Abstract
Cancer vaccines have overall had a record of failure as an adjuvant therapy for malignancies that are treated with alkylating chemotherapy, and the contribution of standard treatment to that failure remains unclear. Vaccines aim to harness the proliferative potential of the immune system by expanding a small number of tumor-specific lymphocytes into a large number of anti-tumor effectors. Clinical trials are often conducted after treatment with alkylating chemotherapy, given either as standard therapy or for immunomodulatory effect. There is mounting evidence for synergy between chemotherapy and adoptive immunotherapy or vaccination against self-antigens; however, the impact of chemotherapy on lymphocytes primed against tumor neo-antigens remains poorly defined. We report here that clinically relevant dosages of standard alkylating chemotherapies such as temozolomide and cyclophosphamide significantly inhibit the proliferative abilities of lymphocytes in mice. This proliferative impairment was long lasting and led to quantitative and qualitative defects in B and T cell responses to neo-antigen vaccines. High affinity responder lymphocytes receiving the strongest proliferative signals from vaccines experienced the greatest DNA damage responses, skewing the response toward lower affinity responders with inferior functional characteristics. Together these defects lead to inferior efficacy and overall survival in murine tumor models treated by neo-antigen vaccines. These results suggest that clinical protocols for cancer vaccines should be designed to avoid exposing responder lymphocytes to alkylating chemotherapy.
Introduction
Immune mediated destruction of solid tumors requires the infiltration of adequate numbers of effector lymphocytes into the tumor site (1). Tumors express mutant proteins termed “neo-antigens” that result from frameshift, gene fusion, and missense mutations (2). These neo-antigens rather than self-antigens tend to dominate the naturally occurring immune responses against cancer (3, 4). The immunogenicity and tumor specificity of the neo-antigens provide a compelling rationale for their identification and targeting with therapeutic cancer vaccines. Recent bioinformatics advances make prospective identification of neo-antigens for personalized cancer vaccines feasible (5). Numerous analyses of individual patients suggest that naturally occurring T cell responses against neo-antigens can be associated with dramatic responses and long-term survival (3, 4, 6). Indeed, it has been suggested that the generation of endogenous anti-tumor responses may be required for durable success of conventional therapies (7). This hypothesis has led to much interest in combining immunotherapy with conventional modalities (8), but the effect of conventional chemotherapy vis-à-vis immunotherapy is incompletely understood.
Numerous reports indicate a synergy between conventional chemotherapy and immune therapy. Synergy is mediated by diverse mechanisms including preferential depletion of regulatory T cells (Treg) (9–11), liberation of homeostatic or inflammatory cytokines (12, 13) and enhanced immunogenicity of chemotherapy treated tumors (14, 15). In the context of vaccines targeting self-antigens, chemotherapy given prior to vaccination can yield synergy and enhanced survival (9, 16). Vaccination against tolerized self-antigens may require Treg depletion to access a latent pool of high avidity self-antigen specific CD8 T cells, whereas high avidity neo-antigen specific T cells can be generated by immunization without Treg depletion (10).
The reported synergy between chemotherapy and vaccines is somewhat paradoxical given that the generation of an adaptive immune response is a highly proliferative process, and chemotherapeutic drugs are given for their selective toxicity to rapidly proliferating cells. The generation of a CD8 T cell response to an acute viral infection involves responder cells doubling ~14 times in a week (17) and cancer vaccines that utilize neo-antigens with potent adjuvants can trigger similar levels of CD8 T cell proliferation (18). The number and proliferative potential of infused effectors have been associated with clinical response to adoptive immunotherapy of metastatic melanoma (19), possibly due to a requirement for local proliferation of lymphocytes to generate sufficient effector: target ratios at the tumor site (20, 21). In adoptive transfer protocols the transferred lymphocytes are cultured ex vivo and therefore are not exposed to chemotherapy (22). By contrast cancer vaccines are administered to drive in vivo proliferation of lymphocytes in pre-treated patients, and the extent to which chemotherapy inhibits vaccine driven immune responses remains unclear.
The lack of understanding of the effect of chemotherapeutic drugs on cancer vaccines is particularly problematic with regard to alkylating chemotherapeutic drugs. Alkylating chemotherapies such as temozolomide and cyclophosphamide covalently modify DNA and inflict cytotoxic damage on exposed cells (23). These drugs are commonly used for their anti-neoplastic effect to treat malignancies that are frequent targets of cancer vaccines such as glioblastoma multiforme (GBM) (24) and metastatic melanoma (25), and additionally to deplete Treg prior to vaccination (16, 26). While case reports suggest that individual patients have benefited from cancer vaccines given after standard alkylating chemotherapy for GBM (24, 27), overall cancer vaccines administered after temozolomide have had a record of failure (28). Alkylating chemotherapeutics have immune inhibitory effects in vitro, specifically via selective toxicity to proliferating lymphocytes (29) and inhibition of differentiation of immune effectors (30). The applicability of these studies to human cancer patients remains unclear: the degree of lymphopenia in temozolomide treated glioblastoma patients is a negative prognostic factor (31), but has also been associated with greater vaccine induced antibody responses (32). To examine the impact of clinically relevant doses of alkylating chemotherapeutics on cancer vaccines, we used controlled animal experiments that minimized the numerous complicating factors encountered in human patients.
Materials and Methods
Cells and culture
GL261 and B16–F10 cells were maintained in DMEM supplemented with 10% FBS. The KM3M14 and O94M2 cell lines were derived from genetically engineered primary murine gliomas and were generated and maintained as described (33). Model antigen expressing tumors (Quad-GL261 and Quad-KM3M14) were generated by stable transfection with Quad antigen cassette (34), a single coding sequence expressing the OT-I and OT-II epitopes of ovalbumin as well as human gp100 and mouse Eα. CD8 T cells were isolated to >90% purity by negative selection kit (Miltenyi). T cells were stimulated with IL-2 (R&D Systems) and CD3/CD28 activator beads (Invitrogen) as previously described (35). Viable cell counts were assessed by trypan blue exclusion periodically after stimulation and are expressed proportionally relative to the number of viable cells at the beginning of the assay.
Mice and animal models
Mouse experiments were performed in accordance with University of Minnesota Animal Care and Use Committee guidelines. C57BL/6J (B6) mice, C57BL/6-Tg(TcraTcrb)1100Mjb/J (OT-I) and B6.PL-Thy1a/CyJ (Thy1.1+) mice were purchased from the Jackson Laboratory and used at 6–10 weeks of age. Nur77GFP reporter mice (36) were courtesy of K. Hogquist (University of Minnesota, Minneapolis, MN). Gliomas were inoculated as described.(34) Cell number inoculated was 15,000 for GL261 and 30,000 for O94M2 and KM3M14. 75,000 B16–F10 cells were inoculated in the right flank. Glioma-bearing mice were euthanized when they became symptomatic; B16–F10-bearing mice were euthanized when tumors became >1000 mm3. For adoptive transfer 2×106 cells OT-I CD8 T cells were transferred into Thy1.1+ mice by retro-orbital injection and allowed to park for 24 hours before drug treatment.
Drug treatments
Temozolomide, carboplatin, doxorubicin and cyclophosphamide were obtained from Toronto Research Chemicals. Carboplatin, doxorubicin and cyclophosphamide were dissolved in PBS and administered by intraperitoneal injection. Temozolomide was well suspended in PBS immediately before being administered via oral gavage. γ-irradiation was administered as a positive control for DNA strand breaks at a dosage of 15 Gy, 30 minutes before experiments. Mouse dosages of temozolomide and cyclophosphamide model relevant human pharmacokinetic exposures based on a calculated equivalence using published pharmacokinetic exposure data (37, 38) detailed in Table S1. Dosages of carboplatin and doxorubicin were selected based on the maximum anti-neoplastic dosages that were previously reported to have an immunostimulatory effect by a Treg depletion dependent mechanism (9).
Vaccinations
Ova vaccinations were performed with 100 µg of whole chicken ovalbumin protein (Fisher) and 10 µg of polyinosinic:polycytidylic acid stabilized with poly-L-lysine(polyICLC, gift of A. Salazar, Oncovir, Washington, DC). All peptide vaccines were given with 50 µg of peptide and 10 µg polyICLC. Vaccinations were administered as subcutaneous injections at the base of the left hind leg.
Peptides and in vitro stimulation
SIINFEKL and variant peptides (Anaspec) were dissolved in sterile water. All other peptides (New England Peptide) were dissolved in minimal DMSO and diluted in sterile water. Splenocytes were incubated with peptides for 8 hours (Nur77GFP induction) or 24 hours (elaborated IFN-γ). Elaborated IFN-γ was measured by cytokine bead array (BD) and normalized to number of antigen specific T cells enumerated with BD counting beads. For B16–F10 stimulation B6 splenocytes were pulsed with 5 µg/mL of the mutant peptide cocktail or irrelevant control (16 and 18 amino acid peptides containing the OT-I and OT-II epitopes). Leukocytes from 100 µL of blood from B16–F10 bearing animals were incubated with 150,000 antigen pulsed splenocytes for 72 hours for IFN-γ elaboration.
The peptide sequences are as follows: GARC-1 RASAALLNKLYAMGL; B16–F10 - mutant peptides: Kif18b-mutant SKPSFQEFVDWENVSPELNSTDQP, Tubb3-mutant RRKAFLHWYTGEAMDEMEFTEAESN, Cpsf3l-mutant FKHIKAFDRTFANNPGPMVVFATPG, Tnpo3-mutant, DRNPQFLDPVLAYLMKGLCEKPLAS, Plod2-mutant, YNTSHLNNDVWQIFENPVDWKEK. (5)
Flow cytometry and ELISA
All antibodies except as indicated were from eBioscience: Phosphorylated ATM (phosphor-Ser1981) antibody was from Millipore. An isotype control for phosphorylated ATM staining was included as a control for background staining in non-temozolomide treated lymphocytes and was the PE-conjugated murine IgG1 κ isotype control from eBioscience. Kb-Ova peptide-MHC multimer staining was performed with dextramer (Immudex). PE conjugated Db-GARC-1 tetramer was synthesized by NIH tetramer core facility (Atlanta, GA). Tetramer falloff assay was performed as described (17) with the following modifications: Kb-Ova dextramer was used to stain and free dextramer was bound with biotinylated anti-Kb-Ova monoclonal antibody. Data were acquired using a BD FACSCanto II and analyzed using Cytobank.org software. Relative affinity and antibody titer were determined by ELISA as described (39).
