Abstract
Background
Strategies for localizing parathyroid pathology preoperatively vary in cost and accuracy. Our purpose was to compute and compare comprehensive costs associated with common localization strategies.
Methods
A decision-analytic model was developed to evaluate comprehensive, short-term costs of parathyroid localization strategies for patients with primary hyperparathyroidism. Eight strategies were compared. Probabilities of accurate localization were extracted from the literature, and costs associated with each strategy were based on 2011 Medicare reimbursement schedules. Differential cost considerations included outpatient versus inpatient surgeries, operative time, and costs of imaging. Sensitivity analyses were performed to determine effects of variability in key model parameters upon model results.
Results
Ultrasound (US) followed by 4D-CT was the least expensive strategy ($5,901), followed by US alone ($6,028), and 4D-CT alone ($6,110). Strategies including sestamibi (SM) were more expensive, with associated expenditures of up to $6,329 for contemporaneous US and SM. Four-gland, bilateral neck exploration (BNE) was the most expensive strategy ($6,824). Differences in cost were dependent upon differences in the sensitivity of each strategy for detecting single-gland disease, which determined the proportion of patients able to undergo outpatient minimally invasive parathyroidectomy. In sensitivity analysis, US alone was preferred over US followed by 4D-CT only when both the sensitivity of US alone for detecting an adenoma was ≥94 %, and the sensitivity of 4D-CT following negative US was ≤39 %. 4D-CT alone was the least costly strategy when US sensitivity was ≤31 %.
Conclusions
Among commonly used strategies for pre-operative localization of parathyroid pathology, US followed by selective 4D-CT is the least expensive.
Primary hyperparathyroidism (PHPT) affects approximately 1 % of the population.1 Parathyroidectomy is accepted as the most durable and cost-effective treatment, with reported cure rates >97 %. Surgical cure prevents complications, such as nephrolithiasis and neurocognitive symptoms, all of which contribute to decreased quality of life.1–4 The majority of affected patients have single-gland disease, which has led to a shift from traditional, four-gland, bilateral neck exploration (BNE) to minimally invasive parathyroidectomy (MIP).5 This change was driven by the potential to achieve decreased patient morbidity and costs, with similar rates of surgical success.6, 7
MIP explorations have similar cure and complication rates as compared to BNE, yet require preoperative localization studies, traditionally with ultrasound (US) and technetium-99 M sestamibi-SPECT (SM) scanning.8–12 Four-dimensional computed tomography (4D-CT), which employs high-resolution multiplanar anatomic and functional imaging to localize abnormal parathyroid glands, has been used recently as an alternative to traditional imaging.13 The accuracy of 4D-CT has been studied in heterogeneous cohorts. Overall, its reported performance is considered superior to standard US and SM approaches.13–17 However, concerns have been raised over its increased associated radiation exposure.14–17
The majority of cost studies support the benefit of MIP, compared with BNE, due to increased operating room and hospitalization costs associated with BNE.18–22 However, preferred strategies for preoperative localization vary greatly from one institution to another,5 and it is unclear which screening strategies, alone, in combination, or in succession, optimize cost savings. Decision analysis is an effective, evidence-based tool for comparing these localization approaches by allowing for integration of key factors that influence the costs of different approaches. Our purpose was to develop a decision-analytic model to compare healthcare costs across the comprehensive spectrum of practiced localization strategies, including 4D-CT, for the treatment of patients with sporadic PHPT.
METHODS
Overview of the Model
We developed a decision-analytic model to compare short-term costs associated with different preoperative localization strategies for patients with PHPT. Costs of imaging and treatment were included in the analysis, but non-healthcare-related costs to the patient (i.e., patient absence from work) were not.23 Input parameters were extracted from the literature.5,7,10,14,20,24–33 The primary cost analysis incorporated best available cost and probability estimates (Table 1). Secondary (sensitivity) analysis was then performed to assess the stability of these results over a range of key model parameters. TreeAge Pro 2009 (TreeAge Software, Williamstown, MA) was used to construct and analyze the model.
TABLE 1.
