction
Highly specific deposition of yellow carotenoid pigments in the macula of the human eye has long been recognized as a fundamental anatomical feature of the primate foveal region.1 While most clinical research has focused on the potential roles of lutein and zeaxanthin in preventing AMD,2–4 much less is known about their function and physiology in the infant's eye. This is due, in part, to the fact that the most commonly used methods to assess macular pigment optical density (MPOD), such as heterochromatic flicker photometry (HFP) and autofluorescence imaging (AFI),5,6 are totally unsuitable for infants and young children because of their inability to participate in the required psychophysical testing and their lack of significant lipofuscin in their RPE. It is clear, however, that these dietary compounds and their metabolites are found in the eye from a very early age by HPLC analysis of autopsy eyes.7 The macular pigment carotenoids could play very important functions in foveal development, enhancement of infant visual acuity, or protection against light-induced oxidative damage.8 But without knowledge of normal macular pigment levels and distributions in infants and children, further progress on these potential functions cannot proceed. In this study, we have developed a noninvasive method to measure peak MPOD and to image macular pigment distributions in infants and children using an FDA-cleared instrument specifically designed to image the infant eye (RetCam; Clarity Medical Systems, Inc., Pleasanton, CA). We then used this technique to measure MPOD in children ranging from premature infants to children aged up to 7 years.
Methods
Human Subjects
Fifty-one infants and children were enrolled in this study with a goal to have a wide distribution of ages ranging from prematurity up to age 7 years. Parental consent was obtained in all cases under an institutional review board–approved protocol that complied with the Declaration of Helsinki. Premature infants were imaged with 0.2% cyclopentolate hydrochloride (HCl) and 1% phenylephrine HCl (Cyclomydril; Alcon, Fort Worth, TX) pupil dilation at the time of scheduled retinopathy of prematurity (ROP) screening. Full-term infants and children had macular pigment imaging performed at the time of examinations under anesthesia (EUAs) scheduled for other medical reasons. These older subjects had their pupils dilated according to the standard of care for their age, typically 2.5% phenylephrine HCl and 1% cyclopentolate HCl. Demographic information including age, sex, race and ethnicity, and ocular and systemic diagnoses was collected at the time of enrollment. For premature infants, the age at the time of measurement was reported relative to the child's predicted full-term 40-week postmenstrual birth date, which means that these infants have a negative age in the tables and graphs.
Macular Pigment Measurement
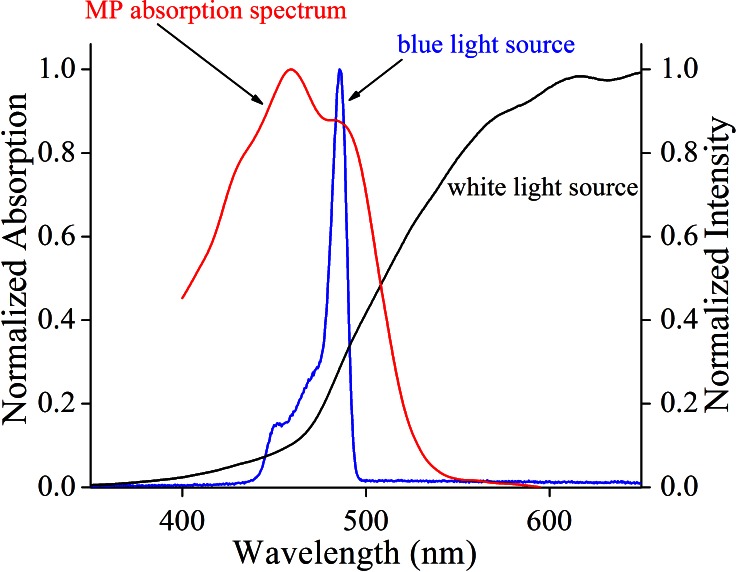
Video capture images from each eye centered on the fovea were collected on a digital fundus camera (RetCam II or RetCam 3; Clarity Medical Systems, Inc.) using its optional blue-light fluorescein angiography light source and an 80° collection lens. Since this is a reflectance method of macular pigment measurement, the matched 510-nm barrier emission filter for fluorescein angiography was purposely omitted. Illumination intensity for the blue light source and the detector gain sensitivity were set at midrange on the instrument's dials and adjusted as necessary to produce usable images. Typical total measurement times were less than 1 minute per eye, well below the 5-minute safety limit for the RetCam's (Clarity Medical Systems, Inc.) blue-light source.9 The best quality blue-light reflectance images (sharp focus and uniform background illumination) were downloaded and transferred to Java-based imaging software (ImageJ; National Institutes of Health [NIH], Bethesda, MD) where the blue-light channel data were converted to grayscale images. The average pixel intensity at designated background regions of the image (Imax) divided by pixel intensity at the fovea (Imin) was then used to calculate peak MPOD using Equation 1, which assumed a double-pass through the macular pigment after reflection from the sclera. A scaling factor, a, was empirically determined to be 1.15 to account for the absorbance of the macular pigment at 480 to 485 nm, the peak wavelength range of the blue-light source measured on two different RetCams (Clarity Medical Systems, Inc.) with a high resolution spectrometer (HR2000+; Ocean Optics, Dunedin, FL), relative to the macular pigment's peak absorbance at 460 nm derived from a published absorption spectrum of the primate macular pigment (Fig. 1).10 In this reflectance model, it is assumed that the media anterior to the retinal surface are optically clear and low in light scattering and that melanin pigmentation of the RPE/choroid is uniform throughout the posterior pole. Two-dimensional and three-dimensional macular pigment plot profiles could also be obtained using the Java-based imaging software (ImageJ; NIH).
