Abstract
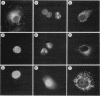
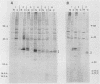
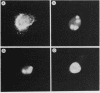
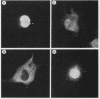
The v-vis gene encodes p28sis, the transforming protein of simian sarcoma virus. This gene resulted from a fusion of the env gene of simian sarcoma-associated virus and the woolly monkey gene for the B chain of platelet-derived growth factor (PDGF). Previous work has shown that the v-sis gene product undergoes signal sequence cleavage, glycosylation, dimerization, and proteolytic processing to yield a secreted form of the protein. It transport across the endoplasmic reticulum is blocked by the introduction of a charged amino acid residue within the signal sequence, the protein does not dimerize, is not secreted, and is no longer transforming as assayed by focus-forming ability in NIH 3T3 cells. Instead, this mutant protein localizes to the nucleus as demonstrated by both indirect immunofluorescence and cell fractionation. Using a series of deletion mutations, we delimited an amino acid sequence within this protein which is responsible for nuclear localization. This region is completely conserved in the predicted human c-sis protein, although it lies outside of regions required for transformation by the v-sis gene product. This nuclear transport signal is contained within amino acid residues 237 to 255, RVTIRTVRVRRPPKGKHRK. An amino acid sequence containing these residues is capable of directing cytoplasmic v-sis mutant proteins to the nucleus. This sequence is also capable of directing less efficient nuclear transport of a normally cytoplasmic protein, pyruvate kinase. Pulse-chase experiments indicate that the half-lives of nuclear and cytoplasmic v-sis mutant proteins are approximately 35 min. Using the heat-inducible hsp70 promoter from Drosophila melanogaster, we showed that the nuclear v-sis protein accumulates in the nucleus within 30 min of induction. The identification of a nuclear transport signal in the v-sis gene product raises interesting questions regarding the possibility of some function for PDGF or PDGF-related molecules in the nucleus.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer E. A., Cooper T. W., Huang J. S., Altman J., Deuel T. F. Stimulation of in vitro human skin collagenase expression by platelet-derived growth factor. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4132–4136. doi: 10.1073/pnas.82.12.4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bold R. J., Donoghue D. J. Biologically active mutants with deletions in the v-mos oncogene assayed with retroviral vectors. Mol Cell Biol. 1985 Nov;5(11):3131–3138. doi: 10.1128/mcb.5.11.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond U., Schlesinger M. J. Ubiquitin is a heat shock protein in chicken embryo fibroblasts. Mol Cell Biol. 1985 May;5(5):949–956. doi: 10.1128/mcb.5.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M. Protein migration into nuclei. I. Frog oocyte nuclei in vivo accumulate microinjected histones, allow entry to small proteins, and exclude large proteins. J Cell Biol. 1975 Feb;64(2):421–430. doi: 10.1083/jcb.64.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran B. H., Reffel A. C., Stiles C. D. Molecular cloning of gene sequences regulated by platelet-derived growth factor. Cell. 1983 Jul;33(3):939–947. doi: 10.1016/0092-8674(83)90037-5. [DOI] [PubMed] [Google Scholar]
- Colledge W. H., Richardson W. D., Edge M. D., Smith A. E. Extensive mutagenesis of the nuclear location signal of simian virus 40 large-T antigen. Mol Cell Biol. 1986 Nov;6(11):4136–4139. doi: 10.1128/mcb.6.11.4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins T., Ginsburg D., Boss J. M., Orkin S. H., Pober J. S. Cultured human endothelial cells express platelet-derived growth factor B chain: cDNA cloning and structural analysis. Nature. 1985 Aug 22;316(6030):748–750. doi: 10.1038/316748a0. [DOI] [PubMed] [Google Scholar]
- Deuel T. F., Huang J. S., Huang S. S., Stroobant P., Waterfield M. D. Expression of a platelet-derived growth factor-like protein in simian sarcoma virus transformed cells. Science. 1983 Sep 30;221(4618):1348–1350. doi: 10.1126/science.6310754. [DOI] [PubMed] [Google Scholar]
