Abstract
The sense of taste is stimulated when nutrients or other chemical compounds activate specialized receptor cells within the oral cavity. Taste helps us decide what to eat and influences how efficiently we digest these foods. Human taste abilities have been shaped, in large part, by the ecological niches our evolutionary ancestors occupied and by the nutrients they sought. Early hominoids sought nutrition within a closed tropical forest environment, probably eating mostly fruit and leaves, and early hominids left this environment for the savannah and greatly expanded their dietary repertoire. They would have used their sense of taste to identify nutritious food items. The risks of making poor food selections when foraging not only entail wasted energy and metabolic harm from eating foods of low nutrient and energy content, but also the harmful and potentially lethal ingestion of toxins. The learned consequences of ingested foods may subsequently guide our future food choices. The evolved taste abilities of humans are still useful for the one billion humans living with very low food security by helping them identify nutrients. But for those who have easy access to tasty, energy-dense foods our sensitivities for sugary, salty and fatty foods have also helped cause over nutrition-related diseases, such as obesity and diabetes.
Introduction
Taste is a sensory modality involving the oral perception of food-derived chemicals that stimulate receptor cells within taste buds. Taste principally serves two functions: it enables the evaluation of foods for toxicity and nutrients while helping us decide what to ingest and it prepares the body to metabolize foods once they have been ingested. Taste percepts are elicited by molecules that stimulate the taste buds in epithelia of the oral cavity and pharynx (back of the throat) [1] (Box 1). Moreover, taste drives a primal sense of ‘acceptable’ or ‘unacceptable’ for what is sampled. Taste combines with smell and tactile sensations to form flavors, which allows us to identify and recognize food items as familiar or novel. If familiar, we can anticipate the metabolic consequences of ingesting the food. If novel, we can use these sensory cues to learn about the physiological outcomes of ingestion. If the outcome is positive, taste will signal pleasure and reward — both directly from the pleasurable quality of the taste itself, as well as from associated metabolic consequences. Some animals also use taste to understand social chemical cues, but there is no evidence presently that it plays this role for humans (Box 2).
Box 1. Glossary.
Perception: the conscious awareness of input from the senses that give rise to experience.
Percept: the conscious experience of an event or stimulation; something that is perceived.
Modality: a particular sensory channel or mode by which something is experienced. Examples include: vision, hearing, smell, taste, touch.
Quality: an attribute of a percept that makes it unlike other sensations. Examples include: bitter, green, minty, sour, purple, hot, C#, sweet, floral, red, etc.
Taste: the perceptual experience of nutrients and other chemicals within the oro-pharynx via receptor cells within taste buds that ultimately cause taste percepts.
Flavor: the unified perceptual experience or ‘Gestalt’ of a food that arises from the integrated sensory signals of several sensory modalities, such as taste, olfaction, oral somatosensation (tactile, temperature, and texture) and oral nociception (pain).
Macronutrient: a metabolically active substance that needs to be ingested in large amounts to sustain growth and health of animals, mainly proteins, fats, and carbohydrates.
Micronutrient: a metabolically active compound or mineral that needs to be ingested in small amounts to sustain growth and health of animals, such as sodium, potassium, iodine, and various vitamins.
Box 2. Taste as a social sense.
In invertebrates, at least, taste has a social function. For example, Drosophila males use taste to differentiate between females and males, as well as to recognize mating status and activities of individual females [102,103]. Whether taste plays a social communication role for vertebrates remains to be determined. For many vertebrates, physical social contact, including licking of social non-volatile chemicals from a conspecific animal’s genitals, urine, sweat, or saliva, serves to help deliver compounds to the vomeronasal organ, a specialized chemosensory pit in the palate or nasal septum of many vertebrate species that responds to conspecific social communication compounds [104]. Whereas signals from the vomeronasal organ are not thought to be part of the gustatory perceptual world, the contact of these social compounds with the tongue, and hence taste buds, provides an opportunity for taste sensations to participate in social communication for vertebrates. Whether ‘gustatory’ social evaluations occur during human interactions, such as kissing, remains to be determined [105].
Taste-stimuli are typically released when food is chewed, dissolved into saliva and pre-digested by oral enzymes, such as amylase, lipase, and proteases [2]. Humans, and possibly many other omnivores, perceive nutrients and toxins qualitatively as sweet, salty, sour, savory, and bitter tasting [1]. Simple carbohydrates are experienced as sweet, the amino acids glutamate, aspartate and selected ribonucleic acids are experienced as savory (or umami), sodium salts, and salts of a few other cations, are experienced as salty, acids are experienced as sour, and many toxic compounds are experienced as bitter. The set of compounds that elicits bitter taste is by far the largest and most structurally diverse, and, consequently, humans possess about 25 functional bitter taste receptor genes (T2Rs). In addition, a variety of other nutrient taste qualities have been suggested, including specific taste percepts from water, starch, malto-dextrins, calcium, and fatty acids [3]. There is, however, presently little agreement on how humans perceive these chemicals and, consequently, on whether we would describe our oral experiences with them as unique tastes.
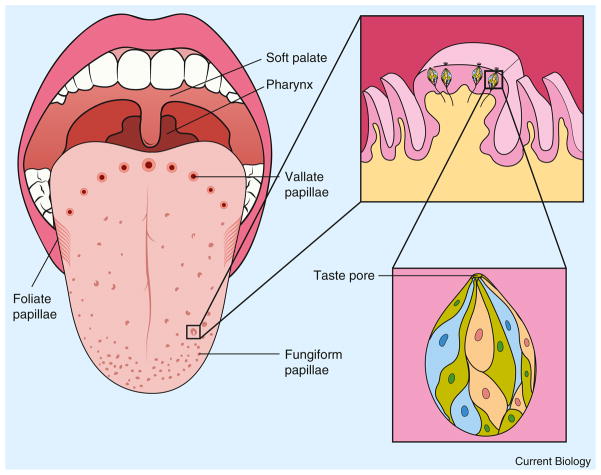
Humans taste with the edges and dorsal surface of the tongue, soft palate (the roof of the mouth toward the back of the oral cavity), and pharynx (Figure 1) [4]. These tissues comprise the gustatory epithelia. We do not taste with our lips, the underside of our tongue, our hard palate (behind our upper incisors), or the inside of our cheeks, although young children may have taste buds in more areas of the oral cavity than do adults [5]. The sensory organ within these epithelia is the taste bud — a microscopic rosette shaped cluster of approximately 80–100 receptor cells, in which chemicals are detected by transmembrane receptors (Figure 1) [4]. The human taste receptors have not all been confirmed in vivo except for selected toxin/bitter and a glutamate/umami receptor. Nevertheless, for many stimuli there are strong hypotheses of the identity of human taste receptors based on mouse and fly research. The principal receptor hypothesized to transduce human sweet stimuli is T1R2/T1R3, for umami stimuli it is T1R1/T1R3 (although mGluR1, mGluR4 and NMDA have been implicated), and for bitter taste stimuli it is the family of T2Rs. For salty stimuli there is growing evidence that the epithelial sodium channel (ENaC), in part, transduces salty taste, and for sour taste stimuli acid sensing ion channels (ASICs) and possibly other proton detectors are involved. Whereas it was once hypothesized that these receptors should be expressed in particular zones according to presumed taste quality regions of the mouth, we now believe that the receptor expression zones are heavily overlapping in most regions of the mouth.
Figure 1. Taste papillae and taste buds of the human tongue.
The human tongue contains three types of taste papillae. Vallate and foliate papillae reside on the middle and sides of the posterior 1/3 of the tongue, respectively, and contain hundreds of taste buds collectively. Circumvallate papillae comprise an arc of small ring-like structures (tiny towers surrounded by motes) in posterior tongue. Foliate papillae are slits (leaves) in the side of posterior tongue and can appear like gills in the tongue. Fungiform papillae look like small bumps or mushrooms and are scattered in the anterior 2/3 of the tongue, each harboring 0–15 taste buds. Taste buds are also located in the soft palate (non-bony palate in front of the uvula) and pharynx (back of the throat) but are in the flat epithelium, rather than in papillae in these locations. The first inset depicts the microscopic taste buds residing within the epithelium (outer layer) of a fungiform papilla. The small structures surrounding the fungiform papilla are called filiform papillae, which do not contain taste buds, and serve to make the surface of the tongue rough and help detect food textures. The second inset depicts a single rosette-shaped taste bud from within this fungiform papilla that contains dozens of taste receptor cells and contacts taste stimuli within the oral cavity via a small epithelial hole called a taste pore.
