Abstract
Diabetic patients taking metformin have lower incidence of breast cancer than those taking other anti-diabetic medications. Additionally, triple negative breast cancer (TNBC), a form of breast cancer disproportionately afflicting premenopausal African American women, shows atypical susceptibility to metformin’s antiproliferative effect. The mechanisms involved in metformin’s function in TNBC has not yet been fully elucidated. Therefore, we sought to identify pathways regulated by metformin in using the MDA-MB-468 TNBC cell model. Metformin dose-dependently caused apoptosis, decreased cell viability, and induced cell morphology/chromatin condensation consistent with the permanent proliferative arrest. Furthermore, gene expression arrays revealed that metformin caused expression of stress markers DDIT3, CYP1A1, and GDF-15 and a concomitant reduction in PTGS1 expression. Our findings show that metformin may affect the viability and proliferative capacity of TNBC by inducing an antiproliferative gene signature, and that metformin may be effective in the treatment/prevention of TNBC.
Keywords: Metformin, breast cancer, ER stress, GDF15, DDIT3, PTGS1
Premenopausal African American women with breast cancer are more likely to die from the disease as compared to their counterparts of other ethnicity.1 Whereas socioeconomic parameters are thought to play a significant role in terms of health inequities in breast cancer patient survival, recent studies suggest that biological factors may play a substantial role in the elevated risk of breast cancer-related mortality among African American women. Indeed, certain clinico-pathological features which are predictive of poor prognosis are more prevalent in African American women. Three prognostic pathological features including expression of estrogen receptor α (ERα), progesterone receptor (PR), and the Her2 oncogene have become the basis by which sporadic (non-hereditary) breast cancer is categorized. Additionally the subtypes of breast cancer, based in part in the expression of these three markers, are predictive of patient outcomes.2,3 The phenotype marked by a lack of expression of all three of these biomarkers, triple negative breast cancer (TNBC), is disproportionately represented in premenopausal African American breast cancer patients and is a marker for shorter survival when compared with other breast cancer phenotypes.4,5 Due to the lack of ERα expression in TNBC, hormone-based therapies such as SERMs (selective estrogen receptor modulators) or aromatase inhibitors are ineffective modes of treatment. Additionally, therapeutic targeting of Her2 is ineffective due to the lack of Her2-expression in these tumors. As such, patients with TNBC are relegated to treatment with non-selective cytotoxic chemotherapy and the associated systemic side effects.2 Accordingly, the development of efficacious targeted therapy for the treatment or chemoprevention of TNBC would have a dramatic impact on the premenopausal African American women who have been diagnosed with or are at risk for breast cancer.
The anti-diabetic biguanide metformin has emerged as a potential therapeutic agent in the prevention and treatment of breast cancer.6 Moreover, it has been suggested that TNBC is specifically susceptible to the anti-proliferative and pro-apoptotic effects of metformin.7 Indeed, both in vivo and in vitro models have shown that metformin has anti-neoplastic effects against breast cancer cells, including TNBC models. Additionally, evidence suggest that metformin inhibits epithelial to mesenchymal transition in certain cancers, a phenotype marked by increased tumor invasiveness and enhance metastatic potential.8
Due to its potential as an anti-neoplastic agent, metformin has garnered much interest and several mechanisms of action have been identified. The canonical pathway involves activation of AMP (adenosine monophosphate)-dependent kinase (AMPK), which functions as a metabolic stress sensor, responding to increased AMP levels in the cell. AMPK activation subsequently leads to a decrease in mTOR (mammalian target of rapamycin) and increased p53 activity.9 However, other studies have suggested that AMPK is not necessary for metformin’s antineoplastic activity, suggesting that several parallel mechanisms may play a role in mediating metformin’s effects.10
Here, we investigated the effects of metformin on breast cancer cell viability/proliferation and stress-related gene expression using the MDA-MB-468 triple negative breast cancer cell model. Metformin impaired proliferation of MDA-MB-468 cells and induced phenotypic changes typically associated with cellular senescence. The induction of the senescent phenotype is prefaced with a metformin-induced gene expression signature composed of genes related to the endoplasmic reticulum stress and metabolic/oxidative stress pathways. These studies contribute to a more detailed understanding of the mechanisms by which metformin may elicit anti-neoplastic activity in TNBC cells, and provide an impetus for its incorporation into the therapeutic armamentarium for treatment of TNBC.