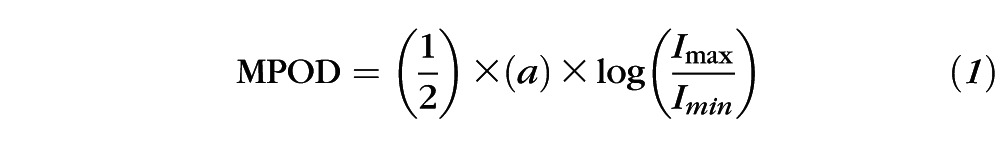 |
Figure 1.
Spectroscopic principles underlying macular pigment measurement with the RetCam. The normalized absorption spectrum of primate macular pigment as reported by Snodderly et al.10 was plotted versus the normalized emission spectra of the RetCam's blue-light and white-light sources as measured by a commercial spectrometer. A correction factor of 1.15 (a) is employed in Equation 1 to compensate for the lower absorbance of MP at the peak of the fundus camera's 480- to 485-nm blue light source relative to the macular pigment's peak absorbance at 460 nm.
Skin Carotenoid Measurement
We measured skin carotenoid levels noninvasively using resonance Raman spectroscopy on the heel of each subject's foot. This method is ideal for use in infants and young children because it is quick and painless and has been extensively validated as a noninvasive biomarker of tissue and serum carotenoid levels.11–13 Briefly, 10-mW, 488-nm low-intensity blue laser light is focused as a 2-mm spot for 30 seconds on the child's skin using a specially designed handheld probe that contains the light delivery and collection optics. The collected backscattered light was analyzed by a custom-built Raman spectrograph. The intensity of the characteristic C = C stretch band at 1525 cm−1 was measured after correcting for background skin fluorescence. Signal intensity is reported in arbitrary units (Raman counts) after calibration at each session against an external industrial diamond calibration standard.
Serum and Milk Carotenoid Measurements
Blood samples (1–5 mL) were obtained at the time of the next routine blood draw for premature infants or at the time of surgery for children undergoing EUAs. Serum was prepared by centrifugation at 3500 rpm (1000 g) at 4°C for 5 to 10 minutes. Carotenoids in 200-μL portions of serum were extracted into ethyl acetate with 0.1% butylated hydroxytoluene as an antioxidant preservative. The dried down material was then resuspended in HPLC running buffer and analyzed by HPLC according to previously published methods.14 Mother's milk samples collected in 12-mL tubes at the time of their infant's examination were treated in a similar manner except larger volumes of sample (1–2 mL) and organic extractants were used because milk carotenoid levels are typically 10-fold lower than serum levels.
Dietary Carotenoid Assessments
A registered dietitian met with the mothers to estimate each subject's daily intake of carotenoids from foods based on 3-day food intake diaries and published US Department of Agriculture and proprietary databases of carotenoid contents of food. Bottled breast milk and formula-fed infant carotenoid intakes were calculated based on volume consumed and HPLC analysis of mother's milk or manufacturers' reports of formula carotenoid content. Breast milk intake for exclusively breast-fed, healthy infants was estimated at 160 mL/kg/d. Carotenoid intake for tube-fed children was calculated from the manufacturers' reports of formula carotenoid content.
Statistics
Linear regression analysis and Pearson's correlation coefficients were used to determine significant associations between measurements. Statistical analyses were performed on statistical software (SAS 9.3; SAS Software, Cary, NC). When MPOD was available on both eyes, the right and left eye values were always averaged for graphical and statistical analyses unless otherwise noted.