Results
An intrinsic proliferation defect of lymphocytes exposed to alkylating chemotherapy
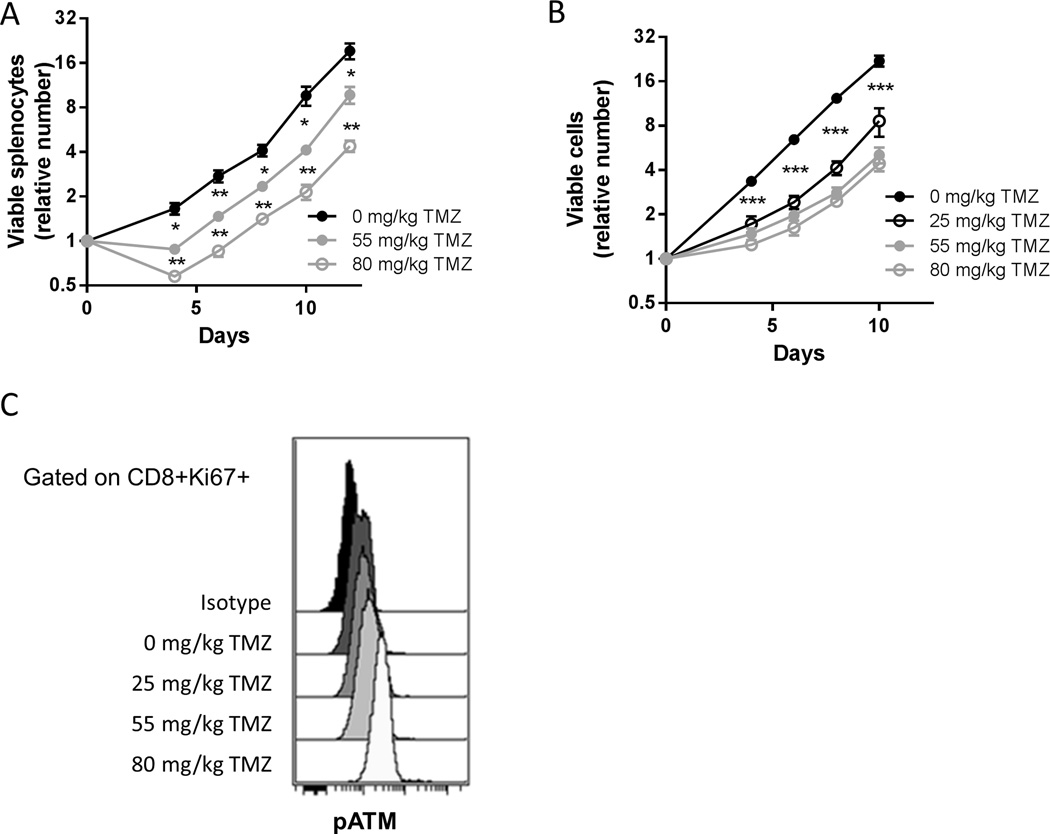
To choose clinically relevant doses of temozolomide with which to treat mice, we selected doses that yielded an equivalent pharmacokinetic exposure as obtained after oral dosing in humans (40) (personal communication James Gallo, Mount Sinai School of Medicine, New York, NY). Low (25 mg/kg), intermediate (55 mg/kg), and high (80 mg/kg) dosages of temozolomide given daily for five days led to a transient reduction in lymphocyte counts similar in magnitude to that seen in patients treated with temozolomide (Table S1). Temozolomide significantly inhibited proliferation of splenic T cells (Figure 1A). This proliferation defect is cell intrinsic because purified CD8 T cells from temozolomide treated mice demonstrated a similar inhibition of proliferation and activation of the DNA damage response as assessed by phosphorylated Ataxia Telangiectasia Mutated (pATM) staining (Figure 1, B and C).
Figure 1. Clinically relevant doses of temozolomide inhibit T lymphocyte proliferation in mice.
(A) C57BL/6 mice (n=3 per group) were treated with the indicated dosages of TMZ daily for 5 days and 2 days after the last dose mice were sacrificed and splenocytes plated with CD3/CD28 beads and IL-2. Viable cell counts were assessed at the indicated time points. Representative of two independent experiments. Error bars indicate SEM. *, p<0.05; **, p<0.01. (B) Purified CD8 T cells from mice treated with the indicated dosages of TMZ (n=3 per group) were stimulated as above. Viable cell counts were assessed at the indicated time points. Data are pooled from two independent experiments. Error bars indicate SEM. ***, p<0.001. (C) Aliquots of CD8 T cells stimulated as in (B) were taken for FACS analysis of phosphorylated ATM on day 4 post-stimulation.
Quantitative defects in immune responses to cancer vaccines after temozolomide treatment
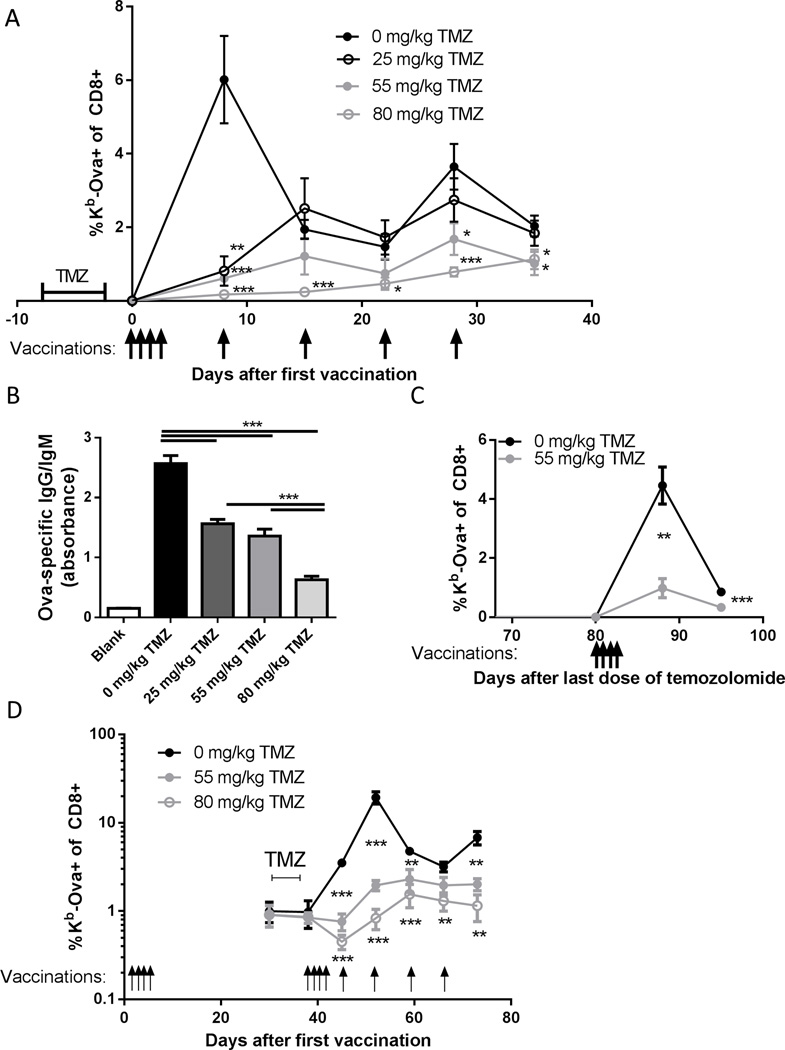
To examine the effects of alkylating chemotherapy on neo-antigen cancer vaccines we vaccinated mice with the model antigen chicken ovalbumin (Ova) and polyICLC (18). A cluster of four daily subcutaneous vaccinations causes a robust CD8 T cell response, with the percentage of Kb-Ova-specific CD8 T cells in the blood expanding from essentially undetectable levels to ~6% of the CD8 compartment in a week (Figure 2A). This expansion from a precursor frequency of ~1/150,000 (41) to a frequency of ~1/20 represents approximately 13 doublings. When followed by weekly boosters, similar to many established clinical protocols, a second peak in Kb-Ova-specific T cell percentage was observed a week after the third booster (Figure 2A). The magnitude of the CD8 T cell response was diminished in a dose dependent fashion by temozolomide treatment (Figure 2A). The levels of Ova-specific antibody circulating in the blood of temozolomide treated mice were also lower in a dose dependent fashion (Figure 2B).
Figure 2. Temozolomide exposure leads to a dose dependent inhibition of adaptive immune responses to vaccination.
(A) C57BL/6 mice (n=7–8 per group) were treated with the indicated dosages of TMZ for 5 days, and 2 days after the last dose were vaccinated daily for 4 days with Ova and poly ICLC followed by weekly booster vaccinations as indicated. Kb-Ova specific CD8 T cells in the blood were assessed by flow cytometry. Error bars indicate SEM. *, p<0.05; **, p<0.01; ***, p<0.001. One representative experiment is shown; this experiment was performed independently three times with similar results. (B) The mice in (A) were terminally bled 70 days after the first vaccination and anti-Ova IgG/IgM was quantified by ELISA. Error bars indicate SEM. ***, p<0.001. (C) C57/BL6 mice (n=9 per group) were treated with TMZ at the indicated dosages for 5 days and 80 days after the last dose of TMZ were vaccinated daily for 4 days, with antigen specific CD8 T cells in blood assessed as above. Error bars indicate SEM. **, p<0.01; ***, p<0.001. The experiment shown is representative of two independent experiments with similar results. (D) C57BL/6 mice (n=5 per group) were vaccinated daily for 4 days and immunological memory was allowed to establish for 1 mo. at which point TMZ was administered daily for 5 days at the indicated dosages. The mice were again vaccinated daily for 4 days and given booster vaccinations weekly as indicated, and throughout antigen specific CD8 T cells in blood were assessed by flow cytometry. Error bars indicate SEM. **, p<0.01; ***, p<0.001.Representative of two independent experiments.