Methods
Tissue culture
MDA-MB-468 cells were acquired from the American Tissue Type Collection (ATCC). Cells were maintained in Dulbecco’s modified eagle medium (DMEM) supplemented with 10% fetal bovine serum and 10% penicillin/streptomycin antibiotic/antimycotic. Cultures were incubated at 37°C and 5% CO2 and sub-cultured twice weekly at a 1:4 ratio.
Crystal violet cell viability assays
MDA-MB-468 cells were plated in six well plates at a density of 1 × 105 cells per well. 24 hours after plating, cells were either mock treated or treated with metformin for 48 hours, cells were washed with PBS (phosphate buffered saline) and subsequently incubated for five days under normal tissue culture conditions. On day seven, cultures were washed with PBS three times and subsequently fixed in 1% formaldehyde in PBS for 10 minutes at room temperature. Plates were washed in distilled water and subsequently stained in crystal violet staining solution (0.1% crystal violet in 50% methanol) for 10 minutes. Plates were subsequently washed in distilled water to remove unbound stain. Images for crystal violet-stained plates were acquired and analyzed on the VersaDoc imaging station (Bio-Rad) as a measure of cell density. Background signal was subtracted and staining intensity normalized to untreated control (100%).
Morphological analysis
Apoptosis was determined by morphological analysis. MDA-MB-468 cells were plated and incubated in the absence or presence of metformin at indicated concentrations for 24 or 48 hours. Cells were fixed with 1% formaldehyde and analyzed by phase-contrast microscopy on an Olympus BX41 inverted microscope. To determine the effect of metformin on chromatin condensation, 2 × 104 MDA-MB-468 cells were plated on chamber slides, incubated with or without metformin for 48 hours, and subsequently cultured in serum free media for five additional days. Cells were fixed in 1% formaldehyde/PBS for 10 minutes, and subsequently stained in DAPI (4′,6-diamidino-2-phenylindole). Images were analyzed for evidence of senescence-associated heterochromatin/chromatin condensation. Fluorescence imaging was performed using a BX51 epifluorescence microscope with a mounted Olympus DP72 color camera.
Gene expression analysis with qrt-PCr and qrt-PCr arrays
MDA-MB-468 cells were treated for 48 hours, and RNA was isolated using Qiagen RNeasy RNA extraction in accordance with the manufacturer’s instructions. RNA quality was determined using Experion automated gel electrophoresis (Bio-Rad, RNA StdSens chip). Complimentary DNA was synthesized from 0.5 μg RNA using the RT2 first strand synthesis kit (SA Biosciences), was incubated with RT2 SYBR green master mix (SA Biosciences), and applied to Stress and Toxicity Pathfinder arrays, version 3.0 (SA Biosciences). Confirmation with specific cDNA sequences was performed in accordance with the manufacturer’s protocols, using optimized qSTAR primer pairs (Origene) qRT-PCR for DDIT3 (HP207450), GDF-15 (HP208119), PTGS1 (HP200899), and CYP1A1 (HP200471) in triplicate. All PCR amplifications were performed using an IQ5 Real Time PCR detection system (Biorad). Gene expression was normalized using the ΔΔ CT method.