Model inputs for base-case analysis and sensitivity analysis
| Parameter | Data | Sensitivity analysis range | Source(s) |
|---|---|---|---|
| Single-gland disease prevalence | 0.879 | 0.68–1.0 | 32,36 |
| Proportion of outpatient cases | |||
| BNE | 13 % | 0–1.0 | 5 |
| MIP | 38 % | 0–1.0 | 5,20,32 |
| Operative time (min) | |||
| MIP | 78 | 36–116 | 7,10 |
| BNE | 144 | 50–168 | 7,10 |
| Imaging test characteristics | |||
| Ultrasound sensitivity | 0.761 | 0.4–1.0 | 14,25,31 |
| Sestamibi sensitivity | 0.789 | 0.4–1.0 | 14,25,27,28 |
| 4D-CT sensitivity | |||
| Weighted average | 0.894 | 0.4–1.0 | 14 |
| Following indeterminate SM and US | 0.718 | 0.4–1.0 | 14 |
| Following indeterminate SM | 0.85 | 0.4–1.0 | 29 |
| Following indeterminate US | 0.85 | 0.4–1.0 | a |
| Ultrasound + sestamibi sensitivity | 0.91 | 0.4–1.0 | 24,26,30 |
| Costs($) | |||
| Hospital cost | |||
| Inpatient (DRG 627b) | $4,367 | 33 | |
| Outpatient (DRG 627b) | $2,064 | 33 | |
| Surgeon cost (CPT 60500) | $1,014 | 33 | |
| Cost of anesthesiac | |||
| MIP (CPT 00320) | $808 | $303–$977 | 7,10,33 |
| BNE (CPT 00320) | $1,566 | $421–$1,566 | 7,10,33 |
| Intraoperative-PTH monitoring (CPT 36522) | $176 | 33 | |
| Imaging (includes physician and hospital costs) | |||
| Neck ultrasound (CPT 76536) | $96 | (0.5–1.5) × BCE | 33 |
| Sestamibi-SPECT (CPT 78070) | $475 | (0.5–1.5) × BCE | 33 |
| 4D-CT (CPT 72127) | $334 | (0.5 × BCE) to thresholdd | 33 |
MIP minimally invasive parathyroidectomy, BNE four-gland, bilateral neck exploration, BCE base case estimate
Given that data were unavailable, sensitivity of 4D-CT following US was assumed to be similar to that for 4D-CT following negative SM
Diagnosis-related group for parathyroid procedures without major comorbidities or complications
Cost of anesthesia was based on average anesthesia time (15 min increments), 2011 CPT Anesthesia Base Units, and the national anesthesia conversion factor
Sensitivity ranges extended to test thresholds beyond which a change in our analysis results (strategy preference) could occur
We designated our base case as a 55 year-old woman with sporadic PHPT who met criteria for surgery based on NIH consensus guidelines for PHPT.34 Patients could either undergo a BNE or a MIP depending on the preoperative localization strategy and attendant imaging results. Test characteristics were defined as follows: true-positives, an abnormal gland identified on both imaging and at surgery; false-negatives, a gland not identified on imaging but found to be abnormal at the time of surgery; true-negative, a gland not identified on imaging and found to be normal at the time of surgery; and false-positive, an abnormal gland identified on imaging but found to be normal at surgery.
Only those patients with localization of a parathyroid adenoma confirmed at surgery (true-positives) and appropriate decrease in intraoperative-PTH (IOPTH) level had MIP; all others had BNE, either planned or following failed MIP. All patients were assumed to undergo IOPTH testing.
Given current practice patterns, patients were assumed to have general anesthesia.5 We also assumed that patients were American Society of Anesthesiologists Physical Status classification class I or II and had no contraindications IV contrast, concurrent thyroid pathology, or prior neck surgery.
MIP patients underwent one of seven localization strategies: (1) ultrasound (US) alone; (2) 4D-CT alone; (3) sestamibi-SPECT (SM) alone; (4) contemporaneous US and SM; (5) US followed by 4D-CT (if US was indeterminate); (6) SM followed by 4D-CT (if SM was indeterminate); and (7) US and SM followed by 4D-CT (if contemporaneous US and SM were indeterminate). Patients undergoing the final strategy (8) went directly to BNE without preoperative imaging.
Because rates of permanent hypoparathyroidism and recurrent laryngeal nerve injury have not been shown to differ between MIP and BNE, this was not factored into the analysis.10,35,6 Preference-based utility measures of the various screening approaches have not been assessed to date and are likely to be nondifferential (i.e., undergoing 4D-CT vs. SM is likely to have minimal effect on health-related quality of life). Therefore, a cost-analysis alone was chosen to compare localization strategies in this study.
Model Inputs and Sources
Probability Estimates
Model inputs are illustrated in Table 1. We used a prevalence estimate from the largest reported series for our primary analysis.32 Practice pattern probabilities, including the differential proportion of outpatient cases in MIP patients versus BNE, were obtained from a comprehensive survey of more than 250 surgeons from various subspecialties.5 Operative times of MIP and BNE, respectively, were based on the results of a prospective, randomized trial comparing the two operative strategies.10 Imaging test characteristics were elicited from a recent meta-analysis.14 We assumed that the sensitivity of 4D-CT was the same, whether followed by indeterminate US or SM, given the absence of reported data to inform this difference.29
Costs
Current Procedural Terminology (CPT) and Diagnosis-Related Group (DRG)–based physician and facility fees were calculated using Medicare national reimbursement data for 2011 for the direct costs of imaging, surgery, and hospitalization.33 Anesthesiology fees were based on average anesthesia time (15 min increments), 2011 CPT Anesthesia Base Units, and the national anesthesia conversion factor.7,37
Sensitivity Analyses
We performed one-way sensitivity analyses to assess effects of varying key model parameters upon our results. In particular, we varied imaging test performance characteristics (for localization of single-gland disease), all cost estimates, and the proportion of patients undergoing out-patient (versus inpatient) surgery (Table 1). Model inputs were tested over ranges reported in the literature when available. Additional threshold analyses were performed, where relevant, to determine at what thresholds our results would change. We also assessed variability in cost without the use of IOPTH during BNE.
