Abstract
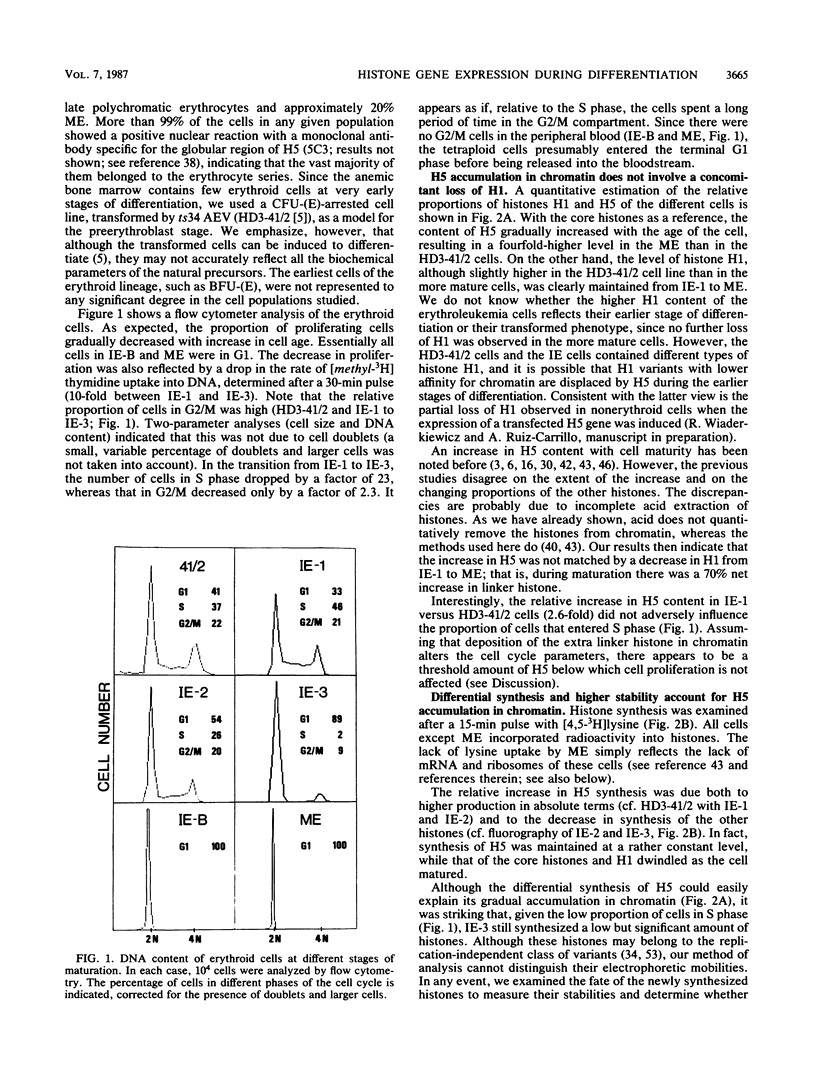
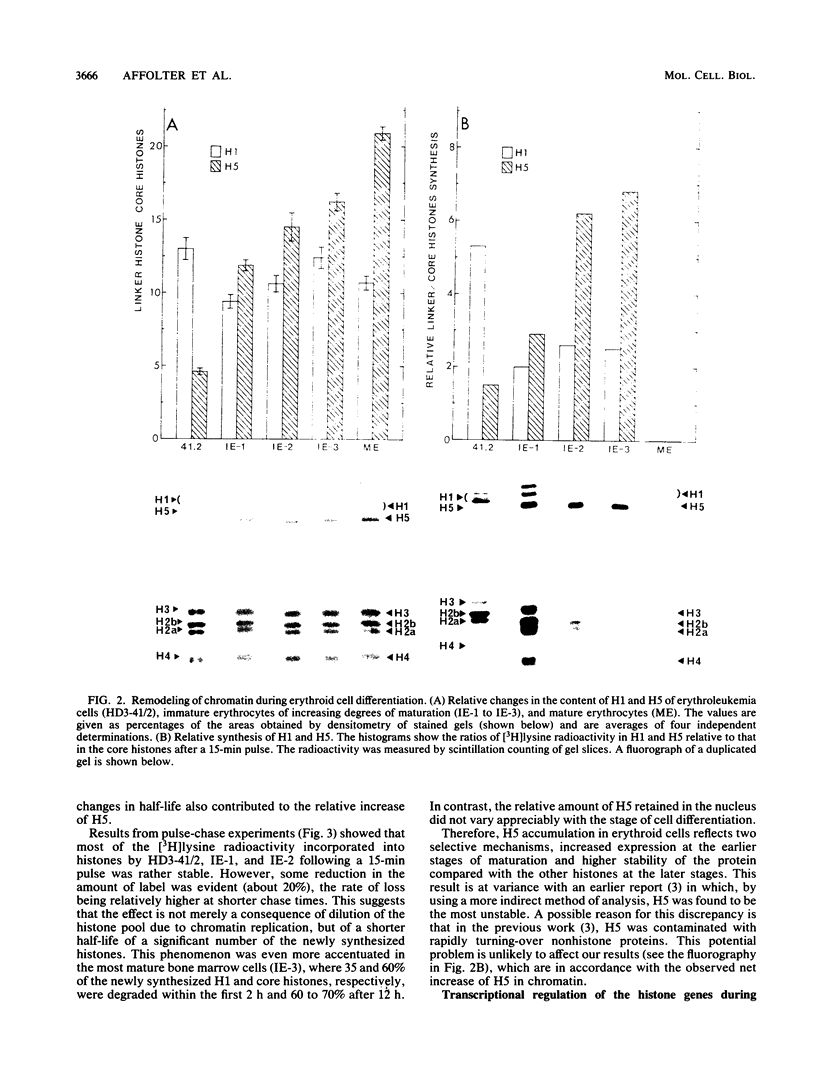
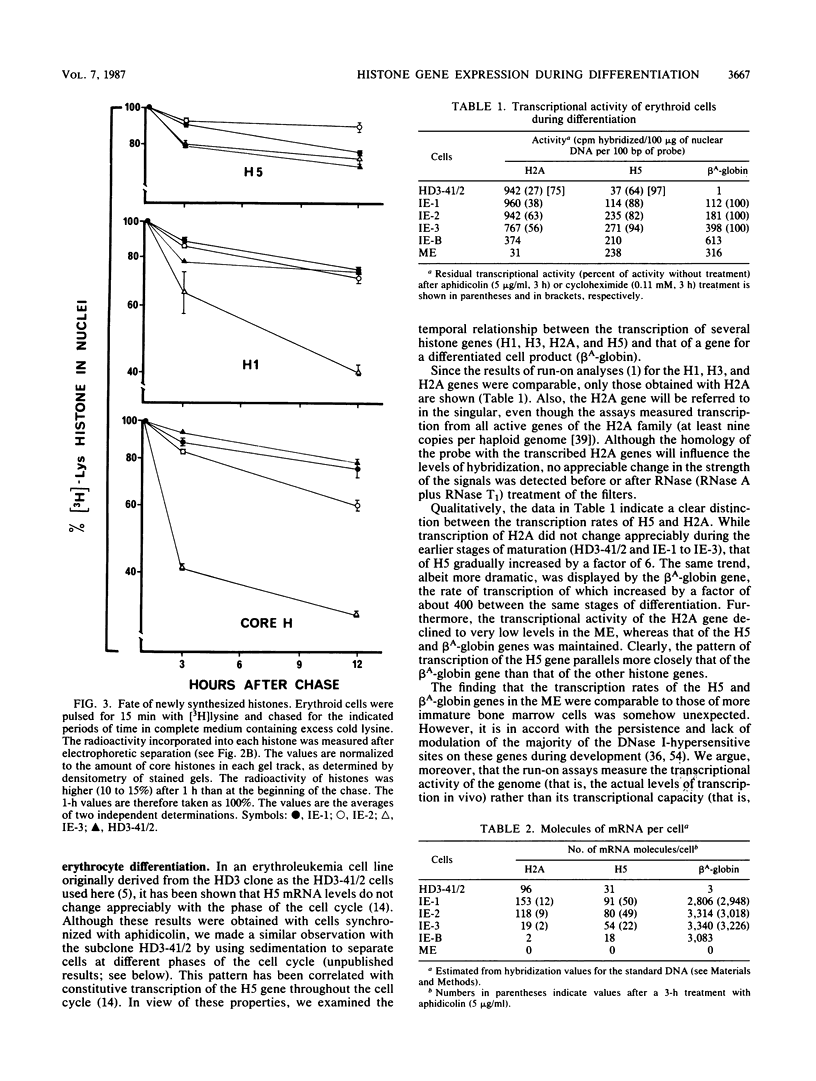
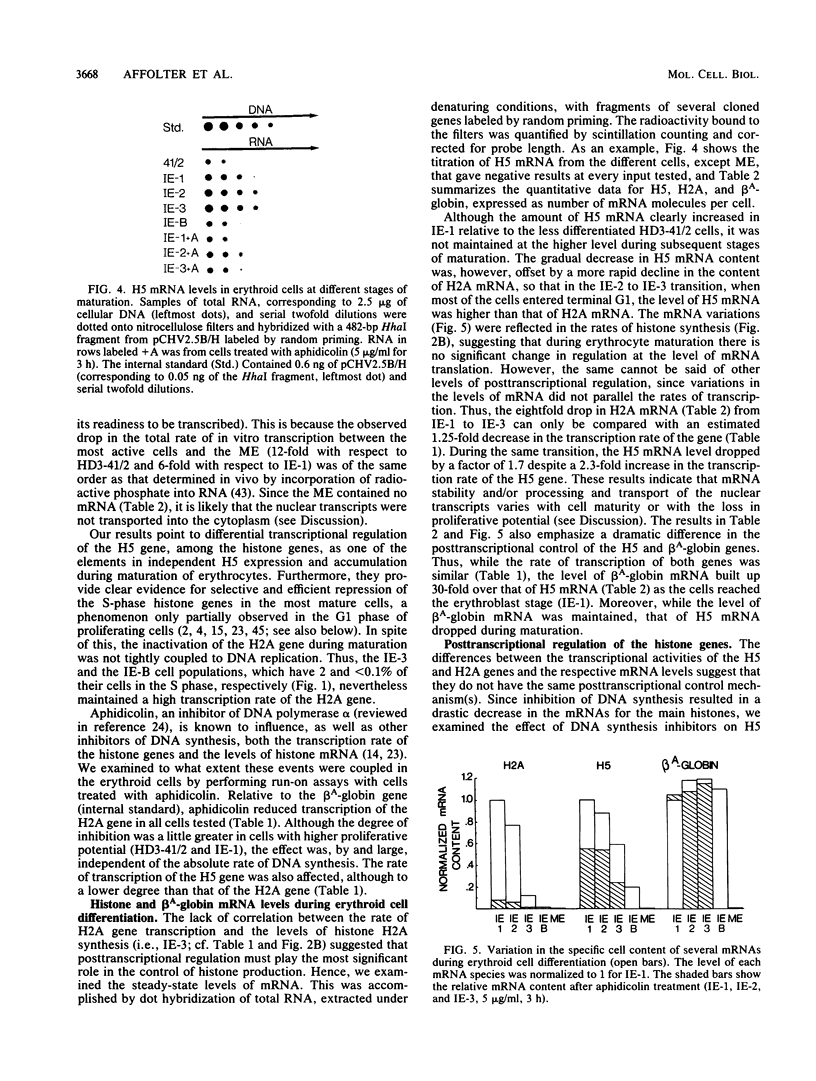
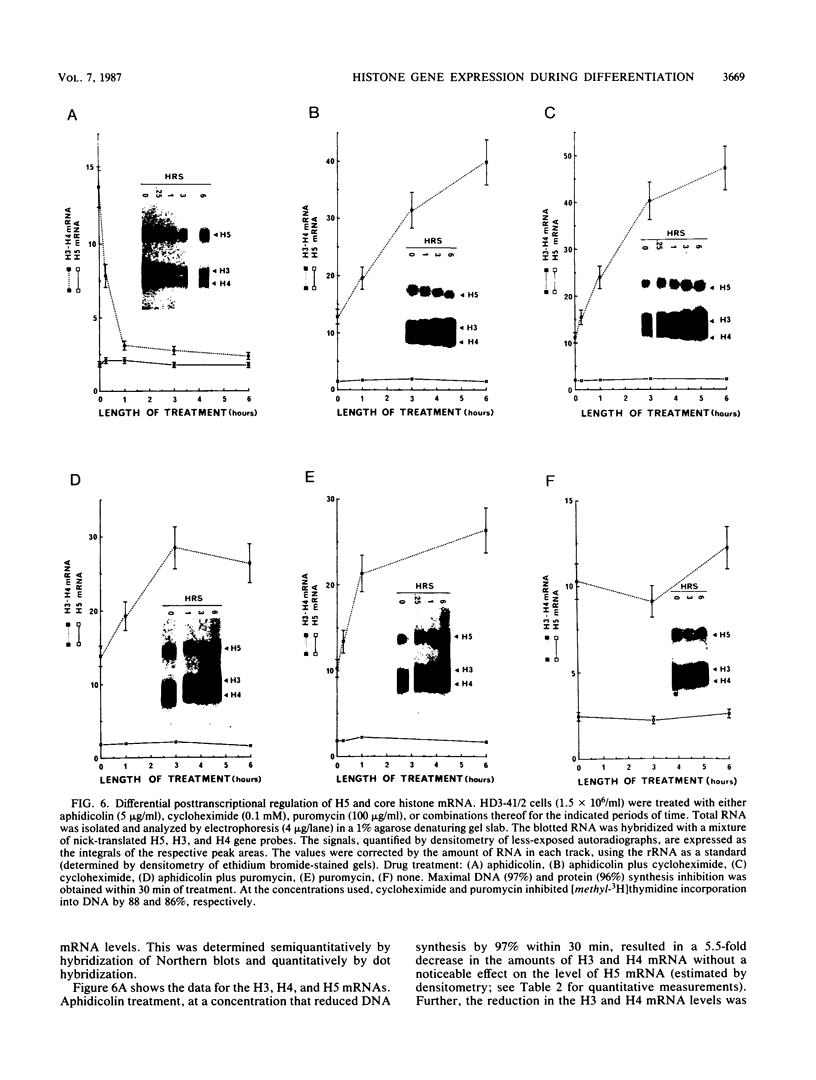
The expression of the genes for several histones and beta A-globin was examined in the chicken erythroid cells lineage. During the transition from CFU-(E) to the mature erythrocyte, histone H5 gradually increased fourfold in nuclei with little concomitant displacement of the H1 histones. This resulted in a 70% net increase in linker histone (H1 plus H5) content. The differential accumulation of H5 reflected (i) an increase in the transcriptional activity of the H5 gene occurring at the erythroblast stage, (ii) an apparent longer half-life of H5 mRNA, and (iii) a higher stability of the protein. Although the transcriptional activity of the histone genes (except H5) decreased with cell age, it was not tightly coupled to the S phase. On the other hand, the mRNA levels for these histones were tightly regulated during the cell cycle. Use of protein and DNA synthesis inhibitors indicated that the content of H5 mRNA was regulated at the posttranscriptional level by a control mechanism(s) differing from those for the other histones. Although the transcription rates of the H5 and beta A-globin genes were comparable, differential accumulation of beta A-globin mRNA led to a 30- to 170-fold-higher copy number of the beta A-globin mRNA as the cell matured.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Affolter M., Ruiz-Carrillo A. Transcription unit of the chicken histone H5 gene and mapping of H5 pre-mRNA sequences. J Biol Chem. 1986 Sep 5;261(25):11496–11502. [PubMed] [Google Scholar]
