Abstract
Oral delivery of peptide and protein drugs faces immense challenge partially due to the gastrointestinal (GI) environment. In spite of considerable efforts by industrial and academic laboratories, no major breakthrough in the effective oral delivery of polypeptides and proteins has been accomplished. Upon oral administration, gastrointestinal epithelium acts as a physical and biochemical barrier for absorption of proteins resulting in low bioavailability (typically less than 1–2%). An ideal oral drug delivery system should be capable of a) maintaining the integrity of protein molecules until it reaches the site of absorption, b) releasing the drug at the target absorption site, where the delivery system appends to that site by virtue of specific interaction, and c) retaining inside the gastrointestinal tract irrespective of its transitory constraints. Various technologies have been explored to overcome the problems associated with the oral delivery of macromolecules such as insulin, gonadotropin-releasing hormones, calcitonin, human growth factor, vaccines, enkephalins, and interferons, all of which met with limited success. This review article intends to summarize the physiological barriers to oral delivery of peptides and proteins and novel pharmaceutical approaches to circumvent these barriers and enhance oral bioavailability of these macromolecules.
Keywords: oral delivery, proteins, peptides, nanoparticles, microparticles, GI tract
1. Introduction
Delivery via oral route remains the most preferred mode of drug administration. More than 60% of conventional small molecule drug products available in the market are administered via oral route (DeVane, 2004). However, this does not hold true for protein and peptide drugs. Effective delivery of peptides and proteins via oral route still remains unaccomplished. The journey of protein drugs has started in 1920, with the use of bovine or porcine insulin for the treatment of diabetes. Recent advancement in the field of molecular biology and biotechnology led to the development of several large molecular weight therapeutic protein and peptides. At present there are more than 40 peptide and protein drugs in the market worldwide with approximately 270 peptides in clinical phase and 400 peptides in advanced preclinical phase testing (Marx, 2005). The 2008 PhRMA report on “Biotechnology Medicines” identified 633 biotechnology products in developmental stages, of which 59 for autoimmune diseases, 254 for cancer, 34 for HIV/AIDS and related conditions, 162 for infectious diseases, 25 for cardiovascular disease, and 19 for diabetes and related conditions.
Typically, a peptide consists of a chain of amino acids with amide bonds. When the peptide chain folds into a three-dimensional configuration, it is called as protein (Lehninger et al., 2005; Sheppard, 1981). Peptides and proteins offer several advantages as compared to conventional drugs. These include high activity, high specificity, low toxicity, and minimal nonspecific and drug-drug interactions. Apart from these peptides and proteins have become the drugs of choice in certain disease states such as enzyme deficiency, genetic and degenerative disease and protein-dysfunction. Developments in the field of biotechnology resulted in large-scale production of peptides, hormones and vaccines in an economical manner (Fix, 1996). Most of the commercially available protein formulations are delivered via traditional routes such as intramuscular (IM), subcutaneous (SC), or intravenous (IV) injections because of their poor oral bioavailability. Oral route of administration has several advantages which include: patient compliance, ease of administration and reasonably low cost of production. Low oral bioavailability of macromolecular drugs stems mainly from pre-systemic enzymatic degradation and poor penetration across the intestinal membrane (Hamman et al., 2005). Development of a viable oral drug delivery system for proteins and peptides requires a careful consideration of their physicochemical properties (molecular weight, pH stability, hydrophobicity, molecular size, and ionization constant) and biological barriers (proteolysis in stomach, variable pH, poor permeation and membrane efflux) that restrict absorption from the gastrointestinal (GI) tract (Mahato et al., 2003). Despite these challenges some polypeptide drugs such as desmopressin and cyclosporin A have been developed as oral dosage forms (Mahato et al., 2003). Major pharmaceutical companies and academic institutions have intensified their research towards oral peptide and protein delivery during the past few decades (Shaji and Patole, 2008). This review article summarizes the physiological barriers to oral delivery of peptides and proteins and provides an insight into novel pharmaceutical approaches to improve oral bioavailability of therapeutic proteins.
2. Barriers to oral delivery of peptide and protein drugs
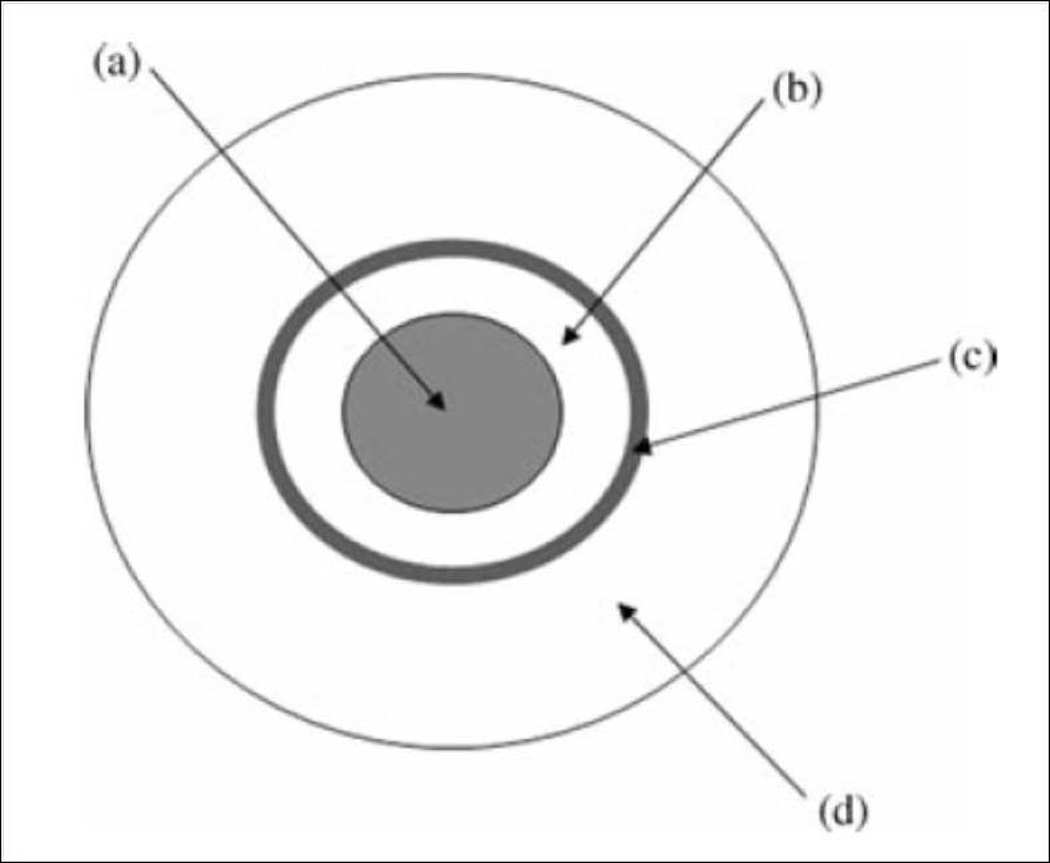
Following oral administration gastrointestinal epithelium acts as a physical and biochemical barrier to absorption of permeants. The physical barrier is mainly represented by impermeable gastrointestinal (GI) epithelium, while the biochemical barrier comprises of enzymatic degradation by peptidases. Indeed it is necessary to understand these barriers for achieving optimal delivery of proteins and peptides (Catnach et al., 1994). GI tract exhibits site specific absorption based on the nature of drugs and regional differences such as pH, enzyme activity, thickness of mucosa, residence time and surface area (Kompella and Lee, 2001). The length of GI tract is ~20 feet and it is broadly divided into two segments. The upper segment mainly consists of mouth, pharynx, esophagus and stomach while the lower segment consists of small intestine (duodenum, jejunum and ileum) large intestine (cecum, colon and rectum) and anus. It consists of 4 concentric layers:
Mucosa: It is the innermost, mucus secreting layer which contains many projections (villi) responsible for absorption of food and drug substances. This layer is further divided into epithelium, lamina propria and muscularis mucosa. These cells mainly secrete pepsinogen, hydrochloric acid, and gastric lipase.
Submucosa: It consists of a connective tissue with large blood vessels, lymphatics, and nerves branching into the mucosa and muscularis externa.
Muscularis externa: It is made up of longitudinal and circular muscle fibers. The longitudinal fibers shorten the tract, while the circular fibers prevent food from traveling backward and propel the balled-up food through the GI tract.
Serosa: It is also known as adventitia. This consists of several epithelial layers and forms an external protective coat.
Bioavailability of protein and peptide molecules depends on their ability to cross the intestinal mucosa and reach the systemic circulation (Johnson, 1994; Kwan, 1997).
The pH of GI tract varies from 1– 7, with stomach pH between 1–3, duodenum pH between 6.0–6.5, and large intestine pH from 5.5–7.0 (Van de Graaff, 1986). Protein absorption through the stomach is limited by several factors such as low surface area, action of pepsin and harsh degradative acidic environment (Kompella and Lee, 2001). Intestinal epithelium is made up of phospholipid bilayer membrane and cholesterol. Upon oral administration drug molecules must traverse through this lipoidal membrane before entering into systemic circulation. Small intestine is responsible for absorption of more than 90% of nutrients (carbohydrates, proteins, lipids, water, vitamins and minerals), while the rest are absorbed in the stomach and large intestine. The microvilli present on the absorptive mucosal cells of small intestine provide extended surface area for nutrient absorption following which they enter the bloodstream or lymphatic circulation (Tortora and Grabowski, 1996). However, capillary drug absorption eventually results in first-pass metabolism by the hepatic enzymes. Therefore, absorption through Peyer’s patches in the ileum that consists of lymph nodes can be explored as a potential alternative for protein and peptide drugs (Mahato et al., 2003; Shakweh et al., 2004). Compounds absorbed through the lymphatic system enter the blood circulation via thoracic duct. By this approach, first pass metabolism by the liver can be mostly eliminated.
The inner wall of small intestine is made of mucosa which consists of ~1 µm long projections or evaginations called microvilli, mucus secreting goblet cells, secretin secreting enteroendocrine cells and lysozyme secreting Paneth cells. Most of the nutrients (lipids, proteins, and carbohydrates) undergo digestion and absorption from the small intestine and hence can be considered as a potential absorptive site for protein and peptide drugs. Moreover, Paneth cells are phagocytic in nature and can aid in the uptake of particulate peptides (Repassy and Lapis, 1979).
Besides goblet cells and enteroendocrine cells, enterocytes and M cells are also important for intestinal transport (Yun et al., 2012). Enterocytes line the gastrointestinal tract and M cells are primarily located within the epithelium of Peyer’s patches. M cells represent only about 5% of the human follicle-associated epithelium. These cells are capable of delivering proteins and peptides from the lumen to the underlying lymphoid tissues and induce immune responses. On the other hand, M cells are also exploited by some pathogens as a means of host invasion. Moreover, the high endocytotic ability of M cells enables oral delivery of proteins and peptides. The high transcytotic capability of M cells allows transport of a wide variety of substances, including nanoparticles, microparticles etc (Yun et al., 2012). Macromolecules, particles and microorganisms are taken up by M cells through adsorptive endocytosis via clathrin-coated pits and vesicles, phagocytosis and fluid phase endocytosis (Buda et al., 2005).
The large intestine consisting of the cecum and colon differs from the small intestine. The wall of the large intestine consists of simple columnar epithelium and mucus secreting goblet cells. Large intestine houses more than 400 species of bacteria that help in digestion of polysaccharides and reduction of azo and nitro compounds which in turn are absorbed by passive diffusion (Chien, 1992; Rafii et al., 1990). The bacteria also generate vitamins such as vitamin K, thiamine, riboflavin, and biotin for absorption into the circulation. The colon acts as a suitable absorption site for protein and peptide drugs due to the absence of digestive enzymes and proteolytic activity besides providing longer residence time (Sinha and Kumria, 2003). Vitamin influx receptors can also be exploited for delivery of macromolecules using surface modified particulate systems. Unique bacterial colonization in the colon also offers a platform for oral protein delivery. Intact protein and peptide molecules can be delivered to the colon with pH sensitive, mucoadhesive or azo polymers that can be degraded by the bacteria in the colon (Chourasia and Jain, 2003).
3. Intestinal drug transport mechanisms
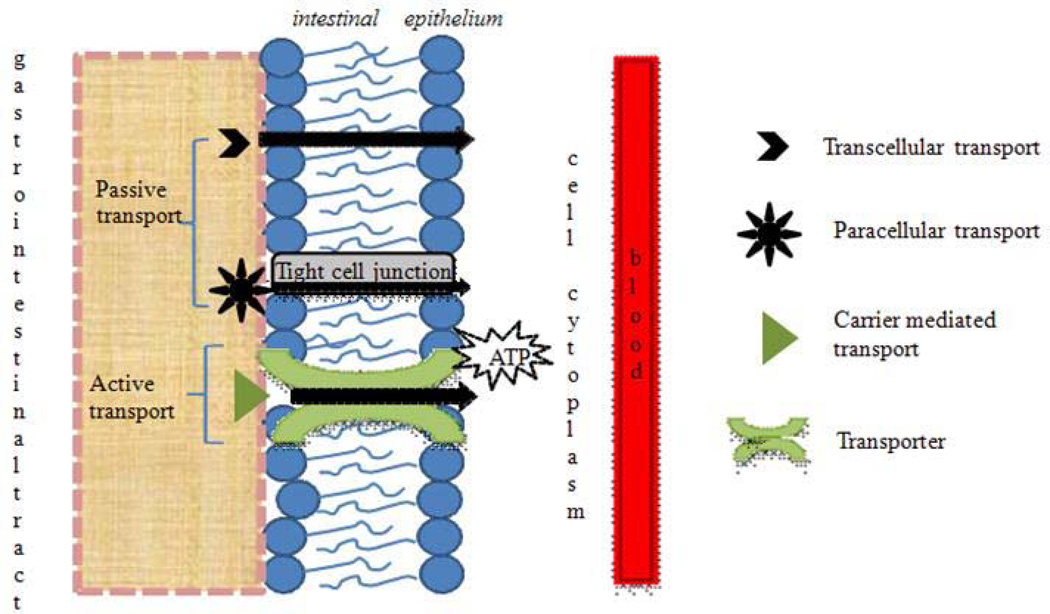
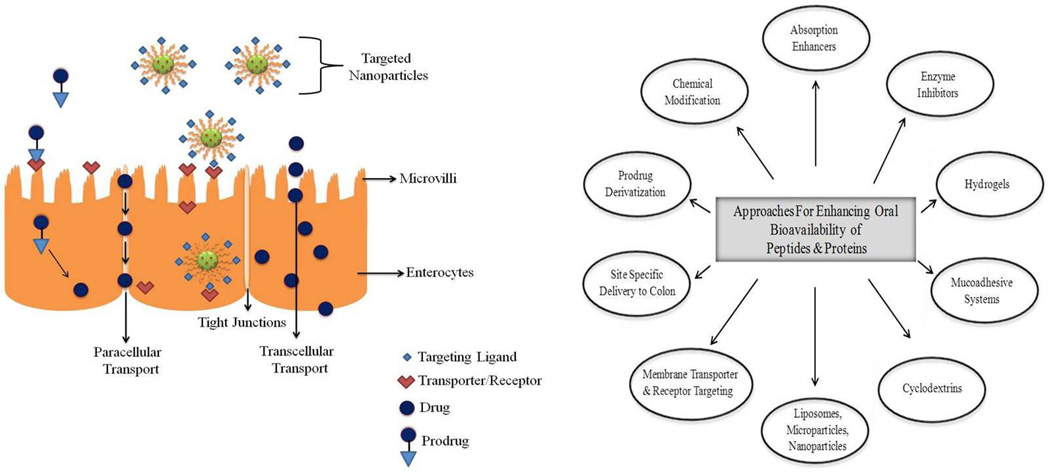
Drug transport across the intestinal epithelium is mediated by active or passive transport processes (Fig. 1). Mechanism of transport depends mainly on the physicochemical properties of drug molecule. Active transport involves the movement of drug molecules against concentration gradient (i.e. from low to high concentration) by transmembrane proteins with expenditure of ATP molecules. Passive transport involves the diffusion of drug molecules in the direction of concentration gradient (Gibaldi, 1991). The rate of drug transfer is governed by Fick’s law of diffusion (Eq. 1).
| (Eq. 1) |
dQ/dt = rate of diffusion
D= diffusion coefficient
K= oil/water partition coefficient of drug
A= surface area of the membrane across which drug transfer occurs
h = thickness of the membrane through which diffusion occurs
(C1− C2)= difference in drug concentrations in area 1 and 2 respectively
Passive diffusion of peptides and proteins can be described by a combination of two processes:
-
Paracellular transport: This process involves the transport of molecules via water filled pores/channels between cells. Approximately 0.01–0.1% of the total intestinal surface area consists of water filled pores. Taking into consideration that the intestinal epithelium has a surface area of ~2 × 106 cm2 (Fasano, 1998), paracellular route corresponds to ~200 to 2000 cm2. This surface area is sufficient for the absorption of small quantities (pM–nM range) of a protein adequate to exert their biological activity (Salamat-Miller and Johnston, 2005). This route is preferred by low molecular weight hydrophilic compounds such as small peptide fragments generated from the breakdown of proteins. Peptide and protein molecules are hydrophilic in nature with logP value < 0. These molecules enter cells mostly via paracellular route (Pappenheimer and Reiss, 1987). However, the presence of tight junctions or zonula occludens between the epithelial cell layer of GIT severely limit penetration ability of polar macromolecules (Stella, 2007). The diffusion of polypeptides via paracellular route depends on their physicochemical properties, molecular dimension and overall ionic charge (Pauletti et al., 1997; Salamat-Miller and Johnston, 2005). The bioavailability of drugs decrease rapidly with increase in molecular weight beyond 700 Da (Antosova et al., 2009). Unfortunately, most of the therapeutic proteins have molecular weight much greater than 700 Da and hence exhibit low bioavailability.
