Abstract
Chaperone proteins and heat shock proteins (HSP) are essential components of cellular protein folding systems under normal conditions; their expression and activities are upregulated during stress. Chronically stressed tumors frequently exhibit high chaperone protein levels, exploiting their anti‐apoptotic mechanisms and general proteome homeostasis amidst a background of genetic instability. Co‐chaperones interact with chaperones as malleable regulatory components of protein folding activity and may represent a conduit for modification of chaperone activity to the detriment of the tumor. We have initially characterized one such co‐chaperone, heat shock protein 70‐binding protein (HspBP) 1 from human brain tumors, their xenografts grown in immune‐compromised mice, and in syngeneic murine models in immune‐competent mice. Immunohistochemical analyses show HspBP1 overexpression (with unusual subcellular localizations) in patient brain tumors relative to normal brain tissue. This holds true for the xenograft and syngeneic murine tumor models. In biochemical affinity chromatography assays, HspBP1 interacts with members of the HSP70 family from brain tumor lysates and from surface‐derived samples, including HSP70, glucose regulated protein (GRP)75, GRP78, and HSP110. From normal brain lysates, only heat shock cognate (HSC)70, GRP75, and HSP110 bind to HspBP1. FACS analyses indicate that HspBP1 binds to brain tumor cell surfaces, possibly via HSP70 family members, and internalizes into cells. This has implications for HspBP1 biology as well as its utility as a tumor‐targeting agent. Our results suggest that HspBP1 may play a role in tumor (dys)regulation of chaperone proteins, and that HspBP1 may have extracellular roles with therapeutic implications. (Cancer Sci 2009; 100: 1870–1879)
Solid tumors are generally stressed tissues, frequently expressing high levels of numerous stress proteins, especially members of the chaperone and heat shock protein (HSP) family.( 1 ) These stresses may take the form of hypoxia, pH or redox imbalances, accelerated metabolism, and immune insult.( 2 , 3 , 4 , 5 ) HSP cytoprotective activities allow tumors to thrive amidst the hostile environments of the host response and of the tumor's own making.( 1 , 6 , 7 ) Surface display of HSP also may occur on tumor cells (but not normal cells), although the biology behind this is unclear.( 8 , 9 , 10 )
Although there have been a number of immunohistochemical studies of HSP in brain tumors (reviewed in Graner and Bigner( 11 )), there are relatively few reports of stress activity characterization in central nervous system (CNS) tumors.( 12 , 13 , 14 ) We have recently demonstrated that brain tumors express high levels of HSP, and are capable of impressive induction of such chaperones, particularly members of the HSP70 family.( 15 ) In addition to their robust HSP expression, brain tumor cells also display HSP and cohorts on their cell surfaces, including the HSP70 co‐chaperone heat shock protein binding protein (HspBP) 1.( 15 )
HspBP1 is a HSP70 co‐chaperone involved in nucleotide exchange during the chaperone cycle of HSP70.( 16 , 17 , 18 ) HspBP1 expression is upregulated in a number of murine tumors,( 19 ) and this expression has been implicated in increased drug sensitivity of tumor cells as an antagonist of HSP70 pro‐survival activity.( 20 ) Studies of co‐chaperones in brain tumors are almost completely lacking in the literature;( 21 ) we show for the first time in this report that HspBP1 is highly expressed in human brain tumors, and that it has the capacity to interact with multiple HSP70 family members, including glucose regulated protein (GRP)75 and GRP78. Furthermore, we demonstrate that HspBP1 can bind directly to brain tumor cell surfaces, both in cell lines and disaggregated xenograft tumors. This binding is partly inhibited by HSP70 antibodies, indicating that other factors may be involved. Certain brain tumor cell surface‐biotinylated HSP70 family members can also bind to HspBP1, and the co‐chaperone is internalized into tumor cells upon exogenous addition to the cells. Thus, HspBP1 may be a means of targeting tumor cells with expression of one or more HSP70 types on their surfaces either as drug‐ or radio‐conjugates or in some other toxified construct.
Materials and Methods
Cells and xenografts, culture and lysate preparations, and LDS‐PAGE and western blotting. The cell lines, xenografts, and syngeneic tumors D54MG, D392MG, D341MED, and SMA560 have all been described before.( 15 ) 12B1 is a syngeneic murine model of chronic myelogenous leukemia,( 22 , 23 ) and GL261 is a syngeneic murine glioma from American Type Culture Collection (Manassas, VA, USA). SK‐MEL‐28 is also from the American Type Culture Collection. The cells/xenografts D247MG, D283MED, D256MG, and NR6M have been described previously.( 24 , 25 , 26 , 27 ) Xenografts H2156 (adult glioma) and H2159MG (pediatric glioma) were from the Duke collection from The Preston Robert Tisch Brain Tumor Center at Duke University, Durham, NC, USA. Cells, xenografts, and syngeneic tumors were grown as described previously, as were lysate preparations.( 15 ) All animal experimentation was carried out under the auspices of Duke Institutional Animal Care and Use Committee (IACUC)‐approved protocols at the Cancer Center Isolation Facility. Sample preparation for lithium dodecyl sulphate (LDS)‐PAGE, electroblotting, and probing of the western blots (including the antibodies used) has been previously described.( 15 ) Anti‐HSP/HSC70 monoclonal antibody was from Assay Designs (SPA‐820; Ann Arbor, MI, USA). Densitometry measurements of HSP/HSC70 expression were made relative to actin staining using Image J (http://rsb.info.nih.gov/ij/).
Tissue microarrays and immunohistochemistry. Human tissue microarrays (TMA) were purchased from Cybrdi (Gaithersburg, MD, USA). Immunohistochemistry (IHC) using the sheep anti‐HspBP1 antibody( 28 ) was carried out as described previously.( 15 ) Scoring was based on an intensity scale of 0 (no staining) to 4 (highest observed on any tumor or tissue). Distributions of the staining patterns were classified as cytoplasmic, nuclear, perinuclear, or mixtures of those patterns.
Chaperone binding to immobilized HspBP1. Lysates from solid tumors or from cell surface‐biotinylated cells or lysates were prepared as described previously.( 15 ) Generation of his‐tagged recombinant HspBP1 and utilization of an immobilized metal affinity chromatography (IMAC) matrix has been described previously( 16 ) with a Co2+ resin (TALON; Clontech, Palo Alto, CA, USA) substituted for a Ni2+ resin. Lysates were passed over the matrix; the matrix was washed, and eluted with imidazole as described in Reference 16. Flow‐through and eluted proteins were separated on LDS‐PAGE, electroblotted, and blots probed as mentioned above. An additional antibody used was anti‐HSP110 (clones 21; BD Biosciences Transduction Laboratories, Lexington, KY, USA). Blots were also probed for HSP90 and HSP27 as negative controls.
