Abstract
Comparatively few studies over the past 30 years have used pharmacological manipulations as a means of understanding processes underlying feeding behavior of nonhuman primates. In the 1970s and early 1980s, four laboratories provided data on the anorexigenic effects of a range of drugs on rhesus monkeys and baboons, and a fifth laboratory studied the effects of neuropeptides on feeding behavior of baboons. There were differences in the way anorexigenic drugs altered eating topography, and those that increased dopamine levels had greater abuse liability than those that increased serotonin levels. Studies in the 1980s and 1990s used foraging models and principles of behavioral economics to understand food–drug interactions. Experimenter-given anorexigenic drugs did not function as economic substitutes for food. Recent studies have examined the effects of a range of drugs on consumption of highly palatable food and model diet-induced obesity. Although some drugs, including stimulants, N-methyl-D-aspartate antagonists, and a cannabinoid antagonist increased the latency to standard food consumption, there was little evidence for a selective effect of any drug on highly palatable food consumption. Results obtained in nonhuman primates did not always confirm those observed in rodents. Future studies looking at sex differences and social factors may provide insight into factors related to human obesity.
Keywords: amphetamine, anorectic, anorexigenic, baboon, binge eating, dexfenfluramine, diazepam, eating, nonhuman primate, rhesus monkey, topography
Introduction
Over the past 30 years, research examining the effects of pharmacological manipulations on feeding behavior in nonhuman primates has focused on three major areas. One set of studies has looked at the efficacy of drugs in decreasing food intake in the context of medication development and has assessed the abuse liability of drugs that affect food intake. A second set of studies has used pharmacological manipulations as tools to understand the processes underlying feeding behavior, including the initiation and termination of meals. The final set of studies, and the largest by far, has used food intake or responding reinforced by food as a measure of the specificity of action of a putative pharmacological intervention for the treatment of drug abuse, or has used operant responding for food as a behavioral baseline for understanding variables affecting responding. As the third set of studies used food intake as either a convenience measure for nonspecific disruption of behavior or as a means of reinforcing operant behavior rather than the primary outcome, this review will focus on the first two sets of studies.
Medication development and abuse liability
Since the mid twentieth century it has been well established that under certain conditions amphetamine increased alertness, improved performance, disrupted sleep, and decreased food intake (Harris et al., 1947; Kornetsky et al., 1959; Weiss and Laties, 1962). It was also known that amphetamine increased ‘positive’ subjective effects and had significant abuse liability (Knapp, 1952; Lasagna et al., 1955). Given the clinical significance of effective weight-loss medications, a considerable amount of research during the latter third of the twentieth century had the goal of chemically creating new possible therapeutic agents for weight loss and evaluating them behaviorally for clinical efficacy, side-effects, and abuse liability. For example Martin et al. (1971) comprehensively assessed the effects, including food intake during a single meal, of five stimulants in 12 volunteers, collecting nearly a 1000 data points. Within this article, Fig. 2 presents the dose–response functions for all five drugs on 12 dependent measures, allowing readers to rapidly comprehend the range of effects of anorexigenic drugs in relation to the doses that decrease food intake.
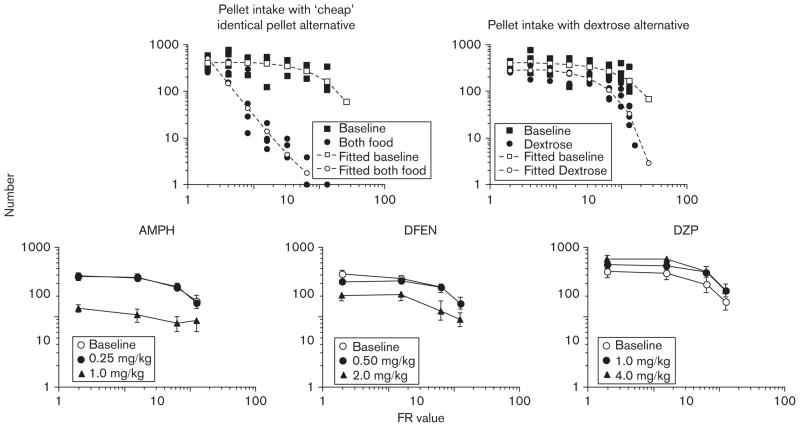
Fig. 2.
Top panels: total pellet intake when only those pellets were available (baseline) and when pellets or a dextrose solution were available on an alternate lever under a FR2 schedule as a function of the number of responses required for a single pellet (adapted from Foltin, 1992). Open symbols with dashed lines indicate the predicted demand curve based on the parameters derived from fitting the data to the equation developed by Hursh et al. (1988). Bottom panels: pellet intake during the 22-h session as a function of the number of responses required for a single pellet and drug dose (adapted from Foltin, 1993: to increase clarity some original data points have been omitted). AMPH, D-amphetamine; DFEN, dexfenfluramine; DZP, diazepam; FR, fixed ratio.
At about the same time, Tang and Kirch (1971) published a study that set a high standard for research examining the effects of pharmacological manipulations on food intake in nonhuman primates. They measured the effects of 15 drugs including amphetamine, methamphetamine, fenfluramine, phenmetrazine, diethylpropion, and chlorphentermine on (i) consumption of standard laboratory chow during a 1-h period, (ii) locomotor activity, and (iii) sleep patterns in 13 rhesus monkeys. Amphetamine and related drugs that predominantly increase central dopamine (Fuchs et al., 2005) produced dose-dependent decreases in food intake, increased locomotor activity and latency to sleep, and decreased sleep integrity. By contrast, fenfluramine and related drugs that predominantly increase central serotonin levels (Blundell et al., 1976) produced dose-dependent decreases in food intake without affecting locomotor activity or sleep.
Griffiths et al. (1976, 1978) approached the issue of assessing anorexigenic efficacy and abuse liability by examining how drugs that were self-administered affected response to food pellets. Baboons could self-administer a dose of drug once every 3 h by responding under a fixed-ratio (FR) schedule in which 160 responses (FR160, an effortful schedule) on one lever resulted in drug delivery. Throughout the day, baboons could also receive a food pellet by responding 15–25 times on a second lever (FR15–FR25, an easy schedule). Amphetamine, diethylpropion, chlorphentermine, phentermine, and clortermine were all self-administered and produced dose-dependent decreases in pellet intake, whereas fenfluramine and phenylpropanolamine also produced dose-dependent decreases in pellet intake but were not self-administered. Thus the drugs that were found to be stimulants in the paper by Tang and Kirch (1971) had abuse potential. Corwin et al. (1987) compared the ability of six anorexigenic drugs to reduce pellet intake during a single 1–4-h meal in one group of rhesus monkeys with the ability to maintain self-administration responding in a second group of rhesus monkeys. Mazindol, phenmetrazine, and benzphetamine were self-administered and decreased pellet intake, whereas phendimetrazine only decreased pellet intake. In contrast to observations made by Griffith et al. (1976), chlorphentermine and clortermine were not self-administered but decreased pellet intake. Species, dose, and procedural differences most likely account for the disparate results.
In summary, this set of studies shows that many drugs that decrease food intake in nonhuman primates are self-administered, but some drugs with limited reinforcing efficacy also decrease food intake. Although providing information about abuse potential relative to an estimate of anorexigenic efficacy, the above studies were generally limited to a single meal and did not provide data on how drugs may affect more naturalistic patterns of eating or food consumption throughout the day.
Pharmacological insight into mechanisms of feeding behavior
At about the same time the studies on the relationship between anorexigenic efficacy and abuse liability described above were being conducted, a series of studies were published, many by Blundell and colleagues, using pharmacological manipulations to probe for mechanisms of feeding behavior in laboratory rodents. In rats given access to food after 16 h of deprivation, amphetamine increased latency to the first meal, decreased meal size and duration, increased eating rate (g/min), and decreased or had no effect on meal number (Blundell et al., 1976, 1979). In contrast, fenfluramine had no effect on latency to initiate feeding or meal number, but decreased meal size, duration, and rate of eating (g/min; Blundell et al., 1976, 1979). Drugs that affected dopaminergic function and those that affected serotonergic function influenced feeding topography in different ways.
Commonly, in the natural ecology, both the location and quality of food vary intermittently (Owen, 1980; Rodman and Cant, 1984). In the 1970s, Collier et al. (1977, 1980) developed procedures that utilized operant methodology to model naturalistic feeding behavior under controlled laboratory settings in nonprimates. Combining aspects of the topography measures used by Blundell and colleagues, the foraging models developed by Collier and colleagues offered a novel approach for studying the behavioral pharmacology of food intake. In the Collier foraging model, searching for a ‘patch’ of food requires laboratory animals to complete a fixed number of responses on an operant manipulandum to gain access to a second manipulandum. Responding on the second manipulandum is reinforced with food until an animal stops responding for 10 min; that is, until a meal has been consumed. At this point, to gain access to another food patch, the animal needs to complete the initial operant requirement again. Thus, food is available continuously under a two-component chained schedule of reinforcement. Completion of the response requirement of the first seeking or procurement component provides access to the second taking or consumption component, and the animal, rather than the experimenter, controls initiation and termination of all eating bouts.
Fischman and Foltin conducted a series of studies extending the Collier model of foraging to a large nonhuman primate, the baboon (Foltin, 1989; Foltin and Fischman, 1989; Foltin et al., 1989, 1990). When eight male baboons had to make only 10 responses to gain access to a meal, they consumed about three hundred and fifty 1-g pellets in a 22-h period, having nearly eight meals per day. Increasing the seeking cost to 200 responses decreased the number of meals to four, but total daily pellet intake remained stable; baboons responded more quickly during the seeking components and consumed fewer, but larger meals. This pattern replicated data observed in a range of vertebrates including chickens, rats, cats, and guinea pigs (Collier et al., 1972; Hirsch and Collier, 1974a, 1974b; Collier and Rovee-Collier, 1980; Collier, 1983).
We then conducted a second series of studies using the Collier model of foraging as the behavioral baseline for the assessment of drug effects. We adjusted the seeking response cost for each of eight baboons such that each baboon maintained a stable daily number of meals (three to four) and consumed a stable number of pellets each day (experimental days were 22 h long: 11:00 h until 09:00 h the following day). Table 1 summarizes the effects of a range of pharmacological manipulations on six measures of feeding topography.
Table 1.