- Devare S. G., Reddy E. P., Law J. D., Robbins K. C., Aaronson S. A. Nucleotide sequence of the simian sarcoma virus genome: demonstration that its acquired cellular sequences encode the transforming gene product p28sis. Proc Natl Acad Sci U S A. 1983 Feb;80(3):731–735. doi: 10.1073/pnas.80.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingwall C., Laskey R. A. Protein import into the cell nucleus. Annu Rev Cell Biol. 1986;2:367–390. doi: 10.1146/annurev.cb.02.110186.002055. [DOI] [PubMed] [Google Scholar]
- Dingwall C., Sharnick S. V., Laskey R. A. A polypeptide domain that specifies migration of nucleoplasmin into the nucleus. Cell. 1982 Sep;30(2):449–458. doi: 10.1016/0092-8674(82)90242-2. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F., Hunkapiller M. W., Hood L. E., Devare S. G., Robbins K. C., Aaronson S. A., Antoniades H. N. Simian sarcoma virus onc gene, v-sis, is derived from the gene (or genes) encoding a platelet-derived growth factor. Science. 1983 Jul 15;221(4607):275–277. doi: 10.1126/science.6304883. [DOI] [PubMed] [Google Scholar]
- Einat M., Resnitzky D., Kimchi A. Inhibitory effects of interferon on the expression of genes regulated by platelet-derived growth factor. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7608–7612. doi: 10.1073/pnas.82.22.7608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldherr C. M., Kallenbach E., Schultz N. Movement of a karyophilic protein through the nuclear pores of oocytes. J Cell Biol. 1984 Dec;99(6):2216–2222. doi: 10.1083/jcb.99.6.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay D. R., Newmeyer D. D., Price T. M., Forbes D. J. Inhibition of in vitro nuclear transport by a lectin that binds to nuclear pores. J Cell Biol. 1987 Feb;104(2):189–200. doi: 10.1083/jcb.104.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett J. S., Coughlin S. R., Niman H. L., Tremble P. M., Giels G. M., Williams L. T. Blockade of autocrine stimulation in simian sarcoma virus-transformed cells reverses down-regulation of platelet-derived growth factor receptors. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7466–7470. doi: 10.1073/pnas.81.23.7466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb D. S., Gariépy J., Schoolnik G., Kornberg R. D. Synthetic peptides as nuclear localization signals. Nature. 1986 Aug 14;322(6080):641–644. doi: 10.1038/322641a0. [DOI] [PubMed] [Google Scholar]
- Hall M. N., Hereford L., Herskowitz I. Targeting of E. coli beta-galactosidase to the nucleus in yeast. Cell. 1984 Apr;36(4):1057–1065. doi: 10.1016/0092-8674(84)90055-2. [DOI] [PubMed] [Google Scholar]
- Hannink M., Donoghue D. J. Biosynthesis of the v-sis gene product: signal sequence cleavage, glycosylation, and proteolytic processing. Mol Cell Biol. 1986 Apr;6(4):1343–1348. doi: 10.1128/mcb.6.4.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannink M., Donoghue D. J. Cell surface expression of membrane-anchored v-sis gene products: glycosylation is not required for cell surface transport. J Cell Biol. 1986 Dec;103(6 Pt 1):2311–2322. doi: 10.1083/jcb.103.6.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannink M., Donoghue D. J. Requirement for a signal sequence in biological expression of the v-sis oncogene. Science. 1984 Dec 7;226(4679):1197–1199. doi: 10.1126/science.6095451. [DOI] [PubMed] [Google Scholar]
- Hannink M., Sauer M. K., Donoghue D. J. Deletions in the C-terminal coding region of the v-sis gene: dimerization is required for transformation. Mol Cell Biol. 1986 Apr;6(4):1304–1314. doi: 10.1128/mcb.6.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B. Growth factors: mechanism of action and relation to oncogenes. Cell. 1984 May;37(1):9–20. doi: 10.1016/0092-8674(84)90296-4. [DOI] [PubMed] [Google Scholar]
- Huang J. S., Huang S. S., Deuel T. F. Human platelet-derived growth factor: radioimmunoassay and discovery of a specific plasma-binding protein. J Cell Biol. 1983 Aug;97(2):383–388. doi: 10.1083/jcb.97.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J. S., Huang S. S., Deuel T. F. Transforming protein of simian sarcoma virus stimulates autocrine growth of SSV-transformed cells through PDGF cell-surface receptors. Cell. 1984 Nov;39(1):79–87. doi: 10.1016/0092-8674(84)90193-4. [DOI] [PubMed] [Google Scholar]