Results
Subject Data
A total of 51 infants and children were enrolled in this cross-sectional study, and their major demographic data and measurement results are shown in Supplementary Table S1. We achieved an even distribution between our four primary age categories (premature infants, infants aged between 0 and 1 year, toddlers aged between 1 and 4 years, and children aged between 4 and 7 years). Some data are absent due to poor quality or missing images (29 of 102 eyes) caused by various congenital abnormalities (11 eyes); intraocular tumors (6 eyes); history of ROP (4 eyes); difficulties at the time of ROP screening due to motion artifacts, small pupils, and media opacities (6 eyes); trauma (1 eye); and unknown (1 eye). Other missing data included malfunctions of the skin Raman instrument (9 subjects); missing blood samples (6 subjects); lack of parental cooperation with dietary assessments (15 subjects); and incomplete hospital records of daily nutritional intake for some premature infants (5 subjects).
Macular Pigment Measurement With the RetCam
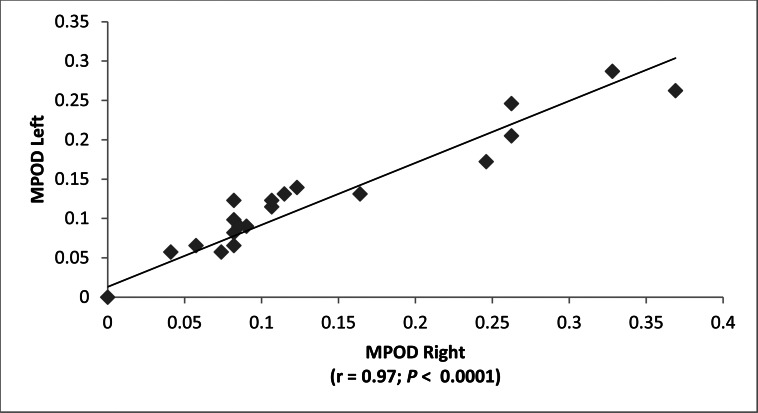
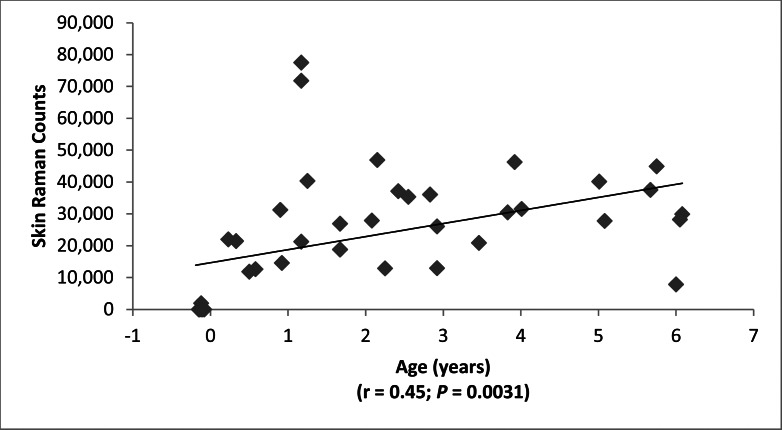
Typical raw and processed macular pigment images are shown in Figure 2. The blue-light source on the RetCam (Clarity Medical Systems, Inc.) is nominally listed as “approximately 471 nm” in its user manual,9 but when we measured it directly with a spectrometer (HR2000+; Ocean Optics) on two RetCams (Clarity Medical Systems, Inc.), it was actually a narrowly filtered band ranging from 480 to 485 nm, while the white-light source has a much wider band ranging from 450 nm to past 900 nm (see Fig. 1). The macular pigment in the living eye peaks at 460 nm, but there is a strong secondary shoulder peak that overlaps well with the 480- to 485-nm blue-light band. It is well established that peak MPOD is highly correlated between eyes in adults.14 In subjects with measurable MPOD in both eyes, we confirmed a strong correlation between the two eyes over a wide range of values (Fig. 3), establishing that our method is likely to be an accurate and reproducible way to measure MPOD in children. Macular pigment distributions in our infants and children were almost always narrow, radially symmetric peaks that are in contrast to more diverse distribution patterns reported in middle-aged and elderly adults.14,15 Macular pigment was always undetectable in premature babies at the time of their ROP screening examinations. Infants and children, on the other hand, had a wide range of MPOD ranging from less than 0.05 to nearly 0.4. For the entire study population, there was a significant rise of MPOD with age, and linear regression analysis predicted an MPOD of 0.0835 at birth (Fig. 4).
