Abstract
Background
Transplanting pancreatic islets is of significant interest for type 1 diabetes mellitus. After intraportal injection of islets, inferior engraftment and eventual loss of transplanted islets constitute major limitations. Therefore, alternative approaches will be helpful. Here, we evaluated in animals whether an isolated venous sac would support survival of transplanted islets, along with correction of hyperglycemia.
Methods
Pancreatic islets isolated from adult Lewis rats were transplanted either into an isolated venous sac made from lumbar vein or into the portal vein of syngeneic rats. The integrity and vascular organization of the venous sac was determined by studies of the local microcirculation. The engraftment, survival, and function of transplanted islets were analyzed by histology, including endocrine function in situ and by glycemic control in rats with streptozotocin-induced diabetes.
Results
Transplanted islets showed normal morphology with insulin expression in isolated venous sac during the long term. Transplanted islets received blood supply from vasa vasorum and had access to drainage through venous tributaries in the venous sac. This resulted in restoration of euglycemia in diabetic rats. Removal of islet graft-bearing venous sac in diabetic rats led to recurrence of hyperglycemia. By contrast, euglycemia was not restored in rats treated by intraportal transplantation of islets.
Conclusions
We demonstrated that pancreatic islets successfully engrafted and functioned in the isolated venous sac with ability to restore euglycemia in diabetic rats. Therefore, the isolated venous sac offers a new site for transplantation of pancreatic islets. This would be clinically beneficial as an alternative to intrahepatic islet transplantation.
Keywords: Islets, Pancreas, Intravascular transplantation
Insulin administration by repeated injections or an insulin pump is the most reliable therapy for type 1 diabetes, but complete normalization of blood glucose concentrations is achieved only rarely. Because of increased variability in blood glucose concentrations, along with greater mean glucose levels during prolonged periods, these patients are at higher risk for complications, for example, nephropathy, retinopathy, and neuropathy. Since the development of methods for large-scale human islet isolation and initial experience of human islet allotransplantation, intraportal site has been preferred for treating people with diabetes (1, 2). Further progress has been made in the past decade with the introduction of glucocorticoid-free immunosuppressive regimens and isolation of higher quality human islets for transplantation (3). However, the requirement of chronic immunosuppression restricts islet transplantation to the most advanced forms of type 1 diabetes, for example, patients with severe and recurrent hypoglycemic episodes. Alternatively, patients currently on chronic immunosuppressants to maintain a functioning kidney transplant are also considered for therapy. A major problem in clinical islet transplantation is the enormous tissue loss occurring within min, hr, and days after transplantation. Although 80% of cases achieve insulin independence at 1 year, typically with two islet infusions, islet function steadily declines with insulin independence in only 10% to 15% after 5 years of islet transplantation (4). More recently, at least five different international islet transplant programs reported 50% long-term 5-year insulin-independence rates, which matches results of whole pancreas transplantation (5, 6). However, donor organ shortages remain problematic. This requires further approaches for transplantation of islets, such that superior engraftment, survival, and function could be achieved.
In view of failure of function in islets during the long term after intraportal transplantation in humans, which is similar to that in rodents (7), alternative implantation sites have been examined for islet transplantation, for example, spleen, kidney, omental pouch, gastric and small intestinal submucosa, vascularized subcutaneous device, and others (8–15). However, results of these studies have been variable.
Here, we hypothesized that an isolated intravascular site will be advantageous for islet transplantation. Such a location will be readily accessible, transplanted islets will be well oxygenated by an abundance of blood supply, endothelial lining of blood vessels will provide helpful growth factors like cytokines and other substances, endocrine functions will be simply maintained, and islets could be conveniently retrans-planted, if necessary. To address these possibilities, we performed studies in rats with an isolated venous sac and demonstrate for the first time that pancreatic islets can be successfully transplanted into such an intravascular site, with maintenance of insulin expression and ability to correct hyperglycemia in diabetic rats.
RESULTS
Islet Quality Assessment
The number of islets obtained per donor pancreas was 1400±98. Viability of freshly isolated islets was greater than 93%. The purity of hand-picked islets was more than 90%. The size of islets ranged from 50 μm to greater than 500 μm in diameter. Most islets were 100 to 250 μm in diameter. The glucose stimulation index of islets was 3.1±0.3 (range, 2.8–3.4) (n=3) (see Materials and Methods).