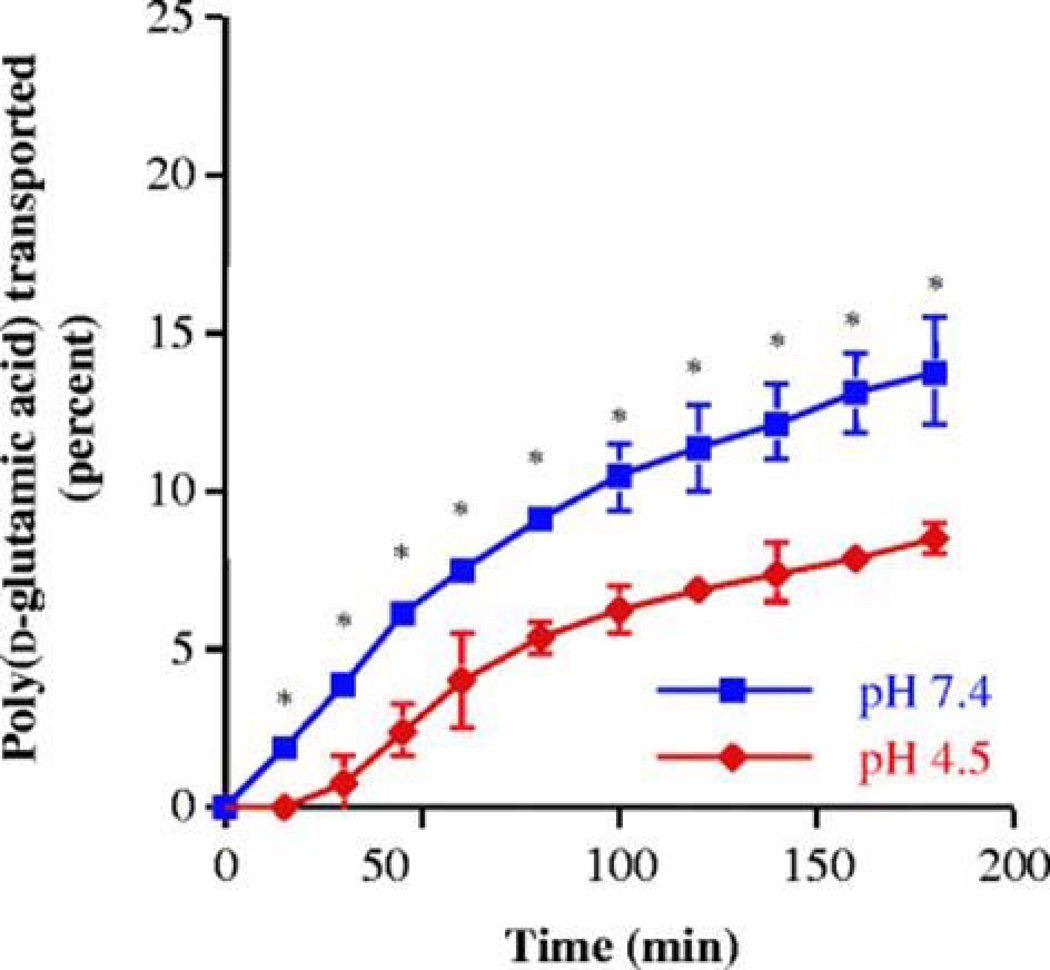
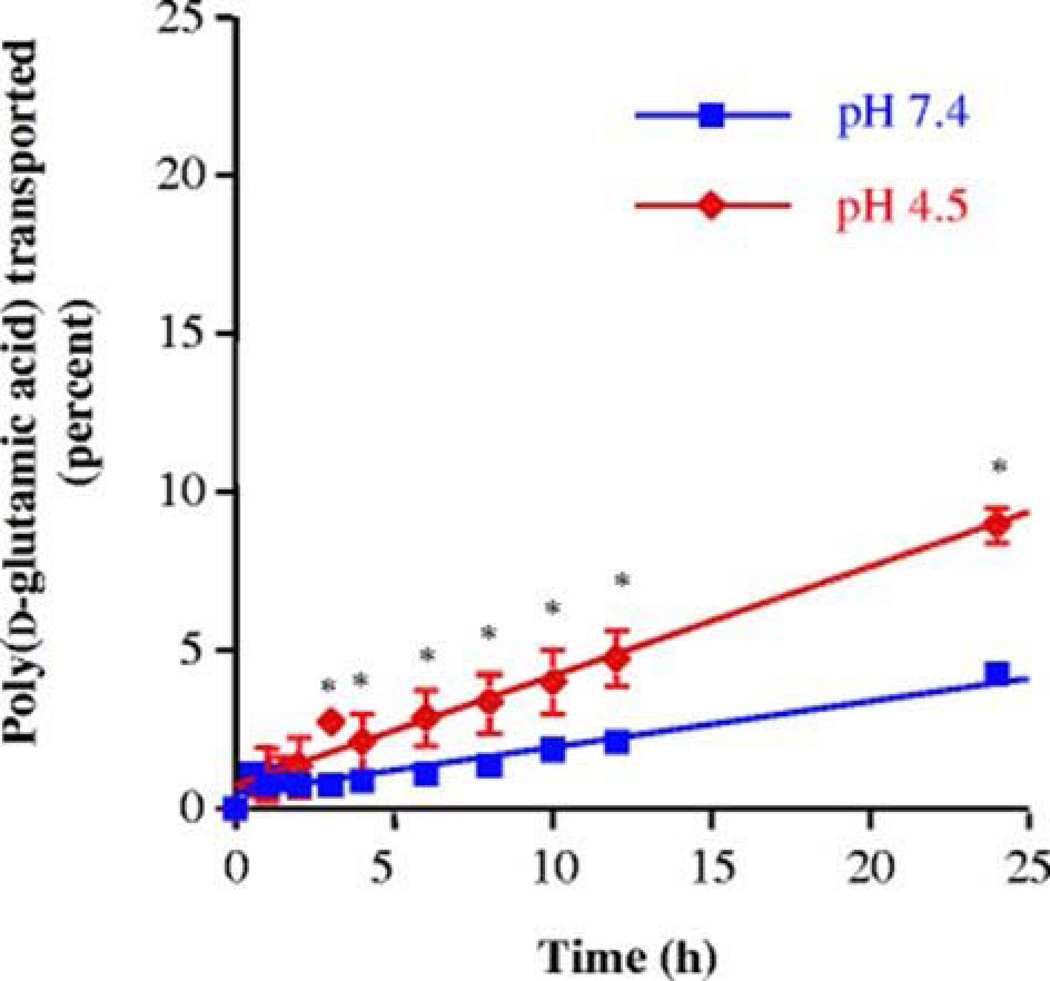
The tight epithelial junctions of colon are impermeable to molecules with radii larger than 8–9 A°. However, in case of polypeptides with high conformational flexibility it is possible that even larger molecules can diffuse through the tight junctions (Tomita et al., 1988). Chittchang et al. (Chittchang et al., 2002) studied the effect of secondary structure on the aqueous diffusion of a model peptide poly(L-lysine) through a microporous membrane. This study concluded that the change in secondary structure of poly(L-lysine) from the random coil to the α-helix did not alter apparent permeability (Papp) and intrinsic diffusion coefficient (Daq). However, the β–sheet conformer significantly lowered Papp and Daq values. This result was attributed to higher solution viscosity and extended β– sheet structure of poly(L-lysine). In another study, Chittchang et al. (Chittchang et al., 2007) examined the effect of secondary structure and charge of a model polypeptide, poly(d-glutamic acid) on its permeability through negatively charged pores of synthetic porous membranes and Caco-2 cell monolayers. Poly(d-glutamic acid) exists as a highly negatively charged random coil conformer at neutral pH and below pH 5.0 it changes to α-helix conformer. Transport studies across Caco-2 cell monolayers revealed higher permeability of poly(d-glutamic acid) at pH of 7.4 (Fig. 2), while a completely opposite trend was observed in the moderately hindered diffusion case (Fig. 3). This observation may be due to the effect of electric field that plays a significant role in the permeation of solutes which are small relative to the pores.
However, for large molecules sieving through the pores is dependent mainly on the molecular size which dominates the influence of electric field. This study concluded that charge and secondary structure of polypeptides play a significant role in determining the rate of aqueous diffusion in a hindered diffusion model. Dodoo et al. (Dodoo et al., 2000) studied the permeability of 14 synthetic model peptides labeled with an amino acid fluorophore on rat alveolar cell monolayers cultured on permeable supports. The results indicated that the peptides entered cells primarily via paracellular route and Papp values were inversely proportional to the molecular size. Scientists have investigated the role of paracellular route in the absorption of peptides such as potent analogs of vasopressin octreotide (Jaehde et al., 1994), thyrotropin-releasing hormone (TRH) (Thwaites et al., 1993), salmon calcitonin (Guggi et al., 2003; Lee and Sinko, 2000) and peptidomimetic renin inhibitors (Walter et al., 1995). Novel strategies such as modification of drug molecule and modulation of tight junctions associated with the paracellular pathway were investigated to increase the penetration of macromolecules (Lane and Corrigan, 2006).
Transcellular transport: This process involves the diffusion of drug molecules through the apical and basolateral membranes. This route is ideal for lipophilic drugs which express relatively high affinity for the lipid bilayer of cell membrane. Many theoretical models based on molecular size, charge, hydrogen bonding, confirmation and lipophilicity have been developed to study transcellular transport of drugs molecules (Rautio et al., 2008). Since cell membrane consists of lipid bilayer, it is widely accepted that lipophilicity plays an important role in determining the transport mechanism. However, early in vivo studies concluded that the intestinal absorption dimishes when lipophilicity is very high (usually log P > 5) (Catnach et al., 1994). Burton et al. (Burton et al., 1996) studied the effect of lipophilicity, chain length and number of polar groups on the transport of model peptides in Caco-2 cell monolayers. Interestingly it was observed that the permeability of peptide depends on the number of polar groups that require desolvation before diffusion of peptide into the cell membrane rather than lipophilicity as observed in small organic molecules. Corandi et al. (Conradi et al., 1991) studied the relationship between structure and permeability of neutral and zwitterionic peptides prepared from D-phenylalanine and glycine across Caco-2 cell monolayers. The lipophilicity (log P) of peptides varied from −2.2 to +2.8. The results indicated no apparent correlation between the apparent lipophilicity and observed flux. Moreover, a strong correlation was noted for the flux of neutral series and the total number of possible hydrogen bonds of the peptide with water molecules. These results clearly indicate that the passive transcellular absorption of a peptide depends on the energy required to break water-peptide hydrogen bonds so the molecules can enter the cell membrane.
Fig. 1.
Intestinal drug transport mechanisms.
Fig. 2.
Percentages of FITC-labeled poly(d-glutamic acid) transported across a Caco-2 cell monolayer at 37°C. Asterisks indicate a significant difference (P < 0.05) compared to pH 4.5. The TEER values before and after the transport experiments of poly(d-glutamic acid) at pH 4.5 were not significantly different from each other (i.e., 189.7 ± 13.8 and 185.6 ± 11.1 Ω cm2, respectively), which confirms that the integrity of Caco-2 cell monolayers was not affected after having been exposed to pH 4.5 for 3 hr (Reproduced with permission from reference (Chittchang et al., 2007).
Fig. 3.
Percentages of poly(d-glutamic acid) transported across a track-etched polycarbonate membrane with an average pore diameter of 0.015 µm at 37°C. Asterisks indicate a significant difference (P<0.05) compared to pH 7.4 (Reproduced with permission from reference (Chittchang et al., 2007).
Carrier mediated transport: This mechanism involves the movement of small molecules, or macromolecules via membrane proteins (transporters). This is also known as facilitated diffusion or active transport process. It has been well established that intestinal absorption of di- and tri-peptides occurs via carrier mediated peptide transport systems. The presence of peptide transport system in mammalian gut was first reported by Newey and Smyth in 1959 (Newey and Smyth, 1959). These oligopeptide transporters also help in the absorption of peptidomimetics such as amino-β-lactam antibiotics, renin-inhibitors, and angiotensin converting enzyme inhibitors (Bai and Amidon, 1992). Detailed understanding about the structural features of the peptide is required to target these transporters for protein delivery.
4. Formulation strategies for increasing the oral bioavailability of peptides and proteins
4.1. Absorption enhancers
Absorption enhancers are substances that enhance or promote absorption of drugs for enhancing oral bioavailability (Aungst, 2012; Checkoway et al., 2012; Jitendra et al., 2011; Williams and Barry, 2004). Peptide and protein drugs are hydrophilic in nature with large molecular weight, hence absorption through transcellular and paracellular routes is severely limited (Shaji and Patole, 2008). Absorption enhancers’ act by several mechanisms: a) temporarily disrupting the structural integrity of the intestinal barrier, b) decreasing the mucus viscosity, c) opening the tight junctions and d) increasing the membrane fluidity (Liu et al., 1999). These compounds allow therapeutic agents to permeate across biological membranes into systemic circulation and reach the site of action to exert pharmacological effect (Shaji and Patole, 2008). However, the selection of absorption enhancer and its efficacy depends on the physicochemical properties of the protein and peptide drugs, regional differences in intestinal membrane, nature of the vehicle and other excipients. Permeation enhancers should be safe and non toxic, pharmacologically and chemically inert, non-irritant, and non-allergenic (Jitendra et al., 2011; Senel and Hincal, 2001). Various absorption enhancers have been investigated for the enhancement of protein and peptide absorption through the intestinal membrane. These can be grouped into surfactants, chelating agents, bile salts, cationic and anionic polymers, acylcarnitines, fatty acids and their derivatives (Table 1). These enhancers in combinations show synergistic effect than the individual enhancers, particularly with specific combinations such as mixture of sodium salicylate and peanut oil (Yang, 1999). Chelators generally act by complex formation with calcium ions and facilitate permeation of proteins through paracellular opening. Protein and peptide delivery can be enhanced by micellar protection or membrane disruption by solubilization of membrane phospholipids. Absorption of human calcitonin in rats was significantly increased by co-administration with surfactants, such as sodium myristate, sodium deoxycholate, sodium taurodeoxycholate, sodium lauryl sulfate, quillaja saponin, and sugar esters. These compounds can be used as absorption enhancers for oral peptide and protein absorption (Nakada et al., 1988). However, absorption enhancers are not protein specific, and risk of toxin or allergen import along with the proteins might be possible. It may result in unwanted side effects. For example, calcium chelators can lead to Ca2+ depletion, which can disrupt actin filaments, modify adherent junctions and reduce cell adhesion (Sood and Panchagnula, 2001).
Table 1.
Commonly used absorption enhancers and their mechanisms of action.
| Category | Examples | Mechanism of action |
|---|---|---|
| Bile salts | Sodium Deoxycholate, Sodium Taurocholate, Sodium Glycodeoxycholate, Sodium Taurodihydrofusidate, Sodium Glycodihydrofudisate | Form reverse micelles and disrupt membrane, open up tight junctions, enzyme inhibition and mucolytic activity. |
| Chelators | EDTA, Citric acid, Salicylates | Interferes with calcium ions, chelation disrupts intracellular junctions and decreases transepithelial electrical resistance. |
| Surfactants | Sodium Lauryl Sulfate, Laureth-9, Sodium Dodecylsulfate, Sodium Taurodihydrofusidate, Poly Oxyethylene Ethers | Perturbation of intercellular lipids, lipid order, orientation and fluidity. Inhibition of efflux mechanisms. |
| Fatty acids and Derivatives | Oleic Acid, Linoleic Acid, Caprylic Acid, Capric Acid, Acyl Carnitines, Mono and Di-Glycerides | Increase fluidity of phospholipid membranes, contraction of actin myofilaments, opening of tight junctions |
| Cationic Polymers | Chitosan and its Derivatives | Combined effect of mucoadhesion and opening of tight junctions via ionic interactions with the cell membrane. |
| Anionic Polymers | Carbopol and Polyacrylic Acid Derivatives | Combined effect of enzyme inhibition and opening of tight junctions through removal of extracellular calcium ions. |
| N-Acetyl Cysteine | Reduce the viscosity of mucus layer by breaking down disulfide bonds. | |
| Acylcarnitines | Lauroyl-L-Carnitine Chloride, Palmitoylcarnitine Chloride | Membrane disruption, Opening of tight junctions with a calcium independent mechanism |
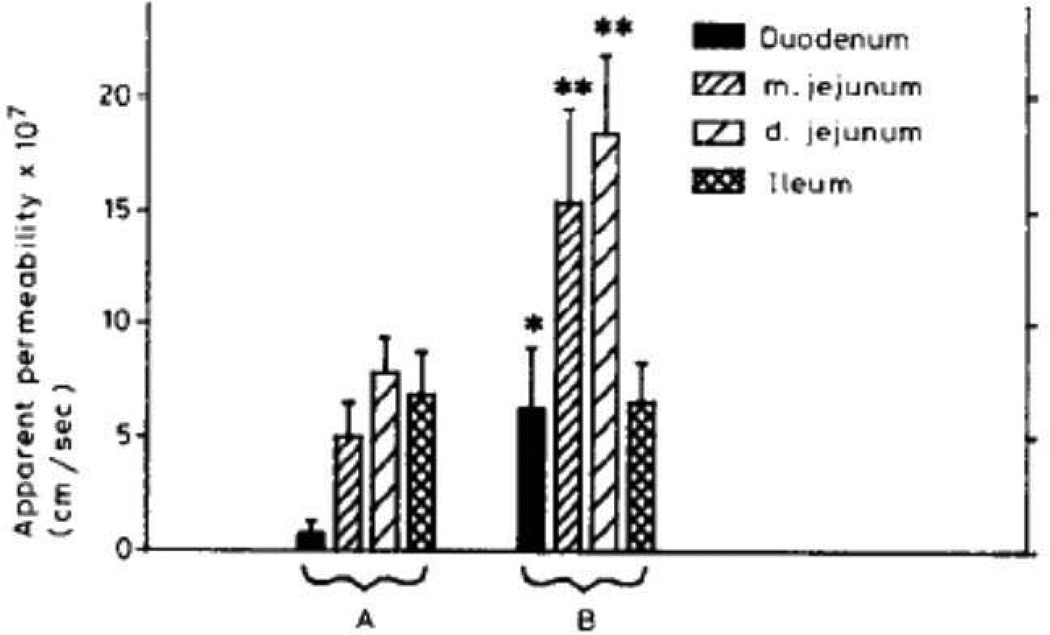
Chitosans have also been studied for their enhanced permeation effect and their effect depends on the charge density and structural features of chitosan salts such as N-trimethyl chitosan chloride (Thanou et al., 2001) . More recently, poly unsaturated fatty acids were added to increase cortisol transport in a BBB model through membrane fluidization (Navarro et al., 2011). Peroral delivery of therapeutics such as salmon calcitonin, interferon α, insulin, and recombinant human growth hormone have been shown to be improved by the use of N-acetylated, non-α aromatic amino acids and N-acylated, α-amino acids. This effect has been attributed to non-covalent interaction between peptide and protein drugs and permeation enhancers (Leone-Bay et al., 2001; Sood and Panchagnula, 2001). Prolonged use of salicylates, chelators, bile salts and surfactants may cause local irritation and mucosal damage (Sithigorngul et al., 1983; Swenson et al., 1994; Yamamoto et al., 1992). Chitosans (Artursson et al., 1994; Schipper et al., 1996; Schipper et al., 1999), acylcarnitines (Lecluyse et al., 1993) and dihydrofusidates (Hurni et al., 1993) appear to be relatively safer than other absorption enhancers. Since the oral route of administration is the most convenient and acceptable method of chronic drug administration, studies have been performed to examine insulin absorption from small intestine. Mixed micellar solutions were examined as a means for enhancing the fluidity of the mucosal membrane and subsequent increase in insulin absorption. Potential role of bile salt/fatty acid mixed micelle in gastrointestinal absorption was also explored. A mixture of sodium glycocholate and linoleic acid in the ratio of 3:1 was used in the preparation of mixed micelles. Use of mixed micellar solutions significantly increased the insulin permeability through the everted gut sacs in the duodenum, medial and distal jejunum but there was insignificant change in the ileum. Insulin absorption was significantly enhanced in the duodenum and jejunum, with the highest overall apparent permeability observed in the jejunum, where addition of mixed micelles to the buffer solution containing insulin increased Papp from about 7 × 10−7 to (1.5–1.8) × 10−6 cm/s (Fig. 4) (Schilling and Mitra, 1990). Interestingly, experiments with intact gut sacs showed no significant degradation of insulin exposed to either the mucosal or serosal tissues. Mixed micelle solutions, employed as absorption enhancers significantly enhanced cumulative amount of insulin transported across the intestinal mucosa.
Fig. 4.
Apparent permeability (mean+S.E.) of insulin across everted gut sac segments, with mucosal solutions of 83.3 µM insulin in: A, modified buffer solution (n = 5); or B, modified buffer solution with 3:1 sodium glycocholate/linoleic acid mixed micelles (n = 4). For A versus B, *p<0.1; **p<0.05 (Reproduced with permission from reference (Schilling and Mitra, 1990).
A recent study investigated the combined effect of absorption enhancers and electrical assistance on transbuccal delivery of salmon calcitonin (sCT) using fresh swine buccal tissue (Oh et al., 2011). The effect of enhancers on sCT buccal permeation was investigated in the presence of 10% of ethanol, various concentrations (1%, 2% and 5%) of N-acetyl-L-cysteine (NAC), sodium deoxyglycocholate (SDGC), and a mixture of enhancers. The absorptive flux of sCT in the ethanol group was enhanced compared to the control group, although not statistically significant. Presence of NAC enhanced sCT permeation, and the enhancing effect was not significant but apparently NAC concentration dependent. Further, the optimal concentration of SDGC for transbuccal sCT delivery was reported to be about 1% SDGC. The combination of 1% SDGC with 10% ethanol and 5% NAC with 10% ethanol showed the maximum drug permeation.
It is well known that oral delivery of proteins and peptides generally involves the coadministration of enzyme inhibitors and/or penetration enhancers, to inhibit proteolytic enzymes and promote the macromolecule permeation, respectively (Sinha et al., 2007). Controlling the release of enzyme inhibitors and/or penetration enhancers prior to the release of a macromolecule might enhance the effectiveness of these adjuvants and establish a more favorable environment. Therefore, a two-pulse delivery system was recently reported which could facilitate adjuvant(s) release prior to the drug release and reduce susceptibility to enzymatic degradation and/or improve mucosal permeation (Del Curto et al., 2011). This delivery platform consisted of (a) drug in a fast disintegrating core, (b) an inner low viscosity HPMC coating, (c) an intermediate layer composed of enzyme inhibitor/absorption enhancer, and (d) an outer additional HPMC coating (Fig. 5). An external gastroresistant film would also be envisaged to overcome the highly variable stomach residence and facilitate time-dependent colon delivery. The outer HPMC coat assists in delaying the adjuvant release from the intermediate layer offering the in vivo lag phase of 3–4 hr in colon, while the inner low viscosity HPMC coating is expected to delay the release of macromolecule with respect to that of the adjuvant.
Fig. 5.
Schematic illustration of the two-pulse delivery system: (a) protein-containing core, (b) inner HPMC coating, (c) intermediate enzyme inhibitor/absorption enhancer, and (d) outer HPMC coating. Reproduced with permission from reference (Del Curto et al., 2011).
4.2. Enzyme Inhibitors
Co-administration of protease inhibitors can lower the enzymatic barrier and prevent degradation of proteins and peptides in the GI tract thereby facilitating intestinal absorption (Mahato et al., 2003; Malik et al., 2007; Werle et al., 2009). Structure of the molecule and their specificity towards enzymes is crucial to evaluate the stability of protein and peptide drugs (Bernkop-Schnurch, 1998). Inhibitors such as aprotinin (trypsin/chymotrypsin inhibitor), amastatin, bestatin, boroleucine, and puromycin (aminopeptidase inhibitors) have been widely employed. Naturally occurring inhibitors are comparatively non-toxic and among them aprotinin has been widely used for oral peptide delivery. Co-administration of insulin along with trypsin and chymotrypsin inhibitors enhanced oral bioavailability of insulin (Danforth and Moore, 1959; Kidron et al., 1982; Laskowski et al., 1958; Yamamoto et al., 1994). Yamamoto et al. studied the effect of enzyme inhibitors such as sodium glycocholate, camostat mesilate, bacitracin, soybean trypsin inhibitor, and aprotinin on the intestinal metabolism of insulin in rats. Sodium glycocholate, camostat mesilate, and bacitracin were found to be more efficient in improving the physiological availability of insulin in the large intestine. A profound increase (22-fold) in the oral bioavailability of pentapeptide enkephalin YAGFL [Tyr-Ala-Gly-Phe-Leu], with D-conformation of alanine and leucine amino acids was shown in the presence of peptidase inhibitor amastatin (Lee and Amidon, 2002). Most natural inhibitors have to be co-administrated excessively in large amounts because these compounds are susceptible to enzymatic degradation in gut. Even large amounts of these inhibitors may not be adequate to reduce protease activity which may necessitate the encapsulation of proteins and peptides in nano-drug delivery systems. On the other hand, chronic and prolonged usage of these inhibitors may result in high toxicity. It may also affect the absorption of other proteins that normally would be degraded. A major drawback associated with these inhibitors is that the non-site specific activity of such compounds will noticeably alter the metabolic pattern in the GI tract and intestinal membrane primarily due to reduced digestion of food proteins (Watanabe et al., 1992).
