Abstract
Autosomal dominant deficiency of signal transducer and activator of transcription 3 (STAT3) is the main genetic etiology of hyper-immunoglobulin (Ig) E syndrome. We documented the molecular, cellular, and clinical features of 60 patients with heterozygous STAT3 mutations from 47 kindreds followed in France. We identified 11 known and 13 new mutations of STAT3. Low levels of interleukin (IL)-6-dependent phosphorylation and nuclear translocation (or accumulation) of STAT3 were observed in Epstein-Barr virus-transformed B lymphocytes (EBV-B cells) from all STAT3-deficient patients tested. The immunologic phenotype was characterized by high serum IgE levels (96% of the patients), memory B-cell lymphopenia (94.5%), and hypereosinophilia (80%). A low proportion of IL-17A-producing circulating T cells was found in 14 of the 15 patients tested. Mucocutaneous infections were the most frequent, typically caused by Staphylococcus aureus (all patients) and Candida albicans (85%). Up to 90% of the patients had pneumonia, mostly caused by Staph. aureus (31%) or Streptococcus pneumoniae (30%). Recurrent pneumonia was associated with secondary bronchiectasis and pneumatocele (67%), as well as secondary aspergillosis (22%). Up to 92% of the patients had dermatitis and connective tissue abnormalities, with facial dysmorphism (95%), retention of decidual teeth (65%), osteopenia (50%), and hyperextensibility (50%). Four patients developed non-Hodgkin lymphoma. The clinical outcome was favorable, with 56 patients, including 43 adults, still alive at the end of study (mean age, 21 yr; range, 1 mo to 46 yr). Only 4 patients died, 3 from severe bacterial infection (aged 1, 15, and 29 yr, respectively). Antibiotic prophylaxis (90% of patients), antifungal prophylaxis (50%), and IgG infusions (53%) improved patient health, as demonstrated by the large decrease in pneumonia recurrence. Overall, the prognosis of STAT3 deficiency may be considered good, provided that multiple prophylactic measures, including IgG infusions, are implemented.
Introduction
Hyper-immunoglobulin E syndrome (HIES) is a complex primary immunodeficiency first described, to our knowledge, by Davis et al [12] in 1966 as Job syndrome (OMIM #147060) in 2 patients. In 1972, Buckley et al [9] reported the same clinical association in 2 boys with very high plasma immunoglobulin (Ig) E levels, which they thus named HIES. Further studies allowed a more precise phenotypic description of the disease and led to the discovery of autosomal dominant (AD) inheritance in multiplex kindreds. [22] AD-HIES confers a selective immunodeficiency, with susceptibility to skin diseases and pneumonia caused by pyogenic bacteria, chronic mucocutaneous candidiasis, [5, 26, 38] and additional features, including facial dysmorphism, impaired shedding of deciduous teeth, bone abnormalities (osteopenia), craniosynostosis, and hyperextensibility. [6, 10, 18, 21, 22, 27, 39, 50, 64] Patients with AD-HIES often display high serum IgE levels and hypereosinophilia. [8, 22] The molecular basis of AD-HIES was identified in 2007. Heterozygous mutations in the signal transducer and activator of transcription 3 (STAT3) gene were identified, affecting the DNA-binding domain, with a dominant negative effect. [47] Mutations affecting the DNA-binding domain and Src homology 2 (SH2) domains of STAT3 were described shortly thereafter. [28]
STAT3 is activated by various Janus kinases in response to the stimulation of the cell with various cytokines, hormones, and growth factors, and down-regulated by proteins of the suppressor of cytokine signaling (SOCS) family. [48] STAT3 is a major downstream transducer for the cytokine receptor gp130, which is involved in the signaling pathway involving the members of the IL-6 family (IL-6, -11, -27, -31), ciliary neurotropic factor, oncostatin M, leukemia inhibitory factor (LIF), and, to a lesser extent, IL-12R [beta]1 (IL-12 and IL-23). [45] STAT3 also mediates cellular responses to other families of cytokines, including the IL-2 family (IL-2, -7, -9, -15, -21), the interferon (IFN) family (IFN- [gamma], IFN- [alpha]/[beta]), the IL-10 family (IL-10, -19, -20, -22, -24, -26, IFN-lambdas), Flt3 ligand, M-CSF, G-CSF, leptin, and growth hormone. [40, 48, 51] This suggests that STAT3 plays a major role in a number of physiologic pathways, including immunologic pathways in particular. Further studies of patients heterozygous for loss-of-function STAT3 alleles (hereafter referred to as STAT3-deficient patients), some of which were shown to be dominant-negative, led to a more detailed description of the immunologic phenotype of this primary immunodeficiency, with the demonstration of a deficiency of IL-17A- and IL-22-producing T cells [13, 43, 44] and a lack of circulating memory B cells. [3, 4]
Since 2007, up to 230 STAT3-deficient patients have been reported in individual case reports [2–4,13, 33, 35, 41, 43, 44, 55, 61, 68, 71] or national or international series, including 8 patients (Japan), [47] 37 (United States and Europe), [60] 12 (Lebanon and Europe), [29] 50 (international), [28] 64 (international), [70] and 48 patients (international). [62] However, most reports have focused on the molecular and cellular defects of the patients, providing little clinical information. The nature and severity of the infectious events, the impact of prophylaxis, age at diagnosis, and clinical outcome remain poorly characterized. We therefore undertook a detailed molecular, cellular, and clinical description of what is to our knowledge the largest national cohort reported to date, comprising 60 patients with STAT3 deficiency living in France.
Patients and Methods
Patients and Clinical Definitions
The patients consulted at various French hospitals and were identified through the Centre de Reference des Deficits Immunitaires Hereditaires (CEREDIH) at Necker Enfants Malades Hospital, Paris, France. A detailed questionnaire was completed by the physicians caring for the patients; clinical and laboratory data were collected for the patients from their birth until February 2011 or their death. Approval for this study was obtained from the institutional review board of Necker Hospital, and informed consent was obtained from all patients, or their families in the case of minors, in accordance with the Helsinki Declaration (Commission Nationale de L'informatique et des Libertes [lsqb] CNIL [rsqb] authorization: no. 908256, October 14, 2008).
We used the National Institutes of Health (NIH) scoring system: each feature was scored separately and all the scores were then added together to give a total point score (scores over 40 points indicate that the subject is likely to carry an AD-HIES phenotype, scores of 20-40 points are inconclusive concerning the presence of AD-HIES, and scores below 20 points indicate that the subject is unlikely to have AD-HIES). [9, 12, 19, 24, 53] The definition of Aspergillus species-related pulmonary invasive fungal disease was based on the European Organization for Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) consensus definitions. [14] Invasive fungal disease was considered proven if the presence of fungus was systematically demonstrated in diseased tissues; probable if a host factor, clinical features, and mycologic evidence were present; and possible if there were appropriate host factors and sufficient clinical evidence consistent with invasive fungal disease, but no mycologic confirmation. Bone mineral density was measured by dual-energy X-ray absorptiometry of the lumbar spine and hip. Osteoporosis was defined as a lumbar spine or hip score of −2.5 standard deviations (SD) or less, and osteopenia as a score between −1.0 and −2.5 SD. [32]
Genetic Analysis
Genomic DNA was prepared from the blood samples of patients and controls by the standard phenol-chloroform extraction method. Exons 1–24 and of STAT3 and their flanking intron sequences were amplified by polymerase chain reaction (PCR) with specific oligonucleotide primers (available on request) and sequenced with the Applied Biosystems Big Dye terminator kit v.1.1 (Applied Biosystems, Foster City, CA) and an ABI Prism 3130×l Analyzer (Applied Biosystems). PolyPhen2 (polymorphism phenotyping) (http://genetics.bwh.harvard.edu/pph2/), a bioinformatics method for predicting the possible impact of an amino acid substitution on the structure and function of a human protein on the basis of physical and comparative considerations, was used to estimate the effect of the identified STAT3 mutations.
