Abstract
Heart failure (HF) is a complex multifaceted problem of abnormal ventricular function and structure. In recent years, new information has been accumulated allowing for a more detailed understanding of the cellular and molecular alterations that are the underpinnings of diverse causes of HF, including myocardial ischemia, pressure-overload, volume-overload or intrinsic cardiomyopathy. Modern pharmacological approaches to treat HF have had a significant impact on the course of the disease, although they do not reverse the underlying pathological state of the heart. Therefore gene-based therapy holds a great potential as a targeted treatment for cardiovascular diseases. Here, we survey the relative therapeutic efficacy of genetic modulation of β-adrenergic receptor signaling, Ca2+ handling proteins and angiogenesis in the most common extrinsic models of HF.
Keywords: angiogenesis, animal models, β-adrenergic signaling, Ca2+ handling proteins, gene therapy, heart failure
Introduction
Heart failure is a major cause of morbidity and mortality in contemporary society. The leading cause of heart failure (HF) in the USA is ischemic heart disease (IHD). Other common conditions that cause HF include extrinsic disorders: pressure-overload, volume overload, ischemia, ischemia/reperfusion; and intrinsic cardiomyopathy, which itself may be a result of toxic, genetic, viral and inflammatory etiologies. Assuming that HF is the final manifestation of various pathological insults, each of which results in an inability of the left ventricle to maintain a stroke volume sufficient to meet metabolic demands [1], effective therapies require an understanding of the pathophysiological mechanisms underlying this disorder. After the initial pathological insult, there is neurohumoral activation and remodeling of the left ventricle (LV), including changes in interstitial collagen and continued loss of myocytes. Therefore, a model that recapitulates these pathways aids in the understanding of the pathophysiology and simultaneously assists in the development of new treatment strategies [2,3]. Irrespective of the etiology, HF is a complex of abnormal LV function and structure. Deterioration in LV pump function prompts adaptive and initially compensatory local and systemic alterations in an attempt to maintain adequate stroke volume [4]. Among these are: (i) the Frank–Starling mechanism to maintain cardiac output by an increase in ventricular preload, by ventricular dilatation and volume expansion; (ii) myocardial hypertrophy of unaffected myocardium to minimize the increase in wall stress as the heart dilates; (iii) peripheral vasoconstriction, which initially maintains perfusion to vital organs; renal sodium and water retention to enhance ventricular preload; (iv) activation of the adrenergic nervous system, which increases heart rate and improves contractile function. These processes are controlled mainly by the activation of various vasoconstrictive neurohormonal systems, including the renin-angiotensin-aldosterone system, the adrenergic nervous system, and the non-osmotic release of arginine-vasopressin. The resultant increase in wall stress along with neurohormonal activation facilitates pathological ventricular remodeling. This process has been closely linked to heart failure disease progression. All of these initially compensatory changes become detrimental over time because they ultimately increase myocardial oxygen consumption and result in a decrement in energy efficiency. Subsequent ventricular changes are accompanied by dilatation, impairment of wall motion, wall thinning and a decrease ejection fraction (EF) related to myocyte loss as a result of necrosis, myocyte slippage and ongoing apoptosis [2]. Eventually, these events in conjunction with increased wall stress result in progressive pump dysfunction, thereby promoting a maladaptive remodeling process.
The general pharmacological approach for heart failure management includes diuretics to reduce extracellular fluid volume excess; vasodilators to lower ventricular filling pressures and systemic vascular resistance; and positive inotropic agents to increase cardiac output in low-flow states of acute HF. These treatments for HF have had a significant impact on the course of the disease, although they do not reverse the underlying pathological state of the heart [5]. Therefore, HF therapy is in need of novel therapeutic approaches. Advances in the understanding of the molecular basis of myocardial dysfunction, together with the evolution in vector technology and methods in cardiac gene transfer, has placed HF with cardiomyocyte dysfunction and maladaptive remodeling within reach of gene-based therapy. The main goal of gene therapy is to treat dysfunctional myocytes and to prevent further subsequent loss of healthy myocytes. At the myocyte level, HF is associated with molecular alterations and likely involves the dysregulation of thousands of genes. There are several mechanisms, however, that appear to be central to the pathogenesis of contractile dysfunction. These include: dysregulation of β-adrenergic signaling, defects in sarcoplasmic reticulum function and abnormal intracellular calcium handling, activation of pro-apoptotic pathways, and electrical remodeling [6,7]. Targeting these pathways by gene transfer appears to improve overall function in failing hearts of diverse etiologies.
The ideal HF gene therapy strategy may indeed depend on the specific cause of the condition (ischemic, valvular, hypertensive, genetic, etc.); thus, a combinatorial approach that targets different mechanisms and signaling pathways is required for optimal therapeutic impact [8]. Ideally, clinically relevant models of heart failure should be used to test the role of the specific molecular pathways in disease pathogenesis, helping to validate the potential targets for clinical therapeutic intervention.
Herein, we focus on recent advances in surgical animal models of HF that have been utilized for gene therapeutic interventions and examine the most important model-specific molecular targets for genetic intervention, as well as the results obtained. Through this analysis, we hope to gain insight into those molecular targets that may be most important as a function of the extrinsic cause of heart failure. We may discover that certain molecular targets are most attractive candidates for gene therapy as a result of volume overload, although these targets may be more relevant for ischemic cardiomyopathy. Regardless of the cause of HF, a necessary goal is to improve the function of the failing cardiomyocytes, as a necessary step towards reversing global myocardial dysfunction and to facilitate reverse (adaptive) remodeling.
Surgical animal models of heart failure
A clinically relevant animal model should mimic both the etiology in an important form of human HF and parallel its natural history. Although earlier studies focused on the hemodynamic, cardiorenal and neurohormonal changes associated with HF, more recently, the emphasis is on the study of processes of myocardial remodeling and the role played by stretch-activated pathways, cytokine activation and molecular changes in cardiomyocytes, as well as in the extracellular matrix [2]. Each animal HF model has advantages and limitations and none of them is ideal (Table 1). Rodents differ substantially in their cardiovascular physiology and pathophysiology from humans and large animals in the rate of metabolism, as well as in the pattern of cardiac contractile dysfunction during the development of HF [3,9]. Sheep and swine have advantages over dogs in that the coronary anatomy closely mimics that of humans, whereas dogs have an extensive coronary collateral supply and much faster heart rates. The relatively large size of sheep and swine, their consistent coronary anatomy and vasomotor responsiveness makes them suitable for multiple diagnostic and therapeutic strategies. Also, similar to humans, relatively few collaterals make the effect of ischemia more predictable and the study of LV metabolism more reliable. Larger species have a heterogeneous genetic background, and their longer lifespans make them ideally suited for long-term evaluation of possible gene therapy candidates for clinical application [3,10] (Table 2).
Table 1.
Surgical Models of Heart Failure
| Model Description | Advantages | Disadvantages |
|---|---|---|
| Ischemia/Infarction | ||
| Coronary Artery Ligation | • Simple method to create transmural MI • Clinically relevant, reproducible in targeted areas •Rapid onset of HF |
• Invasive • High morbidity risk with surgical complications and arrythmias • Difficult to estimate Infarct Size |
| Coronary Artery Embolization | •Minimally invasive • High Incidence in Clinic • Ability to control ischemic response |
• Inconsistent length and size of occlusions • Requires repeat catheterizations and interventions to achieve HF • High cost: equipment and personnel |
| Coronary Artery Narrowing | • Ability to create partial or gradual occlusion • Permits the study of chronic myocardial ischemia |
• Invasive and high risk of myocardial injury from occluders/constrictors • Requires the use of technically complex procedure and flowmeters • Difficult to control degree and progress of stenosis |
| Ischemia/Reperfusion | • Highest rate of clinical occurrence • Minimally Invasive |
•Lack of extensive experimental data • Technical difficulty and requires expensive equipment and personnel |
| Cryoinfarction | •Simple procedure and device use • Ability to control size and location of infarct |
•Invasive • Inconsistency in HF progression • Infarcts typically not transmural |
| Tachycardia-Induced Model | ||
| Ventricular Induced Pacing Supraventricular Induced Pacing |
• Relatively simple and generates predictable degrees of HF • Ability to produce right and left ventricular dysfunction • No impact on coronary vessels |
• Reversible dysfunction after pacing cessation • Mechanisms of HF not similar to the human condition • Delayed onset of HF and requires intensive monitoring |
