Abstract
NRG1 signaling has multiple functions in neurons and glia. The data in this study demonstrate that NRG1 may also possess significant signaling and cytoprotective properties in human brain endothelial cells (BMECs). NRG1 mRNA and protein expression are present in these cells, and NRG1 receptors erbB2 and erbB3 are phosphorylated in response to NRG1. NRG1 triggers clear biological responses in BMECs – elevated phospho-Akt levels, increased ring formation in a Matrigel assay, and decreased cell death after oxidative injury with H2O2. These data suggest that NRG1 signaling is functional and cytoprotective in BMECs.
Keywords: blood-brain barrier, erbB, neuregulin, neuroprotection, neurovascular unit, oxidative stress
INTRODUCTION
Neuregulin-1 (NRG1) and its erbB receptors perform multiple functions in the nervous system. NRG1 isoforms are present in various tissues types, and have been described as oncogenes, glial growth factor, and a promoter of Schwann cell migration (Falls 2003).
NRG1 was found to be neuroprotective in animal models of stroke (Xu et al. 2006). Although NRG1’s actions on neurons and glia during ischemic brain injury has been studied (Xu et al. 2006), its role in brain endothelial cell function has not been characterized. There is evidence that successful neuroprotective strategies must target the entire neurovascular unit consisting of the neuron, its associated glia, endothelium, and matrix (Iadecola 2004; Lok et al. 2007; Park et al. 2003). Because of the importance of the endothelium in neurovascular signaling, we investigated NRG1 function in human brain microvascular endothelial cells (BMECs). NRG1 mRNA, protein expression, and activity were detected in BMECs. Additionally, NRG1 decreased endothelial cell death in an in-vitro model of oxidative stress. Our data suggest that NRG1 signaling is functional and cytoprotective in BMECs.
MATERIALS AND METHODS
Cell Culture
A human brain microvascular endothelial cell line (BMEC) was cultured in RPMI 1640 and characterized for brain endothelial phenotypes as previously described (Callahan et al. 2004).
Treatment with NRG1 for Akt activation
Recombinant human NRG1-β1 (R&D Systems, Minneapolis, MN) was used for all experiments. BMECs were grown on plates coated with 10ug/ml Human Fibronectin (BD Biosciences, San Diego, CA). When 80% confluent the next day, cells were serum-starved for 18 hours. Vehicle or NRG1 +/− PI3K inhibitor LY294002 was then added. At appropriate time points, cells were rinsed with cold phosphate-buffered saline (PBS) and collected into lysis buffer. Cell lysates were centrifuged at 14,000 rpm (10 minutes, 4°C).
Evaluation of mRNA expression
Total RNA extraction was accomplished using RNeasy mini kit (Qiagen Sciences, Germantown, Maryland). The expression of neurotrophins and their receptors were assessed by Oligo GEArray (OHS-031, SuperArray Bioscience Corporation, Frederick, MD) and Oligo GEArray Trial Kit (SuperArray, GA-029), as suggested by the manufacturer. Image acquisition was performed using X-ray film and digital scanner.
Preparation of Brain Homogenates and Microvascular-Enriched Fraction (MVE)
Brain samples were homogenized on ice in lysis buffer. After centrifugation, supernatant was collected. MVE was prepared using standard procedures (Galea and Estrada, 1991). Briefly, meninges, cerebellum, subcortical structures, and choroids plexus were removed and the brain placed in ice-cold PBS. The volume of tissue and PBS was measured and an equal volume of 26% dextran was added. This mixture was homogenized and centrifuged (13,500g, 10 minutes, 4°C). The supernatant was decanted and 3 mg of the pellet containing microvessels was homogenized in lysis buffer and centrifuged (14,000 rpm, 15 minutes).