4.3. Hydrogels
Hydrogels are three-dimensional mesh like networks containing hydrophilic polymers that imbibe large amounts of water and form a gel like matrix as a result of physical or chemical cross-linking of individual polymer chains. Synthetic hydrogels present a possibly efficient and convenient way to administer peptides and proteins (Ichikawa and Peppas, 2003; Peppas et al., 2000; Ridgley and Wilkins, 1991). Hydrogels can be broadly classified into i) neutral hydrogels, and ii) ionic hydrogels. Ionic hydrogels are composed of pendant groups that ionize in response to variation in surroundings, making the hydrogel network bulge as it becomes more hydrophilic. Hydrogels that are capable of responding to surrounding stimuli such as temperature, ionic strength variation and pH alteration are known as physiologically-responsive hydrogels (Lowman et al., 1999; Peppas et al., 2000). Hydrogels made from natural polymers are biodegradable and biocompatible. However, these materials do not have adequate mechanical strength and may contain pathogens which may elicit auto-immune response. Synthetic hydrogels do not exhibit these intrinsic bioactive properties and can be tailored to yield hydrogels with desired degradability and functionality. There are two distinct groups of hydrogels, viz. performed and in-situ forming gels. Performed hydrogels are simple viscous solutions which do not undergo any alterations in their structure or properties after administration. While, in situ forming gels undergo gelation on administration due to changes in physicochemical properties depending on the environment (Gratieri et al., 2010; Nanjawade et al., 2007). The polymer network can be either homopolymers or copolymers, with the chemical structure determining the properties of hydrogel. Monomers widely used in preparation of hydrogels for protein delivery are 2-hydroxyethyl methacrylate, ethylene glycol dimethacrylate, N-isopropyl acrylamide, acrylic acid and methacrylic acid. Poly(ethylene glycol) (PEG) and poly(vinyl alcohol) are two other polymers that have been employed in hydrogels. One important parameter to be considered is the mesh size which is the distance between cross-links in the hydrogel network. Any change in the mesh size may modify the diffusion pattern of a therapeutic protein from the hydrogel matrix (Morishita et al., 2002; Torres-Lugo et al., 2002). Ichikawa et al. (Ichikawa and Peppas, 2003) designed poly [methacrylic acid-grafted-poly (ethylene glycol)] [P(MAA-g-EG)], a complexation hydrogel for oral delivery of insulin. This study examined cytotoxicity and insulin-transport enhancing effect of P(MAA-g-EG) hydrogel microparticles on intestinal epithelial cells. Results from these studies revealed that the P(MAA-g-EG) hydrogel microparticles are cytocompatible and have transport-enhancing effect of insulin on intestinal epithelial cell.
Recent study by Kamei et al. (Kamei et al., 2009a)on complexation of hydrogels P(MAA-g-EG) have shown high insulin encapsulation efficiency and rapid insulin release in the intestine in a pH-dependent manner. Further studies were carried out with other protein drugs such as calcitonin and interferon β. Incorporation efficiency of hydrogels were high with insulin, calcitonin, and interferon β. Moreover, polymeric microparticles loaded with calcitonin and interferon β displayed complexation/decomplexation and pH-sensitive release pattern. A dose-dependent augmentation of plasma interferon β levels and drastic reduction in plasma calcium levels accompanied with calcium absorption were observed after administration of particles loaded with interferon β or calcitonin into closed rat ileal segments. These findings imply that P(MAA-g-EG) hydrogels can be utilized as potential carriers for oral delivery of peptides and proteins.
4.4. Mucoadhesive systems
These systems comprise of synthetic or natural polymers that can bind (adhere) to biological substrates such as mucosal membranes. The phenomenon of bioadhesion allows a greater amount of drug to be available at the target site resulting in desired therapeutic effect. The ability of the mucoadhesive polymers to adhere to mucin layer on the mucosal epithelium can improve oral bioavailability of protein and peptide therapeutics. Drug delivery systems comprising bioadhesive polymers are known to reduce the rate of clearance of drug molecules from the absorption site, thus prolonging the time available for absorption. Bioadhesive drug delivery systems also offer a controlled release of drugs and thus can reduce the frequency of drug administration. Increased oral bioavailability via delayed gastrointestinal transit induced by bioadhesive polymers was shown for the first time by Longer et al (Longer et al., 1985). A mucoadhesive polymer should possess ideal characteristics (Guo and Cooklock, 1996; Peh and Wong, 1999; Shaikh et al., 2011):
It should be hydrophilic in nature and be able to form strong adhesive bonds with mucosal membranes because of the presence of large amounts of water in the mucus layer.
Polymers with a high molecular weight are desirable because they provide more bonding sites.
It should possess optimum surface tension which can enable spreading of polymers onto mucosal/ epithelial cell layer.
It should contain adequate hydrogen bond – forming groups such as -OH and -COOH groups that provide strong adhesive bonds between the entangled polymer chains.
It should be non-irritant, non-toxic and non-allergenic in nature.
It should be chemically inert and may not react with the oral epithelium or the protein/peptide drugs.
The cost of the polymer should not be high, so that the final product remains competitive in the market place.
Mucoadhesive polymers were also found to inhibit proteolytic enzymes and/or modulate the permeability of tight epithelial tissue barriers (Lehr, 1996). Bioadhesive polymers are generally classified into synthetic or semi-natural. Synthetic bioadhesive polymers are either polyacrylic acid or cellulose derivatives. Polyacrylic acid-based polymers include carbopol, polycarbophil, polyacrylic acid, polyacrylate, poly(methylvinylether-co-methacrylic acid), poly(2-hydroxyethyl methacrylate), poly(methacrylate), poly(alkylcyanoacrylate), poly(isohexylcyanoacrylate) and poly(isobutylcyanoacrylate). Examples of cellulose derivatives are carboxymethyl cellulose, hydroxyethyl cellulose, hydroxypropyl cellulose, sodium carboxymethyl cellulose, methyl cellulose, and methylhydroxyethyl cellulose. Chitosan and various gums such as guar, xanthan, crylamide-acrylate polymer (PHPA), poly (vinylpyrrolidone), and poly (vinyl alcohol) constitute semi-natural bioadhesive polymers. A wide range of bioadhesive formulations have been investigated for the oral cavity. For instance, luminal degradation of insulin, calcitonin, and insulin-like growth factor-I (IGF-I) by trypsin and chymotrypsin was inhibited by employing carbopol polymers (Bai et al., 1996). Site specific targeting was achieved with lectins, that possess high affinity for carbohydrate binding with Kd values of 104–106, high diffusion coefficients, and high resistance to proteolytic breakdown. Lectins prefer binding to receptors on the cell surface rather than mucosal gel layer (Haas and Lehr, 2002). A significant enhancement in intestinal absorption of 9-desglycinamide, 8-arginine vasopressin (DGAVP) was observed in rats using the weakly cross-linked poly(acrylate) derivative (polycarbophil) dispersed in physiological saline (Haas and Lehr, 2002). Surface conjugation of the bioadhesive agent, tomato lectin demonstrated higher intestinal uptake of orally administered inert nanoparticles in rats (Hussain et al., 1997). Peptide and protein drugs formulated with chitosan–EDTA conjugates inhibited peptide and protein drugs from enzymatic degradation across the GI tract and greatly enhanced their oral bioavailability (Bernkop-Schnurch and Krajicek, 1998). Binding patterns associated with wheat germ agglutinin (WGA) and peanut agglutinin (PNA) to glycoproteins in human and rodent colon were examined in gastrointestinal diseases. The authors also investigated the feasibility of utilizing N-(2-hydroxypropyl)methacrylamide (HPMA) copolymer-lectin-drug conjugates to deliver therapeutic agents. This report suggested that HPMA copolymer-lectin-drug conjugates provide site-specific treatment of conditions such as colitis and Barrett’s esophagus (Wroblewski et al., 2000).
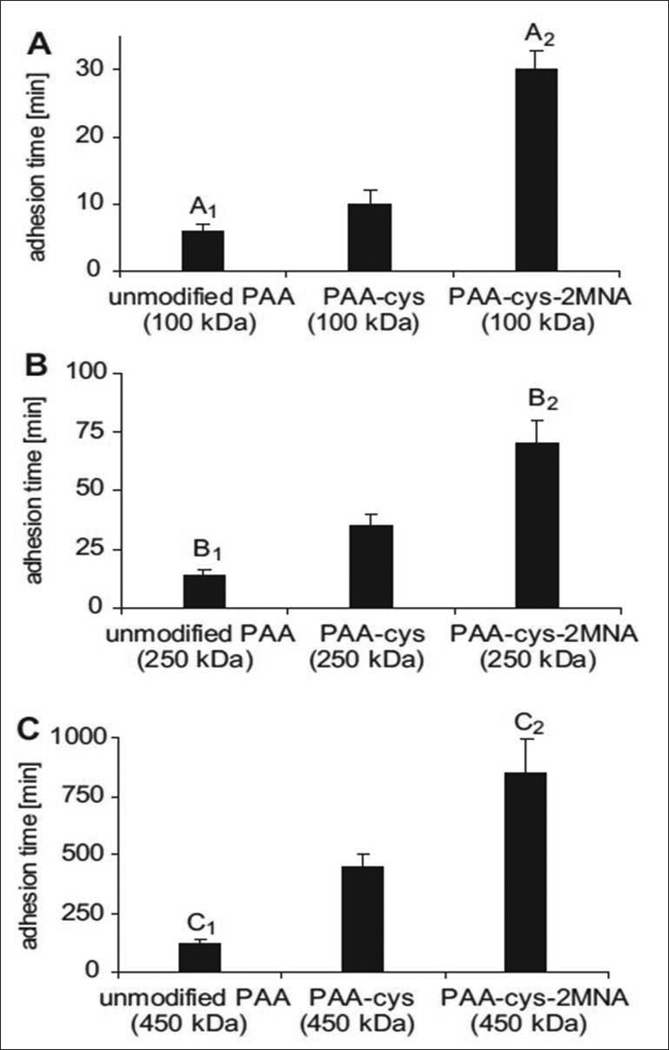
Thiolated polymers (thiomers) have been widely employed as mucoadhesive polymers for enhancing oral delivery of hydrophilic macromolecular drugs (Bernkop-Schnurch et al., 2004). Unlike other mucoadhesive polymers, thiomers form covalent bonds with cysteine-rich subdomains of mucus glycoproteins via thiol/disulfide exchange reactions. These polymers form stronger covalent interactions than other non-covalent bonds such as hydrogen bonds, Van der Waal’s forces and ionic interactions of polymer with anionic substructures of the mucus membranes (Bernkop-Schnurch et al., 2006; Iqbal et al., 2012). Furthermore, thiolated polymers act as enzyme inhibitors, permeation enhancers and efflux pump inhibitors. These polymers are also capable of protecting the incorporated peptides and protein drugs against enzymatic degradation in the intestine. However, stability of thiomers in solutions and gels is a major concern which may reduce the efficacy of thiomers. These polymers are susceptible to early oxidation (pH ≥ 5) unless protected under inert conditions. A recent study aimed at utilizing pre-activated thiol groups to facilitate better stability, prolong retention time of dosage forms, offer mucoadhesion in order to enhance uptake and oral bioavailability (Iqbal et al., 2012). Poly(acrylic acid)-cysteine-2-mercaptonicotinic acid (PAAcys-2MNA) conjugates were synthesized by the oxidative disulfide coupling of PAA-cys (100-, 250- and 450 kDa) with 2-mercaptonicotinic acid (2MNA). In vitro mucoadhesion studies revealed that immobilization of thiol groups on PAA (100, 250 and 450 kDa) exhibited 1.7-, 2.5- and 452-fold improvement in mucoadhesive properties, respectively. Tablets based on PAA-cys-2MNA (100, 250 and 450 kDa) conjugates displayed 5.0-, 5.4- and 960-fold improvement in the mucoadhesion time relative to corresponding unmodified PAAs (Fig. 6). Results from in vitro permeation studies displayed the permeation enhancement ability for preactivated thiomers and was ranked as follows: PAA(450)-Cys-2MNA (h) >PAA(250)-Cys-2MNA (h) > PAA(100)-Cys-2MNA (h) on both Caco-2 cells and rat intestinal mucosa. Also, the apparent permeability of sodium fluorescein was observed to be 5.08-fold higher in Caco-2 cells for PAA(450)-Cys-2MNA (h) and 2.46-fold higher on intestinal mucosa for PAA(450)-Cys-2MNA (m), respectively, relative to sodium fluorescein in buffer only (Wang et al., 2012). Such enhancement in permeability as well as better stability render preactivated thiomers as promising macromolecular permeation enhancers and mucoadhesive polymers and may be suitable for non-invasive drug administration.
Fig. 6.
Comparison of the mucoadhesive properties of unmodified, thiolated and preactivated poly acrylates with an average molecular mass of A: 100 kDa; B: 250 kDa and C: 450 kDa as determined by the rotating cylinder method. Reproduced with permission from reference (Iqbal et al., 2012).
4.5. Liposomes
Liposomes are defined as microscopic vesicles composed of one or more phospholipid bilayer membranes with a diameter ranging from 0.0 −10µm. These are lipid-based delivery systems which offer some degree of protection for peptides and proteins in GI tract (Li et al., 2010). Liposomes have been successfully applied in the delivery of wide range of therapeutics including nucleotides, proteins and plasmids (Kurz and Ciulla, 2002). Degradation of proteins and peptides can be prevented by encapsulation in liposomal bilayers. Depending on the size, liposomes are classified into small uni-lamellar vesicles (SUV) (10–100nm), large uni-lamellar vesicles (LUV) (100–300nm) and multi-lamellar vesicles (more than one bilayer). Liposome consists of aqueous inner core enclosed by a membrane, composed of phospholipids. Hydrophilic drugs can be encapsulated in the inner aqueous core while the hydrophobic drugs tend to stay in lipid bilayer (Kaur et al., 2004). Vesicles are made of naturally derived phospholipids such as egg phosphatidylethanolamine or dioleoylphosphatidylethanolamine (DOPE), phosphotidyl choline or phosphotidyl inositol (Dharma et al., 1986). Among various types of liposomes, dehydrated-rehydrated vesicles are generally used in protein drug delivery due to ease of preparation and low stress exerted on proteins (Kisel et al., 2001). Liposomes can be used for site specific delivery of peptides by decorating surface groups with targeting moieties such as antibodies. Various parameters such as composition of the liposomes, encapsulation efficiency, rate of drug release from lipid vesicles, size and surface charge are important for effective delivery of peptides from liposomes. Though liposomes are generally considered to be stable carriers, some drawbacks exist such as: a) extensive leakage of water-soluble drugs during GIT passage, b) heterogeneity in the vesicular size, and c) instability of formulations. Scientists have come up with new liposomal formulations viz., archeosomes which could address these issues. Archaeosomes are liposomal formulations that are prepared with archaeobacterial membrane lipids mainly composed of diether and/or tetraether lipids (Patel et al., 2000). These archaeobacterial lipids present unique features over conventional liposomes (Sprott, 1992). Archeosomes are proven to be substantially more stable against extreme pH and oxidation, and also against the action of bile salts and lipases (Sprott et al., 2003). Li et.al (Li et al., 2010) studied the potential of archaeosomes as vehicles for oral delivery with insulin as a model peptide. Archaeosomes were prepared from polar lipid fraction E (PLFE) purified from Sulfolobus acidocaldarius. The pharmacodynamics of insulin encapsulated archaeosomes was evaluated in diabetic mice following oral administration. Archaeosomes made of PLFE were comparatively stable in simulated GI tract conditions and also facilitated slow passage of fluorescently labeled peptide in the GI tract in vivo. Archaeosomes controlled blood glucose levels more tightly relative to conventional liposomes. Hence archeosomes can be considered as better carriers for protein and peptide delivery due to higher stability. Mohanraj et.al (Mohanraj et al., 2010) developed hybrid silica-liposome nanocapsule (SNLC) system containing insulin. Capabilities of silica-liposome nanocapsule to shield against lipolytic degradation and prolong insulin release in simulated GI conditions were studied. These new carriers protected insulin from enzymatic degradation in presence of digestive enzymes. Liposomal drug release kinetics and stability of vesicles can be controlled by coating with a specifically tailored nanoparticle layer. SNLC is a promising candidate for storage and delivery of proteins and peptides. Thirawong et.al (Thirawong et al., 2008) prepared self-assembling pectin-liposome nanocomplexes (PLNs) by mixing cationic liposomes with pectin solution for improving intestinal absorption of calcitonin (eCT). The eCT-loaded PLNs exhibited good pharmacological action compared to eCT solution and eCT-loaded liposomes. The enhancing effect was attributed to the ability of pectin to adhere to the mucosal layer and extend the retention with the intestinal mucosa.
4.6. Nanoparticles
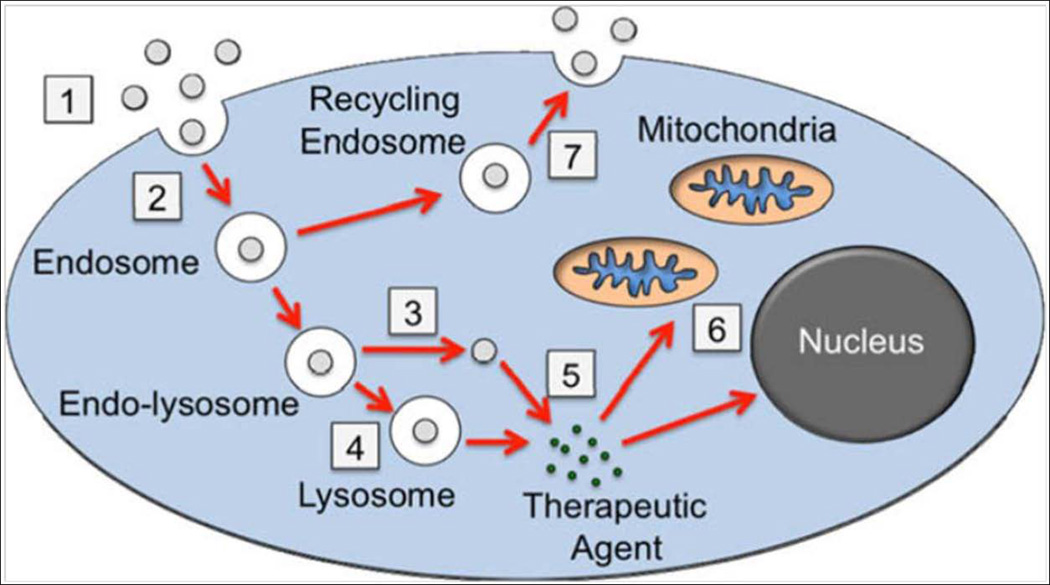
Nanoparticles are colloidal carriers with size ranging between 1 to 1000 nm, nevertheless, particles with size greater than 200 nm are profoundly pursued (Kreuter, 1994; Yun et al., 2012). Nanoparticles can be broadly classified into two types, nanospheres and nanocapsules. Nanospheres are matrix systems in which drug is uniformly physically dispersed, whereas, nanocapsules are vesicles in which drug is encapsulated by a polymeric membrane. The physicochemical and drug release properties of nanoparticles vary with the preparation method (Singh and Lillard, 2009; Yun et al., 2012). Compared to other colloidal carriers such as liposomes and micelles, most of the nanoparticles are stable in the harsh GI environment. They can be tailor made to target certain tissue and achieve controlled drug release by altering the polymer features and surface chemistry (Panyam and Labhasetwar, 2003). Nanoparticles are taken up by cells via endocytosis process, which includes three subtypes phagocytosis, pinocytosis, and receptor-mediated endocytosis. Phagocytosis involves the assimilation of materials up to 10 µm in diameter especially by macrophages, neutrophils, and dendritic cells. Pinocytosis is a cellular uptake mechanism, generally involves absorption of sub-micron material and substances in solution and its conducted by all cell types. Figure 7 explains the process of endocytosis and fate of nanoparticle after internalization into cell cytoplasm. First, the nanoparticles associate with the cell membrane and subsequently are endocytosed. Then nanoparticles escape from endosomes and degrade in the lysosome. Finally, therapeutic agent diffuses out from lysosome into cytoplasm and transport of therapeutic agent to target organelle takes place which is then followed by exocytosis of nanoparticles (Faraji and Wipf, 2009). Captivating the benefit of varying pH in the GI tract, pH sensitive nanoparticles can be tailor-made to deliver peptides and proteins to different parts of the intestine. Such nanoparticles are essentially prepared with either polyanionic or polycationic polymer and their mixtures.. Mechanism of drug release from nanoparticles is mainly based on the drug dissolution property, swelling pattern of polymer or both of these at a particular pH. Nanoparticles can enhance drug stability, augment mucoadhesion, extend the residence time in GI tract, enhance intestinal permeability and improve the saturation solubility and dissolution rate. Most pH sensitive carriers have been widely used as enteric coating materials for a prolonged period of time and their safety has been approved. More recently, diethylene triamine pentaacetic acid (DTPA) is a complexing agent, known to disrupt intestinal tight junctions and prevent intestinal proteases by chelating divalent metal ions. Su et.al has made an attempt to incorporate DTPA in functionalized nanoparticles (NPs) for oral delivery of insulin. DTPA was conjugated to poly(γ-glutamic acid) (γPGA) to maintain the complexing agent concentrated on the intestinal mucosal surface, where enzyme inhibition and paracellular permeation enhancement are vital. NPs were prepared by mixing anionic γPGA-DTPA conjugate and cationic chitosan (CS). The γPGA-DTPA conjugate inhibited the activity of intestinal proteases significantly, and made a transient and reversable enrichment of paracellular permeability. The NPs were responsive to pH alterations, CS/γPGA-DTPA NPs swelled with increasing pH and disintegrated above pH 7.0. Furthermore, the biodistribution of orally delivered insulin by CS/γPGA-DTPA NPs in rats was observed by confocal microscopy and scintigraphy. Experimental results showed higher absorption of insulin from CS/γPGA-DTPA NPs and absorbed insulin was evidently noticed in the kidney and bladder. CS/γPGA-DTPA NPs have produced a prolonged reduction in blood glucose levels; the oral intake of enteric-coated capsule containing CS/γPGA-DTPA NPs had shown maximum insulin levels at 4 hr after treatment. The relative oral bioavailability of insulin was approximately 20%. Results from this study clearly indicated the potential role of NPs in delivering insulin by oral route (Su et al., 2012).