The taste bud serves as the first stage of gustatory signal processing and there are many ways in which cells within a bud communicate with one another, including electric coupling via gap junctions and cell to cell chemical communication via glutamate, serotonin, and ATP among other possible transmitters [6]. Taste buds reside within small bumps or folds on the tongue, called ‘papillae’, in addition to the smooth epithelia of the soft palate and pharynx [1] (Figure 1). Taste receptor cells within the buds are electrically active epithelial cells that can depolarize and release neurotransmitters. Whereas these taste receptor cells are not neurons themselves, they do communicate with nearby neurons via synaptic transmission and intercellular communication using ATP and other neurochemicals [6,7]. Taste receptor cells are continuously replaced in the bud every 9 to 15 days, to compensate for mechanical, thermal, or toxin-induced damage to the gustatory epithelia [8]. Moreover, the entire taste bud and the taste organ, the gustatory epithelium, can be removed or destroyed and will fully regenerate [9], making one of the very few organs in humans capable of total regeneration [10]. The taste system is also highly resistant to senescence and damage [11,12]. It is arguably the most durable and well-defended of all of our sensory systems, as indicated by the observation that humans who truly have no taste are exceedingly rare.
From the oral cavity, taste signals are transmitted to the brain stem along cranial nerves VII (Facial), IX (Glossopharyngeal), and X (Vagus), where there is a topographical representation of the oral cavity within the first nuclear relay, the solitary tract nucleus, in which brainstem reflexes of acceptance and rejection are controlled [13]. Strong sweet tastes are accepted and strong bitter tastes are rejected, even in decerebrate animals and anencephalic humans [14,15]. As afferent taste signals ascend the brain from caudal to rostral, the information flow is split between the ventral forebrain and more dorsal thalamo-cortical regions where primary and secondary gustatory cortices (opercular, insular, orbitofrontal) give rise to conscious taste sensation [1]. The ventral pathways are involved in autonomic and visceral functions, affective and emotional processing, and memory and learning [16,17]. The dorsal pathways involve multiple secondary and tertiary cortices that process taste qualities, attention, reward, valence, multi-modal sensory integration, higher cognitive functions and decision making [18,19]. Ultimately, the informational content and values of the ventral and the dorsal pathways are integrated [20].
This review uses an evolutionary perspective to address the questions: ‘What are the functions of human taste?’, ‘Why do we have the particular set of taste qualities that we perceive?’, and ‘How does taste guide humans to ingest foods?’. As taste is an essential component of all food flavors, the role of multi-modal sensory integration to form flavors is also considered. Exploring the ancestral context for taste is useful to understand how modern humans use taste to live and feed today. Those who live in an environment of very low food security forage using taste to identify nutritious foods to eat. While those who live in an environment of abundant, palatable foods are guided by taste to over consume calorically dense foods, which too often results in diabetes and obesity.
The Importance of Taste in Omnivores
Taste is an especially important sense for omnivorous species given that the potential range of foods, their variation in nutrient content, and the hazards of accidental toxin ingestion increase with the variety and complexity of the feeding strategy. In contrast, species with highly specialized diets, such as the leaf-eating koalas (eating mostly eucalyptus) and giant pandas (eating mostly bamboo), have fewer nutritional decisions to make and face fewer hazards from toxins than do omnivores. Consequently, their gustatory systems appear to have dwindled. Apparently due to the great reduction in their selected food types relative to other bears, giant pandas have lost (pseudogenized) an amino acid taste receptor gene TAS1R1 [21]. By contrast, exclusively carnivorous mammals have retained the amino acid taste receptor, but have lost many other taste receptors from their genome. For example, all cats (felidae) have lost their canonical sweet taste receptor gene, TAS1R2 [22]. Although we cannot know for certain why these genes have been lost in these lineages, probably the receptors were no longer advantageous or necessary for survival. Aquatic carnivorous mammals, such as sea lions, have even more taste receptor pseudogenes and appear to have lost a large number of taste receptors, perhaps because most of their prey are swallowed whole and would not be tasted [23]. In this case, the identification of swimming fish via visual recognition and the body and kinetic senses of pursuing prey may have replaced taste [23]. Therefore, many species appear to have lost some or all of their taste receptors because they do not need the specific nutrient detectors.
Assuming that humans have retained a diversity of functional taste receptors because of the need to taste, an implicit question is — ‘What important functions does taste serve for humans?’ [Note that the questions ‘Of what utility is taste perception?’ and ‘Of what utility are taste receptors?’ are different questions in light of the discovery that taste receptors are expressed in tissues throughout the body and serve multiple functions (Box 3).] First, taste sensory inputs influence our thinking, deciding, and behavior toward sampled foods, both consciously and unconsciously, to guide ingestion [24]. Second, taste inputs influence our physiology and the metabolic processing and signaling of nutrients and toxins once ingested [25]. The former is involved with determining what foods enter our body and the latter with how these nutrients are handled once they enter it. Together these two functions help create our food preferences and feeding habits that sustain and maintain us throughout life and enable our species to reproduce.
Box 3. Taste transduction in non-gustatory gastrointestinal tract.
It is difficult for the human body to determine or estimate macronutrient levels in a food without directly sensing what is ingested. If unfamiliar foods are ingested, there is another level beyond the mouth at which anticipatory metabolic responses can occur. Nutrients will be sensed in the gastrointestinal tract by taste receptors, among several other types of nutrient detectors [106,107]. Although taste receptors in the stomach and intestine do not trigger taste sensations, they can elicit anticipatory metabolic responses. Consequently, ingested macronutrients are monitored throughout the gastrointentinal tract, beginning with the gustatory system, but also in the stomach, and the small and the large intestine. This helps prepare for incoming nutrients and to regulate metabolic responses accordingly. Taste receptors in the intestine may also play a role in monitoring the microbiome status. Probiotic bacteria aid in the digestion of many foods and in the immune defense against infectious bacteria. Taste receptors in the gut may facilitate these processes by monitoring metabolic activities of ‘bad’ bacteria and bacterial signals in the gut [108].
Conscious Taste Perception Guides Ingestion
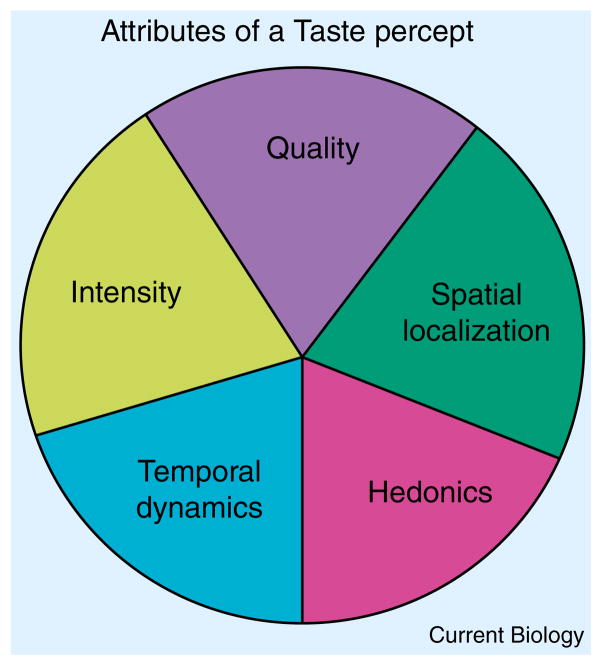
The conscious understanding of ‘taste’ comes from the everyday experiences we have with foods and their taste compounds. Tastes are multi-attribute sensations. Most people appreciate taste percepts as having the traits of quality (salt, sweet, bitter, sour, savory, and possibly others) and intensity, yet we are also aware that tastes can have location and timing cues [26–29], such as when bitter tastes linger too long in the back of our throat (Figure 2). If we combine stimuli representative of multiple taste qualities into a cocktail containing sucrose, monosodium glutamate, sodium chloride, citric acid, and quinine sulfate, subjects experience this cocktail as simultaneously sweet, savory, salty, sour, and bitter [30]. This illustrates that the taste system is able to analyze the individual components of a complex mixture, consistent with the idea that it analyzes foods for nutritional content. This does not, however, preclude the components of a taste mixture interacting with each other to alter perception. Certain stimulus combinations interact in the taste buds and receptor cells, such as salts and toxins that show inhibition [31,32], and many combinations of strong stimuli can interact cognitively to suppress or enhance one another [33]. These taste interactions are functional in foods. Gorillas, for example, have been found to tolerate more bitter plant tannins if the sugar content also present is high [34]. Taste–taste interactions are numerous and have been reviewed elsewhere [35].