| Pressure Overload Model | ||
| Aortic Banding Pulmonary Artery Banding |
• Low morbidity and ease of use • Ability to study the progression of RV or LV hypertrophy |
• Difficult to achieve analogous HF as found in clinical situations • Only a small percentage develop signs of HF |
| Volume Overload Model | ||
| Arteriovenous Fistula Mitral Regurgitation Aortic Regurgitation Tricuspid Regurgitation |
• Effective in evaluating diastolic HF • Adequate to study compensatory mechanisms of HF |
• Requires a complicated surgical procedure • Delayed onset of HF • Does not represent the complete spectrum of HF |
Table 2.
, Large vs. Small Animal Models (Key Differences in Cardiac Physiology and in the Induction of Heart Failure)
| Differences In The Management of Large and Small Animal Models: |
| • Small models have the advantages of higher throughput and lower costs |
| • Larger models demand a substantial investment in resources for execution and postoperative care |
| • Small models have more favorable regulatory guidelines for implementation |
| • Higher degree of flexibility in testing multiple aims in small vs. large |
| Differences In Basic Cardiac Physiology of Large and Small Animal Models: |
| • A number of small models, particularly rodents, exhibit shorter action potential without a plateau phase |
| • The activity of SR Ca2+ ATPase is higher in the rat ventricle and the rate of Ca2+ removal through the Na+/Ca2+ exchange is lower vs. large species |
| • The myocytes or small (mice/rats) and large animal models (pigs/sheep) differ with respect to the quantitative balance of Ca2+ flux. |
| • Large models exhibit much higher activity with respect to Ca2+ removal predominated by the Sarcoplasmic Reticulum pump |
| • Structural difference in myosin heavy chain isoforms involved in contraction |
| • Less control of infarct size in mice/rat models due to greater anatomical variability |
| • Chronotropy – Rodent heart rates are typically 5 times higher in comparison to large models, thus affecting the force-frequency relationship which is inversely proportional |
| • Higher degree of infarct size in small animals required to achieve HF: Rats (40-50%) and Sheep/Pig (15-25%) |
| • Small animal HF progression differs significantly from large models with respect to neurohormonal activation, since this is much more difficult to obtain in rats/mice |
Ischemia/infarction models
Chronic HF primarily of ischemic origin remains a leading cause of mortality and morbidity and is the basis for 70% of HF in the USA. IHD can lead to HF through extensive transmural myocardial necrosis with pump failure or after small infarction with regional contractile dysfunction and adverse remodeling with myocyte hypertrophy, apoptosis and deposition of extracellular matrix [4]. Surgical induction of myocardial infarction (MI) in an animal model has the advantage of facilitating precise timing, location and extent of the coronary event, leading to more reproducible results and, not unexpectedly, it is the most frequently used animal model. It is relatively easy to simulate, is well controlled and has clear methods of evaluation. These advantages provide the ability to create varying degrees of failure, depending on the size of the infarct or the duration of ischemia. MI can be produced by either extravascular devices (occluder, constrictor or ligature), or intravascular occlusion methods such as balloon inflation or microembolization [11].
Coronary artery ligation
Direct ligation of coronary arteries is a reasonably simple method of creating areas of MI, which results in subsequent remodeling and ventricular dysfunction. This approach is very attractive as a result of both its clinical relevance and reproducibility (Figure 1A). The major disadvantage of this technique, however, is its invasiveness because the surgery-related morbidity is significant. Another complicating factor with this model is a priori estimating the percentage of MI by estimating the actual area of myocardium perfused by the vessel, distal to the ligation. The most preferred vessels for ligation are the left anterior descending (LAD) and/or diagonal arteries [12–14] and the circumflex artery and its branches [15–17]. This heart failure model was first applied in dogs, with a mortality of over 50% as a result of malignant ventricular tachycardias. MI was much smaller than expected as a result of an extensive network of collaterals in this species [18]. Subsequently, most studies worked with sheep or swine, creating a replicable model of anteroapical MI involving 22–25% and posterobasal MI involving 24–28% of the LV area [19–21]. Initial application was accompanied by a high mortality rate [22], prompting some studies to recommend using a variety of drugs with the aim of preventing ventricular dysrhythmias, leading to enhanced early term survival [14,17,23,24].
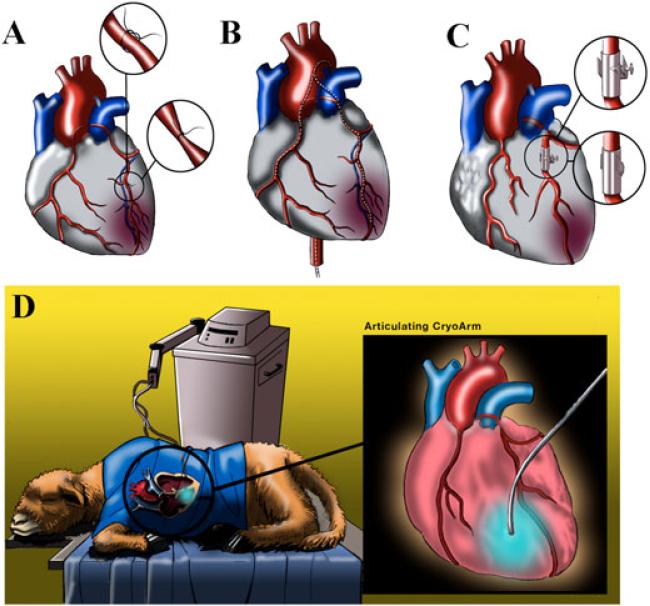
Figure 1.
(A) Coronary artery ligation. Left anterior thoracotomy is usually performed through the fifth intercostal space. After opening the pericardium, the coronary anatomy is inspected. Arteries are ligated based upon the preselected zone of myocardial infarction. (B) Coronary artery embolizations. This model is based on intracoronary embolizations with microspheres, agarose, polystyrene beads, injection of thrombin and autogenous blood with fibrinogen. Animals undergo three to ten percutaneous catheter-mediated intracoronary interventions during 1–3 weeks. (C) Coronary artery narrowing. After left anterolateral thoracotomy, the pericardium is opened and the artery is dissected to allow placement of a hydraulic occluder and ultrasonic flow probe around the vessel. The flow probe is placed distal to the occluder to record downstream flow through the artery. Inflation of occluder induces partial stenosis or complete occlusion. Ameroid constrictors are implanted in a similar way and are made of hydroscopic casein in a stainless ring. At body temperature, the casein swells after absorption of water from the surrounding tissue. Usually, coronary arteries are occluded in 3–6 weeks. (D) Cryoinfarction. After an intercostal thoracotomy and exposition of the heart, cryoinfarction is produced by applying a liquid nitrogen cryoprobe for 10–20 s several times on the left ventricular wall.
Coronary artery embolizations
This model is based on intracoronary embolization with radioactive microspheres [25], polysterene latex microspheres [2,26,27], agarose, polystyrene beads, intracoronary injections of thrombin, autogenous blood with fibrinogen [2,28] or embolic coils [29] (Figure 1B). Sequential selective microembolization is repeated three to ten times during 1–3 weeks, until the animals start to develop clinical signs of HF with a decrease of EF of less than 35–40% [1,2,26,27]. The principal advantages of the microembolization approach include minimal invasiveness, and the model mimics the clinical scenario where there is continual embolization of atherosclerotic and thrombotic debris [11]. Furthermore, the ability to titrate the response after repeated embolization, a lack of recovery of LV function once coronary embolization is discontinued, and relative homogeneity of ischemic damage, certainly complement the attractiveness of this technique [2,26]. A major limitation of the embolization procedure is the lack of control of the exact level or site and length of coronary artery occlusion; others include the need for serial intracoronary interventions (sometimes more than 10), a high rate of malignant dysrhythmias, and the inability to reproduce the changes that are similar to IHD [1,2,30].
Narrowing of coronary arteries
Occluders and constrictors are used to obtain a calibrated degree of narrowing of the coronary arteries. The most common occluders are U-shaped and ring-shaped [11] (Figure 1C). The occluder is simply a hydraulic device placed around the vessel through implantation via thoracotomy. To control the degree of stenosis, an ultrasonic flow probe can be placed distally to the occluded artery [31]. An alternative is an ameroid constrictor constructed from the hydroscopic casein. At body temperature, the constrictor absorbs water and the ring around the artery narrows gradually, compressing the vessel and producing occlusion over a long period of time [32–34]. Most commontly, these constrictors have been placed on the circumflex artery that supplies 20–35% of LV mass [34–37]. The reported mortality rate, however, is quite high [38].
Cryoinfarction model
This method is often used for gene therapy in small animal models. Commonly, to achieve cryoinfarction, studies have used a nitrogen-cooled cryoprobe that is applied on the free LV wall (Figure 1D). However, cryoinjury does not always induce a transmural MI. Using this methodology, the size of the MI was estimated at approximately 30% of total LV area [39–42].
Ischemia/reperfusion model
This model is attractive because it resembles the clinical situation following percutaneous coronary intervention (PCI) in the setting of acute MI. Reperfusion injury after MI is assumed to cause increased oxidative stress, mitochondrial dysfunction and intracellular Ca2+ overload [43]. PCI and intracoronary balloons are needed to create ischemia/reperfusion injury. The LAD and the obtuse marginal branch have each been utilized as the target for this approach. Typically, the artery is occluded for 30–90 min with subsequent coronary angiography after balloon deflation to confirm patency. The size of the MI is approximately 15–20% [29,44].
Tachycardia-induced model