Results
Metformin induces proliferative arrest and apoptosis in a concentration-dependent fashion
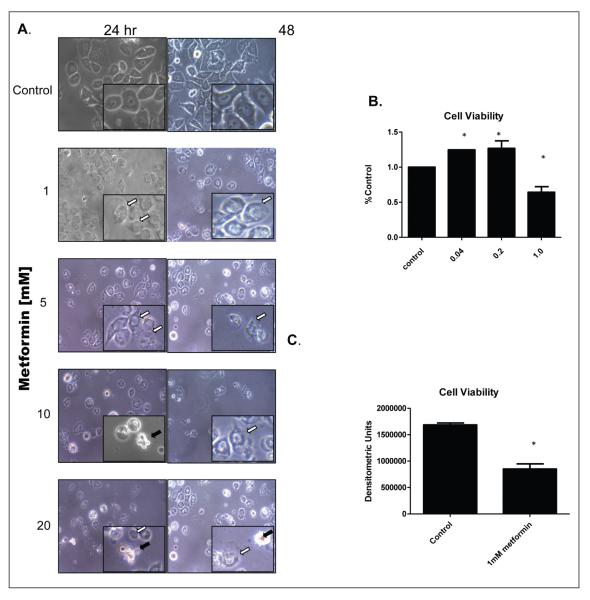
Several studies have demonstrated that metformin has pro-apoptotic effects at concentration of 5 mM and above, a concentration which is thought to correlate with its tissue concentrations in vivo.7 In our studies, 5–20 mM metformin caused a substantial induction of apoptosis as evidenced by the presence of apoptotic bodies at both 24 and 48 hours (Figure 1A, black arrows). Of interest was the fact that 1 mM metformin caused morphological changes (white arrows) at 24 and 48 hours of exposure to metformin, but did not show prominent apoptosis as was evident with greater concentrations of metformin. In subsequent experiments, we determined that 1 mM metformin proved to be a threshold concentration at which impaired cell proliferation was evident at 72 hours, with a significant increase in cell viability at 0.04 mM and 0.2 mM (Figure 1B). Interestingly, when cells were incubated with metformin for 48 hours and incubated for an additional five days, the antiproliferative effect was still apparent, suggesting that metformin exposure caused long-term impairment of the cell proliferative capacity, with a greater than 50% decrease in staining intensity as compared to untreated control (Figure 1C). Still, attached and viable cells were detected even at seven days of exposure. Our studies show that metformin has a multiphase effect on tumor cells, with suboptimal concentrations leading to slightly increased cell viability, moderate concentrations causing a sustained decrease in proliferation and the higher doses initiating apoptotic cell death.
Figure 1.
A) Metformin causes concentration dependent apoptosis and proliferative arrest in MDa-MB-468 breast cancer cells. 1 × 105 MDA-MB-468 cells were exposed to metformin for 24 and 48 hours at indicated concentration, and images captured using phase contrast microscopy (400X). White arrows indicate early senescent cells. Black arrow indicate plasma membrane blebbing, indicative of apoptosis. B, C ) 1 mM metformin is the minimum effective concentra-tion for inhibition of cell viability in MDa-MB-468 cells. Cells were plated at 1 × 105 cells per well and after 24 hours, incubated with increasing concentrations of metformin for 72 hours, quantified and normalized to % control (untreated). In panel C, cells were treated for 48 hours, and cell viability determined at day 7 as described. Error bars represent standard deviation of the mean Statistical analysis ANOVA with post-hoc analysis A) or Student’s T-test B).* denotes where p≤0.05 when compared to control.
Metformin induces a senescence-like phenotype in MDa-MB-468 breast cancer cells
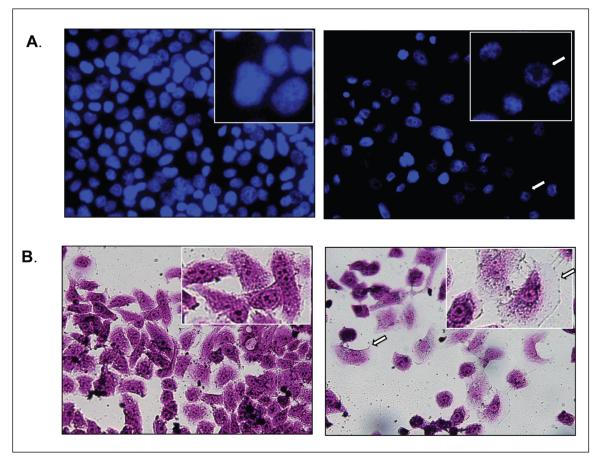
Since 1 mM metformin caused inhibition of cell proliferation without apparent induction of apoptosis, we sought to determine if sub-apoptotic concentration of metformin might mediate these effects by inducing cell senescence. Two hallmarks of cell senescence, cellular hypertrophy and chromatin condensation, were determined in the presence or absence of metformin. MDA-MB-468 cells were exposed to 1 mM metformin for 48 hours and microscopically observed at day seven. Heterochromatic nuclei were observed in metformin treated cells, indicative of senescence in cells previously exposed to 1 mM metformin (figure 2A, white arrows). Morphologically, cells exposed to metformin are enlarged and flattened, consistent with induction of stress-induced senescence (figure2B, white arrows). β galactosidase activity, which is considered a marker but is not required for senescence, was not increased with metformin treatment (data not shown). Together, these findings show that metformin mediates its anti-neoplastic effects in part by permanently inhibiting proliferation through inducing senescence.