Figure 2. The attributes of a taste percept.
Each taste percept may be subdivided into multiple taste attributes that are integrated to form a single taste sensation.
Taste sensations naturally co-occur with other sensory modalities. We feel the solid and liquid foods that deliver taste compounds, which are dissolved into and diluted by saliva, so they can enter the ‘taste pores’ at the apical tip of each taste bud (Figure 1). Taste sensations are integrated with food temperatures, tactile textures, pain sensations from the mouth, and with volatile compounds that are detected by the olfactory epithelium within the nasal cavity. Auditory inputs from foods (fizzes, crunches, and conductions of food sounds to the ear via the jaw while chewing) are also incorporated into the flavor. This multimodal integration leads to a unified flavor ‘gestalt’ [36]. Hence, oral stimulation by food is perhaps the most richly multimodal sensory experience we can have [20]. In addition, the proprioceptive inputs from teeth and jaw as we bite down on foods tell us if the food is dry, flaky, chewy, creamy, brittle, crunchy, etc. [37]. The principal brain regions devoted to these multimodal flavor integrations are insular and orbitofrontal cortex and also amygdala and entorhinal cortex [18,20]. More traditional multisensory regions such as the intraparietal sulcus and superior frontal cortex are also involved with integrating these different sensory signals [38,39]. These combined sensations enable a complex evaluation of the food to facilitate decisions to ingest or reject the food.
The multiple sensory attributes that comprise taste (quality, intensity, oral location, temporal dynamics) are also integrated with another dimension of taste, its affect or palatability. Many tastes have intrinsic affective properties (Figure 2). Moderately strong sweet sensations are innately attractive and accepted by newborns and adults alike, whereas moderate bitter tastes are innately aversive and rejected by newborns [40,41]. The palatability of complex flavors, apart from their nutrient associations, is largely based upon the taste components of flavor, which form the foundation of the experience. Sweet taste lifts most flavors to higher acceptance and strong bitter taste does the opposite [42,43]. The acceptance and rejection of tastes and flavors is mainly governed by brainstem reflexes that drive rhythmic tongue movements accompanied by swallowing for sweet tastes and gapes and shudders (involving rapid head shakes and rhythmic arm flails) for intense bitter tastes [15,40]. Humans also prefer weak salty taste, as well as umami taste, but the latter usually only in the context of food, not when presented as pure MSG [44]. Thus, desired nutrients at appropriate levels can elicit pleasant tastes and harmful levels of toxins elicit very unpleasant tastes. We tend to tolerate low levels of bitterness in foods more readily as they frequently co-occur with nutrients in plants. Moreover, many low level bitter compounds in plants are beneficial due to medicinal properties.
However, learning can reverse these innate responses. The palatability of a taste, flavor, or food is the most labile of the chemosensory attributes. Taste quality cannot change easily with experience; sugar should always taste sweet. But it is relatively easy to make the taste of sugar change from palatable to unpalatable [45]. For example, experimental pairing of sugar taste with upper gastric malaise and nausea can render sugar unpalatable [46], whereas the pairing of bitter tasting quinine with the taste of sugar can render quinine taste palatable [47]. Indeed, many foods contain varying levels of bitter tasting toxins as phytochemicals [43,48]. These cannot be physically separated from the nutrients and so must be ingested regardless of how we perceive them. We also learn to enjoy the taste of mildly bitter foods, if paired with the positive metabolic and pharmacological outcomes, as in the case of chocolate, coffee, or wine.
Tastes can also be positively or negatively palatable depending upon their context in food flavors. A low level of bitterness is desirable, even necessary, in beer, but is less acceptable in milk. Similarly, we find sourness desirable in the context of fruit flavors, but it is unpleasant in coffee flavors. These matches between tastes and flavors are called flavor congruencies [49]. Most taste-odor flavor pairings are learned associatively from food experiences. These associations may be sufficiently strong to conjure the sensory imagery of the stimulus partner in its absence, such as occurs when we refer to ‘sweet’ odors [50]. We may even experience the illusion of sweet taste in the absence of sweetener, such as when an odor, previously paired with a sweetener, elicits tastes [51]. These associations are not only among oral and upper airway sensations but can also be formed with post-ingestive reward and punishment from nutrients, calories, and toxins. Tastes and flavors associated with calories and nutrients are preferred and can become more pleasurable, whereas poisoning and illness will cause flavors to be aversive. The reward and punishment that is triggered by taste activation and associated with post-ingestive outcomes is not necessarily a conscious process. We are aware when brain stem reflexes of acceptance and rejection occur because we are aware of our responses during the reflex, but these reflexes occur independently of the forebrain and do not require any higher processing to occur [14,15].
Unconscious Taste Processes Guide Metabolism
The taste buds also serve as endocrine organs and secrete regulatory hormones in response to nutrient stimulation, including glucagon like peptide-1 (GLP-1) and glucagon, among other endocrine peptides [52]. The secretory responses of digestive hormones by peripheral tissues would signal to digestive organs, such as the pancreas, that nutrients are being ingested and prepare metabolic systems to respond, such as insulin secretion to control elevated blood glucose. These anticipatory processes are essential to optimal metabolism during and after feeding.
Our bodies strive to maintain homeostasis of blood nutrient and metabolite levels. From this perspective, a large meal is an assault on nutritional homeostasis [53]. If our bodies cannot anticipate a large meal, the rise in insulin-dependent macronutrients will be large and an excessive pancreatic release of insulin will be required to return blood sugar and amino acid levels to normal. Both the elevated levels of plasma nutrients and insulin, if repeated frequently, can lead to metabolic syndrome and insulin resistance. If, however, a small amount of insulin is secreted in anticipation of incoming nutrients, labeled pre-absorptive insulin release (PIR) or cephalic phase insulin release, then the system is primed to remove nutrients from the blood immediately upon their arrival. A key factor in anticipating incoming nutrients, particularly sugars, is the taste receptor responses. It is well established that humans show a PIR to oral glucose, activated presumably via a carbohydrate taste receptor, such as T1R2/T1R3 [25,54,55]. This results in a smaller deviation of blood nutrient levels with less overall insulin secretion. Although a PIR comprises a tiny portion of the overall insulin secreted, it is responsible for decreasing blood sugar during a meal by 50% [54]. When the PIR is experimentally blocked in humans during feeding, dysregulation of blood sugar (dysglycemia) ensues and high levels of plasma insulin are attained [55]. A blunted PIR is associated with obesity, exacerbating if not causing metabolic problems [56]. Much less is known about whether similar responses occur for nutrients other than sugars, but they likely do occur in the intestine (Box 3).
Anticipatory responses to ingested toxins minimize poisoning, illness, and death. Oral toxins trigger responses to prevent them from being ingested or to minimize poisoning, including containment in the upper gastrointestinal tract and vomiting. As most naturally occurring bitter tasting stimuli are toxins at some concentration, the body responds to strong bitter tastes as if toxins are about to be ingested [57]. Psychological and physiological anticipatory responses follow. First, those who are susceptible experience nausea, the feeling of sickness and gastrointestinal malaise. This, like pain, is a psychological response to punish our behavior and to protect us. Second, the normal activity of stomach contraction shifts to a more chaotic pattern that prevents the stomach from normal churning, to contain any ingested toxins in the stomach, and to prepare to vomit [57]. Whether the activation of detoxification enzymes is triggered by strong bitter tastes has not been explored, but it is a reasonable hypothesis.
Important to this idea of anticipation of poisoning is that responses are restricted to strong bitter tastes, but not weak ones. Normal foraging behavior requires that we ingest weak to moderate bitter tastes in the course of feeding. Indeed, most naturally occurring foods we eat contain toxins [43]. These do not pose a problem for our physiology, as humans can ingest and detoxify small amounts of toxins. But we usually do not tolerate strong bitter tastes. Foods that contain very small amounts of several distinct bitter tasting toxins tend to grow linearly in bitter taste intensity with the number of toxins present [33]. This suggests that we maintain more-or-less accurate accounting of the total toxin load of a food, which is logical given that we must procure nutrients embedded within foods with differing low levels of multiple toxins.