This model is unique from the ischemia/infarction models and results in dilated cardiomyopathy leading to HF. It allows for study of the effect of rapid pacing on ventricular function, as well as the recovery phase associated with discontinuation of pacing (Figure 2). The advantages of the rapid pacing model are its relative simplicity, the ability of sustained atrial or ventricular pacing to produce biventricular systolic and/or diastolic dysfunction, and the neurohormonal alterations that occur without concomitant changes in the coronary vessels [45,46]. The major deficiency of this model is the fact that the molecular and hemodynamic alterations revert nearly to almost values soon after pacing cessation, suggesting that the pathophysiological derangements are distinct from irreversible HF in humans. Development of end-stage HF usually requires 3–5 weeks of continued pacing at 190–240 beats/min in large animals. By pacing at a slower rate or for a shorter duration, a lesser degree of LV dysfunction can be produced [47–49]. Newer implantable pacemakers designed for mice and rodents may allow for extension of this model to smaller animals, although higher pacing rates will be required.
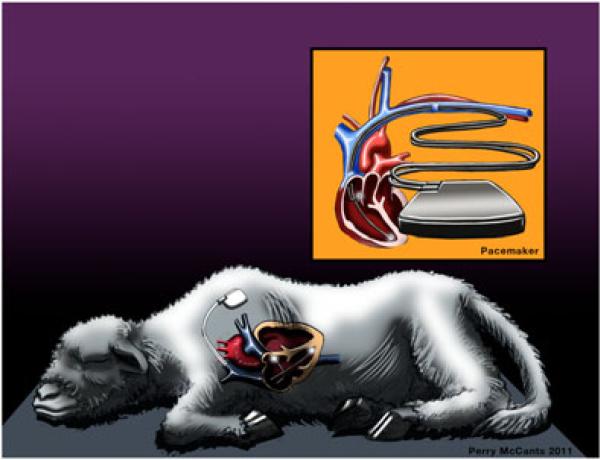
Figure 2.
Tachycardia-induced model. A pacemaker can be surgically placed into the chest or dorsum of animals through a small incision. A bipolar atrial and/or ventricular lead is placed transvenously under fluoroscopic guidance in the right atrium and/or right ventricle and connected to the pacemaker. The most common protocol uses rapid ventricular pacing at 190–240 beats/min at 3–5 weeks.
Pressure-overload model
Pressure-overload is accompanied by reductions in ventricular chamber compliance and diastolic dysfunction. Reduced compliance requires higher pressure to fill the ventricles, and symptoms of HF result from afterload mismatch and decreased contractility. LV pressure-overload can be created with devices narrowing the ascending or descending thoracic aorta with a variety of surgical approaches [49–53]. For right ventricle (LV) pressure-overload, pulmonary artery banding has been employed [54].
Volume overload model
Surgical techniques to induce volume overload involve the creation of mitral, aortic or tricuspid valve regurgitation, or the creation of an arteriovenous fistula [49,55–57]. Altered loading conditions and decreased contractility in cases of mitral and tricuspid regurgitation result in progressive LV and RV dilatation and the development of HF. Increased cardiac filling pressures, reduced cardiac output, excessive peripheral vasoconstriction, and impaired natriuresis and diuresis are the established hallmarks of HF in volume overload models. The mechanisms contributing to ventricular dilatation in this model are considered to include myocyte slippage, elongation of the cardiac cell, extracellular matrix remodeling dropout, and altered intracellular signaling pathways [58].
Combined models
A combination of existing models was developed using serial myocardial infarctions with occluders followed by rapid ventricular pacing. It was considered that coronary artery occlusion with ventricular pacing would increase the imbalance between myocardial energy supply and demand both in ischemic and in non-ischemic zones. Interestingly, unlike the tachycardia-induced model of HF, the LV dysfunction did not reverse after cessation of pacing [30]. This hypothesis has been confirmed using coronary artery stenosis in combination with ventricular pacing [59]. In another model combining moderate mitral regurgitation created by LV to left atrial shunt and anteroapical MI, greater ventricular remodeling was demonstrated compared to MI alone [60].
In this review, we address the issue of inducing heart failure by surgical means, although it should be noted that several genetic models of heart failure have been used extensively [10,46,49,61,62]. Of fundamental importance among them are models of the spontaneously hypertensive heart failure-prone rats; muscle limb protein knockout mice; tumor necrosis factor-α- overexpressing mice; genetically engineered hamsters and mice lacking dystrophin and utrophin; with β-myosin heavy chain mutation, null mutation of the cardiac α-actin gene, etc.
Gene transfer targets in different models of heart failure
Identification of the mechanisms of molecular, neurohumoral and hemodynamic changes revealed that HF is a complex pathological state, consisting of the simultaneous alterations in multiple signaling pathways [63,64]. The most thoroughly investigated potential targets of gene therapy for HF are enhancement of contractility via beta adrenergic receptor (βAR) pathways and Ca2+ handling proteins, and stimulation of cardiac angiogenesis (Figure 3). Gene therapy for the treatment HF as a consequence of IHD has been studied most extensively. Ideally, gene therapy for ischemic HF should result in neoangiogenesis, inhibition of apoptosis, reversal of remodeling and prevention of arrhythmias. It is quite natural that, for the other causes of HF, such as pressure or volume overload, the objectives of gene therapy may be different. Therefore, specific molecular targets should be investigated for selected types of HF, and genetic intervention, respectively, should be aimed in multiple possible directions, including target overexpression, target inhibition, alteration of the target's intracellular route, and/or correction of gene mutation or deletions [8]. In addition, we must remark on the current available methods of cardiac gene delivery. These techniques can be classified by the site of vector injection, interventional approach and method of cardiac circulation during gene transfer. Unfortunately, despite the development of new vectors with significant tropism for the heart, there does not yet exist one practical, efficient and clinically relevant method of gene delivery. However, it is now assumed that the ideal technique may include: (i) a coronary sinus or coronary arteries route of delivery; (ii) extended vector residence time in coronary circulation; (iii) isolation of the cardiac circulation from the systemic; (iv) washout of the vector after gene transfer; (v) no technique-associated morbidity; and (vi) minimization of collateral expression [65].
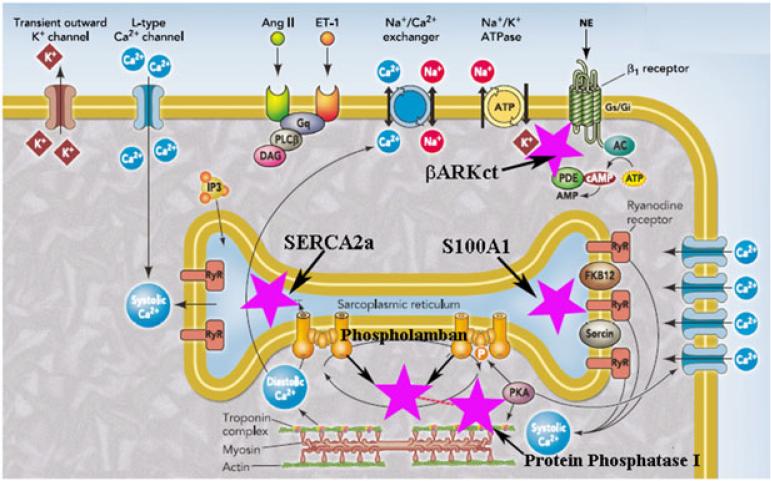
Figure 3.
Simplified representation of the excitation-contraction coupling in cardiomyocytes and main gene therapy targets. The cardiac action potential is initiated in the myocardial cell membrane, and the depolarization results in inward Ca2+ through L-type Ca2+ channels from extracellular to intracellular fluid during plateau. Entry of Ca2+ into the myocardial cell triggers the release of more Ca2+ from stores in the sarcoplasmic reticulum (SR) through ryanodine receptors. Ca2+ binds to troponin C, tropomyosin is moved, and the actin and myosin can bind and tension is produced. Relaxation occurs when Ca2+ reaccumulated in the SR by the action of the Ca2+ ATPase and Ca2+–Na+ exchange in the sarcolemmal membrane. After the onset of HF, most promising targets are: 1. SERCA2a (Lilac Star). Abnormal Ca2+ leak from SR; depressed SR Ca2+ re-uptake caused by low expression of cardiac sarcoplasmic reticulum Ca2+ ATPase (SERCA2a) pump and an increased activity of endogenous inhibitor of SERCA2a, phospholamban. SERCA2a has a critical role in Ca2+ regulation. The overexpression of SERCA2a is one of the targets for the treatment of HF. 2. βARKct (Lilac Star). Molecular abnormalities associated with HF include the uncoupling of the β-adrenergic receptor system, enhanced expression and activity of the G protein-coupled receptor kinase and loss of βAR inotropic reserve. βARKct gene delivery approach has the potential to resolve βAR downregulation and desensitization. 3. Phospholamban (Lilac Star) is an endogenous inhibitor of the SR Ca2+-ATPase. Phosphorylation of phospholamban by cyclic AMP- dependent or calmodulin-dependent protein kinases (PKA or CaMKII) relieves this inhibition, allowing faster twitchrelaxation and decline of intracellular Ca2+. Because the SR Ca2+-ATPase competes better with Na+/Ca2+ exchange, phosphorylation of phospholamban also enhances Ca2+ content in the SR. 4. S100A1 (Lilac Star) plays a role in increasing SERCA2a activity, diminishing diastolic SR Ca2+ leak, and augmenting systolic open probability of the ryanodine receptors, causing an overall gain in SR Ca2+ cycling. Also, S100A1 regulates SERCA2A-phospholamban function, resulting in a balanced enhancement of SR Ca2+ release and uptake. 5. In failing hearts, the downregulation of adrenergic receptor and cAMP-dependent protein kinase signaling leads to the inactivation of inhibitor-1 which, in turn, results in increased activity of Protein Phosphatase 1 (Lilac Star). This activation leads to the dephosphorylation of phospholamban thus reducing calcium uptake by SERCA-2a. PKA, protein kinase A; RyR, ryanodine receptors; FKB12, calstabin 2; AngII, angiotensin II; ET-1, endothelin 1; NE, norepinephrine; ATP, adenosine triphosphate; cAMP, cyclic adenosine monophosphate; AC, adenylyl cyclase; PDE, phosphodiesterase; Gq, class of guanine nucleotide-binding proteins; PLCβ, phospholipase C beta; DAG, diacylglycerol; IP3, inositol trisphosphate; Gs/Gi, stimulatory/inhibitory G protein; L-type, long lasting dihydropyridine receptors. Lilac stars indicate the main gene therapy targets. Reproduced with modifications [64].
The β-adrenergic signaling cascade
Under normal physiological conditions, the β-adrenergic receptor signaling system plays an important role in the control of cardiac function, mediating the inotropic, chronotropic and lusitropic responses to the sympathetic neurotransmitters, epinephrine and norepinephrine [66]. Dysregulation of the pathway, including downregulation of βARs, uncoupling of second-messenger systems, and upregulation of βAR kinase (βARK1, GRK2), has been shown to be a hallmark of HF, and antagonism of the receptor with β-blockers has recently become a standard adjunct therapy for treating the disease. βARs are regulated by GRK2, a member of a G protein-coupled receptor kinase family that phosphorylates agonist-occupied receptors that trigger the binding of β-arrestins and the process of homologous desensitization [67]. GRK2 expression and activity in the heart appear to constitute a critical regulator of heart function in vivo [67]. Enhanced GRK2 expression and activity are present in the failing heart. βARKct, a competitive inhibitor of GRK2, has the potential to resolve βAR downregulation and desensitization associated with HF [39,67,68]. Thus, inhibiting the activity of GRK2 or lowering its expression appears to offer a novel means to enhance cardiac function.