Given that temozolomide and other alkylating drugs covalently modify DNA and that some of the directly produced DNA-alkyl adducts (42) or indirectly produced DNA lesions (43, 44) are long lived, we measured the magnitude of immune responses to vaccines given several weeks after temozolomide treatment. B6 mice that were given intermediate dose temozolomide had significantly lower percentages of Kb-Ova-specific T cells elicited by a cluster of four vaccinations that began 80 days after the last dose of temozolomide relative to untreated, age matched controls, with a peak frequency of ~1% versus ~4.5% (Figure 2C).
Antigen experienced memory T cells have an intrinsic resistance to DNA intercalating chemotherapy with daunorubicin (45), and tumor specific T cell clones may be antigen experienced in cancer patients (46). We therefore measured the effect of vaccine driven T cell expansion in an antigen experienced memory cell population in mice treated with alkylating chemotherapy prior to vaccination. Mice treated with intermediate or high dose temozolomide after an initial cluster of vaccines had a significant inhibition of antigen specific T cell proliferation when vaccinated with a second cluster of vaccines (Figure 2D). The peak percentage of Kb-Ova-specific CD8 T cells was approximately 10 fold lower (~2% versus ~20%) in intermediate dose temozolomide treated mice versus controls, and the percentage of Kb-Ova-specific CD8 T cells decreased immediately following vaccination in high dose treated mice (Figure 2D).
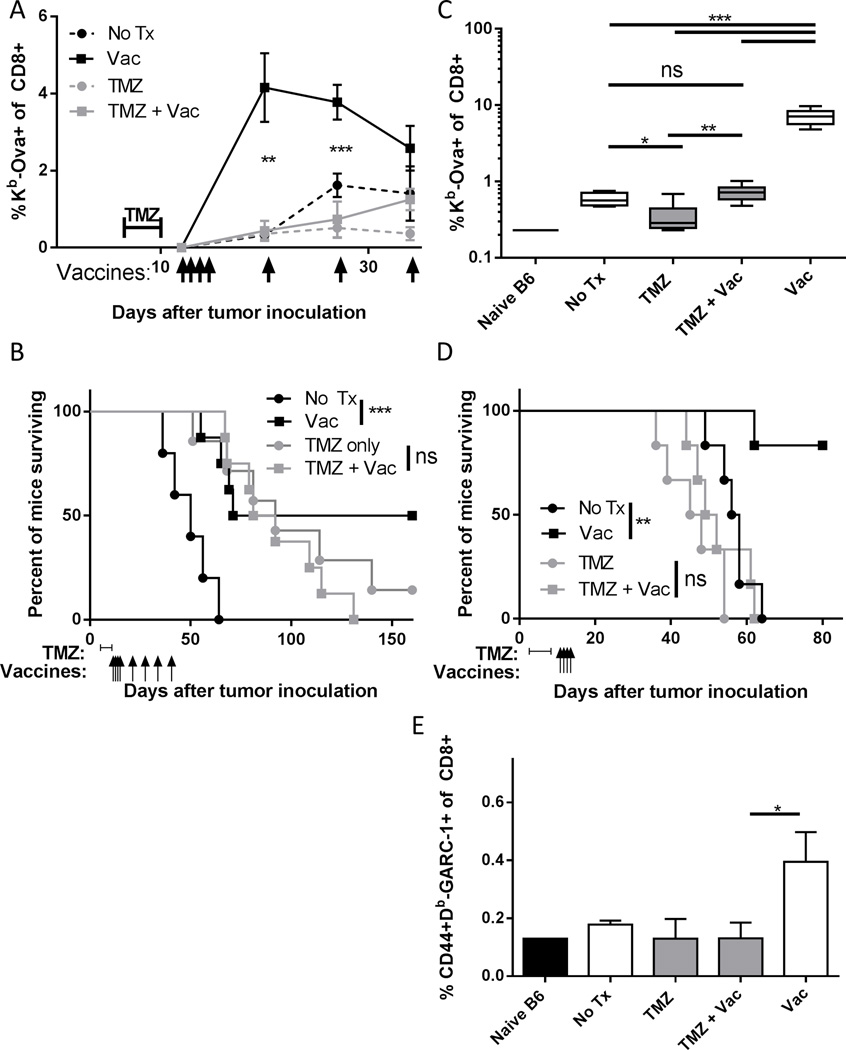
We next quantified the impact of temozolomide on the efficacy of vaccines in tumor bearing animals using both model antigens and mutated tumor specific neo-antigens. B6 mice were implanted orthotopically with the syngeneic, antigen force-expressing Quad-GL261 glioma line (34). The peak percentage of Kb-Ova-specific CD8 T cells in blood elicited by vaccination was significantly lower for high dose temozolomide pre-treated animals, and levels remained lower after numerous booster vaccines (Figure 3A). The GL261 cell line is sensitive to temozolomide (47) and therefore the median survival for all three treated groups was similar (Figure 3B). However, high dose temozolomide treatment abrogated the efficacy of vaccine treatment: i.e. mice treated with vaccine survived significantly longer than untreated controls, but the benefit of vaccination was lost in temozolomide treated mice.
Figure 3. Temozolomide exposure leads to inhibition of immune responses in tumor bearing animals.
C57BL/6 mice (n=5–8 per group) were inoculated with Quad-GL261 cell line and in the indicated groups treated on days 6–10 after tumor inoculation with 80 mg/kg of TMZ and vaccinated with Ova and polyICLC on days 12–15, 19, 26, 33 and 40. (A) The percentage of Kb-Ova specific CD8 T cells in the blood was assessed at the indicated time points before control mice became moribund. Error bars indicate SEM. **, p<0.01; ***, p<0.001. (B) The percentage of mice in each group surviving is shown. P values shown are for log rank test. ns, not significant (p>0.05); ***, p<0.001. Representative of two independent experiments with similar results. (C) C57BL/6 mice (n=5–6 per group) were inoculated with Quad-KM3M14 and in the indicated groups were treated on days 3–7 after tumor inoculation with 55 mg/kg of TMZ and vaccinated with Ova and poly ICLC on days 10–13. Kb-Ova specific CD8 T cells were assessed by flow cytometry on day 17. Error bars indicate SEM. ns, not significant (p>0.05); *, p<0.01; **, p<0.01; ***, p<0.001. (D) The percentage of mice in each group surviving is shown. P values shown are for log rank test. ns, not significant (p>0.05); **, p<0.01. (E) C57BL/6 mice (n=6–8 per group) were inoculated with GL261 tumors and in the indicated groups treated with 55 mg/kg TMZ on days 6–10 after tumor inoculation and vaccinated with GARC-1 peptide and poly ICLC on days 12–15. Db-GARC-1 specific activated CD8 T cells were assessed in blood by flow cytometry on day 19. Error bars indicate SEM. ns, not significant (p>0.05); *, p<0.01. Representative of two independent experiments with similar results.
While temozolomide increases median survival in glioma patients (48), benefit from treatment is not uniform, and patients with O-6-methylguanine-DNA methyltransferase (MGMT) promoter unmethylated tumors are less likely to benefit (49). To model this clinical situation, we inoculated B6 mice with a Quad antigen expressing version of the B6 syngeneic KM3M14 glioma cell line (Quad-KM3M14), which is highly resistant to temozolomide treatment in vitro (data not shown). Intermediate dose temozolomide treatment abrogated the magnitude of Kb-Ova-specific CD8 T cell response to a single cluster of four vaccinations with Ova and polyICLC (Figure 3C). In addition, a spontaneous Kb-Ova-specific CD8 T cell response observed in untreated mice was abrogated in temozolomide treated mice (Figure 3C). For this temozolomide insensitive, immunogenic cell line a single course of vaccination is a largely curative therapy (Figure 3D). This survival benefit is entirely abrogated by temozolomide treatment before vaccination, and median survival for temozolomide treated mice was shorter than non-treated controls (Figure 3D). Similarly, we found that inhibition of spontaneous immune responses by temozolomide treatment could lead to a failure to reject a highly immunogenic B6 glioma line expressing the SV40 Large T antigen (Figure S1).
To examine the impact of temozolomide treatment on mutated self neo-antigens we inoculated mice with the GL261 cell line. This cell line expresses an immunogenic mutant self protein, GARC-1, which forms a Db-binding CD8 T cell epitope based on a single amino acid substitution due to a point mutation (50). We vaccinated using a peptide containing the immunogenic amino acid substitution in glioma bearing mice, with or without temozolomide treatment. A significant reduction in the percentage of activated, Db-GARC-1 specific CD8 T cells in blood elicited by vaccination was observed (Figure 3E).