Evolution of Human Taste Preferences and Aversions
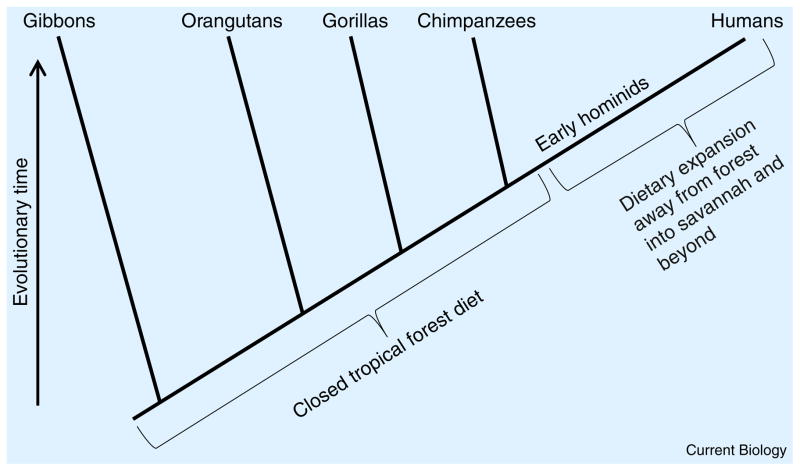
Humans last shared a common ancestor with other great apes approximately 7 to 8 million years ago [58]. If the wild feeding patterns of extant great apes reflect the diet of our last common ancestor, then this species was an omnivore whose diet was rooted in tropical fruits, with leaves and insects [59]. Our closest relatives, the chimpanzees (Pan troglodytes) [60], derive the large majority of their calories from fruit [61]. A small part of their diet is also animal-based, ranging from monkeys to insects. Early hominids drifted away from the forest diet of the apes to more varied, open-terrain diets (Figure 3). Between 4.4 and 2.3 million years ago, the dietary habits and nutritional versatility of hominids expanded dramatically [62]. Despite this dietary expansion we retained our ancestral fruit preference and fondness for sugars and acids, which we share with the other great apes. The principal attraction to fruit nutritionally is the sugars they contain, which are innately satisfying, and the vitamin C, which is necessary for hominoids to sustain life.
Figure 3. Evolutionary dendrogram of apehominid evolution.
The vertical axis represents time and ends in the present with gibbons, orangutans, gorillas, chimpanzees, and humans contemporaneously existing. The vertical distance from the top to a branch point along the dendrogram is the time to coalescence. Where ape lineages join represents when the two species shared a common ancestor. All modern apes live in closed tropical forests, and obtain all of their nutrition there. Their last common ancestor to the apes would presumably have lived in a similar environment. During early hominid evolution, human ancestors left the forest for the savannah and other ecosystems and their dietary repertoire greatly expanded. Eventually their diet is hypothesized to have included more meats, fermented foods, and, most recently, large amounts of starch due to the advent of agriculture.
Why humans are able to taste acids and even prefer sourness has been debated. Sour stimuli are not of great nutritional value, with the exception of vitamin C. This is an important exception, however, as, unlike most mammals, monkeys and apes cannot synthesize vitamin C due to the loss of a functional gluconolactone oxidase gene [63]. The common ancestor of the anthropoids that lost this enzyme must have had sufficiently high ascorbic acid intake from fruits and other plants that the enzyme became dispensable. Presumably, sour taste was necessary as a guide to vitamin C rich fruits. The mixture of acids with sugars also can enable the identification of fruit ripeness via sweet and sour taste combinations. From this perspective, acids were not stimuli which we evolved to respond to alone, but rather we experienced them in the context of fruit sugars. Thus, sweet and sour tastes are perceived as synergistic in fruit flavors [64]. In addition, acids and sour taste are markers of fermentation, which humans around the globe clearly seek and ingest.
The wild great apes derive the majority of their daily protein intake from forest plants, especially young leaves [59]. But early hominids as well as modern humans tended to eat somewhat more digestible forms of proteins, such as meats [65,66]. Umami taste is not obvious in fresh meats, but aged or cooked meats have much stronger umami taste. Consequently, chimpanzees do not appear to have a taste subsystem devoted to processing glutamate or ribonucleotides taste, although they can taste these stimuli [67]. Hydrolyzed protein has a characteristic umami taste carried predominantly by glutamate and ribonucleotides. Humans have developed a preference for glutamate, ribonucleotides and umami taste, perhaps as markers of easily digested protein in slightly aged or cooked meats. Note that many fossil records indicate that cooking predates the origin of modern humans. In addition, humans around the world enjoy a wide range of fermented plants and animal products. Our strong interest in the taste of free amino acids and ribonucleotides may arise from an inclination to ingest fermented foods, including slightly aged and/or cooked meat. This category of food would have multiple advantages to the survival of our species. Fermentation not only provides more ready access to macro- and micronutrients, but it also provides access to probiotic bacteria, which help maintain overall nutritional health, prevent diseases, and fight gastrointestinal infections [68,69]. Although the savory taste of glutamate and ribonucleotides has been hypothesized to be a marker of protein, many high-protein foods are not particularly savory or umami tasting when fresh. It is the fermentation or aging of these foods that releases glutamate and savory taste from protein. Thus, our attraction to amino acids, especially glutamate, and savory taste may be born of a desire for fermented foods and the advantages of the improved nutrition and probiotic bacteria for our species.
The advent of agriculture eight to ten thousand years ago significantly changed our diet by greatly increasing the role that grains and starch played. Starch, a complex glucose polymer of varying forms — principally amylose and amylopectin — is digested by the pancreatic enzyme alpha-amylase, which breaks starch down into intermediate glucose oligomers called maltooligosaccharides (MOS) and isomaltooligosaccharides (IMOS). These in turn are further cleaved into maltose (a glucose disaccharide). Maltase ultimately cleaves maltose into glucose, which then passes into the blood. All mammals produce pancreatic alpha-amylase. But a few mammals, great apes and some commensal rodents (rats, mice, voles), also produce amylase in their salivary glands. In the common ancestor of apes, a retroviral-like insertion directed a copy of the pancreatic amylase gene to be transcribed in the salivary glands [70]. Rodents evolved amylase expression in their salivary glands via a different mechanism. Thus, ‘predigestion’ of starch appears to be of significant value to a wide range of omnivorous animals [71].
Starch that is cleaved by salivary amylase within the oral cavity will form MOS, IMOS, and maltose. Rodents appear to perceive a distinct taste quality from MOS that is distinguished from the taste of sugars [72,73]. Knockout mice lacking functional genes for both components of the canonical saccharide receptor, T1R2/T1R3, show no electrophysiological or behavioral responses to glucose, fructose, or sucrose, but respond normally to MOS [74]. Maltose elicits a taste in rats and mice that possesses qualities of both sucrose and MOS [74]. And rats can be trained to discriminate the taste of sucrose from the taste of maltose [75]. Whereas humans do not have a strong conscious taste perception from MOS, they can discriminate the taste of maltose from that of glucose or fructose [76]. Thus, humans and rodents both seem to perceive maltose as possessing a gustatory quality distinct from glucose and fructose. Why rodents have a strong, distinct taste for larger glucose polymers, such as MOS, and humans do not, is unclear. Yet, oral MOS rinses increase exercise performance in humans relative to oral non-nutritive sweeteners, despite not being consciously tasted [77]. And oral MOS activates brain reward centers in orbitofrontal cortex and striatum similar to oral glucose, unlike non-nutritive sweeteners [77]. Thus, what appears relatively tasteless to us consciously is activating brain areas similar to orally presented glucose and is able to modify our performance.
Humans are unique among mammals in that we have a large-scale copy number polymorphism of the salivary amylase gene, AMY1. Our early history with starchy tubers may have triggered this divergence from the other apes 100 to 200,000 years ago [78]. The recent advances of agriculture appear to have increased the number of copies in traditional agricultural societies [78]. The more copies of salivary amylase one carries in the genome, the more amylase is produced in one’s saliva. Quantities and efficiencies of salivary amylase are sufficiently high that a cooked starch food such as a thick pudding can be transformed from a semi-solid to a liquid within seconds in the oral cavity [79]. Although salivary amylase transforms starch into MOS, IMOS, and maltose, the concentrations of sugar generated are not sufficient to stimulate sweet taste [80]. But they may be generating sufficient quantities of MOS, IMOS, and maltose to activate brain areas of taste and reward. Furthermore, people with high salivary amylase levels respond to the oral presentation of starch physiologically. People who possess high salivary amylase levels secrete insulin preabsorptively in response to oral starch, which helps reduce their glycemic response when ingesting starch, compared to people who produce relatively little salivary amylase [80]. Thus, it appears that a taste receptor is present in the human mouth that responds to glucose polymers but does not necessarily give rise to a conscious taste. This receptor may be stimulated by the breakdown products of oral starch in the presence of high levels of salivary amylase.