Summary of the effects of drugs on feeding topography in baboons
| Latency | 22-h intake | Meal number | Size | First meal duration | Response ratea | |
|---|---|---|---|---|---|---|
| Dexfenfluramineb | ⇔ | ⇓ | ⇓ | ⇔ | ⇓ | ⇔ |
| Amphetamineb | ⇑ | ⇓ | ⇓ | ⇓ | ⇔ | ⇔ |
| Phendimetrazinec | ⇑ | ⇓ | ⇓ | ⇓ | ⇓ | ⇔ |
| Phenmetrazinec | ⇑ | ⇓ | ⇔ | ⇓ | ⇔ | ⇔ |
| Diethylpropionc | ⇑ | ⇓ | ⇓ | ⇓ | ⇓ | ⇔ |
| Phenterminec | ⇑ | ⇓ | ⇔ | ⇓ | ⇓ | ⇔ |
| Chlorphenterminec | ⇔ | ⇓ | ⇔ | ⇔ | ⇔ | ⇔ |
| Clorterminec | ⇑ | ⇓ | ⇔ | ⇓ | ⇔ | ⇔ |
| Mazindolc | ⇑ | ⇓ | ⇓ | ⇔ | ⇔ | ⇔ |
| Phenylpropanolaminec | ⇔ | ⇔ | ⇔ | ⇔ | ⇔ | ⇔ |
| Cocained | ⇑ | ⇔ | ⇔ | ⇔ | ⇔ | ⇔ |
| Desipramined | ⇔ | ⇓ | ⇓ | ⇓ | ⇔ | ⇔ |
| Cholecystokinin analoge | ⇑ | ⇓ | ⇔ | ⇓ | ⇓ | ⇔ |
| Diazepamf | ⇔ | ⇑ | ⇔ | ⇑ | ⇑ | ⇔ |
indicates an increase;
indicates a decrease;
indicates no effect.
Rate of responding (response/s), not rate of food delivery.
Each of the first nine listed drugs produced dose-dependent decreases in 22-h pellet intake, replicating the overall anorexigenic effect described above in other studies on nonhuman primates (Tang and Kirch, 1971; Griffiths et al., 1976; Corwin et al., 1987). The data in Table 1 provide additional information about drug effects on feeding topography. All of these nine anorexigenic drugs, except dexfenfluramine and clortermine, increased the latency to the first meal (Foltin, 1989; Foltin and Fischman, 1989). The anorexigenic drugs also had different effects on the number of meals consumed, with only five drugs (dexfenfluramine, amphetamine, phendimetrazine, diethylpropion, and mazindol) decreasing meal number over the 22-h session. Finally, only six of the anorexigenic drugs decreased the size of the first meal; that is the number of pellets consumed. Phenylpropanolamine, the putative active ingredient in over-the-counter weight-loss aids that were available at that time (e.g. Dexatrim and Accutrim), had no effect in baboons at the dose range tested (maximal dose possible with constraints of solubility and injection volume). Cocaine produced a different pattern of results: it dose dependently increased latency to the first meal from about 1 h after placebo to up to 6 h after administration of 2.0 mg/kg of cocaine; however, it had no effect on pellet intake over the 22-h session (Foltin et al., 1990). The brief duration of action of cocaine does not account for the difference between it and the other anorexigenic drugs, whose duration of action also ended during the daily session. The decrease in eating produced by the other anorexigenic drugs was not accompanied by compensatory overeating after the drug effect wore off. Finally, the antidepressant desipramine had no effect on latency to the first meal, but significantly decreased pellet intake (Foltin et al., 1990).
At about the same time Figlewicz et al. (1996) and Woods (2009) conducted a series of studies examining neuroendocrine and neuropeptide modulation of feeding behavior in baboons. One set of studies focused on how infusions of cholecystokinin and cholecystokinin analogs decreased feeding behavior in male baboons by decreasing meal size (Figlewicz et al., 1986, 1989, 1992, 1995; Stein and Belluzzi, 1986). We also (Foltin and Moran, 1989) examined the effects of a short-acting cholecystokinin analog (U-67) on the pattern of eating using the Collier foraging model in baboons. Indeed, U-67 increased latency to the first meal and decreased first meal size and 22-h pellet intake.
Finally, benzodiazepines reliably increase short-term food intake in a variety of species including laboratory rodents, dogs, cats, horses, and pigeons (Bainbridge, 1968; Brown et al., 1976, 1981; Fratta et al., 1976; Soubrie et al., 1976; Cooper and Posadas-Andrews, 1979) and under certain conditions in rhesus monkeys (Delgado et al., 1976). As shown by the last item in Table 1, diazepam increased pellet intake in baboons by increasing the size of the first meal, but surprisingly, without affecting latency to the first meal (Foltin et al., 1989).
These latter studies on the effects of benzodiazepines and various peptides provided valuable data about behavioral mechanisms underlying food intake under laboratory conditions designed to mimic normal eating patterns. Revisiting this decades-old dataset with the eyes of a modern medicinal chemist might provide insight into possible chemical families that could be manipulated to produce novel anorexigenic agents.
The results obtained in baboons (Table 1) clearly vary from the consistent pattern of results observed in rodents (Blundell et al., 1976, 1979). Although amphetamine, but not dexfenfluramine, increased the latency to the first meal, there were no differences between amphetamine and dexfenfluramine in effects on meal number. There were key differences between the procedures and measures. Feeding topography in rodents was based on a 1-h feeding interval after a 16-h deprivation period: under baseline conditions, there were about 20 meal bouts of less than 0.5 g each, with an interbout interval of about 90 s. Baboons were not food restricted, but the parameters were chosen to engender three to four meals of 75–150 g each day. Despite the quantitative differences between species and in experimental conditions, similar qualitative differences in drug effects were observed with drugs that affected dopaminergic function, whereas drugs that affected serotonergic function influencing feeding slightly differently.
At the beginning of these studies, we had expected that some drug doses would cause disruptions of operant behavior such that decreases in rate of responding would be an index of nonspecific drug effects. None of the above drugs had any significant effects on rate of responding during the first meal. Although not described above, the hallucinogenic drug phencyclidine did disrupt responding during the first meal (Foltin, 1989). Furthermore, baboons ate more pellets later in the day, such that the total daily pellet intake was unchanged. Baboons did not compensate for the pause in eating caused by anorexigenic drugs other than cocaine. These findings emphasize the importance of measuring behavior for a prolonged interval after drug administration. Phencyclidine also produced clear behavioral disruptions including drooling, sensitivity to noise, and staring off into space. With the exception that animals did more grunting after amphetamine administration, there were few clear signs of intoxication after drug administration.
Doses were carefully chosen to be at the low end, so as to not decrease food intake to less than 20–30% of baseline levels. To avoid potential toxicity, individuals conducting behavioral studies are generally reluctant to administer large doses of drugs to nonhuman primates. It is likely that studies using rodents would test a wider range of doses and nonspecific disruptions would be observed at the larger doses.
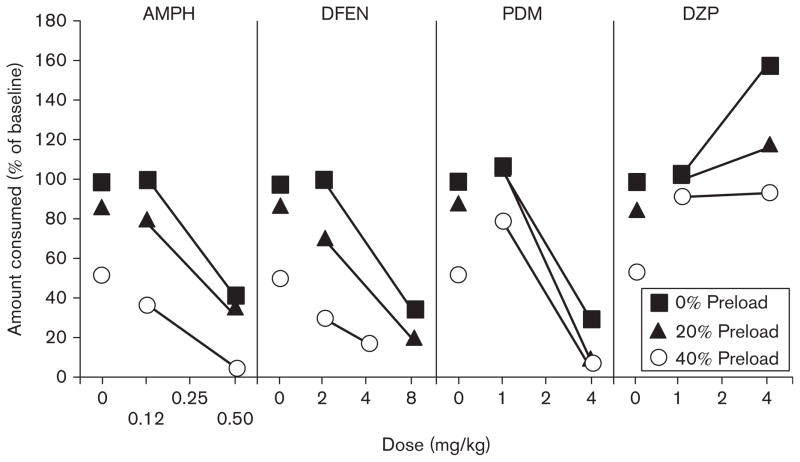
Data obtained with rodents suggest that increases in latency to initiate feeding reflect a decrease in hunger (Blundell et al., 1976), whereas decreases in meal size reflect an increase in satiation, but this is only partially supported by the data described above in nonhuman primates. If accurate, then animals that are partially sated should be more sensitive to the effects of drugs that may increase satiety like dexfenfluramine. Baseline meal size can be decreased by nasogastrically infusing rhesus monkeys with nutrients before giving them access to food (Baile et al., 1971; McHugh and Moran, 1978; Foltin and Schuster, 1983): infusing nutrients produces energy-dependent decreases in meal size during a single meal. A study was conducted to test the hypothesis that infusing rhesus monkeys with nutrients before the session would shift the dose–response function for anorexigenic drugs to the left (Foltin and Schuster, 1983). Dose–response functions for amphetamine, dexfenfluramine, phendimetrazine, cathinone (a stimulant), and diazepam were determined at each of four meal sizes.
As shown in Fig. 1, amphetamine, dexfenfluramine, phendimetrazine, and cathinone (data not shown) produced dose-dependent decreases in food intake when there was no energy preload (0% energy preload). The dose–response functions for amphetamine, dexfenfluramine, and cathinone, but not for phendimetrazine, were shifted to the left in an energy-dependent manner. Diazepam increased food intake under the 0% energy preload condition and the dose–response function was also shifted to the left in an energy-dependent manner. With the exception of those for phendimetrazine, the anorexigenic and orexigenic drug effects were related to the size of the meal. Thus, there was no evidence that effects of amphetamine and dexfenfluramine were differentially affected by energy preloading, and the findings do not support the suggestion that these drugs affect food intake through different behavioral mechanisms.
Fig. 1.
Percent of food consumed, compared with baseline, as a function of drug dose and energy content of intragastric preload (adapted from Foltin and Schuster, 1983: to increase clarity some original data points have been omitted). AMPH, D-amphetamine; DFEN, dexfenfluramine; DZP, diazepam; PDM, phendimetrazine.
In summary, a wide range of drugs that are effective anorexigenic agents in laboratory rodents and humans produce dose-dependent decreases in single-meal and free food intake in nonhuman primates. Although there was some difference in how different drugs affected meal patterns in the series of studies described above, there was scant evidence that drugs that increase dopamine differentially decrease hunger – that is increase latency to eating – whereas drugs that increase serotonin differentially increase satiety – that is decrease meal size – as reported in rats. Clearly a large range of procedural factors such as single versus multiple meal measurements, differences in duration of test session, range of doses tested, drug pharmacokinetics, absorption, and distribution contribute to the differences observed between rats and nonhuman primates. Feeding is a complex process with multiple determinants, which most likely vary across species, size of the species, and ecological niche.
Behavioral economic analysis of anorexigenic drug effects on food intake
Application of the principles of behavioral economics provides another approach to understanding the behavioral mechanism by which anorexigenic drugs decrease food intake (Lea, 1978; Allison, 1981; Hursh, 1984; Hursh and Baumann, 1987). Perhaps anorexigenic drugs affect food intake by altering the reinforcing efficacy of food, which can be quantified using the principles of behavioral economics. Research based on economic models examines response to and consumption of a commodity as a function of cost or, most often in the laboratory, response requirement. Responding will initially increase with increasing cost; however, as cost further increases, responding will eventually reach a maximal amount and then begin to decrease. When responding is measured as a function of increasing cost, it is possible to describe changes in the value in more general ‘demand’ terms. Demand for a commodity can be described by its elasticity – that is the range of costs across which responding increases. Demand for a commodity for which responding increases across a wide range of costs is less elastic than demand for a commodity for which responding increases across a small range of costs. Reinforcers necessary for existence, such as food and water, have less elastic demand compared with nonessential items. The cost at which responding stops increasing and begins to decrease varies across commodities and can be used as an index of the overall demand for a commodity.