Immunologic Investigations
Immunologic investigations were based on those described in previous studies and/or the questionnaires sent to physicians. [54] All antibody determinations were performed before or several months after the end of Ig treatment. Nitroblue tetrazolium reduction tests and chemotaxis were assessed by standard techniques at the various hospitals where the patients were followed. [17]
Statistical Analysis
Survival curves were plotted as a function of age by the Kaplan-Meier method, and, when necessary, were compared in log-rank tests.
Results
Patients and STAT3 Mutations
We studied 60 patients from 47 kindreds (30 female, 30 male) with AD-HIES, diagnosed based on the observation of typical clinical features and the identification of a heterozygous mutation in the STAT3 gene (Table 1). There were no consanguineous families. Twenty-two cases were familial (9 kindreds) (Figure 1); the remaining 38 cases were sporadic. Forty-three kindreds originated from France, 2 from Algeria, 1 from Pakistan, and 1 from Portugal. All patients and their families were living in France at the time of the study. Thirty-six kindreds (55%) carried STAT3 mutations affecting the DNA-binding domain, 15 (32%) carried mutations affecting the SH2 domain, 1 (2%) carried mutations in the linker domain, and 5 (10%) carried mutations affecting the transactivation (TA) domain. We identified 11 known mutations and 13 novel STAT3 mutations (V343F, N472D, I568F, S611G, S614G, G617V, K642E, I665N, S668Y, T708N, K709E, V713M, and T714I) (see Table 1, Figure 2). We sequenced exons 10, 14, 16, and 19-22 from 300 healthy controls, and found none of the new mutations described. Moreover, these mutations were not found in whole-exome sequences from 117 individuals. Most missense mutations (V343F, N472D, G617V, S614G, K642E, I665N, S668Y, T708N, V713M, and T714I) were classified as “probably damaging,” 2 (I568F and S611G) as “possibly damaging,” and only 1 (K709E) as “benign” by polyPhen2. Finally, some mutations were identified in several kindreds: R382W was found in 10 kindreds, R382Q in 6, V637M in 5, Vdel463 in 3, N472D in 2, Y657C in 2, and K709E in 2 kindreds.
Table 1. Characteristics and Outcome in the French Cohort of 60 STAT3-Deficient Patients.
| K | Pt | Age */Sex (yr) |
Country of Origin |
Status |
STAT3 Mutation |
Domain | NIH Score |
Age at 1st Sign (yr) | Age at Diagnosis (yr) | Infection | Dermatitis | Connective Tissue Sign | NHL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||||||||||||
| Skin | Lung | Severe Bacterial | ENT | CMC | Aspergillus | Dysmorphia | Teeth | Osteopenia | Fracture | Hyperextensibility | ||||||||||||
| K1 | P1 | 15/F | France | Alive | V637M | SH2 | 57 | 1 | 2 | + | + | + | + | + | + | + | + | |||||
| K2 | P2 | 11/M | France | Alive | R382W | DNA | 50 | <1 | 3 | + | + | + | + | + | + | + | ||||||
| K3 | P3 | 39/M | France | Alive | F384L | DNA | 72 | <1 | 7 | + | + | + | + | + | + | + | + | + | + | |||
| K3 | P4 | 4/F | France | Alive | F384L | DNA | 56 | 1 | 3 | + | + | + | + | + | + | + | + | |||||
| K4 | P5 | 15/F | France | Alive | T708N | TA | 62 | >5 | 9 | + | + | + | + | + | + | + | + | + | ||||
| K5 | P6 | 19/F | France | Alive | R382Q | DNA | 73 | <1 | 2 | + | + | + | + | + | + | + | + | + | + | + | ||
| K6 | P7 | 29/F | France | Dead | nd | nd | 85 | <1 | ? | + | + | + | + | + | + | + | + | + | + | |||
| K6 | P8 | 29/M | France | Dead | T622I | SH2 | 68 | <1 | 3 | + | + | + | + | + | + | + | + | + | + | + | ||
| K7 | P9 | 12/F | France | Alive | R382Q | DNA | 51 | <1 | 9 | + | + | + | + | + | + | + | + | |||||
| K8 | P10 | 24/F | France | Alive | R382W | DNA | 73 | <1 | ? | + | + | + | + | + | + | + | + | |||||
| K9 | P11 | 18/M | Algeria | Alive | R382W | DNA | 74 | <1 | 5 | + | + | + | + | + | + | + | + | + | + | + | ||
| K10 | P12 | 38/M | France | Alive | K642E | SH2 | 66 | <1 | 7 | + | + | + | + | + | + | + | + | + | + | |||
| K11 | P13 | 19/F | France/Morocco | Alive | T714I | TA | 78 | Birth | Birth | + | + | + | + | + | + | + | + | + | ||||
| K12 | P14 | 36/F | France | Alive | Vdel463 | DNA | 63 | Birth | 30 | + | + | + | + | + | + | + | + | |||||
| K12 | P15 | 11/F | France | Alive | Vdel463 | DNA | 14 | <1 | 7 | + | + | + | ||||||||||
| K12 | P16 | 10/M | France | Alive | Vdel463 | DNA | 59 | <1 | 7 | + | + | + | + | + | + | + | + | + | ||||
| K13 | P17 | 17/F | France | Alive | T620A | SH2 | 52 | <1 | <1 | + | + | + | + | + | + | |||||||
| K14 | P18 | 25/M | French West Indies | Alive | R382W | DNA | 84 | <1 | 10 | + | + | + | + | + | + | + | + | + | ||||
| K15 | P19 | 15/F | France | Alive | S611G | SH2 | 70 | <1 | 4 | + | + | + | + | + | + | + | + | + | + | |||
| K16 | P20 | 23/M | France | Alive | R382W | DNA | 68 | <1 | 2 | + | + | + | + | + | + | + | + | + | ||||
| K17 | P21 | 20/F | French West Indies | Alive | S668Y | SH2 | 74 | <1 | 9 | + | + | + | + | + | + | + | + | |||||
| K18 | P22 | 33/F | France | Alive | R382Q | DNA | 68 | <1 | 15 | + | + | + | + | + | + | + | + | + | ||||
| K18 | P23 | 10/M | France | Alive | R382Q | DNA | 19 | <1 | <1 | + | + | + | + | + | ||||||||
| K19 | P24 | 16/M | France | Dead | V343F | DNA | 70 | <1 | 5y | + | + | + | + | + | + | + | ||||||
