Abstract
Src-like adaptor protein (SLAP) is a hematopoietic adaptor containing Src homology (SH)3 and SH2 motifs and a unique carboxy terminus. Unlike c-Src, SLAP lacks a tyrosine kinase domain. We investigated the role of SLAP in osteoclast development and resorptive function. Employing SLAP-deficient mice, we find lack of the adaptor enhances in vitro proliferation of osteoclast precursors in the form of bone marrow macrophages (BMMs), without altering their survival. Furthermore, osteoclastogenic markers appear more rapidly in SLAP−/− BMMs exposed to RANK ligand (RANKL). The accelerated proliferation of M-CSF-treated, SLAP-deficient precursors is associated with enhanced ERK activation. SLAP’s role as a mediator of M-CSF signaling, in osteoclastic cells, is buttressed by complexing of the adaptor protein and c-Fms in lipid rafts. Unlike c-Src, SLAP does not impact resorptive function of mature osteoclasts, but induces their early apoptosis. Thus, SLAP negatively regulates differentiation of osteoclasts and proliferation of their precursors. Conversely, SLAP decreases osteoclast death by inhibiting activation of caspase 3. These counterbalancing events yield indistinguishable bones of WT and SLAP−/− mice which contain equal numbers of osteoclasts in basal and stimulated conditions.
Keywords: SLAP, osteoclasts, c-Src
Multinucleated osteoclasts, the sole bone-resorbing cells, are critical to skeletal development and maintenance [Teitelbaum, 2000]. They are derived from monocyte/macrophage precursors under the aegis of macrophage colony stimulating factor (M-CSF) and receptor activator of NF-κB ligand (RANKL). While RANKL regulates osteoclast differentiation, M-CSF promotes proliferation of marrow macrophages [Boyle et al., 2003; Pixley and Stanley, 2004; Teitelbaum and Ross, 2003]. RANKL promotes mature osteoclast survival and M-CSF plays the same role for these bone resorbing polykaryons and their precursors [Akiyama et al., 2003; Xaus et al., 2001].
M-CSF binds to its receptor tyrosine kinase (RTK), c-Fms, inducing dimerization and autophosphorylation of specific cytoplasmic domain tyrosine residues. The c-Fms phosphorylated tyrosines recognize SH2 motif-containing proteins, including Src family kinases (SFKs) and PI3K [Alonso et al., 1995; Li and Stanley, 1991; Reedijk et al., 1992]. Recruitment of SH2 domain-bearing proteins to the receptor activates MAP kinases and Akt [Pixley and Stanley, 2004; Ross, 2006]. The MAP kinase cascade, involving Ras/MEK/ERK, is primarily responsible for M-CSF-induced macrophage proliferation [Jaworowski et al., 1999; Takeshita et al., 2007; Valledor et al., 2000], while PI3K/Akt regulates survival [Kelley et al., 1999], proliferation [Zhou et al., 2006] and cytoskeletal organization [Faccio et al., 2007].
The SFK, c-Src, plays a critical role in osteoclast function when activated by M-CSF/c-Fms and/or RANKL/RANK [Insogna et al., 1997; Wang et al., 2004]. The domain structure of SFKs is similar to that of the SLAP (Src-like adaptor protein) family proteins, SLAP and SLAP-2 [Pandey et al., 1995]. Resembling c-Src, SLAP contains a short, unique NH2-terminal region that, when myristoylated, inserts in the plasma membrane. While the protein also contains single SH3 and SH2 domains, unlike c-Src, it lacks a kinase motif, which is replaced by a unique COOH terminus of 104 amino acids.
SLAP family proteins blunt T-cell receptor-mediated signaling [Holland et al., 2001; Loreto et al., 2002; Myers et al., 2005; Myers et al., 2006; Pandey et al., 2002; Sosinowski et al., 2001; Sosinowski et al., 2000]. SLAP and SLAP-2 also negatively modulate B cell development and function through down-regulating B-cell receptors [Dragone et al., 2006a; Dragone et al., 2006b; Holland et al., 2001].
SLAP interacts with the cytoplasmic domain of the Eck receptor tyrosine kinase [Pandey et al., 1995]. It similarly associates with platelet-derived growth factor (PDGF) receptor and suppress PDGF-mediated cell proliferation [Roche et al., 1998]. SLAP-2 negatively regulates M-CSF signaling in macrophages [Manes et al., 2006; Pakuts et al., 2007] which also express SLAP, but whose function, in these cells, is less clear.
We examined the role of SLAP in osteoclast development using mice lacking the adaptor protein. We find increased macrophage proliferation, but not decreased apoptosis, contributes to enhanced osteoclast formation differentiation of SLAP−/− BMMs. In contrast, SLAP suppresses apoptosis of mature polykaryons in vitro. These balancing properties of SLAP are reflected by a normal bone phenotype of mice lacking the adaptor.
MATERIALS AND METHODS
MICE
Generation of SLAP-deficient mice has been described [Sosinowski et al., 2001]. All mice were 6-10 weeks old and maintained at the Animal Facility of Washington University School of Medicine. Experiments were approved by the Animal Ethics Committee of Washington University.
OSTEOCLAST CULTURES
Primary BMMs were prepared as described [Kim et al., 2006]. Marrow was extracted from femora and tibia of 6–8 weeks old mice with α-MEM and cultured in α-MEM containing 10% FBS and M-CSF (100 ng/ml). Cells were incubated at 37°C for 3 days and then washed with PBS and lifted with 1× trypsin/EDTA in PBS. Cells were cultured in α-MEM containing 10% heat-inactivated FBS with GST-RANKL (100 ng/ml) and various concentrations of mouse recombinant M-CSF. Medium was changed every 2 days. Osteoclasts were stained for tartrate-resistant acid phosphatase (TRAP) activity at day 4. For actin ring staining, BMMs were cultured on bone with RANKL (100 ng/ml) and M-CSF (10 ng/ml). After 5 days, cells were fixed in 4% paraformaldehyde, permeabilized in 0.1% Triton X-100, rinsed in PBS, and stained with FITC-phalloidin.