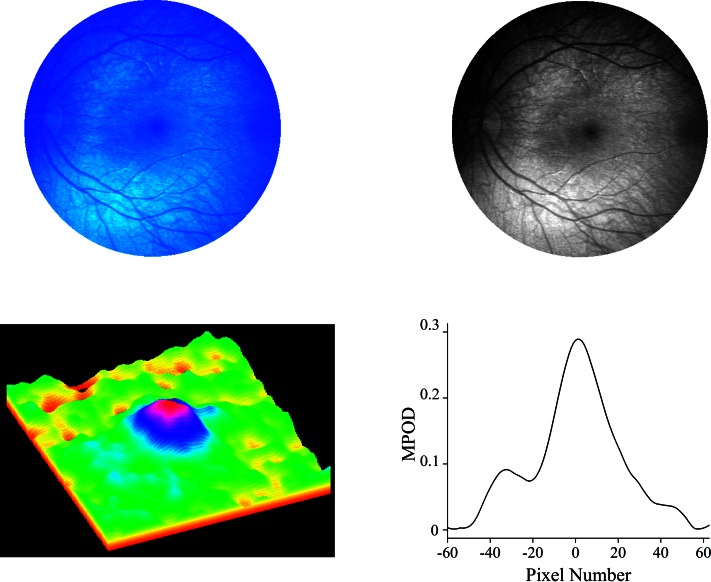
Figure 2.
Blue light reflectance measurement of macular pigment in subject 7. A fundus reflectance image using 485-nm (blue) excitation light was obtained with a RetCam (upper left). The blue fundus image has three components corresponding to the blue, green, and red chips in the RetCam's internal charge-coupled device (CCD) detector. The blue component was converted to grayscale for all calculations and processing (upper right). A corresponding 3-dimensional pseudo-color MP distribution can then be plotted (lower left) along with a horizontal meridian, nasal–temporal intensity profile running through the center of the fovea (lower right).
Figure 3.
Right-left correlation of MPOD measurements of subjects who had both eyes measured.
Figure 4.
MPOD versus age.
Serum and Dietary Measurements of Carotenoid Status
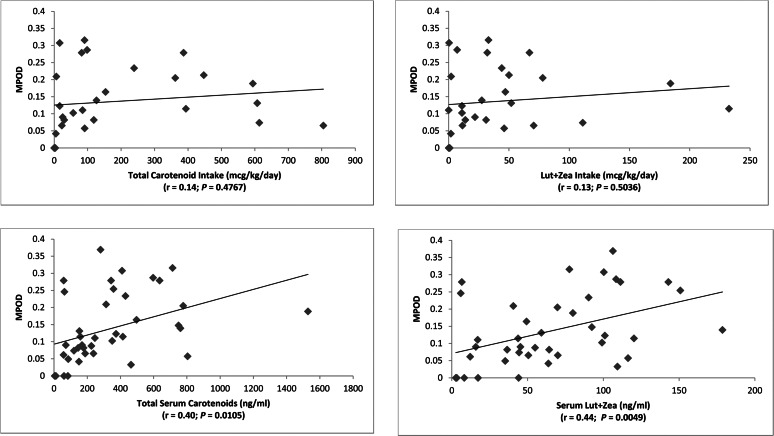
Systemic carotenoid status is traditionally assessed by serum HPLC analysis and dietary surveys. In adults, these two measures are correlated,16 and there is typically a smaller but still significant correlation of them with MPOD.17 We confirmed that these correlations are present in our study population for serum carotenoids (total and Lut + Zea) and MPOD but not for dietary carotenoid intake and MPOD (Fig. 5). There were no substantial changes in these findings when serum lutein and zeaxanthin were analyzed separately versus MPOD (r = 0.45 and P = 0.0043 for serum lutein versus MPOD; r = 0.34 and P = 0.033 for serum zeaxanthin versus MPOD).
Figure 5.
MPOD versus dietary and serum carotenoids.