Figure 2.
a) Metformin induces senescence-associated heterochromatic nuclear staining. 2 × 104 cells were plated on chamber slides and after 24 hours, incubated in the presence or absence of 1 mM metformin for 7 days and stained in DAPI nuclear stain (blue). Punctate nuclear staining is indicative of stress induced cell senescence. B) Metformin causes a senescent phenotype in breast cancer cells. 105 cells were plated each well of a 6 plate. After 24 hours, cells were incubated in presence or absence (control) of 1 mM metformin. Cultures were released from metformin after 2 days and cultured for an additional 5 days, after which they were fixed in formaldehyde and stained with crystal violet. Cell hypertrophy is indicated by arrows.
Metformin induces a gene expression signature involving metabolic stress response and proliferative arrest/senescence-associated genes
Since metformin induced significant changes in cell viability and mediated a senescence-like phenotype, we sought to identify perturbations in the expression of genes involved in stress and toxicity responses using a RT-PCR array format. Arrays consist of 96 well plates containing gene specific primers for genes categorized according to their role in the following: metabolic/oxidative stress, heat shock, proliferation/carcinogenesis, growth arrest/senescence, inflammation, or apoptosis/necrosis. Genes that showed a two-fold or greater change in expression with fewer than 30 cycles of amplification were considered as significantly induced/repressed (Table 1). Of 84 genes, four met the criteria for inclusion. (CYP1A1, PTGS1, DDIT3, and GDF-15). These genes represent two clusters of gene signature response: metabolic/oxidative stress (PTGS1 and CYP1A1), and growth arrest/senescence (GDF15 and DDIT3). CYP1A1 and PTGS1 were conversely regulated by treatment with metformin, with CYP1A1 being induced whereas PTGS1, the gene encoding cyclooxgenase 1, was decreased. DDIT3 and GDF-15, both components of the growth arrest/senescence subset of genes, were each induced by metformin administration. The gene expression signature induced by metformin is consistent with the induction of stress pathways which to culminate in growth arrest and cellular senescence.
Table 1.