FACS using fluorescent HspBP1. Cell line preparations, disaggregated tumors and tissues were prepared for FACS analyses as described previously.( 15 ) Recombinant HspBP1 (full length) and the core domain of HspBP1 (residues 84–359( 29 )) were labeled with FITC as described for labeling of antibodies.( 15 ) There were no discernable differences between the two protein forms when used in FACS. For blocking studies, fixed D54MG cells were incubated with excess amounts of either HspBP1 protein (300 µg/mL) or anti‐HSP70 antibodies (100 µg/mL) for 15 min prior to staining with either FITC‐labeled HspBP1 (30 µg/mL) or with FITC‐labeled anti‐HSP70 monoclonal antibodies (10 µg/mL).
Internalization studies. Unlabeled, 6 × his‐tagged HspBP1 (10 µg/mL) was incubated (‘pulsed’) on D54MG cells for time points of 7.5, 15, 30, 60, and 180 min. At the end of the incubation period, cells were either fixed with paraformaldehyde( 15 ) for surface staining only, or were fixed and permeabilized with 0.1% saponin (Sigma, St Louis, MO, USA) for both surface and intracellular staining (total staining). Cells were washed and incubated with AlexaFluor 488‐labeled anti‐penta‐his antibody (Molecular Probes, Eugene, OR, USA) to detect surface and total exogenously added HspBP1, detectable by its 6 × his tag. Differences in mean fluorescence intensity over various time points are indicative of internalization of HspBP1. An irrelevant his‐tagged protein (an inactive single‐chain variable region fragment molecule) was used as a negative control, and gave the same results as the AlexaFluor 488 anti‐penta‐his antibody alone.
Nomenclature. Although the nomenclature of the chaperone family members is evolving,( 30 ) for this study we will maintain the more common usage for chaperones. Those proteins cited in the text are named as follows under the developing guidelines: HSP70 = HSPA1A; HSC70 = HSPA8; GRP75 = HSPA9; GRP78 = HSPA5; HSP110 = HSPH2; HSP90 = HSPC1, HSPC2, or HSPC3 (the antibodies used did not distinguish between these members); HSP27 = HSPB1; and HSP40 = DNAJB1.
Results
HspBP1 is highly expressed in brain tumors. We have previously shown that HspBP1 is expressed by brain tumor cell lines and xenografts( 15 ) in western blots and IHC. Here, we have extended the tumor repertoire analyzed by western blotting to include additional human tumor xenografts (D256, a glioblastoma multiforme, and the medulloblastoma D283) as well as the widely used murine brain tumor model GL261. We have also used the murine leukemia model 12B1( 23 ) and SK‐MEL‐28 as non‐CNS tumor controls (Fig. 1). All of the tumors were grown in either syngeneic mice (12B1 and GL261) or immune‐incompetent rats (D256MG, D283MED, and SK‐MEL‐28), and rat brain homogenate was used as the normal control tissue. GRP78 and HSP/HSC70 (antibody recognizes both constitutive and inducible forms) are overexpressed by the tumors compared to brain, as is HspBP1, especially the human tumor xenografts. Of note here, HSC70 expression in brain is comparable to that of tumors( 15 ) whereas HSP70 expression is low in brain but high in tumors.( 15 ) Because the antibody used here recognizes both forms of the chaperone, the expression of the constitutive HSC70 tends to dominate the signal. Densitometry measurements of the western blots show that the HSP/HSC70 expression by the brain tumors (but not 12B1) is 1.14–1.25 times higher than brain (data not shown). The antibody used here was raised against a region of human HspBP1 (amino acid residues 84–359) that shares 98% identity to mouse and rat HspBP1. Thus, it is likely that the cross‐reactivity of antibody (i.e. affinity for the different species of HspBP1) is sufficiently high that our comparisons from human xenograft tumors to rodent brain are legitimate. However, we have not definitively determined nor directly measured the antibody's affinity for HspBP1 of different species.
Figure 1.
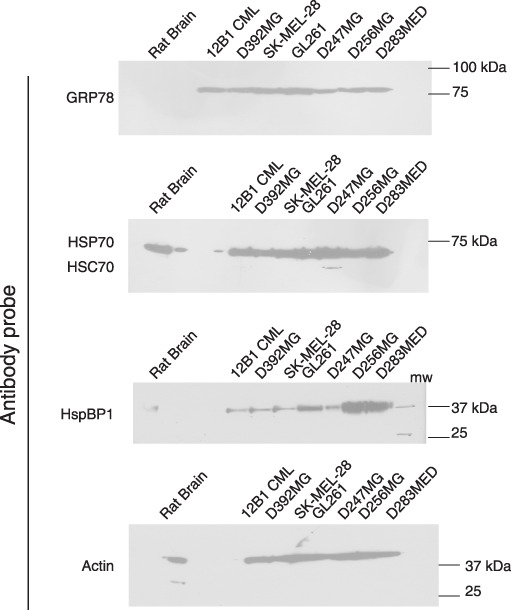
Western blots showing overexpression of heat shock protein binding protein (HspBP) 1 compared to brain tissue. Solid tumors and rat brain were lysed and proteins (20 µg) were separated on lithium dodecyl sulphate (LDS)‐PAGE and blotted to nitrocellulose. Blots were probed with antibodies against the chaperones as listed, with actin consisting as a loading marker. Molecular weight standards are listed at right (in kDa). Results are typical of at least three experiments. GRP, glucose regulated protein; HSC, heat shock cognate; HSP, heat shock protein.
In an IHC assay we probed human high‐grade glioma TMA with a sheep antibody against HspBP1.( 28 ) The TMA consisted of both glioblastoma multiforme (grade IV astrocytomas) and grade III anaplastic astrocytomas, all of which stained positively, and in some cases, very intensely. We saw both nuclear and cytoplasmic staining in both types of high‐grade gliomas, with no apparent relationship to tumor grade (Fig. 2a,b; Table 1); there were unusual patterns of staining of cells in a pattern adjacent to cells that surrounded blood vessels (Fig. 2, lower left panel). We also saw evidence of putative membrane staining (Fig. 2b, lower right panel, arrows in higher magnification). Brain tissues and other CNS structures showed little or no staining (data not shown), except for pyramidal neurons as seen previously.( 15 ) This corroborates the western blot data, and demonstrates for the first time in human brain tumors that HspBP1 is highly expressed. The staining patterns seen here are more varied than was described for HspBP1 staining in IHC of breast tumors.( 31 ) In particular, we did not necessarily see concomitant nuclear and cytoplasmic staining of tumor tissue, but in some cases we even witnessed exclusive cytoplasmic or nuclear staining (Table 1).
Figure 2.


Immunohistochemistry (IHC) of human brain tumor sections using an anti‐heat shock protein binding protein (HspBP) 1 antibody. Representative sections from a tissue microarray are shown. The top two panels on the right are control‐stained sections (normal goat IgG) and correspond to sections to the top left. (a) Black bars = 50 µm. (b) Boxed regions from (a) were magnified for more detail; black bars = 25 µm. Arrows in the lower right panel show possible membrane staining for HspBP1. IHC was carried out twice on this array; numerous other xenograft tumors have been examined with similar results. GBM, glioblastoma multiforme.