Behavioral economic analyses provide a precise definition for commodity substitution. Elasticity of demand for a commodity will be greater when a commodity similar to the original commodity is available – that is responding for one type of food will be more elastic when another similar type of food is available at lower cost. The upper left panel of Fig. 2 compares the number of pellets consumed as cost increased under two conditions (Foltin, 1992): when pellets were only available at that cost (filled squares) and when pellets were also available after two responses (FR2), that is at minimal cost on a second response manipulandum (filled circles). When pellets were available on a single lever, food intake remained stable with increasing cost up to about FR128 for a single pellet. By contrast, when pellets were available under a FR2 schedule on an alternate manipulandum, intake of the more expensive pellets dropped dramatically as cost increased, that is pellets substituted for themselves. Showing substitution of a commodity for itself may appear trivial, but it demonstrates that behavior responds lawfully to changes in cost. The upper right panel of Fig. 2 shows that when an alternative source of energy (isocaloric dextrose solution) was available under a FR2 schedule, intake of the more expensive food also dropped more quickly as cost increased, but not as quickly that as when pellets were available at a lower cost. Thus, it is possible to ask the question ‘Do anorexigenic drugs function as economic substitutes for food?’
To determine whether anorexigenic drugs can function as economic substitutes for food, all dose–response functions for a range of drugs were determined, with each dose being tested across a range of response costs (Foltin, 1993). As shown in the lower two left panels of Fig. 2, amphetamine and dexfenfluramine decreased the level of pellet intake under a FR2 schedule of reinforcement and produced dose-dependent decreases in pellet intake across all response requirements by shifting the demand curve for pellets downward. A similar pattern of results was also obtained for the anorexigenic drugs diethylpropion, phenmetrazine, phenylpropanolamine, and mazindol. Changes in pellet intake were fitted to a theoretical equation derived by Hursh et al. (1988) to describe changes in demand for a commodity. Administration of anorexigenic drugs had no effect on the elasticity of demand for food; that is, none of the anorexigenic drugs were a substitute for food. Given the differences in the effects of amphetamine and dexfenfluramine on feeding topography in baboons and rats, it was expected that dexfenfluramine, perhaps by enhancing satiation, would be an economic substitute for food. Clearly, this was not the case.
Diazepam increased maximal intake when pellets were available under a FR2 schedule, and as with the anorexigenic drugs, produced parallel shifts, albeit upward, in the demand curve for pellets without affecting the elasticity of demand for food. It was expected that diazepam, because of its orexigenic effects, would decrease the elasticity of demand for pellets. Again, the results did not match expectations.
The failure to observe economic substitution of a drug effect for energy intake, although disappointing, is in hindsight not surprising. Perhaps one contributor to the outcome was the fact that the drugs were experimenter-given and not self-administered by the baboon. Perhaps a self-administered drug substitutes for food, whereas an experimenter-given drug does not. This possibility was examined by giving baboons the opportunity to self-administer oral amphetamine by completing the response requirement on one manipulandum and also having pellets available after responding on a second manipulandum (Foltin, 1997).
As shown in the left panel of Fig. 3, when six adult male baboons had nonrestricted access to 1-g pellets 22 h a day under a FR2 schedule, they consumed nearly 550 pellets per day. When the cost was increased to FR128, pellet intake dropped by 50%. Maximal intake under the FR2 schedule was significantly reduced to about 400 pellets per day when baboons had concurrent access to a dextrose solution or an amphetamine solution (0.002 or 0.004mg/kg per delivery). Increasing the response cost for pellets when the fluids were available decreased pellet intake (middle panel of Fig. 3) without a change in slope, that is no change in elasticity of pellet demand. The same manipulations were tested again under conditions of restricted access to pellets, in which each baboon had access to only 70% of the pellets they consumed under the nonrestricted condition. Under these conditions, the slope (calculated using the equation developed by Hursh et al., 1988) for the decrease in pellet intake with increasing cost was greater when a larger dose of amphetamine was available (right panel of Fig. 3); that is, pellet intake decreased more rapidly as cost increased. Thus, self-administered amphetamine served as an economic substitute for food pellets only when food intake was restricted.
Fig. 3.
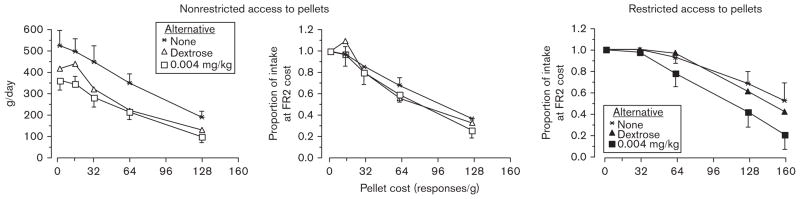
Mean total daily pellet intake as a function of the number of responses required for a single pellet and type of concurrently available fluid when baboons had nonrestricted access to pellets and when access to pellets was restricted (adapted from Foltin, 1997: to increase clarity some original data points have been omitted). The doses of oral D-amphetamine available per fluid delivery were 0.002 and 0.004 mg/kg, respectively. FR, fixed ratio.
In summary, behavioral economic approaches have proven valuable in understanding demand for commodities and how alternatives influence that demand. The hypothesis that anorexigenic drugs would function as economic substitutes for food was not confirmed. One tenet of behavioral economics is that behavior is studied in a ‘closed’ economy – that is the individual is responsible for working for the commodity and none of the commodities are provided for free (Timberlake and Peden, 1987). In many operant procedures, supplemental food is provided outside the session, resulting in an ‘open’ economy. The finding that self-administered amphetamine substituted for food when total food intake was restricted was unexpected. One caveat to this conclusion is based on how food restriction altered the daily pattern of behavior. When animals had nonrestricted access to pellets, they consumed pellets and fluid (dextrose, amphetamine) throughout the day, with bouts of pellet intake interspersed with bouts of fluid intake. When food was restricted, baboons tended to respond for all of their daily pellets followed by multiple bouts of fluid intake. As pellet cost increased, baboons stopped responding for pellets and started responding for fluid earlier within the session; that is, the interval between the single pellet meal and the first fluid bout shortened. The substitution by the larger dose of amphetamine may have been related more to differences in the baseline topography of eating rather than a true economic substitution. Behavioral baselines used in studies on food intake vary from study to study, from laboratory to laboratory, and among species. The differences in baseline, including those related to food received outside the test session, possibly account for more of the variability in results across studies compared with direct drug effects.
Incentive salience
A consistent finding in studies using rodents is that drugs that increase dopamine, such as amphetamine and cocaine, also increase the incentive value of stimuli paired with reinforcement (Robinson and Berridge, 1993; Zhang et al., 2009; Smith et al., 2011). By contrast, increases in serotonin are hypothesized to decrease responding that is reinforced by the presentation of stimuli paired with primary reinforcement (Fletcher, 1995, 1996; Wilson et al., 2000). Because drugs that increase dopamine often decrease food intake, this raises the interesting possibility that a pharmacological manipulation may increase responding reinforced by stimuli paired with food, yet reduce consumption of the same food. Indeed Cohen and Branch (1991) reported that amphetamine decreased responding in pigeons that was reinforced with food, but increased responding that was reinforced with stimuli that had been paired with food; that is, amphetamine increased the reinforcing efficacy of conditioned reinforcers. Thus, data suggest that both amphetamine and dexfenfluramine would decrease food intake, but only amphetamine would increase responding for cues paired with food, whereas dexfenfluramine would decrease responding for cues paired with food.
In the previous sections, food seeking and food taking, as described by Collier (1980), was modeled using FR schedules. To determine the effect of anorexigenic drugs on the reinforcing value of stimuli paired with food, we developed a procedure that used a fixed-interval schedule rather than a ratio schedule of responding, and embedded a second-order FR schedule within the interval such that responding during the seeking component for each meal was reinforced by presentation of stimuli that were also presented with food delivery during the taking component – that is every 10 responses within the seeking interval were reinforced by the flashing of lights paired with food (Kelleher, 1966). Food was available under this schedule 24 h/day (rather than 22 h as in previous studies: from 09:00 h one day until 09:00 h the next day), with the initiation and termination of all eating occasions determined by the baboon (Foltin, 2001).
This group of eight baboons ate about 350 pellets each day during taking components and earned about 50 conditioned reinforcer deliveries during seeking components (Fig. 4). Baboons completed about 3.5 seeking and taking components each day, such that they had essentially three meals a day, as with the FR foraging Collier procedure. Both amphetamine and dexfenfluramine produced dose-dependent decreases in pellet intake during taking components. Amphetamine, however, increased responding during seeking components, whereas dexfenfluramine decreased responding during seeking components (Foltin, 2005, 2006a). In contrast to data obtained using the Collier procedure, both amphetamine and dexfenfluramine increased the latency to the first taking or meal component of the day. Diethylpropion reduced food taking without affecting food-seeking behavior (data not shown). Diazepam increased food taking and food seeking (Fig. 4). In separate studies, the anorexigenic drug sibutramine decreased responding for food during taking components without affecting incentive salience measured during seeking components (Foltin, 2006a), whereas baclofen decreased both seeking and taking responding, most likely due to nonspecific behavioral disruptions (Foltin, 2005).
Fig. 4.
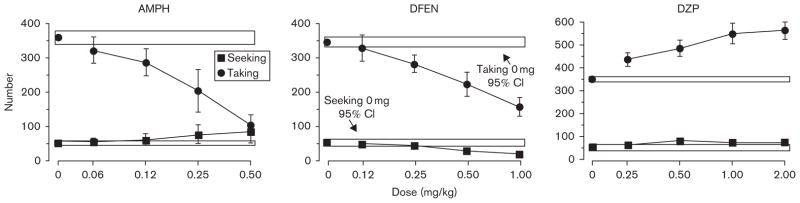
Total number of daily reinforcers delivered during seeking and taking components as a function of drug dose (adapted from Foltin, 2001). The rectangles represent the 95% confidence intervals for the number of pellets delivered during taking components and the number of light flashes delivered during seeking components under placebo conditions. AMPH, D-amphetamine; CI, confidence interval; DFEN, dexfenfluramine; DZP, diazepam.
