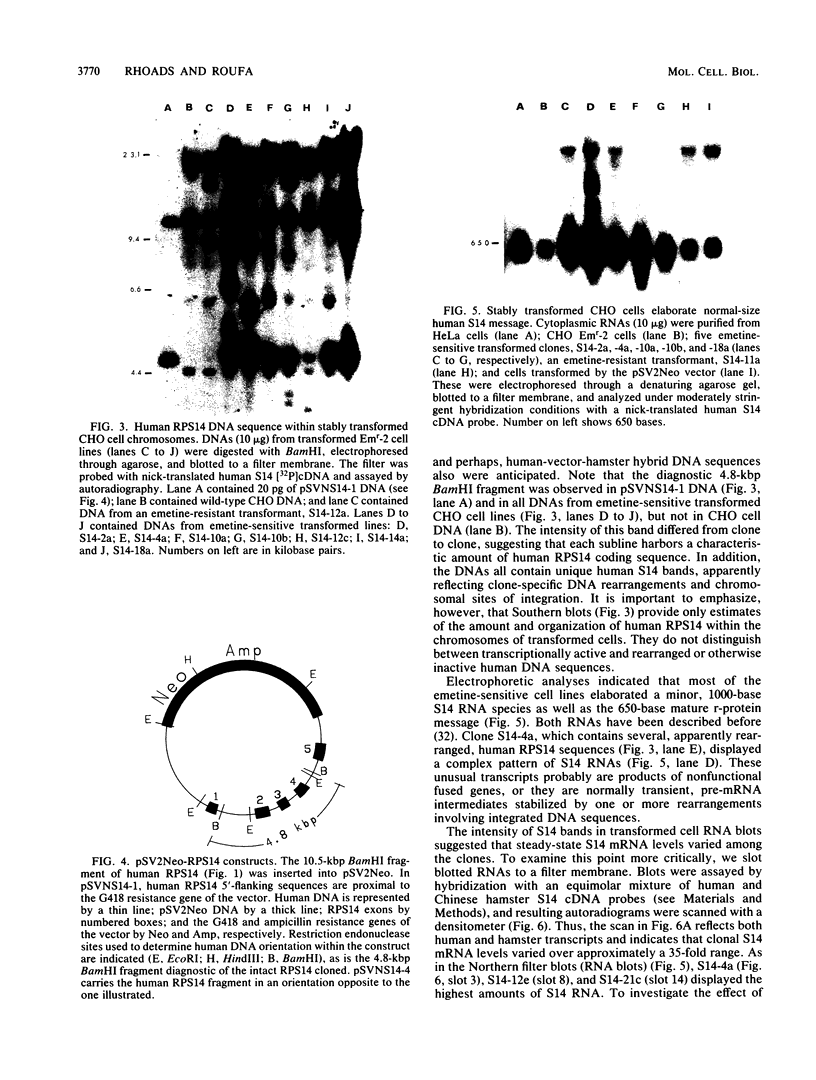
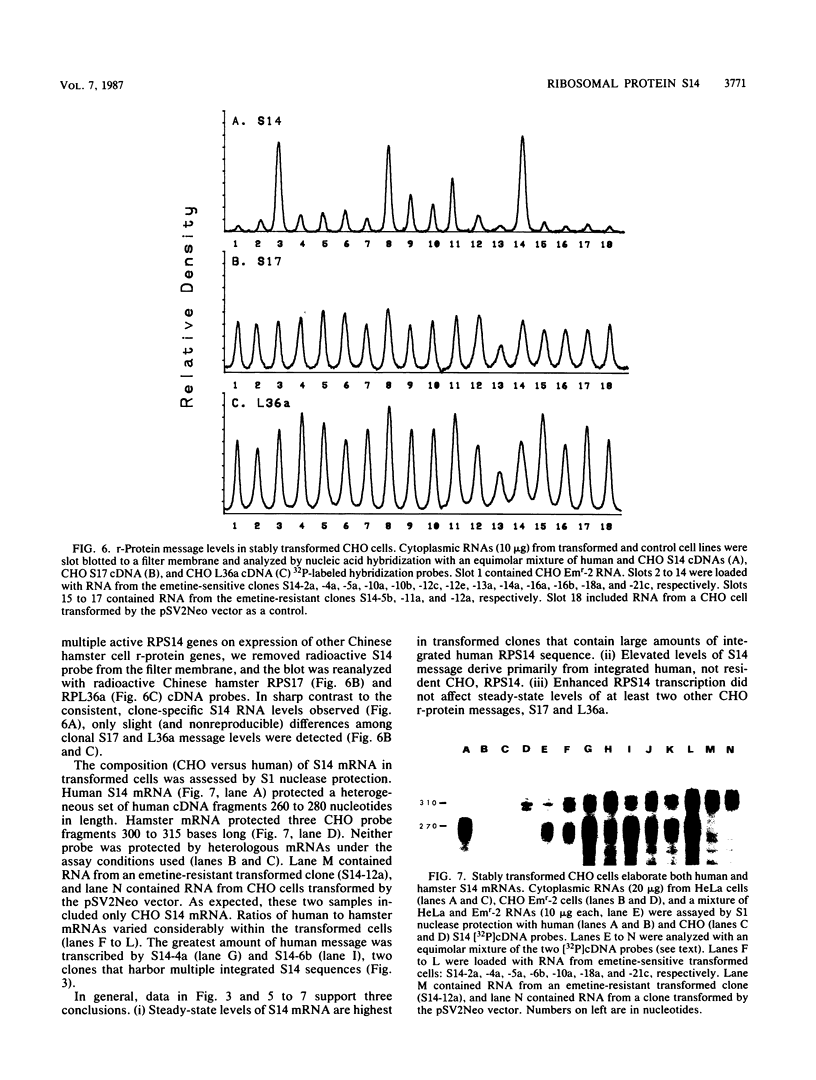
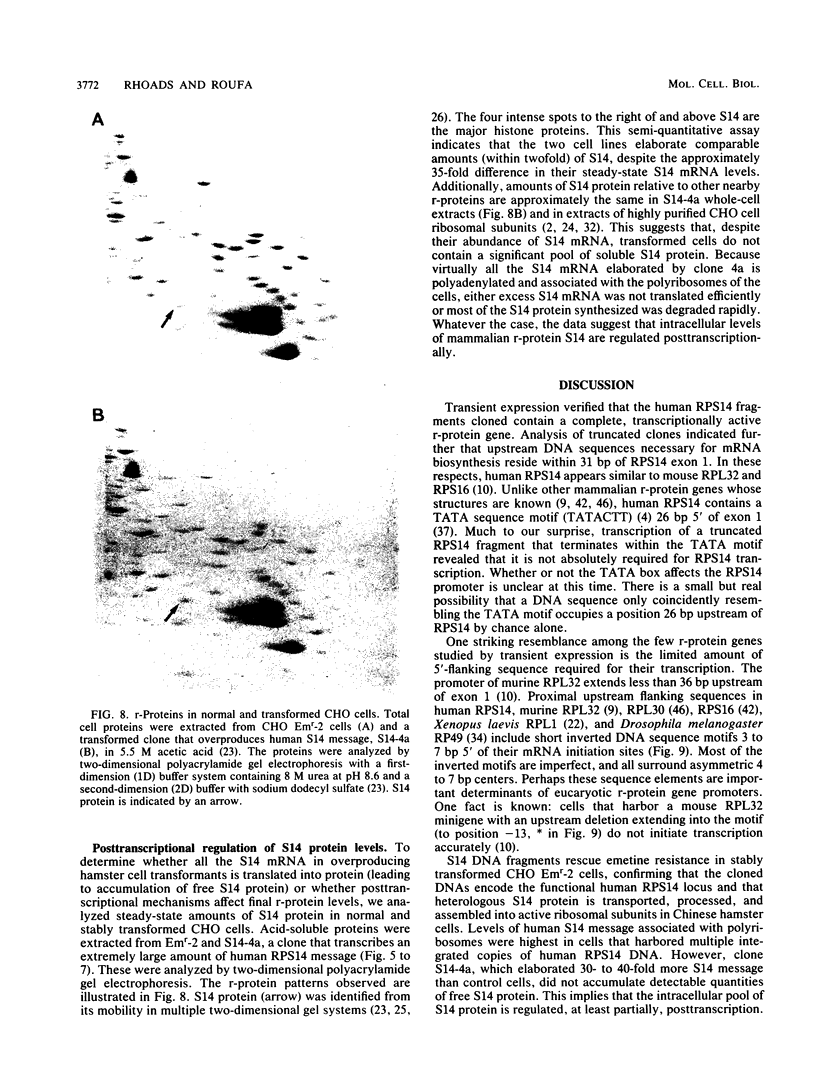
Abstract
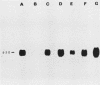
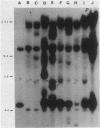
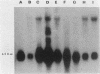
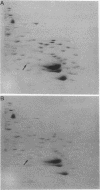
Cloned fragments of human ribosomal protein S14 DNA (RPS14) were transfected into cultured Chinese hamster (CHO) cells. Transient expression assays indicated that DNA with as little as 31 base pairs of upstream flanking sequence was transcribed into a polyadenylated, 650-base mRNA that is largely bound to the polyribosomes. In these respects the exogenous human S14 message appeared to function normally in CHO cells. Interestingly, transcription of human RPS14 did not require the TATA sequence located 26 base pairs upstream of exon 1. Stably transformed clones were selected from cultures of emetine-resistant CHO cells (Emr-2) after transfection with pSV2Neo-human RPS14 constructs. Human RPS14 complemented the mutationally based drug resistance of the Chinese hamster cells, demonstrating that the cloned human ribosomal protein gene is functional in rodent cells. Analysis of transformed cells with different amounts of integrated RPS14 indicated that human S14 mRNA levels are not tightly regulated by CHO cells. In contrast, the steady-state S14 level fluctuated only slightly, if at all, in transformed clones whose S14 message contents differed by more than 30-fold. These data support the conclusion that expression of human RPS14 is regulated, at least partially, posttranscriptionally.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Boersma D., McGill S. M., Mollenkamp J. W., Roufa D. J. Emetine resistance in Chinese hamster cells is linked genetically with an altered 40S ribosomal subunit protein, S20. Proc Natl Acad Sci U S A. 1979 Jan;76(1):415–419. doi: 10.1073/pnas.76.1.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boersma D., McGill S., Mollenkamp J., Roufa D. J. Emetine resistance in Chinese hamster cells. Analysis of ribosomal proteins prepared from mutant cells. J Biol Chem. 1979 Jan 25;254(2):559–567. [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Burke J. F. High-sensitivity S1 mapping with single-stranded [32P]DNA probes synthesized from bacteriophage M13mp templates. Gene. 1984 Oct;30(1-3):63–68. doi: 10.1016/0378-1119(84)90105-7. [DOI] [PubMed] [Google Scholar]
- Chaney W. G., Howard D. R., Pollard J. W., Sallustio S., Stanley P. High-frequency transfection of CHO cells using polybrene. Somat Cell Mol Genet. 1986 May;12(3):237–244. doi: 10.1007/BF01570782. [DOI] [PubMed] [Google Scholar]