βARKct
Coronary artery ligation model
Ad/βARKct delivery through the circumflex artery to ischemic myocardium after ligation of an obtuse marginal branch resulted in a significant increase in adenylyl cyclase activity and improvement of LV dP/dt. It was first demonstrated that the βARKct transgene expression reverses βAR signaling abnormalities and LV systolic dysfunction after MI [16,17], proving a potential βARKct therapeutic benefit in HF. Tevaearai et al. [69] demonstrated that Ad/βARKct delivery before circumflex artery ligation may represent a new strategy for myocardial protection with the prevention of HF. Later, the same approach was used with cardiac-specific inhibition of GRK2 with βARKct before surgical ligation of the LAD artery in mice, resulting in a reduction in infarct-related mortality, and decrease in the extent and progression of cardiac dysfunction and preserved βAR responsiveness. Loss of GRK2 after MI was associated with a lower extent of cardiac hypertrophy [68].
Cryoinfarction model
Rengo et al. [39] studied the rat model of cryoinfarction for delivery of βARKct via adeno-associated virus (AAV) 6-mediated direct intramyocardial injection. They found that long-term (12 weeks) myocardial expression of βARKct accompanied by significant amelioration of the HF-related neurohormonal status decreased the hyperactivity of the sympathetic nervous system and renin-angiotensin-aldosterone signaling axis. Also, the expression of various-related genes and hypertrophic markers was decreased, indicating a reversal of LV remodeling and maladaptive hypertrophy. The authors considered that these findings reflected decreased apoptosis and an increase in cardiomyocyte survival and proliferation.
Ischemia/reperfusion model
The use of mice with overexpression of βARKct in an ischemia/reperfusion model revealed that the inhibition of GRK2 can have an impact on cardioprotection with a reduction of infarct size and the apoptotic index [70].
Pressure-overload model
Emani et al. [71] used a model of increased RV afterload through pulmonary artery banding. Before gene delivery, rabbits underwent placement of a deployable pulmonary artery band. It was found that RV-specific Ad/βARKct expression limits RV dilatation, improves contractility and survival. The physiological changes were associated with normalization of intracellular βAR signaling. A possible mechanism by which βARKct prevents ventricular dilatation is by altering G-protein coupled receptor signaling pathways [54,58]. An improvement in cardiac function and cell morphology was also found in transgenic mice that underwent transverse aortic constriction after βARKct delivery. The high level of βARKct expression preserved both adenylyl cyclase activity and βAR density [71]. These results demonstrated that βARKct expression in the hypertrophied heart mitigates desensitization associated with pressure-overload hypertrophy and preserves in vivo inotropic reserve.
Volume overload model
RV pressure-overload state was caused by surgically created tricuspid insufficiency in a dog model. A decrease of adenylyl cyclase levels and an increase GRK2 activity was demonstrated in both LV and RV samples [55].
There are other therapeutic options that improve the function of desensitized β-adrenergic receptors. One of them is to increase cAMP levels by overexpressing the V2 vasopressin receptor, which normally promotes the activation of adenylate cyclase, and might therefore be expected to have a positive inotropic effect. Adenovirus-mediated transfer of vasopressin resulted in an increase in myocardial contractility [72]. In another study, the intracoronary injection of adenovirus encoding for adenylyl cyclase resulted in an increase in adenylyl cyclase protein content and stimulated cyclic AMP levels, which was accompanied by a prolonged increase in left ventricular contractility [73].
The calcium cycling proteins
Ca2+ cycling has been found to be critically dysregulated in CHF and, similar to β-adrenergic signaling, provides an important role in excitation-contraction coupling. To understand the Ca2+ handling defects in heart failure, we need to briefly describe the processes occurring in cardiac excitation–contraction coupling. During the cardiac action potential, Ca2+ enters the cell through depolarization-activated Ca2+ channels as an inward Ca2+ current, which contributes to the action potential plateau. Ca2+ entry triggers Ca2+ release from the sarcoplasmic reticulum (SR). This allows Ca2+ to bind to the myofilament protein troponin C, which then switches on the contractile process. For relaxation to occur, there is intracellular Ca decline, allowing Ca2+ to dissociate from troponin. This requires Ca2+ transport out of the cytosol by pathways involving SR Ca2+-ATPase, sarcolemmal Na+/Ca2+ exchange, sarcolemmal Ca2+-ATPase or mitochondrial Ca2+. Deficient SR Ca2+ uptake during myocyte relaxation has been identified in failing hearts from both humans and animals, and is associated with a decrease in the expression and activity of sarcoplasmic reticulum calcium ATPase (SERCA2a). This protein is a Ca2+-ATP-dependent pump of the sarcoplasmic or endoplasmic reticulum that has a critical role in Ca2+ regulation. The overexpression of SERCA2a is one of the most promising targets for the treatment of HF [63].
SERCA2a
The activity of SERCA2a is modulated by phospholamban (PLN), a SR transmembrane protein. Increased SERCA2a activity, as a result of PLN phoshorylation, results in increased SR calcium reuptake and release. Increasing SERCA2a function or decreasing the inhibitory effect of PLN has been the subject of gene transfer approaches.
Ischemia/infarction model
The long-term effect of SERCA2a gene transfer was examined in the rat ischemic HF model. A lentiviral vector containing the SERCA2a gene was infused into a rat heart by hypothermic intracoronary delivery 2 weeks after MI. The transduction efficiency was approximately 40%. Six months later, it was revealed that SERCA2a gene transfer protected LV dilatation and improved systolic and diastolic function, resulting in reduction in mortality rates [74].
Ischemia/reperfusion model
del Monte et al. [75] demonstrated that SERCA2a overexpression in a model of ischemia/reperfusion suppressed arrhythmias and reduced MI size. Also, it was shown that SERCA2a overexpression can decrease the number of episodes of ventricular tachycardia after reperfusion in a porcine model [29]. Several mechanisms are described that are vital for this process. Abnormal Ca2+ cycling by the SR post-infarction participates in action potential duration alternans, which leads to T wave alternans and ventricular arrhythmias. By contrast, in the overexpression of SERCA2a, there are two theories that have been found to prevent arrhythmias. One of them is called ‘delayed afterdepolarization’; Ca2+ overload of the SR generates release of Ca2+ by the ryanodine receptors and a depolarizing inward current is mediated by the Na+/Ca2+ exchanger. Another is called ‘early afterdepolarization’ and involves prolonged action potentials allowing excessive Ca2+ entry through L-type Ca2+ channels [63,75].
Tachycardia-induced model
The delivery of SERCA2a in a large animal model led to dose-dependent significant improvements in LV diameter, LV fractional shortening and EF after pacing-induced HF. In addition to favorable hemodynamic effects, brain natriuretic peptide (marker of remodeling) was reduced [76].
Volume-overload model
In studies in pigs HF was induced by percutaneously created mitral regurgitation. Two months later, AAV1/SERCA2a was administered by intracoronary delivery. At 4 months, gene transfer resulted in a positive LV inotropic effect and improvement in LV remodeling based on decreasing systolic diameter. At follow-up, brain natriuretic peptide levels remained stable [56]. As also demonstrated in sheep that upregulate SERCA2a after percutaneous delivery, AAV6/SERCA2a in a model of mitral regurgitation with small apical MI may improve contractility and activate antiapoptotic pathways [77].
Pressure-overload model
Miyamoto et al. [78] investigated whether increasing SERCA2a expression can improve HF in a rat model with aortic constriction. Overexpression of SERCA2a restored ATPase activity to nonfailing levels. Furthermore, rats transfected with Ad/SERCA2a had improvements in LV + dP/dt, –dP/dt and the rate of isovolumic relaxation, normalizing them to sham-operated levels. It was considered that SERCA2a decreased diastolic intracellular Ca2+ by increasing uptake into the SR and enhancing Ca2+ release. This mechanism decreased the stimulation of phosphatases and reduced pro-apoptopic and pro-hypertrophy signaling. The SERCA2a overexpressed in rat heart after abdominal aortic banding revealed improved hemodynamic parameters compared to controls, although there was no difference in mortality [79]. del Monte et al. [80] demonstrated that, in a rat model of ascending aortic banding 4 weeks after gene transfer with Ad/SERCA2a, survival was 63% and LV end diastolic volume was 0.46 ml compared to 9% and 0.64 ml in the failing heart with Ad/βgal/GFP. Moreover overexpression of SERCA2a increased the phosphocreatinine/ATP ratio, thus increasing energy reserve [80].
SERCA2a + parvalbumin
Parvalbumin is a small molecular weight intracellular calcium-binding protein that is expressed in striated muscle fibers but not in the heart. It is ideally designed to speed the rate of decline in intracellular calcium, with advantages of this occurring via a non-ATP dependent process [81].
Pressure-overload model