- Alterman R. B., Ganguly S., Schulze D. H., Marzluff W. F., Schildkraut C. L., Skoultchi A. I. Cell cycle regulation of mouse H3 histone mRNA metabolism. Mol Cell Biol. 1984 Jan;4(1):123–132. doi: 10.1128/mcb.4.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appels R., Wells J. R. Synthesis and turnover of DNA-bound histone during maturation of avian red blood cells. J Mol Biol. 1972 Oct 14;70(3):425–434. doi: 10.1016/0022-2836(72)90550-5. [DOI] [PubMed] [Google Scholar]
- Artishevsky A., Delegeane A. M., Lee A. S. Use of a cell cycle mutant to delineate the critical period for the control of histone mRNA levels in the mammalian cell cycle. Mol Cell Biol. 1984 Nov;4(11):2364–2369. doi: 10.1128/mcb.4.11.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beug H., von Kirchbach A., Döderlein G., Conscience J. F., Graf T. Chicken hematopoietic cells transformed by seven strains of defective avian leukemia viruses display three distinct phenotypes of differentiation. Cell. 1979 Oct;18(2):375–390. doi: 10.1016/0092-8674(79)90057-6. [DOI] [PubMed] [Google Scholar]
- Billett M. A., Hindley J. A study of the quantitative variation of histones, and their relationship to RNA synthesis, during erythropoiesis in the adult chicken. Eur J Biochem. 1972 Aug 4;28(4):451–462. doi: 10.1111/j.1432-1033.1972.tb01932.x. [DOI] [PubMed] [Google Scholar]
- Birnstiel M. L., Busslinger M., Strub K. Transcription termination and 3' processing: the end is in site! Cell. 1985 Jun;41(2):349–359. doi: 10.1016/s0092-8674(85)80007-6. [DOI] [PubMed] [Google Scholar]
- Borun T. W., Gabrielli F., Ajiro K., Zweidler A., Baglioni C. Further evidence of transcriptional and translational control of histone messenger RNA during the HeLa S3 cycle. Cell. 1975 Jan;4(1):59–67. doi: 10.1016/0092-8674(75)90134-8. [DOI] [PubMed] [Google Scholar]