- Chen I. T., Dixit A., Rhoads D. D., Roufa D. J. Homologous ribosomal proteins in bacteria, yeast, and humans. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6907–6911. doi: 10.1073/pnas.83.18.6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dana S. L., Chang S., Wasmuth J. J. Synthesis and incorporation of human ribosomal protein S14 into functional ribosomes in human-Chinese hamster cell hybrids containing human chromosome 5: human RPS14 gene is the structural gene for ribosomal protein S14. Somat Cell Mol Genet. 1985 Nov;11(6):625–631. doi: 10.1007/BF01534727. [DOI] [PubMed] [Google Scholar]
- Dudov K. P., Perry R. P. Properties of a mouse ribosomal protein promoter. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8545–8549. doi: 10.1073/pnas.83.22.8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudov K. P., Perry R. P. The gene family encoding the mouse ribosomal protein L32 contains a uniquely expressed intron-containing gene and an unmutated processed gene. Cell. 1984 Jun;37(2):457–468. doi: 10.1016/0092-8674(84)90376-3. [DOI] [PubMed] [Google Scholar]
- Faliks D., Meyuhas O. Coordinate regulation of ribosomal protein mRNA level in regenerating rat liver. Study with the corresponding mouse cloned cDNAs. Nucleic Acids Res. 1982 Feb 11;10(3):789–801. doi: 10.1093/nar/10.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon A. M., Jinks C. S., Strycharz G. D., Nomura M. Regulation of ribosomal protein synthesis in Escherichia coli by selective mRNA inactivation. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3411–3415. doi: 10.1073/pnas.76.7.3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer P. K., Meyuhas O., Perry R. P., Johnson L. F. Regulation of ribosomal protein mRNA content and translation in growth-stimulated mouse fibroblasts. Mol Cell Biol. 1982 Jun;2(6):685–693. doi: 10.1128/mcb.2.6.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillin F. D., Roufa D. J., Beaudet A. L., Caskey C. T. 8-Azaguanine resistance in mammalian cells. I. Hypoxanthine-guanine phosphoribosyltransferase. Genetics. 1972 Oct;72(2):239–252. doi: 10.1093/genetics/72.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorenstein C., Warner J. R. Coordinate regulation of the synthesis of eukaryotic ribosomal proteins. Proc Natl Acad Sci U S A. 1976 May;73(5):1547–1551. doi: 10.1073/pnas.73.5.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. S., Siminovitch L. The isolation and preliminary characterization of somatic cell mutants resistant to the protein synthesis inhibitor-emetine. Cell. 1976 Oct;9(2):213–219. doi: 10.1016/0092-8674(76)90112-4. [DOI] [PubMed] [Google Scholar]
