Abstract
Purpose of review
We review recent literature with a view to forge an integrative understanding of the molecular, cellular and extracellular milieu of pancreatic cancer, and discuss them in the context of development of novel, personalized therapeutic options.
Recent findings
Pancreatic tumorigenesis, examined using genetically engineered mouse models, appears to be driven by local inflammation, in concert with the ‘big four’ mutations involving oncogenic KRAS, SMAD4, CDKN2A, and TP53, through induction of EMT and cancer stem cells, and accompanied by metastasis. High throughput sequencing of pancreatic ductal adenocarcinoma (PDAC) as well as neuroendocrine tumors and rarer subtypes of cancers of the pancreas have revealed several novel mutations in genes like PALB2, GNAS, DAXX, ATRX, SWI/SNF pathway related, and in genes in the ubiquitin dependent pathways such as USP9X. Therapeutic targeting of the tumor-stroma axis by cytokines and immune response modulators and the role of autophagy in pancreatic cancer are some other salient themes explored in the recent publications.
Summary
Recent publications shed new light on the mutational landscape of pancreatic cancer and further delineate the distinctive pancreatic cancer-stroma ecosystem as determined by the dynamic interplay of inflammation, hallmark mutations, epithelial to mesenchymal transition (EMT) and cancer stem cells.
Keywords: pancreatic ductal adenocarcinoma (PDAC), next generation sequencing, cancer stem cells, genetically engineered mouse models (GEMM) of pancreatic cancer, desmoplastic stroma
Introduction
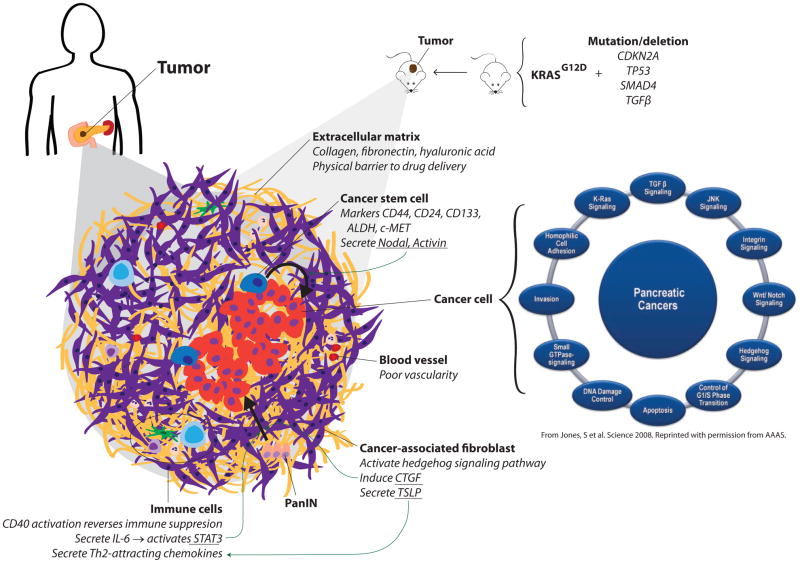
While not the most prevalent of malignancies, pancreatic cancer continues to be a leading cause of cancer related deaths worldwide, due to late detection and lack of specific therapeutic targets and ineffective therapies. With the next generation sequencing of tumors, development of primary tumor xenograft models, elucidation of early events in tumorigenesis and the interplay between tumor and stroma using genetically engineered mouse models, the field of pancreatic cancer research is poised for major breakthroughs in early detection and more effective treatment modalities. Here we review salient, sometimes representative, reports in pancreatic cancer published over the last year, encompassing the broad areas of high throughput sequencing, cancer cell and stromal biology, and cell fate related studies using genetically engineered mouse models (Figure 1) (1).
Figure 1.
Pancreatic cancer in mouse and man: emerging concepts in pancreatic cancer biology.
High throughput sequencing of pancreatic cancers reveals intratumoral heterogeneity
High throughput sequencing has quickly emerged as the primary modality of genomic characterization of cancers (2), with mutational profiles of several major carcinomas being reported over the past several years (3–7), including pancreatic cancer. In a high throughput Sanger sequencing analysis of more than twenty thousand genes from 24 pancreatic cancers, Jones et. al. have identified an average of 63 genetic alterations per tumor, and assigned each of these alterations to a core set of 12 distinct cellular pathways (1). While most tumors had genomic alterations in each of these pathways, individual tumors each seemed to harbored a distinct set of mutations affecting these conserved core pathways. Analyzing genomic rearrangements in pancreatic cancer, Campbell et. al. described evidence of telomere dysfunction and dysregulated G1-to-S-phase transition in early cancer development (8). They also noted ongoing evolution among metastases, and interestingly, phylogenetic trees across metastases showed organ-specific branches. In an independent study analyzing clonal relationships among primary cancer and metastatic cells, Yachida et. al. reported that clonally distinct subpopulations within the primary pancreatic carcinoma presumably gives rise to distant metastases, suggesting that the genetic heterogeneity of metastases is engendered in the primary tumor itself. Recent studies using mouse models of pancreatic cancer progression also appear to support this premise (discussed below). Further, comparing the mutation profiles in the initiating lesions with those in the metastatic tissues, Yachida et. al. estimated that it takes at least 15 years from the initiating mutation in a benign cell to reach a full blown metastasis. This inference, if validated in independent studies, will imply that early detection of pancreatic cancer could potentially afford a broader therapeutic window (9).