Isolated Venous Sac
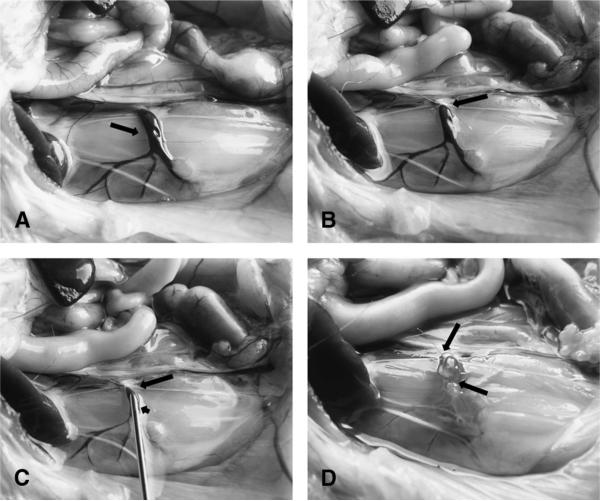
The isolated venous sac used for islet transplantation was typically 2-cm long with an outside diameter of 2 mm. The procedure to prepare the venous sac did not require sacrifice of any tributary to lumbar vein (Fig. 1). This procedure was well tolerated with no surgical morbidity or mortality.
FIGURE 1.
Preparation of isolated venous sac. A, Normal anatomy of lumbar vein on the right side of a rat (arrow). Note venous tributaries draining into the vein. The lumbar artery situated immediately to the right of the lumbar vein is not readily seen. B, Appearance of lumbar vein after placement of a ligature at distal portion (arrow). C, Isolated lumbar vein segment with ligature (long arrow) and insertion of cannula in a proximal position (short arrow). D, Completion of isolated venous segment after injection of pancreatic islets with placement of a second ligature (arrows).
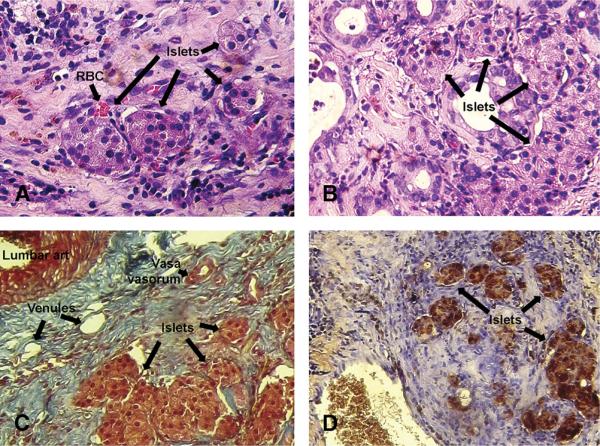
After initial studies, we found isolated venous sac readily accommodated up to 350 rat islets. Therefore, in all subsequent studies, we transplanted 350 islets. Histologic examination of islet graft-bearing venous sacs in rats demonstrated no inflammatory cell infiltration (Fig. 2A, B). Transplanted islets seemed healthy and were embedded within connective tissue matrix (Fig. 2C). We observed presence of venules and vasa vasorum and red blood cells in proximity to islets in venous sacs at various time points after islet transplantation, including after 3, 10, 28, and 60 days (n=3 each). To determine whether transplanted islets were capable of expressing insulin, we performed immunohistochemical analysis in tissues recovered from animals after 10, 28, and 60 days (n=3 each) (Fig. 2D). Clusters of islets with well-preserved morphology and strong immunostaining signals for insulin were present in all grafts.
FIGURE 2.
Histopathologic evaluation of explanted islet-containing venous sacs. Hematoxylin-eosin-stained section from a rat 1 week (A) and 60 days (B) after islet transplantation. Transplanted islets are visible in venous sac (arrows). Red blood cells (arrow) are present in vascular spaces (A). Masson trichrome stain (C) showing arrangement of extra-cellular matrix, vessels, and islets (arrows) in venous sac after 60 days. The lumbar artery is visible on the upper left near the venous sac. Within stroma are seen venules and vasa vasorum (arrows). Immunohistochemistry (D) showing insulin (brown) in transplanted islets (arrows) (original magnification 100).