RT-PCR
Total RNA (1μg) extracted from cultured cells was used as a template for cDNA synthesis. Primers were synthesized on the basis of the reported mouse cDNA sequence. The following primers were used: for SLAP, 5′-AGATTGGTAGCTTCATGATTCG-3′ and 3′-GATTCGTCCACTCTGAGTGG-5′; for SLAP-2, 5′-ATGGGAAGTTTGTCCAGCAGAGGG-3′ and 3′-AGCATCATCCAAGGGGTCCTCAGC; for TRAP, 5′-ACAGCCCCCCACTCCCACCCT-3′ and 3′-TCAGGGTCTGGGTCTCCTTGG-5′; for MMP-9, 5′-CCTGTGTGTTCCCGTTCATCT-3′ and 3′-CGCTGGAATGATCTAAGCCCA-5′; for Cathepsin K, 5′-GGAAGAAGACTCACCAGAAGC-3′ and 3′-GCTATATAGCCGCCTCCACAG-5′; for NFAT2, 5′-ACTGTGCTGGGATCCTGAAG-3′ and 3′-AACGCCTTTCCACCATAGAG-5'; for GAPDH, 5′-ACTTTGTCAAGCTCATTTCC-3′ and 3′-TGCAGCGAACTTTATTGATG-5′. Amplification was conducted for 22-31 cycles, each of 94°C for 30 sec, 55°C for 30 sec, and 72°C for 30 sec. Ten microliters of each reaction mixture was analyzed by 1.5% agarose gel electrophoresis.
PROLIFERATION ASSAY AND CELL DEATH DETECTION ELISA
BMMs plated in 96-well plates were grown for 3 days and labeled with BrdU for the last 4 hrs of culture. The BrdU ELISA was conducted using the cell proliferation Biotrak ELISA system (Amersham Biosciences). Cell death assay was performed using cell death detection ELISAPLUS kit (Roche Applied Science), which detects cytoplasmic histone-associated DNA fragmentation.
WESTERN BLOTTING AND ANTIBODIES
BMMs starved in serum-free α-MEM for 16 hrs or osteoclasts starved for 3 hrs were stimulated with RANKL (100 ng/ml) or M-CSF (10 or 25 ng/ml). At indicated times, cells were washed with cold PBS and harvested with RIPA buffer containing protease and phosphatase inhibitors. c-Fms was immunoblotted with a rabbit polyclonal antibody (Santa Cruz) and immunoprecipitated with a rat anti-mouse mAb (E-Bioscience). SLAP (Santa Cruz) and flotillin (BD Transduction Laboratories) were immunoblotted using mAbs. For JNK, Akt, and ERK immunoblot, we used monoclonal antibodies to phospho-JNK, phospho-Akt, and phospho-ERK (Cell Signaling) and polyclonal antibodies to JNK, Akt and ERK (Cell Signaling). Anti-phosphotyrosine mAb 4G10 was obtained from Upstate Laboratories. Polyclonal antibodies to phospho-P38, PLCγ2 and active caspase-3 and phospho-IκBα mAbs were obtained from Cell Signaling. Flotillin mAb was purchased from BD Transduction Laboratories.
LIPID RAFT PURIFICATION AND ANALYSIS
Lipid rafts were prepared from BMMs by the detergent-free density gradient centrifugation method of Macdonald and Pike [Macdonald and Pike, 2005]. Fractions were immunoblotted for c-Fms, SLAP and flotillin.
HISTOLOGICAL ANALYSIS
Decalcified histological sections of proximal tibiae were prepared and OC number was determined using standard methods and the Osteomeasure system (Osteometrics).
STATISTICS
All data are presented as mean +/− S.D. Statistical significance was determined by 2-tailed Student’s t test.
RESULTS
ABSENCE OF SLAP INCREASES OSTEOCLAST FORMATION
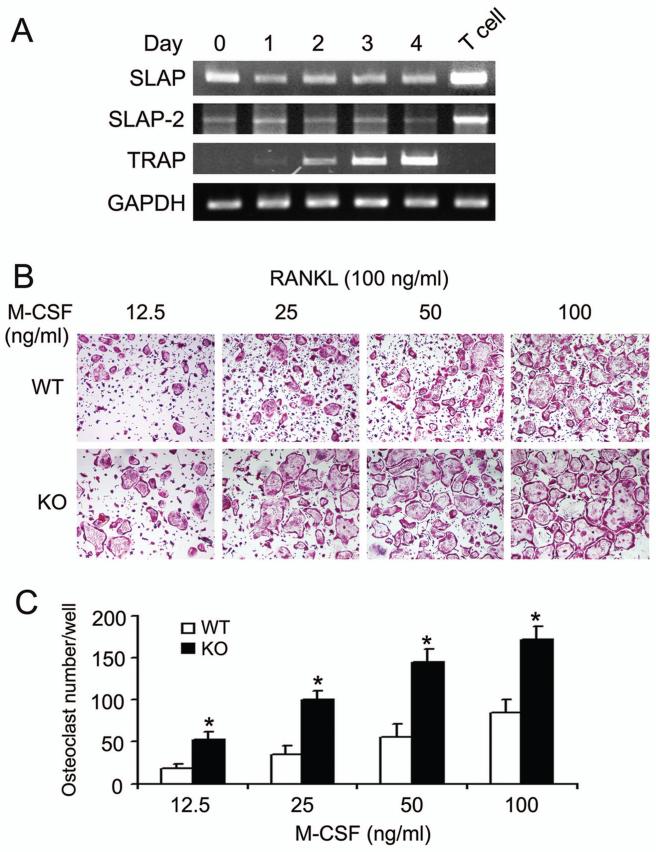
To determine the expression pattern of SLAP family proteins during osteoclast differentiation, BMMs were isolated from WT mice and exposed to RANKL and M-CSF. SLAP and SLAP-2 mRNA are constitutively present in BMMs and unaltered during osteoclast differentiation (Fig. 1A).
Fig. 1.