- Brawerman G. The Role of the poly(A) sequence in mammalian messenger RNA. CRC Crit Rev Biochem. 1981;10(1):1–38. doi: 10.3109/10409238109114634. [DOI] [PubMed] [Google Scholar]
- Brown D. T., Wellman S. E., Sittman D. B. Changes in the levels of three different classes of histone mRNA during murine erythroleukemia cell differentiation. Mol Cell Biol. 1985 Nov;5(11):2879–2886. doi: 10.1128/mcb.5.11.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brush D., Dodgson J. B., Choi O. R., Stevens P. W., Engel J. D. Replacement variant histone genes contain intervening sequences. Mol Cell Biol. 1985 Jun;5(6):1307–1317. doi: 10.1128/mcb.5.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler W. B., Mueller G. C. Control of histone synthesis in HeLa cells. Biochim Biophys Acta. 1973 Feb 4;294(1):481–496. doi: 10.1016/0005-2787(73)90104-4. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dalton S., Coleman J. R., Wells J. R. Transcription of the histone H5 gene is not S-phase regulated. Mol Cell Biol. 1986 Feb;6(2):601–606. doi: 10.1128/mcb.6.2.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisle A. J., Graves R. A., Marzluff W. F., Johnson L. F. Regulation of histone mRNA production and stability in serum-stimulated mouse 3T6 fibroblasts. Mol Cell Biol. 1983 Nov;3(11):1920–1929. doi: 10.1128/mcb.3.11.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick C., Johns E. W. A quantitative comparison of histones from immature and mature erythroid cells of the duck. Biochim Biophys Acta. 1969 Mar;175(2):414–418. doi: 10.1016/0005-2795(69)90020-8. [DOI] [PubMed] [Google Scholar]
- Dolan M., Dodgson J. B., Engel J. D. Analysis of the adult chicken beta-globin gene. Nucleotide sequence of the locus, microheterogeneity at the 5'-end of beta-globin mRNA, and aberrant nuclear RNA species. J Biol Chem. 1983 Mar 25;258(6):3983–3990. [PubMed] [Google Scholar]