DNA damage response induced by high intensity TCR stimulation following alkylating chemotherapy
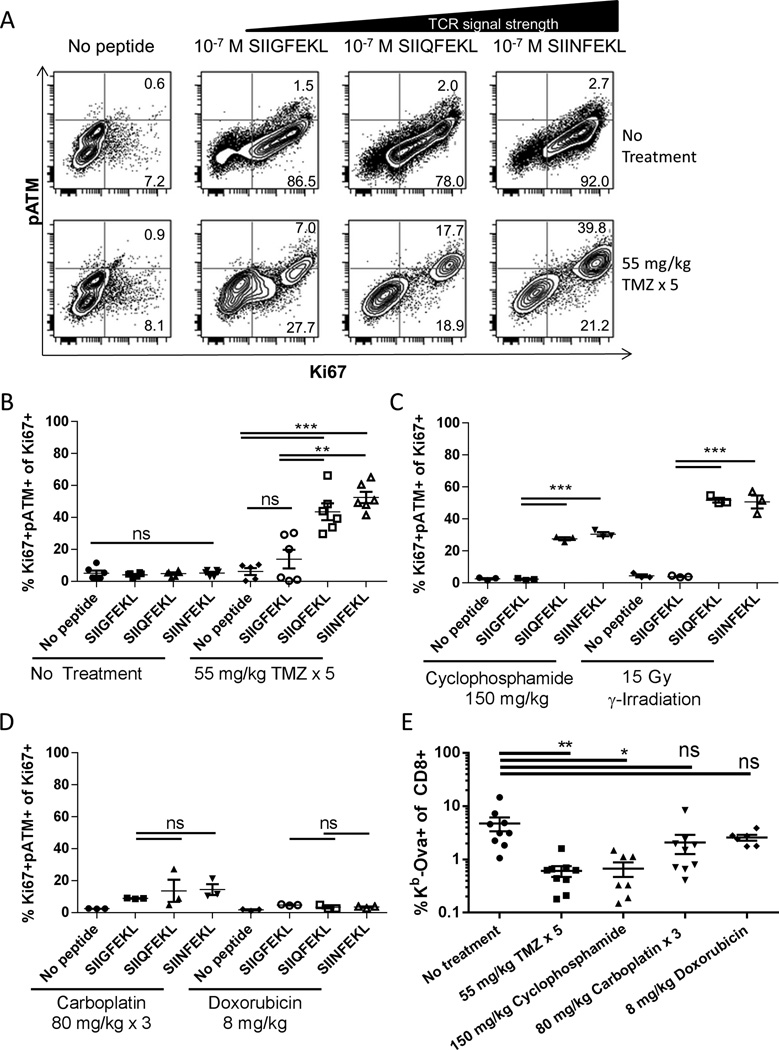
Since we observed activation of the DNA damage response in T cells given a strong stimulation through the TCR (Figure 1), we hypothesized that the degree of DNA damage response would correlate with TCR signal intensity. To dissect this question, OT-I mice were treated drugs and then their splenocytes were stimulated with altered peptide ligands that induce varying TCR signal strengths (36). All three peptides induced similar levels of proliferation and minimal DNA damage response in OT-I cells from untreated control animals as assessed by Ki67 and pATM staining respectively (Figure 4A). In temozolomide treated animals the frequencies of Ki67+ OT-I cells were inhibited for all peptides, but interestingly, the percentage of proliferating cells that had activated the DNA damage response (i.e. were pATM+) increased with increasing strength of TCR stimulation (Figure 4, A and B).
Figure 4. Strong TCR signals induce DNA damage response following alkylating chemotherapy.
(A) OT-I mice were treated with temozolomide for five days and two days later splenocytes were stimulated in vitro with peptide for 48 hours. Cells were stained for CD8, phosphorylated ATM and Ki67 and analyzed by flow cytometry. Representative plots are gated on CD8+ cells. (B) Aggregate data of flow cytometry as performed in (A). Percentage of proliferating (Ki67+) cells that were positive for phosphorylated ATM is plotted. Each experiment was performed with three technical replicates derived from the splenocytes of one mouse treated as indicated. Error bars indicate SEM. ns, not significant (p>0.05); **, p<0.01; ***, p<0.001. Data shown are pooled from two independent experiments with similar outcome. (C) and (D) Aggregate data of flow cytometry performed as in (A) for OT-I mice given the indicated treatments. Error bars indicate SEM. ns, not significant (p>0.05); **, p<0.01; ***, p<0.001. (E) C57BL/6 mice (n=6–9 per group) were given the indicated treatments then vaccinated daily for 4 days with ovalbumin and poly ICLC. 7 days after the first vaccine antigen specific cells in blood were assessed by flow cytometry. Error bars indicate SEM. ns, not significant (p>0.05); *, p<0.05; **, p<0.01. Data are pooled from three independent experiments with similar results.
To assess induction of DNA damage response in lymphocytes after treatment with other DNA damaging cancer therapies, we repeated the above experiments after treatment of OT-I mice with cyclophosphamide, carboplatin, doxorubicin and γ-irradiation. Following treatment with the alkylating chemotherapy cyclophosphamide and γ-irradiation, there were significantly more proliferating cells exhibiting DNA damage in response to the strong antigenic peptides SIIQFEKL and SIINFEKL than with the weak antigenic peptide SIIGFEKL or with no peptide (Figure 4C). The platinum drug carboplatin and the DNA intercalating agent doxorubicin did not lead to this effect, with similar pATM staining in proliferating cells stimulated with all peptides (Figure 4D). The induction of the DNA damage response upon stimulation after drug treatment was mirrored by the defect in CD8 T cell responses to vaccines in B6 mice. Temozolomide and cyclophosphamide lead to markedly and significantly lower peak levels of antigen specific CD8 T cells in the blood following vaccination (Figure 4E). By contrast, in animals treated with carboplatin and doxorubicin the percentage of antigen specific CD8 T cells was incrementally lower and not significantly different from untreated controls. This defect is likely accounted for by responder lymphocytes both failing to enter cell cycle (as in Figure 4A) as well as undergoing apoptosis due to DNA damage response, since a fraction of adoptively transferred OT-I became apoptotic (Annexin V+7-AAD+) after vaccination with SIINFEKL peptide in temozolomide treated mice (Figure S2).
Lower affinity for antigen of vaccine responder lymphocytes after temozolomide treatment
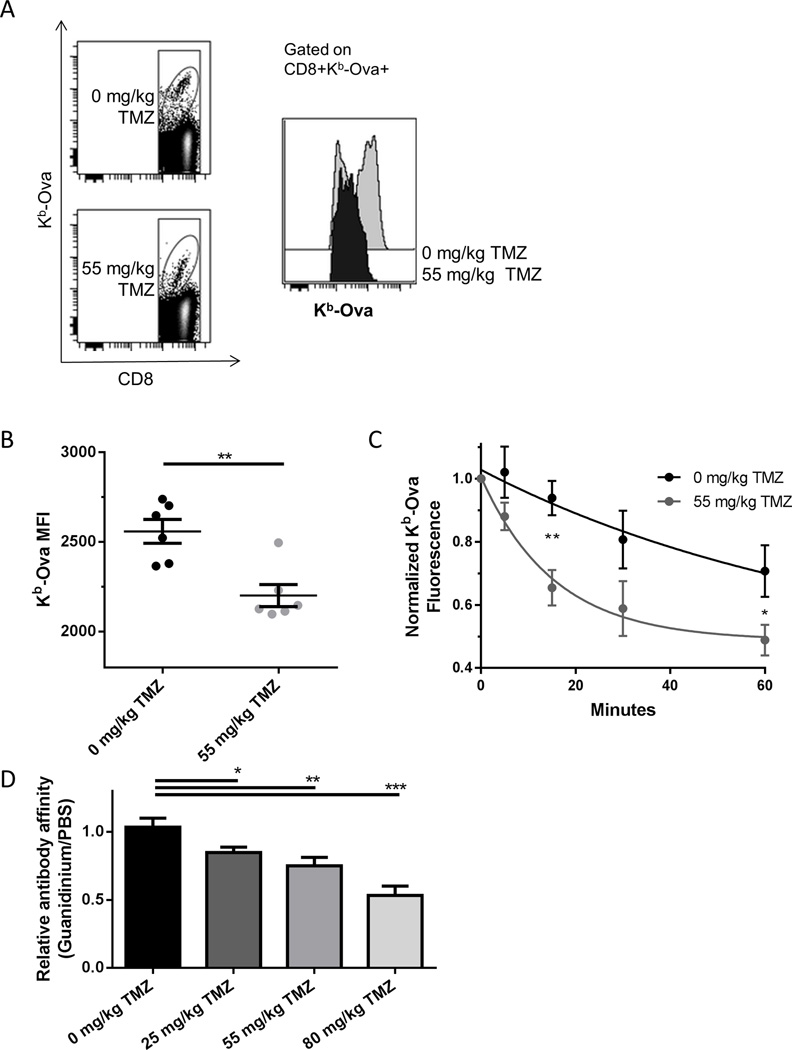
Due to the greater DNA damage response we observed in OT-I T cells stimulated with stronger antigenic peptides, we hypothesized that in vivo vaccine responder cells would be skewed towards lymphocytes with antigen receptors with lower affinity for cognate antigen. We found that the median fluorescent intensity (MFI) of peptide-MHC multimer staining of memory CD8 T cells elicited by vaccination was lower in temozolomide treated mice than in controls (Figure 5, A and B). The higher initial rate of decay of staining in a multimer falloff assay (17) in temozolomide treated animals also suggested a lower avidity of vaccine elicited CD8 T cells for antigen (Figure 5C). Similarly, we measured a lower relative affinity of anti-Ova serum Ig in temozolomide treated animals than in controls (Figure 5D).
Figure 5. The affinity of vaccine responding lymphocytes is lower following temozolomide.
(A) C57BL/6 mice (n=5 per group) were treated with TMZ as indicated and vaccinated daily for 4 days with Ova and poly ICLC. 30 days later splenocytes were stained for Kb-Ova and CD8 and analyzed by flow cytometry. Antigen specific cells were gated as shown. (B) Aggregate data of a representative experiment performed as in (A). Error bars indicate SEM. **, p<0.01. Data shown are representative of two independent experiments with similar results. (C) C57BL/6 mice (n=3–4 per group) were treated with the TMZ as indicated and vaccinated daily for 4 days with Ova and poly ICLC. 7 days later splenocytes were stained for Kb-Ova and CD8. Relative affinity of vaccine responding CD8 T cells was calculated by observing decay in normalized total fluorescence by flow cytometry as described in Materials and Methods. Curves shown are first order exponential decays fit to data. Error bars indicate SEM. *, p<0.05; **, p<0.01. Data shown are pooled data of two independent experiments with similar results. (D) C57BL/6 (n=7–8 per group) mice were treated with the TMZ as indicated and vaccinated daily for 4 days with Ova and poly ICLC followed by 4 weekly boosters (as in Figure 2A) and terminally bled 70 days after the first vaccination. Relative affinity was calculated by dividing the absorbance of ELISA wells plated with PBS (control) by those plated with 1 M guanidinium chloride (chaotropic agent). Error bars indicate SEM. *, p<0.05; **, p<0.01; ***, p<0.001.