SLAP deficiency increases osteoclastogenesis. (A) WT BMMs were cultured in M-CSF (10 ng/ml) and RANKL (100 ng/ml) with time. SLAP and SLAP-2 mRNA was measured by RT-PCR. T cell lysate serves as positive control for expression of both isoforms. TRAP serves as positive control for osteoclastogenesis and GAPDH as loading control. (B) WT and SLAP−/− (KO) BMMs were cultured with 100 ng/ml of RANKL and the indicated concentrations of M-CSF. After 4 days, cells were stained for TRAP activity. (C) Statistical analysis of the number of WT and SLAP−/− TRAP positive multinucleated cells/well at day 4. (*p < 0.005)
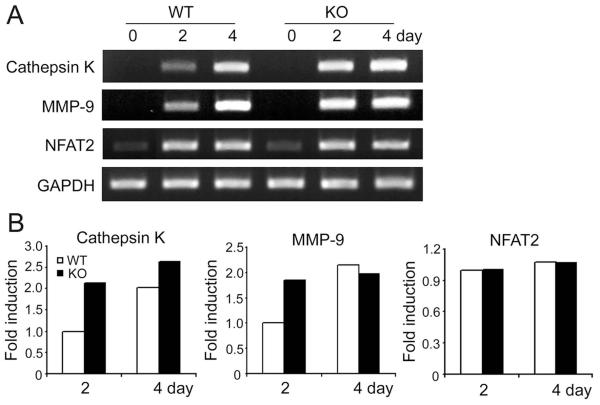
Because it is expressed in osteoclastogenic cells, we asked if SLAP participates in osteoclast differentiation. Hence we cultured WT and SLAP−/− BMMs in RANKL and various amounts of M-CSF, for 4 days, and stained cells for tartrate-resistant acid phosphatase (TRAP). In all concentrations of M-CSF, SLAP deficiency enhances osteoclastogenesis. (Fig. 1B and C). Reflecting the increased numbers of TRAP-positive cells, mRNAs of the osteoclastogenic indicators, cathepsin K and MMP-9 are elevated in day 2 SLAP−/− pre-osteoclasts. Surprisingly, the markers are comparable to those of WT by day 4 (Fig. 2 A,B). SLAP deficiency, however, does not change osteoclastogenesis-associated NFAT2 mRNA abundance.
Fig. 2.
Expression of osteoclastogenic markers is accelerated in SLAP−/− cells. (A) RT-PCR analysis of osteoclastogenic markers in WT and SLAP−/− cells in culture with M-CSF (10 ng/ml) alone (day 0) or with RANKL (100 ng/ml) for 2 or 4 days. (GAPDH serves as loading control) (B) Quantitative analysis of data presented in A.
ABSENCE OF SLAP ENHANCES OSTEOCLAST PRECURSOR PROLIFERATION
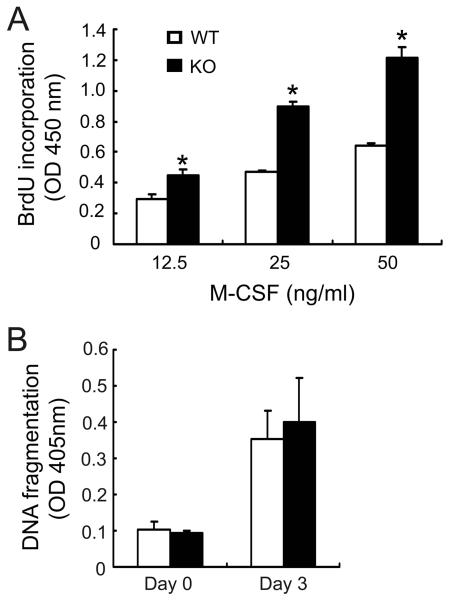
Increased osteoclast formation, in the absence of SLAP, could reflect accelerated precursor proliferation and/or decreased apoptosis [Ross and Teitelbaum, 2005]. To address this issue we cultured WT and SLAP−/− BMMs in increasing concentrations of M-CSF for 3 days. BrdU incorporation, during the last 4 hours, was increased at all doses of the cytokine (Fig. 3A). Next we assessed the apoptotic rate of WT and SLAP−/− BMMs as a function of DNA fragmentation. We find this parameter comparable in both genotypes, at initiation of culture (day 0) or after 3 days with M-CSF plus RANKL (Fig. 3B) at which time the cells express osteoclastogenic markers but have not fused [Faccio et al., 2003]. The increased number of SLAP−/− osteoclasts, therefore, does not represent accelerated apoptosis of naïve BMMs or mononuclear cells exhibiting early commitment to the osteoclast phenotype. Hence, SLAP deficiency influences proliferation of osteoclast precursors, but not their survival.
Fig. 3.
SLAP regulates the proliferation but not survival of osteoclast precursors. (A) Equal numbers of WT and SLAP−/− BMMs were cultured with increasing concentrations of M-CSF for 3 days. Incorporation of BrdU during the last 4 hrs of culture was determined. (*p < 0.001). (B) WT and SLAP−/− BMMs were cultured in M-CSF alone (10 ng/ml) (day 0) or M-CSF and RANKL (100 ng/ml) (day 3). Apoptosis was determined as a function of DNA fragmentation.