- Engel J. D., Sugarman B. J., Dodgson J. B. A chicken histone H3 gene contains intervening sequences. Nature. 1982 Jun 3;297(5865):434–436. doi: 10.1038/297434a0. [DOI] [PubMed] [Google Scholar]
- Graves R. A., Marzluff W. F. Rapid reversible changes in the rate of histone gene transcription and histone mRNA levels in mouse myeloma cells. Mol Cell Biol. 1984 Feb;4(2):351–357. doi: 10.1128/mcb.4.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves R. A., Pandey N. B., Chodchoy N., Marzluff W. F. Translation is required for regulation of histone mRNA degradation. Cell. 1987 Feb 27;48(4):615–626. doi: 10.1016/0092-8674(87)90240-6. [DOI] [PubMed] [Google Scholar]
- Harvey R. P., Whiting J. A., Coles L. S., Krieg P. A., Wells J. R. H2A.F: an extremely variant histone H2A sequence expressed in the chicken embryo. Proc Natl Acad Sci U S A. 1983 May;80(10):2819–2823. doi: 10.1073/pnas.80.10.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintz N., Sive H. L., Roeder R. G. Regulation of human histone gene expression: kinetics of accumulation and changes in the rate of synthesis and in the half-lives of individual histone mRNAs during the HeLa cell cycle. Mol Cell Biol. 1983 Apr;3(4):539–550. doi: 10.1128/mcb.3.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman J. A. New views of the biochemistry of eucaryotic DNA replication revealed by aphidicolin, an unusual inhibitor of DNA polymerase alpha. Cell. 1981 Mar;23(3):647–648. doi: 10.1016/0092-8674(81)90426-8. [DOI] [PubMed] [Google Scholar]
- Kapp L. N., Brown S. L., Klevecz R. R. Detecting small quantities of DNA on CsCl gradients. Biochim Biophys Acta. 1974 Aug 29;361(2):140–143. doi: 10.1016/0005-2787(74)90341-4. [DOI] [PubMed] [Google Scholar]