STRESS RELATED GENE EXPRESSION SIGNATURE INDUCED BY METFORMIN
| Gene | Fold induction | Gene | Fold induction |
|---|---|---|---|
| PTGS1 | −2.60 | HSF1 | 1.47 |
| GDF15 | 25.61 | HSPA1A | −1.12 |
| CYP1A1 | 3.38 | HSPA1L | −1.24 |
| DDIT3 | 3.29 | HSPA2 | −1.12 |
| ANXA5 | 1.15 | HSPA4 | −1.21 |
| ATM | −1.24 | HSPA5 | 1.28 |
| *BAX | 2.03 | HSPA6 | 1.35 |
| BCL2L1 | −1.32 | HSPA8 | −1.10 |
| CASP1 | 1.62 | HSPB1 | 1.37 |
| CASP10 | −1.17 | HSP90AA2 | 1.01 |
| CASP8 | 1.14 | HSP90AB1 | −1.05 |
| CAT | 1.07 | HSPD1 | 1.08 |
| CCL21 | 1.35 | HSPE1 | 1.02 |
| CCL3 | 1.69 | HSPH1 | 1.12 |
| CCL4 | 1.35 | IGFBP6 | −1.03 |
| CCNC | 1.34 | IL18 | 1.61 |
| CCND1 | 1.20 | IL1A | 1.23 |
| CCNG1 | 1.36 | *IL1B | 2.30 |
| CDKN1A | 1.26 | IL6 | 1.45 |
| CHEK2 | 1.32 | *LTA | 2.15 |
| CRYAB | 1.52 | MDM2 | −1.11 |
| *CSF2 | 4.20 | MIF | −1.13 |
| CXCL10 | −1.04 | MT2A | 1.06 |
| *CYP2E1 | − 2.21 | NFKB1 | 1.24 |
| *CYP7A1 | −3.25 | NFKBIA | 1.32 |
| DDB1 | −1.11 | NOS2 | 1.57 |
| DNAJA1 | −1.05 | PCNA | −1.16 |
| DNAJB4 | 1.10 | POR | 1.11 |
| E2F1 | −1.30 | PRDX1 | −1.04 |
| EGR1 | 1.80 | PRDX2 | 1.06 |
| EPHX2 | −1.18 | RAD23A | 1.33 |
| ERCC1 | 1.17 | RAD50 | 1.00 |
| ERCC3 | 1.09 | SERPINE1 | 1.08 |
| FASLG | 1.35 | SOD1 | 1.29 |
MDA-MB-468 cells were cultured for 48 hours in the absence or presence of 1 mM metformin after which cDNA was synthesized and applied to stress and toxicity pathway finder RT-PCR array plates. Data was analyzed by the delta delta CT method. Genes meeting the following criteria were chosen for independent confirmation with separate primer sets: 1) greater than 2 fold induction/repression, 2) CT values less than 30 in at least 1 sample 3) and melt curve profiles consistent with a single gene product. Genes meeting only 2 of these criteria are designated with an *. GDF15, CYP1A1, PTGS1, and DDIT3 are the only four of 84 test genes to meet all three criteria, and are designated in bold type. Gene names are those designated by HGNC (HUGO Gene Nomenclature Committee).
Discussion
The overall goal of this study was to elucidate the mechanisms involved in metformin’s antineoplastic effects in TNBC, and to ascertain the feasibility of metformin being employed as a therapeutic or chemopreventive agent in breast cancer. The findings of this study not only confirm that high concentrations of metformin induce apoptosis in TNBC, but also demonstrates that moderate levels of exposure to metformin induce a senescent phenotype in TNBC cells, and at concentrations below threshold level can actually promote survival. In accordance with this senescent phenotype is the observation that metformin modulates the expression of several genes involved in adaptive stress response in breast cancer cells. These findings lend evidence to the notion that low dose metformin may be a viable, relatively safe option in the treatment and/or prevention of breast cancer in TNBC with a high degree of tolerance for such patients.
Metformin as an option in the treatment/prevention of breast cancer
Recent studies have explored the potential role of metformin as an antineoplastic agent. After the observance that diabetic patients taking metformin experienced lower incidence of breast cancer, the prevailing thought was that treatment with metformin helped to resolve incidence of hyperglycemia and hyperinsulinemia, and thereby reducing the availability of both glucose and insulin for the support of tumorigenesis.6,11 However, Zakikhani et al. showed that metformin, in an AMPK and mTOR dependent manor, decreased proliferation of several breast cancer cells in vitro, leading toward a model involving direct antiproliferative/apoptotic effect on breast cancer.12 However these and most other studies have shown the antiproliferative and proapoptotic effects of metformin occur at concentrations of 5 mM or greater. More recently, studies have suggested that metformin has differential effects on distinct cell population within heterogeneous cell populations, reflecting the cell context-dependence of metformin’s effect in cancer.13–16
Triple negative breast cancer cell lines are differentially responsive to metformin
There has been evidence that metformin is more efficacious against triple negative breast cancer cell lines as compared to luminal and Her2 breast cancer lines.7 A 2009 study comparing triple negative, luminal, and Her2 breast cancer cell types showed that metformin was able to induce apoptosis in triple negative cell lines, but not in those lines which either expressed ERα or overexpressed the Her2 oncogene. As evidenced in our own studies however, the apoptotic effect of metformin was shown at higher concentrations of metformin (20–30 mM). A recent study suggests that the TNBC cells lines are commonly dependent on STAT3, and that metformin inhibits this activity.17 Moreover, it has been shown that metformin’s apoptotic effect is contingent on STAT3 inhibition, in that inhibition of STAT3 activity either pharmacologically or through siRNA-mediated silencing, potentiated the effects of metformin in TNBC cells. However, a retrospective study examining the clinical outcomes of patients with triple negative breast cancer receiving metformin in an adjuvant setting did not show a significant difference in patient survival.18 Though limited by a relatively small number of patient samples, theses finding suggest that metformin may be most efficacious in the chemopreventive setting for TNBC.