Apart from global genomic analyses, recent studies have identified several novel mutations in pancreatic cancers. Through exome sequencing of familial pancreatic cancer patients, a truncating mutation in the BRCA2 interacting protein, PALB2 (Partner and localizer of BRCA2), was identified as a pancreatic cancer susceptibility gene (10). Mutations in PALB2 have since been reported in familial breast and pancreatic cancer in independent cohorts (11–13). Likewise, recurrent mutations in GNAS (guanine nucleotide binding protein, alpha stimulating) were identified in intraductal papillary mucinous neoplasms (IPMN), pancreatic cysts frequently associated with malignant growth (14, 15). Activating mutations in GNAS have been previously described in colorectal cancer(16), thyroid carcinomas(17), adrenocortical lesions(18–21), pituitary tumors(22), and Leydig cell tumors(23), among others. Exomic sequencing of pancreatic neuroendocrine tumors (PanNETs) identified novel recurrent mutations in the transcription/chromatin remodeling complex comprising of DAXX (death-domain-associated protein) and ATRX (α thalassemia/mental retardation syndrome X-linked), as well as mutations in genes in the mTOR (mammalian target of rapamycin) pathway that could potentially be amenable to treatment with mTOR inhibitors (24). Interestingly, as speculated, tumors with ATRX and DAXX mutations were found to harbor altered telomeres in a follow up study (25), and have been associated with good prognosis (26). Focusing on functional grouping of somatic mutations identified in genomic sequencing of pancreatic cancers, Shain et. al. have identified loss of function mutations in genes encoding components of the SWI/SNF chromatin remodeling complex that includes putative DNA binding subunits (ARID1A, ARID1B, and PBRM1) and the enzymatic subunits (SMARCA2 and SMARCA4), that, while rare individually, together are present in one-third of all pancreatic cancers (27).
Mutations identified through high throughput sequencing of tumor samples need further characterization to establish potential functions in tumorigenesis. Taking a converse route, Perez-Mancera et. al., carried out a functional genomic screen for KRAS interactors using transposon-mediated insertional mutagenesis in a mouse model of pancreatic cancer and observed a loss of deubiquitinase Usp9x in over 50% of the murine pancreatic tumors(28). Although no analogous mutations in this gene were observed in human pancreatic cancer tissues, lower USP9X expression level was correlated with poor survival after surgery, and inversely associated with metastatic burden in advanced disease. Thus, USP9X has been proposed to signify an important tumor suppressor gene with prognostic and therapeutic relevance in PDACs.
Thus overall, application of high throughput genomics to pancreatic cancers have yielded several novel leads in a short span of time that are likely to be vigorously followed up in the coming years. With increasing access to low cost, high throughput sequencing and with better understanding of the implications for these genetic alterations in diagnostic and therapeutic management, it is expected that some of these findings may inform personalized therapeutic options in the future.
The increasing footprint of tumor microenvironment in pancreatic cancer
The desmoplastic stroma enveloping PDAC is comprised of cancer-associated fibroblasts (CAFs), infiltrating immune cells, blood vessels, and extracellular matrix, together constituting an elaborate microenvironment that is favorable for tumor growth and may also act as an effective physical barrier to chemotherapeutics. Several studies have explored the role of individual components of stroma in sustaining pancreatic cancer, and provide multiple potential therapeutic avenues. In one such study, Olive et. al. associated poor vasculature of the stroma with the activity of fibroblasts. Using an inhibitor of the hedgehog pathway reduced the proliferation of cancer associated fibroblasts, increased stromal vasculature, leading to improved permeability of the chemotherapeutic agent gemcitabine and reduced tumor burden (29). In another study, the authors targeted the hyaluronic acid (HA) matrix in the stroma that presents a physical barrier to drug delivery, and report that enzymatic ablation of stromal HA expands the stromal microvasculature, improving efficacy of gemcitabine treatment, resulting in a near doubling of overall survival using a genetically engineered mouse model of pancreatic cancer (30).
The fact that oncogenic KRAS (G12D) readily induces PanIN lesions in experimental mouse models but progression to PDAC requires local inflammation indicates the requirement for a permissive environmental milieu. Several cytokine and immune modulators of tumor-stroma interaction have been described recently that help us better understand this dynamic milieu as well as point to potential therapeutic avenues (31). The stroma infiltrating immune cells secrete IL-6 that activates STAT3 and triggers progression of PanINs to PDAC (32). Another study highlights the role of the chemokine CXCR2 in mediating the induction of CTGF by fibroblasts that promotes the progression of PanINs to PDAC (33). Notably, CXCR2 inhibitors were found to inhibit tumor progression by disrupting tumor-stromal interactions and improved survival in a mouse model of PDAC (34). PDAC stroma containing high levels of intratumor T helper type 2 cell infiltrate (Th2 (GATA-3+)) was associated with poor survival and was shown to result from PDAC activated CAFs secreting thymic stromal lymphopoietin (TSLP) that induced myeloid DCs to secrete Th2-attracting chemokines (35). On the other hand, Beatty et. al. reported tumor regression in some patients with surgically incurable PDAC by using an immune-reactivating agonist CD40 antibody in combination with gemcitabine chemotherapy (36). Follow up studies in mouse models of PDAC revealed that tumor regression by CD40 was through recruitment of macrophages that infiltrated and killed the tumor cells as well as depleted the tumor stroma, independent of activated T cells or gemcitabine (36). Further, tumor progression triggered by inflammation has been hypothesized to result from abrogation of the oncogene driven senescence characteristic of low-grade mPanINs. Thus, it was proposed that anti-inflammatory drugs can potentially block mPanIN expansion, reducing the risk of developing PDAC (37).