Immunoprecipitation (IP)/Western Blot analysis
For Western blot, equal amounts of protein were loaded per lane. For IP followed by Western blot, the entire cell lysate from each plate was used and the procedure was performed as previously described (Sardi et al. 2006). Prior to gel electrophoresis, samples were heated in buffer with reducing conditions. After gel electrophoresis, proteins were transferred to polyvinylidene difluroide or nitrocellulose membranes, and treated with blocking buffer (TBS with 0.1% Tween 20, and 0.2% I-block [Tropix, Bedford, MA] or 4% BSA). Membranes were treated overnight (4°C) with primary antibody. After secondary antibody incubation, immune complexes were visualized by enhanced chemiluminescence. Primary antibodies: Neuregulin Ab-1 (7D5) from Lab Vision, Fremont, CA; ErbB2 (Neu), phospho-erbB2 (Tyr1248), erbB3 (C-17), and erbB4 (C-18) from Santa Cruz Biotechnology, Santa Cruz, CA; mouse 4G10 anti-phospho-tyrosine from Gjoerup and Roberts, Dana-Farber Cancer Institute, Boston, MA, and pAkt (serine 473) from Cell Signaling Technology, Danvers, MA. Dilutions: 1:1000 for NRG1, erbB2, erbB3, erbB4, and phospho-erbB2; and 1:5000 for 4G10 (anti-phospho-tyrosine).
Matrigel Tube Formation Assay for angiogenesis
BMECs were plated in 24 well plates in serum-free media in wells coated with Growth Factor Reduced Matrigel Matrix (B-D Biosciences, CA). After 18 hours, photos were taken of 5 locations (4X magnification). The numbers of complete rings in these fields were counted.
Induction of Oxidative Stress with H2O2
Cells were plated in wells coated with 10μg/ml of human fibronectin factor (B-D Biosciences). When 80% confluent, cells were placed in serum-free media with/without 100 μM H2O2 for 18 hours. Vehicle, NRG1, or NRG1+AG825 (an inhibitor of the erbB2 tyrosine kinase activity) were added (AG825 5–10 minutes prior to NRG1, and NRG1 5–10 minutes prior to H2O2). After 18 hours, cell viability was evaluated using MTT assay.
Assessment of Cytoxicity
Cell survival was measured by MTT assay, in which mitochondrial function was assessed by reduction of 3-(4.5-dimethylthiazol-2-yl)2,5-diphenyl-tetrazolium bromide (MTT) (Sigma, St. Louis, MO) to a formazan product. After 18h incubation with H2O2 or vehicle, cells were placed in media with 0.4% MTT. After 60 minutes at 37°C, media was removed, and cells were dissolved in DMSO. Formazan formation was measured by reading absorbance at 570nm with a reference setting of 630nm on a microplate reader (FL600, Bio-Tek Instruments, Inc). Percent cell death was quantified as (100% – % cell survival).
Statistical Analysis
Matrigel ring formation data were analyzed using ANOVA and Tukey-Kramer tests. Statistical significance was set at p<0.05. Data are expressed as mean + SD. H2O2-induced cytotoxicity was analyzed as log-OD using a hierarchical linear mixed model with fixed cell-means for all combinations of H2O2, NRG1, and AG concentrations and random intercepts among plates, among rows and columns within plate, and among replicate OD measurements for each well within each experiment. This is equivalent to a repeated measures strip-plot ANOVA with replicate OD measurements. Effects of experimental conditions relative to controls were estimated by linear contrasts. Point and interval estimates were back-transformed to yield estimates of percent OD of each treatment relative to control OD. Percent cell death was calculated as 1 – percent survival assuming a linear relationship between OD and density of live cells. Reported results are for two-tailed tests. Analyses were performed using SAS (version 9.1.3, SAS Institute, Cary, NC). Statistical significance was set at p<0.05. Data are expressed as mean +/− Confidence Interval.
RESULTS
NRG1 signaling is present in human brain microvascular endothelial cells
Using a DNA microarray for neurotrophins/receptors, NRG1 mRNA was detected in BMECs, along with BDNF, NGF, FGF, and NT-5 (Fig. 1A). NRG1 protein expression was found, with multiple NRG1 immunoreactive-bands corresponding to previously reported isoforms (Cote et al. 2005; Francis et al. 1999; Lebrasseur et al. 2003). BMECs, microvascular-enriched (MVE) fractions, and rat whole brain homogenates all express a similar pattern of NRG-1 immunoreactive bands (Fig. 1B). Although the MVE fraction contains other cell types which express NRG1, enriching for endothelial cells did not decrease NRG1 immunoreactivity, further suggesting that BMECs express NRG1.
Figure 1.