Depriving baboons of a part of their daily food intake by terminating their session early produced deprivation-dependent increases in both seeking and taking behavior during the first seeking and taking components of the following session (Foltin, 2001). The effects of energy prefeeding were determined by providing baboons with free (no response requirement), highly palatable food (e.g. bananas) at the start of the session (09:00 h). Energy prefeeding produced energy-dependent decreases in taking behavior during the first taking component of the day, but also increased food seeking during the first seeking component of the day. Colantuoni et al. (2001)) reported that intermittent consumption of large amounts of a palatable food increased central dopamine, which supports the hypothesis that eating preferred foods, like amphetamine administration, increases the reinforcing value of stimuli paired with pellets, but decreases pellet intake through dopaminergic pathways.
In the same group of baboons, the differential effects of amphetamine and dexfenfluramine on incentive salience were demonstrated when responding occurred under extinction (Foltin, 2004a), the procedure most commonly used with rodents. The fact that increases in responding were only observed when stimuli paired with reinforcement were presented (Foltin, 2004b) shows that the drug effects were not due to nonspecific changes in rate of responding. In summary, as observed in rodents, amphetamine, but not other anorexigenic drugs, increased the incentive salience of stimuli paired with food, but in contrast to the hypothesized role of incentive stimuli in motivating behavior for the primary reinforcer (Robinson and Berridge, 1993), amphetamine decreased consumption of food.
Consumption of highly palatable foods: a model of ‘binge’ eating
Pharmacological manipulations can provide insight into mechanisms associated with food intake and potential avenues for pharmacotherapy development for obesity and eating disorders. Bellisari (2008) suggested several reasons why both human and nonhuman primates are behaviorally and genetically prone to obesity. Primates initially adapted to environments in which quality and quantity of food varied seasonally, and those animals with the ability to store fat preferentially weathered periods of limited food availability. Primates have eclectic diets with a preference for high-carbohydrate sweet food. Many primates also have a preference for variety in their diet. Primates will spend a considerable amount of time foraging for food and preferred foods when available, but will also gorge on a single preferred food when available. Primates can have large energy demands as they can expend large amounts of energy quickly when avoiding a predator or defending territories. Further, their long gestation period necessitates the ability to store energy. Thus, the large energy requirement for a periodically active lifestyle coupled with a long gestation period in the face of seasonally-changing food availability favors overconsumption when foods such as nuts and ripe fruit are available to supplement readily available grasses and corms. Such seasonal overeating can lead to increased fat disposition.
Primates appear to be disposed to eat – that is ‘opportunistic eaters’ (Rowland, 2012) – if food is available, especially if the food is palatable and available at a good ‘cost’. In a sense, overeating is the norm and is easy to model. For example, Altmann et al. (1993) compared body morphology in a troop of baboons living in their natural ecology with that in a troop of baboons living near a human garbage dump. Female baboons that foraged in a garbage dump weighed about 5.5 kg more than wild-foraging females, whereas males who foraged in the garbage dump weighed about 3.5 kg more than wild-foraging males. Such overconsumption of energy-dense food also occurs in the natural ecology based on seasonal availability. When a fruit was in season, energy intake in male orangutans in Indonesia was more than double, from about 3800 to 8400 kcal/day, and that in female orangutans was four times the regular amount, from 1800 to 7400 kcal/day (Knott, 1998). Given the energetic needs of pregnancy, it is not surprising that in the natural ecology female primates appear to be more sensitive to the availability of high-energy foods and more prone to increased fat storage than males.
One pattern of eating that can contribute to obesity is binge eating (Klein and Walsh, 2004). A ‘binge’, is the consumption of large amounts of food in a brief time frame, accompanied by feelings of loss of control over eating and distress. Individuals who attempt to compensate for the energy by vomiting or purging may have bulimia nervosa (APA DSM-IV, 1994), and individuals who do not try to compensate may have binge-eating disorder (APA DSM-IV, 1994). Approximately 3.5% of adult female Americans and 2% of adult male Americans have binge-eating disorder (Hudson et al., 2007).
We developed a model of binge eating in nonhuman primates based on the paradigm used by Corwin and Buda-Levin (2004) in rats: rats given brief (2 h) access to highly palatable fat food on alternate weekdays develop a binge-type eating pattern of fat (Corwin et al., 1998; Dimitriou et al., 2000; Corwin and Buda-Levin, 2004). Using a variation of the Collier foraging procedure on Mondays, Wednesdays, and Fridays, baboons had access to a single morning meal of highly palatable candy food (candy-coated, fruit-flavored jelly Skittles or candy-coated chocolate M&Ms). After completion of a 30-min seeking component, in which every 10 responses were reinforced by flashing the lights associated with candy, baboons could earn as much candy as they wanted in a single candy meal, with candy delivered after every 10 responses. The candy meal ended after a baboon stopped pulling the lever for 10 min. Baboons had access to pellet meals for the remainder of the day, and only pellet meals were available on the remaining 4 days of the week. Foraging for a meal was possible 23.5 h/day: from 09:00 h one day to 08:30 h the next day.
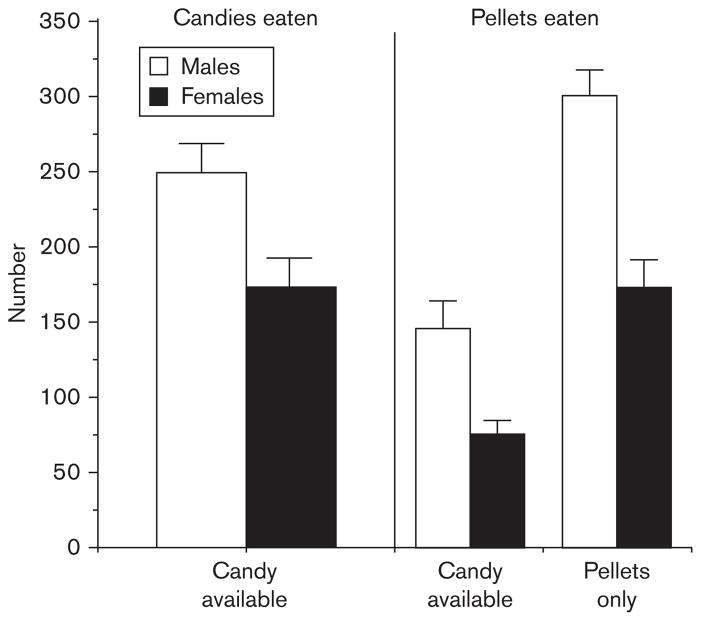
Both male and female baboons rapidly began eating large amounts of candy within the single meal (Foltin, 2006b). Three of four male baboons and two of four female baboons developed the unexpected behavior of responding for but then not eating large numbers of M&Ms. The five baboons who wasted M&Ms earned about 350 candies during a meal, but sucked the candy off and threw the chocolate center away for about 200 candies each meal; that is, they did not eat the chocolate center. This ‘tasting and wasting’ behavior occurred even though it was possible for baboons to save the M&Ms in the food hopper and eat them later. This behavior occurred much less often when the baboons had access to Skittles. This may have been because of the fact that Skittles have five flavors whereas M&Ms have only one flavor (six colors), or the fact that baboons are known to prefer fruit flavors to chocolate flavors (Wene et al., 1982). When Skittles were available, male baboons ate about 250 in a single meal and female baboons ate about 175 in a single meal (Fig. 5). On days when only pellets were available, male baboons ate about 300 pellets and female baboons ate about 175 pellets during the entire day. Thus, both male and female baboons responded for nearly as much candy during a single meal as they did for pellets over 23.5 h. Because Skittles have a greater energy content than pellets (4.3 vs. 3.3 kcal per single item) the baboons derived about the same amount of energy (males) or more (females) from a single meal of candies as they did from pellets during the entire day when candy was not available.
Fig. 5.
The mean number of Skittles candies eaten and pellets consumed by four male and four female baboons on the 3 days each week when candy was available and the 4 days each week when only pellets were available. Adapted from with permission Foltin, 2006a, 2006b. Error bars represent 1SEM.
This procedure provides a model for the consumption of excessive amounts of food within a single meal and can be used to provide a behavioral baseline for evaluating potential pharmacological interventions. However, although the procedure provides a model for eating large amounts of food in a limited time frame, it does not model the psychological variables that contribute to human binge eating. Binge-eating disorder is not caused by the mere intermittent availability of certain foods. Palatability-driven overconsumption, as described here, is analogous to but not homologous with human binge eating.
Given this caveat, the possible specificity of an intervention for eating a large meal of preferred items, compared with ‘normal’ eating, can be evaluated by comparing the effects of drugs on days when a preferred food is available and eaten during a large meal with those on days when only pellets are available and eaten during multiple smaller meals. Protocols for comparing the specificity of drug effects on eating preferred or ‘forbidden’ foods with that on eating ‘healthy alternatives’ require complex designs with multiple measures of eating topography. Such studies benefit from the power obtained with the large number of repeated observations that can be acquired with a long-living, nonhuman primate, as described here. The translational significance of this type of study is large as a medication that specifically decreases consumption of, and perhaps craving for, obesogenic foods would be popular among consumers and of great market value to a pharmaceutical firm.
Both amphetamine (Foltin and Haney, 2007) and dexfenfluramine (Bisaga et al., 2008) decreased the number of candies (Skittles) consumed during the candy meal and the number of pellets consumed in the first pellet meal and the entire day (Table 2). As described above, there were differences in how the two drugs affected eating topography. Both amphetamine and dexfenfluramine increased the latency to the first pellet meal, whereas only amphetamine increased the latency to the first candy meal. A decrease in candy taking without a change in latency supports the concept that dexfenfluramine increases satiety, but the data obtained with pellets do not. Perhaps dexfenfluramine increased the satiety value of the food already consumed when the animals woke up that day, thereby increasing the latency to the first pellet meal. This effect was not seen on candy days because animals were more motivated to work for candy; that is, the satiating effect of food plus dexfenfluramine was less when candy was available. Because there were no external cues telling the baboons whether candy or pellets were first available, it is assumed that baboons learned the sequence of candy delivery as being on Mondays, Wednesdays, and Fridays. Regardless, this dissociation between drug effects and candy and pellet intake was not observed for amphetamine. Both dexfenfluramine and amphetamine produced dose-dependent decreases in responding reinforced by candy or pellet delivery.
Table 2.
Summary of the effects of drugs on ‘binge’ eating in baboons
| Candy
|
Pellet
|
|||||
|---|---|---|---|---|---|---|
| Seekingd
|
Seeking
|
|||||
| Latency | Rewards | Takinge rewards | Latency | Rewards | Taking rewards | |
| Dexfenfluraminea | ⇔ | ⇔ | ⇓ | ⇑ | ⇔ | ⇓ |
| Amphetamineb | ⇑ | ⇔ | ⇓ | ⇑ | ⇔ | ⇓ |
| Memantinea | ⇔ | ⇔ | ⇓ | ⇑ | ⇔ | ⇓ |
| MTEPa | ⇔ | ⇔ | ⇓ | ⇔ | ⇔ | ⇔ |
| Neramexanec | ⇔ | ⇔ | ⇓ | ⇑ | ⇔ | ⇓ |
| CB1 antagonistb | ⇔ | ⇔ | ⇓ | ⇔ | ⇑ ⇓f | ⇓ |
indicates an increase;
indicates a decrease;
indicates no effect.