LOSS OF SLAP ENHANCES M-CSF-INDUCED ERK ACTIVATION
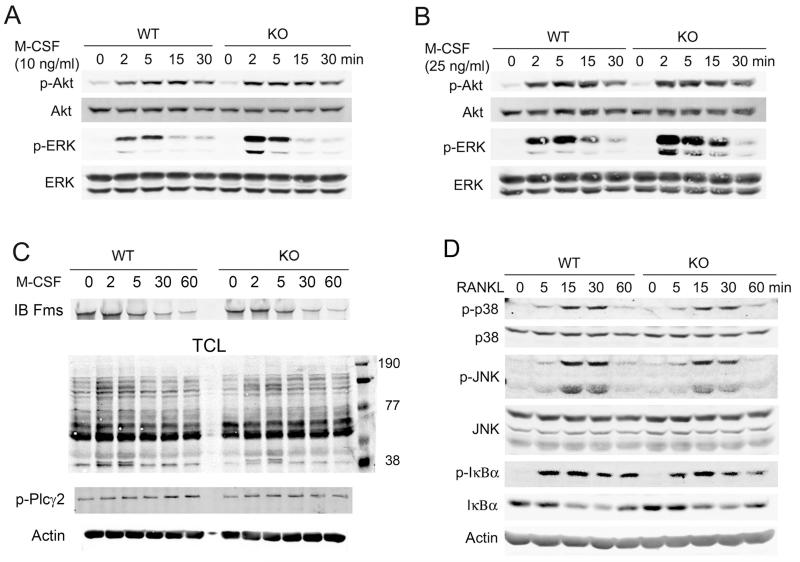
M-CSF binds to c-Fms, its unique receptor tyrosine kinase, and activates MAP kinases and PI3K/Akt, each of which regulate cell proliferation [Zhou et al., 2006]. To determine if the enhanced rate of SLAP−/− BMM replication is mediated by these signals, we assessed M-CSF-stimulated ERK and Akt activation. While SLAP deficiency does not impact BMM Akt phosphorylation, that of ERK is enhanced in cells lacking the adaptor (Fig. 4A and B).
Fig. 4.
M-CSF-induced ERK phosphorylation is enhanced in SLAP−/− BMMs. BMMs were cultured with 10 ng/ml (A) or 25 ng/ml (B) of M-CSF for the indicated times. Akt and ERK phosphorylation was determined by immunoblot. Total ERK and Akt levels serve as loading controls. (C) Cytokine and serum starved WT and SLAP−/− BMMs were exposed to M-CSF (50ng/ml) with time. c-Fms immunoprecipitates were immunoblotted with antibodies to c-Fms. Total cell lysate was immunoblotted for tyrosine phosphorylated proteins and phospho-PLCγ2. Actin serves as loading control (D) BMMs were treated with RANKL (100 ng/ml) with time. Lysates were immunoblotted with indicated antibodies. Actin and total p38, JNK and IκB serve as loading controls.
M-CSF promotes down regulation of surface residing c-Fms, which is autonomous of SLAP (Fig 4C). Similarly, total lysate tyrosine phosphorylation and PLCγ2 activation are unaltered in M-CSF-treated SLAP−/− BMMs. In contrast to M-CSF’s impact on ERK, loss of SLAP does not modify activation of immediate effectors of RANK signaling, including p38, JNK, and IκB, in osteoclast precursors (Fig. 4D). Thus, SLAP selectively regulates M-CSF signaling via the MAP kinase pathway, leading to decreased proliferation of osteoclast precursors.
C-FMS AND SLAP ASSOCIATE IN LIPID RAFTS
These data establish that SLAP mediates M-CSF-stimulated signaling, in osteoclastic cells, suggesting c-Fms/SLAP association. To determine if such is the case we treated BMMs with M-CSF or carrier. We immunoblotted c-Fms immunoprecipitates and whole cell lysates with anti-SLAP mAb but failed to observe a reaction product in either circumstance (not shown).
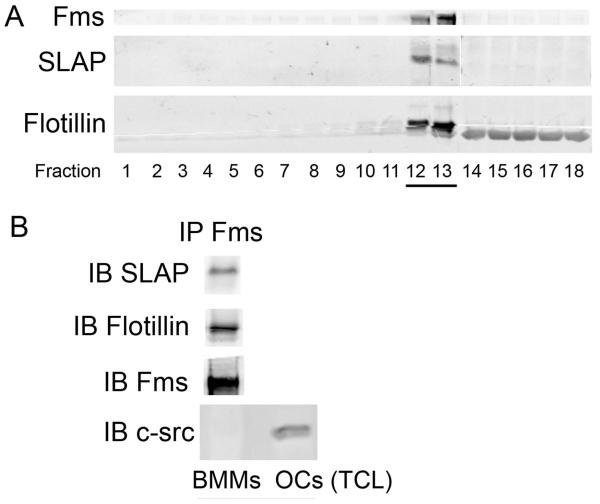
Because lipid rafts accumulate signaling molecules, such as receptor tyrosine kinases, we reasoned that c-Fms and SLAP may accumulate in these plasma membrane-residing structures. To this end we subjected WT BMMs to detergent-free gradient centrifugation designed to isolate lipid rafts [Macdonald and Pike, 2005]. Immunoblotting the resulting fractions establishes that both c-Fms and SLAP concentrate in these microdomains, identified by flotillin co-migration (Fig 5A). This latter conclusion is buttressed by the co-immunoprecipitation of c-Fms and flotillin (Fig 5 B). Importantly, SLAP immunoblots of c-Fms immunoprecipitates, derived from pooled fractions 12 and 13, establish the two proteins associate (Fig 5B).
Fig 5.
c-Fms and SLAP associate in lipid rafts. WT BMM lysates were isolated by density gradient centrifugation. (A) Fractions were immunoblotted for c-Fms and SLAP. Flotillin serves as a lipid raft marker. (B) c-Fms immunoprecipitates of pooled fractions 12 and 13 were immunoblotted for SLAP, flotillin and c-Fms. Immunoblotting with mAb recognizing c-Src, which is absent in BMMs, serves as negative control. Immunoblot of lysate (TCL) derived from c-Src-expressing osteoclasts documents antibody efficacy.