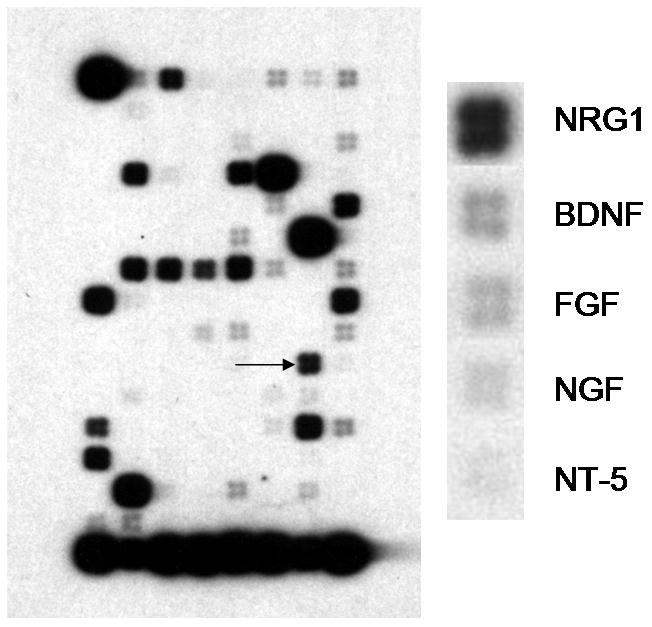



(A) A focused subset (neurotrophin and growth factor) microarray demonstrates the presence of NRG1 (arrow in left panel) in clearly detectable amounts along with many common growth factors, including BDNF, NGF, FGF, and NT-5 (see enlarged right panel). The bottom row of the microarray shows the amount of mRNA encoding various structural genes and genes for routine function, providing a control for loading conditions. (B) Western blots (representative of 3 blots) detected multiple NRG1 isoforms (30, 44, 50, 55, 60, 97, 114 kDa) in whole rat brain homogenate, microvascular-enriched fractions (MVE) from rat brain, and BMECs. (C) Western blots show that BMECs express erbB2 and erbB3. Using immunoprecipitation with the respective erbB antibody followed by western blotting with antibodies against either phospho-erbB2 or phospho-tyrosine, we found that both erbB2 and erbB3 become phosphorylated in BMECs after incubation with 12.5nM (100 ng/ml) NRG1. erbB4 phosphorylation was not found (data not shown). These images are representative of 2–3 immunoblots. (D) Incubation of BMECs with 100 ng/ml (12.5 nM) NRG1 60 minutes resulted in an increase in phosphorylated Akt detected by Western blotting. This increase was abolished by the PI3K inhibitor LY294002 (50 μM). (E) pAkt and actin signal intensities are plotted as mean +/− SD (n=2 experiments).
IP/Western demonstrated the presence of erbB2 and erbB3 receptors that became phosphorylated after NRG1 incubation (Fig. 1C). ErbB4 was not detected (data not shown). NRG1 increased Akt phosphorylation, which was abolished when the PI3-kinase inhibitor LY294002 (50μM) was added (Fig. 1D).
NRG1 is pro-angiogenic and cytoprotective in brain microvascular endothelial cells
NRG1 increased ring formation in a Matrigel assay for angiogenesis (Fig. 2A, 2B). At baseline there was an average of 300 rings per well. In NRG1-treated wells, there was an increase of 30–40 rings per well (p<0.05).
Figure 2.




(A) In a Matrigel assay for angiogenesis, NRG1 (1.2nM and 12.5nM, corresponding to 10ng/ml and 100ng/ml) stimulated an increase in the number of rings in 5 fields counted per well. * p<0.05 versus untreated conditions. N=5 independent experiments performed in triplicate. Baseline ring number ranged from 250–350 rings in the 5 fields counted per well. (B) Images of Matrigel assays: control, NRG1 6.2nM (50ng/ml); NRG1 12.5nM (100ng/ml). (C) Images of BEMC (10X) under various experimental conditions. a: Untreated; b: Incubated with 100 μM H2O2 for 18 h; c: Incubated with 100 μM H2O2 and 12.5nM (100ng/ml) NRG1 for 18 h; d: Incubated with 100 μM H2O2, 12.5nM (100ng/ml) NRG1, and 5μM AG825 for 18 h. (D) H: 100 μM H2O2 for 18 h; H+N: 100 μM H2O2 and 12.5nM (100ng/ml) NRG1 for 18 h; H+N+A: Incubated with 100 μM H2O2, 12.5nM (100ng/ml) NRG1, and 5μM AG825 for 18 h. N=6 independent experiments performed in duplicate or triplicate.