CB1, cannabinoid type 1.
Foltin R.W., Bisaga A., Danysz W., unpublished observations.
Seeking refers to responding reinforced by stimuli paired with pellets or candy that occurs before the delivery of pellets or candy.
Taking refers to responding reinforced by a pellet or a piece of candy.
Increased in males, decreased in females.
Glutamate pathways are an important component of the central reward system, involved in regulating reinforced and consummatory behaviors related to the use of drugs of abuse and preferred foods (Parsons et al., 2005). As the role of glutamatergic pathways in regulating binge-type eating is unknown, we evaluated the effects of glutamatergic compounds, the N-methyl-D-aspartate receptor antagonists, neramexane and memantine (Parsons et al., 2005), and the mGluR5 antagonist 3-((2-methyl-1,3-thiazol-4-yl)ethynyl)pyridine hydrochloride (MTEP; Busse et al., 2004) using our laboratory model of binge eating.
Although the effects of memantine were not as large as those of dexfenfluramine, the pattern of results was the same: memantine decreased the size of the candy meal without affecting latency, and it increased the latency to the pellet meal and decreased the number of pellets consumed (Bisaga et al., 2008). Neramexane decreased the size of first pellet meal and the candy meal and increased the latency to the first pellet meal without affecting the latency to the first candy meal (Foltin R.W., Bisaga A., Danysz W., unpublished observations). The only significant behavioral effect of MTEP was a reduction in the number of candies consumed during the candy meal (Bisaga et al., 2008).
Administration of memantine, a noncompetitive N-methyl- D-aspartate receptor antagonist, and MTEP, an allosteric metabotropic mGlu5 receptor antagonist, produced effects on candy consumption that were comparable with the effect of dexfenfluramine even though their pharmacological effects differ from those of dexfenfluramine, which is primarily a serotonin releaser (Heal et al., 1998). At the same time, dexfenfluramine and memantine decreased standard pellet seeking and consumption, whereas MTEP had a minimal effect on standard pellet seeking and consumption. Memantine and MTEP may reduce the amount and the energy intake from highly palatable food in humans by altering the reinforcing effects of food. These results suggest that mGluR5 antagonists may be more selective in reducing the reinforcing effect of highly palatable food than standard food (Saper et al., 2002). A caveat to this conclusion is that there were no controls for the differing candy and pellet meal sizes or candy and pellet palatabilities, such that any differential effects may reflect a drug effect based on meal size rather than on palatability.
Much work has focused on the potential therapeutic effects of cannabinoid type 1 (CB1) receptor antagonists in the treatment of obesity (Kirkham, 2005). CB1 receptor antagonists decrease intake of standard chow diets in laboratory rodents (e.g. Thornton-Jones et al., 2005; Gardner and Mallet, 2006). Using a choice paradigm in food-restricted rats, Arnone et al. (1997) reported that the CB1 receptor antagonist rimonabant (SR141716) specifically decreased consumption of sucrose pellets but not standard ‘bland’ chow-based pellets. A specific effect of cannabinoids on sweet or palatable food consumption corresponds with the desire for sweets (the ‘munchies’) reported by marijuana users (Abel, 1975) and demonstrated in a controlled laboratory study on human marijuana smokers (Foltin et al., 1988). Thus, we evaluated the effects of the CB1 receptor antagonist SR141716 on candy and pellet intake in baboons (Foltin and Haney, 2007).
Contrary to our expectations, we did not see a specific effect of a CB1 receptor antagonist on candy intake: both the number of candies consumed during the candy meal and pellets consumed during the pellet meal were decreased. Of note, decreases in consumption were observed without any effects of the CB1 receptor antagonist on latency to the first candy or pellet meal. Given that some marijuana users report urges for sweet foods, we had expected the CB1 receptor antagonist to increase the latency to the candy meal or to both meals.
In the above studies, although there were some occasional interactions between a drug dose and sex of the baboon, these interactions were generally accounted for by the baseline differences in pellet or candy intake. The exception to this was the effect of the CB1 receptor antagonist on the number of light flashes received during the seeking component for the first pellet meal: responding increased in males and decreased in females.
Figure 6 compares the effects of five drugs on candy and pellet consumption: data are the means for the eight baboons (four males and four females). Although the effects of MTEP were small, it was the only drug to decrease candy consumption differentially without affecting pellet consumption. All the remaining drugs decreased candy and pellet consumption to a similar extent. Although not statistically analyzed, the graphs for candy consumption are generally shifted to the left of the graphs for pellet consumption, suggesting that candy consumption was decreased at lower doses compared with pellet consumption. Only amphetamine increased the latency to the candy and first pellet meal. Thus, amphetamine affected feeding behavior by different behavioral mechanisms from the other drugs. The number of rewards earned during the seeking phases of the candy and pellet meals was not affected by any drug, indicating that responding during the 30-min intervals was not a sensitive measure of incentive salience. This differs from data we obtained in two previous groups of baboons. The reason for this discrepancy is unclear, but is most likely related to the different behavioral histories of the different groups of animal.
Fig. 6.
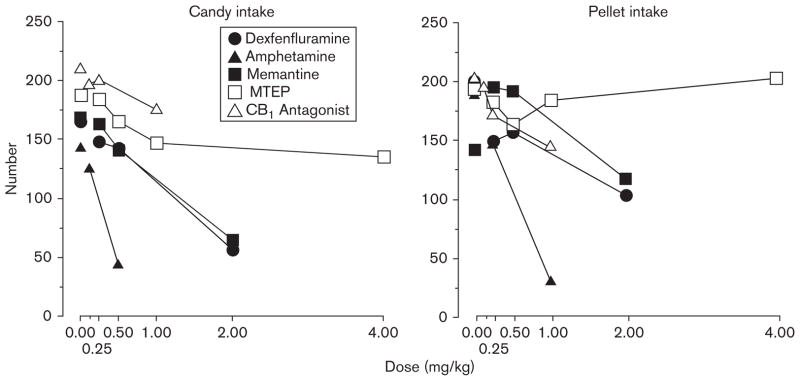
The mean number of Skittles candies eaten and pellets consumed as a function of drug dose (adapted from Bisaga et al., 2008; Foltin and Haney, 2007: to increase clarity some original data points have been omitted).
In summary, the excessive consumption of candy in our model of binge eating is most likely motivated by the highly palatable taste of sweet, high-sugar candies in nonhuman primates and most likely, on the basis of data in laboratory rodents, dependent upon the limited-access schedule. The model does not address the psychological issues reported by patients with eating disorders (Ferriter and Ray, 2011). We are not privy to the emotional state of our animals thus we must rely on behavioral similarities in developing our models.
Effect of sucrose consumption on sensitivity to drug effects
As described above, we saw no evidence that experimenter-administered amphetamine was an economic substitute for food, but we and others have shown that palatable food is an economic substitute for self-administered amphetamine (Foltin, 1997) and cocaine (Foltin, 1999) in nonhuman primates. A number of studies have reported that sucrose consumption in rats either alters or is predictive of the response to amphetamine. For example, rats that had access to sucrose plus chow demonstrated larger place preferences conditioned by a low dose of amphetamine compared with control rats (Vitale et al., 2003). Further, intake of sucrose solutions was predictive of locomotor responses to amphetamine (Sills and Vaccarino, 1994) and the propensity to acquire amphetamine self-administration (DeSousa et al., 2000). Avena and colleagues have shown a reciprocal relationship between excessive sugar intake by rats and the effects of amphetamine. Rats had a larger locomotor response to amphetamine after 21 days of sugar access (Avena and Hoebel, 2003b), and they had a larger locomotor response to sugar after 6 days of amphetamine injections (Avena and Hoebel, 2003a). Rada et al. (2005) and Colantuani et al. (2001) reported that intermittent consumption of large amounts of a palatable food increased central dopamine levels in a manner similar to but less than amphetamine (Fuchs et al., 2005), providing a mechanism that may account for interactions between amphetamine and highly palatable foods such as sucrose. Perhaps, under some circumstances, sucrose and amphetamine can function as economic substitutes for one another [see review on evidence for economic substitution in humans and nonhumans by Bickel et al. (1995)]. In contrast, conflicting data exist on the effects of dietary manipulations on the response to dexfenfluramine (Yeomans and Clifton, 1997; Inam, et al., 2006; Jabeen and Haleem, 2008).
We examined the effects of consumption of a high-sucrose food on the anorexigenic actions of amphetamine, dexfenfluramine, heroin, and naloxone by determining complete dose–response functions before, during, and after a period of access to Skittles candy in eight experimentally-naive male baboons (Foltin, 2011). Baboons were administered with a test dose of drug or placebo on Tuesday and Thursday of each week. During candy access, candies containing 75% of energy as that in sugar were available during the morning on Mondays, Wednesdays, and Fridays; pellets (19% of energy as that in sugar) were available in the afternoon and throughout the remaining days of the week. During candy access, baboons consumed a mean of 177 pieces of candy containing 696 kcal on Monday, Wednesday, and Friday mornings.
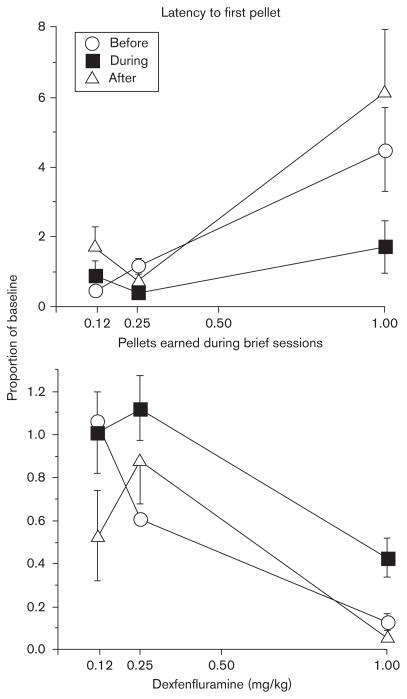
Amphetamine (data not shown) and heroin (Foltin R.W., unpublished observations), but not naloxone (Foltin R.W., unpublished observations), decreased pellet intake in the morning and afternoon, although having no effect on candy consumption (Fig. 7). Amphetamine and heroin also increased the latency to the first pellet meal of the afternoon. Unexpectedly, the anorexigenic effects of dexfenfluramine were altered by consumption of candy (Fig. 8). Dexfenfluramine produced dose-dependent increases in latency to the first pellet meal of the afternoon and decreases in pellet intake during the morning. These drug effects were significantly smaller when baboons had access to candy 3 days a week, but the effects did not differ between the dose–response functions determined before and 6 months after access to candy –that is tolerance to the effects of dexfenfluramine during candy consumption developed.
Fig. 7.