Fig. 7.
Steps detailing the cytosolic delivery of therapeutic agents via nanoparticles carriers. (1) Association of nanoparticles with cell membrane, (2) internalization of nanoparticles by endocytosis, (3) escape of nanoparticles from endosomes, (4) degradation of nanoparticle in lysosome, (5) diffusion of therapeutic agent from lysosome into cytoplasm, (6) cytoplasmic transport of therapeutic agent to target organelle, (7) exocytosis of nanoparticles. Reproduced with permission from reference (Faraji and Wipf, 2009).
Targeted drug delivery is a novel approach for augmenting the oral absorption and hypoglycemic activity of insulin by means of encapsulation in folate-(FA) coupled polyethylene glycol (PEG)ylated polylactide-co-glycolide (PLGA) nanoparticles (NPs; FA-PEG-PLGA NPs). FA-PEG-PLGA NPs (50 U/kg) demonstrated a two-fold surge in the oral bioavailability (twice hypoglycemia) without hypoglycemic shock when compared to subcutaneously administered regular insulin solution. Insulin NPs sustained the blood glucose levels for 24 hr, while, subcutaneous insulin exhibited a transient effect (<8 hr) with a severe hypoglycemic shock. This nanoformulation of insulin is suitable for once-daily administration and would be adequate to regulate blood glucose levels for at least 24 hr (Jain et al., 2012). In a different study, goblet cell-targeting nanoparticles were designed to enhance insulin oral absorption. The insulin loaded NPs were made using trimethyl chitosan chloride (TMC) surface decorated with a CSKSSDYQC (CSK) cell targeting peptide. Rather than unmodified nanoparticles, the CSK peptide on the surface facilitated the uptake process of nanoparticles in villi. Increase in drug permeation across the epithelium and higher internalization of drug was facilitated by clathrin and caveolae mediated endocytosis of goblet cell-like HT29-MTX cells. Orally administrated CSK peptide modified nanoparticles had shown a better hypoglycemic effect with a higher relative bioavailability of 1.5-fold compared to unmodified NPs. Over all, oral delivery of insulin by CSK peptide modified TMC nanoparticles was effective in targeting goblet cells (Jin et al., 2012; Rubinstein, 2012). Insulin loaded PLGA/HP55 nanoparticles were developed to improve the hypoglycemic effect of orally administered insulin. In vivo efficacy of nanoparticles was tested in diabetic rats. Upon oral administration (50 IU/kg) to diabetic rats, nanoparticles were able to decrease the blood glucose level rapidly with a maximal effect between 1 and 8 hr. The relative bioavailability of nanoparticles when compared to subcutaneous injection (5 IU/kg) in diabetic rats was 11.3% ± 1.05%. This study revealed that PLGA/HP55 nanoparticles might be used for oral delivery of insulin. Preactivated thiomers are biocompatible and improve mucoadhesion to a great extent. Thiomer nanoparticles are prepared by simple ionic gelation method. In vivo studies indicated enhanced bioavailability of protein-based drugs due to thiomer nanoparticulate formulations relative to formulations of non-thiolated polymers (Hauptstein and Bernkop-Schnurch, 2012). Numerous thiomers have been developed and studied in terms of nanoparticulate carrier systems. Considering the low bioavailability of protein and peptide based drugs when administrated orally, very encouraging results have been reached with thiomer based nanoparticles. Table 2 shows the outcomes and best features of thiomers for insulin delivery.
Table 2.
Salient features of thiomer nanoparticles in insulin delivery.
| Thiomer | Drug/model drug |
Outcome | Reference |
|---|---|---|---|
| PAA-Cys | Insulin | In vitro degradation studies, nanoparticles protected 44.47% of the initial insulin amount from trypsin, 21.33% from chymotrypsin, 45.01% from elastase compared to insulin solutions | (Perera et al., 2009) |
| Chitosan-6MNAcid | Insulin | AUC in vivo after oral administration to rats fourfold improved compared to unmodified chitosan nanoparticles | (Millotti et al., 2011) |
| TMC-Cys | Insulin | Oral and ileal application in rats blood glucose depression of 35% for oral administration and 70% for ileal application in vivo, hypoglycemic effect higher and longer-lasting compared to TMC-insulin nanoparticles | (Yin et al., 2009) |
Solid lipid nanoparticle (SLN) is another class of nanoparticles, also widely used in oral protein delivery (Almeida and Souto, 2007; Fonte et al., 2012; Yuan et al., 2009). SLN does not involve the use of toxic organic solvent and hence provide improved protein stability during formulation. SLN has demonstrated improved oral bioavailability of several therapeutic proteins such as insulin, calcitonin, and cyclosporine A. After oral administration of insulin loaded SLN to diabetic rats, Sarmento et al. (Sarmento et al., 2007) observed a substantial hypoglycemic effect during 24 hr. Relative bioavailability of insulin increased from 1.6% in oral solution to 5% when administered as loaded SLN. Garcia-Fuentes et al. (Garcia-Fuentes et al., 2005) evaluated ability of surface modified lipid nanostructures for oral delivery of salmon calcitonin (sCT) in rats. Following oral administration of sCT-loaded CS-coated nanoparticles, a significant and prolonged reduction in the serum calcium levels were obtained as compared to those obtained for sCT solution. Ability of nanoparticles to improve oral bioavailability of macromolecules by protection from harsh GI environment makes them a promising tool for oral protein delivery. In spite of encouraging results, requirement of high dose and lack of control over delivery hindered development of marketed nanoparticulate formulations.
4.7. Microparticles
Oral bioavailability of peptides and proteins is extremely low due to extreme pH variation, enzymatic degradation by GI fluid and relatively impermeable intestinal epithelium. Various strategies have been employed to increase oral bioavailability of macromolecules. Among them particulate carrier systems such as microparticles have generated significant interest (Siegel and Langer, 1984). Encapsulation of macromolecules inside polymeric carriers not only protect them from enzymatic and pH degradation but also control their release and augment their absorption. Hagan et al. (O’Hagan et al., 1993; O’Hagan et al., 1991) developed PLGA microparticles loaded with cholera toxin B. Following oral administration in mice specific antibody-secreting cells were observed both in the mesenteric lymph nodes and spleen. Maculotti et al. (Maculotti et al., 2009) developed ovalbumin loaded microspheres using chondroitin sulphate/chitosan (CS/CH) polymers for oral delivery using new emulsion-complex coacervation method. Microspheres released ~30% of ovalbumin in 24 hr, while 100% release was observed in the presence of chondroitinase making them suitable for oral administration. Microspheres of mucin and sodium alginate polymers were developed using coacervation and diffusion filling method. Reduction in blood glucose levels were observed after oral administration to diabetic rabbits. Blood glucose lowering effect following oral administration of insulin-entrapped microparticles was identical to subcutaneously injected insulin solution (Builders et al., 2008). Cheng et al. (Cheng et al., 2006) developed responsive magnetic microparticles and evaluated the effect of prolonged GI transit on the bioavailability of insulin in presence of an external magnetic field. Up to 43.8% reduction in blood glucose levels were observed following administration of 100 U/kg of insulin-magnetite-PLGA microparticles in fasted mice. Based on glucose and ELISA assay, external magnetic field for 20 hr resulted in a bioavailability of 2.77 +/− 0.46 and 0.87 +/− 0.29%, respectively. In the absence of magnetic field, bioavailability values were 0.66 +/− 0.56 and 0.30 +/− 0.06%, for glucose and ELISA assay, respectively. A significant improvement in the hypoglycemic effect was noticed in mice that were orally administered with insulin loaded magnetite-PLGA microparticles in presence of an external magnetic field, implying that magnetic force can be used to enhance the effectiveness of orally delivered protein therapeutics. Kim et al. (Kim et al., 2005) developed biodegradable microparticles with alginate utilizing a piezoelectric ejection process. Then lectin (wheat germ agglutinin, WGA) was conjugated to alginate microparticles to obtain dual benefits of protective action and mucoadhesive effect from WGA for enhanced oral insulin delivery. The authors concluded that alginate-WGA microparticles enhanced the intestinal absorption of insulin to cause significant drop in blood glucose levels. Jones et al. (Jones et al., 1997) utilized biodegradable and biocompatible polymer, PLGA to encapsulate bacterial and viral proteins, synthetic peptides and plasmid DNA in microparticles. They compared the immune responses elicited following oral and parenteral administration in mice. Precise systemic and mucosal humoral immune responses as well as cell-mediated immune responses have confirmed the potential of this polymer as a vehicle for oral delivery of vaccines. Development of a clinically successful microparticulate formulation for long-term protein delivery systems requires improvement in loading efficiency, control of burst release and precise control over protein release kinetics.
4.8. Cyclodextrins
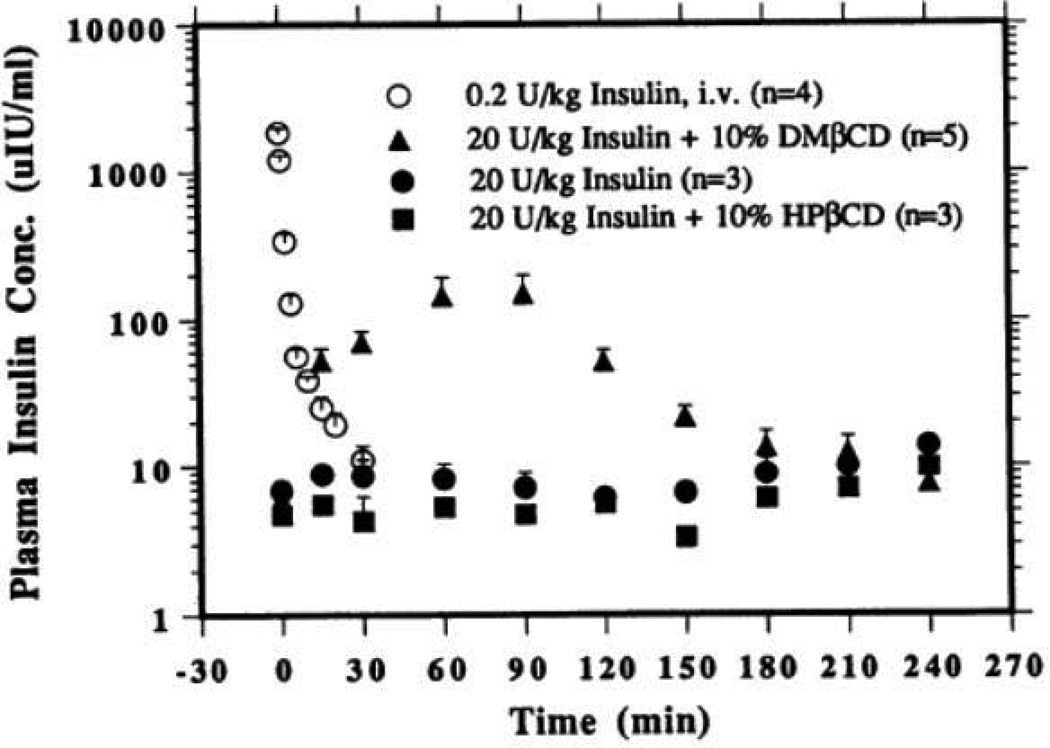
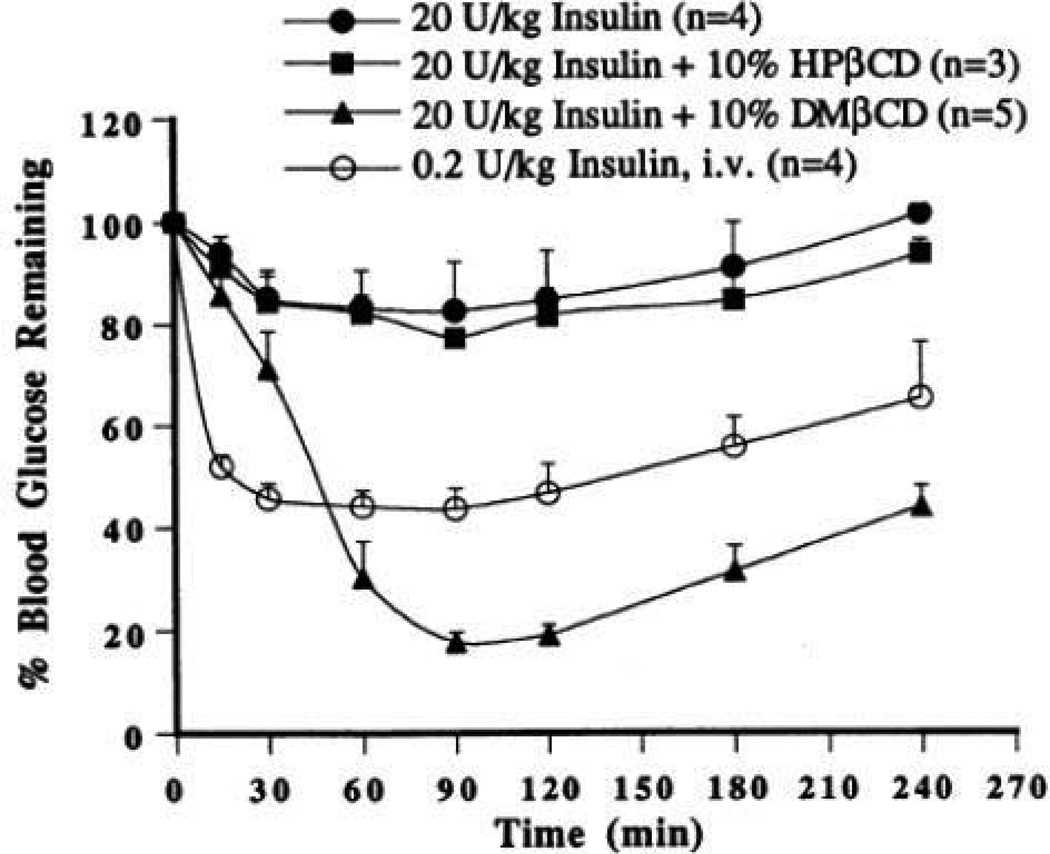
Cyclodextrins (CD) are cyclic oligosaccharides containing at least 6 D-(+) glucopyranose units attached by α-(1, 4) glucosidic bonds. These molecules contain hydrophobic inner cavities and hydrophilic outer surfaces (Challa et al., 2005; Kanwar et al., 2011). These cyclic oligosaccharides contain six (α-CD), seven (β-CD), eight (γ-CD), nine (δ-CD), ten (ε-CD) or more (α−1,4)-linked α-D-glucopyranose units. Cyclodextrins containing less than 6 units cannot be formed due to steric hindrance. Higher homologs containing 9 or more glucose units are difficult to purify. However, several kinds of large ring CDs were isolated and purified to acquire relatively large amount of δ-CD (cyclomaltonose) with 9 glucose units. These compounds are capable of interacting with a large variety of guest molecules to form non-covalent inclusion complexes. The chair conformation of the glucopyranose units shapes the cyclodextrins in the form of a truncated cone or torus rather than a perfect cylinder (Brewster and Loftsson, 2007). Commercially available cyclodextrins include hydroxypropyl derivatives of β-CD and γ-CD, randomly methylated β-cyclodextrin (RM-β-CD), and sulfobutylether β-cyclodextrin sodium salt (SBE-β-CD). The ability of CD to interact with biological membranes allow them to serve as potential carriers for delivery of large molecules such as proteins, peptides, and oligonucleotide drugs (Irie and Uekama, 1999). Efflux pumps such as P-gp serve as a barrier for non-specific uptake of peptides causing low peptide bioavailability. Hydrophobic peptides such as cyclosporin A (Augustijns et al., 1993), D, N-acetyl-leucyl-leucylnorleucinal (Burton et al., 1993), valinomycin (Ueda et al., 1993), gramicidin (Loe and Sharom, 1994), and ditekiren (Takahashi et al., 1997) are known substrates for P-gp. Also, P-gp mediated peptide efflux might play an important role in hindering availability to the central nervous system (Sharom et al., 1996). CD can also inhibit the efflux pumps i.e. P-gp and multidrug resistance associated proteins (MRP2). DM-β-CD significantly impaired efflux function of P-gp and MRP2 in Caco-2 cell monolayers. However, cell viability and membrane integrity of Caco-2 cell monolayers remained unaltered (Arima et al., 2004). Cyclodextrins have been investigated for their potential to deliver glycosylated calcitonin (modified calcitonin) and somatostatin analog octapeptide octreotide (Sandostatin®). Inclusion of peptides in β-cyclodextrin (β-CD) and hydroxypropyl-β-cyclodextrin (HP- β-CD) cavity resulted in increased chemical and enzymatic stability of these peptides besides improving absorption. The reason for improved absorption may be due to impairment of tight junctional integrity which was observed through enhanced permeation of paracellular marker PEG-4000 (Haeberlin et al., 1996). Cyclodextrins have been shown to enhance chemical and physical stability of peptides and proteins through complexation. Thymopentin, a small peptide consisting of five amino acids, was stabilized in aqueous solutions by complexation with (2-hydroxypropyl)-â-cyclodextrin without any loss of pharmacological activity (Brown et al., 1993). Glutathione (GSH) was successfully encapsulated in Eudragit RS-100-based microparticles containing HP- β-CD. Presence of CD resulted in enhanced GSH absorption and sustained release of tripeptide. The authors concluded that GSH-loaded Eudragit RS 100 microparticles containing HP-β-CD may be suitable for sustained oral delivery of GSH (Trapani et al., 2007). Enzymatic degradation of basic fibroblast growth factor was minimized by employing water-soluble β-CD sulfate (Loftsson and Brewster, 1996). β-CD has been employed in the formulation of alginate microspheres containing insulin for oral delivery. Serum sugar and insulin levels following administration of microspheres (multiple oral doses) indicated absorption of insulin from the GI region. With this method, insulin absorption from optimized microspheres was found to take place from GI (Jerry et al., 2001). Shao et al. (Shao et al., 1994) studied the relative effectiveness of two β-CD derivatives, i.e., dimethyl-β-cyclodextrin (DM-β-CD) and HP-β-CD, as mucosal absorption promoters. Insulin absorption was evaluated in the lower jejuna/upper ileal segments of the rat with an in situ closed loop method. Insulin alone and in the presence of 10 % w/v HP-β-CD did not show any significant improvement in the systemic absorption. Figure 8, shows the plasma insulin concentration profiles plotted as a function of time. AUC0–∞ values for insulin alone and in the presence of 10 % HP-β-CD were reported to be 3 ± 2 µU/ml*hr. Incorporation of 10% w/v DM-β-CD to insulin solution showed a significant enhancement in insulin absorption as evidenced by an AUC0–∞ of 255 ± 65 µU/ml*hr and insulin bioavailability dramatically increased from 0.06% (insulin alone) to 5.63% (Fig. 8). Moreover, hypoglycemic effects of insulin were also evaluated in the presence of cyclodextrins. Enteral administration of insulin to lower jejuna/upper ileal segments of the rat resulted in a minimal glucose lowering effect. Addition of 10 % (w/v) HP-β-CD to insulin solution did not result in significant hypoglycemic effect. However, incorporation of 10 % (w/v) DM-β-CD significantly improved insulin hypoglycemic effect (Fig. 9) (Shao et al., 1994). In addition, histopathological evaluations displayed no observable destruction to the overall tissue integrity indicating high tolerance of the intestinal mucosa to HP-β-CD and DM-β-CD favoring their use as absorption promoters for oral delivery of proteins and peptides.
Fig. 8.