Table 1.
Immunohistochemistry scoring for heat shock protein binding protein (HspBP) 1 on high‐grade glioma tissue microarrays
| Tumor no. | Tumor type | Core‐1, score (0–4) | Core‐1, distribution | Core‐2, score (0–4) | Core‐2, distribution |
|---|---|---|---|---|---|
| 1 | GBM | 3 | N > C | 2 | N > C |
| 2 | GBM | 4 | N | 4 | N |
| 3 | GBM | 4 | N > C | 4 | N > C |
| 4 | GBM | 2 | C > N | 4 | N > C |
| 5 | GBM | 4 | N > C | 3 | N |
| 6 | GBM | 4 | N = C | 4 | N = C |
| 7 | GBM | 2 | C | 2 | C |
| 8 | GBM | 2 | C | 2 | C |
| 9 | GBM | 2 | C >> N | 2 | C >> N |
| 10 | GBM | 4 | N | 4 | N |
| 11 | GBM | 3 | C | 3 | C |
| 12 | GBM | 3 | C > N | 3 | C > N |
| 13 | GBM | 2 | C >> N | 2 | C > N |
| 14 | GBM | 2 | C = N | 2 | C = N |
| 15 | GBM | 2 | C > N | 2 | C > N |
| 16 | GBM | 4 | N = C | 4 | N = C |
| 17 | GBM | 4 | C | 3 | C > N |
| 18 | GBM | 2 | C | 2 | C |
| 19 | GBM | 2 | C > N | 2 | C > N |
| 20 | GBM | 1 | N > C | 1 | N > C |
| 21 | GBM | 2 | C | 2 | N > C |
| 22 | GBM | 2 | C >> N | 1 | C >> N |
| 23 | GBM | 3 | C = N | 3 | C = N |
| 24 | GBM | 2 | C | 3 | C |
| 25 | GBM | 3 | C | 3 | C |
| 26 | GBM | 2 | PND | 2 | PND |
| 27 | GBM | 4 | C >> N | 4 | C > N |
| 28 | GBM | 4 | C = N | 4 | C = N |
| 29 | GBM | 4 | C > N | 3 | C > N |
| 30 | GBM | 2 | C = N | 2 | C = N |
| 31 | GBM | 3 | C = N | 3 | C = N |
| 32 | GBM | 1 | N | 1 | N |
| 33 | GBM | 2 | C | 2 | C > N |
| 34 | GBM | 3 | C | 3 | C |
| 35 | GBM | 2 | C >> N | 5 | N |
| 36 | GBM | 1 | C | 1 | C |
| 37 | GBM | 2 | C | 2 | C |
| 38 | GBM | 1 | C | 1 | C |
| 39 | GBM | 1 | C | 1 | C |
| 40 | GBM | 2 | N >> C | 2 | N >> C |
| 41 | GBM | 3 | C | 3 | C |
| 42 | GBM | 2 | C | 2 | C |
| 43 | GBM | 3 | C = N | 2 | C = N |
| 44 | GBM | 2 | PND | 2 | PND |
| 45 | GBM | 2 | C = N | 3 | C |
| 46 | GBM | 4 | C > N | 2 | C = N |
| 47 | GBM | 3 | C | 2 | C |
| 48 | GBM | 2 | C | 2 | C |
| 49 | GBM | 1 | N | 1 | N |
| 50 | GBM | 3 | N | 3 | N |
| 51 | AA | 2 | C | 2 | C |
| 52 | AA | 2 | C | 2 | C |
| 53 | AA | 3 | C | 3 | C |
| 54 | AA | 3 | C | 3 | C |
| 55 | AA | 4 | C > N | 4 | C > N |
| 56 | AA | 3 | C > N | 3 | C > N |
| Normal brain (neurons/cortex) | 0 | 0 |
TMA is spotted from two cores of each patient's tumor. Staining was carried out as in Materials and Methods, using a sheep anti‐HspBP1 polyclonal antibody, followed by a biotinylated mouse anti‐sheep/goat monoclonal antibody, followed by streptavidin–HRP. Tumor types were GBM (glioblastoma multiforme) and grade III ananplastic astrocytomas (AA). Scoring was based on an intensity scale of 0 (no staining) to 4 (highest observed on any tumor or tissue). Distribution of staining pattern:
C = cytoplasmic staining; N = nuclear staining; PND = perinuclear. Where both cytoplasm and nucleus exhibit staining, the intensities are gauged as either equivalent (=), one greater than the other (>), or one much greater than the other (>>).
HspBP1 can interact with multiple members of the HSP70 chaperone family. The abundance of HSP70 family members expressed by brain tumor cells (Fig. 1; see also Reference 15) including GRP75 and GRP78, provoked the question of whether HspBP1 might interact with the entire spectrum of HSP70 members. GRP75 and GRP78 are normally localized to the mitochondria and endoplasmic reticulum, respectively; their presence in the cytoplasm of brain tumor cells( 15 ) (Dodd RD, Dechkovskaia AM, Bigner DD, Nicchitta CV, Graner MW, 2009) suggests that HspBP1 would have access to these chaperones.
We assessed this possibility by carrying out pull‐down assays using immobilized HspBP1 as an affinity matrix( 16 ) for binding proteins from the lysate of murine and human xenograft brain tumors. As seen in Figure 3, HspBP1 is capable of binding most of the HSP70 family members from tumor lysates, including the large HSP70 family member HSP110,( 32 ) though the murine tumor SMA560 does not appear to express HSP110. Curiously, the constitutive HSC70 from brain homogenate does bind to HspBP1, whereas that from the tumors binds either weakly or not at all. Also, GRP78 from brain fails to bind HspBP1 whereas tumor GRP78 does bind. Thus, HspBP1 is capable of binding multiple HSP70 family members, whose presence in the cytoplasm of brain tumor cells may influence the activity of the co‐chaperone. It should be pointed out that the tissues were lysed in RIPA buffer with no alterations made for nucleotide concentrations; these conditions could conceivably affect the bindings of the chaperones to the co‐chaperone.
Figure 3.