| K20 | P25 | 22/F | France | Alive | R382W | DNA | 86 | ? | ? | + | + | + | + | + | + | + | + | + | + | |||
| K21 | P26 | 23/F | France | Alive | V713M | TA | 69 | <1 | 3 | + | + | + | + | + | + | + | + | |||||
| K22 | P27 | 10/F | France | Alive | S614G | SH2 | 53 | <1 | 6 | + | + | + | + | + | + | + | + | |||||
| K23 | P28 | 29/M | France | Alive | R382Q | DNA | 67 | <1 | 1 | + | + | + | + | + | ||||||||
| K23 | P29 | 4/M | France | Alive | R382Q | DNA | 33 | <1 | 1 | + | + | + | + | + | ||||||||
| K23 | P30 | 2/M | France | Alive | R382Q | DNA | 25 | Birth | Birth | + | + | + | + | + | + | |||||||
| K24 | P31 | 7/F | France | Alive | K709E | TA | 64 | <1 | 4 | + | + | + | + | + | + | + | ||||||
| K25 | P32 | 15/M | Algeria | Alive | R382W | DNA | 54 | <1 | <1 | + | + | + | + | + | + | + | + | |||||
| K26 | P33 | 26/F | France | Alive | V637M | SH2 | 73 | <1 | 2y | + | + | + | + | + | + | + | + | + | + | |||
| K27 | P34 | 18/M | France | Alive | V637M | SH2 | 64 | <1 | <1 | + | + | + | + | + | + | + | + | |||||
| K28 | P35 | 30/M | France | Alive | Vdel463 | DNA | 64 | <1 | 3 | + | + | + | + | + | + | + | + | + | ||||
| K29 | P36 | 21/F | France | Alive | T412S | DNA | 44 | >5 | 15 | + | + | + | + | + | + | + | ||||||
| K30 | P37 | 43/M | France | Alive | nd | nd | nd | <1 | ? | + | + | + | + | + | ||||||||
| K30 | P38 | 13/F | France | Alive | N472D | DNA | nd | <1 | ? | + | + | + | + | + | ||||||||
| K31 | P39 | 30/F | France | Alive | R382W | DNA | 74 | <1 | 17 | + | + | + | + | + | + | + | + | + | + | |||
| K32 | P40 | 28/F | France | Alive | R382W | DNA | 73 | <1 | 4 | + | + | + | + | + | + | |||||||
| K33 | P41 | 16/M | France | Alive | Y657C | SH2 | 45 | <1 | ? | + | + | + | + | + | ||||||||
| K34 | P42 | 11/F | France | Alive | R382Q | DNA | 59 | <1 | 7 | + | + | + | + | + | + | + | + | |||||
| K35 | P43 | 38/F | Pakistan | Alive | Vdel463 | DNA | 79 | <1 | 24 | + | + | + | + | + | + | + | + | + | + | |||
| K35 | P44 | 11/M | Pakistan | Alive | Vdel463 | DNA | 55 | <1 | 1 | + | + | + | + | + | + | + | + | |||||
| K35 | P45 | 1/F | Pakistan | Dead | Vdel463 | DNA | 33 | Birth | Birth | + | + | + | + | + | + | |||||||
| K36 | P46 | 19/M | France | Alive | N472D | DNA | 55 | <1 | 14 | + | + | + | + | + | + | + | ||||||
| K37 | P47 | 37/M | France | Alive | V637M | SH2 | 74 | <1 | ? | + | + | + | + | + | + | + | + | + | + | |||
| K38 | P48 | 45/F | France | Alive | I665N | SH2 | 65 | <1 | ? | + | + | + | + | + | + | + | ||||||
| K38 | P49 | 17/F | France | Alive | I665N | SH2 | 26 | >5 | 18 | + | + | + | + | |||||||||
| K39 | P50 | 19/M | France | Alive | K709E | TA | 77 | <1 | <1 | + | + | + | + | + | + | + | + | + | ||||
| K40 | P51 | 19/M | France | Alive | R382W | DNA | 37 | <1 | 2 | + | + | + | + | + | + | + | + | |||||
| K41 | P52 | 8/F | France | Alive | P639S | SH2 | 51 | 1 | 6 | + | + | + | + | + | + | + | + | + | + | |||
| K42 | P53 | 30/M | France | Alive | I568F | Linker | 41 | <1 | 1 | + | + | + | + | + | + | + | + | |||||
| K43 | P54 | 25/F | France | Alive | G617V | SH2 | 67 | <1 | 8 | + | + | + | + | + | + | + | + | + | + | + | ||
| K44 | P55 | 0.3/M | France | Alive | R382Q | DNA | 32 | Birth | Birth | + | + | + | + | + | + | |||||||
| K45 | P56 | 38/M | France | Alive | Y657C | SH2 | 68 | <1 | ? | + | + | + | + | + | + | + | + | + | ||||
| K46 | P57 | 27/M | Portugal | Alive | V637M | SH2 | 79 | <1 | ? | + | + | + | + | + | + | + | + | + | ||||
| K47 | P58 | 46/M | France | Alive | Q469H | DNA | 44 | <1 | ? | + | + | + | + | + | + | + | + | |||||
| K28 | P59 | 0.1/M | France | Alive | Vdel463 | DNA | nd | Birth | Birth | + | ||||||||||||
| K3 | P60 | 7/M | France | Alive | F384L | DNA | 14 | 5 | 7 | + | ||||||||||||
Abbreviations: ? = unknown, Aspergillus = Aspergillus species-related disease, CMC = chronic mucocutaneous candidiasis, K = kindred, nd = not determined, Pt = patient, severe bacterial infection = septicemia, meningitis, deep inner abscess, arthritis, osteomyelitis, Teeth = retention of deciduous teeth.
Age at time of study.
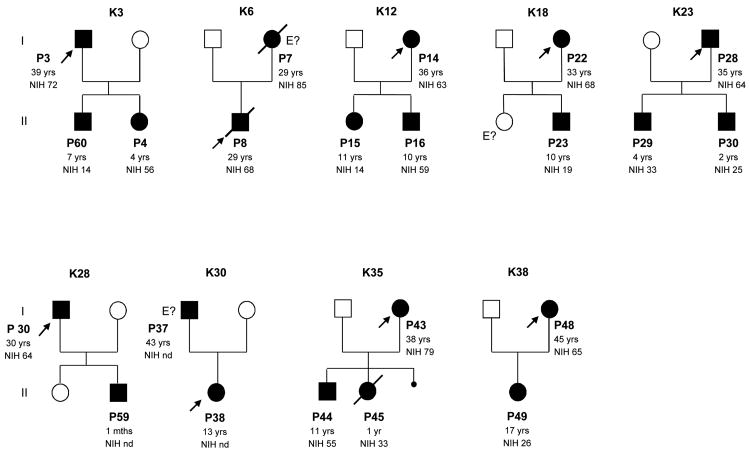
Figure 1.
Pedigrees of the 9 kindreds with STAT3 deficiency (familial cases) identified. Each kindred with STAT3 deficiency is designated by a capital letter (K), each generation is designated by a Roman numeral (I) and each individual is designated by an Arabic numeral (from left to right). Patients with a clinical phenotype are indicated by closed symbols. In each family, the proband is indicated by an arrow. Individuals whose genetic status could not be evaluated are indicated by “E?”. Patient age at last follow-up and NIH score are also provided.
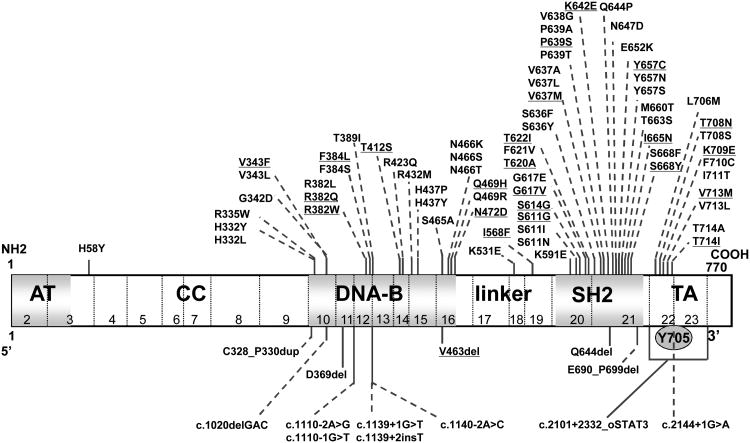
Figure 2.
Schematic representation of the STAT3 gene. Positions of the STAT3 mutations in the DNA binding (DNA) domain, the Src homology 2 (SH2) domain, the transactivation (TA) domain, amino-terminal (AT) domain, or coiled-coil (CC) domain. Underlined mutations were identified in patients from the current study; mutations not underlined were identified in previous studies. [2–4,28,29,33,35,36,41,47,52,55,60-62,70,71] The positions of all known mutations are indicated in amino acid nomenclature, with the exception of mutations starting with c. at the bottom, which are shown in nucleotide nomenclature.