Plots of plasma insulin concentrations versus time following intravenous administration of 0.2 U/kg insulin, enteral administration of 20 U/kg insulin alone, enteral administration of 20 U/kg insulin with 10 % (w/v) HP-β-CD, and enteral administration of 20 U/kg insulin with 10 % (w/v) DM-β-CD. Values denote means ± SE. Reproduced with permission from reference (Shao et al., 1994).
Fig. 9.
Pharmacodynamic response following enteral administration of 20 U/kg porcine-zinc insulin in 0.01 M phosphate buffered saline in the absence and presence of 10 % β-cyclodextrin derivatives. Data from intravenous administration of 0.2 U/kg insulin have also been included. Values represent means ± SE. Reproduced with permission from reference (Shao et al., 1994).
A recent investigation evaluated mechanistic aspects on the uptake and intracellular trafficking of novel CD transfection complexes by intestinal epithelial (Caco-2) cells (MJ et al., 2011). This study was directed at investigating the efficacy of a novel poly-6-cationic amphiphilic CD to transfect intestinal enterocytes; the endocytotic uptake pathways and the intracellular trafficking of the CD•DNA complexes, by changing the orientation of the lipid tail on CD. Inhibitors of clathrin- and caveolae-mediated endocytosis and macropinocytosis were employed to evaluate the mechanisms of CD·DNA uptake by both undifferentiated and differentiated Caco-2 cells. Significant levels of pDNA uptake and gene expression (comparable to PEI) was evident in both undifferentiated and differentiated Caco-2 cells via complexation of plasmid DNA (pDNA) with CD. Also, CDs were capable of transfecting the differentiated Caco-2 cells. The uptake of CD.DNA transfection complexes by both undifferentiated and differentiated Caco-2 cells was found to be mediated by macropinocytosis. On the other hand, heparan sulphate proteoglycans demonstrated binding of complexes to undifferentiated Caco-2 cells. This study provides an insight for the development of optimized CD based transfection complexes for intestinal delivery with enhanced cellular uptake and intracellular trafficking properties.
5. Other approaches to enhance the oral delivery of peptide and proteins
5.1. Site specific delivery to colon
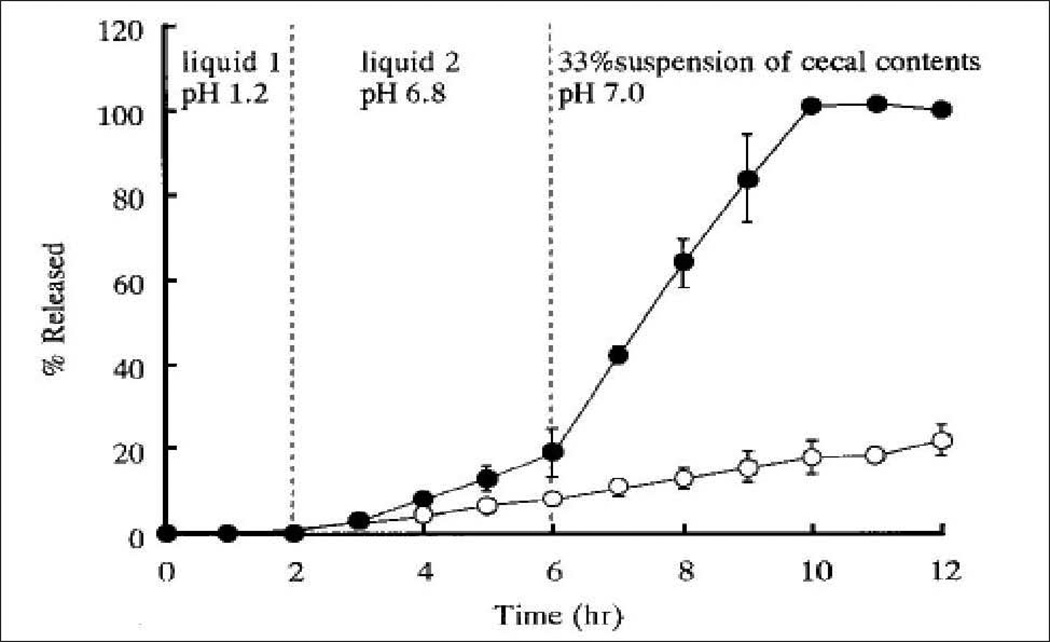
High proteolytic degradation and poor absorption through epithelial barriers pose major challenges to successful oral delivery of protein and peptide therapeutics. One of the approaches to lessen degradation is to deliver protein molecules to a specific region of GI tract such as colon where proteolytic activity is comparatively low (Kumar and Mishra, 2008; Maroni et al., 2012). Though protein and peptides are inactivated in acidic environment of stomach and also by proteolytic enzymes in the small intestine, the colon offers an attractive target for delivery of protein therapeutics due to relatively low abundance of peptidases and presence of alkaline pH. Moreover, prolonged residence time and more responsiveness to absorption enhancer allow higher absorption of proteins through colonic epithelia (Patel, 2011). Colon targeted delivery has been exploited for several protein and peptide drug candidates such as vasopressin, insulin, calcitonin, glucagon and so on (Patel et al., 2007). Recent advances in pharmaceutical technology lead to the development of numerous colon-specific delivery approaches. These approaches include pH and time dependent delivery systems, pressure-induced drug delivery, microflora-activated systems, and particulate drug delivery systems (Gangurde H.H., April–June 2011; Gazzaniga et al., 1994; Hamman et al., 2005). Because of higher pH in the terminal ileum and colon, polymers such as methacrylic acid copolymers, Eudragit L100 and Eudragit S100, which disintegrate preferentially at high pH levels (6.5–7.0), are usually employed for colon-specific delivery. For instance, Touitou et al. (Touitou and Rubinstein, 1986) designed soft gelatin capsules coated with polyacrylic polymer (Eudragit) for colon-specific delivery of insulin. A significant rise in hypoglycemic effect was observed in rats after oral administration of capsule relative to control. In another study, Lowman et al. (Lowman et al., 1999) developed insulin loaded gel particles from pH responsive poly (methacrylic-g-ethylene glycol) hydrogels. In acidic environment, stomach prevents the swelling of gel particles protecting insulin from proteolytic degradation. However, in basic environment of small intestine the gel rapidly swells and dissociates to release the entrapped insulin. A strong hypoglycemic effect was observed in both healthy and diabetic rats within 2 hr of oral administration of insulin loaded gel particles (Lowman et al., 1999). Even though, a pH responsive system can protect protein in the stomach, and proximal small intestine, such systems suffer from poor site-specificity causing dissolution and drug release in the lower part of small intestine prior to the colon. Alternatively, polysaccharides and azo-polymers which are refractory in stomach and small intestine while degraded by the colonic microflora can be used. Polysaccharides such as chitosan, dextran, inulin, pectin, chondroitin sulfate have been exploited for colon-specific delivery (Gulbake and Jain, 2012; Shah et al., 2011). Tozaki et al. developed chitosan capsule for improving insulin absorption from the rat colon. To evaluate effectiveness of chitosan capsule in colon- specific delivery, carboxyfluorescein (CF) (a model water soluble compound) release from chitosan capsule was performed in three different dissolution media: artificial gastric juice (pH 1), an artificial intestinal juice (pH 6.8), and a suspension of rat cecal contents (pH 7.0). Figure 10 illustrates release profile of CF from chitosan capsule. When insulin loaded chitosan capsule was orally administered in rats, a strong hypoglycemic effect was observed relative to insulin solution. This effect was attributed to the protection of insulin from proteolytic degradation as well as the ability of chitosan to increase permeability of insulin through colonic epithelia (Tozaki et al., 1997).
Fig. 10.
Release of CF from chitosan capsules, determined by the Japanese Pharmacopoeia (J. P.) rotating basket method. (●) artificial gastric juice→ an artificial intestinal juice → a suspension of rat cecal contents (O) phosphate buffered saline (pH 6.0). Reproduced with permission from reference (Tozaki et al., 1997).
Fetih et al. have evaluated applicability of chitosan for colon specific delivery of [Asu 1, 7]-eel calcitonin in rats (Fetih et al., 2006). In this study, [Asu 1,7 ]-eel calcitonin (ECT) loaded chitosan capsule was orally administered in rat and the % pharmacological availability (PA%) was calculated. Authors have also studied the effect of incorporation of various additives such as absorption enhancers (S-nitroso-N-acetyl-DL-penicillamine (SNAP) and sodium glycocholate) and protease inhibitors (bacitracin and aprotinin) in the dosage forms on PA%. Following oral administration, PA% for ECT loaded chitosan capsule (PA%: 0.551%) was very high compared to ECT solution (PA %: 0.041%). In addition, the hypocalcemic effect and thereby PA% was further increased significantly by co-administration with various additives and the highest PA % (6.344%) was reached with chitosan capsule loaded with 20 IU ECT, 1.1mg SNAP, 2.5mg sodium glycocholate, 3.5mg bacitracin and 1mg aprotinin. It is evident from the results that chitosan capsule along with various additives significantly improved the efficacy of ECT. In another study, the suitability of chitosan hydrogel beads for colon-specific delivery of larger proteins was evaluated in vitro with FITC-BSA as a model protein. In vitro release studies in simulated intestinal fluid (SIF) (6 hr) followed by rat cecal and colonic enzyme medium (14 hr) revealed 20% release of BSA in SIF while approximately 50% release was observed in cecal and colonic enzyme medium (Zhang et al., 2002). Use of azopolymer is another widely used approach for colon specific delivery of proteins and peptides via oral route. Azopolymer contains an azo-aromatic group R-C6H4-N=N-C6H4-R’ that is specifically reduced by microflora present in the colon which ultimately leads to polymer degradation and release of drug in the colon. The concept of azopolymer was first investigated by Saffran et al in 1986 for oral delivery of vasopressin and insulin (Roldo et al., 2007; Saffran et al., 1986). Later, Tozaki et al. developed azopolymer-coated pellets for colon specific delivery of insulin and ECT in rats. For both peptides, maximum biological effects correspond to the presence of pellet in the large intestine. However, some effects were observed prior to pellets reaching the colon indicating the leakage of drug from pellets. Moreover incorporation of protease inhibitors raised the bioavailability. Pellets containing insulin (12.5 IU) with camostat mesilate (24.2 mg) demonstrated approximately 30% reduction in glucose level as compared to pellets containing insulin alone. Similarly, in case of ECT, a greater hypocalcemic effect was observed after incorporation of camostat mesilate in pellets (Tozaki et al., 2001). Saffran et al. exploited this natural phenomenon by using copolymers of styrene and hydroxyl- ethylmethaacrylate cross-linked with azo-bonds for oral insulin delivery. Oral administration of insulin containing azopolymer coated pellets to diabetic rats showed greater that 40% reduction in initial glucose levels, however, variable results were obtained after oral administration in normal rats. This may be due to the premature release of drug prior to reaching the colon (Saffran et al., 1986). Though the use of azopolymers looks more appealing for colon-specific delivery of proteins, further refinement in this approach is needed in terms of developing and testing other derivatives of azopolymers. Therefore, colon targeted drug delivery represents a novel concept for improving the systemic bioavailability of orally administered protein and peptide therapeutics. However, colon targeted delivery systems pose multiple challenges such as presence of lower surface area along with tight junctions which hinder drug absorption at the colonic site. In addition, certain disease conditions alter the enzymatic activity of microflora present in the colonic site and subsequently affect the drug release from cargo (Vinay Kumar K.V. et al., 2011).
5.2. Chemical modification
Chemical modifications of peptides and proteins have shown exciting results primarily due to enhanced enzymatic stability, intestinal permeability and lesser extent of immunogenicity. Generally therapeutic activity of protein drugs can be optimized up to certain extent by chemical modifications. These chemical modifications may include substitution of existing amino acids with new amino acids (analogue formation), acylation and PEGylation (Frokjaer and Otzen, 2005; Hamman et al., 2005). In analogue approach, modifications are carried out by interchanging of amino acids preferably by substitution of L-amino acid with D-amino acids or by replacement with different amino acids. Desmopressin, a vasopressin analogue, was developed by deamination of N-terminal amino acid and replacement of the C-terminal L649 arginine by D-arginine in vasopressin. Natural vasopressin is orally active only at very large doses while desmopressin exhibits twice the activity of vasopressin only at 1/75 fraction of the dose, which is mainly attributed to enhanced permeability across intestinal epithelia and improved enzymatic stability (Rado et al., 1976; Vavra et al., 1974; Vilhardt and Lundin, 1986). Thytropar, a thyrotropin releasing hormone (THR) is a tripeptide (Glu-His-Pro), which is resistant to proteolytic degradation. However, oral bioavailability is poor due to high metabolic clearance. MK-771 is an analog of THR synthesized by substituting nitrogen atom with sulfur in pyrrolidine ring 2 of the proline residue of THR, which showed two hundred times higher CNS activity with less metabolic clearance. It was also equipotent to THR in causing release of TSH. However oral bioavailability was only 2% and it was attributed to inefficient membrane transport (Hichens, 1983).
Conjugation of fatty acid with protein and peptides has also been attempted to improve their lipophilicity. Lipidization is reported to improve metabolic stability, membrane permeability, bioavailability, and changes pharmacokinetic and pharmacodynamic properties of several peptide therapeutics (Zhang and Bulaj, 2012). Compared to tetragastrin (TG), acyl, caproyl and lauroyl derivatives of TG have shown marked increase in gastric acid secretion following administration into the large intestinal loop. This increased activity is due to improved permeation of TG from the large intestine (Tenma et al., 1993). In another study, Wang et al. synthesized lipidized salmon calcitonin (REAL–sCT) conjugate by ‘reversible aqueous lipidization’ (REAL) technology using N-palmitoyl cysteinyl 2-pyridyl disulfide as a lipidizing reagent (Wang et al., 2003). The pharmacokinetic and pharmacodynamic aspects of REAL–sCT was studied in rats and compared with normal salmon calcitonin. Following oral administration, oral absorption of sCT in rats was minimal and only at 0.5 hr, plasma sCT levels were detectable with concentration of 4.6 ± 2.8 pg/0.2 ml plasma (Fig. 11). On the other hand, lipidization enhanced absorption of sCT and greater than 7 pg/ml plasma level of the intact sCT was detected at 12 hr after the oral administration of REAL–sCT. The AUC of oral REAL–sCT was at least 19 times higher than oral sCT. Moreover, REAL–sCT also demonstrated anti-bone resorption activity in ovariectomized (OVX) rats following oral administration (Wang et al., 2003). Using REAL technology, Wang et al. have chemically modified opioid peptide leu-enkephalin (ENK) by conjugating with a novel amine-reacting lipophilic dimethylmaleic anhydride analog, 3,4-bis(decylthiomethyl)-2,5-furandione. The REAL-ENK demonstrated enhanced stability in mouse small intestinal mucosal homogenate with 12-fold increment in half-life over ENK. Moreover, after oral administration, significantly higher and sustained plasma peptide levels were detected upto 24 hr in normal mice. The peak concentration and area under the curve of REAL-ENK were 4.4 and 21 times higher relative to ENK. Most importantly, REAL-ENK also produced significant and sustained anti-nociception in a rodent inflammatory pain model (Wang et al., 2006).
Fig. 11.
Plasma level–time profile of sCT in rats orally administered with 500 µg/kg of either sCT or REAL–sCT. The concentration of sCT in the plasma was determined by using a commercial RIA kit. Reproduced with permission from reference (Wang et al., 2003).
Since decades, scientists have rationally designed and synthesized several derivatives of insulin in order to improve oral bioavailability by conjugation with either fatty acid or PEG. Hashizume et al. examined absorption of mono and dipalmitoyl insulin. Palmitoylation of insulin not only enhanced transport of insulin across the mucosal membrane of the large intestine but also improved stability against enzymatic degradation in intestine (Hashizume et al., 1992). Development of hexyl-insulin for oral delivery is one of the biggest achievements of the chemical modification approach. NOBEX Corporation has developed hexyl-insulin-monoconjugate-2 (HIM2) by covalent attachment of PEG-amphiphilic oligomer to free amino group on the lysine residue at 20th position in the β-chain of recombinant human insulin (Park et al., 2011). This modification has enhanced the stability of insulin in presence of gastrointestinal enzymes and facilitated greater absorption. Following oral administration of HIM2 (0.5 and 1.0mg/kg) in type-2 diabetes patients, 30 min prior to meal, lower postprandial plasma glucose levels were obtained during a 4 hr post dose evaluation period compared to placebo. Even though lower peripheral insulin concentrations were attained after oral HIM2, control of post postprandial plasma glucose levels during a 4 hr post dose evaluation period was comparable to subcutaneous insulin (8 units). This data is consistent with the hypothesis that oral delivery of insulin may lead to the development of a portal-to-peripheral insulin gradient similar to the state observed in individuals not administered subcutaneously (Kipnes et al., 2003). Following oral administration of HIM2 in type-1 diabetes patients, plasma glucose levels declined or remained stable for 30 min to 2 hr and were <150% of pre-dose levels throughout the post-dose evaluation period. Moreover, no episodes of symptomatic hypoglycemia were observed. Hence there may be less risk of severe hypoglycemia associated with HIM2 as compared to injectable insulin (Clement et al., 2002).
A recent study investigated the combined approach of chemical modification with application of pH responsive hydrogels for oral delivery of insulin (Tuesca et al., 2009). In this study, authors have developed PEG–insulin conjugate by PEGylation at the amino terminus of the B-chain. The biological activity of PEG–insulin conjugate was evaluated and compared with human insulin following intravenous and subcutaneous dosing in rats. The PEG–insulin conjugate retained complete biological activity as human insulin following intravenous administration in rats. On the other hand, the conjugate exhibited a relatively high hypoglycemic effect 127.8% than human insulin after subcutaneous dosing. Later, authors have loaded PEG-insulin conjugate and human insulin into pH responsive poly(methacrylic acid-g-ethylene glycol) (P(MAA-g-EG)) hydrogel and evaluated in vitro release profile. The fractional release of insulin and PEGylated insulin exhibited pH dependant release profile with low release at pH 4.7 and 5.6 which was increased at pH 6.2 and 7.4. However, in all cases, the release of PEGylated insulin was lower than human insulin which was suggested due to a stronger affinity of PEGylated insulin for the hydrogel. The authors suggested that this release pattern could be advantageous for oral insulin delivery because PEGylated insulin would maintain higher concentrations within the hydrogel as it moves down through the GI tract. This combined approach of PEGylated insulin and P(MAA-g-EG) hydrogels have significant promise for improving oral delivery of insulin (Tuesca et al., 2009).
Although PEGylation is considered a gold standard for chemical modifications of proteins drugs, safety and efficacy of newly developed PEGylated proteins need to be considered. PEG polymers are mixture of polymers of different molecular mass and in addition protein contains several accessible sites for PEGylation which allow product heterogeneity. Such product heterogeneities may alter the efficacy of the parent protein drugs. For example, PEGylation of epidermal growth factor at Lys28 and Lys 48 have shown reduced biological activity compared to its parent structure (Lee and Park, 2002).
5.3. Prodrug derivatization
Prodrugs are pharmacologically inactive entities; made by chemical modifications, and able to convert into their active forms by enzymatic or non-enzymatic transformation upon crossing barriers (Hsieh et al., 2009; Jana et al., 2010). An extensive research to improve solubility, stability, targetability and permeability of small molecules by prodrug approach has been carried out in recent years (Hsieh et al., 2009; Rautio et al., 2008). However, structural complexity, lack of methodology for synthesis and poor stability during synthesis limits application of this approach for peptides and proteins (R et al., 1997). In spite of these challenges recent studies have shown exciting results for several protein and peptide therapeutics. One of the promising prodrug approaches is bioreversible cyclization which has been utilized by Borchardt et al. (Borchardt, 1999) for improving oral absorption of opioid peptide. The phenylpropionic acid based cyclic prodrugs of (Leu5)-enkephaline have shown approximately 1680 fold higher permeability through Caco-2 cell monolayer compared to (Leu5)-enkephaline. In the same study, the authors have synthesized coumarinic acid based cyclic prodrugs of (Leu5)-enkephaline which not only exhibited high permeability but also showed good stability in the presence of peptidases. Similar results were obtained with cyclization of kyotrophin. Cyclic kyotrophin showed improved stability and higher permeability on excised small intestine of rat (Mizuma et al., 2000). A recent study documented targeted lipid prodrug strategy which may help in higher absorption of hydrophilic molecules such as peptides and proteins. In this study, a lipid raft has been conjugated to the parent drug molecule to impart lipophilicity. Simultaneously a targeting moiety that can be recognized by a specific transporter on the cell membrane has also been tethered to the other terminal of lipid raft to facilitate active targeting. Results from this study demonstrated that both the targeting and the lipid moiety act synergistically toward cellular uptake. The authors concluded that this novel prodrug design strategy may help in higher absorption of hydrophilic therapeutics such as genes, siRNA, antisense RNA, DNA, oligonucleotides, peptides and proteins (Vadlapudi et al., 2012a).