Humans seek out salt in foods. We find the taste of moderate salt concentrations, near isotonicity (150 mM) highly attractive, as do other omnivores [81]. High concentrations of salt, however, are aversive to us, as they challenge the osmotic balance of body fluids. Salt is added to food globally, and humans from many different cultures ingest roughly the same amount of salt daily. The similarity in salt intake cross-culturally suggests that humans consume this amount of salt because of biologically determined reasons [82]. While a carnivorous animal will ingest salt with every meal, a herbivorous animal can easily become sodium depleted [83]. This generates a salt appetite in herbivores, who will then seek out natural ‘salt licks’. An omnivore’s exposure to dietary salt would logically be between that of carnivores and herbivores. Humans, however, lose salt through sweating, which may explain why humans prefer a higher salt intake than other omnivores.
Our unique history has shaped us to carry taste preferences for sugars and acids that provide energy and vitamin C, as well as newly developed preferences for higher intakes of salt and starch. In addition, we have developed a taste for umami tasting fermented foods, which have the benefit of introducing more digestible nutrients and probiotic bacteria to our diet.
Taste and Human Reproduction
Taste also plays an important role during human development as it can ensure proper growth and development through acquired nutrients, as well as the avoidance of toxins harmful during development. This is true both of women during pregnancy as well as in the young child. Consequently, pregnancy alters taste responses and feeding patterns in women. Chief among these changes are increased sensitivity to bitter stimuli and feelings of nausea in response to bitter foods [84–86]. One hypothesis claims this is a protective response at a time when major fetal organs are first forming and are highly sensitive to low levels of toxins [84–86]. Since many foods we eat contain low levels of toxins [48], an increase in sensitivity of the brain’s nausea processing regions, such as area postrema, to toxins would make usually innocuous foods potentially nauseating. Maternal vomiting may thus benefit the fetus during this period. In support of this idea, several studies have shown that women who experience nausea during their first trimester experience fewer miscarriages [87–90] and tend to have larger and healthier babies [91–93]. Bitter taste appears linked to this system. At the time when fetal toxin sensitivity is greatest, women’s sensitivity to bitter compounds is greater, perceived bitterness intensity is higher [94], and more foods taste bitter to them relative to before pregnancy [85]. Pregnant women generally find a greater variety of flavors more aversive than do non-pregnant women [84,85]. Finally, there is a relationship between nausea and bitter taste among pregnant women. Women who are nauseous during pregnancy are more sensitive to bitter stimuli [86].
Children seem to continue the maternal-fetal nutritional strategy of avoiding toxins. Children are notoriously averse to eating vegetables that are dense in micronutrients and phytonutrients, but are low in calories and macronutrients. Vegetables children tend to prefer are often high in sugar and free glutamate and, thus, taste sweet and savory. Infants come into the world drinking mother’s milk which is very high in both free sugars and free glutamate, so perhaps they imprint upon these tastes [95] or simply learn to like them [96]. Infants naturally prefer sugars and glutamate in soup, but they do not prefer free glutamate in pure water [44]. There may be important nutritional information conveyed to infants by the tastes of foods. Protein malnourished children and malnourished infants find soup fortified with added glutamate more appealing than unfortified soup and even more appealing than soup fortified with added sugar [97,98]. Perhaps the association of umami taste with amino acid ingestion has enabled protein-energy malnourished children to understand implicitly the link between nutrient deficit and the taste of the target nutrient. Thus, the overall interaction of taste with development and hormonal state are beneficial for survival by altering feeding strategies that minimize the risk of toxicosis while ensuring proper macronutrient intake.
Associating Flavors with Metabolic Consequences
For omnivorous species, it is essential that many different foods are sampled, and their post-ingestive consequences, the nutritional rewards or punishments, are associated with their sensory properties. These associations are what ultimately shape our food likes and dislikes and guide our future foraging decisions. In this way, taste serves as a marker, especially in the context of complex flavors, for the nutrient and toxic load of foods. Rats can learn very rapid associations between tastes and flavors and metabolic and physiological consequences that result in an utter rejection of a food if associated with nausea and upper gastrointestinal malaise or a strong preference for it if paired with MOS in the stomach [99]. These associations occur after only a single trial and are salient enough to resist extinction (ignoring the association) even after multiple presentations of the stimulus with no metabolic or physiological consequences [100]. Learning about foods and metabolic consequences is necessary for omnivores to survive. In the evolution of our own species, these abilities would have become increasingly important as early hominids ventured into novel terrains and ecological niches. Moreover, it is these associations that enable the anticipatory physiological responses to ingested nutrients. Foods that are unfamiliar are less likely to induce anticipatory metabolic reflexes, and thus are less efficiently utilized.
All of these benefits of our highly evolved taste system are relevant and necessary to our species today. About 1 billion people are presently experiencing severe food insecurity. A lack of energy and protein and the loss of water and minerals from diarrhea are the top two causes of death in children worldwide. For these people, the taste evaluation, nutrient reward, and learning systems are vital for survival, if foods can be identified and ingested from the environment. Yet, at the same time, more and more countries face an obesity epidemic. But obesity and over nourishment are a modern problem and evolution would not necessarily have selected against such an epidemic. In the developed world obesity is caused, in part, by the creation of foods that are hyper-appealing — foods high in salt, glutamate, sugar and fat. These foods may be thought of as Tinbergian ‘super stimuli’, after the ethologist Niko Tinbergen who showed that by greatly enhancing the sensory properties of already attractive objects one can generate ‘super stimuli’, such as giant eggs on which birds would sit and not remove themselves. Developed societies face an overabundance of such gustatory super stimuli. This unfortunately may create a situation in which people will be obese but be malnourished from lack of micronutrients. That is, the variety of their diet and the intake of nutrient rich foods will be poor, but their intake of insulin-dependent macronutrients may be exceedingly high. It is possible that the deliberate overconsumption of these calorically dense foods is the body’s way of searching for nutrients, particularly protein, at the expense of becoming obese [101]. Since evolution has shaped our taste system to guide us to seek and eat energy dense foods, we may need to impose cognitive heuristics to focus on what is nutritious for humans today, which may be similar to some of our species’ earlier diets.
Conclusion
Our taste system plays many roles in ensuring survival. As an omnivorous, foraging species, taste helps us identify safe and nutritious foods in a complex environment. Different tastes within foods interact with each other, as well as with other sensory stimuli, to form flavors and provide an overall profile of a food that helps us determine whether to ingest. These sensory clues also signal to the metabolic organs to prepare to digest the food that is entering our bodies. Our particular history, beginning as ape-like creatures millions of years ago living in tropical forests and ending with a global dispersion of humans to every known climate and environment, has resulted in our specific taste abilities and preferences for sweet, sour, salty, fatty, umami, and starchy foods. We also have an adaptive taste system that can modify its sensitivities with internal state in times of high nutritional need, and in times of high sensitivity to toxins, such as during pregnancy. Ultimately, all of these taste traits and properties are integrated and utilized during feeding, and the metabolic consequences of food form associations with the tastes and flavors of the food that guide future feeding decisions. But, in modern environments taste-driven decisions of what to eat must be checked, as our sense of taste may lead us to eat highly palatable foods that are high in calories but low in nutrients, an action that if repeated often will cause disease.