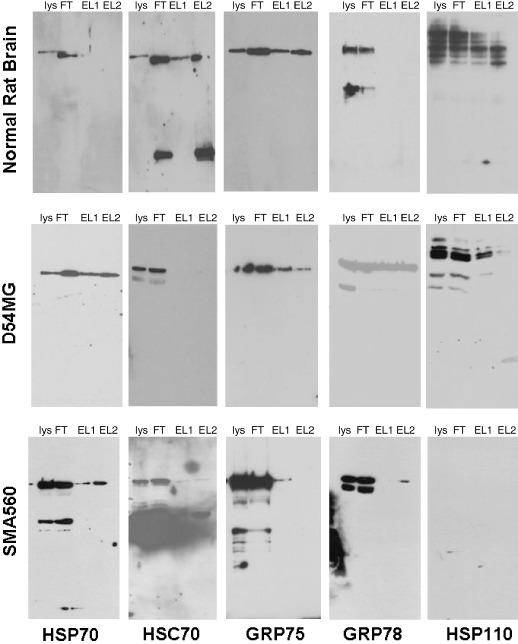
Affinity chromatography using immobilized heat shock protein binding protein (HspBP) 1 as a matrix. Recombinant his‐tagged HspBP1 was immobilized to a TALON IMAC resin, and equal amounts of lysates (5 mg) prepared from solid tumors (D54MG and SMA560) and from rat brain (listed at the left side) were passed over the columns. After extensive washing, HspBP1 and bound components were eluted with 1 M imidazole. Starting material (lysate = lys), flow‐through (FT), and the first two elution samples (EL1, EL2) were analyzed by western blots probed with the antibodies listed (at bottom). These results are typical of three experiments. GRP, glucose regulated protein; HSC, heat shock cognate; HSP, heat shock protein.
HspBP1 binds to brain tumor cell surfaces. We have previously shown that most of the aforementioned HSP70 family members are present on the surfaces of brain tumor cells;( 15 ) that information coupled with the binding studies above prompted us to ask if HspBP1 itself could bind to brain tumor cell surfaces, possibly via externalized HSP70. We labeled recombinantly produced HspBP1 with FITC and used it in FACS analyses for surface staining of brain tumor cell lines and disaggregated tumors. As seen in Figure 4, brain tumor cell lines and disaggregated xenograft cells all were bound by HspBP1–FITC, including the murine tumor lines SMA560 and GL261; the murine leukemia 12B1 did not react. Curiously, NR6M, a 3T3 line transfected with the truncation mutant epidermal growth factor receptor variant III (EGFRvIII), also reacts with HspBP1–FITC. Although human brain tumor xenografts bound HspBP1–FITC, disaggregated rat brain did not. HspBP1 thus can adhere to brain tumor cells, both from tissue culture and when grown as solid tumors, perhaps through interactions with surface‐exposed HSP.
Figure 4.

Binding of heat shock protein binding protein (HspBP) 1 to cell surfaces as analyzed by FACS. HspBP1 was FITC labeled and used as a probe on cultured human brain tumor cells, on cultured murine tumor cells, and on disaggregated human xenografts (and rat brain), as seen with the solid lines. Control stains are shown in gray or dark fill. FITC–IgG was used as a control; irrelevant his‐tagged, FITC‐labeled proteins showed no specific staining either (data not shown). Results are typical of many experiments.
To address the idea that HspBP1 may bind tumor cell surfaces via HSP, we attempted to block that interaction by pre‐treating the brain tumor cell line D54MG with monoclonal antibodies specific for the inducible HSP70 prior to FACS staining with labeled HspBP1–FITC. Figure 5(a) shows that this pre‐treatment reduced the co‐chaperone's binding to cells by approximately one log. The inverse experiment (Fig. 5b), pre‐treating cells with unlabeled HspBP1 before staining with FITC‐labeled anti‐HSP70, also diminished antibody binding, but only slightly. These results indicate that HSP70 is likely one of the targets for HspBP1 binding, but is presumably not the only one, given that significant binding of both HSP70 antibodies and HspBP1 occurred despite the reciprocal blocking efforts.
Figure 5.
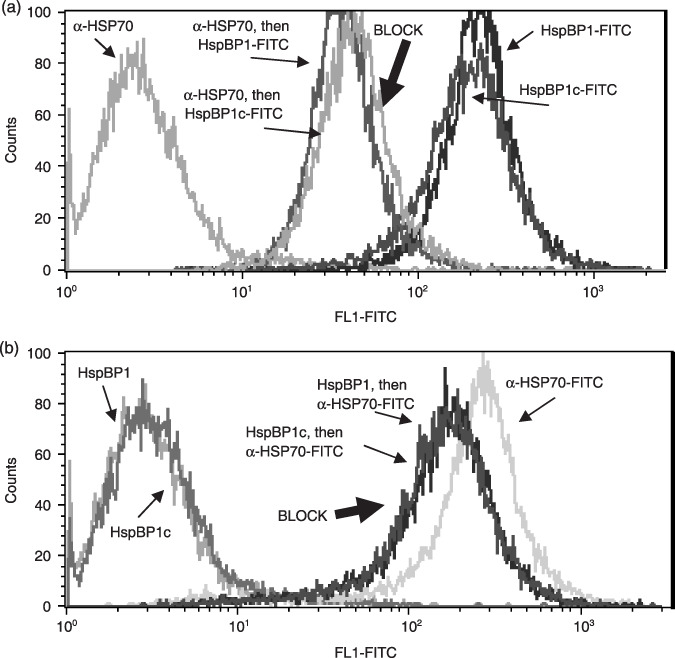
(a) Reduced binding of heat shock protein binding protein (HspBP) 1–FITC to D54MG cell surfaces after blocking with antibodies against heat shock protein (HSP) 70. D54MG cells were formalin‐fixed and incubated with unlabeled anti‐HSP70 monoclonal antibodies (100 µg/mL) for 15 min. After washing, cells were then incubated with FITC‐labeled HspBP1, or with HspBP1 core protein (HspBP1c), and analyzed by flow cytometry. HspBP1–FITC‐stained cells without blocking are shown for comparison, and the unlabeled HSP70 antibody itself had no effect on the fluorescence of the cells (trace to the far left). (b) The reciprocal experiment was carried out, where unlabeled HspBP1 or core protein was used to block (at 300 µg/mL), followed by staining with FITC‐labeled anti‐HSP70 (unblocked staining is show in the far right trace). Again, the unlabeled HspBP1 had no effect on the cell fluorescence (far left traces). Control staining in both cases, whether unlabeled antibody or protein, was identical to the isotype control staining. Data shown have been replicated twice.
HspBP1 can bind multiple HSP70 family members derived from brain tumor cell surfaces. As it appeared that other proteins may be involved in the tumor cell surface interactions of HspBP1, we asked if similar affinity pull‐down assays as used to obtain the data for Figure 3 could also be used for cell surface‐biotinylated proteins. We biotinylated proteins on the surfaces of D54MG cells and D54MG disaggregated xenograft tumor cells using a membrane‐impermeant compound as we had done previously to demonstrate the presence of surface HSP on tumor cells.( 15 ) Cells were lysed, and biotinylated proteins were harvested by streptavidin affinity chromatography. Following elution, the labeled proteins were then passed over a HspBP1 affinity resin (his‐tagged HspBP1 bound to an IMAC resin). After washing, the entire protein complex was then eluted with imidazole, and subjected to gel electrophoresis and western blotting. Blots were probed with antibodies to HSC70, HSP70, GRP75, and GRP78, and streptavidin–HRP was used as a positive control. In Figure 6(a), one sees that HSP70, GRP75, GRP78 bind to immobilized HspBP1, whereas HSC70 does not, replicating what was seen in Figure 3. Streptavidin–HRP staining indicates that the proteins eluted from the HspBP1 affinity column are clustered in the 70–80 kDa molecular weight range, which mirrors the sizes of HSP70 family members. Figure 6(b) shows that HSP70 and GRP75, biotinylated on the surfaces of disaggregated tumor cells, also bind to the HspBP1 affinity resin. Thus, the surface localization of the HSP does not apparently affect their abilities to bind HspBP1 in this type of pull‐down assay (the same caveats mentioned for Fig. 3 apply here as well).