Radiation Exposure
Estimates of the lifetime attributable risk of cancer incidence and death were calculated from the Biological Effects of Ionizing Radiation (BEIR) VII report (Tables 12-D 1 and 2) based on age and reported ranges of radiation exposure from SM and 4D-CT.38
RESULTS
The results of the primary analysis are shown in Table 2. Of the strategies evaluated, US followed by 4D-CT (if US was indeterminate) was the least costly strategy ($5,901) and US alone was the next least costly. BNE was the most costly ($6,824), with substantially higher associated costs ($450) compared with the next most costly strategy. Importantly, BNE remained the most expensive strategy even when IOPTH costs were excluded (cost = $6,648).
TABLE 2.
Differential cost and cost by rank of localization strategies
| Rank | Strategy | Cost ($) | Incremental cost ($)(compared with US → 4D-CT) |
|---|---|---|---|
| 1 | US → 4D-CT (if US indeterminate) | 5,901 | – |
| 2 | US | 6,028 | 127 |
| 3 | 4D-CT | 6,110 | 209 |
| 4 | SM → 4D-CT (if SM indeterminate) | 6,266 | 365 |
| 5 | US + SM → 4D-CT (if US and SM indeterminate or discordant) | 6,319 | 418 |
| 6 | US/SM | 6,329 | 428 |
| 7 | SM | 6,374 | 473 |
| 8 | BNE | 6,824 | 923 |
US ultrasound, SM sestamibi-SPECT, BNE four-gland, bilateral neck exploration
Sensitivity Analysis Results
US followed by 4D-CT remained the preferred strategy across the majority of sensitivity ranges tested, including those pertaining to test performance characteristics, prevalence of single gland disease, imaging costs, clinical practice patterns (e.g., related to preference for proportion of outpatient procedures), and operative times.
Test Characteristics
In one-way sensitivity analyses using a threshold analysis approach, US followed by 4D-CT (if US indeterminate) remained the least costly strategy even when each of the following one-way sensitivity analyses was performed: (1) increasing SM sensitivity to 100 %, (2) increasing combined US + SM sensitivity to 100 %, and (3) increasing 4D-CT sensitivity to 100 %. These results are driven by the lower comparative cost of US relative to other imaging modalities but comparable sensitivity for detecting single-gland disease.
Varying the sensitivity of US did not affect the optimal strategy within a probable range of this parameter. When varying the sensitivity of US to determine at what point strategy preferences would change (e.g., in a threshold analysis), we found that: (1) US alone became the least costly strategy at an US sensitivity of ≥94 %; and (2) 4D-CT alone was the least costly strategy when US sensitivity was ≤31 %. US alone also became the least costly strategy when the sensitivity of 4D-CT following indeterminate US was ≤39 %.
Prevalence of Single Gland Disease
Higher rates of multigland disease have been reported in some series.36 Given that a decrease in the pretest probability of single-gland disease would influence the value of localization studies (and ability to perform MIP alone), we assessed our results over a wide range of parathyroid adenoma prevalence reported in the literature (Fig. 1). As expected, the cost of all localization strategies increased with decreasing single-gland disease prevalence, given increased requirements of BNE. However, US followed by 4D-CT (if US indeterminate) remained the least costly strategy ($6,206) and no localization strategy exceeded the cost of BNE at the lowest reported prevalence of single-gland disease (68 %).36
FIG. 1.
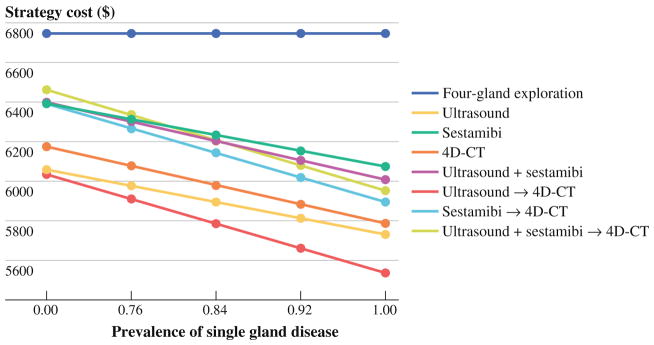
Plot of one-way sensitivity analysis of a reported range of prevalence of single gland disease. Ultrasound followed by 4D-CT (if US indeterminate) is the least costly strategy throughout the range tested. Base-case analysis single-gland disease prevalence input = 0.897
Imaging Costs
The preferred strategy was not sensitive to fluctuating short-term costs of the various localization studies: US followed by 4D-CT (if US indeterminate) remained the least costly strategy for a wide range of US and SM costs. It also remained the least costly strategy when the cost of 4D-CT<$722. US alone was the least costly when the cost of 4D-CT exceeded $722.