Metformin-induced gene expression signature
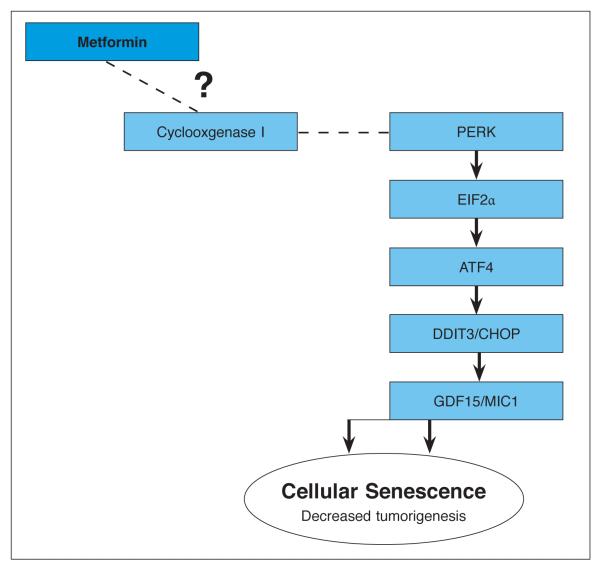
Quantitative RT-PCR Stress and Toxicity pathway finder arrays were used to identify potential candidate pathways involved in the response of MDA-MB-468 cells to metformin. Four genes met the predetermined criteria for changes in expression in response to 1 mM metformin, representing two functional groupings (metabolic/oxidative stress and growth arrest/ senescence). The induction of GDF-15 and DDIT3 are associated with decreased cell proliferation. In the oxidative/metabolic stress functional grouping, metformin induces countervailing trends, with the repression of PTGS1 and simultaneous induction of CYP1A1. CYP1A1 might be expected to increase reactive oxygen species generation through enhanced oxidative metabolism. PTGS1 however, is reduced upon metformin treatment, and might be expected to result in a decrease in inflammation-related reactive oxygen species formation by breast cancer cells. However, it should not be overlooked that the cycloxygenase 1, the gene product of PTGS1, is involved in the synthesis of both inflammatory and anti-inflammatory prostanoids. As such it is difficult to determine which is the predominant effect on cellular physiology, or how that might affect cell viability and/or proliferation.19 These arrays however suggest that suppression of PTGS1 is potentially a key signaling hub in the induction of endoplasmic reticulum stress response by metformin. It has been shown that salicylates, which are inhibitors of COX-1 enzymattic activity, result in the induction of DDIT3 downstream of the EIF2a-PERK-DDIT3 pathway.20 Furthermore, GDF15 is strongly induced by nonsteroidal antiinflammatory drugs (NSAIDs) which target COX enzymatic activity.21,22 Finally, NSAID-induced activation of endoplasmic reticulum stress response, including induction of DDIT3, results in increased GDF15 expression.23 A rational extension of these findings then, is that metformin causes transcriptional downregulation of PTGS1 and subsequent COX1 activity, resulting in DDIT3 activation and impaired proliferation. Of equal importance is the fact that, with the exception of Bax, no upregulation of apoptosis or necrosis associated genes was observed, highlighting the fact that the effect of metformin on gene expression is specific to this pathway. Thus, our proposed model for metformin effects in breast cancer cells is initiated with a reduction in PTGS1 expression, and culminating with an increased expression of DDIT and GDF15, thereby contributing to proliferative arrest and senescence (Figure 3). Our findings, when taken into the context of previously published data, suggest that suppression of PTGS1 is a key signaling event in the induction of endoplasmic reticulum stress and subsequent cell senescence in breast cancer in response to metformin.