Figure 6.
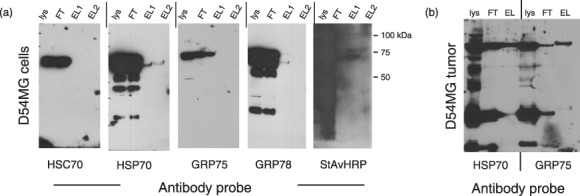
Affinity chromatography using immobilized heat shock protein binding protein (HspBP) 1 as a matrix, but using cell surface‐biotinylated proteins as binding partners. (a) D54MG cell surface proteins were biotinylated with a membrane‐impermeant compound. Biotinylated proteins were purified via avidin chromatography. Biotinylated cell surface proteins were then passed over a HspBP1 affinity matrix as described for Figure 3; proteins were eluted in imidazole; starting material lysates (lys), flow‐through (FT), and two elution fraction samples (EL1, EL2) were analyzed by western blotting with the antibody probes shown. To verify biotinylation, a separate blot was probed with streptavdin–horseradish peroxidase (StAvHRP). Molecular weight markers in kDa are shown at the far right. (b) D54MG xenograft tumor was resected from an immune‐compromised rat, disaggregated, and surface biotinylated as in (a). Biotinylated cell surface proteins were chromatographed as in (a); fractions (lysate = lys; flow‐through = FT, pooled elution fractions = EL) were analyzed by western blotting and probed with antibodies to heat shock protein (HSP) 70 and GRP75. These experiments have been carried out twice.
Extracellular HspBP1 can internalize into brain tumor cells. The possibility of multiple HSP binding partners on the surfaces of brain tumor cells invites the concept of targeting tumor cells that express cell surface HSP via proteins or peptides that specifically bind to such chaperones.( 33 , 34 , 35 ) The utility of such targeting agents is enhanced if the agent internalizes into the intended cell. We analyzed the internalization of HspBP1 into unfixed cells by a ‘pulse‐chase’ FACS assay. Here, his‐tagged HspBP1 was applied to cells for various periods of time. The cells were harvested at these time points and split into two groups, one of which was fixed with paraformaldehyde, and the other was fixed and permeabilized with saponin. Both groups were then incubated with AlexaFluor‐labeled anti‐penta‐his antibody as a secondary reagent and subjected to FACS analyses. The first group represents the surface‐stained cell population only, whereas the second group corresponds to the ‘total’ staining for HspBP1 (i.e. both surface and internalized protein). The difference between the mean fluorescence intensities (MFI) is considered the internalized fraction. Figure 7 shows typical results from two experiments where the upper panel demonstrates what appears to be rapid internalization of the co‐chaperone (based on the differences between the 7.5‐min time point – the earliest obtainable value – and the 30‐min time point). The 60‐min time point – with an MFI nearly the same as the starting value – suggests that there may be recycling of the internalized HspBP1. After 3 h, there is a substantial reduction in surface staining; however, that essentially matches the 3‐h value for the total HspBP1 staining (lower panel), suggesting that it may indicate degradation. As expected, most of the values accounting for the total HspBP1 staining (lower panel) remained constant up to 60 min (we regard the 15‐min profile as an anomaly, although we have seen this occur in both experiments). The internalized fraction, visualized as the amounts between the dotted lines dropped down, were consistently approximately a threefold reduction in MFI between the total and the surface remnants. Thus, some portion of HspBP1 internalizes into target cells, yielding the possibility that the co‐chaperone could be a means of targeted drug, toxin, or radiotherapy.
Figure 7.

Heat shock protein binding protein (HspBP) 1 internalizes into D54MG cells. Unfixed D54MG cells were incubated with 10 µg/mL of his‐tagged HspBP1 for the time points shown (arrows). Cells were then either fixed only (for surface HspBP1 staining, top panel), or were fixed and permeabilized with saponin (for total stain, both surface and internalized HspBP1, bottom panel). After washing, AlexFluor 488‐labeled anti‐penta‐his antibody was used as a probe for the his‐tagged HspBP1. Differences in the mean fluorescence intensity (peaks of traces shown for AlexaFluor 488) are indicative of the internalized fraction (dotted lines dropped down). AlexaFluor 488‐anti‐his antibody staining of an irrelevant his‐tagged protein is shown in blue shaded fill. Abscissas are elongated to accentuate differences in the staining intensity over the time courses. The 15‐min time point staining for the total stain (bottom panel) appears artifactual, but has been reproduced in two independent experiments.
Discussion
Brain tumors such as high‐grade gliomas are extremely difficult to treat and are associated with dismal prognoses; patients experience median survival times of less than 15 months despite maximal surgical, radiological, and chemotherapeutic interventions.( 36 ) New therapies are desperately needed, and will come from an improved understanding of brain tumor biology. The stress responses in brain tumor cells have been documented but not heavily studied,( 12 , 13 , 14 ) and even less is known about co‐chaperones in these tumors. This work presents an initial characterization of the HSP70 family co‐chaperone HspBP1, originally discovered as an inhibitor of HSP70 chaperone activity.( 16 ) HspBP1 is one of the HSP70 nucleotide exchange factors( 17 ) that has complex interactions with HSP70 in the presence of other co‐chaperones and chaperone adaptors.( 37 , 38 ) As such we have shown high expression levels of HspBP1 in samples from patients with high‐grade gliomas, in numerous brain tumor cell lines and xenografts, as well as murine brain tumor models, via western blots and IHC (1, 2; Table 1). These results suggest that HspBP1 may be used as a tumor marker. We demonstrated that HspBP1 can bind multiple HSP70 family constituents both from tumor lysates and from cell surface‐localized populations of the family (3, 6). As we previously showed that many chaperones are displayed on brain tumor cell surfaces, as well as HspBP1 itself,( 15 ) these results begged the question, can HspBP1 bind tumor cell surface components? We found this to be true and likely in part due to reactivity with surface HSP70 (4, 5). In addition, HspBP1 can internalize into tumor cells, thus complicating the biology of the co‐chaperone as well as alluding to its potential as a tumor‐targeting ligand.