We then investigated the molecular impact of various mutations affecting the STAT3 DNA-binding domain, SH2, or TA domains in Epstein-Barr virus-transformed B lymphocytes (EBV-B cells) stimulated with IL-6, IL-10, IL-21, or IFN-[alpha] (see Supplementary Figure 1a-d). Intracellular flow cytometry analysis of STAT3 tyrosine phosphorylation (pY705) showed that pY705-STAT3 levels in response to stimulation with IL-6, IL-10, and IL-21 were significantly lower in STAT3-deficient patients than in healthy controls (n = 6). This difference was particularly marked in EBV-B cells carrying TA domain mutations. Levels of nuclear translocation and DNA binding by STAT3 in response to stimulation with IL-6, IL-10, IL-21, or IFN- [alpha], evaluated by EMSA ELISA (electrophoretic mobility shift assay/enzyme-linked immunosorbent assay), were significantly lower in STAT3-deficient patients than in healthy controls (n = 8) (see Supplementary Figure 2a-d), but similar amounts of STAT1 DNA-binding complexes were observed in patients and healthy controls upon IFN- [alpha] treatment (data not shown). Finally, levels of mRNA for SOCS3, a STAT3 target gene, in response to stimulation with IL-6, IL-10, IL-21, or IFN- [alpha] were significantly lower in STAT3-deficient cells than in control cells, as shown by reverse transcription PCR (RT-PCR) analysis (see Supplementary Figure 3a-d).
Immunologic and Hematologic Investigations
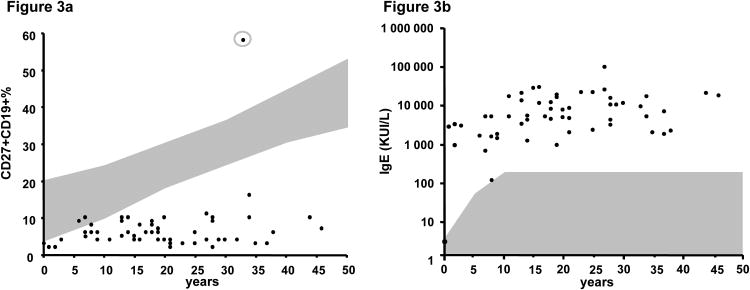
We analyzed blood leukocyte subsets in 56 patients with STAT3 deficiency (Table 2). Polymorphonuclear neutrophil counts were in normal range for all patients, except during infectious events. Hypereosinophilia ([mt] 0.5 × 10[sup]9 eosinophils/L) was found in 80% of patients (range, 0.5-10 × 10[sup]9 eosinophils/L). Phagocyte functions (oxidative burst and chemotaxis) were normal in all patients studied. T-cell subsets were present in normal numbers in 98% of patients tested; 1 patient (Patient 3) had persistent global lymphopenia (0.8 × 10[sup]9 lymphocytes/L). We previously showed that IL-17-producing T-lymphocyte levels were low in 14 of the 15 patients tested, [13] with 86% of the tested patients also having low proportions of CCR6+CCR4 -CD4 T cells, CCR6 being a putative marker for Th17 cells. NK cells were present in normal numbers in 92% of patients studied. T cells proliferated normally in response to mitogens and recall antigens in vitro, for all the patients studied. The number of B cells was normal in 98% of patients, but 94.5% of the patients tested had low levels of memory CD19+CD27+ B cells (Figure 3a), and an excess of transitional CD19+IgM+CD38+B cells was found in 4 of the 7 patients tested, consistent with previous reports. [3] The only patient (Patient 3) with a high percentage of CD27+CD19+ cells displayed global lymphopenia, including a lack of B cells. High serum IgE levels were found in 96% of patients, with a median level of [mt]10,108 IU/mL (range, 3-93,900 IU/mL). IgE levels were normal in 2 patients (1 at age 2 mo and the other at age 7 yr) (see Table 2, Figure 3b). High serum IgD levels were also found in 8 of the 9 patients tested as previously described. [30] IgG, IgA, and IgM levels were normal to high in most patients, but IgG levels were slightly low in 1 patient, and another patient had low IgG2 levels. The production of antibodies against protein antigens was detected in 78% of the patients tested. Thirteen of the 14 patients tested had detectable IgG antibodies against pneumococcus after infection and/or immunization with nonconjugated vaccine. The production of IgM allohemagglutinins against erythrocyte AB antigens was normal in the 8 patients tested. In conclusion, the immunologic phenotype of patients with STAT3 deficiency is characterized by low levels of circulating IL-17-producing T cells and memory B cells, hypereosinophilia, and high serum IgE and IgD levels.
Table 2. Immunologic Investigations in 55 STAT3-Deficient Patients.
| Patients Tested (n) | Normal (%) | Low (%) | High (%) | |
|---|---|---|---|---|
| Lymphocytes (109 lymphocytes/L) | 54 | 98 | 2 | - |
| CD4 T lymphocytes (%) | 54 | 98 | 2 | - |
| CD8 T lymphocytes (%) | 54 | 96 | 4 | - |
| CD4 CCR4-CCR6+ T lymphocytes (N >2%) | 35 | 14 | 86 | - |
| Th17 lymphocytes (%) | 15 | 7 | 93 | - |
| B lymphocytes (%) | 55 | 98 | 2 | - |
| CD27+CD19+ memory B lymphocytes (%) | 55 | 5.5 | 94.5 | - |
| NK lymphocytes (%) | 26 | 92 | 8 | - |
| T-cell proliferation (cpm) | 18 | 100 | - | - |
| IgG levels (g/L) | 45 | 71 | 2 | 27 |
| IgG1 levels (g/L) | 14 | 100 | - | - |
| IgG2,3,4 levels (g/L) | 14 | 86 | 14 | - |
| IgA levels (g/L) | 45 | 69 | 13 | 18 |
| IgM levels (g/L) | 45 | 69 | 31 | |
| IgD levels | 9 | 11 | - | 89 |
| IgE levels (KUI/L) | 56 | 4 | 96 | |
| Antibodies against protein antigens (tetanus, diphtheria, or polio) | 24 | 79 | 21 | |
| Antibody against H. influenzae type b | 5 | 100 | - | - |
| Antibody against S. pneumoniae | 14 | 93 | 7 | |
| Allohemagglutinin | 8 | 100 | - | - |
| Nitroblue tetrazolium or dihydrorhodamine test | 23 | 100 | - | - |
| Neutrophil chemotaxis | 16 | 100 | - | - |
Figure 3.
A, B-cell immunophenotyping in 55 STAT3-deficient patients. Percentage of CD27+CD19+ B cells in STAT3-deficient patients, with the gray area representing the normal values for these cells according to age. In the gray circle, the only patient (Patient 3) with a high percentage of CD27+CD19+ cells displayed global lymphopenia, including B-cell lymphopenia. B, IgE levels in 56 STAT3-deficient patients (KUI/L). The gray area indicates the normal values of IgE as a function of age.
Demographic Features
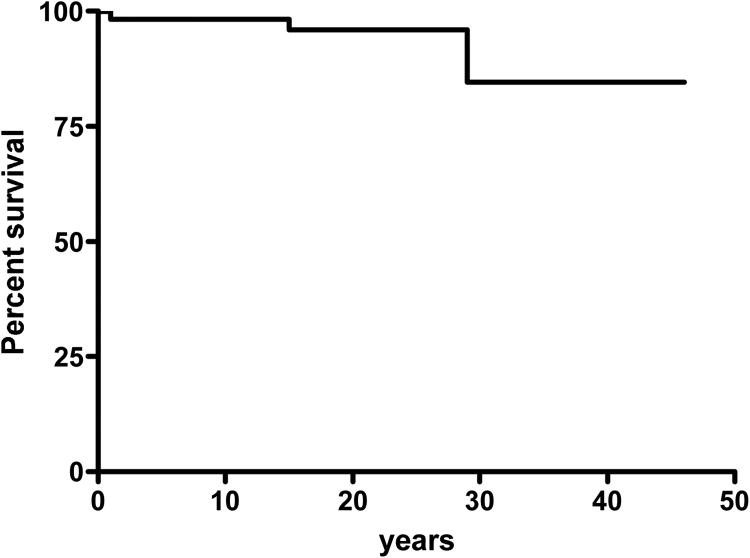
The mean age of the cohort at the time of the study was 21 years (range, 1 mo to 46 yr), with a mean age at clinical diagnosis of 6.8 years (range, 0-30 yr). The mean age at the identification of STAT3 mutation was 17 years (range, 1 mo to 46 yr). The observation time was 1214 years for the 60 patients of the cohort, with a mean of 21 years/patient. The mean age at first infection was 10.5 months (range, 0-180 mo; SD, 28 mo). Bacterial and fungal infections were the most prominent clinical feature of the disease. The first clinical signs in most patients were skin rash and lung infections in 70% (42/60) and 38% (23/60) of patients, respectively. An NIH HIES score [mt]40 was found in 82% (47/57) of patients, and half the patients with scores [lt]40 were under the age of 10 years (see Table 1) (mean NIH score, 60). We observed considerable clinical variability in individuals bearing the same heterozygous STAT3 mutation, including those from the same kindred (see Figure 1). Four (7%) patients died (Table 3): 1 in an accident and 3 from uncontrolled bacterial infection (Figure 4). Patient 7 died at age 29 years, from rapidly extensive purulent mediastinitis following esophageal perforation. Patient 19 died at age 15 years from pneumococcal meningitis. Patient 45 died at age 14 months from septic shock and acute respiratory distress syndrome secondary to Streptococcus pneumoniae lung infection.