5.4. Membrane transporter and receptor targeting
Covalent conjugation of carrier molecules to protein and peptide represent a feasible approach for enhancing their intestinal absorption. Presence of a transport-carrier molecule enables recognition by a specific endogenous membrane transporter or a receptor. This strategy has been explored by many researchers to improve oral drug bioavailability. Various transporters have been identified on the intestinal epithelial cells (Kramer et al., 1997; Sood and Panchagnula, 2001; Tsuji and Tamai, 1996; Vadlapudi et al., 2012b) . These include (i) amino acid and oligopeptide transporters, (ii) bile acid transporters, (iii) water-soluble vitamin transport systems, (iv) carbohydrate transporters, (v) monocarboxylic acid transporters, and (vi) phosphate transporters. The driving force for these carrier systems is provided through electrochemical gradients of Na+ and H+ ions. Inwardly directed ion gradients are a result of Na+/K+-ATPase (localized on the basolateral membrane) and Na+/H+ exchanger (localized on luminal surface), which are responsible for lower intracellular Na+ and H+ ion concentrations in intestinal epithelial cells. Most transport systems are characterized to be sodium (amino acid, vitamin, bile acid, phosphate transporters) and proton (oligopeptide, short chain fatty acid transporters) dependent. For instance, protein or peptide therapeutics conjugated to an amino acid or a dipeptide carrier moiety can aid in recognition of the conjugates by an amino acid or peptide transporter which in turn facilitates enhanced oral absorption. Bile acid and oligopeptide transporter have shown to possess greater capacity (>10g/day), which can be utilized for delivery of protein or peptide therapeutics. However, biological barriers such as efflux transporters may limit the absorption of orally administered drugs including peptides. In such a case, simultaneous co-administration of efflux inhibitors may contribute to significant enhancement in the absorption of peptide drugs that are substrates of efflux proteins (Katragadda et al., 2005; Varma et al., 2003). Membrane transporters generally limit their ability to transport only small molecule drugs. On the other hand, receptors translocate drug molecules via receptor mediated endocytosis and do not limit their translocation based on the size of permeant. Receptor ligands such as lectins, toxins, viral haemagglutinins, invasins, transferrin and vitamins (vitamin B12, folate, riboflavin and biotin), can be conjugated or covalently attached to protein or peptide drug to achieve site specific targeting (Lim and Shen, 2005). Vitamin B12 absorption pathway has been shown to play a prominent role in the absorption of the cobalamin–protein conjugates (Russell-Jones, 2001). Transferrin receptor has been utilized for oral delivery of protein drugs such as insulin and granulocyte colony-stimulating factor (G-CSF) (Russell-Jones, 2004). An expression construct consisting of granulocyte colony-stimulating factor (G-CSF)-transferrin fusion protein (G-CSF-Tf) was developed by fusing human cDNAs encoding G-CSF and transferrin. With the use of transferrin based recombinant fusion protein, the biological activity of orally administered protein was not destroyed by proteolytic enzymes as compared to that of subcutaneously administered G-CSF. This technology may be valuable for the future development of orally effective biologicals (Bai et al., 2005).
5.5. Cell penetrating peptides
Recently, cell penetrating peptides (CPP) have gained a lot of attention in the delivery of small molecules as well as macromolecules, liposomes and nanoparticles into cells by chemical modifications (Chen et al., 2012; Khafagy el and Morishita, 2012; Koren and Torchilin, 2012). CPPs act by transporting their cargoes into the cytoplasm via perturbation of the lipid bilayer of the cell membrane or by endocytosis (Trehin and Merkle, 2004; Zorko and Langel, 2005). CPP linked to insulin (insulin-CPP hybrids) have demonstrated an increase in intestinal absorption efficiency of insulin compared to normal insulin across Caco-2 cell monolayers (Liang and Yang, 2005). Though this strategy lacks supportive in vivo data, it can be explored for enhancing therapeutic potential (Vives, 2005).
CPPs such as HIV-1 Tat, penetratin and oligoarginine are considered as a valuable tool for the intracellular delivery of therapeutic peptides and proteins (biodrugs) (Khafagy el and Morishita, 2012). Hence, CPPs may enhance the absorption of biodrugs through intestinal epithelium. CPPs are considered to be powerful tools for overcoming the low permeability of therapeutic macromolecules through the intestinal epithelial membrane, the major barrier to their oral delivery. This promising strategy appears to be advantageous in improving intestinal absorption especially when CPPs are coadministered with drugs. It is postulated that the intermolecular binding interactions between a drug and a CPP are important factors for enhancing the intestinal absorption of macromolecular drugs (Fig. 12). However, the mechanism of uptake of CPP-biodrug conjugate by epithelial cells and its subsequent enzymatic degradation and release into circulation are unclear. Further studies are warranted to delineate the complete mechanisms for the absorption-enhancing effect of CPPs (Khafagy el and Morishita, 2012).
Fig. 12.
Mechanism of intermolecular interaction between drug and CPP. Reproduced with permission from reference (Khafagy el and Morishita, 2012).
The in vivo absorption of bioactive macromolecules conjugated to CPPs was evaluated in mice by Schwarze et al. (Schwarze et al., 1999). Intraperitoneal injection of β-galactosidase protein (120-kDa), fused to the protein transduction domain from the HIV TAT protein, facilitated delivery of the biologically active fusion protein to all tissues including the brain. Also, the potential of CPPs as a permeation enhancer to improve the penetrating ability of poorly permeable macromolecules have been explored (Kamei et al., 2008; Kamei et al., 2009b; Khafagy el et al., 2009a; Khafagy el et al., 2009b; Morishita et al., 2007). Previous reports have shown that CPPs were capable of enhancing peptide and protein delivery across mucosal membranes safely by non-covalent cargo interaction approach. Moreover, CPPs have successfully delivered the most challenging peptide and protein cargos through intestinal mucosa with greater bioavailability. The assembly of cargo/CPP interaction via non-covalent interaction strategy appears to be advantageous because it simplifies chemical conjugation protocols. Various cargos can be delivered by simply mixing the CPP with biomacromolecules without the need of optimizing individual synthetic process.
6. Conclusion
Oral delivery of peptides and proteins is currently a topic of intense research. Advancements in the biopharmaceutical industry have resulted in the development of several new protein and peptide based therapeutics. Oral administration is most preferred because of patient compliance and acceptability, but the physiological, enzymatic and chemical barriers for this route pose a significant challenge to the delivery of peptide and protein drugs. Better understanding of the routes of drug absorption (paracellular and transcellular), proteolytic enzyme activity, stability and degradation of macromolecules during the intestinal transport process is important for the development of protein and peptide drug delivery systems. Several approaches including chemical modifications, use of absorption and penetration enhancers, use of mucoadhesive polymers, formulation design, and use of enzyme inhibitors have been investigated to improve their bioavailability. Although these strategies have marginally improved intestinal absorption of macromolecules, they often resulted in undesirable effects. For example, permeation enhancers may cause potential toxicity by modifying the phospholipid bilayers, leaching of proteins from the mucous membrane or destroying the epithelial cell membranes there by leading to irritation and inflammation. Also opening up the tight junctions may allow the entry of unwanted xenobiotics or pathogens. Therefore, there is an unmet need in the development of novel approaches or modification of existing approaches to achieve site specific and controlled delivery of protein and peptide drugs besides preserving their biological and therapeutic properties. Oral delivery of peptides and proteins can be facilitated by a thorough understanding of the primary, secondary and tertiary structures of macromolecules on a case-by-case basis.
ACKNOWLEDGEMENTS
This work was supported by NIH grants R01 GM064320-03, R01EY09171, R01EY010659 and R01 AI071199-01A2.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almeida AJ, Souto E. Solid lipid nanoparticles as a drug delivery system for peptides and proteins. Adv Drug Deliv Rev. 2007;59:478–490. doi: 10.1016/j.addr.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Antosova Z, Mackova M, Kral V, Macek T. Therapeutic application of peptides and proteins: parenteral forever? Trends Biotechnol. 2009;27:628–635. doi: 10.1016/j.tibtech.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Arima H, Yunomae K, Morikawa T, Hirayama F, Uekama K. Contribution of cholesterol and phospholipids to inhibitory effect of dimethyl-beta-cyclodextrin on efflux function of P-glycoprotein and multidrug resistance-associated protein 2 in vinblastine-resistant Caco-2 cell monolayers. Pharm Res. 2004;21:625–634. doi: 10.1023/b:pham.0000022409.27896.d4. [DOI] [PubMed] [Google Scholar]
- Artursson P, Lindmark T, Davis SS, Illum L. Effect of chitosan on the permeability of monolayers of intestinal epithelial cells (Caco-2) Pharm Res. 1994;11:1358–1361. doi: 10.1023/a:1018967116988. [DOI] [PubMed] [Google Scholar]
- Augustijns PF, Bradshaw TP, Gan LS, Hendren RW, Thakker DR. Evidence for a polarized efflux system in CACO-2 cells capable of modulating cyclosporin A transport. Biochem Biophys Res Commun. 1993;197:360–365. doi: 10.1006/bbrc.1993.2487. [DOI] [PubMed] [Google Scholar]
- Aungst BJ. Absorption enhancers: applications and advances. AAPS J. 2012;14:10–18. doi: 10.1208/s12248-011-9307-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai JP, Amidon GL. Structural specificity of mucosal-cell transport and metabolism of peptide drugs: implication for oral peptide drug delivery. Pharm Res. 1992;9:969–978. doi: 10.1023/a:1015885823793. [DOI] [PubMed] [Google Scholar]
- Bai JP, Chang LL, Guo JH. Effects of polyacrylic polymers on the degradation of insulin and peptide drugs by chymotrypsin and trypsin. J Pharm Pharmacol. 1996;48:17–21. doi: 10.1111/j.2042-7158.1996.tb05869.x. [DOI] [PubMed] [Google Scholar]
- Bai Y, Ann DK, Shen WC. Recombinant granulocyte colony-stimulating factor-transferrin fusion protein as an oral myelopoietic agent. Proc Natl Acad Sci U S A. 2005;102:7292–7296. doi: 10.1073/pnas.0500062102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernkop-Schnurch A. The use of inhibitory agents to overcome the enzymatic barrier to perorally administered therapeutic peptides and proteins. J Control Release. 1998;52:1–16. doi: 10.1016/s0168-3659(97)00204-6. [DOI] [PubMed] [Google Scholar]
- Bernkop-Schnurch A, Hoffer MH, Kafedjiiski K. Thiomers for oral delivery of hydrophilic macromolecular drugs. Expert Opin Drug Deliv. 2004;1:87–98. doi: 10.1517/17425247.1.1.87. [DOI] [PubMed] [Google Scholar]
- Bernkop-Schnurch A, Krajicek ME. Mucoadhesive polymers as platforms for peroral peptide delivery and absorption: synthesis and evaluation of different chitosan-EDTA conjugates. J Control Release. 1998;50:215–223. doi: 10.1016/s0168-3659(97)00136-3. [DOI] [PubMed] [Google Scholar]
- Bernkop-Schnurch A, Weithaler A, Albrecht K, Greimel A. Thiomers: preparation and in vitro evaluation of a mucoadhesive nanoparticulate drug delivery system. Int J Pharm. 2006;317:76–81. doi: 10.1016/j.ijpharm.2006.02.044. [DOI] [PubMed] [Google Scholar]
- Borchardt RT. Optimizing oral absorption of peptides using prodrug strategies. J Control Release. 1999;62:231–238. doi: 10.1016/s0168-3659(99)00042-5. [DOI] [PubMed] [Google Scholar]
- Brewster ME, Loftsson T. Cyclodextrins as pharmaceutical solubilizers. Adv Drug Deliv Rev. 2007;59:645–666. doi: 10.1016/j.addr.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Brown ND, Butler DL, Chiang PK. Stabilization of thymopentin and preservation of its pharmacological properties by 2-hydroxypropyl-beta-cyclodextrin. J Pharm Pharmacol. 1993;45:666–667. doi: 10.1111/j.2042-7158.1993.tb05675.x. [DOI] [PubMed] [Google Scholar]
- Buda A, Sands C, Jepson MA. Use of fluorescence imaging to investigate the structure and function of intestinal M cells. Adv Drug Deliv Rev. 2005;57:123–134. doi: 10.1016/j.addr.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Builders PF, Kunle OO, Okpaku LC, Builders MI, Attama AA, Adikwu MU. Preparation and evaluation of mucinated sodium alginate microparticles for oral delivery of insulin. Eur J Pharm Biopharm. 2008;70:777–783. doi: 10.1016/j.ejpb.2008.06.021. [DOI] [PubMed] [Google Scholar]
- Burton PS, Conradi RA, Hilgers AR, Ho NF. Evidence for a polarized efflux system for peptides in the apical membrane of Caco-2 cells. Biochem Biophys Res Commun. 1993;190:760–766. doi: 10.1006/bbrc.1993.1114. [DOI] [PubMed] [Google Scholar]
- Burton PS, Conradi RA, Ho NF, Hilgers AR, Borchardt RT. How structural features influence the biomembrane permeability of peptides. J Pharm Sci. 1996;85:1336–1340. doi: 10.1021/js960067d. [DOI] [PubMed] [Google Scholar]
- Catnach SM, Fairclough PD, Hammond SM. Intestinal absorption of peptide drugs: advances in our understanding and clinical implications. Gut. 1994;35:441–444. doi: 10.1136/gut.35.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challa R, Ahuja A, Ali J, Khar RK. Cyclodextrins in drug delivery: an updated review. AAPS PharmSciTech. 2005;6:E329–E357. doi: 10.1208/pt060243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checkoway H, Boffetta P, Mundt DJ, Mundt KA. Critical review and synthesis of the epidemiologic evidence on formaldehyde exposure and risk of leukemia and other lymphohematopoietic malignancies. Cancer Causes Control. 2012;23:1747–1766. doi: 10.1007/s10552-012-0055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Yuan L, Zhou L, Zhang ZH, Cao W, Wu Q. Effect of cell-penetrating peptide-coated nanostructured lipid carriers on the oral absorption of tripterine. Int J Nanomedicine. 2012;7:4581–4591. doi: 10.2147/IJN.S34991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Teply BA, Jeong SY, Yim CH, Ho D, Sherifi I, Jon S, Farokhzad OC, Khademhosseini A, Langer RS. Magnetically responsive polymeric microparticles for oral delivery of protein drugs. Pharm Res. 2006;23:557–564. doi: 10.1007/s11095-005-9444-5. [DOI] [PubMed] [Google Scholar]
- Chien Y. Oral drug delivery and delivery systems. In: YW C, editor. Novel drug delivery systems. New York: Marcel Dekker Inc; 1992. pp. 139–196. [Google Scholar]
- Chittchang M, Mitra AK, Johnston TP. Interplay of secondary structure and charge on the diffusion of a polypeptide through negatively charged aqueous pores. Pharm Res. 2007;24:502–511. doi: 10.1007/s11095-006-9166-3. [DOI] [PubMed] [Google Scholar]
- Chittchang M, Salamat-Miller N, Alur HH, Vander Velde DG, Mitra AK, Johnston TP. Poly(L-lysine) as a model drug macromolecule with which to investigate secondary structure and microporous membrane transport, part 2: diffusion studies. J Pharm Pharmacol. 2002;54:1497–1505. doi: 10.1211/002235702108. [DOI] [PubMed] [Google Scholar]
- Chourasia MK, Jain SK. Pharmaceutical approaches to colon targeted drug delivery systems. J Pharm Pharm Sci. 2003;6:33–66. [PubMed] [Google Scholar]
- Clement S, Still JG, Kosutic G, McAllister RG. Oral insulin product hexyl-insulin monoconjugate 2 (HIM2) in type 1 diabetes mellitus: the glucose stabilization effects of HIM2. Diabetes Technol Ther. 2002;4:459–466. doi: 10.1089/152091502760306544. [DOI] [PubMed] [Google Scholar]
- Conradi RA, Hilgers AR, Ho NF, Burton PS. The influence of peptide structure on transport across Caco-2 cells. Pharm Res. 1991;8:1453–1460. doi: 10.1023/a:1015825912542. [DOI] [PubMed] [Google Scholar]
- Danforth E, Jr, Moore RO. Intestinal absorption of insulin in the rat. Endocrinology. 1959;65:118–123. doi: 10.1210/endo-65-1-118. [DOI] [PubMed] [Google Scholar]
- Del Curto MD, Maroni A, Palugan L, Zema L, Gazzaniga A, Sangalli ME. Oral delivery system for two-pulse colonic release of protein drugs and protease inhibitor/absorption enhancer compounds. J Pharm Sci. 2011;100:3251–3259. doi: 10.1002/jps.22560. [DOI] [PubMed] [Google Scholar]
- DeVane LC. Principles of pharmacokinetics and pharmacodynamics. In: Schatzberg AF, Nemeroff CB, editors. The American Psychiatric Publishing textbook of psychopharmacology. 3rd ed. Washington, DC: American Psychiatric Pub.; 2004. pp. 181–200. [Google Scholar]
- Dharma SK, Fishman PH, Peyman GA. A preliminary study of corneal penetration of 125I–labelled idoxuridine liposome. Acta Ophthalmol (Copenh) 1986;64:298–301. doi: 10.1111/j.1755-3768.1986.tb06923.x. [DOI] [PubMed] [Google Scholar]
- Dodoo AN, Bansal S, Barlow DJ, Bennet FC, Hider RC, Lansley AB, Lawrence MJ, Marriott C. Systematic investigations of the influence of molecular structure on the transport of peptides across cultured alveolar cell monolayers. Pharm Res. 2000;17:7–14. doi: 10.1023/a:1007514121527. [DOI] [PubMed] [Google Scholar]
- Faraji AH, Wipf P. Nanoparticles in cellular drug delivery. Bioorg Med Chem. 2009;17:2950–2962. doi: 10.1016/j.bmc.2009.02.043. [DOI] [PubMed] [Google Scholar]
- Fasano A. Modulation of intestinal permeability: an innovative method of oral drug delivery for the treatment of inherited and acquired human diseases. Mol Genet Metab. 1998;64:12–18. doi: 10.1006/mgme.1998.2667. [DOI] [PubMed] [Google Scholar]
- Fetih G, Fausia H, Okada N, Fujita T, Attia M, Yamamoto A. Colon-specific delivery and enhanced colonic absorption of [Asu(1,7)]-eel calcitonin using chitosan capsules containing various additives in rats. J Drug Target. 2006;14:165–172. doi: 10.1080/10611860600648494. [DOI] [PubMed] [Google Scholar]
- Fix JA. Oral controlled release technology for peptides: status and future prospects. Pharm Res. 1996;13:1760–1764. doi: 10.1023/a:1016008419367. [DOI] [PubMed] [Google Scholar]
- Fonte P, Andrade F, Araujo F, Andrade C, Neves J, Sarmento B. Chitosan-coated solid lipid nanoparticles for insulin delivery. Methods Enzymol. 2012;508:295–314. doi: 10.1016/B978-0-12-391860-4.00015-X. [DOI] [PubMed] [Google Scholar]
- Frokjaer S, Otzen DE. Protein drug stability: a formulation challenge. Nat Rev Drug Discov. 2005;4:298–306. doi: 10.1038/nrd1695. [DOI] [PubMed] [Google Scholar]
- Gangurde HH, CMA, Tamizharasi S, Sivakumar T, Upasani CD. Approaches for Peptides and Proteins by Colon Specific Delivery: Review. International Journal of Pharmaceutical Frontier Research. 2011 Apr-Jun;1:110–125. [Google Scholar]