OSTEOCLASTS LACKING SLAP UNDERGO ENHANCED APOPTOSIS
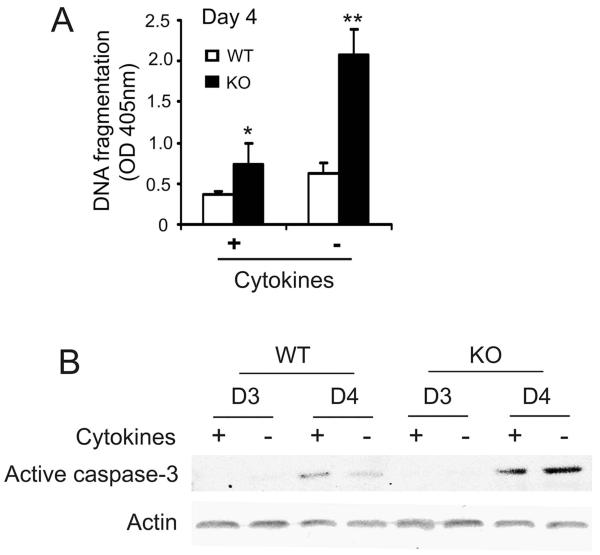
Osteoclasts are terminally differentiated, multinucleated cells prone to undergo programmed death [Hughes and Boyce, 1997]. Fig. 6A shows that apoptosis is increased in day 4 cultures of SLAP−/− mature osteoclasts in the presence of M-CSF and RANKL and this difference is more evident after withdrawal of cytokines for 4 hours. In agreement with these observations, activation of caspase-3 is enhanced in day 4 SLAP−/− cells, cultured with or without cytokines, as compared to their WT counterparts (Fig 6B). Thus, while SLAP deficiency does not modify death of its precursors, it increases that of mature osteoclasts.
Fig. 6.
SLAP-deficient osteoclasts are predisposed to apoptosis. WT and SLAP−/− BMMs were cultured in M-CSF and RANKL. At day 4, cells were incubated with (+) or without (−) cytokines for the last 4 hours. (A) The magnitude of apoptosis was measured as a function of DNA fragmentation (B) Activated caspase-3 was measured by immunoblot on day 3 and day 4. (*p < 0.05, **p < 0.005).
SLAP DOES NOT REGULATE OSTEOCLAST FUNCTION IN VITRO OR IN VIVO
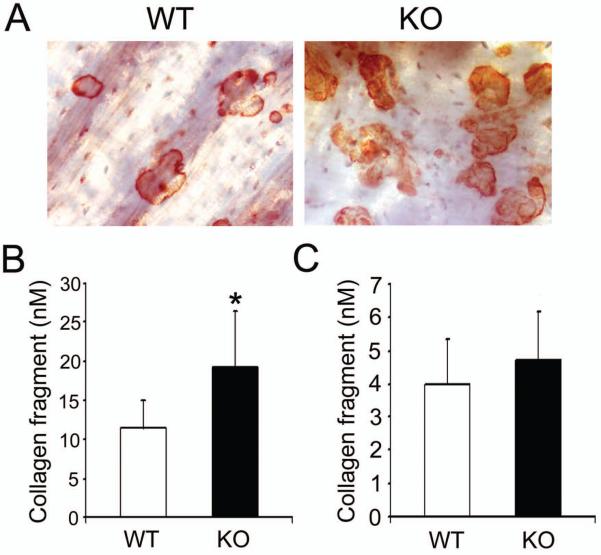
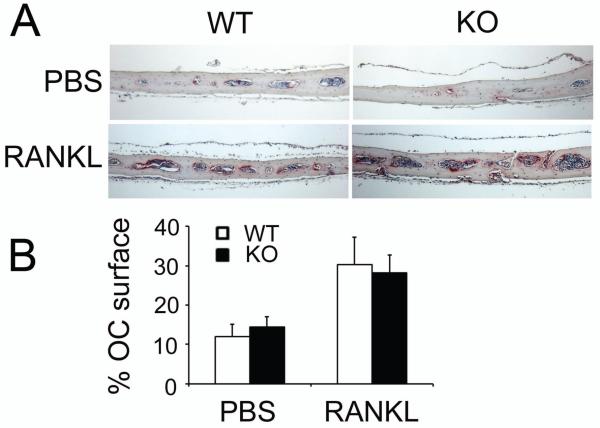
SLAP is structurally similar to c-Src, which modulates osteoclast function. To determine if SLAP also regulates the resorptive capacity of individual osteoclasts, WT and SLAP−/− BMMs were maintained on bone with M-CSF and RANKL. After 5 days, cells were removed. As detailed in Fig 7A, B, the number of resorptive pits and the quantity of Type1 collagen fragments (CTx-1) released into the culture medium is enhanced by SLAP−/− osteoclasts. These results however, may reflect increased numbers of SLAP/−osteoclasts due to precursor proliferation or augmented resorptive capacity of individual polykaryons. To distinguish between these possibilities we asked if SLAP deficiency impacts mature osteoclast function in circumstances that do not influence precursor replication. Thus, we cultured BMMs on plastic for 3 days, with RANKL and M-CSF, at which time the cells are non-proliferative [Zhou et al., 2006]. The committed precursors were lifted and replated on bone, for 24 hr, in the presence of osteoclastogenic cytokines. In this circumstance, no differences exist in CTx-1 content of WT and SLAP−/− cultures (Fig. 7C). Finally, the increase in osteoclast number and bone resorption, measured by histomorphometry, is the same basally and after five daily supra-calvarial injections of RANKL to WT or SLAP null mice (Fig 8). Thus, SLAP deficiency enhances osteoclast formation and apoptosis of the mature polykaryons without altering resorptive capacity of the individual cell.
Fig. 7.
SLAP does not regulate function of individual osteoclasts in vitro. WT and SLAP−/− BMMs were cultured on bone with M-CSF (10 ng/ml) and RANKL (100 ng/ml) for 5 days. (A) Resorptive lacuna formation was examined on day 5. (B) Bone resorptive activity of the cultures in A was determined by collagen type I fragment ELISA of culture media on day 5. (*p < 0.05). (C) WT and SLAP−/− BMMs were cultured on plastic in the presence of M-CSF and RANKL. After 3 days, the cells were lifted and replated on bone in M-CSF and RANKL. One day later, resorptive activity was determined by collagen type I fragment ELISA.
Fig. 8.
SLAP deficiency does not alter osteoclast number in vivo. WT and SLAP−/− mice were injected, supracalvarially, with PBS or RANKL (100 μg) daily, for 7 days. (A) TRAP-(red reaction product) stained histological sections of calvaria. (B) Percentage of osteoclast surface per unit bone volume.