While tumor microenvironment research largely pertains to the components of stroma and the tumor stroma interaction, the oxidative state of tumor cells constitutes a local environmental cue as well. One study exploring ROS metabolism in the context of oncogene expression in murine cells found that ROS are actively suppressed in K-RAS (G12D) and B-RAF (V619E) expressing cells and in human pancreatic cancer through activation of the NRF2 mediated antioxidant program (38). Interestingly, abrogation of the NRF2 pathway impaired K-RAS (G12D)-induced proliferation and tumorigenesis in vivo, thus presenting yet another potential avenue for therapeutic intervention (38). Overall, these recent studies suggest that each component of the tumor-stroma ecosystem contributes to pancreatic tumorigenesis and sustenance, and in fact present multiple layers of vulnerabilities that may need to be integrated for successful therapeutic strategies.
The driving force of pancreatic cancer stem cells
The precise identity of the cell of origin for pancreatic ductal adenocarcinoma (PDAC) continues to be debated. The hypothesis that a subset of cells that renews the adult pancreas such as stem cells, progenitor cells, facultative stem cells or trans-differentiated bone marrow cells might also serve as cancer stem cells (CSCs) has merit, but needs to be demonstrated (39). In that light the report showing a role for TGF-β superfamily members Nodal and Activin that are known regulators of embryonic stem cell fate in pancreatic cancer stem cells is of significant interest. Nodal and Activin were found to be overexpressed in pancreatic CSCs, and their knockdown abrogated self-renewal capacity and in vivo tumorigenicity (40). Impressively, the combination of a stroma-targeting hedgehog pathway inhibitor and inhibitor of Nodal/Activin in mouse tumors provided long-term, progression-free survival, suggesting a route to pancreatic CSC therapy (41).
In another independent study the potential CSC function of a known pancreatic stem cell marker c-MET was analyzed (42). In this study, pancreatic cancer cells from low passage primary human pancreatic cancer xenografts were sorted on the basis of c-MET expression and were found to form tumorspheres and tumor xenografts in NOD-SCID mice more readily as compared with c-MET negative populations. The combination of c-MET and CD44 cell surface expression identified the most highly tumorigenic population studied, when compared to other CSC markers, such as ALDH and CD133. Significantly, c-MET inhibition through shRNAs or use of the c-MET inhibitor XL184, inhibited tumor sphere formation as well as reduced tumor growth in vivo and knocked down the CSC population (42). In addition, the c-MET inhibitor XL-184 was able to completely block the development of metastasis using an intra-cardiac injection model, highlighting the potential utility of targeting c-MET in the neoadjuvant or adjuvant setting, where patients who undergo surgical resection are at high risk for metastatic recurrence.
Evidence of metastasis as an early event in pancreatic tumorigenesis
Two recent publications independently examining early events in pancreatic tumorigenesis- one using mouse model of PDAC (43) and the other involving computational modeling of human pancreatic cancer (44), have arrived at a provocative common conclusion that metastasis is likely an early event during tumorigenesis (45), not the last step in a series of sequential acquisitions of mutations (46, 47). Rhim et. al. utilized a YFP labeled, in vivo lineage tracking system to follow the fate of pancreatic epithelial cells expressing mutant KRAS and TP53 in mouse models of PDAC (43). In presence of inflammation induced by cerulein, PanIN lesions progressed to PDAC, with pancreatic cells displaying surface markers of EMT and CSCs found in the circulation of mice harboring PDAC, as well as interestingly, in mice with only PanIN lesions. This suggests that metastatic cells may bud off from the primary tumor/preneoplastic lesions much sooner than previously believed.
Pancreatic cancer cells and autophagy
Autophagy describes the phenomenon of systematic degradation of cell organelles and macromolecules through recruitment of lysosomes. Autophagy has been associated with oncogenic K-RAS-induced malignant cell transformation in cell culture models (48), and recently, pancreatic cancer cells were shown to display elevated levels of autophagy (49). Inhibition of autophagy led to growth suppression of pancreatic cancer cells in vitro, and treatment with the common anti-malarial drug chloroquine, that is also a potent inhibitor of autophagy, led to tumor regression and prolonged survival in pancreatic cancer xenografts and genetic mouse models (49). Another study with experimental mouse models has reported an autophagy-dependent anticancer immune response following treatment with chemotherapeutic agents (50). These are exciting new leads that may provide additional avenues for pancreatic cancer therapy.
Improved mouse models of pancreatic cancer
Genetically engineered mouse models (GEMMs) of PDAC harboring combinations of KRAS, TP53 (and p16INK4/SMAD4) mutations have enabled precise analyses of PDAC biology, tumor development and progression, and more recently, the role of inflammation and the tumor microenvironment (51). Mouse models of PDAC are also being used to develop new therapeutic and diagnostic approaches (52). While, transgenic mice harboring mutations in KRAS and TP53 provide a powerful model of pancreatic cancer, aberrant expression of the transgenes commences in embryonic pancreas, which does not mimic human pancreatic cancer development, where somatic mutations affect the adult pancreas. A recent publication describing transgenic mice with tetracycline inducible expression of the oncogenic KrasG12D in combination with active/inactive alleles of the tumor suppressor gene p53 provides a very useful addition to the existing PDAC models (53). Using reversible induction experiments, mutant KRAS was found to be required for both the initiation and maintenance of pancreatic cancer in mice. Another study using inducible KRAS has elaborated the role of anabolic glucose metabolism in the maintenance of pancreatic cancer that could potentially help delineate a molecular link between diabetes and pancreatic cancer (54). The inducible model of PDAC may emerge as the preferred model to tease out the contributions of individual mutations in pancreatic cancer.