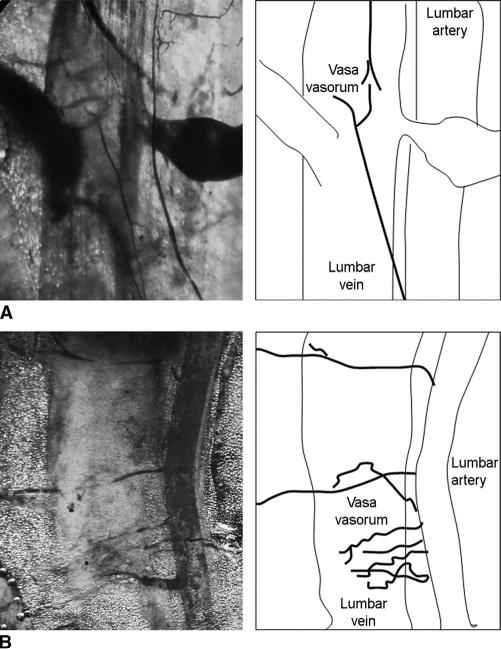
Vascular Integrity of the Venous Sac
We determined the source of blood supply to the venous sac by visualizing capillary microcirculation. The lumbar vein and artery were situated in close proximity (Fig. 3A). Vasa vasorum emanating from lumbar artery were visible in lumbar vein. Similarly, in rats 3 days after islet transplantation in the venous sac, lumbar vein, and artery were visualized, along with vasa vasorum that were patent (Fig. 3B). Moreover, this finding was consistent with presence of red blood cells, venules, and vasa vasorum identified by histologic examination of explanted venous sacs after islet transplantation (Fig. 2).
FIGURE 3.
Integrity and vascular supply of venous sacs. A, Vascular casts obtained with latex dye of lumbar vessels showing vasa vasorum in a healthy rat. B, Vascular casts in rat 3 days after islet transplantation showing patent vessels and vasa vasorum. Line drawings (right) identify specific structures in corresponding microphotographs (left).
Islet Transplantation-Corrected Hyperglycemia
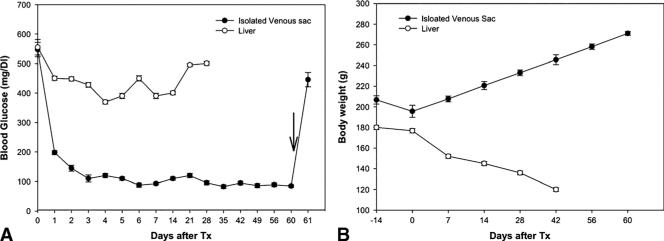
To determine whether islets transplanted into the isolated venous sac would restore euglycemia, we performed studies in streptozotocin-treated diabetic rats. Transplantation of 350 islets in isolated venous sac normalized blood glucose levels (mean blood glucose levels before treatment, 519±45 mg/dL; after islet transplantation, 120±35; P<0.05). This was followed by improvements in general clinical condition, as reflected by steady gains in body weight (before treatment, 205±10 g; after islet transplantation, 265±15 g; P<0.05) (Fig. 4A, B) (n=12). When the islet graft-bearing venous sac was surgically removed, all such animals reverted to hyperglycemia within 24 hr (mean blood glucose levels before excision, 120±35 mg/dL; after excision, 450±53; P<0.05) (n=12).
FIGURE 4.
Regulation of blood glucose levels and body weight in diabetic rats after islet transplantation. A, Animals with islet transplants in isolated vascular sac (group 1, -●-) or intraportally (group 2, -○-). Note that hyperglycemia promptly recurred when islet-bearing venous sac was removed in animals (arrow) (P<0.05), which confirmed that glycemic control was caused by transplanted islets. B, Showing gains in body weight in diabetic rats with improvement in blood glucose levels. Tx, transplantation.