Inferior functional characteristics of vaccine responder lymphocytes after temozolomide treatment
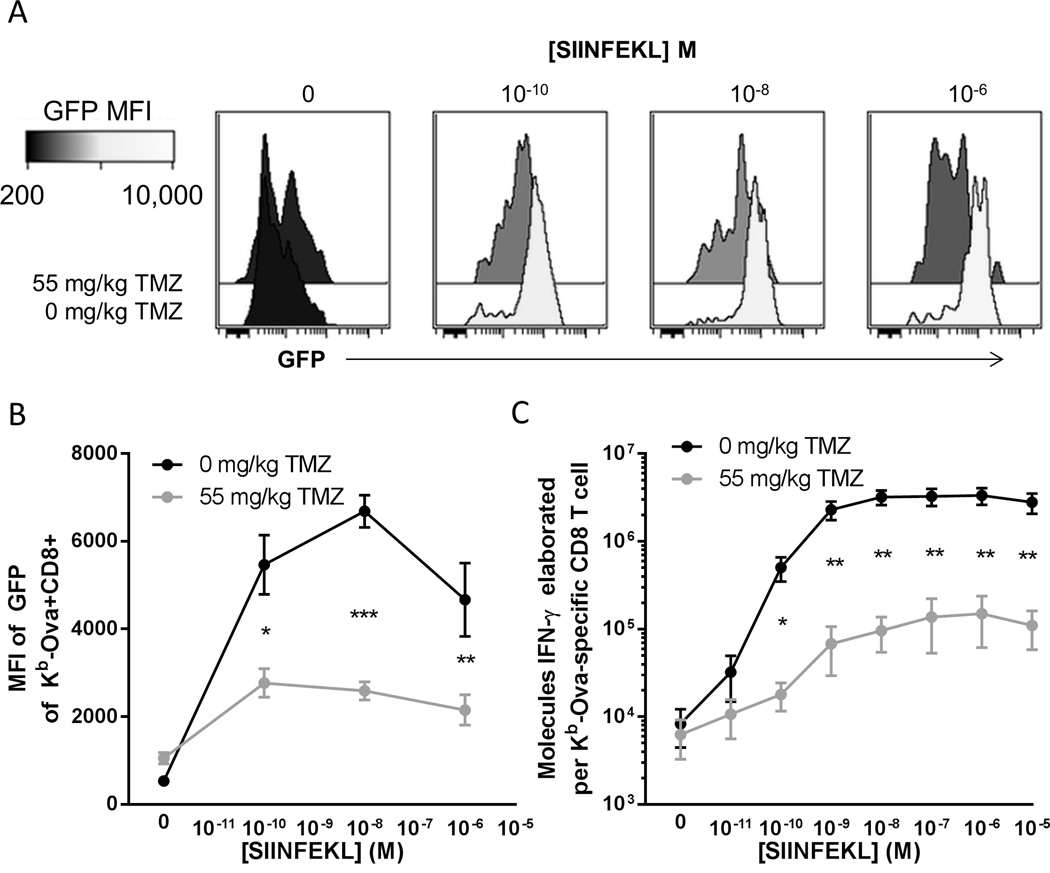
The lower affinity of responder lymphocytes for antigen after alkylating chemotherapy suggests that post-temozolomide vaccine responder cells are less sensitive towards antigenic targets. We hypothesized that these cells would receive lower intensity proliferative signals in temozolomide treated mice than controls and display inferior effector function upon stimulation. Using the Nur77GFP TCR signal strength reporter mouse (36), we directly tested this hypothesis by measuring GFP fluorescence intensity in vaccine expanded CD8 T cells upon antigenic stimulation. Vaccine expanded Kb-Ova-specific CD8 T cells from untreated control mice displayed high intensity GFP fluorescence upon culture with SIINFEKL peptide (Figure 6, A and B). In temozolomide pre-treated mice antigen specific GFP fluorescence upon stimulation was significantly lower (Figure 6B). We next measured IFN-γ elaboration upon antigenic stimulation in culture and enumerated antigen specific cells per well. We calculate that in temozolomide pre-treated animals vaccine activated CD8 T cells elaborated 10 fold fewer IFN-γ molecules on a per cell basis (Figure 6C).
Figure 6. CD8 T cells expanded by vaccines following temozolomide have inferior functional characteristics.
(A) Nur77GFP TCR signal strength reporter mice (n=4 per group) were treated with the TMZ as indicated and vaccinated daily for 4 days with Ova and poly ICLC. 7 days later splenocytes were plated with the indicated amount of SIINFEKL peptide for 8 hours. Antigen specific T cells were identified by staining for Kb-Ova and CD8, and GFP intensity of antigen specific cells was assessed by flow cytometry. (B) Aggregate data of experiments performed as described in (A). Error bars indicate SEM. *, p<0.05; **, p<0.01; ***, p<0.001. Data shown are pooled from two independent experiments with similar results. (C) C57BL/6 mice (n=3–4 per group) were treated with the TMZ as indicated and vaccinated daily for 4 days with ovalbumin and poly ICLC. 7 days later mice splenocytes were plated for 24 hours with the indicated amount of SIINFEKL peptide. Elaborated IFN-γ was measured by cytokine bead array and normalized to antigen specific T cells as indicated in Materials and Methods. Error bars indicate SEM. *, p<0.05; **, p<0.01. Data shown are pooled data from two independent experiments with similar results.
Diminished efficacy of neo-antigen cancer vaccines after alkylating chemotherapy
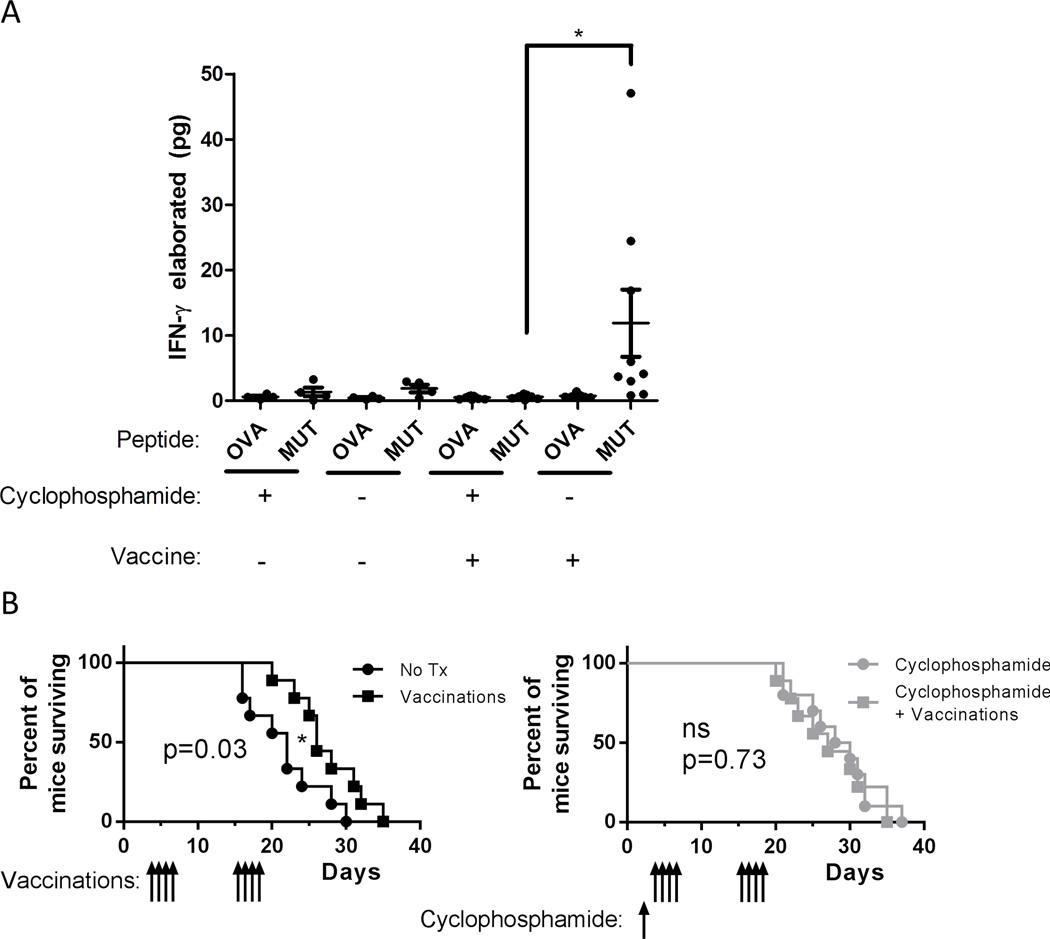
To examine the efficacy of neo-antigen cancer vaccines after Treg depleting alkylating chemotherapy we used a peptide vaccine targeting five immunogenic point mutations expressed by the B16–F10 melanoma cell line (5). Despite the fact that mice treated with low dose cyclophosphamide had a depletion of Treg cells as both a percentage of CD4 T cells and in absolute numbers (data not shown), we found that circulating leukocytes from mice vaccinated after cyclophosphamide elaborated significantly less IFN-γ upon peptide stimulation than in mice receiving vaccine only (Figure 7A). Similarly, the survival benefit of vaccinated animals relative to untreated controls was lost in animals pre-treated with cyclophosphamide (Figure 7B).