Proportion of Outpatient Cases
In two-way sensitivity analysis (in which we varied two model parameters simultaneously to determine the effects on model results), we assessed the effect of varying the proportion of patients admitted following (1) MIP and (2) direct BNE, on strategy preference. Assuming that the proportion of patients being admitted by a particular surgeon after BNE will always equal or exceed the proportion of patients being admitted following MIP, US followed by 4D-CT (if US indeterminate) remained the preferred strategy.
Anesthesia Costs
Surgeon reimbursement for MIP and BNE is the same. Therefore, differences in operative times are reflected in differences in time-based anesthesia costs. One-way sensitivity analysis of anesthesia costs for BNE showed that below $850 (equivalent to 1.7 h of anesthesia time) US alone became the preferred strategy. Figure 2 illustrates the effect of varying the anesthesia costs of the two surgical procedures. Within a wide range of anesthesia costs reported in the literature, localization strategies are preferred over direct BNE.
FIG. 2.
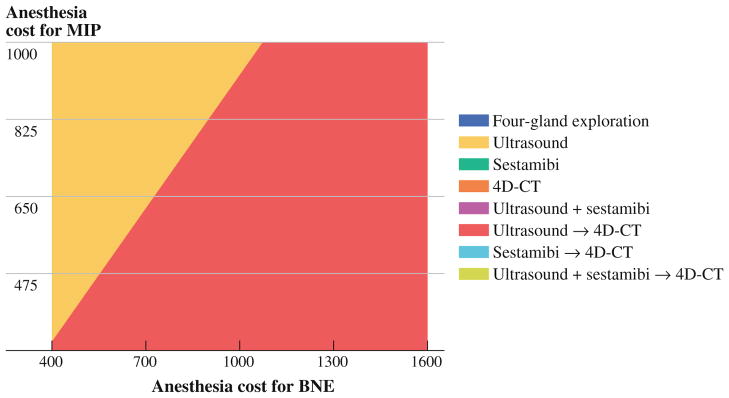
Strategy graph of two-way sensitivity analysis of time-based anesthesia costs for MIP and BNE. When costs are based on average reported anesthesia times ($808 for MIP and $1,566 for BNE), ultrasound followed by 4D-CT remains the preferred strategy
Radiation Exposure
There continues to be concern about increased radiation exposure from 4D-CT. Given that the large majority (>97 %) of patients with PHPT are cured, patients usually only require one-time preoperative localization. Reported average effective dose ranges from nuclear parathyroid (99mTc-sestamibi) scans and 4D-CT are 6–11 mSv and 10–24 mSv, respectively, depending on the protocol utilized (i.e., some centers do not perform delayed CT images).39–41 For comparison, background environmental radiation on average is 3 mSv/year.41 Based on estimates of radiation-induced cancer risk from a widely accepted reference source, the Biological Effects of Ionizing Radiation (BEIR) VII report, projected lifetime radiation-induced cancer incidence and mortality rates remain low (Table 3).38
TABLE 3.
Lifetime attributable risk of cancer
| Radiation source | Effective dose estimate (mSv) | Age (year)/sex | Lifetime attributable risk of cancer incidence (%) | Lifetime attributable risk of cancer death (%) | Years needed to get same dose from background radiation* |
|---|---|---|---|---|---|
| Sestamibi-SPECT | 6–11 | 50F | 0.04–0.08 | 0.03–0.05 | 2.0–3.7 |
| 6–11 | 50M | 0.04–0.07 | 0.02–0.04 | 2.0–3.7 | |
| 4D-CT | 10–24 | 50F | 0.07–0.18 | 0.05–0.11 | 3.3–6.0 |
| 10–24 | 50M | 0.06–0.14 | 0.04–0.09 | 3.3–6.0 | |
| Sestamibi-SPECT | 6–11 | 55F | 0.04–0.07 | 0.03–0.05 | 2.0–3.7 |
| 6–11 | 55M | 0.03–0.06 | 0.02–0.04 | 2.0–3.7 | |
| 4D-CT | 10–24 | 55F | 0.07–0.16 | 0.04–0.11 | 3.3–6.0 |
| 10–24 | 55M | 0.05–0.13 | 0.04–0.08 | 3.3–6.0 | |
| Sestamibi-SPECT | 6–11 | 60F | 0.04–0.06 | 0.02–0.04 | 2.0–3.7 |
| 6–11 | 60M | 0.03–0.05 | 0.02–0.04 | 2.0–3.7 | |
| 4D-CT | 10–24 | 60F | 0.06–0.14 | 0.04–0.10 | 3.3–6.0 |
| 10–24 | 60M | 0.05–0.12 | 0.03–0.08 | 3.3–6.0 |
Background radiation 3 mSv/year
DISCUSSION
Prior cost-effectiveness analyses of treatment strategies for PHPT have found that surgery is preferable to observation or pharmacological therapy.4,42–45 Reported cure rates range from 95 to 98 % for both MIP and BNE approaches.3,7,10,12 Cost-utility analyses comparing MIP to BNE largely support the value of MIP; this was supported by our work.18–20,22 In keeping with the reported success of MIP, the majority of parathyroidectomies now performed in the United States are minimally invasive.5 Preoperative localization is required for MIP, traditionally with US and SM. Recent reports indicate 4D-CT to have improved sensitivity over US and SM; however, it is unclear if this modality should supplant or support standard imaging with ultrasound and sestamibi.13,14,16,17,39 Moreover, comparative costs of various screening strategies, vital in a potentially resource-constrained environment, are relevant.