- Gupta R. S., Siminovitch L. The molecular basis of emetine resistance in Chinese hamster ovary cells: alteration in the 40S ribosomal subunit. Cell. 1977 Jan;10(1):61–66. doi: 10.1016/0092-8674(77)90140-4. [DOI] [PubMed] [Google Scholar]
- Haralson M. A., Roufa D. J. A temperature-sensitive mutation affecting the mammalian 60 S ribosome. J Biol Chem. 1975 Nov 25;250(22):8618–8623. [PubMed] [Google Scholar]
- Ignotz G. G., Hokari S., DePhilip R. M., Tsukada K., Lieberman I. Lodish model and regulation of ribosomal protein synthesis by insulin-deficient chick embryo fibroblasts. Biochemistry. 1981 Apr 28;20(9):2550–2558. doi: 10.1021/bi00512a029. [DOI] [PubMed] [Google Scholar]
- Kief D. R., Warner J. R. Hierarchy of elements regulating synthesis of ribosomal proteins in Saccharomyces cerevisiae. Mol Cell Biol. 1981 Nov;1(11):1016–1023. doi: 10.1128/mcb.1.11.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauter K. S., Soeiro R., Nadal-Ginard B. Unco-ordinate regulation of ribosomal RNA and ribosomal protein synthesis during L6E9 myoblast differentiation. J Mol Biol. 1980 Sep 15;142(2):145–159. doi: 10.1016/0022-2836(80)90042-x. [DOI] [PubMed] [Google Scholar]
- Loreni F., Ruberti I., Bozzoni I., Pierandrei-Amaldi P., Amaldi F. Nucleotide sequence of the L1 ribosomal protein gene of Xenopus laevis: remarkable sequence homology among introns. EMBO J. 1985 Dec 16;4(13A):3483–3488. doi: 10.1002/j.1460-2075.1985.tb04107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madjar J. J., Arpin M., Buisson M., Reboud J. P. Spot position of rat liver ribosomal proteins by four different two-dimensional electrophoreses in polyacrylamide gel. Mol Gen Genet. 1979 Mar 20;171(2):121–134. doi: 10.1007/BF00269998. [DOI] [PubMed] [Google Scholar]
- Madjar J. J., Frahm M., McGill S., Roufa D. J. Ribosomal protein S14 is altered by two-step emetine resistance mutations in Chinese hamster cells. Mol Cell Biol. 1983 Feb;3(2):190–197. doi: 10.1128/mcb.3.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madjar J. J., Michel S., Cozzone A. J., Reboud J. P. A method to identify individual proteins in four different two-dimensional gel electrophoresis systems: application to Escherichia coli ribosomal proteins. Anal Biochem. 1979 Jan 1;92(1):174–182. doi: 10.1016/0003-2697(79)90641-9. [DOI] [PubMed] [Google Scholar]