Figure 7. Cyclophosphamide pre-treatment is associated with less survival benefit from melanoma vaccines.
(A) C57BL/6 mice (n=9 per group) were implanted subcutaneously with 7.5*104 B16–F10 melanoma cells. Mice were given cyclophosphamide as indicated day 2 after tumor inoculation and vaccinated with B16–F10 cell line mutant peptides and poly ICLC on days 3–6 and17–20 after tumor inoculation. On day 10 after tumor inoculation leukocytes from 100 µL blood were incubated with splenocyte APC pulsed either with irrelevant (Ova-derived) peptides or B16–F10 mutant peptide cocktail. Elaborated IFN-γ was measured by cytokine bead array for all 9 mice per group for vaccinated groups, 4 per group for non-vaccinated groups. Error bars indicate SEM. *, p<0.05. (B) Survival of mice in (A). Mice were sacrificed when tumors reached >1000 mm3 in volume, survival is depicted by Kaplan-Meier plot. P values shown are for log rank test. ns, not significant (p>0.05); *, p<0.05.
Discussion
We found that alkylating chemotherapy has a long lasting anti-proliferative effect on lymphocytes in mice, and this effect leads to inferior responses to cancer vaccines targeting mutated self antigens. Animals pre-treated with alkylating chemotherapeutic drugs had lower peak numbers of vaccine responding CD8 T cells and lower antibody titers. This impairment corresponds to the activation of DNA damage responses in proliferating cells, and this activation of DNA damage responses is greatest in responder cells receiving the strongest TCR signals from the vaccine. In turn, this selective toxicity in the cells with the highest affinity for cognate antigen leads to impairment of CD8 T cell and antibody responses. These responses consist of lymphocytes with on average lower affinity antigen receptors that have inferior effector function. Importantly, these effects occur were observed even at low, Treg depleting doses of alkylating chemotherapeutics (9).
The defects we observed are likely general to all populations rapidly proliferating immune responder cells expanded by vaccination (e.g. CD4 T cells, B cells, etc.). Activated lymphocytes implement a metabolic and anti-apoptotic program that allows for sustained synthesis of macromolecules and cell division (51), dividing up to twice a day during the peak of adaptive immune responses (17). Alkylating chemotherapy covalently modifies DNA with methyl adducts for methylating drugs like temozolomide or dacarbazine (43) or inter- and intra-strand alkyl crosslinks for nitrogen mustard derivatives like cyclophosphamide (52). These lesions cause stalling of replication forks and double strand DNA breaks in proliferating cells (53). This DNA damage is detected by proteins such as ATM which binds to double strand DNA breaks and autophosphorylates, in turn activating numerous downstream effectors involved in cell cycle arrest and apoptosis such as Chk2 kinase (54) and p53 (55). Proliferation driven toxicity in vaccine responder cells is therefore a side effect of alkylating chemotherapy that must be balanced against its reported immunomodulatory effects. In the case of neo-antigen vaccines for which Treg depletion is not required for efficacy, our data suggest that the negative anti-proliferative effect of chemotherapy is dominant over the immunomodulatory effect.
We observed that the immune inhibitory effect of alkylating chemotherapy was long lived, with significant defects in CD8 T cell priming persisting >10 weeks after cessation of temozolomide treatment (Figure 2C). The persistence of this effect could be due to the fact that DNA repair is induced by proliferative signals (56), so quiescent naive lymphocytes may not fully repair DNA damage. This damage is then “activated” by replication fork read through during DNA synthesis in response to proliferative signals like vaccines.
Numerous studies have examined the effect of alkylating chemotherapy on immunotherapeutic modalities. The predominant finding reported has been depletion of Treg and induction of lymphopenia (11, 57), although high doses have been associated with peripheral Treg expansion in rodents (58) and humans (32). Several studies that have reported an immunostimulatory effect of alkylating chemotherapy due to Treg depletion have been conducted using transferred cells not exposed to drug (59–61). The clinical application of this strategy is complicated by the difficulty of generating large numbers of tumor specific lymphocytes ex vivo for human patients (62), and cancer vaccines are typically administered after standard chemotherapies (7). Conversely, other studies of endogenous anti-tumor immune responses following Treg depletion have focused on self-antigens for which breaking tolerance is required (9, 10, 16, 63) or have not directly compared immune responses in exposed and non-exposed lymphocytes (58, 64).
We have focused on tumor specific neo-antigens derived from mutated self proteins as well as exogenous model antigens, both of which are inherently immunogenic, i.e. can readily be targeted by vaccination without additional therapy to break tolerance. Such neo-antigens are technically challenging to predict from patient tumor samples, but have been retrospectively identified in clinically responding patients using tumor cell lines and patient lymphocytes in several studies (3). Similarly, clinical experience from vaccination with idiotypic immunoglobulin for lymphoma suggests that non-germline encoded epitopes from hypervariable regions are more immunogenic and stimulate CD4 and CD8 cells preferentially over framework regions (65). Due to their generation de novo in neoplastic cells, such mutant antigenic targets are less likely to cause autoimmune side effects and are not subjected to central tolerance that can cause negative selection of high-avidity T cells (66). Recent advances in bioinformatics have made prospective identification of immunogenic mutations possible, and is an active area of further research into personalized cancer vaccines (5). However, the experience of adoptive immunotherapy suggests that the proliferative potential of effector cells is a critical variable (19). For personalized cancer vaccines targeting tumor specific mutations to be successful, they should be administered in a protocol designed to maximize the quality and proliferative ability of responder lymphocytes.
We demonstrated that the generation of T cell responses against mutated self proteins by cancer vaccines was inhibited by temozolomide in a mouse model of glioma (Figure 3E) and by cyclophosphamide in a mouse model of melanoma (Figure 7A). In addition, using the model antigen ovalbumin we found that T cell clones that did expand after alkylating chemotherapy had lower affinity for cognate antigen, and lower TCR signal strength and inferior effector function upon antigenic stimulation (Figures 5 and 6). These differences seem sufficient to account for the loss of survival benefit from vaccination that we observed in both temozolomide and cyclophosphamide treated mice (Figs. 3B, 3D and 7B).
In conclusion, we found that vaccine driven and spontaneous adaptive anti-tumor immune responses were inhibited by the direct anti-proliferative effect of alkylating chemotherapy. These findings are particularly noteworthy since alkylating chemotherapy is a standard treatment for several malignancies that have been the target of vaccine immunotherapy, including temozolomide for GBM (48) and dacarbazine for metastatic melanoma (25). These findings suggest that easily implemented modifications of conventional clinical protocols for cancer vaccine trials, such as banking unexposed PBMC prior to chemotherapy for use in later immunotherapy, could yield improved results. It has been reported, for instance, that 500 mL of blood contains sufficient numbers of naïve precursor CD8 T cells to allow large numbers of T cells specific to multiple tumor and viral antigens to be expanded in vitro (67). Thus, easily extracted quantities of lymphocytes could be frozen and stored, either as source material for the in vitro expansion of anti-tumor T cells or as a banked pool of non-drug exposed naïve T cells to be infused prior to vaccination.
Furthermore, future trials of immune therapy could use such prognostic markers to stratify patients based on their relative likelihood to benefit from conventional alkylating chemotherapy versus cancer vaccines, and prioritize immune therapy over chemotherapy in those most likely to benefit. MGMT promoter methylation status in glioblastoma is prognostic of response to temozolomide and is widely measured clinically (49), whereas tumors with the mesenchymal gene expression pattern have a poor survival prognosis but appear to be more sensitive to active immune therapy than glioblastomas with other gene expression patterns (24). Altering clinical protocols and basing patient treatment on known prognostic indicators of treatment response could minimize harm of conventional therapies to cancer vaccines and maximize efficacy, leading to improved outcomes for patients treated with these vaccines.
Supplementary Material
Acknowledgments
The authors wish to thank D.A. Largaespada, C. A. Pennell, T. S. Griffith, and B.M. Andersen for editorial assistance and critical comments.
Funding: This work was supported by grants from the National Institutes of Health (R01-CA154345, R01-CA160782), the American Cancer Society (RSG-09-189-01-LIB) and the Children’s Cancer Research Fund.
Abbreviations used
- ATM
Ataxia telangiectasia mutated
- GBM
Glioblastoma multiforme
- MFI
median fluorescent intensity
- MGMT
O-6-methylguanine-DNA methyltransferase
- Poly ICLC
polyinosinic:polycytidylic acid stabilized with poly-L-lysine
- TMZ
temozolomide
Footnotes
Competing Interests: The authors declare no competing financial interests.