Even as mouse models are useful, these are no substitute for analysis of human tumors. Hidalgo’s group established the experimental protocol for engraftment of primary human PDACs in nude mice followed by generation of low passage xenografts and primary tumor cell lines for therapeutic testing (55). Primary human tumor xenografts provide a powerful tool for genomic/proteomic analysis of individual tumors, as well as it enabling pre-clinical testing of novel drugs and targets in vivo to explore personalized therapeutic options. In a recent report, they systematically evaluated the factors affecting engraftment and the predictability of clinical outcome based on experiments with low-passage xenografts (56). We expect to see this experimental approach being adopted by more researchers, particularly in conjunction with clinical sequencing, to test the efficacy of personalized therapeutic targets(57).
Advances in imaging of early pancreatic cancer lesions
Highly sensitive and specific early detection of pancreatic cancer is needed to extend the available therapeutic time window, but a major limitation is availability of biomarkers for early detection of the disease. Recently two independent studies using mouse models of PDAC have demonstrated the potential of non-invasive detection of pancreatic cancer cells by coupling serine protease cathepsin activity in preinvasive mPanIN lesions and PDAC as compared to benign or inflamed pancreatic tissue with fluorescent substrate probes (58, 59). Eser et. al. identified the serine protease cathepsin to be specifically overexpressed in preinvasive mouse PanIN lesions and PDAC as compared to benign or inflamed pancreatic tissue. They combined a cathepsin-activated near-infrared probe with confocal fluorescence laser microscopy to detect and grade murine PanIN lesions in real time in vivo (59). Elsewhere, in vivo imaging in mice using Plec-1 targeting peptides as a contrast agent for single photon emission computed tomography could distinguish primary and metastatic PDACs from benign tissues (60, 61). Follow up studies testing these leads in a human clinical trial setting are in order.
Conclusions
Pancreatic cancer research is gathering steam with several conceptual breakthroughs made in the field of mutational profiles of tumors, tumor-stroma interactions, cancer stem cells, epithelial to mesenchymal transition, and metastasis using high throughput sequencing applications, robust transgenic mouse models as well as primary tumor xenografts and cell lines (Figure 1). We envision further maturation of the field in the near future will lead to the much needed therapeutic breakthroughs in this ‘jewel in the crown of the ‘emperor of all maladies’.
Key points.
The pancreatic ductal adenocarcinoma (PDAC) niche in the pancreas is comprised cancer cells, cancer cells undergoing epithelial to mesenchymal transition (EMT), cancer stem cells (CSCs), and cancer associated stroma.
Most PDACs are characterized by the presence of hallmark mutations in KRAS, p16INK4/CDKN2A, TP53, and SMAD4.
High throughput sequencing has led to the discovery of novel recurrent mutations in PDACs as well as rarer subtypes of benign/neoplastic lesions of pancreas.
Genetically engineered mice with inducible KRAS mutation provide a powerful tool to model pancreatic tumorigenesis.
Future therapeutic strategies need to target tumor, stromal, and stem cells, engaging tumor immune response and autophagy.
Acknowledgments
CKS is supported by University of Michigan GI SPORE Career Development Award and National Institute of Health 5-R21-CA-155992-02. IW is supported by NIH 2T32CA009672-21. DMS is supported by NIH R01CA131045-01 and P50CA130810-1A and the Rich Rogel Fund for Pancreatic Cancer Research.
We apologize to all the authors whose work could not be cited due to space limitation.
Footnotes
Authors declare no conflict of interest.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
**of outstanding interest
- 1.Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, Hong SM, Fu B, Lin MT, Calhoun ES, Kamiyama M, Walter K, Nikolskaya T, Nikolsky Y, Hartigan J, Smith DR, Hidalgo M, Leach SD, Klein AP, Jaffee EM, Goggins M, Maitra A, Iacobuzio-Donahue C, Eshleman JR, Kern SE, Hruban RH, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–6. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mardis ER, Wilson RK. Cancer genome sequencing: a review. Hum Mol Genet. 2009;18:R163–8. doi: 10.1093/hmg/ddp396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee W, Jiang Z, Liu J, Haverty PM, Guan Y, Stinson J, Yue P, Zhang Y, Pant KP, Bhatt D, Ha C, Johnson S, Kennemer MI, Mohan S, Nazarenko I, Watanabe C, Sparks AB, Shames DS, Gentleman R, de Sauvage FJ, Stern H, Pandita A, Ballinger DG, Drmanac R, Modrusan Z, Seshagiri S, Zhang Z. The mutation spectrum revealed by paired genome sequences from a lung cancer patient. Nature. 2010;465:473–7. doi: 10.1038/nature09004. [DOI] [PubMed] [Google Scholar]