Skin Carotenoid Levels
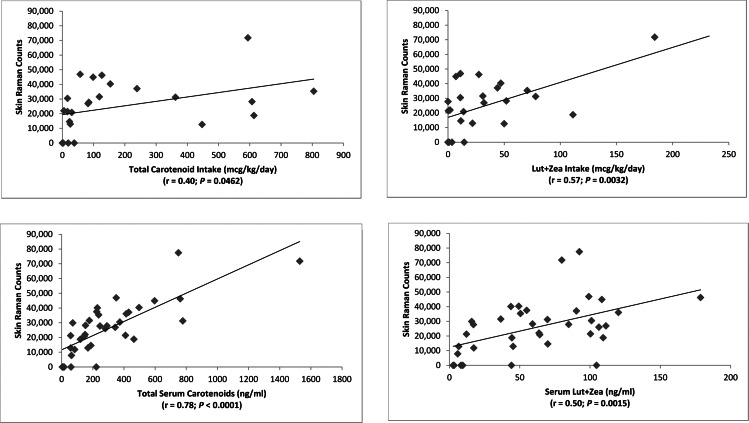
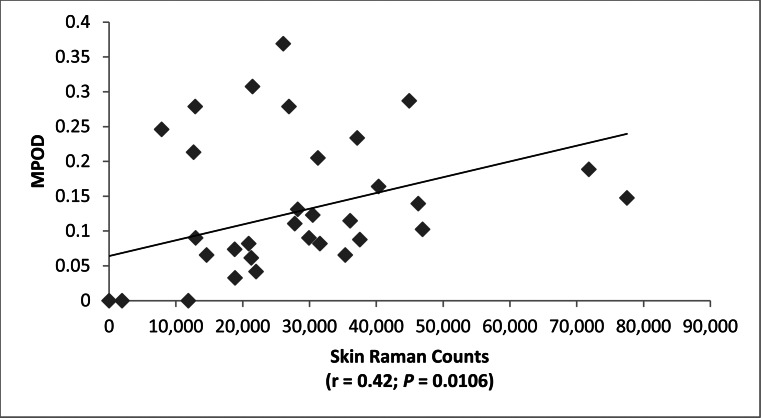
Resonance Raman spectroscopy (RRS) measurement of skin carotenoids is a particularly useful method to assess nutritional status noninvasively in infants and children.11,12 RRS skin carotenoid levels were very low in premature infants and then rose significantly with age in the study population (Fig. 6). RRS correlated significantly with serum carotenoids (total and Lut + Zea) and with dietary carotenoid intake (Fig. 7). There were no substantial changes in these findings when serum lutein and zeaxanthin were analyzed separately versus skin RRS (r = 0.52 and P = 0.0012 for serum lutein versus skin RRS; r = 0.39 and P = 0.0183 for serum zeaxanthin versus skin RRS). Skin RRS was a significant predictor of peak MPOD (Fig. 8).
Figure 6.
Resonance Raman measurement of skin carotenoids versus age.
Figure 7.
Resonance Raman measurement of skin carotenoids versus dietary and serum carotenoids.
Figure 8.
MPOD versus skin carotenoids measured by resonance Raman spectroscopy (RRS).
Discussion
The macular pigment carotenoids, lutein, zeaxanthin, and lutein's metabolite meso-zeaxanthin, are specifically concentrated in the macula of the human eye where they are thought to provide protection against light-induced oxidative damage associated with AMD.1–4 More recently, attention has also focused on possible functional roles of the macular pigment carotenoids in improving visual acuity, enhancing contrast sensitivity, and alleviating glare symptoms.18 While there is considerable interest in determining the value of carotenoid supplementation in adults in studies such as Age-Related Eye Disease Study 2 (AREDS2),19 there is a paucity of information on the potential roles of carotenoids in infant and child ocular health.8 Specifically, the macular pigment carotenoids may exert a beneficial influence on foveal and visual development or vice versa, as conditions associated with a hypoplastic fovea and poor central vision such as albinism generally have no macular pigment,20 and our one patient with oculocutaneous albinism and nystagmus (subject 44) had no detectable macular pigment in either eye. The macular pigment carotenoids also might modulate risk of ROP through antioxidant effects,21–23 and diseases of aging such as AMD may depend on light damage and oxidative stress that accumulate over a lifetime,1–4 including the childhood years. Thus, more complete knowledge of infant ocular carotenoid status could have considerable value in enhancing the diagnosis and treatment of a variety of ocular disorders throughout the lifespan.