- Garcia-Fuentes M, Prego C, Torres D, Alonso MJ. A comparative study of the potential of solid triglyceride nanostructures coated with chitosan or poly(ethylene glycol) as carriers for oral calcitonin delivery. Eur J Pharm Sci. 2005;25:133–143. doi: 10.1016/j.ejps.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Gazzaniga A, Iamartino P, Maffione G, Sangalli ME. Oral delayed-release system for colonic specific delivery. Int J Pharm. 1994;108:77–83. [Google Scholar]
- Gibaldi M. Biopharmaceutics and clinical pharmacokinetics. 4th ed. Philadelphia: Lea & Febiger; 1991. [Google Scholar]
- Gratieri T, Gelfuso GM, Rocha EM, Sarmento VH, de Freitas O, Lopez RF. A poloxamer/chitosan in situ forming gel with prolonged retention time for ocular delivery. Eur J Pharm Biopharm. 2010;75:186–193. doi: 10.1016/j.ejpb.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Guggi D, Kast CE, Bernkop-Schnurch A. In vivo evaluation of an oral salmon calcitonin-delivery system based on a thiolated chitosan carrier matrix. Pharm Res. 2003;20:1989–1994. doi: 10.1023/b:pham.0000008047.82334.7d. [DOI] [PubMed] [Google Scholar]
- Gulbake A, Jain SK. Chitosan: a potential polymer for colon-specific drug delivery system. Expert Opin Drug Deliv. 2012;9:713–729. doi: 10.1517/17425247.2012.682148. [DOI] [PubMed] [Google Scholar]
- Guo JH, Cooklock KM. The effects of backing materials and multilayered systems on the characteristics of bioadhesive buccal patches. J Pharm Pharmacol. 1996;48:255–257. doi: 10.1111/j.2042-7158.1996.tb05912.x. [DOI] [PubMed] [Google Scholar]
- Haas J, Lehr CM. Developments in the area of bioadhesive drug delivery systems. Expert Opin Biol Ther. 2002;2:287–298. doi: 10.1517/14712598.2.3.287. [DOI] [PubMed] [Google Scholar]
- Haeberlin B, Gengenbacher T, Meinzer A, Fricker G. Cyclodextrins -- Useful excipients for oral peptide administration? Int J Pharm. 1996;137:103–110. [Google Scholar]
- Hamman JH, Enslin GM, Kotze AF. Oral delivery of peptide drugs: barriers and developments. BioDrugs. 2005;19:165–177. doi: 10.2165/00063030-200519030-00003. [DOI] [PubMed] [Google Scholar]
- Hashizume M, Douen T, Murakami M, Yamamoto A, Takada K, Muranishi S. Improvement of large intestinal absorption of insulin by chemical modification with palmitic acid in rats. J Pharm Pharmacol. 1992;44:555–559. doi: 10.1111/j.2042-7158.1992.tb05463.x. [DOI] [PubMed] [Google Scholar]
- Hauptstein S, Bernkop-Schnurch A. Thiomers and thiomer-based nanoparticles in protein and DNA drug delivery. Expert Opin Drug Deliv. 2012;9:1069–1081. doi: 10.1517/17425247.2012.697893. [DOI] [PubMed] [Google Scholar]
- Hichens M. A comparison of thyrotropin-releasing hormone with analogs: influence of disposition upon pharmacology. Drug Metab Rev. 1983;14:77–98. doi: 10.3109/03602538308991382. [DOI] [PubMed] [Google Scholar]
- Hsieh PW, Hung CF, Fang JY. Current prodrug design for drug discovery. Curr Pharm Des. 2009;15:2236–2250. doi: 10.2174/138161209788682523. [DOI] [PubMed] [Google Scholar]
- Hurni MA, Noach AB, Blom-Roosemalen MC, de Boer AG, Nagelkerke JF, Breimer DD. Permeability enhancement in Caco-2 cell monolayers by sodium salicylate and sodium taurodihydrofusidate: assessment of effect-reversibility and imaging of transepithelial transport routes by confocal laser scanning microscopy. J Pharmacol Exp Ther. 1993;267:942–950. [PubMed] [Google Scholar]
- Hussain N, Jani PU, Florence AT. Enhanced oral uptake of tomato lectin-conjugated nanoparticles in the rat. Pharm Res. 1997;14:613–618. doi: 10.1023/a:1012153011884. [DOI] [PubMed] [Google Scholar]
- Ichikawa H, Peppas NA. Novel complexation hydrogels for oral peptide delivery: in vitro evaluation of their cytocompatibility and insulin-transport enhancing effects using Caco-2 cell monolayers. J Biomed Mater Res A. 2003;67:609–617. doi: 10.1002/jbm.a.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal J, Shahnaz G, Dunnhaupt S, Muller C, Hintzen F, Bernkop-Schnurch A. Preactivated thiomers as mucoadhesive polymers for drug delivery. Biomaterials. 2012;33:1528–1535. doi: 10.1016/j.biomaterials.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie T, Uekama K. Cyclodextrins in peptide and protein delivery. Adv Drug Deliv Rev. 1999;36:101–123. doi: 10.1016/s0169-409x(98)00057-x. [DOI] [PubMed] [Google Scholar]
- Jaehde U, Masereeuw R, De Boer AG, Fricker G, Nagelkerke JF, Vonderscher J, Breimer DD. Quantification and visualization of the transport of octreotide, a somatostatin analogue, across monolayers of cerebrovascular endothelial cells. Pharm Res. 1994;11:442–448. doi: 10.1023/a:1018929508018. [DOI] [PubMed] [Google Scholar]
- Jain S, Rathi VV, Jain AK, Das M, Godugu C. Folate-decorated PLGA nanoparticles as a rationally designed vehicle for the oral delivery of insulin. Nanomedicine (Lond) 2012;7:1311–1337. doi: 10.2217/nnm.12.31. [DOI] [PubMed] [Google Scholar]
- Jana S, Mandlekar S, Marathe P. Prodrug design to improve pharmacokinetic and drug delivery properties: challenges to the discovery scientists. Curr Med Chem. 2010;17:3874–3908. doi: 10.2174/092986710793205426. [DOI] [PubMed] [Google Scholar]
- Jerry N, Anitha Y, Sharma CP, Sony P. In vivo absorption studies of insulin from an oral delivery system. Drug Deliv. 2001;8:19–23. doi: 10.1080/107175401300002711. [DOI] [PubMed] [Google Scholar]
- Jin Y, Song Y, Zhu X, Zhou D, Chen C, Zhang Z, Huang Y. Goblet cell-targeting nanoparticles for oral insulin delivery and the influence of mucus on insulin transport. Biomaterials. 2012;33:1573–1582. doi: 10.1016/j.biomaterials.2011.10.075. [DOI] [PubMed] [Google Scholar]
- Jitendra, Sharma PK, Bansal S, Banik A. Noninvasive routes of proteins and peptides drug delivery. Indian J Pharm Sci. 2011;73:367–375. doi: 10.4103/0250-474X.95608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LR. Physiology of the gastrointestinal tract. 3rd ed. New York: Raven Press; 1994. [Google Scholar]
- Jones DH, Partidos CD, Steward MW, Farrar GH. Oral delivery of poly(lactide-co-glycolide) encapsulated vaccines. Behring Inst Mitt. 1997:220–228. [PubMed] [Google Scholar]
- Kamei N, Morishita M, Chiba H, Kavimandan NJ, Peppas NA, Takayama K. Complexation hydrogels for intestinal delivery of interferon beta and calcitonin. J Control Release. 2009a;134:98–102. doi: 10.1016/j.jconrel.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei N, Morishita M, Eda Y, Ida N, Nishio R, Takayama K. Usefulness of cell-penetrating peptides to improve intestinal insulin absorption. J Control Release. 2008;132:21–25. doi: 10.1016/j.jconrel.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Kamei N, Morishita M, Takayama K. Importance of intermolecular interaction on the improvement of intestinal therapeutic peptide/protein absorption using cell-penetrating peptides. J Control Release. 2009b;136:179–186. doi: 10.1016/j.jconrel.2009.02.015. [DOI] [PubMed] [Google Scholar]
- Kanwar JR, Long BM, Kanwar RK. The use of cyclodextrins nanoparticles for oral delivery. Curr Med Chem. 2011;18:2079–2085. doi: 10.2174/092986711795656243. [DOI] [PubMed] [Google Scholar]
- Katragadda S, Budda B, Anand BS, Mitra AK. Role of efflux pumps and metabolising enzymes in drug delivery. Expert Opin Drug Deliv. 2005;2:683–705. doi: 10.1517/17425247.2.4.683. [DOI] [PubMed] [Google Scholar]
- Kaur IP, Garg A, Singla AK, Aggarwal D. Vesicular systems in ocular drug delivery: an overview. Int J Pharm. 2004;269:1–14. doi: 10.1016/j.ijpharm.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Khafagy el S, Morishita M. Oral biodrug delivery using cell-penetrating peptide. Adv Drug Deliv Rev. 2012;64:531–539. doi: 10.1016/j.addr.2011.12.014. [DOI] [PubMed] [Google Scholar]
- Khafagy el S, Morishita M, Isowa K, Imai J, Takayama K. Effect of cell-penetrating peptides on the nasal absorption of insulin. J Control Release. 2009a;133:103–108. doi: 10.1016/j.jconrel.2008.09.076. [DOI] [PubMed] [Google Scholar]
- Khafagy el S, Morishita M, Kamei N, Eda Y, Ikeno Y, Takayama K. Efficiency of cell-penetrating peptides on the nasal and intestinal absorption of therapeutic peptides and proteins. Int J Pharm. 2009b;381:49–55. doi: 10.1016/j.ijpharm.2009.07.022. [DOI] [PubMed] [Google Scholar]
- Kidron M, Bar-On H, Berry EM, Ziv E. The absorption of insulin from various regions of the rat intestine. Life Sci. 1982;31:2837–2841. doi: 10.1016/0024-3205(82)90673-7. [DOI] [PubMed] [Google Scholar]
- Kim BY, Jeong JH, Park K, Kim JD. Bioadhesive interaction and hypoglycemic effect of insulin-loaded lectin-microparticle conjugates in oral insulin delivery system. J Control Release. 2005;102:525–538. doi: 10.1016/j.jconrel.2004.10.032. [DOI] [PubMed] [Google Scholar]
- Kipnes M, Dandona P, Tripathy D, Still JG, Kosutic G. Control of postprandial plasma glucose by an oral insulin product (HIM2) in patients with type 2 diabetes. Diabetes Care. 2003;26:421–426. doi: 10.2337/diacare.26.2.421. [DOI] [PubMed] [Google Scholar]
- Kisel MA, Kulik LN, Tsybovsky IS, Vlasov AP, Vorob’yov MS, Kholodova EA, Zabarovskaya ZV. Liposomes with phosphatidylethanol as a carrier for oral delivery of insulin: studies in the rat. Int J Pharm. 2001;216:105–114. doi: 10.1016/s0378-5173(01)00579-8. [DOI] [PubMed] [Google Scholar]
- Kompella UB, Lee VH. Delivery systems for penetration enhancement of peptide and protein drugs: design considerations. Adv Drug Deliv Rev. 2001;46:211–245. doi: 10.1016/s0169-409x(00)00137-x. [DOI] [PubMed] [Google Scholar]
- Koren E, Torchilin VP. Cell-penetrating peptides: breaking through to the other side. Trends Mol Med. 2012;18:385–393. doi: 10.1016/j.molmed.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Kramer W, Wess G, Enhsen A, Falk E, Hoffmann A, Neckermann G, Schubert G, Urmann M. Modified bile acids as carriers for peptides and drugs. Journal of Controlled Release. 1997;46:17–30. [Google Scholar]
- Kreuter J. Nanoparticles. In: Kreuter J, editor. Colloidal Drug Delivery Systems. New York: M. Dekker; 1994. pp. 219–342. [Google Scholar]
- Kumar P, Mishra B. Colon targeted drug delivery systems--an overview. Curr Drug Deliv. 2008;5:186–198. doi: 10.2174/156720108784911712. [DOI] [PubMed] [Google Scholar]
- Kurz D, Ciulla TA. Novel approaches for retinal drug delivery. Ophthalmol Clin North Am. 2002;15:405–410. doi: 10.1016/s0896-1549(02)00034-2. [DOI] [PubMed] [Google Scholar]
- Kwan KC. Oral bioavailability and first-pass effects. Drug Metab Dispos. 1997;25:1329–1336. [PubMed] [Google Scholar]
- Lane ME, Corrigan OI. Paracellular and transcellular pathways facilitate insulin permeability in rat gut. J Pharm Pharmacol. 2006;58:271–275. doi: 10.1211/jpp.58.2.0016. [DOI] [PubMed] [Google Scholar]
- Laskowski M, Jr, Haessler HA, Miech RP, Peanasky RJ, Laskowski M. Effect of trypsin inhibitor on passage of insulin across the intestinal barrier. Science. 1958;127:1115–1116. doi: 10.1126/science.127.3306.1115. [DOI] [PubMed] [Google Scholar]
- Lecluyse EL, Sutton SC, Fix JA. In vitro effects of long-chain acylcarnitines on the permeability, transepithelial electrical resistance and morphology of rat colonic mucosa. J Pharmacol Exp Ther. 1993;265:955–962. [PubMed] [Google Scholar]
- Lee H, Park TG. Preparation and characterization of mono-PEGylated epidermal growth factor: evaluation of in vitro biologic activity. Pharm Res. 2002;19:845–851. doi: 10.1023/a:1016113117851. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Amidon GL. The effect of enzyme inhibitor and absorption site following [D-ala2, D-leu5]enkephalin oral administration in rats. Biopharm Drug Dispos. 2002;23:131–141. doi: 10.1002/bdd.302. [DOI] [PubMed] [Google Scholar]
- Lee YH, Sinko PJ. Oral delivery of salmon calcitonin. Adv Drug Deliv Rev. 2000;42:225–238. doi: 10.1016/s0169-409x(00)00063-6. [DOI] [PubMed] [Google Scholar]
- Lehninger AL, Nelson DL, Cox MM. Lehninger principles of biochemistry. 4th ed. New York: W.H. Freeman; 2005. [Google Scholar]
- Lehr CM. From sticky stuff to sweet receptors--achievements, limits and novel approaches to bioadhesion. Eur J Drug Metab Pharmacokinet. 1996;21:139–148. doi: 10.1007/BF03190262. [DOI] [PubMed] [Google Scholar]
- Leone-Bay A, Sato M, Paton D, Hunt AH, Sarubbi D, Carozza M, Chou J, McDonough J, Baughman RA. Oral delivery of biologically active parathyroid hormone. Pharm Res. 2001;18:964–970. doi: 10.1023/a:1010936227570. [DOI] [PubMed] [Google Scholar]
- Li Z, Chan J, Sun W, Xu Y. Investigation of archaeosomes as carriers for oral delivery of peptides. Biochem Biophys Res Commun. 2010;394:412–417. doi: 10.1016/j.bbrc.2010.03.041. [DOI] [PubMed] [Google Scholar]
- Liang JF, Yang VC. Insulin-cell penetrating peptide hybrids with improved intestinal absorption efficiency. Biochem Biophys Res Commun. 2005;335:734–738. doi: 10.1016/j.bbrc.2005.07.142. [DOI] [PubMed] [Google Scholar]
- Lim CJ, Shen WC. Comparison of monomeric and oligomeric transferrin as potential carrier in oral delivery of protein drugs. J Control Release. 2005;106:273–286. doi: 10.1016/j.jconrel.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Liu DZ, LeCluyse EL, Thakker DR. Dodecylphosphocholine-mediated enhancement of paracellular permeability and cytotoxicity in Caco-2 cell monolayers. J Pharm Sci. 1999;88:1161–1168. doi: 10.1021/js990094e. [DOI] [PubMed] [Google Scholar]
- Loe DW, Sharom FJ. Interaction of multidrug-resistant Chinese hamster ovary cells with the peptide ionophore gramicidin D. Biochim Biophys Acta. 1994;1190:72–84. doi: 10.1016/0005-2736(94)90035-3. [DOI] [PubMed] [Google Scholar]
- Loftsson T, Brewster ME. Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization. J Pharm Sci. 1996;85:1017–1025. doi: 10.1021/js950534b. [DOI] [PubMed] [Google Scholar]
- Longer MA, Ch'ng HS, Robinson JR. Bioadhesive polymers as platforms for oral controlled drug delivery III: oral delivery of chlorothiazide using a bioadhesive polymer. J Pharm Sci. 1985;74:406–411. doi: 10.1002/jps.2600740408. [DOI] [PubMed] [Google Scholar]
- Lowman AM, Morishita M, Kajita M, Nagai T, Peppas NA. Oral delivery of insulin using pH-responsive complexation gels. J Pharm Sci. 1999;88:933–937. doi: 10.1021/js980337n. [DOI] [PubMed] [Google Scholar]
- Maculotti K, Tira EM, Sonaggere M, Perugini P, Conti B, Modena T, Pavanetto F. In vitro evaluation of chondroitin sulphate-chitosan microspheres as carrier for the delivery of proteins. J Microencapsul. 2009;26:535–543. doi: 10.1080/02652040802485725. [DOI] [PubMed] [Google Scholar]
- Mahato RI, Narang AS, Thoma L, Miller DD. Emerging trends in oral delivery of peptide and protein drugs. Crit Rev Ther Drug Carrier Syst. 2003;20:153–214. doi: 10.1615/critrevtherdrugcarriersyst.v20.i23.30. [DOI] [PubMed] [Google Scholar]
- Malik DK, Baboota S, Ahuja A, Hasan S, Ali J. Recent advances in protein and peptide drug delivery systems. Curr Drug Deliv. 2007;4:141–151. doi: 10.2174/156720107780362339. [DOI] [PubMed] [Google Scholar]
- Maroni A, Zema L, Del Curto MD, Foppoli A, Gazzaniga A. Oral colon delivery of insulin with the aid of functional adjuvants. Adv Drug Deliv Rev. 2012;64:540–556. doi: 10.1016/j.addr.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Marx M. Watching peptide drugs grow up. Chem. Eng. News. 2005;83:17–24. [Google Scholar]
- Millotti G, Perera G, Vigl C, Pickl K, Sinner FM, Bernkop-Schnurch A. The use of chitosan-6-mercaptonicotinic acid nanoparticles for oral peptide drug delivery. Drug Deliv. 2011;18:190–197. doi: 10.3109/10717544.2010.522611. [DOI] [PubMed] [Google Scholar]
- Mizuma T, Koyanagi A, Awazu S. Intestinal transport and metabolism of glucose-conjugated kyotorphin and cyclic kyotorphin: metabolic degradation is crucial to intestinal absorption of peptide drugs. Biochim Biophys Acta. 2000;1475:90–98. doi: 10.1016/s0304-4165(00)00051-9. [DOI] [PubMed] [Google Scholar]
- MJ ON, Guo J, Byrne C, Darcy R, CM OD. Mechanistic studies on the uptake and intracellular trafficking of novel cyclodextrin transfection complexes by intestinal epithelial cells. Int J Pharm. 2011;413:174–183. doi: 10.1016/j.ijpharm.2011.04.021. [DOI] [PubMed] [Google Scholar]
- Mohanraj VJ, Barnes TJ, Prestidge CA. Silica nanoparticle coated liposomes: a new type of hybrid nanocapsule for proteins. Int J Pharm. 2010;392:285–293. doi: 10.1016/j.ijpharm.2010.03.061. [DOI] [PubMed] [Google Scholar]
- Morishita M, Kamei N, Ehara J, Isowa K, Takayama K. A novel approach using functional peptides for efficient intestinal absorption of insulin. J Control Release. 2007;118:177–184. doi: 10.1016/j.jconrel.2006.12.022. [DOI] [PubMed] [Google Scholar]
- Morishita M, Lowman AM, Takayama K, Nagai T, Peppas NA. Elucidation of the mechanism of incorporation of insulin in controlled release systems based on complexation polymers. J Control Release. 2002;81:25–32. doi: 10.1016/s0168-3659(02)00019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakada Y, Awata N, Nakamichi C, Sugimoto I. The effect of additives on the oral mucosal absorption of human calcitonin in rats. J Pharmacobiodyn. 1988;11:395–401. doi: 10.1248/bpb1978.11.395. [DOI] [PubMed] [Google Scholar]
- Nanjawade BK, Manvi FV, Manjappa AS. In situ-forming hydrogels for sustained ophthalmic drug delivery. J Control Release. 2007;122:119–134. doi: 10.1016/j.jconrel.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Navarro C, Gonzalez-Alvarez I, Gonzalez-Alvarez M, Manku M, Merino V, Casabo VG, Bermejo M. Influence of polyunsaturated fatty acids on Cortisol transport through MDCK and MDCK-MDR1 cells as blood-brain barrier in vitro model. Eur J Pharm Sci. 2011;42:290–299. doi: 10.1016/j.ejps.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Newey H, Smyth DH. The intestinal absorption of some dipeptides. J Physiol. 1959;145:48–56. doi: 10.1113/jphysiol.1959.sp006125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hagan DT, McGee JP, Holmgren J, Mowat AM, Donachie AM, Mills KH, Gaisford W, Rahman D, Challacombe SJ. Biodegradable microparticles for oral immunization. Vaccine. 1993;11:149–154. doi: 10.1016/0264-410x(93)90011-l. [DOI] [PubMed] [Google Scholar]
- O'Hagan DT, Rahman D, McGee JP, Jeffery H, Davies MC, Williams P, Davis SS, Challacombe SJ. Biodegradable microparticles as controlled release antigen delivery systems. Immunology. 1991;73:239–242. [PMC free article] [PubMed] [Google Scholar]
- Oh DH, Chun KH, Jeon SO, Kang JW, Lee S. Enhanced transbuccal salmon calcitonin (sCT) delivery: effect of chemical enhancers and electrical assistance on in vitro sCT buccal permeation. Eur J Pharm Biopharm. 2011;79:357–363. doi: 10.1016/j.ejpb.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003;55:329–347. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- Pappenheimer JR, Reiss KZ. Contribution of solvent drag through intercellular junctions to absorption of nutrients by the small intestine of the rat. J Membr Biol. 1987;100:123–136. doi: 10.1007/BF02209145. [DOI] [PubMed] [Google Scholar]
- Park K, Kwon IC, Park K. Oral protein delivery: Current status and future prospect. Reactive and Functional Polymers. 2011;71:280–287. [Google Scholar]