Table 3. Causes and Age of Death in the French Cohort of STAT3-Deficient Patients.
| K | Pt | NIH Score | STAT3 Mutation | Age at Death | Cause of Death |
|---|---|---|---|---|---|
| K6 | P7 | 85 | T622I | 29 yr | Purulent mediastinitis after eosophagal perforation and paravertebral abscess caused pyogenic bacteria |
| K6 | P8 | 68 | T622I | 29 yr | Accident |
| K15 | P19 | 70 | S611G | 15 yr | Meningitis caused by S. pneumoniae |
| K35 | P45 | 33 | Vdel463 | 14 mo | Septic shock and acute respiratory distress syndrome caused by S. pneumoniae |
Abbreviations: See Table 1.
Figure 4.
Survival curve of the 60 STAT3-deficient patients.
Eczema and Staphylococcal Skin Diseases
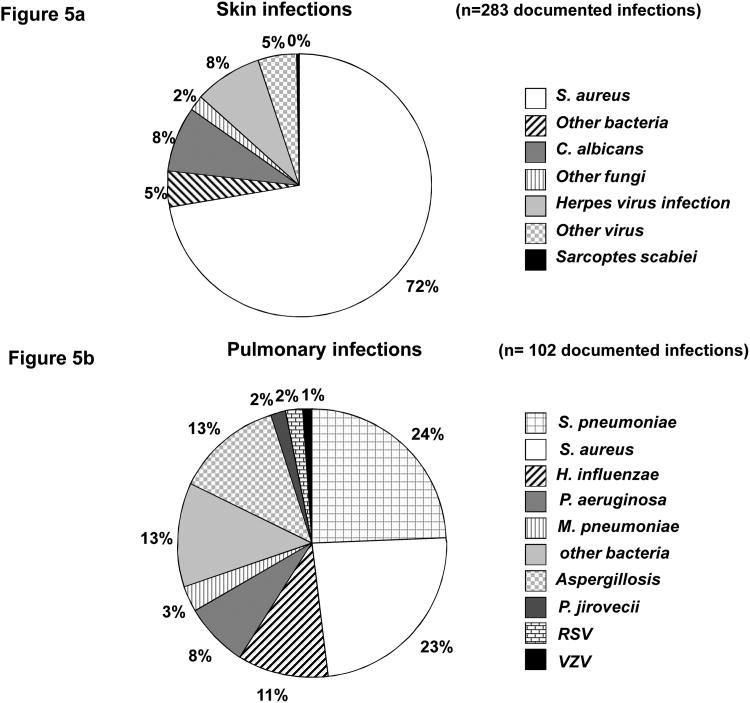
Most patients (93%, n = 56/60) developed noninfectious dermatitis (see Table 1). A neonatal papulopustular rash was observed in 48% (29/60) of patients, occurring within the first 2 weeks of life in 69% (20/29) of these patients. Skin biopsy revealed eosinophilic spongiotic dermatitis in 4 patients. Chronic eczematiform dermatitis developed in 92% (55/60) of patients before the age of 18 months. The skin features consisted of crusting and extensive papular (prurigo-like) or papulopustular (folliculitis-like) eruptions, initially on face, scalp, chest, and buttocks in particular. Remarkably, subsequent Staph. aureus infection was consistently observed and led to chronic impetiginized dermatitis. All STAT3-deficient patients developed bacterial infections of the skin, whether or not they had dermatitis, with a mean age at onset of 11.5 months (range, 0 to 96 mo); such infections were present in 14 (24%) patients at birth. Cold abscesses of the skin, with minor inflammatory symptoms, were observed in 73% (44/60) of patients (ranging from 1 to [mt]60 episodes), most frequently on the neck and trunk, including recurrent cold abscesses on the breast. The other types of bacterial skin infections observed included impetigo, pustulosis, and pyodermatitis in 45% of patients (n = 27), folliculitis and/or furunculosis in 35% (n = 21), paronychia in 23% (n = 14), lymph node abscesses in 20% (n = 12), and cellulitis in 16% of patients (n = 10). Evidence for the presence of bacteria was documented for 70% (42/60) of patients. Staph. aureus was found in 94% of the documented bacterial episodes (204 of 217 episodes, 0.2 episodes/patient.year), including 1 case of thoracic wall cellulitis due to a Panton-Valentine leukocidin-positive strain of Staph. aureus. Other bacteria were isolated more rarely and included gram-negative bacilli for 8 patients and gram-positive bacteria for 4 patients (Figure 5a).
Figure 5.
A, Documented skin infections. Skin bacterial infections were caused by Staph. aureus in 204 episodes and by other bacteria in 13 episodes (E. coli in 3, Proteus mirabilis in 2, P. aeruginosa in 2, Enterobacter aerogenes in 1, Serratia marcescens in 1, [alpha] or [beta]-hemolytic Streptococcus in 3, and Propionibacterium acnes in 1). Skin fungal infections were caused by C. albicans in 23 episodes and by other fungi in 6 episodes (C. parapsilosis in 2, C. glabrata in 1, T. rubrum in 1, T. mentagrophytes in 1, and Cryptococcus laurentii in 1). Skin viral infections were caused by varicella zoster virus in 16, herpes simplex virus in 8, human papillomavirus in 9, and the Molluscum contagiosum poxvirus in 4 episodes. Isolated Sarcoptes scabiei pediculosis was reported in 1 episode. B, Documented lung infections. S. pneumoniae and Staph. aureus were the most frequently involved, in 25 and 24 episodes of documented infections, respectively. Three cases of M. pneumoniae infections were also reported. The other bacteria isolated were H. influenzae isolated in 11, P. aeruginosa in 8, Stenotrophomonas maltophilia in 2, E. coli in 2, K. pneumoniae in 2, Serratia marcescens in 2, Fusobacterium species in 1, Bacteroides fragilis in 1, Prevotella oralis in 1, and Moraxella catarrhalis in 2 episodes. Lung fungal infections were secondary aspergillosis in 13 episodes, and 2 cases of pneumocystosis were also identified. Two patients also presented respiratory infections caused by respiratory syncytial virus (RSV), and 1 displayed a respiratory infection caused by varicella zoster virus (VZV).