- 4.Hudson TJ, Anderson W, Artez A, Barker AD, Bell C, et al. International network of cancer genome projects. Nature. 2010;464:993–8. doi: 10.1038/nature08987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bignell GR, Greenman CD, Davies H, Butler AP, Edkins S, Andrews JM, Buck G, Chen L, Beare D, Latimer C, Widaa S, Hinton J, Fahey C, Fu B, Swamy S, Dalgliesh GL, Teh BT, Deloukas P, Yang F, Campbell PJ, Futreal PA, Stratton MR. Signatures of mutation and selection in the cancer genome. Nature. 2010;463:893–8. doi: 10.1038/nature08768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pleasance ED, Cheetham RK, Stephens PJ, McBride DJ, Humphray SJ, Greenman CD, Varela I, Lin ML, Ordonez GR, Bignell GR, Ye K, Alipaz J, Bauer MJ, Beare D, Butler A, Carter RJ, Chen L, Cox AJ, Edkins S, Kokko-Gonzales PI, Gormley NA, Grocock RJ, Haudenschild CD, Hims MM, James T, Jia M, Kingsbury Z, Leroy C, Marshall J, Menzies A, Mudie LJ, Ning Z, Royce T, Schulz-Trieglaff OB, Spiridou A, Stebbings LA, Szajkowski L, Teague J, Williamson D, Chin L, Ross MT, Campbell PJ, Bentley DR, Futreal PA, Stratton MR. A comprehensive catalogue of somatic mutations from a human cancer genome. Nature. 2010;463:191–6. doi: 10.1038/nature08658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stratton MR, Campbell PJ, Futreal PA. The cancer genome. Nature. 2009;458:719–24. doi: 10.1038/nature07943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell PJ, Yachida S, Mudie LJ, Stephens PJ, Pleasance ED, Stebbings LA, Morsberger LA, Latimer C, McLaren S, Lin ML, McBride DJ, Varela I, Nik-Zainal SA, Leroy C, Jia M, Menzies A, Butler AP, Teague JW, Griffin CA, Burton J, Swerdlow H, Quail MA, Stratton MR, Iacobuzio-Donahue C, Futreal PA. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature. 2010;467:1109–13. doi: 10.1038/nature09460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B, Kamiyama M, Hruban RH, Eshleman JR, Nowak MA, Velculescu VE, Kinzler KW, Vogelstein B, Iacobuzio-Donahue CA. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114–7. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones S, Hruban RH, Kamiyama M, Borges M, Zhang X, Parsons DW, Lin JC, Palmisano E, Brune K, Jaffee EM, Iacobuzio-Donahue CA, Maitra A, Parmigiani G, Kern SE, Velculescu VE, Kinzler KW, Vogelstein B, Eshleman JR, Goggins M, Klein AP. Exomic sequencing identifies PALB2 as a pancreatic cancer susceptibility gene. Science. 2009;324:217. doi: 10.1126/science.1171202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hofstatter EW, Domchek SM, Miron A, Garber J, Wang M, Componeschi K, Boghossian L, Miron PL, Nathanson KL, Tung N. PALB2 mutations in familial breast and pancreatic cancer. Fam Cancer. 2011;10:225–31. doi: 10.1007/s10689-011-9426-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peterlongo P, Catucci I, Pasquini G, Verderio P, Peissel B, Barile M, Varesco L, Riboni M, Fortuzzi S, Manoukian S, Radice P. PALB2 germline mutations in familial breast cancer cases with personal and family history of pancreatic cancer. Breast Cancer Res Treat. 2011;126:825–8. doi: 10.1007/s10549-010-1305-1. [DOI] [PubMed] [Google Scholar]
- 13.Villarroel MC, Rajeshkumar NV, Garrido-Laguna I, De Jesus-Acosta A, Jones S, Maitra A, Hruban RH, Eshleman JR, Klein A, Laheru D, Donehower R, Hidalgo M. Personalizing cancer treatment in the age of global genomic analyses: PALB2 gene mutations and the response to DNA damaging agents in pancreatic cancer. Mol Cancer Ther. 2011;10:3–8. doi: 10.1158/1535-7163.MCT-10-0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu J, Matthaei H, Maitra A, Dal Molin M, Wood LD, Eshleman JR, Goggins M, Canto MI, Schulick RD, Edil BH, Wolfgang CL, Klein AP, Diaz LA, Jr, Allen PJ, Schmidt CM, Kinzler KW, Papadopoulos N, Hruban RH, Vogelstein B. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med. 2011;3:92ra66. doi: 10.1126/scitranslmed.3002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furukawa T, Kuboki Y, Tanji E, Yoshida S, Hatori T, Yamamoto M, Shibata N, Shimizu K, Kamatani N, Shiratori K. Whole-exome sequencing uncovers frequent GNAS mutations in intraductal papillary mucinous neoplasms of the pancreas. Sci Rep. 2011;1:161. doi: 10.1038/srep00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Idziaszczyk S, Wilson CH, Smith CG, Adams DJ, Cheadle JP. Analysis of the frequency of GNAS codon 201 mutations in advanced colorectal cancer. Cancer Genet Cytogenet. 202:67–9. doi: 10.1016/j.cancergencyto.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 17.Collins MT, Sarlis NJ, Merino MJ, Monroe J, Crawford SE, Krakoff JA, Guthrie LC, Bonat S, Robey PG, Shenker A. Thyroid carcinoma in the McCune-Albright syndrome: contributory role of activating Gs alpha mutations. J Clin Endocrinol Metab. 2003;88:4413–7. doi: 10.1210/jc.2002-021642. [DOI] [PubMed] [Google Scholar]