Sakata et al. [82] examined whether short- and long-term gene transfer of Ca2+ handling proteins restores LV mechanoenergetics in aortic banding hearts. They found that the slope of the relationship between cardiac oxygen consumption and systolic pressure-volume area and the O2 cost of total mechanical energy were twice the normal values, whereas these parameters normalized in failing hearts receiving SERCA2a + parvalbumin. Therefore enhancement of calcium handling by resequestration into SR or by intracellular buffering improves not only mechanical, but also energetic function [82]. A large animal model with diastolic dysfunction up to 1 year after thoracic aortic coarctation was tested by Hirsch et al. [83]. Gene transfer of the SERCA2a + parvalbumin restored isolated cardiac myocyte relaxation in a dose-dependent manner.
S100A1
S100A1 is a member of the S100 protein family that has approximately 20 members. In cardiac myocytes, S100A1 is localized at the SR, myofilaments and mitochondria. [41]. Physiological functions of S100A1 at a molecular level in cardiomyocytes primarily play a role in increasing SERCA2a activity, diminishing diastolic SR Ca2+ leak, and augmenting systolic open probability of the ryanodine receptors, leading to an overall gain in SR Ca2+ cycling. Also, S100A1 regulates SERCA2A-PLN function, resulting in a balanced enhancement of SR Ca2+ release and uptake. Therefore, S100A1 protein concentration appears to critically determine the responsiveness of Ca2+ cycling to trigger contraction to sympathetic stimulation [41,84].
Cryoinfarction model
The earliest studies utilized Ad/S100A1 gene delivery administrated via rat coronary arteries after cryothermia-induced MI. This strategy resulted in a 30–40% transfection rate, preventing postischemic deterioration with attenuation of cardiac remodeling [85]. Using the same model in vivo after 7 days of Ad/S100A1 gene delivery, S100A1 protein was normalized in failing myocardium, resulting in an improved contractile function of failing hearts. The peak rate of LV pressure rise almost to normal levels, reflecting a rescue of in vivo cardiac systolic function. Similarly, S100A1 gene transfer subsequently decreased end-diastolic pressure to levels seen in nonfailing hearts, demonstrating improved diastolic function [41]. Functional cardiac recovery after 8 weeks in AAV6/S100A1-treated HF rats was accompanied by decreased LV chamber dilatation and improved function, as assessed by EF. These data might be explained by an indirect effect resulting from enhanced contractile function of the heart, thus resulting in reverse remodeling. Improved Ca2+ handling in failing cardiomyocytes might beneficially affect myocardial apoptosis, and hypertrophy or modulate various Ca-dependent signaling pathways, involving calcineurin, calmodulin kinase or protein kinase C isoforms [40].
Ischemia/reperfusion model
A study in a postischemic pig HF model revealed long-term improvement of systolic and diastolic indices, reversed remodeling and restored cardiac energy homeostasis. This study used AAV9/S100A1 and MI was established by PCI balloon occlusion of circumflex artery [86].
Phospholamban
PLN plays a very important role in the regulation of SR Ca2+ homeostasis mediating slower cytosolic Ca2+ decay in cardiomyocytes, which translates into diastolic relaxation. It has been shown that PLN is relatively hypophoshorylated in HF, which contributes to the reduction in SERCA activity [87,88].
Coronary artery ligation model
AAV-mediated overexpression of PLN-S16E (mutant form of PLN) improved LV function and mitigated adverse remodeling in post-MI (LAD occlusion) HF rats. The improvement in LV function was demonstrated by higher EF, limitation of LV dilatation and reduction of hypertrophy [89].
Ischemia-reperfusion model
Removal of PLN in ischemia should result in increased SERCA2a activity and thus mimic catecholamine-mediated phosphorylation of PLN. Subsequently, in a study using PLN-knockout mice, there was 20 min of ischemia and 40 min of reperfusion. This resulted in a decrease in ATP and pH values, and a decline in post-ischemic contractility compared to controls. The exact mechanism of increased ischemia-reperfusion injury is unclear. However, there are some energy changes that may play a role, such as increased oxygen consumption, a decreased phosphocreatine level, or an increase in the active fraction of mitochondrial pyruvate dehydrogenase [90].
Tachycardia-induced model
After 4 weeks of rapid pacing in sheep, the animals receive Ad/PLN-S16E. Two weeks after gene delivery, EF increased from 27% to 50% and LV end-diastolic area decreased from 37 to 32 cm2 [91].
Pressure-overload model
Kiriazis et al. [92] evaluated the effect of PLN-knockout in aortic banded mice. They found that knockout mice and wild-type mice had a similar degree of LV hypertrophy but, paradoxically, the knockout mice had an earlier onset of a decline in fractional shortening.
Volume-overload model
In study by Tsuji et al. [93] rat hearts with combined volume and calcium overload that had been transfected with adenovirus encoding antisence PLN were rescued from LV dysfunction. Ad/PLN gene therapy was associated with preserved LV contractility and normalization of LV mechanoenergetics.
Protein phosphatase 1
The inhibitory action of PLN on SERCA2A and regulation of the β-agonist response is subject to tight secondary control mediated by protein phosphatase 1 (PPI) dephosphorylation [8]. This enzyme is localized to SR membranes and is regulated via the actions of the phosphatase inhibitors. Transgenic cardiac overexpression of PPI results in reduced cardiac contractility and decreased βAR-mediated Ca2+ signaling [94].
Pressure-overload model
Transgenic mice overexpressing a PPI inhibitor display enhanced cardiac contractility, a delay in the development of hypertrophy and a reduction in maladaptive remodeling after transaortic constriction [95].
Cardiac angiogenesis
From a clinical point of view, the main focus of gene therapy for ischemic HF is enhancement of contractile function of cardiomyocytes by an improvement of perfusion and replacement of fibrous post-infarct tissue by newly-formed contractile cells [96]. Numerous studies have demonstrated that stimulation of cardiac angiogenesis is beneficial to ischemic and infarcted heart [97,98]. Currently, gene transfer for therapeutic angiogenesis comprises a well studied area, with several clinical trials having been completed. It was shown that therapeutic vasculogenesis can be achieved by gene transfer of vascular endothelial growth factor (VEGF), hepatocyte growth factor, fibroblast growth factor, and hypoxia-inducible factor 1α [99]. Much of the research is devoted to the study of VEGF.
VEGF
The mammalian genome encodes five isoforms of the VEGF family. VEGF-A and VEGF-B play a key role in angiogenesis in the heart. Transcripts encoding its isoforms VEGF-A121 and VEGF-A165 are detected in the majority of cells and tissues expressing the VEGF gene [97].
Coronary artery ligation model
VEGF-A165 through nonviral delivery systems in rabbits with circumflex artery ligation induces significant neovascularization and improves fractional shortening after MI [98]. AAV/VEGF-A165 delivery during acute MI (LAD occlusion) increased the number of a-smooth muscle actin positive arterioles and improved myocardial viability [99]. Javanel et al. [14] injected VEGF-A165 intramyocardially in the sheep model 1 h after creation of an anteroapical MI. After 15 days, they found a reduction in infarct size (11% versus 18%), an increase in arteriogenesis, a decrease in peri-infarct fibrosis and myofibroblast proliferation, and enhanced mitosis of cardiomyocytes with cytokinesis. Lähteenvuo et al. [100] demonstrated that VEGF-B186 also represents a promising treatment for CAD. In large animals after acute infarction (coil occlusion of LAD), it was shown that intramyocardial Ad/VEGF-B186 increased ejection fraction, collateral artery formation and the appearance of apoptosis-resistant cardiomyocytes around the infarction border zone [100].
Narrowing of coronary arteries model
Laguens et al. [37] found that VEGF-165 transfection in ameroid-ischemic porcine hearts induced an increase in cardiomyocyte hyperplasia in the ischemic zone resulting in division of native myocytes. Ad/VEGF-165 administration also increased EF and regional wall motion in this model [101].
Tachycardia-induced model
Leotta et al. [102] administered Ad/VEGF 121 to the LV. After a 1-week recovery, animals were paced at a rate of 230 beats/min for 7 days to induce HF. It was found that fractional wall thickening and segmental shortening were significantly improved within 7 days after gene delivery.
Conclusions
Clinical cardiac gene therapy experience is limited [7,8]. This is partly a result of the need for more detailed knowledge of the mechanism-specific targets as a function of the extrinsic and intrinsic causes of clinical heart failure. Among the potential targets, the most-well studied are βAR signaling and Ca2+ handling proteins. Currently, it has been well established that the βARKct peptide is an effective inhibitor of GRK2-mediated receptor phosphorylation and desensitization in ischemia/infarction, volume overload and pressure-overload models. In all of the above models, βARKct improved cardiac contractility, reversed LV remodeling, and normalized the neurohumoral status. Gene transfer of SERCA2a has been tested on most of the categories of animal heart failure models and it consistently results in an enhancement of cardiac performance, increased animal survival and preserved energetic status. Other targets are less well studied and need continued investigation (Table 3). Importantly, because a wide variety of molecular changes occur in the failing heart, each potential target should be investigated to assess its therapeutic efficacy in a variety of HF models. Mechanism-specific investigations of genetic manipulation are critically needed to further develop advanced strategies to treat HF.
Table 3.
Gene transfer targets in different models of heart failure
| Model Type | Coronary Artery Ligation |
Coronary Artery Narrowing |
Cryoinfarction | Ischemia/Reperfusion | Tachycardia Induced | Volume Overload | Pressure Overload | |
|---|---|---|---|---|---|---|---|---|
| Cardiac Transgene | βARKct |