DISCUSSION
SLAP is constitutively expressed throughout osteoclastogenesis and its absence yields increased osteoclast numbers, in vitro. This enhanced recruitment of SLAP−/− osteoclasts is due, in part, to accelerated proliferation of precursors, rather than their decreased apoptosis. Because M-CSF is the major cytokine modulating osteoclast progenitor proliferation [Pixley and Stanley, 2004; Ross, 2006], we asked if signals emanating from its occupied receptor are altered by loss of SLAP. We document that increased ERK activity, in the absence of SLAP, is associated with osteoclast precursor proliferation, while Akt plays no detectable role.
Down-regulation of activated RTKs, including c-Fms, governs cell division. Like SLAP, a number of adaptors are negative regulators of M-CSF/c-Fms signaling [Bourette et al., 1998; Bourette et al., 2001; Lee et al., 1999; Ross, 2006; Suzu et al., 2000]. For example, the RING-finger protein, c-Cbl, is involved in c-Fms internalization and degradation Because M-CSF induces c-Cbl phosphorylation, in osteoclasts and their precursors [Faccio et al., 2007], and c-Cbl negatively modulates macrophage division [Lee et al., 1999], we cannot exclude the possibility that SLAP regulates proliferation of osteoclast precursors in a c-Cbl-dependent manner.
SLAP-2 is expressed in hematopoietic cells including macrophages [Holland et al., 2001; Loreto et al., 2002; Manes et al., 2006; Pandey et al., 2002], wherein it acts as a negative regulator of M-CSF/c-Fms signaling [Pakuts et al., 2007] and we find it present throughout osteoclastogenesis. SLAP-2 plays a role in c-Cbl-dependent down-regulation of M-CSF signaling in immortalized fibroblasts expressing exogenous c-Fms [Pakuts et al., 2007]. In contrast, over-expression of SLAP-2, in macrophages, does not affect M-CSF-induced ERK or Akt activation [Manes et al., 2006]. Thus, while the impact of SLAP-2 on c-Fms signaling differs from that of SLAP, one cannot exclude, with certainty, reciprocal compensation in vivo.
In other cells SLAP associates with c-Fms [Pakuts et al., 2007] but we failed to detect this interaction in BMM lysates. Reasoning that c-Fms/SLAP recognition may be focal, we turned to lipid rafts. These structures are plasma membrane-residing, cholesterol- and flotillin-enriched microdomains, with an abundance of signaling molecules. In fact, lipid rafts, isolated by detergent-free gradient centrifugation contain c-Fms in complex with SLAP. Thus, c-Fms joins a variety of other receptors whose signaling activity reflects differential localization within the plasma membrane.
While the COOH terminus of c-Src has kinase activity, that of SLAP is a unique peptide sequence with poorly defined function. On the other hand, the SH3 and SH2 domains of SLAP are similar to the corresponding regions of c-Src. Because the resorptive capacity of c-Src is dependent on its SH2 and SH3 domains [Schwartzberg, 1998], we reasoned that SLAP may antagonizes the tyrosine kinase, in osteoclasts, but find that unlike c-Src, SLAP does not regulate resorptive function. Consistent with our observations, expression of SLAP in NIH 3T3 cells fails to reverse Src-induced cytoskeletal organization, while inhibiting DNA synthesis [Manes et al., 2000]. Congruently, SLAP suppresses proliferation of osteoclast precursors. In this regard, a combination of SFKs, PI3K/Akt, PLC, and ERK activation mediate mitosis in response to M-CSF [Takeshita et al., 2007; Zhou et al., 2006]. Because SFKs modulate macrophage proliferation [Takeshita et al., 2007] and SLAP negatively regulates Src mitogenic function [Manes et al., 2000], it is possible that SLAP antagonizes c-Src during M-CSF-driven cell proliferation.
RANKL governs osteoclast differentiation and longevity. SLAP deficiency, on the other hand, impacts survival of neither naïve macrophages nor day 3 pre-fusion osteoclasts. Therefore, the enhanced osteoclast development in SLAP-deficient cells most likely reflects a combination of increased proliferation, via M-CSF, and accelerated differentiation in response to RANKL. Although loss of SLAP does not impact programmed death in BMMs and pre-fusion osteoclasts, apoptosis of mature resorptive SLAP-deficient polykaryons is enhanced as reflected by DNA fragmentation and activation of executioner caspase 3 [Okahashi et al., 1998]. The augmented programmed cell death of SLAP null osteoclasts explains our findings that the early increase in markers of differentiation is lost by day four in culture. Hence, the normal bone architecture of SLAP null mice likely reflects balanced and opposing changes in differentiation and death of osteoclastic cells.