- 18.Wilson CH, McIntyre RE, Arends MJ, Adams DJ. The activating mutation R201C in GNAS promotes intestinal tumourigenesis in Apc(Min/+) mice through activation of Wnt and ERK1/2 MAPK pathways. Oncogene. 29:4567–75. doi: 10.1038/onc.2010.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsiao HP, Kirschner LS, Bourdeau I, Keil MF, Boikos SA, Verma S, Robinson-White AJ, Nesterova M, Lacroix A, Stratakis CA. Clinical and genetic heterogeneity, overlap with other tumor syndromes, and atypical glucocorticoid hormone secretion in adrenocorticotropin-independent macronodular adrenal hyperplasia compared with other adrenocortical tumors. J Clin Endocrinol Metab. 2009;94:2930–7. doi: 10.1210/jc.2009-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bimpaki EI, Nesterova M, Stratakis CA. Abnormalities of cAMP signaling are present in adrenocortical lesions associated with ACTH-independent Cushing syndrome despite the absence of mutations in known genes. Eur J Endocrinol. 2009;161:153–61. doi: 10.1530/EJE-09-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nies C, Bartsch DK, Ehlenz K, Wild A, Langer P, Fleischhacker S, Rothmund M. Familial ACTH-independent Cushing’s syndrome with bilateral macronodular adrenal hyperplasia clinically affecting only female family members. Exp Clin Endocrinol Diabetes. 2002;110:277–83. doi: 10.1055/s-2002-34590. [DOI] [PubMed] [Google Scholar]
- 22.Landis CA, Masters SB, Spada A, Pace AM, Bourne HR, Vallar L. GTPase inhibiting mutations activate the alpha chain of Gs and stimulate adenylyl cyclase in human pituitary tumours. Nature. 1989;340:692–6. doi: 10.1038/340692a0. [DOI] [PubMed] [Google Scholar]
- 23.Fragoso MC, Latronico AC, Carvalho FM, Zerbini MC, Marcondes JA, Araujo LM, Lando VS, Frazzatto ET, Mendonca BB, Villares SM. Activating mutation of the stimulatory G protein (gsp) as a putative cause of ovarian and testicular human stromal Leydig cell tumors. J Clin Endocrinol Metab. 1998;83:2074–8. doi: 10.1210/jcem.83.6.4847. [DOI] [PubMed] [Google Scholar]
- 24*.Jiao Y, Shi C, Edil BH, de Wilde RF, Klimstra DS, Maitra A, Schulick RD, Tang LH, Wolfgang CL, Choti MA, Velculescu VE, Diaz LA, Jr, Vogelstein B, Kinzler KW, Hruban RH, Papadopoulos N. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011;331:1199–203. doi: 10.1126/science.1200609. This study identifies novel recurrent mutations in pancreatic neuroendocrine tumors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heaphy CM, de Wilde RF, Jiao Y, Klein AP, Edil BH, Shi C, Bettegowda C, Rodriguez FJ, Eberhart CG, Hebbar S, Offerhaus GJ, McLendon R, Rasheed BA, He Y, Yan H, Bigner DD, Oba-Shinjo SM, Marie SK, Riggins GJ, Kinzler KW, Vogelstein B, Hruban RH, Maitra A, Papadopoulos N, Meeker AK. Altered telomeres in tumors with ATRX and DAXX mutations. Science. 2011;333:425. doi: 10.1126/science.1207313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yachida S, Vakiani E, White CM, Zhong Y, Saunders T, Morgan R, de Wilde RF, Maitra A, Hicks J, Demarzo AM, Shi C, Sharma R, Laheru D, Edil BH, Wolfgang CL, Schulick RD, Hruban RH, Tang LH, Klimstra DS, Iacobuzio-Donahue CA. Small cell and large cell neuroendocrine carcinomas of the pancreas are genetically similar and distinct from well-differentiated pancreatic neuroendocrine tumors. Am J Surg Pathol. 2012;36:173–84. doi: 10.1097/PAS.0b013e3182417d36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shain AH, Giacomini CP, Matsukuma K, Karikari CA, Bashyam MD, Hidalgo M, Maitra A, Pollack JR. Convergent structural alterations define SWItch/Sucrose NonFermentable (SWI/SNF) chromatin remodeler as a central tumor suppressive complex in pancreatic cancer. Proc Natl Acad Sci U S A. 2012;109:E252–9. doi: 10.1073/pnas.1114817109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28*.Pérez-Mancera PA, Rust AG, Weyden Lvd, Glen Kristiansen, Allen Li, Sarver Aaron L, Silverstein Kevin AT, Grützmann Robert, Aust Daniela, Rümmele Petra, Knösel Thomas, Herd Colin, Stemple Derek L, Kettleborough Ross, Brosnan Jacqueline A, Li Ang, Morgan Richard, Knight Spencer, Yu Jun, Stegeman Shane, Collier Lara S, ten Hoeve Jelle J, de Ridder Jeroen, Klein Alison P, Goggins Michael, Hruban Ralph H, Chang David K, Biankin Andrew V, Grimmond Sean M, Wessels Lodewyk FA, Wood Stephen A, Iacobuzio-Donahue Christine A, Pilarsky Christian, Largaespada David A, Adams DJ, Tuveson DA Australian Pancreatic Cancer Genome Initiative. The deubiquitinase USP9X suppresses pancreatic ductal adenocarcinoma. Nature. 2012 doi: 10.1038/nature11114. This study describes loss of deubiquitinase USP9X expression (apparently without involving mutations) in pancreatic tumorigenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, Frese KK, Denicola G, Feig C, Combs C, Winter SP, Ireland-Zecchini H, Reichelt S, Howat WJ, Chang A, Dhara M, Wang L, Ruckert F, Grutzmann R, Pilarsky C, Izeradjene K, Hingorani SR, Huang P, Davies SE, Plunkett W, Egorin M, Hruban RH, Whitebread N, McGovern K, Adams J, Iacobuzio-Donahue C, Griffiths J, Tuveson DA. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–61. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu M, Tannock IF. Targeting tumor architecture to favor drug penetration: a new weapon to combat chemoresistance in pancreatic cancer? Cancer Cell. 2012;21:327–9. doi: 10.1016/j.ccr.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Li N, Grivennikov SI, Karin M. The unholy trinity: inflammation, cytokines, and STAT3 shape the cancer microenvironment. Cancer Cell. 2011;19:429–31. doi: 10.1016/j.ccr.