Figure 3.
Model of metformin’s affect on TNBC cells.
In this model, PTGS1, which encode COX1 (cyclooxygenase 1), is transcriptionally repressed by metformin. As COX1 activity is decreased, PERK is activated, and phosphorylates EIF2a. Phosphorylated EIF2α in turn causes selective translation of ATF4, which in turn contributes to DDIT3 gene expression. DDIT3 then regulates expression of antiproliferative genes. Among these is GDF15, which has been associated with senescence in cancer.
In conclusion, we have demonstrated that exposure of MDA-MB-468 cells, a model for TNBC breast cancer, to the antidiabetic drug metformin results in the induction of senescence with concomitant induction of antiproliferative genes DDIT3 and GDF-1 and repression of PTGS1. These findings are significant in that several key intermediates in metformin signaling in TNBC can be pharmacologically manipulated to synergize or otherwise enhance the anti-proliferative effects of metformin.
Acknowledgments
This publication was made possible by funding from the Louisiana Cancer Research Consortium and the NIH-RCMI grant 5G12RR026260 from the National Institute on Minority and Minority Health Disparities, and NIH grant 5K01 CA129078 from the National Cancer Institute. The contents are solely the responsibility of the author and does not necessarily represent the official views of the LCRC or the NIH.
Notes
- 1.Walker B, Figgs LW, Zahm SH. Differences in cancer incidence, mortality, and survival between African Americans and whites. Environ Health Perspect. 1995 Nov;103(Suppl 8):275–81. doi: 10.1289/ehp.95103s8275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anders CK, Carey LA. Biology, metastatic patterns, and treatment of patients with triple-negative-breast cancer. Clin Breast Cancer. 2009 Jun;(9 Suppl 2):S73–81. doi: 10.3816/CBC.2009.s.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morris SR, Carey LA. Molecular profiling in breast cancer. Rev Endocr Metab Disord. 2007 Sep;8(3):185–98. doi: 10.1007/s11154-007-9035-3. 2007. [DOI] [PubMed] [Google Scholar]
- 4.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295(2):2492–502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 5.Millikan RC, Newman B, Tse CK, et al. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat. 2008 May;109(1):123–39. doi: 10.1007/s10549-007-9632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Decensi A, Puntoni M, Goodwin P, et al. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2010 Nov;3(11):1451–61. doi: 10.1158/1940-6207.CAPR-10-0157. [DOI] [PubMed] [Google Scholar]
- 7.Liu B, Fan Z, Edgerton SM, et al. Metformin induces unique biological and molecular responses in triple negative breast cancer cells. Cell Cycle. 2009 Jul;8(13):2031–40. doi: 10.4161/cc.8.13.8814. [DOI] [PubMed] [Google Scholar]
- 8.Cufí S, Vazquez-Martin A, Oliveras-Ferraros C, et al. Metformin against TGFβ-induced epithelial-to-mesenchymal transition (EMT): from cancer stem cells to aging-associated fibrosis. Cell Cycle. 2010 Nov;9(22):4461–8. doi: 10.4161/cc.9.22.14048. [DOI] [PubMed] [Google Scholar]
- 9.Martin-Castillo B, Vazquez-Martin A, Oliveras-Ferraros C, et al. Metformin and cancer: doses, mechanisms and the dandelion and hormetic phenomena. Cell Cycle. 2010 Mar;9(6):1057–64. doi: 10.4161/cc.9.6.10994. [DOI] [PubMed] [Google Scholar]