It was noteworthy that diabetic rats treated with intra portal transplantation of 350 pancreatic islets showed no improvements in hyperglycemia, and the animals remained diabetic (mean blood glucose levels, 508±38 mg/dL) (n=6). Because of sustained hyperglycemia at high levels, these animals exhibited significant weight loss and impairment in general body condition. Therefore, all animals in this group were sacrificed, according to the institutional policy, and were considered to have died.
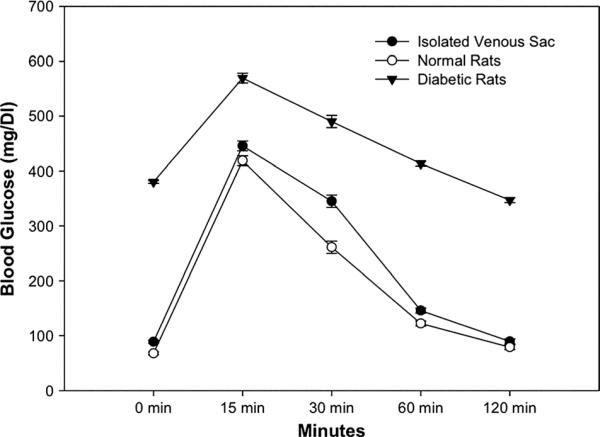
Glucose Tolerance Tests
To verify resolution of hyperglycemia in animals treated with pancreatic islets, we challenged animals with intravenous glucose load (Fig. 5). This glucose tolerance test (GTT) was normal in healthy control rats (blood glucose levels were 67.6±8.2 mg/dL at 0 min, 418.8±73.8 mg/dL at 15 min, 261.0±39.3 mg/dL at 30 min, 122.2±12.2 mg/dL at 60 min, and 78.8±11.3 mg/dL at 120 min) (n=6). GTT was abnormal in diabetic rats with no islet transplantation (380.2±16.9 mg/dL at 0 min, 569.5±21.2 mg/dL at 15 min, 490.2±73.2 mg/dL at 30 min, 413.5±72.7 mg/dL at 60 min, and 347.00±60 mg/dL at 120 min) (n=6). By contrast, GTT was normal in diabetic rats with islets in isolated venous sac (88.8±9.2 mg/dL at 0 min, 445.7±44.4 mg/dL at 15 min, 345.0±26.2 mg/dL at 30 min, 145.8±68.2 mg/dL at 60 min, and 89.5±9.8 mg/dL at 120 min) (n=6), P=not significant.
FIGURE 5.
Glucose tolerance tests in animals. Shows results in groups of animals challenged with intravenous glucose. Healthy rats showed normal glucose tolerance (n=6). In control untreated diabetic rats (n=6), glucose tolerance was impaired. By contrast, in rats treated with islet transplantation in venous sacs (n=6), glucose tolerance was normal.
DISCUSSION
Transplantation of pancreatic islets into the liver is an acceptable and effective therapy for patients with type 1 diabetes mellitus and unstable glycemic control. Islet transplantation is far more attractive than the alternative of vascularized pancreas transplantation from the perspectives of surgical risk and recovery. However, the hepatic microenvironment is hostile to islet grafts. In the early loss of intraportally transplanted islets, contributors include low oxygen tension, cytokines/chemokines released by immunologically active cells, such as Kupffer cells (macrophages) (16), and instant blood-mediated inflammatory reaction (17, 18). Most experts agree appropriate microenvironments supporting islet engraftment will be very important for long-term viability and function of transplanted islets. Therefore, our identification of a more favorable endogenous environment for successful islet transplantation should be of considerable interest.
In our study, even a minimal mass of transplanted islets in isolated venous sac restored normoglycemia in streptozotocin (STZ)-treated diabetic rats, whereas, no diabetic rat achieved normoglycemia after intraportal transplantation of islets in the same number. Previously, we found that diabetes could be corrected in rats by intraportal transplantation of islets, although this required larger numbers of islets (11). By contrast, glucose homeostasis in diabetic rats with normoglycemia after islet transplantation in isolated venous sac was indistinguishable from healthy control rats. This was in agreement with morphologic integrity of islets and continued expression of insulin in islets in isolated venous sac.