In this analysis, we found that US followed by 4D-CT was the least costly strategy followed by US alone. In our study, differences in cost were largely based on improved sensitivity for detecting single gland disease and, therefore, on the proportion of patients able to undergo MIP with shorter operative time and same-day discharge. US followed by 4D-CT remained the best strategy over realistic ranges of SM, US, and 4D-CT costs and test performance for detecting single-gland disease. The percentage of patients admitted to the hospital following surgery has a large influence on cost. This decision will likely be primarily influenced by undergoing MIP versus BNE and surgeon preference. As anesthesia cost is time-based, small changes in OR times had a significant influence on cost. For instance, a 15 min interval of anesthesia cost ($126) is more than the cost of an US ($96).
To date, there is only one published study, by Wang and colleagues, comparing currently utilized screening strategies, including 4D-CT.46 In comparing our studies, there are a number of key differences in model parameters, strategies, and study design that warrant mention. In their study, all patients with MIP were assumed to have outpa-tient procedures, and all BNE were assumed to stay overnight. It is recognized, however, that the distinction between inpatient and outpatient surgery also is informed by practitioner preferences.5 As a result, we incorporated the proportion of cases likely to be managed as inpatient versus outpatient surgeries based on reported clinical practice patterns.5 Ultrasound alone was reported as the least costly strategy by Wang and colleagues; however, the strategy of US followed by 4D-CT, our preferred strategy, was not assessed in their study. Moreover, the authors chose to perform a cost-utility analysis using a disutility reported for patients undergoing bilateral versus unilateral surgery (utility difference of 0.006).42 Given that more research is still needed to better define utility differences between MIP versus BNE, or between localization strategies, we chose to limit our study to a cost analysis. In keeping with their study, we found strategies that integrated SM, alone or with other modalities, were more costly, and that direct BNE was the most costly.
On a clinical practice level, initial assessment with US is practical as increasing numbers of endocrine surgeons perform intraoffice ultrasound. Additionally, US allows for assessment of concurrent thyroid disease prior to surgery. Furthermore, the use of 4D-CT only in cases where US fails minimizes radiation compared to utilizing 4D-CT or SM as the first-line examination.
Our study has expected limitations, common to decision-analytic methods, which arise when using simplifying assumptions to model complex disease processes and care pathways. First, we did not include non-health care (e.g., time off from work) related costs to the patient in our analysis. Differential time off from work—in particular due to postoperative recovery from MIP versus BNE—may be possible. Incorporating time off from work, including a potential increased recovery time for BNE, would likely increase the magnitude of our results as this would make strategies with a higher proportion of patients undergoing BNE more costly. Second, to our knowledge, there are no reported data to inform the sensitivity of 4D-CT after negative US and assumed these tests to be conditionally independent in our base-case analysis. We addressed this limitation using sensitivity analysis—we found that US followed by 4D-CT remained the preferred strategy when sensitivity of 4D-CT after negative US was >39 %. Probabilities of single-gland disease prevalence and accurate parathyroid localization were extracted from the literature. Many of these estimates are based on retrospective data, and therefore, limited by selection bias and errors in collecting the data (i.e., misclassification). Furthermore, the survey data used for clinical practice patterns are limited by the sampling of survey responders, which may impact the generalizability of the results, as well as how accurately the responders answered (i.e., respondent and recall biases). To address these limitations, we performed a number sensitivity analyses to assess the stability of our results to varying model inputs.