Oxidative stress was induced using H2O2, and cell viability assessed with MTT assay (Figure 2C, 2D). 18h exposure to 100 μM H2O2 resulted in 44% cell death compared to untreated cells (p<0.001). Addition of NRG1 reduced cell death to 33% (p<0.001). Further addition of the erbB2 tyrosine kinase inhibitor AG825 negated the cytoprotective effect of NRG1 and cell death increased back to 42% (p<0.002). AG825 alone did not affect cell survival.
DISCUSSION
Our data show that NRG1 and its erbB2 and erbB3 receptors may mediate important biological functions in BMECs. NRG1 increases pAkt, which may enhance survival of endothelial cells. Alternatively, NRG1 may protect BMECs by upregulating other trophic factors. These pro-angiogenic and cytoprotective actions may also contribute to neuronal survival.
The presence of erbB receptors in BMECs parallel the finding of these receptors in other types of endothelial cells (Russell et al. 1999). Although erbB2 lacks ligand-binding activity and erbB3 lacks efficient kinase activity, together the erbB2-erbB3 pair is the most active erbB heterodimer in terms of cell growth and differentiation. Downstream pathways include the Ras-Erk pathway for proliferation, the PI3 kinase pathway for survival, as well as MAPK, phospholipase-Cγ, protein kinase C, and the Janus kinase (Jak-STAT) (Citri et al. 2003). The ability to activate multiple pathways suggests that NRG1 signaling may play a central role in endothelial homeostasis.
There are several caveats in this study. First, our cell culture data are obtained in a cell line rather than primary cells. This cell line was shown previously (Callahan et al. 2004) to possess cerebral endothelial phenotypes. Furthermore, the presence of NRG1 in microvascular-enriched fractions of rat brain supports the cell culture data. Second, many NRG-1 immunoreactive bands are detected by Western blotting. The finding of multiple isoforms is a well-known property of NRG1 (Falls 2003). The reader is referred to a recent manuscript (Cote et al. 2005) for a study of the specificity of NRG1-immunoreactive bands in cardiac endothelial cells. Thirdly, our biologic assays are limited to two endpoints, angiogenesis and cytoprotection. Perhaps NRG1 might have other effects on endothelium. Additionally, our cytoprotection is relatively modest. Larger effects were seen in PC-12 cells transfected with erbB4 receptors (Goldshmit et al. 2001), possibly due to the higher number of erbB4 receptors in these cells. Alternatively, the modest effects of exogenous NRG1 in our study could be due to high levels of endogenous NRG1, although the lack of significant baseline erbB and Akt phosphorylation argues against this hypothesis. It is possible that endogenous NRG1 is subjected to post-translational processing resulting in isoforms that do not act within the endothelial cell. Lastly, although AG825 is relatively specific for erbB2 at the concentration (5μM) used, it may inhibit other types of tyrosine kinases. However, its IC-50 is 0.35 for erbB2, 19 for EGFR, 40 for PDGFR, and >100 for InsR (Levitski et al. 1991). So at 5 uM, its specificity for erbB2 is 4 times more than that for EGFR, and 100 times more than that for PDGFR and for insulin receptor.
NRG1 is shown here to protect BMECs against oxidative stress. Further study is required to determine whether NRG1 is more broadly cytoprotective against other insults, and whether it mediates both acute neuroprotection and post-injury repair. It is likely that endothelial function and neuronal health are linked, and cytoprotection of the brain endothelium may increase neuronal survival during both processes.
In summary, our data suggest that the NRG1 signaling is active in BMEC biology, including the promotion of angiogenesis and protection against oxidative stress. NRG1’s potential role in endothelial signaling merits further study, as neurovascular homeostasis is essential for salvaging brain function in stroke, brain trauma, and neurodegeneration.
Acknowledgments
Supported in part by NIH grants R01-NS37074, R01-NS48422, R01-NS53560, R01-NS35884, P50-NS10828, P01-NS55104, and P30-NS045776. The authors thank Dr. Eric Macklin for statistical analysis of the cytotoxicity data, and Drs. Klaus van Leyen, Douglas Sawyer, Xiaoying Wang, Duy Minh Ha, and Rui-Ying Zhu for many helpful discussions.