Other Mucocutaneous Infections
Chronic mucocutaneous candidiasis of various mucosal sites and nails was observed in 85% (51/60) of patients, including 64% of patients with oral candidiasis reported in the neonatal period (see Table 1). The affected sites were oral (thrush, glossitis, and/or cheilitis) in 63% of patients (n = 32), chronic onychomycosis in 57% (n = 29), genital in 18% (n = 9), cutaneous in 16% (n = 8), and esophageal in 8% (n = 4) of patients. Candida albicans was the most frequently isolated infectious agent and was obtained from 88% (23/26) of samples (see Figure 5a). C. glabrata and C. parapsilosis were documented in only 1 and 2 children, respectively. Skin infections due to Trichophyton rubrum, T. mentagrophytes, or Cryptococcus laurentii were identified in 1 patient each. Viral skin infections occurred in only a few patients: 1 patient had a profuse cutaneous herpes simplex virus (HSV)-1 infection during the neonatal period (Kaposi-Juliusberg syndrome), and 7 other patients had HSV infection with the usual clinical presentation (stomatitis, vulvitis, or keratitis). Sixteen patients developed chickenpox; none of them had been immunized with varicella zoster vaccine. Three of the 16 patients received intravenous antiviral treatment: for hemorrhagic chickenpox in 1 case, for chickenpox pneumonia in 1 case, and for a cutaneous Staph. aureus superinfection in 1 case. Five patients had zoster virus infection (at 2, 16, 16, 21, and 22 years of age, respectively), including 1 with a multimetameric distribution; all these infections resolved favorably with treatment. Nine patients had warts with no unusual extension or outcome. Four other patients had molluscum contagiosum of both infection types, with limited extension and a favorable outcome. Only 1 patient had Sarcoptes scabiei pediculosis, with a good outcome once treated.
Pulmonary Infections
In this cohort, 90% (54/60) of patients had bacterial pneumonia, with a mean of 3 episodes per patient (range, 1-30 episodes) (Tables 1 and 4), and a mean age at onset of 39.5 months (range, 0-192 mo). The severity of the infections depended on their extension, and associated pleural effusion was reported in 38% (23/60) of patients. Bacteria were detected in 44% of acute episodes of pneumonia (84/188 episodes, 0.15 episodes/patient.year). S. pneumoniae and Staph. aureus were the most frequently implicated bacteria, in 30% (25/82) and 29% (24/82) of documented infections, respectively. Other bacteria were also isolated, such as Haemophilus influenzae in 13% of cases (n = 11), Pseudomonas aeruginosa and Stenotrophomonas maltophilia in 12% of cases (n = 8 and 2, respectively), enterobacteria in 7% of cases, and anaerobic bacteria in 6% of cases (Figure 5b). Finally, 3 cases of Mycoplasma pneumoniae infection were reported. Two cases of Pneumocystis jirovecii pneumonia were reported, in Patient 51 at age 5 months and Patient 39 at age 30 years, both with a favorable outcome. Two cases of viral pneumonia due to respiratory syncytial virus (RSV) infection in 1 patient and coinfection with RSV, influenza virus, and pneumococcus in another, were identified. This second patient died at the age of 14 months, from associated S. pneumoniae -related septic shock, attesting to the higher frequency and severity of bacterial infections in this population. P. aeruginosa was isolated almost exclusively from patients with a history of recurrent pneumonia, bronchiectasis, and cystic pulmonary lesions, whereas anaerobic bacteria were isolated from patients with dental and/or ear, nose, and throat (ENT) sources of infection.
Table 4. Documented Bacterial Pneumonia in the French Cohort of STAT3-Deficient Patients.
| Pt | Age at Time of Study (yr) |
STAT3 Mutation |
Age at Time of Pneumonia (yr) |
No. | Bacteria Detected | Complications | Other Complications | Lobectomy | ||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Pleural Effusions | Bronchiectasis | Pneumatocyst | ||||||||
| P1 | 15 | V637M | 1, 3, 5, 6, 7, 10, 14 | 6 | S. aureus, S. pneumoniae | + | + | + | + | |
| P2 | 11 | R382W | 0.7, 2, 7 | >3 | S. pneumoniae | + | + | + | Bronchial fistula | |
| P3 | 39 | F384L | *, 27, 28, 33 | >4 | S. aureus, H. influenzae, P. aeruginosa | |||||
| P4 | 4 | F384L | 2, 2.5 | 2 | NA | + | + | |||
| P5 | 15 | T708N | 5, 6, 7, 9, 11, 12 | 6 | S. pneumoniae | + | + | + | ||
| P6 | 19 | R382Q | 0.3, 8, 8, 13 | 4 | S. pneumoniae, H. influenzae, P. aeruginosa, Branhamella catarrhalis, Klebsiella spp. | + | + | + | ||
| P8 | 29 | T622I | 0.25, 1, 2.5, 4, 4.5, 4.75, 5, 10, 13, 14 | 8 | S. aureus | + | + | + | ||
| P9 | 12 | R382Q | 9 | >2 | S. pneumoniae, H. influenzae | + | + | + | Pneumothorax, bronchopleural fistula | |
| P10 | 24 | R382W | 20, 23 | >4 | S. pneumoniae | + | + | |||
| P11 | 18 | R382W | 3, 17 | 3 | P. aeruginosa, S. pneumoniae | + | + | |||
| P12 | 38 | K642E | 7, 16 | >3 | S. aureus, H. influenzae | |||||
| P13 | 19 | T714I | 3, 14 | 2 | S. aureus, S. pneumoniae | + | ||||
| P14 | 36 | Vdel463 | 0 | NA | + | + | COPD, diffuse interstitial syndrome | |||
| P15 | 11 | Vdel463 | 0 | 0 | ||||||
| P16 | 10 | Vdel463 | 2.5, 3, 3.5, 4, 7 | 5 | S. pneumoniae, H. influenzae | + | + | + | ||
| P17 | 17 | T620A | 0.7, 8, 11, 12, 14, 15 | 6 | H. influenzae | NA | + | + | Pleural fistula, pneumothorax/pneumo-mediastinum | + |
| P18 | 25 | R382W | Birth to 23 | >10 | S. aureus, S. pneumoniae, H. influenzae | + | + | + | + | |
| P19 | 15 | S611G | 4, 5, 14 | 3 | NA | + | + | |||
| P20 | 23 | R382W | 3, 9 | >2 | S. aureus | + | ||||
| P21 | 20 | S668Y | 0.1, 9 | >3 | S. pneumoniae | + | Chronic bronchorrhea | |||
| P22 | 33 | R382Q | 5, 5.5, 30 | 3 | Prevotella oralis | + | + | |||
| P23 | 10 | R382Q | 0 | 0 | ||||||
| P24 | 16 | V343F | 4 | 1 | Mycoplasma pneumoniae | + | + | + | ||
| P25 | 22 | R382W | 8, 10, 11, 14, 17 | >6 | S. pneumoniae, H. influenzae | |||||
| P26 | 23 | V713M | 3, 20 | >3 | S. aureus | + | + | + | ||
| P27 | 10 | S614G | 5, 6, 7 | 4 | S. pneumoniae | |||||
| P28 | 29 | R382Q | 1, 4, 6, 16 | 4 | S. aureus | + | + | + | ||
| P29 | 4 | R382Q | 2, 3 | 2 | S. aureus, P. aeruginosa | |||||
| P30 | 2 | R382Q | 0.4 | 1 | NA | |||||
| P31 | 7 | K709E | 3, 3.5, 6, 7 | 4 | S. pneumoniae, P. aeruginosa, S. aureus | + | + | + | Bronchopleural fistula | + |
| P32 | 15 | R382W | 3.5, 1, 11, 12 | 5 | M. pneumoniae, E. coli, P. aeruginosa, S. aureus | Tracheal stenosis | ||||
| P33 | 26 | V637M | 2, 11, 15, 19 | 4 | S. aureus, E. coli, P. aeruginosa | + | + | + | Hemoptysis | + |
| P34 | 18 | V637M | 4, 6, 12 | >3 | P. aeruginosa | |||||
| P35 | 30 | Vdel463 | 3, 5, 5.5,11 | 4 | S. aureus, S. pneumoniae | + | + | |||
| P37 | 43 | N472D | NA | 1 | S. aureus | + | Encysted pleural effusion | |||
| P39 | 30 | R382W | 1, 3, 15 | >3 | NA | + | + | + | Pneumothorax, hemoptysis | + |
| P40 | 28 | R382W | 0.75, 4, 5, 17, 28 | >5 | S. aureus, H. influenzae | + | + | + | + | |
| P42 | 11 | R382Q | 5, 5.5, 7 | >3 | S. aureus, S. pneumoniae, M. pneumoniae | + | + | |||
| P43 | 38 | Vdel463 | *, 24, 29, 34, 36, 37 | 7 | S. aureus, Enterobacteria | + | + | |||
| P44 | 11 | Vdel463 | 3 | 2 | S. pneumoniae | + | + | + | ||
| P45 | 1 | Vdel463 | 1 | 1 | S. pneumoniae | + | + | |||
| P46 | 19 | N472D | 9, 13 | 2 | NA | + | ||||
| P47 | 37 | V637M | 3 | >3 | S. aureus | NA | + | + | Hemoptysis | |
| P48 | 45 | I665N | 16, 28 | >3 | NA | + | + | |||
| P49 | 17 | I665N | 7 | 1 | NA | |||||
| P50 | 19 | K709E | 13 | >4 | NA | + | + | |||
| P51 | 19 | R382W | 2, 14, 16 | >3 | NA | + | NA | NA | ||
| P52 | 8 | P639S | 1, 2 | 2 | S. aureus, S. pneumoniae | + | + | |||
| P54 | 25 | G617V | 1, 2, 4 | 4 | S. aureus, S. pneumoniae | + | + | + | Pyopneumothorax | + |
| P56 | 38 | Y657C | NA | 3 | S. aureus, S. pneumoniae, H. influenzae | + | ||||
| P57 | 27 | V637M | 0.6, * | 3 | S. aureus | + | + | + | ||
| P58 | 46 | Q649H | NA | NA | S. pneumoniae | + | ||||
| P59 | 0.1 | Vdel463 | 0 | 0 | ||||||
| P60 | 7 | F384L | 0 | 0 | ||||||
Abbreviations: COPD = chronic obstructive pulmonary disease, NA = not available.