References
- 1.Chen DS, Davis MM. Cellular immunotherapy: antigen recognition is just the beginning. Semin Immunopathol. 2005;27:119–127. doi: 10.1007/s00281-005-0200-z. [DOI] [PubMed] [Google Scholar]
- 2.Thomas RK, Baker AC, Debiasi RM, Winckler W, Laframboise T, Lin WM, Wang M, Feng W, Zander T, MacConaill L, Macconnaill LE, Lee JC, Nicoletti R, Hatton C, Goyette M, Girard L, Majmudar K, Ziaugra L, Wong K-K, Gabriel S, Beroukhim R, Peyton M, Barretina J, Dutt A, Emery C, Greulich H, Shah K, Sasaki H, Gazdar A, Minna J, Armstrong SA, Mellinghoff IK, Hodi FS, Dranoff G, Mischel PS, Cloughesy TF, Nelson SF, Liau LM, Mertz K, Rubin MA, Moch H, Loda M, Catalona W, Fletcher J, Signoretti S, Kaye F, Anderson KC, Demetri GD, Dummer R, Wagner S, Herlyn M, Sellers WR, Meyerson M, Garraway LA. High-throughput oncogene mutation profiling in human cancer. Nat Genet. 2007;39:347–351. doi: 10.1038/ng1975. [DOI] [PubMed] [Google Scholar]
- 3.Sensi M, Anichini A. Unique tumor antigens: evidence for immune control of genome integrity and immunogenic targets for T cell-mediated patient-specific immunotherapy. Clin Cancer Res. 2006;12:5023–5032. doi: 10.1158/1078-0432.CCR-05-2682. [DOI] [PubMed] [Google Scholar]
- 4.Lennerz V, Fatho M, Gentilini C, Frye RA, Lifke A, Ferel D, Wolfel C, Huber C, Wolfel T. The response of autologous T cells to a human melanoma is dominated by mutated neoantigens. Proc Natl Acad Sci USA. 2005;102:16013–16018. doi: 10.1073/pnas.0500090102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castle JC, Kreiter S, Diekmann J, Löwer M, van de Roemer N, de Graaf J, Selmi A, Diken M, Boegel S, Paret C, Koslowski M, Kuhn AN, Britten CM, Huber C, Türeci O, Sahin U. Exploiting the mutanome for tumor vaccination. Cancer Res. 2012;72:1081–1091. doi: 10.1158/0008-5472.CAN-11-3722. [DOI] [PubMed] [Google Scholar]
- 6.Huang J, El-Gamil M, Dudley ME, Li YF, Rosenberg SA, Robbins PF. T cells associated with tumor regression recognize frameshifted products of the CDKN2A tumor suppressor gene locus and a mutated HLA class I gene product. J Immunol. 2004;172:6057–6064. doi: 10.4049/jimmunol.172.10.6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zitvogel L, Apetoh L, Ghiringhelli F, Andre F, Tesniere A, Kroemer G. The anticancer immune response: indispensable for therapeutic success? J Clin Invest. 2008;118:1991–2001. doi: 10.1172/JCI35180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell MS. Combinations of anticancer drugs and immunotherapy. Cancer Immunol Immunother. 2003;52:686–692. doi: 10.1007/s00262-003-0427-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Machiels JP, Reilly RT, Emens LA, Ercolini AM, Lei RY, Weintraub D, Okoye FI, Jaffee EM. Cyclophosphamide, doxorubicin, and paclitaxel enhance the antitumor immune response of granulocyte/macrophage-colony stimulating factor-secreting whole-cell vaccines in HER-2/neu tolerized mice. Cancer Res. 2001;61:3689–3697. [PubMed] [Google Scholar]
- 10.Ercolini AM, Ladle BH, Manning EA, Pfannenstiel LW, Armstrong TD, Machiels J-PH, Bieler JG, Emens LA, Reilly RT, Jaffee EM. Recruitment of latent pools of high-avidity CD8(+) T cells to the antitumor immune response. J Exp Med. 2005;201:1591–1602. doi: 10.1084/jem.20042167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banissi C, Ghiringhelli F, Chen L, Carpentier AF. Treg depletion with a low-dose metronomic temozolomide regimen in a rat glioma model. Cancer Immunol Immunother. 2009;58:1627–1634. doi: 10.1007/s00262-009-0671-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schiavoni G, Mattei F, Di Pucchio T, Santini SM, Bracci L, Belardelli F, Proietti E. Cyclophosphamide induces type I interferon and augments the number of CD44(hi) T lymphocytes in mice: implications for strategies of chemoimmunotherapy of cancer. Blood. 2000;95:2024–2030. [PubMed] [Google Scholar]
- 13.Asavaroengchai W, Kotera Y, Mulé JJ. Tumor lysate-pulsed dendritic cells can elicit an effective antitumor immune response during early lymphoid recovery. Proc Natl Acad Sci USA. 2002;99:931–936. doi: 10.1073/pnas.022634999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramakrishnan R, Assudani D, Nagaraj S, Hunter T, Cho H-I, Antonia S, Altiok S, Celis E, Gabrilovich DI. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J Clin Invest. 2010;120:1111–1124. doi: 10.1172/JCI40269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, Shen S, Kepp O, Scoazec M, Mignot G, Rello-Varona S, Tailler M, Menger L, Vacchelli E, Galluzzi L, Ghiringhelli F, di Virgilio F, Zitvogel L, Kroemer G. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011;334:1573–1577. doi: 10.1126/science.1208347. [DOI] [PubMed] [Google Scholar]
- 16.Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, Staehler M, Brugger W, Dietrich PY, Mendrzyk R, Hilf N, Schoor O, Fritsche J, Mahr A, Maurer D, Vass V, Trautwein C, Lewandrowski P, Flohr C, Pohla H, Stanczak JJ, Bronte V, Mandruzzato S, Biedermann T, Pawelec G, Derhovanessian E, Yamagishi H, Miki T, Hongo F, Takaha N, Hirakawa K, Tanaka H, Stevanovic S, Frisch J, Mayer-Mokler A, Kirner A, Rammensee HG, Reinhardt C, Singh-Jasuja H. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med. 2012;29 doi: 10.1038/nm.2883. [DOI] [PubMed] [Google Scholar]
- 17.Blattman JN, Antia R, D. Sourdive DJ, Wang X, Kaech SM, Murali-Krishna K, Altman JD, Ahmed R. Estimating the Precursor Frequency of Naive Antigen-specific CD8 T Cells. J Exp Med. 2002;195:657–664. doi: 10.1084/jem.20001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wick DA, Martin SD, Nelson BH, Webb JR. Profound CD8+ T cell immunity elicited by sequential daily immunization with exogenous antigen plus the TLR3 agonist poly(I:C) Vaccine. 2011;29:984–993. doi: 10.1016/j.vaccine.2010.11.036. [DOI] [PubMed] [Google Scholar]
- 19.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, Citrin DE, Restifo NP, Robbins PF, Wunderlich JR, Morton KE, Laurencot CM, Steinberg SM, White DE, Dudley ME. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.June C, Rosenberg SA, Sadelain M, Weber JS. T-cell therapy at the threshold. Nat Biotechnol. 2012;30:611–614. doi: 10.1038/nbt.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grange M, Buferne M, Verdeil G, Leserman L, Schmitt-Verhulst AM, Auphan-Anezin N. Activated STAT5 promotes long-lived cytotoxic CD8+ T cells that induce regression of autochthonous melanoma. Cancer Res. 2012;72:76–87. doi: 10.1158/0008-5472.CAN-11-2187. [DOI] [PubMed] [Google Scholar]
- 22.Rosenberg SA. Cell transfer immunotherapy for metastatic solid cancer--what clinicians need to know. Nat Rev Clin Oncol. 2011;8:577–585. doi: 10.1038/nrclinonc.2011.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu D, Calvo JA, Samson LD. Balancing repair and tolerance of DNA damage caused by alkylating agents. Nat Rev Cancer. 2012;12:104–120. doi: 10.1038/nrc3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prins RM, Soto H, Konkankit V, Odesa SK, Eskin A, Yong WH, Nelson SF, Liau LM. Gene expression profile correlates with T-cell infiltration and relative survival in glioblastoma patients vaccinated with dendritic cell immunotherapy. Clin Cancer Res. 2011;17:1603–1615. doi: 10.1158/1078-0432.CCR-10-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chapman PB, Einhorn LH, Meyers ML, Saxman S, Destro AN, Panageas KS, Begg CB, Agarwala SS, Schuchter LM, Ernstoff MS, Houghton AN, Kirkwood JM. Phase III multicenter randomized trial of the Dartmouth regimen versus dacarbazine in patients with metastatic melanoma. J Clin Oncol. 1999;17:2745–2751. doi: 10.1200/JCO.1999.17.9.2745. [DOI] [PubMed] [Google Scholar]
- 26.Dudek AZ, Mescher MF, Okazaki I, Math VT, Luo X, Curtsinger JM, Miller JS. Autologous large multivalent immunogen vaccine in patients with metastatic melanoma and renal cell carcinoma. Am J Clin Oncol. 2008;31:173–181. doi: 10.1097/COC.0b013e3181573e6b. [DOI] [PubMed] [Google Scholar]
- 27.Okada H, Kalinski P, Ueda R, Hoji A, Kohanbash G, Donegan TE, Mintz AH, Engh JA, Bartlett DL, Brown CK, Zeh H, Holtzman MP, Reinhart TA, Whiteside TL, Butterfield LH, Hamilton RL, Potter DM, Pollack IF, Salazar AM, Lieberman FS. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {alpha}-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J Clin Oncol. 2011;29:330–336. doi: 10.1200/JCO.2010.30.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okada H, Kohanbash G, Zhu X, Kastenhuber ER, Hoji A, Ueda R, Fujita M. Immunotherapeutic approaches for glioma. Crit Rev Immunol. 2009;29:1–42. doi: 10.1615/critrevimmunol.v29.i1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roos W, Baumgartner M, Kaina B. Apoptosis triggered by DNA damage O6-methylguanine in human lymphocytes requires DNA replication and is mediated by p53 and Fas/CD95/Apo-1. Oncogene. 2004;23:359–367. doi: 10.1038/sj.onc.1207080. [DOI] [PubMed] [Google Scholar]
- 30.Alvino E, Pepponi R, Pagani E, Lacal PM, Nunziata C, Bonmassar E, D'Atri S. O(6)-benzylguanine enhances the in vitro immunotoxic activity of temozolomide on natural or antigen-dependent immunity. J Pharmacol Exp Ther. 1999;291:1292–1300. [PubMed] [Google Scholar]