ACKNOWLEDGMENTS
The project described was supported by Grant Number AR032788, AR046523, AR048853 (SLT), AR046852, AR054190 (FPR) from the National Institutes of Health, and the Korea Health 21 R&D Project, Ministry of Health & Welfare, the Government of Korea (Project Number: A010252). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
REFERENCES
- Akiyama T, Bouillet P, Miyazaki T, Kadono Y, Chikuda H, Chung UI, Fukuda A, Hikita A, Seto H, Okada T, Inaba T, Sanjay A, Baron R, Kawaguchi H, Oda H, Nakamura K, Strasser A, Tanaka S. Regulation of osteoclast apoptosis by ubiquitylation of proapoptotic BH3-only Bcl-2 family member Bim. EMBO J. 2003;22:6653–6664. doi: 10.1093/emboj/cdg635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso G, Koegl M, Mazurenko N, Courtneidge SA. Sequence requirements for binding of Src family tyrosine kinases to activated growth factor receptors. J Biol Chem. 1995;270:9840–9848. doi: 10.1074/jbc.270.17.9840. [DOI] [PubMed] [Google Scholar]
- Bourette RP, Arnaud S, Myles GM, Blanchet JP, Rohrschneider LR, Mouchiroud G. Mona, a novel hematopoietic-specific adaptor interacting with the macrophage colony-stimulating factor receptor, is implicated in monocyte/macrophage development. EMBO J. 1998;17:7273–7281. doi: 10.1093/emboj/17.24.7273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourette RP, De Sepulveda P, Arnaud S, Dubreuil P, Rottapel R, Mouchiroud G. Suppressor of cytokine signaling 1 interacts with the macrophage colony-stimulating factor receptor and negatively regulates its proliferation signal. J Biol Chem. 2001;276:22133–22139. doi: 10.1074/jbc.M101878200. [DOI] [PubMed] [Google Scholar]
- Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- Dragone LL, Myers MD, White C, Gadwal S, Sosinowski T, Gu H, Weiss A. Src-like adaptor protein (SLAP) regulates B cell receptor levels in a c-Cbl-dependent manner. Proc Natl Acad Sci USA. 2006a;103:18202–18207. doi: 10.1073/pnas.0608965103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragone LL, Myers MD, White C, Sosinowski T, Weiss A. SRC-like adaptor protein regulates B cell development and function. J Immunol. 2006b;176:335–345. doi: 10.4049/jimmunol.176.1.335. [DOI] [PubMed] [Google Scholar]
- Faccio R, Takeshita S, Colaianni G, Chappel JC, Zallone A, Teitelbaum SL, Ross FP. M-CSF regulates the cytoskeleton via recruitment of a multimeric signaling complex to c-Fms Tyr-Y559/697/721. J Biol Chem. 2007;282:18991–18999. doi: 10.1074/jbc.M610937200. [DOI] [PubMed] [Google Scholar]
- Faccio R, Zou W, Colaianni G, Teitelbaum SL, Ross FP. High dose M-CSF partially rescues the Dap12−/− osteoclast phenotype. J Cell Biochem. 2003;90:871–883. doi: 10.1002/jcb.10694. [DOI] [PubMed] [Google Scholar]
- Holland SJ, Liao XC, Mendenhall MK, Zhou X, Pardo J, Chu P, Spencer C, Fu A, Sheng N, Yu P, Pali E, Nagin A, Shen M, Yu S, Chan E, Wu X, Li C, Woisetschlager M, Aversa G, Kolbinger F, Bennett MK, Molineaux S, Luo Y, Payan DG, Mancebo HS, Wu J. Functional cloning of Src-like adapter protein-2 (SLAP-2), a novel inhibitor of antigen receptor signaling. J Exp Med. 2001;194:1263–1276. doi: 10.1084/jem.194.9.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes DE, Boyce BF. Apoptosis in bone physiology and disease. Mol Pathol. 1997;50:132–137. doi: 10.1136/mp.50.3.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insogna KL, Sahni M, Grey AB, Tanaka S, Horne WC, Neff L, Mitnick M, Levy JB, Baron R. Colony-stimulating factor-1 induces cytoskeletal reorganization and c-src-dependent tyrosine phosphorylation of selected cellular proteins in rodent osteoclasts. J Clin Invest. 1997;100:2476–2485. doi: 10.1172/JCI119790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworowski A, Wilson NJ, Christy E, Byrne R, Hamilton JA. Roles of the Mitogen-activated Protein Kinase Family in Macrophage Responses to Colony Stimulating Factor-1 Addition and Withdrawal. J Biol Chem. 1999;274:15127–15133. doi: 10.1074/jbc.274.21.15127. [DOI] [PubMed] [Google Scholar]
- Kelley TW, Graham MM, Doseff AI, Pomerantz RW, Lau SM, Ostrowski MC, Franke TF, Marsh CB. Macrophage colony-stimulating factor promotes cell survival through Akt/protein kinase B. J Biol Chem. 1999;274:26393–26398. doi: 10.1074/jbc.274.37.26393. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Zhao H, Kitaura H, Bhattacharyya S, Brewer JA, Muglia LJ, Ross FP, Teitelbaum SL. Glucocorticoids suppress bone formation via the osteoclast. J Clin Invest. 2006;116:2152–2160. doi: 10.1172/JCI28084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PSW, Wang Y, Dominguez MG, Yeung Y-G, Murphy MA, Bowtell DDL, Stanley ER. The Cbl protooncoprotein stimulates CSF-1 receptor multiubiquitination and endocytosis, and attenuates macrophage proliferation. EMBO J. 1999;18:3616–3628. doi: 10.1093/emboj/18.13.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Stanley ER. Role of dimerization and modification of the CSF-1 receptor in its activation and internalization during the CSF-1 response. EMBO J. 1991;10:277–288. doi: 10.1002/j.1460-2075.1991.tb07948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto MP, Berry DM, McGlade CJ. Functional cooperation between c-Cbl and Src-like adaptor protein 2 in the negative regulation of T-cell receptor signaling. Mol Cell Biol. 2002;22:4241–4255. doi: 10.1128/MCB.22.12.4241-4255.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald JL, Pike LJ. A simplified method for the preparation of detergent-free lipid rafts. J Lipid Res. 2005;46:1061–1067. doi: 10.1194/jlr.D400041-JLR200. [DOI] [PubMed] [Google Scholar]