- Johnsson A., Betsholtz C., Heldin C. H., Westermark B. Antibodies against platelet-derived growth factor inhibit acute transformation by simian sarcoma virus. Nature. 1985 Oct 3;317(6036):438–440. doi: 10.1038/317438a0. [DOI] [PubMed] [Google Scholar]
- Johnsson A., Heldin C. H., Wasteson A., Westermark B., Deuel T. F., Huang J. S., Seeburg P. H., Gray A., Ullrich A., Scrace G. The c-sis gene encodes a precursor of the B chain of platelet-derived growth factor. EMBO J. 1984 May;3(5):921–928. doi: 10.1002/j.1460-2075.1984.tb01908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs S. F., Guo C., Ratner L., Wong-Staal F. Human-proto-oncogene nucleotide sequences corresponding to the transforming region of simian sarcoma virus. Science. 1984 Feb 3;223(4635):487–491. doi: 10.1126/science.6318322. [DOI] [PubMed] [Google Scholar]
- Kaiser C. A., Preuss D., Grisafi P., Botstein D. Many random sequences functionally replace the secretion signal sequence of yeast invertase. Science. 1987 Jan 16;235(4786):312–317. doi: 10.1126/science.3541205. [DOI] [PubMed] [Google Scholar]
- Kalderon D., Richardson W. D., Markham A. F., Smith A. E. Sequence requirements for nuclear location of simian virus 40 large-T antigen. Nature. 1984 Sep 6;311(5981):33–38. doi: 10.1038/311033a0. [DOI] [PubMed] [Google Scholar]
- Kalderon D., Roberts B. L., Richardson W. D., Smith A. E. A short amino acid sequence able to specify nuclear location. Cell. 1984 Dec;39(3 Pt 2):499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- Kohase M., May L. T., Tamm I., Vilcek J., Sehgal P. B. A cytokine network in human diploid fibroblasts: interactions of beta-interferons, tumor necrosis factor, platelet-derived growth factor, and interleukin-1. Mol Cell Biol. 1987 Jan;7(1):273–280. doi: 10.1128/mcb.7.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruijer W., Cooper J. A., Hunter T., Verma I. M. Platelet-derived growth factor induces rapid but transient expression of the c-fos gene and protein. Nature. 1984 Dec 20;312(5996):711–716. doi: 10.1038/312711a0. [DOI] [PubMed] [Google Scholar]
- Lanford R. E., Kanda P., Kennedy R. C. Induction of nuclear transport with a synthetic peptide homologous to the SV40 T antigen transport signal. Cell. 1986 Aug 15;46(4):575–582. doi: 10.1016/0092-8674(86)90883-4. [DOI] [PubMed] [Google Scholar]
- Laughon A., Gesteland R. F. Primary structure of the Saccharomyces cerevisiae GAL4 gene. Mol Cell Biol. 1984 Feb;4(2):260–267. doi: 10.1128/mcb.4.2.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonberg N., Gilbert W. Primary structure of chicken muscle pyruvate kinase mRNA. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3661–3665. doi: 10.1073/pnas.80.12.3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Moreland R. B., Nam H. G., Hereford L. M., Fried H. M. Identification of a nuclear localization signal of a yeast ribosomal protein. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6561–6565. doi: 10.1073/pnas.82.19.6561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R., Bravo R., Burckhardt J., Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984 Dec 20;312(5996):716–720. doi: 10.1038/312716a0. [DOI] [PubMed] [Google Scholar]
- Owen A. J., Pantazis P., Antoniades H. N. Simian sarcoma virus--transformed cells secrete a mitogen identical to platelet-derived growth factor. Science. 1984 Jul 6;225(4657):54–56. doi: 10.1126/science.6328659. [DOI] [PubMed] [Google Scholar]
- Paine P. L., Feldherr C. M. Nucleocytoplasmic exchange of macromolecules. Exp Cell Res. 1972 Sep;74(1):81–98. doi: 10.1016/0014-4827(72)90483-1. [DOI] [PubMed] [Google Scholar]
- Paine P. L., Moore L. C., Horowitz S. B. Nuclear envelope permeability. Nature. 1975 Mar 13;254(5496):109–114. doi: 10.1038/254109a0. [DOI] [PubMed] [Google Scholar]