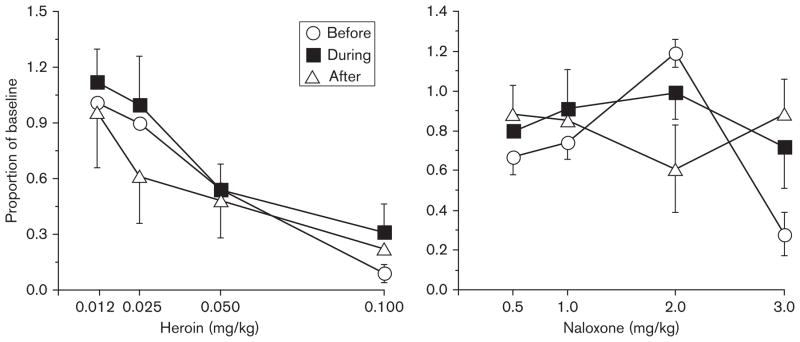
The mean number of pellets earned during morning brief sessions as a function of drug dose (Foltin R.W., unpublished observations). Data were obtained before, during, and after a period of access to candy. Error bars represent 1 SEM.
Fig. 8.
The mean latency to the first pellet and the number of pellets earned during morning brief sessions as a function dexfenfluramine dose. Adapted with permission from Foltin, 2011: to increase clarity some original data points have been omitted. Data were obtained before, during, and after a period of access to candy. Error bars represent 1 SEM.
As described above (Blundell et al., 1976), satiation may be mediated by an increase in central serotonin levels. Thus, the administration of dexfenfluramine has been hypothesized to increase satiation by increasing serotonin levels (Halford et al., 2007). It is tempting to hypothesize that candy required greater serotonin levels than pellets for satiation to be achieved, such that over time baboons became less sensitive to endogenous serotonin; that is, they became ‘tolerant’. A difficulty with this hypothesis is that candy intake was relatively stable across the study and baboons decreased pellet intake on the noncandy days. Of course, numerous other mechanisms may account for the shift in response to dexfenfluramine administration.
In summary, we hypothesize that tolerance to the effects of dexfenfluramine is a proxy measure for a decrease in the satiety signal produced by serotonin release during meals of highly palatable food. This hypothesis suggests a novel approach to treating obesity based on the use of drugs to reverse serotonergic desensitization.
Future directions
Over the past 30 years there have been comparatively few studies on the effects of pharmacological manipulations on feeding behavior of nonhuman primates. A literature search of biological or psychological databases using terms related to normal or disordered eating indicated that for any research term (e.g. hunger) there are between 1 and 5% of the number of articles on nonhuman primates as there are on laboratory rodents. There are procedural difficulties and significant expenses when working with nonhuman primates, and researchers working with nonhuman primates have chosen not to focus on pharmacological studies of food intake.
As described above, relatively complicated experimental procedures can be accomplished with nonhuman primates, which provide detailed measurements of feeding topography as well as other behavioral measures, including sleep and activity. Work with nonhuman primates has most often involved operant responding, using a variety of schedules of reinforcement that provide measures of nonspecific disruptions in rate of responding. The vast majority of studies with rodents involved delivery of food in bulk without a response requirement and measured weight of food consumed at multiple time points. The use of operant schedules better mimics the natural ecology, in which there is some behavioral requirement before eating – for example purchasing and preparing foods, wherein costs that vary across food types such as ready-made snack items versus complex gourmet-prepared foods. Eating behavior involves a series of choices, and these choices can be modeled using operant choice methodologies. Surprisingly little work examining factors affecting food choice has been carried out in nonhuman primates.
Another understudied area is the analysis of how the consequences of food choice affect eating behavior. Just as there is a cost associated with acquiring foods for consumption, there is a cost associated with consequences of eating. At the simple end someone has to clean up the kitchen and dining area after a meal and at the complicated end some food choices (both type and amount) have long-term negative consequences such as obesity and increased blood pressure. Long-lived nonhuman primates offer the opportunity to study food choices in the face of changing consequences as a model of the human condition.
The data described above indicate some similarities and differences relative to the results of pharmacological studies conducted mainly in rats, indicating that data obtained with rodents may not predict outcome with nonhuman or human primates. Jandacek (2012) reached a similar conclusion in his review on the effects of nonabsorbable fats on energy intake in humans and rodents. Rodents accurately compensate for energy dilution by eating greater amounts of food, whereas humans do not. Work is needed to determine whether nonhuman primates adapt to energy dilution in a manner similar to rodents or human primates. We have reported that nonhuman primates do not compensate for energy deprivation as well as rodents (Foltin and Fischman, 1990), suggesting that nonhuman primate models will be important in studying how dietary manipulations affect food intake.
Exciting recent well-controlled work has shown that social factors affect the response of nonhuman primates to palatable food. Arce et al. (2010) compared consumption of a highly palatable food between subordinate and dominant ovariectomized female rhesus monkeys. Submissive animals ate more of the palatable food compared with dominant animals (also see Wilson et al., 2008). This finding parallels the work of Morgan et al. (2002) according to which submissive male cynomolgus monkeys self-administered more intravenous cocaine compared with dominant monkeys. On the basis of these findings, we examined the effect of social status (assigned by three independent observers) on Skittle consumption and response to pharmacological agents in the group of eight male baboons that were studied in the investigation by Foltin (2011). Although not significantly different, the four submissive male baboons consumed on average 18% more candy than the four dominant baboons. However, there were no differences in the effects of amphetamine, dexfenfluramine, heroin, and naloxone between submissive and dominant male baboons, determined before the period of candy availability.
Altmann et al. (1993) and Knott (1998) both reported a greater intake of palatable food by female baboons and orangutans, respectively, compared with males. The findings by Arce et al. (2010) further suggest that social status within sex is an additional factor influencing feeding behavior in nonhuman primates. By my estimate, in the past 30 years only four different female rhesus monkeys and four different female baboons were studied in all the investigations using the controlled laboratory procedures described above. In the case of the four female baboons, they consumed less energy compared with the four male baboons in the same group. They also ate more candy, relative to pellets and as a proportion of total energy intake compared with the males. Finally, they also differed from males with respect to the effects of some anorexigenic drugs on measures of eating topography – that is females were not simply small males. Clearly sex differences are an under-explored area of research.
Recently, Rowland (2012) argued that social, environmental, and economic factors, especially those associated with ready access to highly palatable foods, can overwhelm inhibitory control of eating by physiological factors, leading to obesity. I would argue that these factors can best be modeled in nonhuman primates that, like human primates, evolved within complex social environments.
Conclusion
Despite the number of studies published in the past 30 years on the behavioral pharmacology of feeding behavior in nonhuman primates, significant areas remain unexplored, including factors affecting food choice, dietary factors, social factors, and sex differences.
Acknowledgments
Research was supported by DA-04130 from The National Institute on Drug Abuse, and approved by either the Johns Hopkins Institutional Animal Care and Use Committee or the New York State Psychiatric Institute’s Animal Care and Use Committee. The quality of the research was based on the excellent skills of (i) research assistants: Nondita Bhaduri, James Thomas, Rafael Salazar, Jose Espinal, Julian Perez, April Modranowski, Jean Willi, and Angel Ramirez; and (ii) veterinarians: Robert Adams, Mohamed Osman, Moshe Shalev, Rodolfo Ricart, and Amy Cassano. The assistance of Drs Suzette Evans and Margaret Haney is also gratefully acknowledged.
Footnotes
Conflicts of interest
Neramexane, MTEP, and memantine were provided by Merz Pharmaceuticals, Frankfurt am Main, Germany. Merz Pharmaceuticals also provided partial financial support for studies using these compounds. Sibutramine hydrochloride monohydrate was provided by Abbott Laboratories, Abbott Park, IL. There are no other conflicts of interest.