References
- 1.Breslin PA, Spector AC. Mammalian taste perception. Curr Biol. 2008;18:R148–R155. doi: 10.1016/j.cub.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 2.Pedersen AM, Bardow A, Jensen SB, Nauntofte B. Saliva and gastrointestinal functions of taste, mastication, swallowing and digestion. Oral Dis. 2002;8:117–129. doi: 10.1034/j.1601-0825.2002.02851.x. [DOI] [PubMed] [Google Scholar]
- 3.Mattes RD. Accumulating evidence supports a taste component for free fatty acids in humans. Physiol Behav. 2011;104:624–631. doi: 10.1016/j.physbeh.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breslin PA, Huang L. Human taste: peripheral anatomy, taste transduction, and coding. Adv Otorhinolaryngol. 2006;63:152–190. doi: 10.1159/000093760. [DOI] [PubMed] [Google Scholar]
- 5.Von Skramli E. Handbuch der Physiologie de niederen Sinne. Leipzig: G. Thieme; 1926. [Google Scholar]
- 6.Roper SD. Taste buds as peripheral chemosensory processors. Semin Cell Dev Biol. 2013;24:71–79. doi: 10.1016/j.semcdb.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310:1495–1499. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- 8.Yee KK, Li Y, Redding KM, Iwatsuki K, Margolskee RF, Jiang P. Lgr5-EGFP marks taste bud stem/progenitor cells in posterior tongue. Stem Cells. 2013 doi: 10.1002/stem.1338. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spielman AI, Pepino MY, Feldman R, Brand JG. Technique to collect fungiform (taste) papillae from human tongue. J Vis Exp. 2010:2201. doi: 10.3791/2201. pii. http://dx.doi.org/10.3791/2201. [DOI] [PMC free article] [PubMed]
- 10.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. J Hepatol. 2012;57:692–694. doi: 10.1016/j.jhep.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 11.Cowart BJ, Yokomukai Y, Beauchamp GK. Bitter taste in aging: compound-specific decline in sensitivity. Physiol Behav. 1994;56:1237–1241. doi: 10.1016/0031-9384(94)90371-9. [DOI] [PubMed] [Google Scholar]
- 12.Cowart BJ. Taste dysfunction: a practical guide for oral medicine. Oral Dis. 2011;17:2–6. doi: 10.1111/j.1601-0825.2010.01719.x. [DOI] [PubMed] [Google Scholar]
- 13.Topolovec JC, Gati JS, Menon RS, Shoemaker JK, Cechetto DF. Human cardiovascular and gustatory brainstem sites observed by functional magnetic resonance imaging. J Comp Neurol. 2004;471:446–461. doi: 10.1002/cne.20033. [DOI] [PubMed] [Google Scholar]
- 14.Steiner JE. The gustofacial response: observation on normal and anencephalic newborn infants. Symp Oral Sens Percept. 1973:254–278. [PubMed] [Google Scholar]
- 15.Grill HJ, Norgren R. Neurological tests and behavioral deficits in chronic thalamic and chronic decerebrate rats. Brain Res. 1978;143:299–312. doi: 10.1016/0006-8993(78)90570-x. [DOI] [PubMed] [Google Scholar]
- 16.Piette CE, Baez-Santiago MA, Reid EE, Katz DB, Moran A. Inactivation of basolateral amygdala specifically eliminates palatability-related information in cortical sensory responses. J Neurosci. 2012;32:9981–9991. doi: 10.1523/JNEUROSCI.0669-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tandon S, Simon SA, Nicolelis MA. Appetitive changes during salt deprivation are paralleled by widespread neuronal adaptations in nucleus accumbens, lateral hypothalamus, and central amygdala. J Neurophysiol. 2012;108:1089–1105. doi: 10.1152/jn.00236.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Veldhuizen MG, Albrecht J, Zelano C, Boesveldt S, Breslin P, Lundstrom JN. Identification of human gustatory cortex by activation likelihood estimation. Human Brain Mapping. 2011;32:2256–2266. doi: 10.1002/hbm.21188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zald DH. Orbitofrontal cortex contributions to food selection and decision making. Ann Behav Med. 2009;38(Suppl 1 ):S18–S24. doi: 10.1007/s12160-009-9117-4. [DOI] [PubMed] [Google Scholar]
- 20.Small DM. Flavor is in the brain. Physiol Behav. 2012;107:540–552. doi: 10.1016/j.physbeh.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 21.Zhao H, Yang JR, Xu H, Zhang J. Pseudogenization of the umami taste receptor gene Tas1r1 in the giant panda coincided with its dietary switch to bamboo. Mol Biol Evol. 2010;27:2669–2673. doi: 10.1093/molbev/msq153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, Li W, Wang H, Cao J, Maehashi K, Huang L, Bachmanov AA, Reed DR, Legrand-Defretin V, Beauchamp GK, et al. Pseudogenization of a sweet-receptor gene accounts for cats’ indifference toward sugar. PLoS Genet. 2005;1:27–35. doi: 10.1371/journal.pgen.0010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang P, Josue J, Li X, Glaser D, Li W, Brand JG, Margolskee RF, Reed DR, Beauchamp GK. Major taste loss in carnivorous mammals. Proc Natl Acad Sci USA. 2012;109:4956–4961. doi: 10.1073/pnas.1118360109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maccarthy-Leventhal EM. Post-radiation mouth blindness. Lancet. 1959;2:1138–1139. doi: 10.1016/s0140-6736(59)90117-5. [DOI] [PubMed] [Google Scholar]
- 25.Pavlov IP. The Work of the Digestive Glands. London: Charles Griffin and Co., Ltd; 1902. [Google Scholar]
- 26.Nakamura Y, Goto TK, Tokumori K, Yoshiura T, Kobayashi K, Honda H, Ninomiya Y, Yoshiura K. The temporal change in the cortical activations due to salty and sweet tastes in humans: fMRI and time-intensity sensory evaluation. Neuroreport. 2012;23:400–404. doi: 10.1097/WNR.0b013e32835271b7. [DOI] [PubMed] [Google Scholar]
- 27.Lugaz O, Pillias AM, Boireau-Ducept N, Faurion A. Time-intensity evaluation of acid taste in subjects with saliva high flow and low flow rates for acids of various chemical properties. Chem Senses. 2005;30:89–103. doi: 10.1093/chemse/bji004. [DOI] [PubMed] [Google Scholar]
- 28.Shikata H, McMahon DB, Breslin PA. Psychophysics of taste lateralization on anterior tongue. Percept Psychophys. 2000;62:684–694. doi: 10.3758/bf03206915. [DOI] [PubMed] [Google Scholar]
- 29.Delwiche JF, Lera MF, Breslin PA. Selective removal of a target stimulus localized by taste in humans. Chem Senses. 2000;25:181–187. doi: 10.1093/chemse/25.2.181. [DOI] [PubMed] [Google Scholar]
- 30.Stevens JC. Detection of tastes in mixture with other tastes: issues of masking and aging. Chem Senses. 1996;21:211–221. doi: 10.1093/chemse/21.2.211. [DOI] [PubMed] [Google Scholar]
- 31.Breslin PA, Beauchamp GK. Suppression of bitterness by sodium: variation among bitter taste stimuli. Chem Senses. 1995;20:609–623. doi: 10.1093/chemse/20.6.609. [DOI] [PubMed] [Google Scholar]
- 32.Breslin PA, Beauchamp GK. Salt enhances flavour by suppressing bitterness. Nature. 1997;387:563. doi: 10.1038/42388. [DOI] [PubMed] [Google Scholar]
- 33.Keast RS, Bournazel MM, Breslin PA. A psychophysical investigation of binary bitter-compound interactions. Chem Senses. 2003;28:301–313. doi: 10.1093/chemse/28.4.301. [DOI] [PubMed] [Google Scholar]
- 34.Remis MJ, Kerr ME. Taste Responses to Fructose and Tannic Acid Among Gorillas (Gorilla gorilla gorilla) Int J Primatol. 2002;23:251–261. [Google Scholar]
- 35.Breslin PAS. Interactions among salty, sour and bitter compounds. Trends Food Sci Tech. 1996;7:390–399. [Google Scholar]
- 36.Lim J, Green BG. Tactile interaction with taste localization: influence of gustatory quality and intensity. Chem Senses. 2008;33:137–143. doi: 10.1093/chemse/bjm070. [DOI] [PubMed] [Google Scholar]
- 37.Foster KD, Woda A, Peyron MA. Effect of texture of plastic and elastic model foods on the parameters of mastication. J Neurophysiol. 2006;95:3469–3479. doi: 10.1152/jn.01003.2005. [DOI] [PubMed] [Google Scholar]