It has been reported that the macula lutea (yellow spot) is visible ophthalmoscopically at a young age,1 and autopsy studies have shown that lutein and zeaxanthin are detectable in the retina and vitreous by HPLC in the second and third trimesters of pregnancy7,24; however, there are no prior published reports of noninvasive quantification of MPOD in infants and young children. This is due, in part, to the fact that most MPOD measurement methods routinely used in adults are unsuitable for this younger population. HFP, the most commonly employed clinical measurement technique in adults, is a psychophysical technique that is clearly unsuitable for infants and young children who cannot be expected to perform tasks requiring attention, cooperation, and report of a subjective observation.5,6 AFI of the macular pigment's attenuation of lipofuscin's fluorescence5,6 will not work in children because they have very low levels of lipofuscin relative to adults. Indeed, when we tried to measure infant and child fundus autofluorescence with the RetCam (Clarity Medical Systems, Inc.), the fluorescence intensity signals were too low to record any useful images. Resonance Raman measurement of macular carotenoids in an integral or an imaging mode could potentially measure macular pigment levels in our study population,25–27 but we had safety concerns about the intensity of laser light that would need to be directed at the developing eye's fovea to measure the generally low levels of infant and child macular pigment. We therefore chose blue-light reflectometry as our preferred method for infant and young child MPOD measurement because the ocular media are very clear in subjects in this age range, which means that correction factors for lens absorbance and vitreous scattering required in adults are unnecessary,28,29 and the RetCam is an FDA-cleared platform specifically designed for imaging of the retina of infants and children, so there are no ocular safety concerns as long as the device is used as intended. The RetCam's (Clarity Medical Systems, Inc.) optional blue-light source has a peak wavelength of 480 to 485 nm that overlaps with a major vibronic peak of lutein and zeaxanthin, and its video capture mode allows for efficient bleaching of potentially interfering rod and short wavelength cone photopigments. In order to best record blue-light reflectance, we omitted the fluorescein angiography 510-nm barrier emission filter in the light collection path, and we used the data from the blue-light channel of the RetCam's (Clarity Medical Systems, Inc.) internal CCD detector for image processing. Premature babies and young infants could be swaddled and imaged with topical anesthesia, but older children required general anesthesia due to excessive eye and head movement.
Our results demonstrate a number of new findings related to macular carotenoids in infants and children. First, although we observed the typical ∼10-fold scatter in macular pigment levels at any particular age, there was a steady rise in MPOD over the first 7 years of life, eventually approaching average levels found in adult populations, and nearly all of the subjects with measurable MPOD had narrow, radially symmetric macular pigment patterns, in contrast to the more variable patterns reported in adults.14,15 Total serum carotenoids and serum lutein + zeaxanthin were significantly associated with MPOD; and individually, serum lutein and zeaxanthin also correlated with MPOD. But this correlation with MPOD did not extend to dietary intakes of these nutrients, possibly as a result of the high percentage of subjects whose mothers who did not return 3-day dietary intake surveys of their children (29%) or who had incomplete newborn intensive care unit feeding records (10%). This was confounded further by the challenges of performing accurate assessments of dietary carotenoid intakes in children through nutritional surveys, by incomplete food carotenoid content databases, and by analyses of single samples of mother's milk, which did not account for the known daily and long-term variability of milk carotenoid content.
Skin carotenoid measurement by RRS is an increasingly popular method for assessment of systemic carotenoid status and as a biomarker of fruit and vegetable intake because it is rapid, reproducible, and noninvasive.13 These characteristics make it ideal in young children and infants where blood draws and dietary surveys can be particularly challenging.11,12 As with MPOD, we found a wide scatter of skin RRS values with a significant increase with age. As has been previously reported in adults,13 skin RRS in our study population correlates significantly with total serum carotenoid levels and total dietary carotenoid intake, reflecting the diversity of carotenoids found in skin biopsies of adult humans and newborn male foreskins.13,30 In contrast to adult humans,14 we even found a significant correlation between skin RRS and MPOD in our enrolled subjects.
Our 11 premature infants consistently had undetectable MPOD, and all other measures of carotenoid status in the skin and blood were likewise remarkably low relative to the rest of the study population. The premature infants' undetectable MPOD could be due to their relative foveal immaturity or to systemic carotenoid depletion, which is likely due to the high levels of oxidative stress that they encounter in the newborn intensive care unit and to the near absence of carotenoids in their diets. A recent study in preterm infants showed that supplementing preterm formula with lutein/zeaxanthin (∼0.07 mg/d) raises plasma lutein concentrations to those of healthy milk-fed infants and suggested improved rod photoreceptor function31; however, this study and three other recently published randomized, controlled studies using 0.14 mg of lutein and 0.006 mg of zeaxanthin per day failed to prevent incidence or progression of ROP.21–23 Further studies specifically designed to test the hypotheses that lutein/zeaxanthin supplementation at different dosages or formulations can affect infant visual development or ROP outcomes are still warranted.