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lesina M, Kurkowski MU, Ludes K, Rose-John S, Treiber M, Kloppel G, Yoshimura A, Reindl W, Sipos B, Akira S, Schmid RM, Algul H. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell. 2011;19:456–69. doi: 10.1016/j.ccr.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 33.Ijichi H, Chytil A, Gorska AE, Aakre ME, Bierie B, Tada M, Mohri D, Miyabayashi K, Asaoka Y, Maeda S, Ikenoue T, Tateishi K, Wright CV, Koike K, Omata M, Moses HL. Inhibiting Cxcr2 disrupts tumor-stromal interactions and improves survival in a mouse model of pancreatic ductal adenocarcinoma. J Clin Invest. 2011;121:4106–17. doi: 10.1172/JCI42754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seton-Rogers S. Pancreatic cancer: Fibroblast co-conspirators. Nat Rev Cancer. 2011;11:758. doi: 10.1038/nrc3157. [DOI] [PubMed] [Google Scholar]
- 35.De Monte L, Reni M, Tassi E, Clavenna D, Papa I, Recalde H, Braga M, Di Carlo V, Doglioni C, Protti MP. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J Exp Med. 2011;208:469–78. doi: 10.1084/jem.20101876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36**.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, Torigian DA, O’Dwyer PJ, Vonderheide RH. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–6. doi: 10.1126/science.1198443. An interesting ‘bedside to bench’ study highlighting the potential clinical application of CD40 agonists through its effect on tumor stroma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guerra C, Collado M, Navas C, Schuhmacher AJ, Hernandez-Porras I, Canamero M, Rodriguez-Justo M, Serrano M, Barbacid M. Pancreatitis-induced inflammation contributes to pancreatic cancer by inhibiting oncogene-induced senescence. Cancer Cell. 2011;19:728–39. doi: 10.1016/j.ccr.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCarthy N. Tumorigenesis: oncogene detox programme. Nat Rev Cancer. 2011;11:622–3. doi: 10.1038/nrc3119. [DOI] [PubMed] [Google Scholar]
- 39.Kong B, Michalski CW, Erkan M, Friess H, Kleeff J. From tissue turnover to the cell of origin for pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2011;8:467–72. doi: 10.1038/nrgastro.2011.114. [DOI] [PubMed] [Google Scholar]
- 40*.Lonardo E, Hermann PC, Mueller MT, Huber S, Balic A, Miranda-Lorenzo I, Zagorac S, Alcala S, Rodriguez-Arabaolaza I, Ramirez JC, Torres-Ruiz R, Garcia E, Hidalgo M, Cebrian DA, Heuchel R, Lohr M, Berger F, Bartenstein P, Aicher A, Heeschen C. Nodal/Activin signaling drives self-renewal and tumorigenicity of pancreatic cancer stem cells and provides a target for combined drug therapy. Cell Stem Cell. 2011;9:433–46. doi: 10.1016/j.stem.2011.10.001. This study describes a role for pancreatic stem cell markers in pancreatic cancer stem cells. [DOI] [PubMed] [Google Scholar]
- 41.Donahue TR, Dawson DW. Nodal/Activin signaling: a novel target for pancreatic cancer stem cell therapy. Cell Stem Cell. 2011;9:383–4. doi: 10.1016/j.stem.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 42*.Li C, Wu JJ, Hynes M, Dosch J, Sarkar B, Welling TH, Pasca di Magliano M, Simeone DM. c-Met is a marker of pancreatic cancer stem cells and therapeutic target. Gastroenterology. 2011;141:2218–27. e5. doi: 10.1053/j.gastro.2011.08.009. This study highlights the role of c-Met kinase, a therapeutic target with several inhibitors in clinical trials, as a pancreatic cancer stem cell marker that is required for tumor growth. [DOI] [PubMed] [Google Scholar]
- 43**.Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK, Vonderheide RH, Leach SD, Stanger BZ. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–61. doi: 10.1016/j.cell.2011.11.025. This study provides compelling evidence that pre-neoplastic lesions of pancreatic cancer (in genetically engineered mouse models) disseminate transformed pancreatic epithelial cells into blood circulation that display epithelial to mesenchymal transition as well as cancer stem cell like features. This observation is contrary to the long held assumption that posits metastasis as an endpoint in the gradual progression of tumors through accumulation of enabling mutations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44*.Haeno H, Gonen M, Davis MB, Herman JM, Iacobuzio-Donahue CA, Michor F. Computational modeling of pancreatic cancer reveals kinetics of metastasis suggesting optimum treatment strategies. Cell. 2012;148:362–75. doi: 10.1016/j.cell.2011.11.060. This study uses clinical data from human pancreatic cancer patients to carry out mathematical modeling of progression to metastasis, and supports an ‘early dissemination’ model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tuveson DA, Neoptolemos JP. Understanding metastasis in pancreatic cancer: a call for new clinical approaches. Cell. 2012;148:21–3. doi: 10.1016/j.cell.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 47.Nanda S. Cancer: Pancreatic carcinomas metastasize at a late stage in their development. Nat Rev Gastroenterol Hepatol. 2011;8:4. doi: 10.1038/nrgastro.2010.199. [DOI] [PubMed] [Google Scholar]