- Patel GB, Agnew BJ, Deschatelets L, Fleming LP, Sprott GD. In vitro assessment of archaeosome stability for developing oral delivery systems. Int J Pharm. 2000;194:39–49. doi: 10.1016/s0378-5173(99)00331-2. [DOI] [PubMed] [Google Scholar]
- Patel M, Shah T, Amin A. Therapeutic opportunities in colon-specific drug-delivery systems. Crit Rev Ther Drug Carrier Syst. 2007;24:147–202. doi: 10.1615/critrevtherdrugcarriersyst.v24.i2.20. [DOI] [PubMed] [Google Scholar]
- Patel MM. Cutting-edge technologies in colon-targeted drug delivery systems. Expert Opin Drug Deliv. 2011;8:1247–1258. doi: 10.1517/17425247.2011.597739. [DOI] [PubMed] [Google Scholar]
- Pauletti GM, Okumu FW, Borchardt RT. Effect of size and charge on the passive diffusion of peptides across Caco-2 cell monolayers via the paracellular pathway. Pharm Res. 1997;14:164–168. doi: 10.1023/a:1012040425146. [DOI] [PubMed] [Google Scholar]
- Peh KK, Wong CF. Polymeric films as vehicle for buccal delivery: swelling, mechanical, and bioadhesive properties. J Pharm Pharm Sci. 1999;2:53–61. [PubMed] [Google Scholar]
- Peppas NA, Bures P, Leobandung W, Ichikawa H. Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm. 2000;50:27–46. doi: 10.1016/s0939-6411(00)00090-4. [DOI] [PubMed] [Google Scholar]
- Perera G, Greindl M, Palmberger TF, Bernkop-Schnurch A. Insulin-loaded poly(acrylic acid)-cysteine nanoparticles: stability studies towards digestive enzymes of the intestine. Drug Deliv. 2009;16:254–260. doi: 10.1080/10717540902937471. [DOI] [PubMed] [Google Scholar]
- R TB, Jeffrey A, Siahaan TJ, Gangwar S, Pauletti GM. Improvement of oral peptide bioavailability: Peptidomimetics and prodrug strategies. Adv Drug Deliv Rev. 1997;27:235–256. doi: 10.1016/s0169-409x(97)00045-8. [DOI] [PubMed] [Google Scholar]
- Rado JP, Marosi J, Szende L, Borbely L, Tako J, Fischer J. The antidiuretic action of 1-deamino-8-D-arginine vasopressin (DDAVP) in man. Int J Clin Pharmacol Biopharm. 1976;13:199–209. [PubMed] [Google Scholar]
- Rafii F, Franklin W, Cerniglia CE. Azoreductase activity of anaerobic bacteria isolated from human intestinal microflora. Appl Environ Microbiol. 1990;56:2146–2151. doi: 10.1128/aem.56.7.2146-2151.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautio J, Kumpulainen H, Heimbach T, Oliyai R, Oh D, Jarvinen T, Savolainen J. Prodrugs: design and clinical applications. Nat Rev Drug Discov. 2008;7:255–270. doi: 10.1038/nrd2468. [DOI] [PubMed] [Google Scholar]
- Repassy G, Lapis K. Ultrastructural characteristics, secretory and phagocytotic activity of Paneth cells. Acta Morphol Acad Sci Hung. 1979;27:21–24. [PubMed] [Google Scholar]
- Ridgley CD, Jr, Wilkins JR. A comparison of median hearing thresholds from U.S. Navy and U.S. Army audiometric data bases. Mil Med. (3rd) 1991;156:343–345. [PubMed] [Google Scholar]
- Roldo M, Barbu E, Brown JF, Laight DW, Smart JD, Tsibouklis J. Azo compounds in colon-specific drug delivery. Expert Opin Drug Deliv. 2007;4:547–560. doi: 10.1517/17425247.4.5.547. [DOI] [PubMed] [Google Scholar]
- Rubinstein I. Oral insulin: deja vu? Nanomedicine (Lond) 2012;7:631–632. doi: 10.2217/nnm.12.38. [DOI] [PubMed] [Google Scholar]
- Russell-Jones GJ. The potential use of receptor-mediated endocytosis for oral drug delivery. Adv Drug Deliv Rev. 2001;46:59–73. doi: 10.1016/s0169-409x(00)00127-7. [DOI] [PubMed] [Google Scholar]
- Russell-Jones GJ. Use of targeting agents to increase uptake and localization of drugs to the intestinal epithelium. J Drug Target. 2004;12:113–123. doi: 10.1080/10611860410001693760. [DOI] [PubMed] [Google Scholar]
- Saffran M, Kumar GS, Savariar C, Burnham JC, Williams F, Neckers DC. A new approach to the oral administration of insulin and other peptide drugs. Science. 1986;233:1081–1084. doi: 10.1126/science.3526553. [DOI] [PubMed] [Google Scholar]
- Salamat-Miller N, Johnston TP. Current strategies used to enhance the paracellular transport of therapeutic polypeptides across the intestinal epithelium. Int J Pharm. 2005;294:201–216. doi: 10.1016/j.ijpharm.2005.01.022. [DOI] [PubMed] [Google Scholar]
- Sarmento B, Martins S, Ferreira D, Souto EB. Oral insulin delivery by means of solid lipid nanoparticles. Int J Nanomedicine. 2007;2:743–749. [PMC free article] [PubMed] [Google Scholar]
- Schilling RJ, Mitra AK. Intestinal mucosal transport of insulin. Int J Pharm. 1990;62:53–64. [Google Scholar]
- Schipper NG, Varum KM, Artursson P. Chitosans as absorption enhancers for poorly absorbable drugs. 1: Influence of molecular weight and degree of acetylation on drug transport across human intestinal epithelial (Caco-2) cells. Pharm Res. 1996;13:1686–1692. doi: 10.1023/a:1016444808000. [DOI] [PubMed] [Google Scholar]
- Schipper NG, Varum KM, Stenberg P, Ocklind G, Lennernas H, Artursson P. Chitosans as absorption enhancers of poorly absorbable drugs. 3: Influence of mucus on absorption enhancement. Eur J Pharm Sci. 1999;8:335–343. doi: 10.1016/s0928-0987(99)00032-9. [DOI] [PubMed] [Google Scholar]
- Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- Senel S, Hincal AA. Drug permeation enhancement via buccal route: possibilities and limitations. J Control Release. 2001;72:133–144. doi: 10.1016/s0168-3659(01)00269-3. [DOI] [PubMed] [Google Scholar]
- Shah N, Shah T, Amin A. Polysaccharides: a targeting strategy for colonic drug delivery. Expert Opin Drug Deliv. 2011;8:779–796. doi: 10.1517/17425247.2011.574121. [DOI] [PubMed] [Google Scholar]
- Shaikh R, Raj Singh TR, Garland MJ, Woolfson AD, Donnelly RF. Mucoadhesive drug delivery systems. J Pharm Bioallied Sci. 2011;3:89–100. doi: 10.4103/0975-7406.76478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaji J, Patole V. Protein and Peptide drug delivery: oral approaches. Indian J Pharm Sci. 2008;70:269–277. doi: 10.4103/0250-474X.42967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakweh M, Ponchel G, Fattal E. Particle uptake by Peyer's patches: a pathway for drug and vaccine delivery. Expert Opin Drug Deliv. 2004;1:141–163. doi: 10.1517/17425247.1.1.141. [DOI] [PubMed] [Google Scholar]
- Shao Z, Li Y, Chermak T, Mitra AK. Cyclodextrins as mucosal absorption promoters of insulin. II. Effects of beta-cyclodextrin derivatives on alpha-chymotryptic degradation and enteral absorption of insulin in rats. Pharm Res. 1994;11:1174–1179. doi: 10.1023/a:1018997101542. [DOI] [PubMed] [Google Scholar]
- Sharom FJ, Yu X, DiDiodato G, Chu JW. Synthetic hydrophobic peptides are substrates for P-glycoprotein and stimulate drug transport. Biochem J. 1996;320(Pt 2):421–428. doi: 10.1042/bj3200421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard RC. Amino Acids, Peptides, and Proteins. Royal Society of Chemistry. 1981 [Google Scholar]
- Siegel RA, Langer R. Controlled Release of Polypeptides and Other Macromolecules. Pharmaceutical Research. 1984;1:2–10. doi: 10.1023/A:1016318423563. [DOI] [PubMed] [Google Scholar]
- Singh R, Lillard JW., Jr Nanoparticle-based targeted drug delivery. Exp Mol Pathol. 2009;86:215–223. doi: 10.1016/j.yexmp.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha V, Singh A, Kumar RV, Singh S, Kumria R, Bhinge J. Oral colon-specific drug delivery of protein and peptide drugs. Crit Rev Ther Drug Carrier Syst. 2007;24:63–92. doi: 10.1615/critrevtherdrugcarriersyst.v24.i1.30. [DOI] [PubMed] [Google Scholar]
- Sinha VR, Kumria R. Microbially triggered drug delivery to the colon. Eur J Pharm Sci. 2003;18:3–18. doi: 10.1016/s0928-0987(02)00221-x. [DOI] [PubMed] [Google Scholar]
- Sithigorngul P, Burton P, Nishihata T, Caldwell L. Effects of sodium salicylate on epithelial cells of the rectal mucosa of the rat: a light and electron microscopic study. Life Sci. 1983;33:1025–1032. doi: 10.1016/0024-3205(83)90656-2. [DOI] [PubMed] [Google Scholar]
- Sood A, Panchagnula R. Peroral route: an opportunity for protein and peptide drug delivery. Chem Rev. 2001;101:3275–3303. doi: 10.1021/cr000700m. [DOI] [PubMed] [Google Scholar]
- Sprott GD. Structures of archaebacterial membrane lipids. J Bioenerg Biomembr. 1992;24:555–566. doi: 10.1007/BF00762348. [DOI] [PubMed] [Google Scholar]
- Sprott GD, Sad S, Fleming LP, Dicaire CJ, Patel GB, Krishnan L. Archaeosomes varying in lipid composition differ in receptor-mediated endocytosis and differentially adjuvant immune responses to entrapped antigen. Archaea. 2003;1:151–164. doi: 10.1155/2003/569283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella VJ. Prodrug approaches to enhancing the oral delivery of poorly permeable drugs. In: Stella VJ, Borchardt RT, Hageman MJ, Maag H, Oliyai R, Tilley JW, SpringerLink (Online service), editors. Prodrugs Challenges and Rewards Part 1. New York, NY: American Association of Pharmaceutical Scientists; 2007. pp. 37–82. [Google Scholar]
- Su FY, Lin KJ, Sonaje K, Wey SP, Yen TC, Ho YC, Panda N, Chuang EY, Maiti B, Sung HW. Protease inhibition and absorption enhancement by functional nanoparticles for effective oral insulin delivery. Biomaterials. 2012;33:2801–2811. doi: 10.1016/j.biomaterials.2011.12.038. [DOI] [PubMed] [Google Scholar]
- Swenson ES, Milisen WB, Curatolo W. Intestinal permeability enhancement: structure-activity and structure-toxicity relationships for nonylphenoxypolyoxyethylene surfactant permeability enhancers. Pharm Res. 1994;11:1501–1504. doi: 10.1023/a:1018920728800. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Kim RB, Perry PR, Wilkinson GR. Characterization of the hepatic canalicular membrane transport of a model oligopeptide: ditekiren. J Pharmacol Exp Ther. 1997;281:297–303. [PubMed] [Google Scholar]
- Tenma T, Yodoya E, Tashima S, Fujita T, Murakami M, Yamamoto A, Muranishi S. Development of new lipophilic derivatives of tetragastrin: physicochemical characteristics and intestinal absorption of acyl-tetragastrin derivatives in rats. Pharm Res. 1993;10:1488–1492. doi: 10.1023/a:1018983511247. [DOI] [PubMed] [Google Scholar]
- Thanou M, Verhoef JC, Junginger HE. Oral drug absorption enhancement by chitosan and its derivatives. Adv Drug Deliv Rev. 2001;52:117–126. doi: 10.1016/s0169-409x(01)00231-9. [DOI] [PubMed] [Google Scholar]
- Thirawong N, Thongborisute J, Takeuchi H, Sriamornsak P. Improved intestinal absorption of calcitonin by mucoadhesive delivery of novel pectin-liposome nanocomplexes. J Control Release. 2008;125:236–245. doi: 10.1016/j.jconrel.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Thwaites DT, Hirst BH, Simmons NL. Passive transepithelial absorption of thyrotropin-releasing hormone (TRH) via a paracellular route in cultured intestinal and renal epithelial cell lines. Pharm Res. 1993;10:674–681. doi: 10.1023/a:1018947430018. [DOI] [PubMed] [Google Scholar]
- Tomita M, Shiga M, Hayashi M, Awazu S. Enhancement of colonic drug absorption by the paracellular permeation route. Pharm Res. 1988;5:341–346. doi: 10.1023/a:1015999309353. [DOI] [PubMed] [Google Scholar]
- Torres-Lugo M, Garcia M, Record R, Peppas NA. pH-Sensitive hydrogels as gastrointestinal tract absorption enhancers: transport mechanisms of salmon calcitonin and other model molecules using the Caco-2 cell model. Biotechnol Prog. 2002;18:612–616. doi: 10.1021/bp0101379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortora GJ, Grabowski SR. Principles of anatomy and physiology. 8th ed. New York, NY: HarperCollins College; 1996. [Google Scholar]
- Touitou E, Rubinstein A. Targeted enteral delivery of insulin to rats. Int J Pharm. 1986;30:95–99. [Google Scholar]
- Tozaki H, Komoike J, Tada C, Maruyama T, Terabe A, Suzuki T, Yamamoto A, Muranishi S. Chitosan capsules for colon-specific drug delivery: improvement of insulin absorption from the rat colon. J Pharm Sci. 1997;86:1016–1021. doi: 10.1021/js970018g. [DOI] [PubMed] [Google Scholar]
- Tozaki H, Nishioka J, Komoike J, Okada N, Fujita T, Muranishi S, Kim SI, Terashima H, Yamamoto A. Enhanced absorption of insulin and (Asu(1,7))eel-calcitonin using novel azopolymer-coated pellets for colon-specific drug delivery. J Pharm Sci. 2001;90:89–97. doi: 10.1002/1520-6017(200101)90:1<89::aid-jps10>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Trapani A, Laquintana V, Denora N, Lopedota A, Cutrignelli A, Franco M, Trapani G, Liso G. Eudragit RS 100 microparticles containing 2-hydroxypropyl-beta-cyclodextrin and glutathione: physicochemical characterization, drug release and transport studies. Eur J Pharm Sci. 2007;30:64–74. doi: 10.1016/j.ejps.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Trehin R, Merkle HP. Chances and pitfalls of cell penetrating peptides for cellular drug delivery. Eur J Pharm Biopharm. 2004;58:209–223. doi: 10.1016/j.ejpb.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Tsuji A, Tamai I. Carrier-mediated intestinal transport of drugs. Pharm Res. 1996;13:963–977. doi: 10.1023/a:1016086003070. [DOI] [PubMed] [Google Scholar]
- Tuesca AD, Reiff C, Joseph JI, Lowman AM. Synthesis, characterization and in vivo efficacy of PEGylated insulin for oral delivery with complexation hydrogels. Pharm Res. 2009;26:727–739. doi: 10.1007/s11095-008-9816-8. [DOI] [PubMed] [Google Scholar]
- Ueda K, Shimabuku AM, Konishi H, Fujii Y, Takebe S, Nishi K, Yoshida M, Beppu T, Komano T. Functional expression of human P-glycoprotein in Schizosaccharomyces pombe. FEBS Lett. 1993;330:279–282. doi: 10.1016/0014-5793(93)80888-2. [DOI] [PubMed] [Google Scholar]
- Vadlapudi AD, Vadlapatla RK, Kwatra D, Earla R, Samanta SK, Pal D, Mitra AK. Targeted lipid based drug conjugates: A novel strategy for drug delivery. International journal of pharmaceutics. 2012a;434:315–324. doi: 10.1016/j.ijpharm.2012.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadlapudi AD, Vadlapatla RK, Mitra AK. Sodium dependent multivitamin transporter (SMVT): a potential target for drug delivery. Curr Drug Targets. 2012b;13:994–1003. doi: 10.2174/138945012800675650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Graaff KM. Anatomy and physiology of the gastrointestinal tract. Pediatr Infect Dis. 1986;5:S11–S16. doi: 10.1097/00006454-198601001-00005. [DOI] [PubMed] [Google Scholar]
- Varma MV, Ashokraj Y, Dey CS, Panchagnula R. P-glycoprotein inhibitors and their screening: a perspective from bioavailability enhancement. Pharmacol Res. 2003;48:347–359. doi: 10.1016/s1043-6618(03)00158-0. [DOI] [PubMed] [Google Scholar]
- Vavra I, Machova A, Krejci I. Antidiuretic action of 1-deamino-(8-D-arginine)-vasopressin in unanesthetized rats. J Pharmacol Exp Ther. 1974;188:241–247. [PubMed] [Google Scholar]
- Vilhardt H, Lundin S. In vitro intestinal transport of vasopressin and its analogues. Acta Physiol Scand. 1986;126:601–607. doi: 10.1111/j.1748-1716.1986.tb07861.x. [DOI] [PubMed] [Google Scholar]
- Vinay Kumar KV, Sivakumar T, T Tm. Colon targeting drug delivery system: A review on recent approaches. Int J Pharm Biomed Sci. 2011;2:11–19. [Google Scholar]
- Vives E. Present and future of cell-penetrating peptide mediated delivery systems: “is the Trojan horse too wild to go only to Troy?”. J Control Release. 2005;109:77–85. doi: 10.1016/j.jconrel.2005.09.032. [DOI] [PubMed] [Google Scholar]
- Walter E, Kissel T, Raddatz P. Transport of peptidomimetic renin inhibitors across monolayers of a human intestinal cell line (Caco-2): evidence for self-enhancement of paracellular transport route. Pharm Res. 1995;12:1801–1805. doi: 10.1023/a:1016246629130. [DOI] [PubMed] [Google Scholar]
- Wang J, Chow D, Heiati H, Shen WC. Reversible lipidization for the oral delivery of salmon calcitonin. J Control Release. 2003;88:369–380. doi: 10.1016/s0168-3659(03)00008-7. [DOI] [PubMed] [Google Scholar]
- Wang J, Hogenkamp DJ, Tran M, Li WY, Yoshimura RF, Johnstone TB, Shen WC, Gee KW. Reversible lipidization for the oral delivery of leu-enkephalin. J Drug Target. 2006;14:127–136. doi: 10.1080/10611860600648221. [DOI] [PubMed] [Google Scholar]
- Wang X, Iqbal J, Rahmat D, Bernkop-Schnurch A. Preactivated thiomers: permeation enhancing properties. Int J Pharm. 2012;438:217–224. doi: 10.1016/j.ijpharm.2012.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Takeuchi T, Chey WY. Mediation of trypsin inhibitor-induced pancreatic hypersecretion by secretin and cholecystokinin in rats. Gastroenterology. 1992;102:621–628. doi: 10.1016/0016-5085(92)90111-b. [DOI] [PubMed] [Google Scholar]
- Werle M, Makhlof A, Takeuchi H. Oral protein delivery: a patent review of academic and industrial approaches. Recent Pat Drug Deliv Formul. 2009;3:94–104. doi: 10.2174/187221109788452221. [DOI] [PubMed] [Google Scholar]
- Williams AC, Barry BW. Penetration enhancers. Adv Drug Deliv Rev. 2004;56:603–618. doi: 10.1016/j.addr.2003.10.025. [DOI] [PubMed] [Google Scholar]
- Wroblewski S, Berenson M, Kopeckova P, Kopecek J. Biorecognition of HPMA copolymer-lectin conjugates as an indicator of differentiation of cell-surface glycoproteins in development, maturation, and diseases of human and rodent gastrointestinal tissues. J Biomed Mater Res. 2000;51:329–342. doi: 10.1002/1097-4636(20000905)51:3<329::aid-jbm6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Hayakawa E, Kato Y, Nishiura A, Lee VH. A mechanistic study on enhancement of rectal permeability to insulin in the albino rabbit. J Pharmacol Exp Ther. 1992;263:25–31. [PubMed] [Google Scholar]
- Yamamoto A, Taniguchi T, Rikyuu K, Tsuji T, Fujita T, Murakami M, Muranishi S. Effects of various protease inhibitors on the intestinal absorption and degradation of insulin in rats. Pharm Res. 1994;11:1496–1500. doi: 10.1023/a:1018968611962. [DOI] [PubMed] [Google Scholar]
- Yang HPA, Nguyen Vu Anh, Dong Liang C, Wong Patrick SL. In: Peptide formulation. States U, editor. Palo Alto, CA: Alza Corporation; 1999. CA), (San Jose, CA), (Mountain View, CA), (Palo Alto, CA) [Google Scholar]
- Yin L, Ding J, He C, Cui L, Tang C, Yin C. Drug permeability and mucoadhesion properties of thiolated trimethyl chitosan nanoparticles in oral insulin delivery. Biomaterials. 2009;30:5691–5700. doi: 10.1016/j.biomaterials.2009.06.055. [DOI] [PubMed] [Google Scholar]
- Yuan H, Jiang SP, Du YZ, Miao J, Zhang XG, Hu FQ. Strategic approaches for improving entrapment of hydrophilic peptide drugs by lipid nanoparticles. Colloids Surf B Biointerfaces. 2009;70:248–253. doi: 10.1016/j.colsurfb.2008.12.031. [DOI] [PubMed] [Google Scholar]
- Yun Y, Cho YW, Park K. Nanoparticles for oral delivery: Targeted nanoparticles with peptidic ligands for oral protein delivery. Adv Drug Deliv Rev. 2012 doi: 10.1016/j.addr.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Alsarra IA, Neau SH. An in vitro evaluation of a chitosan-containing multiparticulate system for macromolecule delivery to the colon. Int J Pharm. 2002;239:197–205. doi: 10.1016/s0378-5173(02)00112-6. [DOI] [PubMed] [Google Scholar]
- Zhang L, Bulaj G. Converting peptides into drug leads by lipidation. Curr Med Chem. 2012;19:1602–1618. doi: 10.2174/092986712799945003. [DOI] [PubMed] [Google Scholar]
- Zorko M, Langel U. Cell-penetrating peptides: mechanism and kinetics of cargo delivery. Adv Drug Deliv Rev. 2005;57:529–545. doi: 10.1016/j.addr.2004.10.010. [DOI] [PubMed] [Google Scholar]