- Paine P. L. Nucleocytoplasmic movement of fluorescent tracers microinjected into living salivary gland cells. J Cell Biol. 1975 Sep;66(3):652–657. doi: 10.1083/jcb.66.3.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parag H. A., Raboy B., Kulka R. G. Effect of heat shock on protein degradation in mammalian cells: involvement of the ubiquitin system. EMBO J. 1987 Jan;6(1):55–61. doi: 10.1002/j.1460-2075.1987.tb04718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. A regulatory upstream promoter element in the Drosophila hsp 70 heat-shock gene. Cell. 1982 Sep;30(2):517–528. doi: 10.1016/0092-8674(82)90249-5. [DOI] [PubMed] [Google Scholar]
- Rakowicz-Szulczynska E. M., Rodeck U., Herlyn M., Koprowski H. Chromatin binding of epidermal growth factor, nerve growth factor, and platelet-derived growth factor in cells bearing the appropriate surface receptors. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3728–3732. doi: 10.1073/pnas.83.11.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson W. D., Roberts B. L., Smith A. E. Nuclear location signals in polyoma virus large-T. Cell. 1986 Jan 17;44(1):77–85. doi: 10.1016/0092-8674(86)90486-1. [DOI] [PubMed] [Google Scholar]
- Robbins K. C., Antoniades H. N., Devare S. G., Hunkapiller M. W., Aaronson S. A. Structural and immunological similarities between simian sarcoma virus gene product(s) and human platelet-derived growth factor. Nature. 1983 Oct 13;305(5935):605–608. doi: 10.1038/305605a0. [DOI] [PubMed] [Google Scholar]
- Robbins K. C., Leal F., Pierce J. H., Aaronson S. A. The v-sis/PDGF-2 transforming gene product localizes to cell membranes but is not a secretory protein. EMBO J. 1985 Jul;4(7):1783–1792. doi: 10.1002/j.1460-2075.1985.tb03851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer M. K., Hannink M., Donoghue D. J. Deletions in the N-terminal coding region of the v-sis gene: determination of the minimal transforming region. J Virol. 1986 Aug;59(2):292–300. doi: 10.1128/jvi.59.2.292-300.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz L. D., Friesen J. D. Nucleotide sequence of the tcml gene (ribosomal protein L3) of Saccharomyces cerevisiae. J Bacteriol. 1983 Jul;155(1):8–14. doi: 10.1128/jb.155.1.8-14.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver P. A., Keegan L. P., Ptashne M. Amino terminus of the yeast GAL4 gene product is sufficient for nuclear localization. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5951–5955. doi: 10.1073/pnas.81.19.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. E., Kalderon D., Roberts B. L., Colledge W. H., Edge M., Gillett P., Markham A., Paucha E., Richardson W. D. The nuclear location signal. Proc R Soc Lond B Biol Sci. 1985 Oct 22;226(1242):43–58. doi: 10.1098/rspb.1985.0078. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Autocrine growth factors and cancer. 1985 Feb 28-Mar 6Nature. 313(6005):745–747. doi: 10.1038/313745a0. [DOI] [PubMed] [Google Scholar]
- Sprague J., Condra J. H., Arnheiter H., Lazzarini R. A. Expression of a recombinant DNA gene coding for the vesicular stomatitis virus nucleocapsid protein. J Virol. 1983 Feb;45(2):773–781. doi: 10.1128/jvi.45.2.773-781.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theilen G. H., Gould D., Fowler M., Dungworth D. L. C-type virus in tumor tissue of a woolly monkey (Lagothrix spp.) with fibrosarcoma. J Natl Cancer Inst. 1971 Oct;47(4):881–889. [PubMed] [Google Scholar]
- Waterfield M. D., Scrace G. T., Whittle N., Stroobant P., Johnsson A., Wasteson A., Westermark B., Heldin C. H., Huang J. S., Deuel T. F. Platelet-derived growth factor is structurally related to the putative transforming protein p28sis of simian sarcoma virus. Nature. 1983 Jul 7;304(5921):35–39. doi: 10.1038/304035a0. [DOI] [PubMed] [Google Scholar]
- Yeh H. J., Pierce G. F., Deuel T. F. Ultrastructural localization of a platelet-derived growth factor/v-sis-related protein(s) in cytoplasm and nucleus of simian sarcoma virus-transformed cells. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2317–2321. doi: 10.1073/pnas.84.8.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zullo J. N., Cochran B. H., Huang A. S., Stiles C. D. Platelet-derived growth factor and double-stranded ribonucleic acids stimulate expression of the same genes in 3T3 cells. Cell. 1985 Dec;43(3 Pt 2):793–800. doi: 10.1016/0092-8674(85)90252-1. [DOI] [PubMed] [Google Scholar]