Several bouts of pneumonia during childhood at undetermined ages.
Lung Complications
Lung sequelae were reported in 67% (40/60) of patients, taking the form of bronchiectasis in 65% (39/60) of patients or pneumatocele in 52% (31/60) of patients, at a mean age of 21 years (range, 1-45 yr). Other complications also occurred: bronchopleural fistulas, hemoptysis, pneumothorax and/or pneumomediastinum, and tracheal stenosis (see Table 4). As a consequence, 22% (13/60) of patients required atleast 1 lung lobectomy (range, 1-3) at a median age of 10 years (range, 1-17 yr), to control a bacterial infection or an invasive pulmonary fungal infection, and/or to cure a lung complication. Aspergillus species caused disease in 13 of 60 (22%) patients in the cohort (Table 5), with a favorable outcome in all cases. Eight of the 13 patients were diagnosed with an Aspergillus species-related pulmonary invasive fungal infection, based on EORTC/MSG consensus definitions. Invasive fungal infection was considered proven in 4 cases and possible (due to the absence of microbiologic confirmation) in another 4 cases, with a mean age at onset of 11 years (range, 3-16 yr). Four patients developed a pulmonary aspergilloma within pneumatocele cavities, at a mean age at onset of 12.7 years (range, 10-16 yr). Bronchial Aspergillus species colonization was observed in 5 patients, and it preceded the onset of invasive aspergillosis in 4 other patients. A. fumigatus was isolated in 7 of 9 cases. All of the patients with Aspergillus species-related manifestations had already suffered from atleast 3 episodes of recurrent bacterial pneumonia, complicated with secondary bronchiectasis and/or pneumatoceles, suggesting that these complications may be an essential underlying condition for Aspergillus colonization.
Table 5. Aspergillus Species-Related Manifestations in the French Cohort of STAT3-Deficient Patients.
| Pt | STAT3 Mutation | Age* (yr) | >3 Previous Pneumonia Episodes | Bronchiectasis | Pneumatocele | Bronchial Colonization | Aspergilloma | Invasive Pulmonary Aspergillosis | Culture | Aspergillus Species | Surgery |
|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | V637M | 10 | + | + | Bilateral | + | Possible | - | A. fumigatus | ||
| P3 | F384L | 30 | + | + | Unilateral | + | + | Aspergillus spp. | |||
| P6 | R382Q | 13, 16 | + | + | Bilateral | + | + | Proven | + | A. fumigatus | + |
| P11 | R382W | 16 | + | + | Possible | - | NA | ||||
| P18 | R382W | 10 | + | + | Unilateral | + | Proven | + | A. fumigatus | + | |
| P20 | R382W | 9 | + | + | - ** | Aspergillus spp. | |||||
| P32 | R382W | 3 | + | + | + | + | Possible | + | A. fumigatus | ||
| P33 | V637M | 15, 19 | + | + | Bilateral | + | Proven | + | A. fumigatus | + | |
| P39 | R382W | 14 | + | + | Bilateral | + | Suspected | + | A. fumigatus | ||
| P40 | R382W | 10, 11 | + | + | Bilateral | + | + | Proven | + | A. fumigatus | + |
| P52 | P639S | 1.5 | + | + | + | + | A. fumigatus, A. versicolor | ||||
| P54 | G617V | NA | + | + | + | + | - | Aspergillus spp. | + | ||
| P56 | Y657C | NA | + | + | Possible | - | NA |
Abbreviations: See previous tables.
Age at time of Aspergillus spp.-related disease.
Direct examination of bronchoalveolar lavage revealed Aspergillus filaments, whereas culture remained negative.
Ear, Nose, and Throat Infections
ENT infections had occurred since very early childhood but were also frequently reported in adults. They affected 90% (54/60) of patients and consisted of recurrent otitis media in 73% (44/60) of patients (ranging from 0 to 10 episodes), usually complicated with chronic otorrhea, external otitis in 27% (16/60) of patients, and cholesteatoma in 1 patient at age 17 years (see Table 1). Recurrent and chronic sinusitis were observed in 40% (24/60) of patients; severe pharyngitis, laryngitis, and/or rhinitis in 20% (12/60); mastoiditis in 10% (6/60); and tonsillitis and parapharyngeal abscesses in 10% (6/60) of patients. The bacteria most frequently isolated were P. aeruginosa in 40% (14/35) of episodes and Staph. aureus in 37% (13/35) of episodes, mostly corresponding to recurrent and/or chronic ENT infections. Other bacteria (8/34 documented episodes) were less frequently isolated due to the lack of systematic microbiologic documentation in ENT infections: S. pneumoniae, Escherichia coli, Klebsiella pneumoniae, H. influenzae, and Moraxella catarrhalis. P. aeruginosa bacteria were detected in all cases of external otitis. No case of ENT infections with filamentous fungi was documented for this cohort.
Other Infections
Septicemia was reported in 8 patients, with a median age at onset of 5.5 years (range, 1 mo to 39 yr). It was caused by Staph. aureus in 5 cases and S. pneumoniae in 3 cases, with the lung identified as the source of infection in half the cases. Two patients had central nervous system infections: Staph. aureus meningitis occurred secondary to a neurosurgical procedure in 1 patient at age 26 years, and pneumococcal meningitis occurred at age 15 years in 1 patient who had undergone surgery in early childhood for craniosynostosis. Deep-seated abscesses occurred in 7 patients, at a median age at onset of 9.5 years (range, 1-32 yr): liver abscesses (n = 5) with Staph. aureus isolated in 1 and S. pneumoniae in another case, purulent mediastinitis (n = 2), and pararectal abscess caused by Staph. aureus (n = 1). Osteoarticular infections were reported, with a median age at onset of 14.5 years (range, 1-39 yr), in 22% (13/59) of patients, with a total of 18 episodes of osteitis (n = 7), osteomyelitis (n = 3), spondylodiscitis (n = 3), osteoarthritis (n = 2), arthritis (n = 1), and subperiosteal abscess (n = 1). Staph. aureus was isolated from 76% (10/13) of osteoarticular infections. There were 3 cases of acute E. coli pyelonephritis, 5 patients had recurrent bacterial conjunctivitis, 3 had blepharitis, and 4 had recurrent chalazion (due to Streptococcus agalactiae in 1 case). Finally, bacillus Calmette-Guerin vaccination was performed in 60.5% (20/33) of informative patients, with no adverse effects.