No rupture or bleeding from tributaries was observed as a consequence of lumbar vein ligation. This suggested that blood was drained by alternative venous channels. Absence of gross blood clots immediately after the venous sac was created and was in agreement with that possibility. Moreover, in explanted venous sacs containing transplanted islets, we did not find evidence of thrombus formation. Therefore, transplantation of islets in isolated venous sacs should have lessened the probability of instant blood-mediated inflammatory reaction-type events. We believe that presence of vasa vasorum in venous sac provided an inlet for blood supply and glucose sensing to transplanted islets. Similarly, the presence of venous channels should have permitted entry into systemic circulation of insulin released by transplanted islets. Restoration of vascular supply is necessary for islet survival, especially because the vascular network supplying islets becomes disrupted during the islet isolation process. Therefore, opportunities for oxygenation, nutrient exchange, glucose sensing, and secretion of hormones into blood were available in the venous sac. The normal compact morphology and maintenance of endocrine function in islets transplanted into isolated venous sac were in agreement with these opportunities. Recently, endothelial cells originating from recipient tissues themselves were found to make major contributions in islet integrity.
For instance, 30% to 40% of endothelial cells in nascent islet vascular system may originate from the donor (19-21), but the rest are from the recipient.
Biologic differences in endothelial cells in portal vein versus other veins, for example, lumbar or peripheral limb veins, are unknown. Greater blood flow in the portal vein could have been one difference. This might expose transplanted islets to deleterious shear forces. We do not know whether local release of angiocrine or paracrine factors from venous endothelium in various locations contribute in engraftment, survival, and revascularization of transplanted islets. Unlike the portal vein, absence of Kupffer cells and natural killer cells in isolated venous sac should have avoided exposure of islets to locally released inflammatory cytokines/chemokines. Greater relative oxygenation levels coupled with nutrient-rich blood in the portal vein compared with isolated venous sac could potentially increase deleterious oxidative stress in transplanted islets. Understanding the role of these site-specific mechanistic differences will require further study.
The principle of venous sacs for islet transplantation can be tested in larger animals. The anatomic difference in vascular structures of rats versus larger animals need not be a confounding variable because integrity of the isolated venous sac was maintained by blood supply from vasa vasorum. Therefore, isolated venous sacs in larger animals should remain intact, including after islet transplantation. Transplantation of more islets for achieving normoglycemia in larger animals should also be feasible because vascular space will be proportionately greater.
For potential clinical applicability, studies of additional vessels will be appropriate. It should be noteworthy that the greater saphenous vein (GSV) is routinely sacrificed in people for coronary artery bypass grafting. GSV segments removed for coronary artery bypass grafting have been estimated to typically measure 34.4±10.8 cm in length with a mean diameter of 4.2±0.6 mm (22). Applying the formula for cylinder volume indicates such GSV segments will provide 22.7±10.8 mL of anatomic space, which will be sufficient for large numbers of islets. It should be noteworthy that GSV has a robust network of vasa vasorum (23), which is far more extensive than vasa vasorum in the lumbar veins, as shown in our studies. The arterial supply to GSV includes contributions from external pudendal, superficial femoral, superior genicular, and posterior tibial arteries (24). This should be helpful in revascularization of transplanted islets in GSV similar to our findings in the venous sac. Moreover, such a peripherally accessible site should be highly convenient for clinical applications. We recognize that GSV is connected with multiple venous tributaries, some larger than the size of the islets. This should require appropriate preparation of the vessel to avoid translocation of transplanted islets elsewhere, for example, into the lungs. Alternatively, lumbar or other veins may be appropriate for developing applications in people, although accessing these vessels will obviously require more invasive techniques.
In conclusion, we demonstrated that the venous sac permitted engraftment, survival, and function of transplanted pancreatic islets over the long term. Even a minimal mass of pancreatic islets in isolated venous sacs were successful in restoring euglycemia in STZ-treated diabetic rats. This offers new avenues for cell therapy in type 1 diabetes. Moreover, the venous sac should be useful for testing the fate and function of stem cell-derived pancreatic beta cells or islets in the future. Other conditions may also be amenable to transplantation of cell types in the venous sac. The simplicity of transplanting islets in the venous sac should advance studies for clinical development. This will obviously need studies with transplantation of allogeneic islets. We do not anticipate that immunologic responses to allogeneic islets will be different in the venous sac versus in the portal vein. The significant divergences in the nature of animal and human allograft responses suggest that these studies will be most informative when allogeneic islets are transplanted in people.