The novel aspects of this work include the identification of HspBP1 as a highly expressed protein in human brain tumors, the demonstration of HspBP1's binding potentially to multiple members of the Hsp70 family, and its ability to adhere to brain tumor cell surfaces if exposed exogenously to those cells. Extracellular HspBP1 is present in human serum under normal conditions,( 39 ) as well as in that of patients with breast cancer,( 31 ) whereas serum antibodies against HspBP1 are elevated in human immunodeficiency virus (HIV) patients,( 28 ) suggesting that extracellular HspBP1 may be related to pathological states. The implications of extracellular co‐chaperones are numerous; it is plausible that such proteins are involved in cell surface and extracellular regulation of chaperone activity, and implies that such localization of chaperones and co‐chaperones is not a random event in tumor biology. Indeed, the co‐chaperones HSP40 and Bag‐4 have been identified on tumor cell surfaces,( 40 ) and the sarcoma‐derived HSP90 co‐chaperones p23 and HOP are found associated with HSP90 extracellularly.( 8 ) Cell surface HSP90 may play a role in tumor cell migration and metastasis,( 41 , 42 ) and it is likely that other chaperones carry out functions during tumor cell migration and invasion as well.( 43 , 44 )
The competition of various factors and co‐chaperones for binding to HSP/HSC70 during its chaperone cycle determines the nucleotide exchange rate and therefore the ‘on–off’ rate of the chaperone for its substrates. Excess HspBP1, depending on the ratios of other co‐chaperones, can essentially inhibit the ‘foldase’ activity of HSP/HSC70.( 16 , 37 ) In murine tumors, the ratio of HspBP1 to HSP70 is below the level necessary for 50% inhibition of refolding of a denatured client protein in rabbit reticulocyte lysate, a molar ratio of approximately 4 : 1 (HspBP1 : HSP70( 19 )). However, in a recent report, molar ratios as high as 12 were seen in a number of human carcinoma cell lines, but this compared HspBP1 to the constitutive HSC70 and did not account for the inducible HSP70 contribution to that ratio.( 20 ) That paper related high expression levels of HspBP1 to increased sensitivity of the cell lines to various chemotherapeutic agents. This would be in agreement with HspBP1's role as an ‘inhibitor’ of HSP/HSC70 activity, given the pro‐survival/anti‐apoptotic cant of the chaperone. However, as we have shown here, there is the formal possibility that HspBP1 interacts with a number of HSP70 family members, who are also highly expressed in brain tumor cells and display cytoplasmic localizations. Such ‘extra’ interactions could functionally titrate out HspBP1, and thus allow for the maintenance of the anti‐apoptotic activities of the HSP70 family chaperones. In addition, HspBP1's utility in the chaperone cycle is likely to prepare the chaperone for its next interaction with a client protein by allowing for nucleotide exchange in anticipation of the next binding or folding event. Thus, high levels of HspBP1 may actually assist in the proper folding of nascent proteins to maintain high translational capacity, which is essentially chronic in tumor cells.( 45 ) The functional reduction in the HspBP1 : HSP70 family member ratio (i.e. increased amounts of cytoplasmic HSP/HSC70, HSP110, GRP75, and GRP78) may contribute to such activity.
The differential binding capacities of the various chaperones curiously showed a relatively weak reactivity between tumor HSC70 and HspBP1 (3, 6). Whether this is a true affinity relationship remains to be determined. The competition between the various HSP70 family members and their molar quantities, interactions with other co‐chaperones, and the nucleotide bound or unbound state of the chaperones undoubtedly plays a role in this chaperone–co‐chaperone interaction.
The cell surface binding and internalization activity of HspBP1 (4, 7) is interesting on many levels. Recently it was shown that extracellular HspBP1 could bind and co‐precipitate with extracellular HSP70 (HSP72, the stress‐inducible canonical member of the HSP70 family), and that this pair could influence the phosphorylation state and downstream activity of epidermal growth factor receptor (EGFR).( 46 ) As we have demonstrated the presence of HSP70‐displaying exosomes – which also display EGFR and EGFRvIII – in brain tumor cell lines,( 15 , 47 ) this puts another wrinkle in the potential mechanistic features of extracellular and cell surface chaperones and co‐chaperones. Because EGFR and the mutant EGFRvIII are frequently overexpressed in high‐grade gliomas,( 48 ) the impact of surface and extracellular HspBP1 on EGFR signaling certainly invites additional investigation. Extracellular and internalizable HspBP1 is potentially a means of passing the co‐chaperone to other cells, perhaps using cell surface chaperones as a conduit, thereby altering the aforementioned co‐chaperone : chaperone ratio. Although it is unclear how the initial release of HspBP1 occurs, intracellular vesicular trafficking via the endolysosomal system may be one means of accomplishing this release, as has been proposed for HSP70.( 49 ) As mentioned, exosomes, membrane‐enclosed vesicular derivatives of endosomes that are released extracellularly, contain many HSP,( 15 , 50 ) and could presumably also transport co‐chaperones. We have recently characterized these vesicles from brain tumor cells and from the sera of patients with high‐grade gliomas, where we have identified numerous chaperones.( 47 ) These are clearly avenues of further research.
This report suggests the potential utility of the inherent tumor‐binding capacity of HspBP1 as a tumor‐targeting moiety, with an expanded repertoire of “armaments” due to its internalizing properties. In neuro‐oncology, internalizing antibodies and antibody constructs such as single‐chain variable region fragments, and specific ligands have been combined effectively with radioactive compounds, drug molecules, and toxins, with some of these therapies entering clinical trials.( 51 , 52 , 53 , 54 , 55 ) HspBP1 as a tumor‐targeting biotherapeutic could follow a similar strategy, except that instead of specifically targeting only one protein or surface component, HspBP1 could target several simultaneously, given the co‐expression of many of the HSP70 family members on brain tumor cell surfaces( 15 ) (M. W. Graner, 2009). This enhanced targeting capacity could make it a very attractive entity that shows no off‐target binding to normal brain (Fig. 4). Validation and efficacy testing are currently underway.
In summary, we have previously shown that the HSP70 co‐chaperone HspBP1 is highly expressed in a variety of murine tumors and xenograft models, including on the cell surface.( 15 , 19 ) Here we demonstrate high expression levels of HspBP1 in patient high‐grade gliomas, as well as other xenograft and murine tumor models. We show that HspBP1 can bind with several of the overexpressed HSP70 family members from tumor lysates, as well as their tumor surface cohorts, and that HspBP1 itself can bind to tumor cell surfaces and internalize into those cells. As the biology of HspBP1 becomes more apparent, we suspect that it will define a critical role in chaperone function, which in turn plays a major part in the chronic stress responses that enable tumors to survive and thrive in their hosts. Thus, HspBP1 may be an attractive drug target candidate in that functions of multiple chaperones – which may have redundant, overlapping roles if targeted individually – may be disrupted by inhibiting (or overactivating) one co‐chaperone. As alluded, HspBP1 may itself be a drug‐targeting agent against cells displaying chaperones on their surfaces. Neuro‐oncology needs a clearer understanding of the processes that enable tumor cells to proliferate, invade, and resist attack, and HspBP1 is one molecule that is positioned at such a node.