References
- Abel EL. Cannabis: effects on hunger and thirst. Behav Biol. 1975;15:255–281. doi: 10.1016/s0091-6773(75)91684-3. [DOI] [PubMed] [Google Scholar]
- Allison J. Economics and operant conditioning. In: Harzem P, Zeiler MD, editors. Predictability, correlation, and contiguity. New York: John Wiley; 1981. pp. 321–353. [Google Scholar]
- Altmann J, Schoeller D, Altmann SA, Muruthi P, Sapolsky RM. Body size and fatness of free-living baboons reflect food availability and activity levels. Am J Primatol. 1993;30:149–161. doi: 10.1002/ajp.1350300207. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 2000. text rev. [Google Scholar]
- Arce M, Michopoulos V, Shepard KN, Ha Q-C, Wilson ME. Diet choice, cortisol reactivity, and emotional feeding in socially housed rhesus monkeys. Physiol Behav. 2010;101:446–455. doi: 10.1016/j.physbeh.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone M, Maruani J, Chaperon F, Thiebot MH, Poncelet M, Soubrie P, et al. Selective inhibition of sucrose and ethanol intake by SR141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology. 1997;132:104–106. doi: 10.1007/s002130050326. [DOI] [PubMed] [Google Scholar]
- Avena NM, Hoebel BG. Amphetamine-sensitized rats show sugar-induced hyperactivity (cross-sensitization) and sugar hyperphagia. Pharmacol Biochem Behav. 2003a;74:635–639. doi: 10.1016/s0091-3057(02)01050-x. [DOI] [PubMed] [Google Scholar]
- Avena NM, Hoebel BG. A diet promoting sugar dependency causes behavioral cross-sensitization to a low dose of amphetamine. Neuroscience. 2003b;122:17–20. doi: 10.1016/s0306-4522(03)00502-5. [DOI] [PubMed] [Google Scholar]
- Baile CA, Zinn W, Mayer J. Feeding behavior of monkeys: glucose utilization rate and site of glucose entry. Physiol Behav. 1971;6:537–541. doi: 10.1016/0031-9384(71)90201-0. [DOI] [PubMed] [Google Scholar]
- Bainbridge JG. The effect of psychotropic drugs on food-reinforced behaviour and on food consumption. Psychopharmacologia. 1968;12:204–213. [PubMed] [Google Scholar]
- Bellisari A. Evolutionary origins of obesity. Obes Rev. 2008;9:165–180. doi: 10.1111/j.1467-789X.2007.00392.x. [DOI] [PubMed] [Google Scholar]
- Bickel WK, DeGrandpre RJ, Higgins ST. The behavioral economics of concurrent drug reinforcers: a review and reanalysis of drug self-administration research. Psychopharmacology (Berl) 1995;118:250–259. doi: 10.1007/BF02245952. [DOI] [PubMed] [Google Scholar]
- Bisaga A, Danysz W, Foltin RW. Antagonism of glutamatergic NMDA and mGluR5 receptors decreases consumption of food in baboon model of binge-eating disorder. Eur Neuropsychopharmacol. 2008;18:794–802. doi: 10.1016/j.euroneuro.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell JE, Latham CJ, Leshem MB. Differences between the anorexic actions of amphetamine and fenfluramine – possible effects on hunger and satiety. J Pharm Pharmacol. 1976;28:471–477. doi: 10.1111/j.2042-7158.1976.tb02768.x. [DOI] [PubMed] [Google Scholar]
- Blundell JE, Latham CJ, Moniz E, McArthur RA, Rogers PJ. Structural analysis of the actions of amphetamine and fenfluramine on food intake and feeding behaviour in animals and in man. Curr Med Res Opin. 1979;6 (Suppl 1):34–54. [Google Scholar]
- Brown DE, Fottler HJ, Pugh JL, Fahey GC, Jr, Corbin JE. Effects of elfazepam on feeding behavior, feed intake and nutrient utilization by the dog. Nutr Rep Int. 1981;24:785–789. [Google Scholar]
- Brown RF, Houpt KA, Schryver HF. Stimulation of food intake in horses by diazepam and promazine. Pharmacol Biochem Behav. 1976;5:495–497. doi: 10.1016/0091-3057(76)90116-7. [DOI] [PubMed] [Google Scholar]
- Busse CS, Brodkin J, Tattersall D, Anderson JJ, Warren N, Tehrani L, et al. The behavioral profile of the potent and selective mGlu5 receptor antagonist 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine (MTEP) in rodent models of anxiety. Neuropsychopharmacology. 2004;29:1971–1979. doi: 10.1038/sj.npp.1300540. [DOI] [PubMed] [Google Scholar]
- Cohen SL, Branch MN. Food-paired stimuli as conditioned reinforcers: effects of D-amphetamine. J Exp Anal Behav. 1991;56:277–288. doi: 10.1901/jeab.1991.56-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colantuoni C, Schwenker J, McCarthy J, Rada P, Ladenheim B, Cadet JL, et al. Excessive sugar intake alters binding to dopamine and mu-opioid receptors in the brain. Neuroreport. 2001;12:3549–3552. doi: 10.1097/00001756-200111160-00035. [DOI] [PubMed] [Google Scholar]
- Collier G, Hirsch E, Hamlin PH. The ecological determinants of reinforcement in the rat. Physiol Behav. 1972;9:705–716. doi: 10.1016/0031-9384(72)90038-8. [DOI] [PubMed] [Google Scholar]
- Collier G, Hirsch E, Kanarek R. The operant revisited. Englewood Cliffs, New Jersey: Prentice Hall; 1977. [Google Scholar]
- Collier GH. An ecological analysis of motivation. In: Toates FM, Halliday TR, editors. Analysis of motivational processes. London: Academic Press; 1980. pp. 124–151. [Google Scholar]
- Collier GH. Life in a closed economy: the ecology of learning and motivation. Vol. 3. New York: John Wiley and Sons Ltd; 1983. [Google Scholar]
- Collier GH, Rovee-Collier CK. A comparative analysis of optimal foraging behavior: laboratory stimulations. In: Kamil AC, Sargent TD, editors. Foraging behavior: ecological, ethological, and psychological approaches. New York: Garland STPM Press; 1980. pp. 38–76. [Google Scholar]
- Cooper SJ, Posadas-Andrews A. Food and water intake in the non-deprived pigeon after chlordiazepoxide administration. Psychopharmacology (Berl) 1979;65:99–101. doi: 10.1007/BF00491987. [DOI] [PubMed] [Google Scholar]
- Corwin RL, Buda-Levin A. Behavioral models of binge-type eating. Physiol Behav. 2004;82:123–130. doi: 10.1016/j.physbeh.2004.04.036. [DOI] [PubMed] [Google Scholar]
- Corwin RL, Woolverton WL, Schuster CR, Johanson CE. Anorectics: effects on food intake and self-administration in rhesus monkeys. Alcohol Drug Res. 1987;7:351–361. [PubMed] [Google Scholar]
- Corwin RL, Wojnicki FH, Fisher JO, Dimitriou SG, Rice HB, Young MA. Limited access to a dietary fat option affects ingestive behavior but not body composition in male rats. Physiol Behav. 1998;65:545–553. doi: 10.1016/s0031-9384(98)00201-7. [DOI] [PubMed] [Google Scholar]
- Delgado JMR, Grau C, Delgado-Garcia JM, Rodero JM. Effects of diazepam related to social hierarchy in rhesus monkeys. Neuropharmacology. 1976;15:409–414. doi: 10.1016/0028-3908(76)90118-0. [DOI] [PubMed] [Google Scholar]
- DeSousa NJ, Bush DE, Vaccarino FJ. Self-administration of intravenous amphetamine is predicted by individual differences in sucrose feeding in rats. Psychopharmacology (Berl) 2000;148:52–58. doi: 10.1007/s002130050024. [DOI] [PubMed] [Google Scholar]
- Dimitriou SG, Rice HB, Corwin RL. Effects of limited access to a fat option on food intake and body composition in female rats. Int J Eat Disord. 2000;28:436–445. doi: 10.1002/1098-108x(200012)28:4<436::aid-eat12>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Ferriter C, Ray LA. Binge eating and binge drinking: an integrative review. Eating Behav. 2011;12:99–107. doi: 10.1016/j.eatbeh.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Stein LJ, West D, Porte D, Jr, Woods SC. Intracisternal insulin alters sensitivity to CCK-induced meal suppression in baboons. Am J Physiol Regul Integr Comp Physiol. 1986;250:R856–R860. doi: 10.1152/ajpregu.1986.250.5.R856. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Sipols AJ, Porte D, Jr, Woods SC, Liddle RA. Intraventricular CCK inhibits food intake and gastric emptying in baboons. Am J Physiol Regul Integr Comp Physiol. 1989;256:R1313–R1317. doi: 10.1152/ajpregu.1989.256.6.R1313. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Nadzan AM, Sipols AJ, Green PK, Liddle RA, Porte D, et al. Intraventricular CCK-8 reduces single meal size in the baboon by interaction with type-A CCK receptors. Am J Physiol. 1992;263:R863–R867. doi: 10.1152/ajpregu.1992.263.4.R863. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Sipols AJ, Seeley RJ, Chavez M, Woods SC, Porte D. Intraventricular insulin enhances the meal-suppressive efficacy of intraventricular cholecystokinin octapeptide in the baboon. Behav Neurosci. 1995;109:567–569. doi: 10.1037//0735-7044.109.3.567. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Schwartz MW, Seeley RJ, Chavez M, Baskin DG, Woods SC, et al. Endocrine regulation of food intake and body weight. J Lab Clin Med. 1996;127:328–332. doi: 10.1016/s0022-2143(96)90179-1. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ. Effects of D-fenfluramine and metergoline on responding for conditioned reward and the response potentiating effect of nucleus accumbens D-amphetamine. Psychopharmacology (Berl) 1995;118:155–163. doi: 10.1007/BF02245834. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ. Injection of 5-HT into the nucleus accumbens reduces the effects of D-amphetamine on responding for conditioned reward. Psychopharmacology (Berl) 1996;126:62–69. doi: 10.1007/BF02246412. [DOI] [PubMed] [Google Scholar]
- Foltin RW. Effects of anorectic drugs on the topography of feeding behavior in baboons. J Pharmacol Exp Ther. 1989;249:101–109. [PubMed] [Google Scholar]
- Foltin RW. Economic analysis of the effects of caloric alternatives and reinforcer magnitude on ‘demand’ for food in baboons. Appetite. 1992;19:255–271. doi: 10.1016/0195-6663(92)90166-4. [DOI] [PubMed] [Google Scholar]
- Foltin RW. Effects of pharmacological manipulations on ‘demand’ for food by baboons. Behav Pharmacol. 1993;4:586–596. [PubMed] [Google Scholar]
- Foltin RW. Food and amphetamine self-administration by baboons: effects of alternatives. J Exp Anal Behav. 1997;68:47–66. doi: 10.1901/jeab.1997.68-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltin RW. Food and cocaine self-administration by baboons: effects of alternatives. J Exp Anal Behav. 1999;72:215–234. doi: 10.1901/jeab.1999.72-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltin RW. Effects of amphetamine, dexfenfluramine, diazepam, and other pharmacological and dietary manipulations on food ‘seeking’ and ‘taking’ behavior in non-human primates. Psychopharmacology (Berl) 2001;158:28–38. doi: 10.1007/s002130100865. [DOI] [PubMed] [Google Scholar]
- Foltin RW. Effects of amphetamine, dexfenfluramine, and diazepam on responding during extinction in nonhuman primates. Pharmacol Biochem Behav. 2004a;79:325–330. doi: 10.1016/j.pbb.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Foltin RW. Effects of amphetamine, dexfenfluramine, diazepam, and dietary manipulations on responding reinforced by stimuli paired with food in nonhuman primates. Pharmacol Biochem Behav. 2004b;77:471–479. doi: 10.1016/j.pbb.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Foltin RW. Baclofen decreases feeding in non-human primates. Pharmacol Biochem Behav. 2005;82:608–614. doi: 10.1016/j.pbb.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Foltin RW. Effects of sibutramine on the appetitive and consummatory aspects of feeding in non-human primates. Physiol Behav. 2006a;87:280–286. doi: 10.1016/j.physbeh.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Foltin RW. ‘Tasting and wasting’ behavior in non-human primates: aberrant behavior or normal behavior in ‘times of plenty’. Physiol Behav. 2006b;89:587–597. doi: 10.1016/j.physbeh.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Foltin RW. Consumption of palatable food decreases the anorectic effects of serotonergic, but not dopaminergic drugs in baboons. Physiol Behav. 2011;103:493–500. doi: 10.1016/j.physbeh.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. Food intake in baboons: effects of d-amphetamine and fenfluramine. Pharmacol Biochem Behav. 1989;31:585–592. doi: 10.1016/0091-3057(88)90234-1. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. Effects of caloric manipulations on food intake in baboons. Appetite. 1990;15:135–149. doi: 10.1016/0195-6663(90)90046-b. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Haney M. Effects of the cannabinoid antagonist SR141716 (rimonabant) and D-amphetamine on palatable food and food pellet intake in non-human primates. Pharmacol Biochem Behav. 2007;86:766–773. doi: 10.1016/j.pbb.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltin RW, Moran TH. Food intake in baboons: effects of a long-acting cholecystokinin analog. Appetite. 1989;12:145–152. doi: 10.1016/0195-6663(89)90103-7. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Schuster CR. Interaction between the effects of intragastric meals and drugs on feeding in rhesus monkeys. J Pharmacol Exp Ther. 1983;226:405–410. [PubMed] [Google Scholar]