|

|

|

|

|
||
| SERCA2a |

|

|

|

|

|
|||
| SERCA2a & Parvalbumin |

|
|||||||
| S100A1 |

|

|

|
|||||
| VEGF |

|

|

|
|||||
| Phospholamban |

|

|

|

|
||||
| Protein Phosphatase 1 |

|
|||||||
Acknowledgements
We would like to thank Perry McCants for the excellent illustrations. This work was supported by grants from the National Heart Lung and Blood Institute, NIH 1-R01-HL083078-01A2 (CR Bridges); and the Gene Therapy Resource Program (GTRP) of the National Heart, Lung, and Blood Institute, NIH P30-DK047757 (J. Wilson).
Footnotes
The authors have no proprietary disclosures to report.
References
- 1.Halapas A, Papalois A, Stauropoupou A, et al. In vivo models for heart failure research. In Vivo. 2008;22:767–780. [PubMed] [Google Scholar]
- 2.Huang Y, Hunyor SN, Jiang L, et al. Remodeling of the chronic severely failing ischemic sheep heart after coronary embolization: functional, energetic, structural, and cellular responces. Am J Physiol Heart Circ Physiol. 2004;286:H2141–H2150. doi: 10.1152/ajpheart.00829.2003. [DOI] [PubMed] [Google Scholar]
- 3.Dixon JA, Spinale FG. Large animal models of heart failure. A critical link in the translation of basic science to clinical practice. Circ Heart Fail. 2009;2:262–271. doi: 10.1161/CIRCHEARTFAILURE.108.814459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krum H, Abraham WT. Heart failure. Lancet. 2009;373:941–955. doi: 10.1016/S0140-6736(09)60236-1. [DOI] [PubMed] [Google Scholar]
- 5.Chaudhri BB, del Monte F, Harding SE, et al. Gene transfer in cardiac myocytes. Surg Clin N Am. 2004;84:141–159. doi: 10.1016/S0039-6109(03)00209-3. [DOI] [PubMed] [Google Scholar]
- 6.Hajjar RJ, Samulski RJ. A silver bullet to treat heart failure. Gene Ther. 2006;13:997. doi: 10.1038/sj.gt.3302747. [DOI] [PubMed] [Google Scholar]
- 7.Isner JM. Myocardial gene therapy. Nature. 2002;415:234–239. doi: 10.1038/415234a. [DOI] [PubMed] [Google Scholar]
- 8.Vinge LE, Raake PW, Koch WJ. Gene therapy in heart failure. Circ Res. 2008;102:1458–1470. doi: 10.1161/CIRCRESAHA.108.173195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Harsdorf R, Schott RJ, Shen YT, et al. Gene injection into canine myocardium as a useful model for studying gene expression in the heart of large mammals. Circ Res. 1993;72:688–695. doi: 10.1161/01.res.72.3.688. [DOI] [PubMed] [Google Scholar]
- 10.Sleeper MM, Bish LT, Sweeney HL. Gene therapy in large animal models of human cardiovascular genetic disease. ILAR J. 2009;50:199–205. doi: 10.1093/ilar.50.2.199. [DOI] [PubMed] [Google Scholar]
- 11.Klocke R, Tian W, Kuhlmann MT, et al. Surgical animals models of heart failure related to coronary artery disease. Cardiovasc Res. 2007;74:29–38. doi: 10.1016/j.cardiores.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 12.Kim WG, Shin YC, Hwang SW, et al. Comparison of myocardial infarction with sequential ligation of the left anterior descending artery and its diagonal branch in dogs and sheep. Int J Artif Organs. 2003;26:351–357. doi: 10.1177/039139880302600411. [DOI] [PubMed] [Google Scholar]
- 13.Rabbani S, Ahmani H, Fayazzadeh E, et al. Development of an ovine model of myocardial infarction. ANZ J Surg. 2008;78:78–81. doi: 10.1111/j.1445-2197.2007.04359.x. [DOI] [PubMed] [Google Scholar]
- 14.Javanel GV, Crottogini A, Meckert PC, et al. Plasmid-mediated VEGF gene transfer induces cardiomyogenesis and reduces myocardial infarct size in sheep. Gene Ther. 2006;13:1133–1142. doi: 10.1038/sj.gt.3302708. [DOI] [PubMed] [Google Scholar]
- 15.Gorman JH, Gorman RC, Jackson MB, et al. Annuloplasty ring selection for chronic ischemic mitral regurgitation: lessons from the ovine model. Ann Thorac Surg. 2003;76:1556–1563. doi: 10.1016/s0003-4975(03)00891-9. [DOI] [PubMed] [Google Scholar]
- 16.Shah AS, White DC, Emani S, et al. In vivo ventricular gene delivery of a β-adrenergic receptor kinase inhibitor to the failing heart reverses cardiac dysfunction. Circulation. 2001;103:1311–1316. doi: 10.1161/01.cir.103.9.1311. [DOI] [PubMed] [Google Scholar]
- 17.White DC, Hata JA, Shah AS, et al. Preservation of myocardial β-adrenergic receptor signaling delays the development of heart failure after myocardial infarction. Proc Natl Acad Sci USA. 2000;97:5428–5433. doi: 10.1073/pnas.090091197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hood WB, McCarthy B, Lown B. Myocardial infarction following coronary ligation in dogs. Circ Res. 1967;21:191–199. doi: 10.1161/01.res.21.2.191. [DOI] [PubMed] [Google Scholar]
- 19.Enomoto Y, Gorman JH, Moainie SL, et al. Early ventricular restraint after myocardial infarction: extent of the wrap determines the outcome of remodeling. Ann Thorac Surg. 2005;79:881–887. doi: 10.1016/j.athoracsur.2004.05.072. [DOI] [PubMed] [Google Scholar]
- 20.Crick SJ, Sheppard MN, Ho SE, et al. Anatomy of the pig heart: comparisons with normal human cardiac structure. J Anat. 1998;193:105–119. doi: 10.1046/j.1469-7580.1998.19310105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frink RJ, Merrick B. The sheep heart: coronary and conduction system anatomy with special reference to the presence of an Os Cordis. Anat Rec. 1974;179:189–200. doi: 10.1002/ar.1091790204. [DOI] [PubMed] [Google Scholar]
- 22.Iwanaga K, Takano H, Ohtsuka M, et al. Effects of G-CSF on cardiac remodeling after acute myocardial infarction in swine. Bioch Biophys Res Com. 2004;325:1353–1359. doi: 10.1016/j.bbrc.2004.10.149. [DOI] [PubMed] [Google Scholar]
- 23.Muller CA, Opie LH, Pineda CA, et al. Bucindolol, a beta blocker, decreased ventricular fibrillation and maintained mechanical function in a pig model of acute myocardial ischemia. Cardiovasc Drugs Ther. 1992;6:233–237. doi: 10.1007/BF00051144. [DOI] [PubMed] [Google Scholar]
- 24.Moainie SL, Gorman JH, Guy TS, et al. An ovine model of postinfarction dilated cardiomyopathy. Ann Thorac Surg. 2002;74:753–760. doi: 10.1016/s0003-4975(02)03827-4. [DOI] [PubMed] [Google Scholar]
- 25.Kuroda H, Suga H. Cardiac function of an acute ischemic heart failure model produced by microsphere injection into the left coronary artery. ASAIO J. 1995;41:855–862. [PubMed] [Google Scholar]
- 26.Sabbah HN, Stein PD, Kono T, et al. A canine model of chronic heart failure produced by multiple sequential coronary microembolizations. Am J Physiol. 1991;260:H1379–H1384. doi: 10.1152/ajpheart.1991.260.4.H1379. [DOI] [PubMed] [Google Scholar]
- 27.Schmitto JD, Ortmann P, Wachter R, et al. Chronic heart failure induced by multiple sequential coronary microembolization in sheep. Int J Artif Organs. 2008;31:348–353. doi: 10.1177/039139880803100412. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki M, Asano H, Tanaka H, et al. Development and evaluation of a new canine myocardial infarction model using a closed-chest injection of thrombogenic material. Jpn Circ J. 1999;63:900–905. doi: 10.1253/jcj.63.900. [DOI] [PubMed] [Google Scholar]
- 29.Prunier F, Kawase Y, Gianni D, et al. Prevention of ventricular arrhythmias with sarcoplasmic reticulum Ca2+ ATPase pump overexpression in a porcine model of ischemia reperfusion. Circulation. 2008;118:614–624. doi: 10.1161/CIRCULATIONAHA.108.770883. [DOI] [PubMed] [Google Scholar]
- 30.Shen Y-T, Lynch JJ, Shannon RP, et al. A novel heart failure model induced by sequential coronary artery occlusions and tachycardiac stress in awake pigs. Am J Physiol. 1999;277:H388–H398. doi: 10.1152/ajpheart.1999.277.1.H388. [DOI] [PubMed] [Google Scholar]
- 31.St Louis JD, Hugnes GC, Kypson AP, et al. An experimental model of chronic myocardial hibernation. Ann Thorac Surg. 2000;69:1351–1357. doi: 10.1016/s0003-4975(00)01130-9. [DOI] [PubMed] [Google Scholar]
- 32.Roth DM, White FC, Mathieu-Costello O, et al. Effects of left circumflex artery ameroid constrictor placement on adrenergic innervations of myocardium. Am J Physiol. 1987;253:H1425–H1434. doi: 10.1152/ajpheart.1987.253.6.H1425. [DOI] [PubMed] [Google Scholar]
- 33.Harada K, Grossman W, Friedman M, et al. Basic fibroblast growth factor improves myocardial function in chronically ischemic porcine hearts. J Clin Invest. 1994;94:623–630. doi: 10.1172/JCI117378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Konski MS, White FC, Longhurst J, et al. Ameroid constriction of the proximal left circumflex coronary artery in swine. A model of limited coronary collateral circulation. Am J Cardiovasc Pathol. 1987;1:69–77. [PubMed] [Google Scholar]
- 35.Hugnes HC. Swine in cardiovascular research. Lab Anim Sci. 1986;36:348–350. [PubMed] [Google Scholar]