- Krieg P. A., Robins A. J., D'Andrea R., Wells J. R. The chicken H5 gene is unlinked to core and H1 histone genes. Nucleic Acids Res. 1983 Feb 11;11(3):619–627. doi: 10.1093/nar/11.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lüscher B., Stauber C., Schindler R., Schümperli D. Faithful cell-cycle regulation of a recombinant mouse histone H4 gene is controlled by sequences in the 3'-terminal part of the gene. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4389–4393. doi: 10.1073/pnas.82.13.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molgaard H. V., Perucho M., Ruiz-Carrillo A. Histone H5 messenger RNA is polyadenylated. Nature. 1980 Jan 31;283(5746):502–504. doi: 10.1038/283502a0. [DOI] [PubMed] [Google Scholar]
- Moss B. A., Joyce W. G., Ingram V. M. Histones in chick embryonic erythropoiesis. J Biol Chem. 1973 Feb 10;248(3):1025–1031. [PubMed] [Google Scholar]
- NEELIN J. M., CALLAHAN P. X., LAMB D. C., MURRAY K. THE HISTONES OF CHICKEN ERYTHROCYTE NUCLEI. Can J Biochem. 1964 Dec;42:1743–1752. doi: 10.1139/o64-185. [DOI] [PubMed] [Google Scholar]
- Perucho M., Molgaard H. V., Shevack A., Pataryas T., Ruiz-Carrillo A. An improved method for the preparation of undegraded polysomes and active messenger RNA from immature chicken erythrocytes. Anal Biochem. 1979 Oct 1;98(2):464–471. doi: 10.1016/0003-2697(79)90168-4. [DOI] [PubMed] [Google Scholar]
- Pestka S. Inhibitors of ribosome functions. Annu Rev Microbiol. 1971;25:487–562. doi: 10.1146/annurev.mi.25.100171.002415. [DOI] [PubMed] [Google Scholar]
- Puigdomènech P., Ruiz-Carrillo A. Effect of histone composition on the stability of chromatin structure. Biochim Biophys Acta. 1982 Mar 29;696(3):267–274. doi: 10.1016/0167-4781(82)90057-4. [DOI] [PubMed] [Google Scholar]