Acknowledgments
The authors wish to thank Ian Cumming, Shelley Davis, Nichole Satterwhite, Emily Disney, Ling Wang, and Tim Parrett for wonderful technical assistance. This work was supported by: the Duke University Brain Cancer Specialized Programs of Research Excellence 5 P50 CA108786‐02; SPORE Career Development Award (to MWG); National Institute of Neurological Disorders and Stroke SRC 5 P50 20023‐21; the Pediatric Brain Tumor Foundation; the Brian Cless Research Foundation; the Southeast Brain Tumor Foundation; and generous sources from the University of Colorado Cancer Center (to MWG).
References
- 1. Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones 2005; 10: 86–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Edinger AL. Growth factors regulate cell survival by controlling nutrient transporter expression. Biochem Soc Trans 2005; 33: 225–7. [DOI] [PubMed] [Google Scholar]
- 3. Griguer CE, Oliva CR, Gillespie GY. Glucose metabolism heterogeneity in human and mouse malignant glioma cell lines. J Neurooncol 2005; 74: 123–33. [DOI] [PubMed] [Google Scholar]
- 4. Harguindey S, Orive G, Luis Pedraz J, Paradiso A, Reshkin SJ. The role of pH dynamics and the Na+/H+ antiporter in the etiopathogenesis and treatment of cancer. Two faces of the same coin – one single nature. Biochim Biophys Acta 2005; 1756: 1–24. [DOI] [PubMed] [Google Scholar]
- 5. Griguer CE, Oliva CR, Kelley EE, Giles GI, Lancaster JR Jr, Gillespie GY. Xanthine oxidase‐dependent regulation of hypoxia‐inducible factor in cancer cells. Cancer Res 2006; 66: 2257–63. [DOI] [PubMed] [Google Scholar]
- 6. Garrido C, Schmitt E, Cande C, Vahsen N, Parcellier A, Kroemer G. HSP27 and HSP70: potentially oncogenic apoptosis inhibitors. Cell Cycle 2003; 2: 579–84. [PubMed] [Google Scholar]
- 7. Kamal A, Boehm MF, Burrows FJ. Therapeutic and diagnostic implications of Hsp90 activation. Trends Mol Med 2004; 10: 283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eustace BK, Jay DG. Extracellular roles for the molecular chaperone, hsp90. Cell Cycle 2004; 3: 1098–100. [PubMed] [Google Scholar]
- 9. Radons J, Multhoff G. Immunostimulatory functions of membrane‐bound and exported heat shock protein 70. Exerc Immunol Rev 2005; 11: 17–33. [PubMed] [Google Scholar]
- 10. Schmitt E, Gehrmann M, Brunet M, Multhoff G, Garrido C. Intracellular and extracellular functions of heat shock proteins: repercussions in cancer therapy. J Leukoc Biol 2007; 81: 15–27. [DOI] [PubMed] [Google Scholar]
- 11. Graner MW, Bigner DD. Chaperone proteins and brain tumors: potential targets and possible therapeutics. Neuro-Oncol 2005; 7: 260–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fuse T. [Alterations in cytokinetics and heat shock protein (70 kDa) expression of glial cell by hyperthermia]. No to Shinkei 1991; 43: 843–50 (in Japanese). [PubMed] [Google Scholar]
- 13. Hermisson M, Strik H, Rieger J, Dichgans J, Meyermann R, Weller M. Expression and functional activity of heat shock proteins in human glioblastoma multiforme. Neurology 2000; 54: 1357–65. [DOI] [PubMed] [Google Scholar]
- 14. Wang J, Koyama S, Komatsubara Y, Suzuki Y, Taki M, Miyakoshi J. Effects of a 2450 MHz high‐frequency electromagnetic field with a wide range of SARs on the induction of heat‐shock proteins in A172 cells. Bioelectromagnetics 2006; 27: 479–86. [DOI] [PubMed] [Google Scholar]
- 15. Graner MW, Cumming RI, Bigner DD. The heat shock response and chaperones/heat shock proteins in brain tumors: surface expression, release, and possible immune consequences. J Neurosci 2007; 27: 11214–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Raynes DA, Guerriero V Jr Inhibition of Hsp70 ATPase activity and protein renaturation by a novel Hsp70‐binding protein. J Biol Chem 1998; 273: 32883–8. [DOI] [PubMed] [Google Scholar]
- 17. Kabani M, McLellan C, Raynes DA, Guerriero V, Brodsky JL. HspBP1, a homologue of the yeast Fes1 and Sls1 proteins, is an Hsc70 nucleotide exchange factor. FEBS Lett 2002; 531: 339–42. [DOI] [PubMed] [Google Scholar]
- 18. Shomura Y, Dragovic Z, Chang HC et al . Regulation of Hsp70 function by HspBP1: structural analysis reveals an alternate mechanism for Hsp70 nucleotide exchange. Mol Cell 2005; 17: 367–79. [DOI] [PubMed] [Google Scholar]
- 19. Raynes DA, Graner MW, Bagatell R, McLellan C, Guerriero V. Increased expression of the Hsp70 cochaperone HspBP1 in tumors. Tumour Biol 2003; 24: 281–5. [DOI] [PubMed] [Google Scholar]
- 20. Tanimura S, Hirano AI, Hashizume J et al . Anticancer drugs up‐regulate HspBP1 and thereby antagonize the prosurvival function of Hsp70 in tumor cells. J Biol Chem 2007; 282: 35430–9. [DOI] [PubMed] [Google Scholar]
- 21. Garcia‐Morales P, Carrasco‐Garcia E, Ruiz‐Rico P et al . Inhibition of Hsp90 function by ansamycins causes downregulation of cdc2 and cdc25c and G2/M arrest in glioblastoma cell lines. Oncogene 2007; 26: 7185–93. [DOI] [PubMed] [Google Scholar]
- 22. He L, Feng H, Raymond A et al . Dendritic‐cell‐peptide immunization provides immunoprotection against bcr‐abl‐positive leukemia in mice. Cancer Immunol Immunother 2001; 50: 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Graner MW, Zeng Y, Feng H, Katsanis E. Tumor‐derived chaperone‐rich cell lysates are effective therapeutic vaccines against a variety of cancers. Cancer Immunol Immunother 2003; 52: 226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Garg PK, John CS, Zalutsky MR. Preparation and preliminary evaluation of 4‐[211At]astato‐N‐piperidinoethyl benzamide. Nucl Med Biol 1995; 22: 467–73. [DOI] [PubMed] [Google Scholar]
- 25. Friedman HS, Burger PC, Bigner SH et al . Establishment and characterization of the human medulloblastoma cell line and transplantable xenograft D283 Med. J Neuropathol Exp Neurol 1985; 44: 592–605. [DOI] [PubMed] [Google Scholar]
- 26. Foulon CF, Welsh PC, Bigner DD, Zalutsky MR. Positively charged templates for labeling internalizing antibodies: comparison of N‐succinimidyl 5‐iodo‐3‐pyridinecarboxylate and the d‐amino acid peptide KRYRR. Nucl Med Biol 2001; 28: 769–77. [DOI] [PubMed] [Google Scholar]