In summary, we found that US followed by 4D-CT (if US indeterminate) was the least costly preoperative localization strategy for patients with PHPT. This finding was robust across a wide range of inputs. Differences in cost were influenced primarily by differences in the sensitivity of each strategy, which determined the proportion of patients able to undergo MIP with shorter operative times and same-day discharge. To our knowledge, this is the first study to assess comprehensive comparative costs of pre-operative localization strategies, including the 4D-CT following indeterminate US approach. Incorporating patient preferences and comparative effects of these strategies on health-related quality of life in this patient population will be essential for future comparative effectiveness research.
Acknowledgments
The authors thank Ekin Turan for help verifying radiation exposure estimates. This research is supported in part by the Program in Cancer Outcomes Research Training Grant (NCI R25CA092203) (Lubitz), and by Award Number K07CA133097 from the National Cancer Institute (Pandharipande). The research content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
DISCLOSURES Dr. Pandharipande receives funding from the Medical Imaging and Technology Alliance, for unrelated research.
References
- 1.The American Association of Clinical Endocrinologists and the American Association of Endocrine Surgeons position statement on the diagnosis and management of primary hyperparathyroidism. Endocr Pract. 2005;11:49–54. doi: 10.4158/EP.11.1.49. [DOI] [PubMed] [Google Scholar]
- 2.Silverberg SJ, Shane E, Jacobs TP, Siris E, Bilezikian JP. A 10-year prospective study of primary hyperparathyroidism with or without parathyroid surgery. N Engl J Med. 1999;341:1249–55. doi: 10.1056/NEJM199910213411701. [DOI] [PubMed] [Google Scholar]
- 3.Udelsman R, Pasieka JL, Sturgeon C, Young JE, Clark OH. Surgery for asymptomatic primary hyperparathyroidism: proceedings of the third international workshop. J Clin Endocrinol Metab. 2009;94:366–72. doi: 10.1210/jc.2008-1761. [DOI] [PubMed] [Google Scholar]
- 4.Zanocco K, Angelos P, Sturgeon C. Cost-effectiveness analysis of parathyroidectomy for asymptomatic primary hyperparathyroidism. Surgery. 2006;140:874–81. doi: 10.1016/j.surg.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 5.Greene AB, Butler RS, McIntyre S, et al. National trends in parathyroid surgery from 1998 to 2008: a decade of change. J Am Coll Surg. 2009;209:332–43. doi: 10.1016/j.jamcollsurg.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 6.Beyer TD, Solorzano CC, Starr F, Nilubol N, Prinz RA. Parathyroidectomy outcomes according to operative approach. Am J Surg. 2007;193:368–72. doi: 10.1016/j.amjsurg.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 7.Udelsman R. Six hundred fifty-six consecutive explorations for primary hyperparathyroidism. Ann Surg. 2002;235:665–70. doi: 10.1097/00000658-200205000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Irvin GL, 3rd, Solorzano CC, Carneiro DM. Quick intraoperative parathyroid hormone assay: surgical adjunct to allow limited parathyroidectomy, improve success rate, and predict outcome. World J Surg. 2004;28:1287–92. doi: 10.1007/s00268-004-7708-6. [DOI] [PubMed] [Google Scholar]
- 9.Ruda JM, Hollenbeak CS, Stack BC., Jr A systematic review of the diagnosis and treatment of primary hyperparathyroidism from 1995 to 2003. Otolaryngol Head Neck Surg. 2005;132:359–72. doi: 10.1016/j.otohns.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Russell CF, Dolan SJ, Laird JD. Randomized clinical trial comparing scan-directed unilateral versus bilateral cervical exploration for primary hyperparathyroidism due to solitary adenoma. Br J Surg. 2006;93:418–21. doi: 10.1002/bjs.5250. [DOI] [PubMed] [Google Scholar]
- 11.Sidhu S, Neill AK, Russell CF. Long-term outcome of unilateral parathyroid exploration for primary hyperparathyroidism due to presumed solitary adenoma. World J Surg. 2003;27:339–42. doi: 10.1007/s00268-002-6695-8. [DOI] [PubMed] [Google Scholar]
- 12.Westerdahl J, Bergenfelz A. Unilateral versus bilateral neck exploration for primary hyperparathyroidism: five-year follow-up of a randomized controlled trial. Ann Surg. 2007;246:976–80. doi: 10.1097/SLA.0b013e31815c3ffd. [DOI] [PubMed] [Google Scholar]