- Rave N., Crkvenjakov R., Boedtker H. Identification of procollagen mRNAs transferred to diazobenzyloxymethyl paper from formaldehyde agarose gels. Nucleic Acids Res. 1979 Aug 10;6(11):3559–3567. doi: 10.1093/nar/6.11.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud J., Ruiz-Carrillo A. Fine analysis of the active H5 gene chromatin of chicken erythroid cells at different stages of differentiation. J Mol Biol. 1986 May 5;189(1):217–226. doi: 10.1016/0022-2836(86)90392-x. [DOI] [PubMed] [Google Scholar]
- Robbins E., Borun T. W. The cytoplasmic synthesis of histones in hela cells and its temporal relationship to DNA replication. Proc Natl Acad Sci U S A. 1967 Feb;57(2):409–416. doi: 10.1073/pnas.57.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Carrillo A., Affolter M., Renaud J. Genomic organization of the genes coding for the six main histones of the chicken: complete sequence of the H5 gene. J Mol Biol. 1983 Nov 15;170(4):843–859. doi: 10.1016/s0022-2836(83)80191-0. [DOI] [PubMed] [Google Scholar]
- Ruiz-Carrillo A., Jorcano J. L. An octamer of core histones in solution: central role of the H3-H4 tetramer in the self-assembly. Biochemistry. 1979 Mar 6;18(5):760–768. doi: 10.1021/bi00572a004. [DOI] [PubMed] [Google Scholar]
- Ruiz-Carrillo A., Puigdomènech P., Eder G., Lurz R. Stability and reversibility of higher ordered structure of interphase chromatin: continuity of deoxyribonucleic acid is not required for maintenance of folded structure. Biochemistry. 1980 Jun 10;19(12):2544–2554. doi: 10.1021/bi00553a002. [DOI] [PubMed] [Google Scholar]
- Ruiz-Carrillo A., Wangh L. J., Allfrey V. G. Selective synthesis and modification of nuclear proteins during maturation of avian erythroid cells. Arch Biochem Biophys. 1976 May;174(1):273–290. doi: 10.1016/0003-9861(76)90346-5. [DOI] [PubMed] [Google Scholar]