- 36.Robich MP, Matyal R, Chu LM, et al. Effects of neuropeptide Y on collateral development in a swine model of chronic myocardial ischemia. J Mol Cell Cardiol. 2010;49:1022–1030. doi: 10.1016/j.yjmcc.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laguens R, Meckert PC, Javanel GV, et al. Cardiomyocyte hyperplasia after plasmid-mediated VEGF gene transfer in pigs with chronic myocardial ischemia. J Gene Med. 2004;6:222–227. doi: 10.1002/jgm.478. [DOI] [PubMed] [Google Scholar]
- 38.Hugnes GC, Post MJ, Simons M, et al. Translational physiology: porcine models of human coronary artery disease: implications for preclinical trials of therapeutic angiogenesis. J Appl Physiol. 2003;94:1689–1701. doi: 10.1152/japplphysiol.00465.2002. [DOI] [PubMed] [Google Scholar]
- 39.Rengo G, Lymperopoulos A, Zincarelli C, et al. Myocardial adeno-associated virus serotype 6-βARKct gene therapy improves cardiac function and normalizes the neurohormonal axis in chronic heart failure. Circulation. 2009;119:89–98. doi: 10.1161/CIRCULATIONAHA.108.803999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pleger ST, Most P, Boucher M, et al. Stable myocardial-specific AAV-S100A1 gene therapy results in chronic functional heart failure rescue. Circulation. 2007;115:2506–2515. doi: 10.1161/CIRCULATIONAHA.106.671701. [DOI] [PubMed] [Google Scholar]
- 41.Most P, Pleger ST, Vőlkers M, et al. Cardiac adenoviral S100A1 gene transfer rescues failing myocardium. J Clin Invest. 2004;114:1550–1563. doi: 10.1172/JCI21454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li RK, Jia ZQ, Weisel RD, et al. Smooth muscle cell transplantation into myocardial scar tissue improves heart function. J Mol Cell Cardiol. 1999;31:513–522. doi: 10.1006/jmcc.1998.0882. [DOI] [PubMed] [Google Scholar]
- 43.Kawase Y, Hajjar RJ. The cardiac sarcoplasmic/endoplasmic reticulum calcium ATPase: a potent target for cardiovascular diseases. Nature Clin Prac. 2008;5:554–556. doi: 10.1038/ncpcardio1301. [DOI] [PubMed] [Google Scholar]
- 44.Angeli FS, Shapiro M, Amabile N, et al. Left ventricular remodeling after myocardial infarction: characterization of a swine model on β-blocker therapy. Compar Med. 2009;59:272–279. [PMC free article] [PubMed] [Google Scholar]
- 45.Yarbrough WM, Spinale FG. Large animal models of congestive heart failure: a critical step in translating basic observations into clinical applications. J Nucl Cardiol. 2003;10:77–86. doi: 10.1067/mnc.2003.16. [DOI] [PubMed] [Google Scholar]
- 46.Recchia FA, Lionetti V. Animal models of dilated cardiomyopathy for translational research. Vet Res Com. 2007;31:35–41. doi: 10.1007/s11259-007-0005-8. [DOI] [PubMed] [Google Scholar]
- 47.Shinbane JS, Wood MA, Jensen DN, et al. Tachycardia-induced cardiomyopathy: a review of animal models and clinical studies. J Am Coll Cardiol. 1997;29:709–715. doi: 10.1016/s0735-1097(96)00592-x. [DOI] [PubMed] [Google Scholar]
- 48.Byrne MJ, Raman JS, Alferness CA, et al. An ovine model of tachycardia-induced degenerative dilated cardiomyopathy and heart failure with prolonged onset. J Card Fail. 2002;8:108–115. doi: 10.1054/jcaf.2002.32323. [DOI] [PubMed] [Google Scholar]
- 49.Monnet E, Chachques JC. Animal models of heart failure: what is new? Ann Thorac Surg. 2005;79:1445–14. doi: 10.1016/j.athoracsur.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 50.Wang X, Li F, Gerdes AM. Chronic pressure overload cardiac hypertrophy and failure in guinea pigs: regional hemo-dynamics and myocyte remodeling. J Mol Cell Cardiol. 1999;31:307–317. doi: 10.1006/jmcc.1998.0884. [DOI] [PubMed] [Google Scholar]
- 51.Moorjani N, Catarino P, El-Sayed R, et al. A pressure overload model to track the molecular biology of heart failure. Eur J Cardiothorac Surg. 2003;24:920–925. doi: 10.1016/s1010-7940(03)00514-1. [DOI] [PubMed] [Google Scholar]
- 52.Aoyagi T, Fujii AM, Flanagan MF, et al. Transition from compensated hyper-trophy to intrinsic myocardial dysfunction during development of left ventricular pressure-overload hypertrophy in conscious sheep. Systolic dysfunction precedes diastolic dysfunction. Circulation. 1993;88:2415–2425. doi: 10.1161/01.cir.88.5.2415. [DOI] [PubMed] [Google Scholar]
- 53.del Monte F, Butler K, Boecker W, et al. Novel technique of aortic banding followed by gene transfer during hypertrophy and heart failure. Physiol Genomics. 2002;9:49–56. doi: 10.1152/physiolgenomics.00035.2001. [DOI] [PubMed] [Google Scholar]
- 54.Emani SM, Shah AS, White DC, et al. Right ventricular gene therapy with a β-adrenergic receptor kinase inhibitor improves survival after pulmonary artery banding. Ann Thorac Surg. 2001;72:1657–1661. doi: 10.1016/s0003-4975(01)03130-7. [DOI] [PubMed] [Google Scholar]
- 55.Shah AS, Atkins BZ, Hata JA, et al. Early effects of right ventricular volume overload on ventricular performance and β-adrenergic signaling. J Thorac Cardiovasc Surg. 2000;119:342–349. doi: 10.1067/mtc.2000.107278. [DOI] [PubMed] [Google Scholar]
- 56.Kawase Y, Ly HQ, Prunier F, et al. Reversal of cardiac dysfunction after long-term expression of SERCA2A by gene transfer in a pre-clinical model of heart failure. J Am Coll Cardiol. 2008;51:1112–1119. doi: 10.1016/j.jacc.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 57.Kleaveland JP, Kussmaul WG, Vinciguerra T, et al. Volume overload hypertrophy in a closed-chest model of mitral regurgitation. Am J Physiol. 1988;254:H1034–H1041. doi: 10.1152/ajpheart.1988.254.6.H1034. [DOI] [PubMed] [Google Scholar]
- 58.Emani SM, Shah AS, Bowman MK, et al. Right ventricular targeted gene transfer of a β-adrenergic receptor kinase inhibitor improves ventricular performance after pulmonary artery banding. J Thorac Cardiovasc Surg. 2004;127:787–793. doi: 10.1016/s0022-5223(03)01189-9. [DOI] [PubMed] [Google Scholar]
- 59.Mihaylov D, Reintke H, Blanksma P, et al. Development of acute ischemic heart failure in sheep. Int J Artif Organs. 2000;23:325–330. [PubMed] [Google Scholar]
- 60.Beeri R, Yosefy C, Guerrero JL, et al. Mitral regurgitation augments post-myocardial infarction remodeling failure of hypertrophic compensation. J Am Coll Cardiol. 2008;51:476–486. doi: 10.1016/j.jacc.2007.07.093. [DOI] [PubMed] [Google Scholar]
- 61.Patten RD, Hall-Porter MR. Small animal models of heart failure: development of novel therapies, past and present. Circ Heart Fail. 2009;2:138–144. doi: 10.1161/CIRCHEARTFAILURE.108.839761. [DOI] [PubMed] [Google Scholar]
- 62.Ikeda Y, Ross JJ. Models of dilated cardiomyopathy in the mouse and the hamster. Curr Opin Cardiol. 2000;15:197–201. doi: 10.1097/00001573-200005000-00013. [DOI] [PubMed] [Google Scholar]
- 63.Hajjar RJ, del Monte F, Matsui T, et al. Prospects for gene therapy for heart failure. Circ Res. 2000;86:616–621. doi: 10.1161/01.res.86.6.616. [DOI] [PubMed] [Google Scholar]
- 64.Ly H, Kawase Y, Yoneyama R, et al. Gene therapy in the treatment of heart failure. Physiology. 2007;22:81–96. doi: 10.1152/physiol.00037.2006. [DOI] [PubMed] [Google Scholar]
- 65.Katz MG, Swain JD, Tomasulo CE, et al. Current strategies for myocardial gene delivery. J Mol Cell Cardiol. 2011;50:766–776. doi: 10.1016/j.yjmcc.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brodde OE. Beta-adrenoreceptors in cardiac disease. Pharmacol Ther. 1993;60:405–430. doi: 10.1016/0163-7258(93)90030-h. [DOI] [PubMed] [Google Scholar]
- 67.Koch WJ, Rockman HA, Samama P, et al. Cardiac function in mice overex-pressing the β-adrenergic receptor kinase or a βARK inhibitor. Science. 1995;268:1350–1353. doi: 10.1126/science.7761854. [DOI] [PubMed] [Google Scholar]
- 68.Raake PW, Vinge LE, Gao E, et al. G Protein-coupled receptor kinase 2 ablation in cardiac myocytes before or after myocardial infarction prevents heart failure. Circ Res. 2008;103:413–422. doi: 10.1161/CIRCRESAHA.107.168336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tevaearai HT, Walton GB, Keys JR, et al. Acute ischemic cardiac dysfunction is attenuated via gene transfer of a peptide inhibitor of the β-adrenergic receptor kinase (βARK1). J Gene Med. 2005;7:1172–1177. doi: 10.1002/jgm.770. [DOI] [PubMed] [Google Scholar]
- 70.Brinks HL, Boucher M, Gao E, et al. G-protein-coupled receptor kinase-2 is deterious in myocardial injury: its inhibition mediates cardioprotection. Circ Res. 2009;105:E51. [Google Scholar]
- 71.Tachibana H, Naga Prasad SV, Lefkowitz RJ, et al. Level of b-adrenergic receptor kinase 1 inhibition determines degree of cardiac dysfunction after chronic pressure-overload-induced heart failure. Circulation. 2005;111:591–597. doi: 10.1161/01.CIR.0000142291.70954.DF. [DOI] [PubMed] [Google Scholar]