MATERIALS AND METHODS
Animals
Inbred male Lewis rats were from Charles River Deutschland (Sulzfeld, Germany). Animals had free access to tap water and pelleted food and were housed under 12-hr light/dark cycles with humidity of 70%. All experimental procedures were approved by the Institutional Animal Care and Use Committee.
Islet Isolation, Culture, and In Vitro Function Assessment
Rat pancreatic islets were isolated by collagenase digestion followed by separations on density gradient, as described previously (11). Briefly, pancreas was distended by intraductal injection of 10 mL Hank's balanced salt solution containing 20 PZ-units of NB1 collagenase and 0.4 DMC-U of neutral protease (SERVA Electrophoresis GmbH, Germany), excised, and incubated for 15 to 19 min at 37°-C in shaking water bath. After three washes with Hank's balanced salt solution containing 10% newborn calf serum, the tissue pellet was resuspended in ice-cold University of Wisconsin solution and kept on ice for 30 min. Islets were sedimented in Ficoll (Biochrom, Berlin, Germany). Dissociated tissue was resuspended in 1.090 g/mL Ficoll and placed at the bottom followed by a layer of 1.077 mg/mL and 1.040 g/mL of Ficoll and centrifuged at 800G for 5 min. Islets were collected from interface of 1.077 and 1.040 g/mL Ficoll. After two washes, islets were picked by hand irrespective of their size. Isolated islets were counted manually. Islet viability was determined by trypan blue dye exclusion. Purity of islets was verified by dithiocarbazone staining. One isolation using 6 donors was performed for the entire study. Aliquots from same isolation were transplanted in parallel in the isolated venous sac and intraportally.
Groups of 350 islets were cultured free floating (37°-C, 5% CO2) in 5-mL culture medium consisting of CMRL 1066 (Biochrom) supplemented with 2-mM/L l-glutamine (Biochrom), penicillin-streptomycin (1000 U/mL-10 mg/mL) (Sigma-Aldrich, Schnelldorf, Germany), and 10% (vol/vol) fetal bovine serum (Biochrom) for 20 to 24 hr before transplantation.
After overnight culture, glucose-stimulated insulin secretion was determined during static glucose incubation of 20 islets for 120 min and expressed as stimulation index calculated as ratio of insulin released at 2.8 and 20-mM glucose. Insulin was measured by rat insulin enzyme-linked immunosorbent assay kit (Mercodia, Uppsala, Sweden).
Creation of Isolated Venous Sac and Islet Transplantation
A midline laparotomy was performed in anesthetized rats to expose the right lumbar vein. Ligatures (8-0 nylon suture) were placed at distal and proximal ends of the exposed vessel. Initially, all branches of the isolated vessel were ligated with 8-0 nylon suture. The tributaries were not ligated and remained open. Moreover, vasa vasorum remained intact. The proximal end of the vein was ligated, and distal end was cannulated through a small incision and flushed by normal saline to remove blood. Subsequently, groups of 350 islets drawn in a butterfly needle (25 gauge) were implanted in isolated venous sac, followed by ligation of the distal end of the sac. In control animals, groups of 350 islets were infused by means of the portal vein into the liver. Hemostasis was secured by compressing the injection site for 3 to 4 min. All studies included 10 to 15 rats per group.
Analysis of Vessel Integrity
To demonstrate the patency of capillaries supplying blood to the venous sac, we injected latex-containing dye. The abdominal aorta was cannulated with a 25-gauge needle and flushed with normal saline. This was followed by an injection of 5-mL latex over several min. Microphotographs were taken immediately under transillumination conditions.
Histopathologic Evaluation of Transplanted Islets
Graft-bearing venous segments were collected, fixed in 10% buffered formalin, and embedded in paraffin. Tissue sections of 5 μm thickness were stained with hematoxylin-eosin or Masson trichrome for morphologic evaluation. Expression of insulin was analyzed by immunohistochemistry with mouse monoclonal anti-insulin (1:1000) (ab6995; Abcam plc, Cambridge, UK). Antibody binding was detected with mouse-specific rabbit IgG (ab6728; Abcam plc) by using diaminobenzidine substrate (Sigma-Aldrich).