Blue-light reflectance measurement of MPOD in the young human eye can be readily performed using the RetCam (Clarity Medical Systems, Inc.), a widely available clinical instrument. The major challenges of our technique lie in the requirement for sedation or general anesthesia in older infants and children and in the need for an experienced RetCam operator to provide evaluable images centered on the fovea because the raw blue-light images are dim and indistinct. While our study included a broad range of subjects from prematurity up to age 7, we currently know little about MPOD in normal full-term infants at the time of birth, and this topic will be the focus of an upcoming study. Our novel approach to assessment of infant and child ocular and systemic carotenoid status should prove invaluable in future investigations of the role of lutein, zeaxanthin, and other carotenoids in normal anatomical and functional eye development and in monitoring dietary intervention studies for ocular and systemic diseases that may be associated with carotenoid insufficiencies.
Supplementary Material
Acknowledgments
Supported by National Eye Institute Grant EY-11600 and Core Grant EY-14800, Research to Prevent Blindness, and Abbott Nutrition (Columbus, Ohio).
Disclosure: P.S. Bernstein, None; M. Sharifzadeh, None; A. Liu, None; I. Ermakov, None; K. Nelson, None; X. Sheng, None; C. Panish, None; B. Carlstrom, None; R.O. Hoffman, None; W. Gellermann, None
References
- 1. Nussbaum JJ, Pruett RC, Delori FC. Historic perspectives. Macular yellow pigment. The first 200 years. Retina. 1981; 1: 296–310 [PubMed] [Google Scholar]
- 2. SanGiovanni JP, Neuringer M. The putative role of lutein and zeaxanthin as protective agents against age-related macular degeneration: promise of molecular genetics for guiding mechanistic and translational research in the field. Am J Clin Nutr. 2012; 96: 1223S–1233S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ma L, Dou HL, Wu YQ, et al. Lutein and zeaxanthin intake and the risk of age-related macular degeneration: a systematic review and meta-analysis. Br J Nutr. 2012; 107: 350–359 [DOI] [PubMed] [Google Scholar]
- 4. Loane E, Nolan JM, O'Donovan O, Bhosale P, Bernstein PS, Beatty S. Transport and retinal capture of lutein and zeaxanthin with reference to age-related macular degeneration. Surv Ophthalmol. 2008; 53: 68–81 [DOI] [PubMed] [Google Scholar]
- 5. Howells O, Eperjesi F, Bartlett H. Measuring macular pigment optical density in vivo: a review of techniques. Graefes Arch Clin Exp Ophthalmol. 2011; 249: 315–347 [DOI] [PubMed] [Google Scholar]
- 6. Bernstein PS, Delori FC, Richer S, van Kuijk FJ, Wenzel AJ. The value of measurement of macular carotenoid pigment optical densities and distributions in age-related macular degeneration and other retinal disorders. Vision Res. 2010; 50: 716–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bone RA, Landrum JT, Fernandez L, Tarsis SL. Analysis of the macular pigment by HPLC: retinal distribution and age study. Invest Ophthalmol Vis Sci. 1988; 29: 843–849 [PubMed] [Google Scholar]
- 8. Hammond BR. The dietary carotenoids lutein and zeaxanthin in pre-and-postnatal development. Funct Food Rev. 2012; 4: 130–137 [Google Scholar]
- 9. Clarity Medical Systems RetCam 3 User Manual. PN 21-100277 Rev. C. Pleasanton, CA: Clarity Medical Systems; 2008: 53–56 [Google Scholar]
- 10. Snodderly DM, Brown PK, Delori FC, Auran JD. The macular pigment. I. Absorbance spectra, localization, and discrimination from other yellow pigments in primate retinas. Invest Ophthalmol Vis Sci. 1984; 25: 660–673 [PubMed] [Google Scholar]
- 11. Ermakov IV, Ermakova MR, Bernstein PS, Chan GM, Gellermann W. Resonance Raman based skin carotenoid measurements in newborns and infants[ published online ahead of print November 29, 2012]. J Biophotonics. doi:10.1002/jbio.201200195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scarmo S, Henebery K, Peracchio H, et al. Skin carotenoid status measured by resonance Raman spectroscopy as a biomarker of fruit and vegetable intake in preschool children. Eur J Clin Nutr. 2012; 66: 555–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mayne ST, Cartmel B, Scarmo S, et al. Noninvasive assessment of dermal carotenoids as a biomarker of fruit and vegetable intake. Am J Clin