- 72.Weig HJ, Laugwitz KL, Moretti A, et al. Enhanced cardiac contractility after gene transfer of V2 vasopressin receptors in vivo by ultrasound-guided injection of transcoronary delivery. Circulation. 2000;101:1578–1585. doi: 10.1161/01.cir.101.13.1578. [DOI] [PubMed] [Google Scholar]
- 73.Lai NC, Roth DM, Gao MH, et al. Intracoronary delivery of adenovirus encoding adenylyl cyclase VI increases left ventricular function and cAMP-generating capacity. Circulation. 2000;102:2396–2401. doi: 10.1161/01.cir.102.19.2396. [DOI] [PubMed] [Google Scholar]
- 74.Niwano K, Arai M, Koitabashi N, et al. Lentiviral vector-mediated SERCA2 gene transfer protects against heart failure and left ventricular remodeling after myocardial infarction in rats. Mol Ther. 2008;16:1002–1004. doi: 10.1038/mt.2008.61. [DOI] [PubMed] [Google Scholar]
- 75.del Monte F, Lebeche D, Guerrero JL, et al. Abrogation of ventricular arrhythmias in a model of ischemia and reper-fusion by targeting myocardial calcium cycling. Proc Natl Acad Sci USA. 2004;101:5622–5627. doi: 10.1073/pnas.0305778101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Byrne MJ, Power JM, Preovolos A, et al. Recirculating cardiac delivery of AAV2/1SERCA2a improves myocardial function in an experimental model of heart failure in large animals. Gene Ther. 2008;15:1550–1557. doi: 10.1038/gt.2008.120. [DOI] [PubMed] [Google Scholar]
- 77.Beeri R, Chaput M, Guerrero JL, et al. Gene delivery of sarcoplasmic reticulum calcium ATPase inhibits ventricular remodeling in ischemic mitral regurgitation. Circ Heart Fail. 2010;3:627–634. doi: 10.1161/CIRCHEARTFAILURE.109.891184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Miyamoto MI, del Monte F, Schmidt U, et al. Adenoviral gene transfer of SERCA2a improves left-ventricular function in aortic-banded rats in transition to heart failure. Proc Natl Acad Sci USA. 2000;97:793–798. doi: 10.1073/pnas.97.2.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mϋller OJ, Lange M, Rattunde H, et al. Transgenic rat hearts overexpressing SERCA2a show improved contractility under baseline conditions and pressure overload. Cardiovasc Res. 2003;59:380–389. doi: 10.1016/s0008-6363(03)00429-2. [DOI] [PubMed] [Google Scholar]
- 80.del Monte F, Williams E, Lebeche D, et al. Improvement in survival and cardiac metabolism after gene transfer of sarcoplasmic reticulum Ca2+-ATPase in a rat model of heart failure. Circulation. 2001;104:1424–1429. doi: 10.1161/hc3601.095574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Szatkowski ML, Westfall MV, Gomez CA, et al. In vivo acceleration of heart relaxation performance by parvalbumin gene delivery. J Clin Invest. 2001;107:191–198. doi: 10.1172/JCI9862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sakata S, Lebeche D, Sakata N, et al. Restoration of mechanical and energetic function in failing aortic-banded rat hearts by gene transfer of calcium cycling proteins. J Mol Cell Cardiol. 2007;42:852–861. doi: 10.1016/j.yjmcc.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hirsch JC, Borton AR, Albayya FP, et al. Comparatice analysis of parvalbumin and SERCA2 A cardiac myocyte gene transfer in a large animal model of diastolic dysfunction. Am J Physiol Heart Circ Physiol. 2004;286:H2314–H232168. doi: 10.1152/ajpheart.01137.2003. [DOI] [PubMed] [Google Scholar]
- 84.Most P, Remppis A, Pleger ST, et al. S100A1: a novel inotropic regulator of cardiac performance. Transition from molecular physiology to patho-physiological relevance. Am J Physiol Regul Integr Comp Physiol. 2007;293:R568–R577. doi: 10.1152/ajpregu.00075.2007. [DOI] [PubMed] [Google Scholar]
- 85.Pleger ST, Remppis A, Heidt B, et al. S100A1 gene therapy preserves in vivo cardiac function after myocardial infarction. Mol Ther. 2005;12:1120–1129. doi: 10.1016/j.ymthe.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 86.Pleger ST, Shan C, Klienzyk J, et al. Cardiac AAV9-S100A1 Gene therapy rescues post-ischemic heart failure in a preclinical large animal model. Sci Transl Med. 2011;3:92ra64. doi: 10.1126/scitranslmed.3002097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schwinger RH, Munch G, Bolck B, et al. Reduced Ca(2+)-sensitivity of SERCA 2a in failing human myocardium due to reduced serin-16 phospholamban phosphorylation. J Mol Cell Cardiol. 1999;31:479–491. doi: 10.1006/jmcc.1998.0897. [DOI] [PubMed] [Google Scholar]
- 88.del Monte F, Harding SE, Dec GW, et al. Targeting phospholamban by gene transfer in human heart failure. Circulation. 2002;105:904–907. doi: 10.1161/hc0802.105564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Iwanaga Y, Hoshijima M, Gu Y, et al. Chronic phospholamban inhibition prevents progressive cardiac dysfunction and pathological remodeling after infarction in rats. J Clin Invest. 2004;113:727–736. doi: 10.1172/JCI18716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cross HR, Kranias EG, Murphy E, et al. Ablation of PLB exacerbates ischemic injury to a lesser extent in female than male mice: protective role of NO. Am J Physiol Heart Circ Physiol. 2003;284:H683–H690. doi: 10.1152/ajpheart.00567.2002. [DOI] [PubMed] [Google Scholar]
- 91.Kaye DM, Preovolos A, Marshall BS, et al. Percutaneous cardiac recirculation-mediated gene transfer of an inhibitory phospholamban peptide reverses advanced heart failure in large animals. J Am Coll Cardiol. 2007;50:253–260. doi: 10.1016/j.jacc.2007.03.047. [DOI] [PubMed] [Google Scholar]
- 92.Kiriazis H, Sato Y, Kadambi YJ, et al. Hypertrophy and functional alterations in hyperdynamic phospholamban-knockout mouse hearts under chronic aortic stenosis. Cardiovasc Res. 2002;53:372–381. doi: 10.1016/s0008-6363(01)00487-4. [DOI] [PubMed] [Google Scholar]
- 93.Tsuji T, del Monte F, Yoshikawa Y, et al. Rescue of Ca2+ overload-induced left ventricular dysfunction by targeted ablation of phospholamban. Am J Physiol Heart Circ Physiol. 2009;296:H310–H317. doi: 10.1152/ajpheart.00975.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Carr AN, Schmidt AG, Suzuki Y, et al. Type 1 phosphatase, a negative regulator of cardiac function. Mol Cell Biol. 2002;22:4124–4135. doi: 10.1128/MCB.22.12.4124-4135.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pathak A, del Monte F, Zhao W, et al. Enhancement of cardiac function and suppression of heart failure progression by inhibition of protein phosphatase 1. Circ Res. 2005;96:756–766. doi: 10.1161/01.RES.0000161256.85833.fa. [DOI] [PubMed] [Google Scholar]
- 96.Rissanen TT, Ylä-Herttuala S. Current status of cardiovascular gene therapy. Mol Ther. 2007;v15:1233–1247. doi: 10.1038/sj.mt.6300175. [DOI] [PubMed] [Google Scholar]
- 97.Zhao T, Zhao W, Chen Y, et al. Vascular endothelial growth factor (VEGF)-A: role on cardiac angiogenesis following myocardial infarction. Microvasc Res. 2010;80:188–194. doi: 10.1016/j.mvr.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bull DA, Bailey SH, Rentz JJ, et al. Effect of Terplex/VEGF-165 gene therapy on left ventricular function and structure following myocardial infarction. VEGF gene therapy for myocar-dial infarction. J Control Rel. 2003;93:175–181. doi: 10.1016/j.jconrel.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 99.Ferrarini M, Arsic N, Recchia FA, et al. Adeno-associated virus-mediated transduction of VEGF 165 improves cardiac tissue viability and functional recovery after permanent coronary occlusion in conscious dogs. Circ Res. 2006;98:954–961. doi: 10.1161/01.RES.0000217342.83731.89. [DOI] [PubMed] [Google Scholar]
- 100.Lähteenvuo JE, Lähteenvuo MT, Kivelä A, et al. Vascular endothelial growth factor-B induces myocardium-specific angiogenesis and arteriogenesis via vascular endothelial growth factor receptor-1- and neuropilin receptor-1-dependent mechanisms. Circulation. 2009;119:845–856. doi: 10.1161/CIRCULATIONAHA.108.816454. [DOI] [PubMed] [Google Scholar]
- 101.Zhang D, Gai L, Fan R, et al. Efficacy and safety of therapeutic angiogenesis from direct myocardial administration of an adenoviral vector expressing VEGF 165. Chin Med J (Engl) 2002;115:643–648. [PubMed] [Google Scholar]
- 102.Leotta E, Patejunas G, Murphy G, et al. Gene therapy with adenovirus-mediated myocardial transfer of VEGF 121 improves cardiac performance in a pacing model of congestive heart failure. J Thorac Cardiovasc Surg. 2002;123:1101–1113. doi: 10.1067/mtc.2002.121044. [DOI] [PubMed] [Google Scholar]