- Ruiz-Carrillo A., Wangh L. J., Littau V. C., Allfrey V. G. Changes in histone acetyl content and in nuclear non-histone protein composition of avian erythroid cells at different stages of maturation. J Biol Chem. 1974 Nov 25;249(22):7358–7368. [PubMed] [Google Scholar]
- Ruiz-Vazquez R., Ruiz-Carillo A. Construction of chimeric plasmids containing histone H5 cDNA from hen erythrocyte. DNA sequence of a fragment derived from the 5' region of H5 mRNA. Nucleic Acids Res. 1982 Mar 25;10(6):2093–2108. doi: 10.1093/nar/10.6.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rózalski M., Lafleur L., Ruiz-Carrillo A. Monoclonal antibodies against histone H5. Epitope mapping and binding to chromatin. J Biol Chem. 1985 Nov 15;260(26):14379–14386. [PubMed] [Google Scholar]
- Sittman D. B., Graves R. A., Marzluff W. F. Histone mRNA concentrations are regulated at the level of transcription and mRNA degradation. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1849–1853. doi: 10.1073/pnas.80.7.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotirov N., Johns E. W. Quantitative differences in the content of the histone f2c between chicken erythrocytes and erythroblasts. Exp Cell Res. 1972 Jul;73(1):13–16. doi: 10.1016/0014-4827(72)90095-x. [DOI] [PubMed] [Google Scholar]
- Spohr G., Kayibanda B., Scherrer K. Polyribosome-bound and free-cytoplasmic-hemoglobin-messenger RNA in differentiating avian erythroblasts. Eur J Biochem. 1972 Nov 21;31(1):194–208. doi: 10.1111/j.1432-1033.1972.tb02519.x. [DOI] [PubMed] [Google Scholar]
- Stiles C. D., Lee K. L., Kenney F. T. Differential degradation of messenger RNAs in mammalian cells. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2634–2638. doi: 10.1073/pnas.73.8.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimac E., Groppi V. E., Jr, Coffino P. Inhibition of protein synthesis stabilizes histone mRNA. Mol Cell Biol. 1984 Oct;4(10):2082–2090. doi: 10.1128/mcb.4.10.