Footnotes
Disclosure/Conflict of Interest: None.
References
- Callahan MK, Williams KA, Kivisak P, Pearce D, Stins MF, Ransohoff RM. CXCR3 marks CD4+ memory T lymphocytes that are competent to migrate across a human brain microvascular endothelial cell layer. J Neuroimmunol. 2004;153:150–157. doi: 10.1016/j.jneuroim.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Citri A, Skaria KB, Yarden Y. The deaf and the dumb: the biology of ErbB-2 and ErbB-3. Exp Cell Res. 2003;284:54–65. doi: 10.1016/s0014-4827(02)00101-5. [DOI] [PubMed] [Google Scholar]
- Cote GM, Miller TA, Lebrasseur NK, Kuramochi Y, Sawyer DB. Neuregulin-1alpha and beta isoform expression in cardiac microvascular endothelial cells and function in cardiac myocytes in vitro. Exp Cell Res. 2005;311:135–146. doi: 10.1016/j.yexcr.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Falls DL. Neuregulins: functions, forms, and signaling strategies. Exp Cell Res. 2003;284:14–30. doi: 10.1016/s0014-4827(02)00102-7. [DOI] [PubMed] [Google Scholar]
- Francis A, Raabe TD, Wen D, DeVries GH. Neuregulins and ErbB receptors in cultured neonatal astrocytes. J Neurosci Res. 1999;57:487–494. [PubMed] [Google Scholar]
- Galea E, Estrada C. Periendothelial acetylcholine synthesis and release in bovine cerebral cortex capillaries. JCBFM. 1991;11(5):868–874. doi: 10.1038/jcbfm.1991.147. [DOI] [PubMed] [Google Scholar]
- Goldshmit Y, Erlich S, Pinkas-Kramarski R. Neuregulin rescues PC12-ErbB4 cells from cell death induced by H2O2Regulation of reactive oxygen species levels by phosphatidylinositol 3-kinase. J Bio Chem. 2001;276(49):46379–46385. doi: 10.1074/jbc.M105637200. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- Lebrasseur NK, Cote GM, Miller TA, Fielding RA, Sawyer DB. Regulation of neuregulin/ErbB signaling by contractile activity in skeletal muscle. Am J Physiol Cell Physiol. 2003;284:C1149–1155. doi: 10.1152/ajpcell.00487.2002. [DOI] [PubMed] [Google Scholar]
- Levitzki A, Gazit A. Tyrosine Kinase Inhibition: An Approach to Drug Development. Science. 1995;267:1782–1788. doi: 10.1126/science.7892601. [DOI] [PubMed] [Google Scholar]
- Lok J, Gupta P, Guo S, Kim WJ, Whalen MJ, van Leyen K, Lo EH. Cell-cell Signaling in the Neurovascular Unit. Neurochem Res. 2007;32:2032–2045. doi: 10.1007/s11064-007-9342-9. [DOI] [PubMed] [Google Scholar]
- Park JA, Choi KS, Kim SY, Kim KW. Coordinated interaction of the vascular and nervous systems: from molecule- to cell-based approaches. Biochem Biophys Res Commun. 2003;311:247–253. doi: 10.1016/j.bbrc.2003.09.129. [DOI] [PubMed] [Google Scholar]
- Russell KS, Stern DF, Polverini PJ, Bender JR. Neuregulin activation of ErbB receptors in vascular endothelium leads to angiogenesis. Am J Physiol. 1999;277:H2205–2211. doi: 10.1152/ajpheart.1999.277.6.H2205. [DOI] [PubMed] [Google Scholar]
- Sardi SP, Murtie J, Koirala S, Patten BA, Corfas G. Presenilin-dependent ErbB4 nuclear signaling regulates the timing of astrogenesis in the developing brain. Cell. 2006;127:185–197. doi: 10.1016/j.cell.2006.07.037. [DOI] [PubMed] [Google Scholar]
- Xu Z, Croslan DR, Harris AE, Ford GD, Ford BD. Extended therapeutic window and functional recovery after intraarterial administration of neuregulin-1 after focal ischemic stroke. J Cereb Blood Flow Metab. 2006;26:527–535. doi: 10.1038/sj.jcbfm.9600212. [DOI] [PubMed] [Google Scholar]