- Manes G, Bello P, Roche S. Slap negatively regulates Src mitogenic function but does not revert Src-induced cell morphology changes. Mol Cell Biol. 2000;20:3396–3406. doi: 10.1128/mcb.20.10.3396-3406.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manes GA, Masendycz P, Nguyen T, Achuthan A, Dinh H, Hamilton JA, Scholz GM. A potential role for the Src-like adapter protein SLAP-2 in signaling by the colony stimulating factor-1 receptor. FEBS J. 2006;273:1791–1804. doi: 10.1111/j.1742-4658.2006.05199.x. [DOI] [PubMed] [Google Scholar]
- Myers MD, Dragone LL, Weiss A. Src-like adaptor protein down-regulates T cell receptor (TCR)-CD3 expression by targeting TCRzeta for degradation. J Cell Biol. 2005;170:285–294. doi: 10.1083/jcb.200501164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MD, Sosinowski T, Dragone LL, White C, Band H, Gu H, Weiss A. Src-like adaptor protein regulates TCR expression on thymocytes by linking the ubiquitin ligase c-Cbl to the TCR complex. Nat Immunol. 2006;7:57–66. doi: 10.1038/ni1291. [DOI] [PubMed] [Google Scholar]
- Okahashi N, Koide M, Jimi E, Suda T, Nishihara T. Caspases (interleukin-1β-converting enzyme family proteases) are involved in the regulation of the survival of osteoclasts. Bone. 1998;23:33–41. doi: 10.1016/s8756-3282(98)00069-6. [DOI] [PubMed] [Google Scholar]
- Pakuts B, Debonneville C, Liontos LM, Loreto MP, McGlade CJ. The Src-like adaptor protein 2 regulates colony-stimulating factor-1 receptor signaling and down-regulation. J Biol Chem. 2007;282:17953–17963. doi: 10.1074/jbc.M701182200. [DOI] [PubMed] [Google Scholar]
- Pandey A, Duan H, Dixit VM. Characterization of a novel Src-like adapter protein that associates with the Eck receptor tyrosine kinase. J Biol Chem. 1995;270:19201–19204. doi: 10.1074/jbc.270.33.19201. [DOI] [PubMed] [Google Scholar]
- Pandey A, Ibarrola N, Kratchmarova I, Fernandez MM, Constantinescu SN, Ohara O, Sawasdikosol S, Lodish HF, Mann M. A novel Src homology 2 domain-containing molecule, Src-like adapter protein-2 (SLAP-2), which negatively regulates T cell receptor signaling. J Biol Chem. 2002;277:19131–19138. doi: 10.1074/jbc.M110318200. [DOI] [PubMed] [Google Scholar]
- Pixley FJ, Stanley ER. CSF-1 regulation of the wandering macrophage: complexity in action. Trends Cell Biol. 2004;14:628–638. doi: 10.1016/j.tcb.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Reedijk M, Liu X, van der Geer P, Letwin K, Waterfield MD, Hunter T, Pawson T. Tyr721 regulates specific binding of the CSF-1 receptor kinase insert to PI 3′-kinase SH2 domains: a model for SH2-mediated receptor-target interactions. EMBO J. 1992;11:1365–1372. doi: 10.1002/j.1460-2075.1992.tb05181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche S, Alonso G, Kazlauskas A, Dixit VM, Courtneidge SA, Pandey A. Src-like adaptor protein (Slap) is a negative regulator of mitogenesis. Curr Biol. 1998;8:975–978. doi: 10.1016/s0960-9822(98)70400-2. [DOI] [PubMed] [Google Scholar]
- Ross FP. M-CSF, c-Fms, and signaling in osteoclasts and their precursors. Ann N Y Acad Sci. 2006;1068:110–116. doi: 10.1196/annals.1346.014. [DOI] [PubMed] [Google Scholar]
- Ross FP, Teitelbaum SL. αvβ3 and macrophage colony-stimulating factor: partners in osteoclast biology. Immunol Rev. 2005;208:88–105. doi: 10.1111/j.0105-2896.2005.00331.x. [DOI] [PubMed] [Google Scholar]
- Schwartzberg PL. The many faces of Src: multiple functions of a prototypical tyrosine kinase. Oncogene. 1998;17:1463–1468. doi: 10.1038/sj.onc.1202176. [DOI] [PubMed] [Google Scholar]
- Sosinowski T, Killeen N, Weiss A. The Src-like adaptor protein downregulates the T cell receptor on CD4+CD8+ thymocytes and regulates positive selection. Immunity. 2001;15:457–466. doi: 10.1016/s1074-7613(01)00195-9. [DOI] [PubMed] [Google Scholar]
- Sosinowski T, Pandey A, Dixit VM, Weiss A. Src-like adaptor protein (SLAP) is a negative regulator of T cell receptor signaling. J Exp Med. 2000;191:463–474. doi: 10.1084/jem.191.3.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzu S, Tanaka-Douzono M, Nomaguchi K, Yamada M, Hayasawa H, Kimura F, Motoyoshi K. p56(dok-2) as a cytokine-inducible inhibitor of cell proliferation and signal transduction. EMBO J. 2000;19:5114–5122. doi: 10.1093/emboj/19.19.5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita S, Faccio R, Chappel J, Zheng L, Feng X, Weber JD, Teitelbaum SL, Ross FP. c-Fms tyrosine 559 is a major mediator of M-CSF-induced proliferation of primary macrophages. J Biol Chem. 2007;282:18980–18990. doi: 10.1074/jbc.M610938200. [DOI] [PubMed] [Google Scholar]
- Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003;4:638–649. doi: 10.1038/nrg1122. [DOI] [PubMed] [Google Scholar]
- Valledor AF, Comalada M, Xaus J, Celada A. The Differential Time-course of Extracellular-regulated Kinase Activity Correlates with the Macrophage Response toward Proliferation or Activation. J Biol Chem. 2000;275:7403–7409. doi: 10.1074/jbc.275.10.7403. [DOI] [PubMed] [Google Scholar]
- Wang MW, Wei S, Faccio R, Takeshita S, Tebas P, Powderly WG, Teitelbaum SL, Ross FP. The HIV protease inhibitor ritonavir blocks osteoclastogenesis and function by impairing RANKL-induced signaling. J Clin Invest. 2004;114:206–213. doi: 10.1172/JCI15797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xaus J, Comalada M, Valledor AF, Cardo M, Herrero C, Soler C, Lloberas J, Celada A. Molecular mechanisms involved in macrophage survival, proliferation, activation or apoptosis. Immunobiology. 2001;204:543–550. doi: 10.1078/0171-2985-00091. [DOI] [PubMed] [Google Scholar]
- Zhou P, Kitaura H, Teitelbaum SL, Krystal G, Ross FP, Takeshita S. SHIP1 negatively regulates proliferation of osteoclast precursors via Akt-dependent alterations in D-type cyclins and p27. J Immunol. 2006;177:8777–8784. doi: 10.4049/jimmunol.177.12.8777. [DOI] [PubMed] [Google Scholar]