Studies of Islet Function in Diabetic Rats
Diabetes was induced in inbred male Lewis rats of 6 to 9 weeks of age, weighing 180 to 220 g. Animals were given one intraperitoneal dose of 65-mg/kg STZ (Sigma-Aldrich) dissolved in 0.01 M citrate pH 4.5. Random nonfasting blood glucose levels were measured daily with a glucometer in blood obtained from the tail vein. Presence of hyperglycemia was defined as high blood glucose levels 350 mg/dL or higher for 2 consecutive days.
Islets were transplanted in diabetic animals 2 weeks after STZ treatment. Graft function was defined as nonfasting glycemic levels less than 200 mg/dL. Graft failure was defined as blood glucose levels 350 mg/dL or higher for at least 2 consecutive days.
To exclude residual function of the native pancreas, graft-bearing venous segments were removed 3, 7, 14, and 60 days (n=12) after surgery followed by monitoring of blood glucose levels during the subsequent days to verify the therapeutic effects of islet transplantation.
Glucose tolerance test was performed 58 days after islet transplantation to assess metabolic activity of transplanted islets in the isolated venous sac. Fasting animals were given 2 g/kg glucose in normal saline intravenously followed by blood glucose measurements after 15, 30, 60, and 120 min.
Statistical Analysis
Data were expressed as mean±SE as appropriate. The differences were considered significant when P value was <0.05 using the Mann-Whitney U test.
Footnotes
The authors declare no funding or conflicts of interest.
Z.K., E.B., C.R., S.G., and A.M.J.S. participated in making the research design. Z.K., C.R., A.M.J.S., S.G., and E.B. participated in writing the article. Z.K., E.B., and K.S. participated in performing the research. Z.K., C.R., A.M.J.S., S.G., and E.B. participated in analyzing data.
REFERENCES
- 1.Ricordi C. Islet transplantation: A brave new world. Diabetes. 2003;52:1595. doi: 10.2337/diabetes.52.7.1595. [DOI] [PubMed] [Google Scholar]
- 2.Ricordi C, Strom TB. Clinical islet transplantation: advances and immunological challenges. Nat Rev Immunol. 2004;4:259. doi: 10.1038/nri1332. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 4.Harlan DM, Kenyon NS, Korsgren O, et al. Immunology of Diabetes Society. Current advances and travails in islet transplantation. Diabetes. 2009;58:2175. doi: 10.2337/db09-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vantyghem MC, Kerr-Conte J, Arnalsteen L, et al. Primary graft function, metabolic control, and graft survival after islet transplantation. Diabetes Care. 2009;32:1473. doi: 10.2337/dc08-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellin MD, Barton FB, Heitman A, et al. Potent induction immuno-therapy promotes long-term insulin independence after islet transplantation in type 1 diabetes. Am J Transplant. 2012;12:1576. doi: 10.1111/j.1600-6143.2011.03977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hiller WF, Klempnauer J, Luck R, et al. Progressive deterioration of endocrine function after intraportal but not kidney subcapsular rat islet transplantation. Diabetes. 1991;40:134. doi: 10.2337/diab.40.1.134. [DOI] [PubMed] [Google Scholar]
- 8.Scharp DW, Marchetti P, Swanson C, et al. The effect of transplantation site and islet mass on long-term survival and metabolic and hormonal function of canine purified islet autografts. Cell Transplant. 1992;1:245. doi: 10.1177/0963689792001002-306. [DOI] [PubMed] [Google Scholar]
- 9.Mattsson G, Jansson L, Carlsson PO. Decreased vascular density in mouse pancreatic islets after transplantation. Diabetes. 2002;51:1362. doi: 10.2337/diabetes.51.5.1362. [DOI] [PubMed] [Google Scholar]