Nutr. 2010; 92: 794–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bernstein PS, Ahmed F, Liu A, et al. Macular pigment imaging in AREDS2 participants: an ancillary study of AREDS2 subjects enrolled at the Moran Eye Center. Invest Ophthalmol Vis Sci. 2012; 53: 6178–6186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sharifzadeh M, Bernstein PS, Gellermann W. Nonmydriatic fluorescence-based quantitative imaging of human macular pigment distributions. J Opt Soc Am A Opt Image Sci Vis. 2006; 23: 2373–2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gruber M, Chappell R, Millen A, et al. Correlates of serum lutein + zeaxanthin: findings from the Third National Health and Nutrition Examination Survey. J Nutr. 2004; 134: 2387–2394 [DOI] [PubMed] [Google Scholar]
- 17. Nolan JM, Stack J, O'Connell E, Beatty S. The relationships between macular pigment optical density and its constituent carotenoids in diet and serum. Invest Ophthalmol Vis Sci. 2007; 48: 571–582 [DOI] [PubMed] [Google Scholar]
- 18. Stringham JM, Bovier ER, Wong JC, Hammond BR Jr. The influence of dietary lutein and zeaxanthin on visual performance. J Food Sci. 2010; 75: R24–R29 [DOI] [PubMed] [Google Scholar]
- 19. AREDS2 Research Group, Chew EY,, Clemons T,, et al. The Age-Related Eye Disease Study 2 (AREDS2): study design and baseline characteristics (AREDS2 report number 1). Ophthalmology. 2012; 119: 2282–2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abadi RV, Cox MJ. The distribution of macular pigment in human albinos. Invest Ophthalmol Vis Sci. 1992; 33: 494–497 [PubMed] [Google Scholar]
- 21. Romagnoli C, Giannantonio C, Cota F, et al. A prospective, randomized, double blind study comparing lutein to placebo for reducing occurrence and severity of retinopathy of prematurity. J Matern Fetal Neonatal Med. 2011; 24 (suppl 1): 147–150 [DOI] [PubMed] [Google Scholar]
- 22. Dani C, Lori I, Favelli F, et al. Lutein and zeaxanthin supplementation in preterm infants to prevent retinopathy of prematurity: a randomized controlled study. J Matern Fetal Neonatal Med. 2012; 25: 523–527 [DOI] [PubMed] [Google Scholar]
- 23. Manzoni P, Guardione R, Bonetti P, et al. Lutein and zeaxanthin supplementation in preterm very low-birth-weight neonates in neonatal intensive care units: a multicenter randomized controlled trial [ published online ahead of print January 30, 2013]. Am J Perinatol. doi:10.1055/s-0032-1321494 [DOI] [PubMed] [Google Scholar]
- 24. Iakovleva MA, Panova IG, Feldman TB, et al. Detection of carotenoids in the vitreous body of the human eye during prenatal development [in Russian]. Ontogenez. 2007; 38: 380–385 [PubMed] [Google Scholar]
- 25. Gellermann W, Ermakov IV, Ermakova MR, McClane RW, Zhao DY, Bernstein PS. In vivo resonant Raman measurement of macular carotenoid pigments in the young and the aging human retina. J Opt Soc Am A Opt Image Sci Vis. 2002; 19: 1172–1286 [DOI] [PubMed] [Google Scholar]
- 26. Bernstein PS, Zhao DY, Sharifzadeh M, Ermakov IV, Gellermann W. Resonance Raman measurement of macular carotenoids in the living human eye. Arch Biochem Biophys. 2004; 430: 163–169 [DOI] [PubMed] [Google Scholar]
- 27. Sharifzadeh M, Zhao DY, Bernstein PS, Gellermann W. Resonance Raman imaging of macular pigment distributions in the human retina. J Opt Soc Am A Opt Image Sci Vis. 2008; 25: 947–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Berendschot TT, Goldbohm RA, Klöpping WA, van de Kraats J, van Norel J, van Norren D. Influence of lutein supplementation on macular pigment, assessed with two objective techniques. Invest Ophthalmol Vis Sci. 2000; 41: 3322–3326 [PubMed] [Google Scholar]
- 29. Berendschot TT, van Norren D. Objective determination of the macular pigment optical density using fundus reflectance spectroscopy. Arch Biochem Biophys. 2004; 430: 149–155 [DOI] [PubMed] [Google Scholar]
- 30. Chan GM, Chan MM, Gellermann W, et al. Resonance Raman Spectroscopy and the Preterm Infant Carotenoid Status. J Pediatr Gastroenterol Nutr. 2012; 56: 556–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rubin LP, Chan GM, Barrett-Reis BM, et al. Effect of carotenoid supplementation on plasma carotenoids, inflammation and visual development in preterm infants. J Perinatol. 2012; 32: 418–424 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.