- Madjar J. J., Nielsen-Smith K., Frahm M., Roufa D. J. Emetine resistance in chinese hamster ovary cells is associated with an altered ribosomal protein S14 mRNA. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1003–1007. doi: 10.1073/pnas.79.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Moreland R. B., Nam H. G., Hereford L. M., Fried H. M. Identification of a nuclear localization signal of a yeast ribosomal protein. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6561–6565. doi: 10.1073/pnas.82.19.6561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabeshima Y. I., Ogata K. Stimulation of the synthesis of ribosomal proteins in regenerating rat liver with special reference to the increase in the amounts of effective mRNAs for ribosomal proteins. Eur J Biochem. 1980 Jun;107(2):323–329. doi: 10.1111/j.1432-1033.1980.tb06032.x. [DOI] [PubMed] [Google Scholar]
- Nakamichi N. N., Kao F. T., Wasmuth J., Roufa D. J. Ribosomal protein gene sequences map to human chromosomes 5, 8, and 17. Somat Cell Mol Genet. 1986 May;12(3):225–236. doi: 10.1007/BF01570781. [DOI] [PubMed] [Google Scholar]
- Nakamichi N., Rhoads D. D., Roufa D. J. The Chinese hamster cell emetine resistance gene. Analysis of cDNA and genomic sequences encoding ribosomal protein S14. J Biol Chem. 1983 Nov 10;258(21):13236–13242. [PubMed] [Google Scholar]
- Nomura M., Yates J. L., Dean D., Post L. E. Feedback regulation of ribosomal protein gene expression in Escherichia coli: structural homology of ribosomal RNA and ribosomal protein MRNA. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7084–7088. doi: 10.1073/pnas.77.12.7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell P. O., Rosbash M. Sequence, structure, and codon preference of the Drosophila ribosomal protein 49 gene. Nucleic Acids Res. 1984 Jul 11;12(13):5495–5513. doi: 10.1093/nar/12.13.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson N. J., Fried H. M., Warner J. R. Yeast use translational control to compensate for extra copies of a ribosomal protein gene. Cell. 1982 Jun;29(2):347–355. doi: 10.1016/0092-8674(82)90151-9. [DOI] [PubMed] [Google Scholar]
- Pierandrei-Amaldi P., Beccari E., Bozzoni I., Amaldi F. Ribosomal protein production in normal and anucleolate Xenopus embryos: regulation at the posttranscriptional and translational levels. Cell. 1985 Aug;42(1):317–323. doi: 10.1016/s0092-8674(85)80127-6. [DOI] [PubMed] [Google Scholar]