- 10.Carlsson PO, Palm F, Andersson A, et al. Chronically decreased oxygen tension in rat pancreatic islets transplanted under the kidney capsule. Transplantation. 2000;69:761. doi: 10.1097/00007890-200003150-00015. [DOI] [PubMed] [Google Scholar]
- 11.Kakabadze Z, Gupta S, Brandhorst D, et al. Long-term engraftment and function of transplanted pancreatic islets in vascularized segments of small intestine. Transpl Int. 2011;24:175. doi: 10.1111/j.1432-2277.2010.01160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Echeverri GJ, McGrath K, Bottino R, et al. Endoscopic gastric submucosal transplantation of islets (ENDO-STI): technique and initial results in diabetic pigs. Am J Transplant. 2009;9:2485. doi: 10.1111/j.1600-6143.2009.02815.x. [DOI] [PubMed] [Google Scholar]
- 13.Caiazzo R, Gmyr V, Hubert T, et al. Evaluation of alternative sites for islet transplantation in the minipig: interest and limits of the gastric submucosa. Transplant Proc. 2007;39:2620. doi: 10.1016/j.transproceed.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 14.Kriz J, Jirak D, Vilk GJ, et al. Vascularization of artificial beds for pancreatic islet transplantation in a rat model. Transplant Proc. 2010;42:2097. doi: 10.1016/j.transproceed.2010.05.088. [DOI] [PubMed] [Google Scholar]
- 15.Pileggi A, Molano RD, Ricordi C, et al. Reversal of diabetes by pancreatic islet transplantation into a subcutaneous, neovascularized device. Transplantation. 2006;81:1318. doi: 10.1097/01.tp.0000203858.41105.88. [DOI] [PubMed] [Google Scholar]
- 16.Bottino R, Fernandez LA, Ricordi C, et al. Transplantation of allogeneic islets of Langerhans in the rat liver: effects of macrophage depletion on graft survival and microenvironment activation. Diabetes. 1998;47:316. doi: 10.2337/diabetes.47.3.316. [DOI] [PubMed] [Google Scholar]
- 17.Bennet W, Sundberg B, Groth CG, et al. Incompatibility between human blood and isolated islets of Langerhans: a finding with implications for clinical intraportal islet transplantation? Diabetes. 1999;48:1907. doi: 10.2337/diabetes.48.10.1907. [DOI] [PubMed] [Google Scholar]
- 18.Moberg L, Johansson H, Lukinius A, et al. Production of tissue factor by pancreatic islet cells as a trigger of detrimental thrombotic reactions in clinical islet transplantation. Lancet. 2002;360:2039. doi: 10.1016/s0140-6736(02)12020-4. [DOI] [PubMed] [Google Scholar]
- 19.Brissova M, Fowler M, Wiebe P, et al. Intraislet endothelial cells contribute to revascularization of transplanted pancreatic islets. Diabetes. 2004;53:1318. doi: 10.2337/diabetes.53.5.1318. [DOI] [PubMed] [Google Scholar]
- 20.Linn T, Schneider K, Hammes HP, et al. Angiogenic capacity of endothelial cells in islets of Langerhans. FASEB J. 2003;17:881. doi: 10.1096/fj.02-0615fje. [DOI] [PubMed] [Google Scholar]
- 21.Nyqvist D, Kohler M, Wahlstedt H, et al. Donor islet endothelial cells participate in formation of functional vessels within pancreatic islet grafts. Diabetes. 2005;54:2287. doi: 10.2337/diabetes.54.8.2287. [DOI] [PubMed] [Google Scholar]
- 22.Human P, Franz T, Scherman J, et al. Dimensional analysis of human saphenous vein grafts: implications for external mesh support. J Thorac Cardiovasc Surg. 2009;137:1101. doi: 10.1016/j.jtcvs.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 23.Lametschwandtner A, Minnich B, Kachlik D, et al. Three-dimensional arrangement of the vasa vasorum in explanted segments of the aged human great saphenous vein: scanning electron microscopy and three-dimensional morphometry of vascular corrosion casts. Anat Rec A Discov Mol Cell Evol Biol. 2004;281:1372. doi: 10.1002/ar.a.20098. [DOI] [PubMed] [Google Scholar]
- 24.Lescalié F, Germouty I, Chevalier JM, et al. Extrinsic arterial supply of the great saphenous vein: an anatomic study. Ann Vasc Surg. 1986;1:273. doi: 10.1016/S0890-5096(06)61994-8. [DOI] [PubMed] [Google Scholar]