- Foltin RW, Fischman MW, Byrne MF. Effects of smoked marijuana on food intake and body weight of humans living in a residential laboratory. Appetite. 1988;11:1–14. doi: 10.1016/s0195-6663(88)80017-5. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW, Byrne MF. Food intake in baboons: effects of diazepam. Psychopharmacology (Berl) 1989;97:443–447. doi: 10.1007/BF00439545. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW, Nautiyal C. The effects of cocaine on food intake of baboons before, during, and after a period of repeated desipramine. Pharmacol Biochem Behav. 1990;36:869–874. doi: 10.1016/0091-3057(90)90092-v. [DOI] [PubMed] [Google Scholar]
- Fratta W, Mereu G, Chessa P, Paglietti E, Gessa G. Benzodiazepine-induced voraciousness in rats and inhibition of amphetamine-anorexia. Life Sci. 1976;18:1157–1166. doi: 10.1016/0024-3205(76)90152-1. [DOI] [PubMed] [Google Scholar]
- Fuchs H, Nagel J, Hauber W. Effects of physiological and pharmacological stimuli on dopamine release in the rat globus pallidus. Neurochem Int. 2005;47:474–481. doi: 10.1016/j.neuint.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Gardner A, Mallet PE. Suppression of feeding, drinking, and locomotion by a putative cannabinoid receptor ‘silent antagonist’. Eur J Pharmacol. 2006;530:103–106. doi: 10.1016/j.ejphar.2005.11.032. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Snell JD, Brady JV. Progressive-ratio performance in baboons and the assessment of the abuse liability of drugs: comparison of fenfluramine, chlorphentermine, diethylpropion and cocaine. Problems of Drug Dependence (Committee on Problems of Drug Dependence NRCNAS) 1976:546–551. [Google Scholar]
- Griffiths RR, Brady JV, Snell JD. Relationship between anoretic and reinforcing properties of appetite suppresant drugs: implications for assessment of abuse liability. Biol Psychiatry. 1978;13:283–289. [PubMed] [Google Scholar]
- Halford JC, Harrold JA, Boyland EJ, Lawton CL, Blundell JE. Serotonergic drugs: effects on appetite expression and use for the treatment of obesity. Drugs. 2007;67:27–55. doi: 10.2165/00003495-200767010-00004. [DOI] [PubMed] [Google Scholar]
- Harris SC, Ivy AC, Searle LM. The mechanism of amphetamine-induced loss of weight. J Am Med Assoc. 1947;134:1468–1475. doi: 10.1001/jama.1947.02880340022005. [DOI] [PubMed] [Google Scholar]
- Heal DJ, Cheetham SC, Prow MR, Martin KF, Buckett WR. A comparison of the effects on central 5-HT function of sibutramine hydrochloride and other weight-modifying agents. Br J Pharmacol. 1998;125:301–308. doi: 10.1038/sj.bjp.0702067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch E, Collier G. The ecological determinants of reinforcement in the guinea pig. Physiol Behav. 1974a;12:239–249. doi: 10.1016/0031-9384(74)90178-4. [DOI] [PubMed] [Google Scholar]
- Hirsch E, Collier G. Effort as determinant of intake and patterns of drinking in the guinea pig. Physiol Behav. 1974b;12:647–655. doi: 10.1016/0031-9384(74)90215-7. [DOI] [PubMed] [Google Scholar]
- Hudson JI, Hiripi E, Pope HG, Jr, Kessler RC. The prevalence and correlates of eating disorders in the National comorbidity survey replication. Biol Psychiatry. 2007;61:348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR. Behavioral economics. J Exp Anal Behav. 1984;42:435–452. doi: 10.1901/jeab.1984.42-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, Baumann RA. The behavioral analysis of demand. In: Green L, Kagel JH, editors. Advances in behavioral economics. Vol. 1. Norwood, New Jersey: Ablex Publishing Corporation; 1987. pp. 117–159. [Google Scholar]
- Hursh SR, Raslear TG, Shurtleff D, Bauman R, Simmons L. A cost-benefit analysis of demand for food. J Exp Anal Behav. 1988;50:419–440. doi: 10.1901/jeab.1988.50-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inam QU, Haleem MA, Haleem DJ. Effects of long term consumption of sugar as part of meal on serotonin 1-a receptor dependent responses. Pak J Pharm Sci. 2006;19:94–98. [PubMed] [Google Scholar]
- Jabeen B, Haleem DJ. Desensitization of pre and post synaptic 5-HT-1A receptor responses following long term consumption of sugar rich diet: implications for sugar-induced obesity. Pak J Pharm Sci. 2008;21:327–332. [PubMed] [Google Scholar]
- Jandacek RJ. Review of the effects of dilution of dietary energy with olestra on energy intake. Physiol Behav. 2012;105:1124–1131. doi: 10.1016/j.physbeh.2011.12.018. [DOI] [PubMed] [Google Scholar]
- Kelleher R. Conditioned reinforcement in second-order schedules. J Exp Anal Behav. 1966;9:475–485. doi: 10.1901/jeab.1966.9-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham TC. Endocannabinoids in the regulation of appetite and body weight. Behav Pharmacol. 2005;16:297–313. doi: 10.1097/00008877-200509000-00004. [DOI] [PubMed] [Google Scholar]
- Klein DA, Walsh BT. Eating disorders: clinical features and pathophysiology. Physiol Behav. 2004;81:359–374. doi: 10.1016/j.physbeh.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Knapp PH. Amphetamine and addiction. J Nerv Ment Dis. 1952;115:406–432. [PubMed] [Google Scholar]
- Knott CD. Changes in orangutan caloric intake, energy balance, and ketones in response to fluctuating fruit availability. Int J Primatol. 1998;19:1061–1079. [Google Scholar]
- Kornetsky C, Mirsky AF, Kessler EK, Dorff JE. The effects of dextro-amphetamine on behavioral deficits produced by sleep loss in humans. J Pharmacol Exp Ther. 1959;127:46–50. [PubMed] [Google Scholar]
- Lasagna L, Von Felsinger JM, Beecher HK. Drug-induced mood changes in man. 1. Observations on healthy subjects, chronically ill patients, and ‘postaddicts’. J Am Med Assoc. 1955;157:1006–1020. doi: 10.1001/jama.1955.02950290026009. [DOI] [PubMed] [Google Scholar]
- Lea SEG. The psychology and economics of demand. Psychol Bull. 1978;85:441–466. [Google Scholar]
- Martin WR, Sloan JW, Sapira JD, Jasinski DR. Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther. 1971;12:245–258. doi: 10.1002/cpt1971122part1245. [DOI] [PubMed] [Google Scholar]
- McHugh PR, Moran TH. Accuracy of the regulation of caloric ingestion in the rhesus monkey. Am J Physiol. 1978;235:R29–R34. doi: 10.1152/ajpregu.1978.235.1.R29. [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, et al. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5:169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Owen J. Feeding strategy. Chicago: The University of Chicago Press; 1980. [Google Scholar]
- Parsons CG, Danysz W, Zieglgansberger W. Excitatory amino acid neurotransmission. Handb Exp Pharmacol. 2005;169:249–303. doi: 10.1007/3-540-28082-0_10. [DOI] [PubMed] [Google Scholar]
- Rada P, Avena NM, Hoebel BG. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience. 2005;134:737–744. doi: 10.1016/j.neuroscience.2005.04.043. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rodman PS, Cant JGH. Adaptations for foraging in nonhuman primates. New York: Columbia University Press; 1984. [Google Scholar]
- Rowland NE. Order and disorder: temporal organization of eating. Behav Brain Res. 2012;231:272–278. doi: 10.1016/j.bbr.2011.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36:199–211. doi: 10.1016/s0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- Sills TL, Vaccarino FJ. Individual differences in sugar intake predict the locomotor response to acute and repeated amphetamine administration. Psychopharmacology (Berl) 1994;116:1–8. doi: 10.1007/BF02244864. [DOI] [PubMed] [Google Scholar]
- Smith M, Walker K, Cole K, Lang K. The effects of aerobic exercise on cocaine self-administration in male and female rats. Psychopharmacology (Berl) 2011;218:357–369. doi: 10.1007/s00213-011-2321-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soubrie P, DeAngelis L, Simon P, Boisser JR. Effects of the anxiolytics on fluid intake in novel and familiar situations. Psychopharmacologia. 1976;50:41–45. [Google Scholar]
- Stein L, Belluzzi J. Second messengers, natural rewards, and drugs of abuse. Clin Neuropharmacol. 1986;9:205–207. [PubMed] [Google Scholar]
- Tang AH, Kirch JD. Appetite suppression and central nervous system stimulation in the rhesus system. Psychopharmacology (Berl) 1971;21:139–146. doi: 10.1007/BF00572271. [DOI] [PubMed] [Google Scholar]
- Thornton-Jones ZD, Vickers SP, Clifton PG. The cannabinoid CB1 receptor antagonist SR141716A reduces appetitive and consummatory responses for food. Psychopharmacology. 2005;179:452–460. doi: 10.1007/s00213-004-2047-8. [DOI] [PubMed] [Google Scholar]
- Timberlake W, Peden BF. On the distinction between open and closed economies. J Exp Anal Behav. 1987;48:35–60. doi: 10.1901/jeab.1987.48-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale MA, Chen D, Kanarek RB. Chronic access to a sucrose solution enhances the development of conditioned place preferences for fentanyl and amphetamine in male Long–Evans rats. Pharmacol Biochem Behav. 2003;74:529–539. doi: 10.1016/s0091-3057(02)01034-1. [DOI] [PubMed] [Google Scholar]
- Weiss B, Laties VG. Enhancement of human performance by caffeine and the amphetamines. Pharmacol Rev. 1962;14:1–36. [PubMed] [Google Scholar]
- Wene JD, Barnwell GM, Mitchell DS. Flavor preferences, food intake, and weight gain in baboons (Papio sp.) Physiol Behav. 1982;28:569–573. doi: 10.1016/0031-9384(82)90155-x. [DOI] [PubMed] [Google Scholar]
- Wilson AW, Costall B, Neill JC. Manipulation of operant responding for an ethanol-paired conditioned stimulus in the rat by pharmacological alteration of the serotonergic system. J Psychopharmacol. 2000;14:340–346. doi: 10.1177/026988110001400402. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Fisher J, Fischer A, Lee V, Harris RB, Bartness TJ. Quantifying food intake in socially housed monkeys: social status effects on caloric consumption. Physiol Behav. 2008;94:586–594. doi: 10.1016/j.physbeh.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SC. The control of food intake: behavioral versus molecular perspectives. Cell Metab. 2009;9:10. doi: 10.1016/j.cmet.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeomans M, Clifton P. Exposure to sweetened solutions enhances the anorectic effect of naloxone but not D-fenfluramine. Physiol Behav. 1997;62:255–262. doi: 10.1016/s0031-9384(97)00112-1. [DOI] [PubMed] [Google Scholar]
- Zhang J, Berridge KC, Tindell AJ, Smith KS, Aldridge JW. A Neural Computational Model of incentive salience. PLoS Comput Biol. 2009;5:e1000437. doi: 10.1371/journal.pcbi.1000437. [DOI] [PMC free article] [PubMed] [Google Scholar]