- Rhoads D. D., Dixit A., Roufa D. J. Primary structure of human ribosomal protein S14 and the gene that encodes it. Mol Cell Biol. 1986 Aug;6(8):2774–2783. doi: 10.1128/mcb.6.8.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads D. D., Roufa D. J. Emetine resistance of Chinese hamster cells: structures of wild-type and mutant ribosomal protein S14 mRNAs. Mol Cell Biol. 1985 Jul;5(7):1655–1659. doi: 10.1128/mcb.5.7.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosbash M., Harris P. K., Woolford J. L., Jr, Teem J. L. The effect of temperature-sensitive RNA mutants on the transcription products from cloned ribosomal protein genes of yeast. Cell. 1981 Jun;24(3):679–686. doi: 10.1016/0092-8674(81)90094-5. [DOI] [PubMed] [Google Scholar]
- Santon J. B., Pellegrini M. Expression of ribosomal proteins during Drosophila early development. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5649–5653. doi: 10.1073/pnas.77.10.5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Wagner M., Perry R. P. Characterization of the multigene family encoding the mouse S16 ribosomal protein: strategy for distinguishing an expressed gene from its processed pseudogene counterparts by an analysis of total genomic DNA. Mol Cell Biol. 1985 Dec;5(12):3560–3576. doi: 10.1128/mcb.5.12.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner J. R., Mitra G., Schwindinger W. F., Studeny M., Fried H. M. Saccharomyces cerevisiae coordinates accumulation of yeast ribosomal proteins by modulating mRNA splicing, translational initiation, and protein turnover. Mol Cell Biol. 1985 Jun;5(6):1512–1521. doi: 10.1128/mcb.5.6.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wejksnora P. J., Warner J. R. Regulation of ribosomal RNA and proteins in mouse-hamster hybrid cells. J Biol Chem. 1981 Sep 25;256(18):9406–9413. [PubMed] [Google Scholar]
- Wiedemann L. M., Perry R. P. Characterization of the expressed gene and several processed pseudogenes for the mouse ribosomal protein L30 gene family. Mol Cell Biol. 1984 Nov;4(11):2518–2528. doi: 10.1128/mcb.4.11.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates J. L., Arfsten A. E., Nomura M. In vitro expression of Escherichia coli ribosomal protein genes: autogenous inhibition of translation. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1837–1841. doi: 10.1073/pnas.77.4.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates J. L., Nomura M. E. coli ribosomal protein L4 is a feedback regulatory protein. Cell. 1980 Sep;21(2):517–522. doi: 10.1016/0092-8674(80)90489-4. [DOI] [PubMed] [Google Scholar]
- Zengel J. M., Mueckl D., Lindahl L. Protein L4 of the E. coli ribosome regulates an eleven gene r protein operon. Cell. 1980 Sep;21(2):523–535. doi: 10.1016/0092-8674(80)90490-0. [DOI] [PubMed] [Google Scholar]