- 13.Lubitz CC, Hunter GJ, Hamberg LM, et al. Accuracy of 4-dimensional computed tomography in poorly localized patients with primary hyperparathyroidism. Surgery. 2010;148:1129–37. doi: 10.1016/j.surg.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Cheung K, Wang TS, Farrokhyar F, Roman SA, Sosa JA. A meta-analysis of preoperative localization techniques for patients with primary hyperparathyroidism. Ann Surg Oncol. 2012;19(2):577–83. doi: 10.1245/s10434-011-1870-5. [DOI] [PubMed] [Google Scholar]
- 15.Mortenson MM, Evans DB, Lee JE, et al. Parathyroid exploration in the reoperative neck: improved preoperative localization with 4D-computed tomography. J Am Coll Surg. 2008;206:888–95. doi: 10.1016/j.jamcollsurg.2007.12.044. discussion 895. [DOI] [PubMed] [Google Scholar]
- 16.Rodgers SE, Hunter GJ, Hamberg LM, et al. Improved preoperative planning for directed parathyroidectomy with 4-dimensional computed tomography. Surgery. 2006;140:932–40. doi: 10.1016/j.surg.2006.07.028. discussion 940. [DOI] [PubMed] [Google Scholar]
- 17.Starker LF, Mahajan A, Bjorklund P, Sze G, Udelsman R, Carling T. 4D parathyroid CT as the initial localization study for patients with de novo primary hyperparathyroidism. Ann Surg Oncol. 2011;18:1723–8. doi: 10.1245/s10434-010-1507-0. [DOI] [PubMed] [Google Scholar]
- 18.Chen H, Sokoll LJ, Udelsman R. Outpatient minimally invasive parathyroidectomy: a combination of sestamibi-SPECT localization, cervical block anesthesia, and intraoperative parathyroid hormone assay. Surgery. 1999;126:1016–21. doi: 10.1067/msy.2099.101433. discussion 1021–2. [DOI] [PubMed] [Google Scholar]
- 19.Fahy BN, Bold RJ, Beckett L, Schneider PD. Modern parathyroid surgery: a cost-benefit analysis of localizing strategies. Arch Surg. 2002;137:917–22. doi: 10.1001/archsurg.137.8.917. discussion 922–3. [DOI] [PubMed] [Google Scholar]
- 20.Goldstein RE, Blevins L, Delbeke D, Martin WH. Effect of minimally invasive radioguided parathyroidectomy on efficacy, length of stay, and costs in the management of primary hyperparathyroidism. Ann Surg. 2000;231:732–42. doi: 10.1097/00000658-200005000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mihai R, Weisters M, Stechman MJ, Gleeson F, Sadler G. Cost-effectiveness of scan-directed parathyroidectomy. Langenbecks Arch Surg. 2008;393:739–43. doi: 10.1007/s00423-008-0383-6. [DOI] [PubMed] [Google Scholar]
- 22.Zanocco K, Heller M, Sturgeon C. Cost-effectiveness of para-thyroidectomy for primary hyperparathyroidism. Endocr Pract. 2011;17(Suppl 1):69–74. doi: 10.4158/EP10311.RA. [DOI] [PubMed] [Google Scholar]
- 23.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the panel on cost-effectiveness in health and medicine. JAMA. 1996;276:1253–8. [PubMed] [Google Scholar]
- 24.Arici C, Cheah WK, Ituarte PH, et al. Can localization studies be used to direct focused parathyroid operations? Surgery. 2001;129:720–9. doi: 10.1067/msy.2001.114556. [DOI] [PubMed] [Google Scholar]
- 25.Bergenfelz AO, Jansson SK, Wallin GK, Martensson HG, Rasmussen L, Eriksson HL, Reihner EI. Impact of modern techniques on short-term outcome after surgery for primary hyperparathyroidism: a multicenter study comprising 2,708 patients. Langenbecks Arch Surg. 2009;394:851–60. doi: 10.1007/s00423-009-0540-6. [DOI] [PubMed] [Google Scholar]
- 26.Casara D, Rubello D, Pelizzo MR, Shapiro B. Clinical role of 99mTcO4/MIBI scan, ultrasound and intra-operative gamma probe in the performance of unilateral and minimally invasive surgery in primary hyperparathyroidism. Eur J Nucl Med. 2001;28:1351–9. [PubMed] [Google Scholar]
- 27.Civelek AC, Ozalp E, Donovan P, Udelsman R. Prospective evaluation of delayed technetium-99m sestamibi SPECT scintigraphy for preoperative localization of primary hyperparathyroidism. Surgery. 2002;131:149–57. doi: 10.1067/msy.2002.119817. [DOI] [PubMed] [Google Scholar]
- 28.Gotthardt M, Lohmann B, Behr TM, et al. Clinical value of parathyroid scintigraphy with technetium-99m methoxyisobutylisonitrile: discrepancies in clinical data and a systematic metaanalysis of the literature. World J Surg. 2004;28:100–7. doi: 10.1007/s00268-003-6991-y. [DOI] [PubMed] [Google Scholar]