- 38.Nakashita S, Saito DN, Kochiyama T, Honda M, Tanabe HC, Sadato N. Tactile-visual integration in the posterior parietal cortex: a functional magnetic resonance imaging study. Brain Res Bull. 2008;75:513–525. doi: 10.1016/j.brainresbull.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 39.Grefkes C, Fink GR. The functional organization of the intraparietal sulcus in humans and monkeys. J Anat. 2005;207:3–17. doi: 10.1111/j.1469-7580.2005.00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steiner JE, Glaser D, Hawilo ME, Berridge KC. Comparative expression of hedonic impact: affective reactions to taste by human infants and other primates. Neurosci Biobehav Rev. 2001;25:53–74. doi: 10.1016/s0149-7634(00)00051-8. [DOI] [PubMed] [Google Scholar]
- 41.Ganchrow JR, Steiner JE, Canetto S. Behavioral displays to gustatory stimuli in newborn rat pups. Dev Psychobiol. 1986;19:163–174. doi: 10.1002/dev.420190303. [DOI] [PubMed] [Google Scholar]
- 42.Davidson JM, Linforth RS, Hollowood TA, Taylor AJ. Effect of sucrose on the perceived flavor intensity of chewing gum. J Agric Food Chem. 1999;47:4336–4340. doi: 10.1021/jf9901082. [DOI] [PubMed] [Google Scholar]
- 43.Drewnowski A, Gomez-Carneros C. Bitter taste, phytonutrients, and the consumer: a review. Am J Clin Nutr. 2000;72:1424–1435. doi: 10.1093/ajcn/72.6.1424. [DOI] [PubMed] [Google Scholar]
- 44.Okiyama A, Beauchamp GK. Taste dimensions of monosodium glutamate (MSG) in a food system: role of glutamate in young American subjects. Physiol Behav. 1998;65:177–181. doi: 10.1016/s0031-9384(98)00160-7. [DOI] [PubMed] [Google Scholar]
- 45.Palmerino CC, Rusiniak KW, Garcia J. Flavor-illness aversions: the peculiar roles of odor and taste in memory for poison. Science. 1980;208:753–755. doi: 10.1126/science.7367891. [DOI] [PubMed] [Google Scholar]
- 46.Spector AC, Breslin P, Grill HJ. Taste reactivity as a dependent measure of the rapid formation of conditioned taste aversion: a tool for the neural analysis of taste-visceral associations. Behav Neurosci. 1988;102:942–952. doi: 10.1037//0735-7044.102.6.942. [DOI] [PubMed] [Google Scholar]
- 47.Breslin PA, Davidson TL, Grill HJ. Conditioned reversal of reactions to normally avoided tastes. Physiol Behav. 1990;47:535–538. doi: 10.1016/0031-9384(90)90122-k. [DOI] [PubMed] [Google Scholar]
- 48.Liener IE. Toxic Constituents of Plant Foodstuffs. 2. New York: Academic Press; 1980. [DOI] [PubMed] [Google Scholar]
- 49.Schifferstein HN, Verlegh PW. The role of congruency and pleasantness in odor-induced taste enhancement. Acta Psychol (Amst) 1996;94:87–105. doi: 10.1016/0001-6918(95)00040-2. [DOI] [PubMed] [Google Scholar]
- 50.Stevenson RJ, Mahmut M. Differential context effects between sweet tastes and smells. Atten Percept Psychophys. 2010;72:2304–2313. doi: 10.3758/bf03196703. [DOI] [PubMed] [Google Scholar]
- 51.Levy LM, Henkin RI, Lin CS, Finley A, Schellinger D. Taste memory induces brain activation as revealed by functional MRI. J Comput Assist Tomogr. 1999;23:499–505. doi: 10.1097/00004728-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 52.Dotson CD, Geraedts MC, Munger SD. Peptide regulators of peripheral taste function. Semin Cell Dev Biol. 2013;24:232–239. doi: 10.1016/j.semcdb.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woods SC. The eating paradox: how we tolerate food. Psychol Rev. 1991;98:488–505. doi: 10.1037/0033-295x.98.4.488. [DOI] [PubMed] [Google Scholar]
- 54.Siegel EG, Trimble ER, Renold AE, Berthoud HR. Importance of preabsorptive insulin release on oral glucose tolerance: studies in pancreatic islet transplanted rats. Gut. 1980;21:1002–1009. doi: 10.1136/gut.21.11.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Teff KL, Engelman K. Oral sensory stimulation improves glucose tolerance in humans: effects on insulin, C-peptide, and glucagon. Am J Physiol. 1996;270:R1371–R1379. doi: 10.1152/ajpregu.1996.270.6.R1371. [DOI] [PubMed] [Google Scholar]
- 56.Teff KL, Mattes RD, Engelman K, Mattern J. Cephalic-phase insulin in obese and normal-weight men: relation to postprandial insulin. Metabolism. 1993;42:1600–1608. doi: 10.1016/0026-0495(93)90157-j. [DOI] [PubMed] [Google Scholar]
- 57.Peyrot des Gachons C, Beauchamp GK, Stern RM, Koch KL, Breslin PA. Bitter taste induces nausea. Curr Biol. 2011;21:R247–R248. doi: 10.1016/j.cub.2011.02.028. [DOI] [PubMed] [Google Scholar]
- 58.Kunimatsu Y, Nakatsukasa M, Sawada Y, Sakai T, Hyodo M, Hyodo H, Itaya T, Nakaya H, Saegusa H, Mazurier A, et al. A new Late Miocene great ape from Kenya and its implications for the origins of African great apes and humans. Proc Natl Acad Sci USA. 2007;104:19220–19225. doi: 10.1073/pnas.0706190104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Milton K. Nutritional characteristics of wild primate foods: do the diets of our closest living relatives have lessons for us? Nutrition. 1999;15:488–498. doi: 10.1016/s0899-9007(99)00078-7. [DOI] [PubMed] [Google Scholar]
- 60.Gagneux P, Amess B, Diaz S, Moore S, Patel T, Dillmann W, Parekh R, Varki A. Proteomic comparison of human and great ape blood plasma reveals conserved glycosylation and differences in thyroid hormone metabolism. Am J Phys Anthropol. 2001;115:99–109. doi: 10.1002/ajpa.1061. [DOI] [PubMed] [Google Scholar]
- 61.Goodall J. The Chimpanzees of Gombe: Patterns of Behavior. Belknap Press; 1986. [Google Scholar]
- 62.Teaford MF, Ungar PS. Diet and the evolution of the earliest human ancestors. Proc Natl Acad Sci USA. 2000;97:13506–13511. doi: 10.1073/pnas.260368897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Milton K, Jenness R. Ascorbic acid content of neotropical plant parts available to wild monkeys and bats. Experientia. 1987;43:339–342. doi: 10.1007/BF01945577. [DOI] [PubMed] [Google Scholar]
- 64.Bonnans S, Noble AC. Effect of sweetener type and of sweetener and acid levels on temporal perception of sweetness, sourness, fruitiness. Chem Senses. 1993;18:273–283. [Google Scholar]
- 65.Sponheimer M, Lee-Thorp JA. Isotopic evidence for the diet of an early hominid, Australopithecus africanus. Science. 1999;283:368–370. doi: 10.1126/science.283.5400.368. [DOI] [PubMed] [Google Scholar]
- 66.Milton K. A hypothesis to explain the role of meat-eating in human evolution. Evol Anthropol. 1999;8:11–21. [Google Scholar]
- 67.Hellekant G, Ninomiya Y. On the taste of umami in chimpanzee. Physiol Behav. 1991;49:927–934. doi: 10.1016/0031-9384(91)90205-3. [DOI] [PubMed] [Google Scholar]
- 68.Chang JH, Shim YY, Cha SK, Chee KM. Probiotic characteristics of lactic acid bacteria isolated from kimchi. J Appl Microbiol. 2010;109:220–230. doi: 10.1111/j.1365-2672.2009.04648.x. [DOI] [PubMed] [Google Scholar]
- 69.Won TJ, Kim B, Lim YT, Song DS, Park SY, Park ES, Lee DI, Hwang KW. Oral administration of Lactobacillus strains from Kimchi inhibits atopic dermatitis in NC / Nga mice. J Appl Microbiol. 2011;110:1195–1202. doi: 10.1111/j.1365-2672.2011.04981.x. [DOI] [PubMed] [Google Scholar]
- 70.Meisler MH, Ting CN. The remarkable evolutionary history of the human amylase genes. Crit Rev Oral Biol Med. 1993;4:503–509. doi: 10.1177/10454411930040033501. [DOI] [PubMed] [Google Scholar]
- 71.Waterhouse DF. Digestion in insects. Annu Rev Entomol. 1957;2:1–18. [Google Scholar]
- 72.Ramirez I. Does starch taste like Polycose? Physiol Behav. 1991;50:389–392. doi: 10.1016/0031-9384(91)90083-z. [DOI] [PubMed] [Google Scholar]
- 73.Ackroff K, Manza L, Sclafani A. The rat’s preference for sucrose, polycose and their mixtures. Appetite. 1993;21:69–80. doi: 10.1006/appe.1993.1037. [DOI] [PubMed] [Google Scholar]