- 31.Grossman SA, Ye X, Lesser G, Sloan A, Carraway H, Desideri S, Piantadosi S, Consortium NC. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res. 2011;17:5473–5480. doi: 10.1158/1078-0432.CCR-11-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sampson JH, Aldape KD, Archer GE, Coan A, Desjardins A, Friedman AH, Friedman HS, Gilbert MR, Herndon JE, McLendon RE, Mitchell DA, Reardon DA, Sawaya R, Schmittling R, Shi W, Vredenburgh JJ, Bigner DD, Heimberger AB. Greater chemotherapy-induced lymphopenia enhances tumor-specific immune responses that eliminate EGFRvIII-expressing tumor cells in patients with glioblastoma. Neuro-Oncology. 2011;13:324–333. doi: 10.1093/neuonc/noq157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiesner SM, Decker SA, Larson JD, Ericson K, Forster C, Gallardo JL, Long C, Demorest ZL, Zamora EA, Low WC, SantaCruz K, Largaespada DA, Ohlfest JR. De novo induction of genetically engineered brain tumors in mice using plasmid DNA. Cancer Res. 2009;69:431–439. doi: 10.1158/0008-5472.CAN-08-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohlfest JR, Andersen BM, Litterman AL, Xia J, Pennell CA, Swier LE, Salazar AM, Olin MR. Vaccine Injection Site Matters: Qualitative and Quantitative Defects in CD8 T Cells Primed as a Function of Proximity to the Tumor in a Murine Glioma Model. J Immunol. 2013;190:613–620. doi: 10.4049/jimmunol.1201557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trickett A, Kwan YL. T cell stimulation and expansion using anti-CD3/CD28 beads. J Immunol Meth. 2003;275:251–255. doi: 10.1016/s0022-1759(03)00010-3. [DOI] [PubMed] [Google Scholar]
- 36.Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, Hogquist KA. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med. 2011;208:1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Struck RF, Alberts DS, Horne K, Phillips JG, Peng YM, Roe DJ. Plasma pharmacokinetics of cyclophosphamide and its cytotoxic metabolites after intravenous versus oral administration in a randomized, crossover trial. Cancer Res. 1987;47:2723–2726. [PubMed] [Google Scholar]
- 38.Genka S, Deutsch J, Stahle PL, Shetty UH, John V, Robinson C, Rapoport SI, Greig NH. Brain and plasma pharmacokinetics and anticancer activities of cyclophosphamide and phosphoramide mustard in the rat. Cancer Chemother Pharmacol. 1990;27:1–7. doi: 10.1007/BF00689268. [DOI] [PubMed] [Google Scholar]
- 39.Re MC, Schiavone P, Vitone F, Bon I, De Crignis E, Biagetti C, Alessandrini F, Gibellini D. Low avidity antibody: a reliable method to diagnose a recent HIV-1 infection. New Microbiol. 2008;31:19–26. [PubMed] [Google Scholar]
- 40.Newlands ES, Blackledge GR, Slack JA, Rustin GJ, Smith DB, Stuart NS, Quarterman CP, Hoffman R, Stevens MF, Brampton MH. Phase I trial of temozolomide (CCRG 81045: M&B 39831: NSC 362856) Brit J Cancer. 1992;65:287–291. doi: 10.1038/bjc.1992.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Obar JJ, Khanna KM, Lefrancois L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 2008;28:859–869. doi: 10.1016/j.immuni.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Degan P, Montesano R, Wild CP. Antibodies against 7-methyldeoxyguanosine: its detection in rat peripheral blood lymphocyte DNA and potential applications to molecular epidemiology. Cancer Res. 1988;48:5065–5070. [PubMed] [Google Scholar]
- 43.Marchesi F, Turriziani M, Tortorelli G, Avvisati G, Torino F, De Vecchis L. Triazene compounds: mechanism of action and related DNA repair systems. Pharmacol Res. 2007;56:275–287. doi: 10.1016/j.phrs.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 44.Hengstler JG, Hengst A, Fuchs J, Tanner B, Pohl J, Oesch F. Induction of DNA crosslinks and DNA strand lesions by cyclophosphamide after activation by cytochrome P450 2B1. Mutat Res. 1997;373:215–223. doi: 10.1016/s0027-5107(96)00200-x. [DOI] [PubMed] [Google Scholar]
- 45.Turtle CJ, Swanson HM, Fujii N, Estey EH, Riddell SR. A distinct subset of self-renewing human memory CD8+ T cells survives cytotoxic chemotherapy. Immunity. 2009;31:834–844. doi: 10.1016/j.immuni.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anichini A, Molla A, Vegetti C, Bersani I, Zappasodi R, Arienti F, Ravagnani F, Maurichi A, Patuzzo R, Santinami M, Pircher H, Di Nicola M, Mortarini R. Tumor-reactive CD8+ early effector T cells identified at tumor site in primary and metastatic melanoma. Cancer Res. 2010;70:8378–8387. doi: 10.1158/0008-5472.CAN-10-2028. [DOI] [PubMed] [Google Scholar]
- 47.Zhu X, Fujita M, Snyder LA, Okada H. Systemic delivery of neutralizing antibody targeting CCL2 for glioma therapy. J Neurooncol. 2011;104:83–92. doi: 10.1007/s11060-010-0473-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 49.Hegi ME, Liu L, Herman JG, Stupp R, Wick W, Weller M, Mehta MP, Gilbert MR. Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J Clin Oncol. 2008;26:4189–4199. doi: 10.1200/JCO.2007.11.5964. [DOI] [PubMed] [Google Scholar]
- 50.Iizuka Y, Kojima H, Kobata T, Kawase T, Kawakami Y, Toda M. Identification of a glioma antigen, GARC-1, using cytotoxic T lymphocytes induced by HSV cancer vaccine. Int J Cancer. 2006;118:942–949. doi: 10.1002/ijc.21432. [DOI] [PubMed] [Google Scholar]
- 51.Frauwirth KA, Riley JL, Harris MH, Parry RV, Rathmell JC, Plas DR, Elstrom RL, June CH, Thompson CB. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16:769–777. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 52.Lawley PD, Brookes P. Molecular mechanism of the cytotoxic action of difunctional alkylating agents and of resistance to this action. Nature. 1965;206:480–483. doi: 10.1038/206480a0. [DOI] [PubMed] [Google Scholar]
- 53.Roos WP, Kaina B. DNA damage-induced apoptosis: From specific DNA lesions to the DNA damage response and apoptosis. Cancer Lett. 2012;17:17. doi: 10.1016/j.canlet.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 54.Matsuoka S, Rotman G, Ogawa A, Shiloh Y, Tamai K, Elledge SJ. Ataxia telangiectasia-mutated phosphorylates Chk2 in vivo and in vitro. Proc Natl Acad Sci USA. 2000;97:10389–10394. doi: 10.1073/pnas.190030497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Canman CE, Lim DS, Cimprich KA, Taya Y, Tamai K, Sakaguchi K, Appella E, Kastan MB, Siliciano JD. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science. 1998;281:1677–1679. doi: 10.1126/science.281.5383.1677. [DOI] [PubMed] [Google Scholar]
- 56.Gupta PK, Sirover MA. Sequential stimulation of DNA repair and DNA replication in normal human cells. Mutat Res. 1980;72:273–284. doi: 10.1016/0027-5107(80)90042-1. [DOI] [PubMed] [Google Scholar]
- 57.Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J, Sabzevari H. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105:2862–2868. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- 58.Hirschhorn-Cymerman D, Rizzuto GA, Merghoub T, Cohen AD, Avogadri F, Lesokhin AM, Weinberg AD, Wolchok JD, Houghton AN. OX40 engagement and chemotherapy combination provides potent antitumor immunity with concomitant regulatory T cell apoptosis. J Exp Med. 2009;206:1103–1116. doi: 10.1084/jem.20082205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mitchell DA, Cui X, Schmittling RJ, Sanchez-Perez L, Snyder DJ, Congdon KL, Archer GE, Desjardins A, Friedman AH, Friedman HS, Herndon JE, 2nd, McLendon RE, Reardon DA, Vredenburgh JJ, Bigner DD, Sampson JH. Monoclonal antibody blockade of IL-2 receptor α during lymphopenia selectively depletes regulatory T cells in mice and humans. Blood. 2011;118:3003–3012. doi: 10.1182/blood-2011-02-334565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C, Chauffert B, Solary E, Bonnotte B, Martin F. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34:336–344. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 61.Salem ML, Díaz-Montero CM, Al-Khami AA, El-Naggar SA, Naga O, Montero AJ, Khafagy A, Cole DJ. Recovery from cyclophosphamide-induced lymphopenia results in expansion of immature dendritic cells which can mediate enhanced prime-boost vaccination antitumor responses in vivo when stimulated with the TLR3 agonist poly(I:C) J Immunol. 2009;182:2030–2040. doi: 10.4049/jimmunol.0801829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yee C. Adoptive T cell therapy: Addressing challenges in cancer immunotherapy. J Transl Med. 2005;3:17. doi: 10.1186/1479-5876-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.MacLean GD, Miles DW, Rubens RD, Reddish MA, Longenecker BM. Enhancing the effect of THERATOPE STn-KLH cancer vaccine in patients with metastatic breast cancer by pretreatment with low-dose intravenous cyclophosphamide. J Immunother Emphasis Tumor Immunol. 1996;19:309–316. doi: 10.1097/00002371-199607000-00006. [DOI] [PubMed] [Google Scholar]
- 64.Vaishampayan U, Abrams J, Darrah D, Jones V, Mitchell MS. Active immunotherapy of metastatic melanoma with allogeneic melanoma lysates and interferon alpha. Clin Cancer Res. 2002;8:3696–3701. [PubMed] [Google Scholar]
- 65.Baskar S, Kobrin CB, Kwak LW. Autologous lymphoma vaccines induce human T cell responses against multiple, unique epitopes. J Clin Invest. 2004;113:1498–1510. doi: 10.1172/JCI20312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.von Herrath MG, Dockter J, Oldstone MB. How virus induces a rapid or slow onset insulin-dependent diabetes mellitus in a transgenic model. Immunity. 1994;1:231–242. doi: 10.1016/1074-7613(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 67.Oelke M, Maus MV, Didiano D, June CH, Mackensen A, Schneck JP. Ex vivo induction and expansion of antigen-specific cytotoxic T cells by HLA-Ig-coated artificial antigen-presenting cells. Nat Med. 2003;9:619–624. doi: 10.1038/nm869. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.