- 48.Kim MJ, Woo SJ, Yoon CH, Lee JS, An S, Choi YH, Hwang SG, Yoon G, Lee SJ. Involvement of autophagy in oncogenic K-Ras-induced malignant cell transformation. J Biol Chem. 2011;286:12924–32. doi: 10.1074/jbc.M110.138958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang S, Wang X, Contino G, Liesa M, Sahin E, Ying H, Bause A, Li Y, Stommel JM, Dell’antonio G, Mautner J, Tonon G, Haigis M, Shirihai OS, Doglioni C, Bardeesy N, Kimmelman AC. Pancreatic cancers require autophagy for tumor growth. Genes Dev. 2011;25:717–29. doi: 10.1101/gad.2016111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50*.Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, Shen S, Kepp O, Scoazec M, Mignot G, Rello-Varona S, Tailler M, Menger L, Vacchelli E, Galluzzi L, Ghiringhelli F, di Virgilio F, Zitvogel L, Kroemer G. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011;334:1573–7. doi: 10.1126/science.1208347. This study links autophagy and pancreatic tumorigenesis and highlights its clinical implications. [DOI] [PubMed] [Google Scholar]
- 51.Perez-Mancera PA, Guerra C, Barbacid M, Tuveson DA. What we have learned about pancreatic cancer from mouse models. Gastroenterology. 2012;142:1079–92. doi: 10.1053/j.gastro.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 52.Tuveson D, Hanahan D. Translational medicine: Cancer lessons from mice to humans. Nature. 2011;471:316–7. doi: 10.1038/471316a. [DOI] [PubMed] [Google Scholar]
- 53*.Collins MA, Bednar F, Zhang Y, Brisset JC, Galban S, Galban CJ, Rakshit S, Flannagan KS, Adsay NV, Pasca di Magliano M. Oncogenic Kras is required for both the initiation and maintenance of pancreatic cancer in mice. J Clin Invest. 2012;122:639–53. doi: 10.1172/JCI59227. This study introduces the use of inducibly expressed oncogenic KRAS in genetically engineered mouse model of PDAC, highlighting the requirement of KRAS is tumor initiation as well as sustenance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ying H, Kimmelman AC, Lyssiotis CA, Hua S, Chu G, Fletcher-Sananikone E, Locasale J, Son J, Zhang H, Coloff JL, Yan H, Wang W, Chen SH, Viale A, Zheng H, Paik JH, Lim C, Guimaraes AR, Martin ES, Chang J, Hezel AF, Perry SR, Hu J, Gan B, Xiao Y, Asara JM, Weissleder R, Wang YA, Chin L, Cantley LC, Depinho RA. Oncogenic Kras Maintains Pancreatic Tumors through Regulation of Anabolic Glucose Metabolism. Cell. 2012;149:15. doi: 10.1016/j.cell.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rubio-Viqueira B, Jimeno A, Cusatis G, Zhang X, Iacobuzio-Donahue C, Karikari C, Shi C, Danenberg K, Danenberg PV, Kuramochi H, Tanaka K, Singh S, Salimi-Moosavi H, Bouraoud N, Amador ML, Altiok S, Kulesza P, Yeo C, Messersmith W, Eshleman J, Hruban RH, Maitra A, Hidalgo M. An in vivo platform for translational drug development in pancreatic cancer. Clin Cancer Res. 2006;12:4652–61. doi: 10.1158/1078-0432.CCR-06-0113. [DOI] [PubMed] [Google Scholar]
- 56*.Garrido-Laguna I, Uson M, Rajeshkumar NV, Tan AC, de Oliveira E, Karikari C, Villaroel MC, Salomon A, Taylor G, Sharma R, Hruban RH, Maitra A, Laheru D, Rubio-Viqueira B, Jimeno A, Hidalgo M. Tumor engraftment in nude mice and enrichment in stroma- related gene pathways predict poor survival and resistance to gemcitabine in patients with pancreatic cancer. Clin Cancer Res. 2011;17:5793–800. doi: 10.1158/1078-0432.CCR-11-0341. This study describes factors affecting primary pancreatic tumor engraftment in mice that provide a powerful tool to test personalized therapeutic targets in an in vivo setting. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dennis C. Mouse ‘avatars’ could aid pancreatic cancer therapy. Nature. 2012 doi: 10.1038/nature.2012.10259. [DOI] [Google Scholar]
- 58.Cruz-Monserrate Z, Abd-Elgaliel WR, Grote T, Deng D, Ji B, Arumugam T, Wang H, Tung CH, Logsdon CD. Detection of pancreatic cancer tumours and precursor lesions by cathepsin E activity in mouse models. Gut. 2011;2011 doi: 10.1136/gutjnl-2011-300544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59**.Eser S, Messer M, Eser P, von Werder A, Seidler B, Bajbouj M, Vogelmann R, Meining A, von Burstin J, Algul H, Pagel P, Schnieke AE, Esposito I, Schmid RM, Schneider G, Saur D. In vivo diagnosis of murine pancreatic intraepithelial neoplasia and early-stage pancreatic cancer by molecular imaging. Proc Natl Acad Sci U S A. 2011;108:9945–50. doi: 10.1073/pnas.1100890108. This report highlights a potential approach for highly sensitive, non-invasive detection of early stage pancreatic cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bausch D, Thomas S, Mino-Kenudson M, Fernandez-del CC, Bauer TW, Williams M, Warshaw AL, Thayer SP, Kelly KA. Plectin-1 as a novel biomarker for pancreatic cancer. Clin Cancer Res. 2011;17:302–9. doi: 10.1158/1078-0432.CCR-10-0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Collisson E, Tempero M. Blinded by the light: molecular imaging in pancreatic adenocarcinoma. Clin Cancer Res. 2011;17:203–5. doi: 10.1158/1078-0432.CCR-10-2825. [DOI] [PubMed] [Google Scholar]