- 27. Batra SK, Castelino‐Prabhu S, Wikstrand CJ et al . Epidermal growth factor ligand‐independent, unregulated, cell‐transforming potential of a naturally occurring human mutant EGFRvIII gene. Cell Growth Differ 1995; 6: 1251–9. [PubMed] [Google Scholar]
- 28. Papp D, Prohaszka Z, Kocsis J et al . Development of a sensitive assay for the measurement of antibodies against heat shock protein binding protein 1 (HspBP1): increased levels of anti‐HspBP1 IgG are prevalent in HIV infected subjects. J Med Virol 2005; 76: 464–9. [DOI] [PubMed] [Google Scholar]
- 29. McLellan CA, Raynes DA, Guerriero V. HspBP1, an Hsp70 cochaperone, has two structural domains and is capable of altering the conformation of the Hsp70 ATPase domain. J Biol Chem 2003; 278: 19017–22. [DOI] [PubMed] [Google Scholar]
- 30. Kampinga HH, Hageman J, Vos MJ et al . Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones 2009; 14: 105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Souza AP, Albuquerque C, Torronteguy C et al . HspBP1 levels are elevated in breast tumor tissue and inversely related to tumor aggressiveness. Cell Stress Chaperones 2009; 14(3): 301–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Easton DP, Kaneko Y, Subjeck JR. The hsp110 and Grp1 70 stress proteins: newly recognized relatives of the Hsp70s. Cell Stress Chaperones 2000; 5: 276–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Piselli P, Vendetti S, Poccia F et al . In vitro and in vivo efficacy of heat shock protein specific immunotoxins on human tumor cells. J Biol Regul Homeost Agents 1995; 9: 55–62. [PubMed] [Google Scholar]
- 34. Arap MA, Lahdenranta J, Mintz PJ et al . Cell surface expression of the stress response chaperone GRP78 enables tumor targeting by circulating ligands. Cancer Cell 2004; 6: 275–84. [DOI] [PubMed] [Google Scholar]
- 35. Davidson DJ, Haskell C, Majest S et al . Kringle 5 of human plasminogen induces apoptosis of endothelial and tumor cells through surface‐expressed glucose‐regulated protein 78. Cancer Res 2005; 65: 4663–72. [DOI] [PubMed] [Google Scholar]
- 36. Stupp R, Mason WP, Van Den Bent MJ et al . Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005; 352: 987–96. [DOI] [PubMed] [Google Scholar]
- 37. Oh WK, Song J. Cooperative interaction of Hsp40 and TPR1 with Hsp70 reverses Hsp70–HspBp1 complex formation. Mol Cells 2003; 16: 84–91. [PubMed] [Google Scholar]
- 38. Alberti S, Bohse K, Arndt V, Schmitz A, Hohfeld J. The cochaperone HspBP1 inhibits the CHIP ubiquitin ligase and stimulates the maturation of the cystic fibrosis transmembrane conductance regulator. Mol Biol Cell 2004; 15: 4003–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Raynes DA, Thomson CA, Stroster J, Newton T, Cuneo P, Guerriero V. Human serum contains detectable levels of the Hsp70 cochaperone HspBP1 and antibodies bound to HspBP1. J Immunoassay Immunochem 2006; 27: 251–64. [DOI] [PubMed] [Google Scholar]
- 40. Gehrmann M, Marienhagen J, Eichholtz‐Wirth H et al . Dual function of membrane‐bound heat shock protein 70 (Hsp70), Bag‐4, and Hsp40: protection against radiation‐induced effects and target structure for natural killer cells. Cell Death Differ 2005; 12: 38–51. [DOI] [PubMed] [Google Scholar]
- 41. Tsutsumi S, Neckers L. Extracellular heat shock protein 90: a role for a molecular chaperone in cell motility and cancer metastasis. Cancer Sci 2007; 98: 1536–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tsutsumi S, Scroggins B, Koga F et al . A small molecule cell‐impermeant Hsp90 antagonist inhibits tumor cell motility and invasion. Oncogene 2008; 27: 2478–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Barazi HO, Zhou L, Templeton NS, Krutzsch HC, Roberts DD. Identification of heat shock protein 60 as a molecular mediator of alpha 3 beta 1 integrin activation. Cancer Res 2002; 62: 1541–8. [PubMed] [Google Scholar]
- 44. Lee KJ, Kim YM, Kim DY et al . Release of heat shock protein 70 (Hsp70) and the effects of extracellular Hsp70 on matric metalloproteinase‐9 expression in human monocytic U937 cells. Exp Mol Med 2006; 38: 364–74. [DOI] [PubMed] [Google Scholar]
- 45. Pandolfi PP. Aberrant mRNA translation in cancer pathogenesis: an old concept revisited comes finally of age. Oncogene 2004; 23: 3134–7. [DOI] [PubMed] [Google Scholar]
- 46. Evdonin AL, Kinev AV, Tsupkina N, Guerriero V, Raynes DA, Medvedeva ND. Extracellular HspBP1 and Hsp72 synergistically activate EGF receptor. Biol Cell 2009; 101(6): 351–60. [DOI] [PubMed] [Google Scholar]
- 47. Graner MW, Alzate O, Dechkovskaia AM et al . Proteomic and immunologic analyses of brain tumor exosomes. FASEB J 2009; 23(5): 1541–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kuan CT, Wikstrand CJ, Bigner DD. EGFRvIII as a promising target for antibody‐based brain tumor therapy. Brain Tumor Pathol 2000; 17: 71–8. [DOI] [PubMed] [Google Scholar]
- 49. Mambula SS, Stevenson MA, Ogawa K, Calderwood SK. Mechanisms for Hsp70 secretion: crossing membranes without a leader. Methods 2007; 43: 168–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Asea A. Hsp72 release: mechanisms and methodologies. Methods 2007; 43: 194–8. [DOI] [PubMed] [Google Scholar]
- 51. Newton HB. Advances in strategies to improve drug delivery to brain tumors. Expert Rev Neurother 2006; 6: 1495–509. [DOI] [PubMed] [Google Scholar]
- 52. Rainov NG, Soling A. Clinical studies with targeted toxins in malignant glioma. Rev Recent Clin Trials 2006; 1: 119–31. [DOI] [PubMed] [Google Scholar]
- 53. Vandergrift WA, Patel SJ, Nicholas JS, Varma AK. Convection‐enhanced delivery of immunotoxins and radioisotopes for treatment of malignant gliomas. Neurosurg Focus 2006; 20: E13. [DOI] [PubMed] [Google Scholar]
- 54. Reardon DA, Zalutsky MR, Bigner DD. Antitenascin‐C monoclonal antibody radioimmunotherapy for malignant glioma patients. Expert Rev Anticancer Ther 2007; 7: 675–87. [DOI] [PubMed] [Google Scholar]
- 55. Gerber DE, Laterra J. Emerging monoclonal antibody therapies for malignant gliomas. Expert Opin Invest Drugs 2007; 16: 477–94. [DOI] [PubMed] [Google Scholar]