- 10.Ben Sahra I, Regazzetti C, Robert G, et al. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res. 2011 Jul;71(13):4366–72. doi: 10.1158/0008-5472.CAN-10-1769. [DOI] [PubMed] [Google Scholar]
- 11.Hede K. Doctors seek to prevent breast cancer recurrence by lowering insulin levels. J Natl Cancer Inst. 2008 Jul;100(8):530–2. doi: 10.1093/jnci/djn119. [DOI] [PubMed] [Google Scholar]
- 12.Zakikhani M, Dowling R, Fantus IG, et al. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 2006 Nov;66(2):10269–73. doi: 10.1158/0008-5472.CAN-06-1500. [DOI] [PubMed] [Google Scholar]
- 13.Song CW, Lee H, Dings RP, et al. Metformin kills and radiosensitizes cancer cells and preferentially kills cancer stem cells. Sci Rep. 2012;2:362. doi: 10.1038/srep00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vazquez-Martin A, Oliveras-Ferraros C, Cufí S, et al. Metformin regulates breast cancer stem cell ontogeny by transcriptional regulation of the epithelial-mesenchymal transition (EMT) status. Cell Cycle. 2010 Sep;9(18):3807–14. [PubMed] [Google Scholar]
- 15.Bednar F, Simeone DM. Metformin and cancer stem cells: old drug, new targets. Cancer Prev Res (Phila) 2012 Mar;5(3):351–4. doi: 10.1158/1940-6207.CAPR-12-0026. [DOI] [PubMed] [Google Scholar]
- 16.Cufi S, Corominas-Faja B, Vazquez-Martin A, et al. Metformin-induced preferential killing of breast cancer initiating CD44+CD24−/low cells is sufficient to overcome primary resistance to trastuzumab in HER2+ human breast cancer xenografts. Oncotarget. 2012 Apr;3(4):395–8. doi: 10.18632/oncotarget.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng XS, Wang S, Deng A, et al. Metformin targets Stat3 to inhibit cell growth and induce apoptosis in triple-negative breast cancers. Cell Cycle. 2012 Jan;11(2):367–76. doi: 10.4161/cc.11.2.18813. [DOI] [PubMed] [Google Scholar]
- 18.Bayraktar S, Hernadez-Aya LF, Lei X, et al. Effect of metformin on survival outcomes in diabetic patients with triple receptor-negative breast cancer. Cancer. 2012 Mar;118(5):1202–11. doi: 10.1002/cncr.26439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams CS, Mann M, DuBois RN. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene. 1999 Dec;18(55):7908–16. doi: 10.1038/sj.onc.1203286. [DOI] [PubMed] [Google Scholar]
- 20.Silva AM, Wang D, Komar AA, et al. Salicylates trigger protein synthesis inhibition in a protein kinase R-like endoplasmic reticulum kinase-dependent manner. J Biol Chem. 2007 Apr;282(14):10164–71. doi: 10.1074/jbc.M609996200. [DOI] [PubMed] [Google Scholar]
- 21.Baek SJ, Kim KS, Nixon JB, et al. Cyclooxygenase inhibitors regulate the expression of a TGF-beta superfamily member that has proapoptotic and antitumorigenic activities. Mol Pharmacol. 2001 Apr;59(4):901–8. [PubMed] [Google Scholar]
- 22.Baek SJ, Wilson LC, Lee CH, et al. Dual function of nonsteroidal anti-inflammatory drugs (NSAIDs): inhibition of cyclooxygenase and induction of NSAID-activated gene. J Pharmacol Exp Ther. 2002 Jun;301(3):1126–31. doi: 10.1124/jpet.301.3.1126. [DOI] [PubMed] [Google Scholar]
- 23.Yang H, Park SH, Choi HJ, et al. The integrated stress response-associated signals modulates intestinal tumor cell growth by NSAID-activated gene 1 (NAG-1/MIC-1/ PTGF-beta) Carcinogenesis. 2010 Apr;31(4):703–11. doi: 10.1093/carcin/bgq008. [DOI] [PubMed] [Google Scholar]