- 29.Harari A, Zarnegar R, Lee J, Kazam E, Inabnet WB, 3rd, Fahey TJ., 3rd Computed tomography can guide focused exploration in select patients with primary hyperparathyroidism and negative sestamibi scanning. Surgery. 2008;144:970–6. doi: 10.1016/j.surg.2008.08.029. discussion 976–9. [DOI] [PubMed] [Google Scholar]
- 30.Tresoldi S, Pompili G, Maiolino R, et al. Primary hyperparathyroidism: can ultrasonography be the only preoperative diagnostic procedure? Radiol Med. 2009;114:1159–72. doi: 10.1007/s11547-009-0447-x. [DOI] [PubMed] [Google Scholar]
- 31.Van Husen R, Kim LT. Accuracy of surgeon-performed ultrasound in parathyroid localization. World J Surg. 2004;28:1122–6. doi: 10.1007/s00268-004-7485-2. [DOI] [PubMed] [Google Scholar]
- 32.Udelsman R, Lin Z, Donovan P. The superiority of minimally invasive parathyroidectomy based on 1650 consecutive patients with primary hyperparathyroidism. Ann Surg. 2011;253:585–91. doi: 10.1097/SLA.0b013e318208fed9. [DOI] [PubMed] [Google Scholar]
- 33.Medicare reimbursement schedule. Centers for medicare and medicaid services; [Google Scholar]
- 34.Bilezikian JP, Khan AA, Potts JT., Jr Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the third international workshop. J Clin Endocrinol Metab. 2009;94:335–9. doi: 10.1210/jc.2008-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bergenfelz A, Lindblom P, Tibblin S, Westerdahl J. Unilateral versus bilateral neck exploration for primary hyperparathyroidism: a prospective randomized controlled trial. Ann Surg. 2002;236:543–51. doi: 10.1097/00000658-200211000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siperstein A, Berber E, Barbosa GF, Tsinberg M, Greene AB, Mitchell J, Milas M. Predicting the success of limited exploration for primary hyperparathyroidism using ultrasound, sestamibi, and intraoperative parathyroid hormone: analysis of 1158 cases. Ann Surg. 2008;248:420–8. doi: 10.1097/SLA.0b013e3181859f71. [DOI] [PubMed] [Google Scholar]
- 37.Udelsman R. Surgery in primary hyperparathyroidism: the patient without previous neck surgery. J Bone Miner Res. 2002;17(Suppl 2):N126–32. [PubMed] [Google Scholar]
- 38.Council NR, editor. BEIR VII. Washington DC: National Academies Press; 2007. BEIR biological effects of ionizing radiation, Report BEIR VII. [Google Scholar]
- 39.Kutler DI, Moquete R, Kazam E, Kuhel WI. Parathyroid localization with modified 4D-computed tomography and ultrasonography for patients with primary hyperparathyroidism. Laryngoscope. 2011;121:1219–24. doi: 10.1002/lary.21783. [DOI] [PubMed] [Google Scholar]
- 40.Mahajan A, Starker LF, Ghita M, Udelsman R, Brink JA, Carling T. Parathyroid four-dimensional computed tomography: evaluation of radiation dose exposure during preoperative localization of parathyroid tumors in primary hyperparathyroidism. World J Surg. 2012;36(6):1335–9. doi: 10.1007/s00268-011-1365-3. [DOI] [PubMed] [Google Scholar]
- 41.Mettler FAJ, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology. 2006;248:254–64. doi: 10.1148/radiol.2481071451. [DOI] [PubMed] [Google Scholar]
- 42.Sejean K, Calmus S, Durand-Zaleski I, et al. Surgery versus medical follow-up in patients with asymptomatic primary hyperparathyroidism: a decision analysis. Eur J Endocrinol. 2005;153:915–27. doi: 10.1530/eje.1.02029. [DOI] [PubMed] [Google Scholar]
- 43.Baliski C, Nosyk B, Melck A, Bugis S, Rosenberg F. The cost-effectiveness of three strategies for the surgical treatment of symptomatic primary hyperparathyroidism. Ann Surg Oncol. 2008;15:2653–60. doi: 10.1245/s10434-008-0066-0. [DOI] [PubMed] [Google Scholar]
- 44.Ruda J, Stack BC, Jr, Hollenbeak CS. The cost-effectiveness of sestamibi scanning compared to bilateral neck exploration for the treatment of primary hyperparathyroidism. Otolaryngol Clin North Am. 2004;37:855–70. x–xi. doi: 10.1016/j.otc.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 45.Ruda JM, Stack BC, Jr, Hollenbeak CS. The cost-effectiveness of additional preoperative ultrasonography or sestamibi-SPECT in patients with primary hyperparathyroidism and negative findings on sestamibi scans. Arch Otolaryngol Head Neck Surg. 2006;132:46–53. doi: 10.1001/archotol.132.1.46. [DOI] [PubMed] [Google Scholar]
- 46.Wang TS, Cheung K, Farrokhyar F, Roman SA, Sosa JA. Would scan, but which scan? A cost-utility analysis to optimize preoperative imaging for primary hyperparathyroidism. Surgery. 2011;150:1286–94. doi: 10.1016/j.surg.2011.09.016. [DOI] [PubMed] [Google Scholar]