- 74.Treesukosol Y, Spector AC. Orosensory detection of sucrose, maltose, and glucose is severely impaired in mice lacking T1R2 or T1R3, but Polycose sensitivity remains relatively normal. Am J Physiol Regul Integr Comp Physiol. 2012;303:R218–R235. doi: 10.1152/ajpregu.00089.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Spector AC, Markison S, St John SJ, Garcea M. Sucrose vs. maltose taste discrimination by rats depends on the input of the seventh cranial nerve. Am J Physiol. 1997;272:R1210–R1218. doi: 10.1152/ajpregu.1997.272.4.R1210. [DOI] [PubMed] [Google Scholar]
- 76.Breslin PA, Beauchamp GK, Pugh EN., Jr Monogeusia for fructose, glucose, sucrose, and maltose. Percept Psychophys. 1996;58:327–341. doi: 10.3758/bf03206809. [DOI] [PubMed] [Google Scholar]
- 77.Chambers ES, Bridge MW, Jones DA. Carbohydrate sensing in the human mouth: effects on exercise performance and brain activity. J Physiol. 2009;587:1779–1794. doi: 10.1113/jphysiol.2008.164285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Perry GH, Dominy NJ, Claw KG, Lee AS, Fiegler H, Redon R, Werner J, Villanea FA, Mountain JL, Misra R, et al. Diet and the evolution of human amylase gene copy number variation. Nat Genet. 2007;39:1256–1260. doi: 10.1038/ng2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mandel AL, Peyrot des Gachons C, Plank KL, Alarcon S, Breslin PA. Individual differences in AMY1 gene copy number, salivary alpha-amylase levels, and the perception of oral starch. PloS one. 2010;5:e13352. doi: 10.1371/journal.pone.0013352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mandel AL, Breslin PA. High endogenous salivary amylase activity is associated with improved glycemic homeostasis following starch ingestion in adults. J Nutr. 2012;142:853–858. doi: 10.3945/jn.111.156984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Breslin PA, Spector AC, Grill HJ. Sodium specificity of salt appetite in Fischer-344 and Wistar rats is impaired by chorda tympani nerve transection. Am J Physiol. 1995;269:R350–R356. doi: 10.1152/ajpregu.1995.269.2.R350. [DOI] [PubMed] [Google Scholar]
- 82.Brown IJ, Tzoulaki I, Candeias V, Elliott P. Salt intakes around the world: implications for public health. Int J Epidemiol. 2009;38:791–813. doi: 10.1093/ije/dyp139. [DOI] [PubMed] [Google Scholar]
- 83.Denton D. The Hunger for Salt: An Anthropological, Physiological, and Medical Analysis. New York: Springer-Verlag; 1982. [Google Scholar]
- 84.Kolble N, Hummel T, von Mering R, Huch A, Huch R. Gustatory and olfactory function in the first trimester of pregnancy. Eur J Obstet Gynecol Reprod Biol. 2001;99:179–183. doi: 10.1016/s0301-2115(01)00408-0. [DOI] [PubMed] [Google Scholar]
- 85.Nordin S, Broman DA, Olofsson JK, Wulff M. A longitudinal descriptive study of self-reported abnormal smell and taste perception in pregnant women. Chem Senses. 2004;29:391–402. doi: 10.1093/chemse/bjh040. [DOI] [PubMed] [Google Scholar]
- 86.Sipiora ML, Murtaugh MA, Gregoire MB, Duffy VB. Bitter taste perception and severe vomiting in pregnancy. Physiol Behav. 2000;69:259–267. doi: 10.1016/s0031-9384(00)00223-7. [DOI] [PubMed] [Google Scholar]
- 87.Fenster L, Swan SH, Windham GC, Neutra RR. Assessment of reporting consistency in a case-control study of spontaneous abortions. Am J Epidemiol. 1991;133:477–488. doi: 10.1093/oxfordjournals.aje.a115915. [DOI] [PubMed] [Google Scholar]
- 88.Petitti DB. Nausea and pregnancy outcome. Birth. 1986;13:223–226. doi: 10.1111/j.1523-536x.1986.tb01052.x. [DOI] [PubMed] [Google Scholar]
- 89.Weigel RM, Weigel MM. Nausea and vomiting of early pregnancy and pregnancy outcome. A meta-analytical review. Br J Obstet Gynaecol. 1989;96:1312–1318. doi: 10.1111/j.1471-0528.1989.tb03229.x. [DOI] [PubMed] [Google Scholar]
- 90.Weigel MM, Weigel RM. Nausea and vomiting of early pregnancy and pregnancy outcome. An epidemiological study. Br J Obstet Gynaecol. 1989;96:1304–1311. doi: 10.1111/j.1471-0528.1989.tb03228.x. [DOI] [PubMed] [Google Scholar]
- 91.Brandes JM. First-trimester nausea and vomiting as related to outcome of pregnancy. Obstet Gynecol. 1967;30:427–431. [PubMed] [Google Scholar]
- 92.Little RE. Maternal alcohol and tobacco use and nausea and vomiting during pregnancy: relation to infant birthweight. Acta Obstet Gynecol Scand. 1980;59:495–497. doi: 10.3109/00016348009155438. [DOI] [PubMed] [Google Scholar]
- 93.Tierson FD, Olsen CL, Hook EB. Nausea and vomiting of pregnancy and association with pregnancy outcome. Am J Obstet Gynecol. 1986;155:1017–1022. doi: 10.1016/0002-9378(86)90337-6. [DOI] [PubMed] [Google Scholar]
- 94.Duffy VB, Bartoshuk LM, Striegel-Moore R, Rodin J. Taste changes across pregnancy. Ann NY Acad Sci. 1998;855:805–809. doi: 10.1111/j.1749-6632.1998.tb10663.x. [DOI] [PubMed] [Google Scholar]
- 95.Burrin DG, Stoll B. Metabolic fate and function of dietary glutamate in the gut. Am J Clin Nutr. 2009;90:850S–856S. doi: 10.3945/ajcn.2009.27462Y. [DOI] [PubMed] [Google Scholar]
- 96.Beauchamp GK, Mennella JA. Flavor perception in human infants: development and functional significance. Digestion. 2011;83(Suppl 1 ):1–6. doi: 10.1159/000323397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Beauchamp GK, Pearson P. Human development and umami taste. Physiol Behav. 1991;49:1009–1012. doi: 10.1016/0031-9384(91)90215-a. [DOI] [PubMed] [Google Scholar]
- 98.Vazquez M, Pearson PB, Beauchamp GK. Flavor preferences in malnourished Mexican infants. Physiol Behav. 1982;28:513–519. doi: 10.1016/0031-9384(82)90148-2. [DOI] [PubMed] [Google Scholar]
- 99.Lucas F, Azzara AV, Sclafani A. Flavor preferences conditioned by intragastric polycose in rats: more concentrated polycose is not always more reinforcing. Physiol Behav. 1997;63:7–14. doi: 10.1016/s0031-9384(97)00364-8. [DOI] [PubMed] [Google Scholar]
- 100.Elizalde G, Sclafani A. Flavor preferences conditioned by intragastric polycose infusions: a detailed analysis using an electronic esophagus preparation. Physiol Behav. 1990;47:63–77. doi: 10.1016/0031-9384(90)90043-4. [DOI] [PubMed] [Google Scholar]
- 101.Simpson SJ, Raubenheimer D. A multi-level analysis of feeding behaviour: The geometry of nutritional feeding. Philos Trans Roy Soc B Biol Sci. 1993;342:381–402. [Google Scholar]
- 102.Bray S, Amrein H. A putative Drosophila pheromone receptor expressed in male-specific taste neurons is required for efficient courtship. Neuron. 2003;39:1019–1029. doi: 10.1016/s0896-6273(03)00542-7. [DOI] [PubMed] [Google Scholar]
- 103.Wang L, Han X, Mehren J, Hiroi M, Billeter JC, Miyamoto T, Amrein H, Levine JD, Anderson DJ. Hierarchical chemosensory regulation of male-male social interactions in Drosophila. Nat Neurosci. 2011;14:757–762. doi: 10.1038/nn.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chamero P, Leinders-Zufall T, Zufall F. From genes to social communication: molecular sensing by the vomeronasal organ. Trends Neurosci. 2012;35:597–606. doi: 10.1016/j.tins.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 105.Breslin PAS. Multi-modal sensory integration: Evaluating foods and mates. Chemosens Percept. 2008;1:92–94. [Google Scholar]
- 106.Rozengurt E. Taste receptors in the gastrointestinal tract. I Bitter taste receptors and alpha-gustducin in the mammalian gut. Am J Physiol Gastrointest Liver Physiol. 2006;291:G171–G177. doi: 10.1152/ajpgi.00073.2006. [DOI] [PubMed] [Google Scholar]
- 107.Rozengurt E, Sternini C. Taste receptor signaling in the mammalian gut. Curr Opin Pharmacol. 2007;7:557–562. doi: 10.1016/j.coph.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lee RJ, Xiong G, Kofonow JM, Chen B, Lysenko A, Jiang P, Abraham V, Doghramji L, Adappa ND, Palmer JN, et al. T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. J Clin Invest. 2012;122:4145–4159. doi: 10.1172/JCI64240. [DOI] [PMC free article] [PubMed] [Google Scholar]