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugarman B. J., Dodgson J. B., Engel J. D. Genomic organization, DNA sequence, and expression of chicken embryonic histone genes. J Biol Chem. 1983 Jul 25;258(14):9005–9016. [PubMed] [Google Scholar]
- Sung M. T., Freedlender E. F. Sites of in vivo phosphorylation of histone H5. Biochemistry. 1978 May 16;17(10):1884–1890. doi: 10.1021/bi00603a013. [DOI] [PubMed] [Google Scholar]
- Urban M. K., Franklin S. G., Zweidler A. Isolation and characterization of the histone variants in chicken erythrocytes. Biochemistry. 1979 Sep 4;18(18):3952–3960. doi: 10.1021/bi00585a017. [DOI] [PubMed] [Google Scholar]
- Urban M. K., Zweidler A. Changes in nucleosomal core histone variants during chicken development and maturation. Dev Biol. 1983 Feb;95(2):421–428. doi: 10.1016/0012-1606(83)90043-x. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Beug H., Groudine M., Graf T. Temperature-sensitive changes in the structure of globin chromatin in lines of red cell precursors transformed by ts-AEV. Cell. 1982 Apr;28(4):931–940. doi: 10.1016/0092-8674(82)90072-1. [DOI] [PubMed] [Google Scholar]
- Wu R. S., Bonner W. M. Separation of basal histone synthesis from S-phase histone synthesis in dividing cells. Cell. 1981 Dec;27(2 Pt 1):321–330. doi: 10.1016/0092-8674(81)90415-3. [DOI] [PubMed] [Google Scholar]
- Wu R. S., Panusz H. T., Hatch C. L., Bonner W. M. Histones and their modifications. CRC Crit Rev Biochem. 1986;20(2):201–263. doi: 10.3109/10409238609083735. [DOI] [PubMed] [Google Scholar]
- Wu R. S., Tsai S., Bonner W. M. Patterns of histone variant synthesis can distinguish G0 from G1 cells. Cell. 1982 Dec;31(2 Pt 1):367–374. doi: 10.1016/0092-8674(82)90130-1. [DOI] [PubMed] [Google Scholar]