Prophylaxis to Prevent Infections
Antibacterial prophylaxis was administered to 90% (54/60) of patients of this cohort after diagnosis of the primary immunodeficiency, 50% (30/60) of patients received antifungal prophylaxis, and 53% (32/60) of patients underwent polyvalent IgG infusions (Table 6). Preventive treatment included antibiotic prophylaxis with oral cotrimoxazole, penicillin, macrolides, and/or cephalosporin in most cases. Antibiotic prophylaxis began at a mean age of 7.8 years (range, 2 mo to 39 yr). Cotrimoxazole was replaced by cloxacillin, with a high degree of efficacy, in 6 of 7 adult patients with recurrent bacterial infections despite prophylactic cotrimoxazole treatment. Azithromycin was added to the pre-existing prophylactic regimen in 3 patients, for the purposes of immune modulation. Antibiotic prophylaxis was discontinued in 5 patients with chronic infections. Four patients did not receive antibiotic prophylaxis, 1 patient (Patient 45) died from septic shock due to S. pneumoniae and a second (Patient 47) had bilateral chronic otorrhea and chronic obstructive pulmonary disease with bronchiectasis and pulmonary cyst lesions. The remaining 2 patients had few infections.
Table 6. Prophylaxis Against Infections in the French Cohort of STAT3-Deficient Patients.
| No. of Patients | ||
|---|---|---|
| Before 2007* | After 2007* | |
| No prophylaxis | 3 | 8 |
| Antibacterial prophylaxis | 43/46 | 50/60 |
| Cotrimoxazole alone | 22 | 29 |
| Penicillins (Cloxacillin) | 3 (1) | 8 (7) |
| Alternating antibiotic prophylaxis | 18 | 12 |
| +/- Cotrimoxazole | NA | 7 |
| +/- Pristinamycin | NA | 6 |
| +/- Macrolide | NA | 3 |
| +/- Cephalosporin or penicillin | NA | 8 |
| +/- Other (quinolone, rifampicin, fucidic acid) | NA | 4 |
| Azithromycin† | 1 | 3 |
| IgG treatment (IV or SC) | 12/46 | 32/60 |
| IgG treatment alone | 2 | 2ss |
| Antibiotic prophylaxis and IgG treatment | 10 | 30 |
| Antifungal prophylaxis | 16/42 | 30/60 |
| Primary | 11 | 20 |
| Itraconazole | 10 | 18 |
| Voriconazole | 1 | 2 |
| Secondary | 5 | 10 |
| Itraconazole | 4 | 5 |
| Voriconazole | 1 | 5 |
| Voriconazole + caspofungin | 0 | 1 |
Abbreviations: IgG = immunoglobulins, IV = intravenous, SC = subcutaneous.
2007 is considered the date of reference for the STAT3 mutation identification period.
Azithromycin was always added to a preexisting prophylactic regimen and was included for immune modulation rather than antibacterial prophylaxis.
Preventive treatment included antifungal prophylaxis in 50% of patients, with a mean age at initiation of 12 years (range, 2 mo to 28 yr). Twenty patients received primary antifungal prophylaxis in the form of itraconazole or voriconazole, and 10 patients received secondary antifungal prophylaxis in the form of itraconazole, voriconazole, and/or caspofungin (see Table 6). Four of these patients developed invasive pulmonary aspergillosis despite appropriate antifungal prophylaxis.
Thirty-two (54%) patients received IgG treatment (400-500 mg/kg every 3 or 4 wk by the intravenous route, or 100 mg/kg per week by the subcutaneous route); all but 2 young patients under the age of 4 years had an NIH score above 40. The mean age for the initiation of IgG infusions was 14 years (range, 1-39 yr) (Table 7). We evaluated the impact of IgG infusions on the incidence of bacterial pneumonia in the 32 patients who received IgG. We had access to data for 449 years and 140 years of follow-up without and with IgG treatment, respectively. The incidence of bacterial pneumonia was 27.8/100 patients per year in the absence of IgG treatment and 9.3/100 patients per year with IgG treatment; this difference was highly significant, with a [chi][sup]2 value of 20.47 (p = 6 × 10[sup] -6). Thus, prophylaxis combining oral antibiotics and IgG injections seems to be particularly beneficial in patients with recurrent pulmonary infections, and antifungal prophylaxis should be offered to patients with pneumatocele or bronchiectasis.
Table 7. IgG Treatment in the French Cohort of STAT3-Deficient Patients.
| Pt | Age at Initiation (yr) | NIH Score | Year at Initiation | Route of Injection | Frequency of Ig Treatment | No. of Pneumonia Episodes | ||
|---|---|---|---|---|---|---|---|---|
| Birth to IgG | Age 5 yr to IgG | After IgG Therapy | ||||||
| P1 | 9 | 57 | 2004 | IV then SC | Continuous | 5 | 3 | 1 |
| P2 | 9 | 50 | 2009 | SC | Continuous | >3 | 1 | 0 |
| P3 | 39 | 72 | 2010 | IV then SC | Continuous | >4 | 2 | 0 |
| P5 | 11 | 62 | 2006 | IV | Continuous | 5 | 4 | 1 |
| P6 | 9 | 73 | 2000 | IV then SC | Continuous | 3 | 2 | 1 |
| P9 | 11 | 51 | 2009 | IV | Continuous | >2 | 2 | 0 |
| P10 | 20 | 73 | 2006 | IV | Continuous | >3 | 2 | 1 |
| P11 | 13 | 74 | 2005 | IV | Continuous | >3 | 1 | 1 |
| P16 | 7 | 59 | 2007 | IV | Continuous | 5 | 5 | 0 |
| P17 | 9 | 52 | 2002 | IV | Continuous | 2 | 2 | 4 |
| P18 | 25 | 84 | 2010 | IV | Continuous | >10 | 2 | 0 |
| P19 | 13 | 70 | 2009 | IV | Continuous | 3 | 1 | 0 |
| P21 | 18 | 74 | 2008 | IV then SC | Discontinuous | >3 | 2 | 0 |
| P25 | 11-16 | 86 | 1999-2003 | IV | Discontinuous | 3 | 3 | 2 |
| P26 | 20 | 69 | 2007 | IV | Continuous | 3 | 1 | 0 |
| P27 | 8 | 53 | 2008 | IV | Continuous | 4 | 3 | 0 |
| P28 | 29 | 67 | 2009 | IV | Continuous | 4 | 0 | 0 |
| P29 | 3 | 33 | 2009 | IV | Continuous | 2 | 2 | 0 |
| P30 | 1 | 25 | 2009 | IV | Continuous | 1 | 1 | 0 |
| P31 | 6 | 64 | 2009 | IV then SC | Continuous | 3 | 3 | 1 |
| P32 | 14 | 54 | 2009 | NA | Continuous | 5 | 3 | 0 |
| P33 | 24 | 73 | 2007 | IV | Continuous | >3 | 1 | 0 |
| P34 | 14 | 64 | 2007 | IV | Stopped in 2008 | >3 | 2 | 0 |
| P36 | 20 | 44 | 2009 | Continuous | 0 | 0 | 0 | |
| P39 | 22 | 74 | 2002 | IV | Continuous | >3 | 0 | 0 |
| P40 | 28 | 73 | 2010 | IV | Continuous | >5 | 2 | 0 |
| P42 | 7 | 59 | 2005 | IV | Continuous | >3 | 3 | 0 |
| P43 | 37 | 79 | 2008 | IV | Continuous | 7 | 3 | 0 |
| P44 | 4 | 55 | 2003 | NA | Continuous | 2 | 2 | 0 |
| P50 | 4 | 77 | 1995 | IV | Continuous | >4 | 3 | 1 |
| P52 | <1 | 51 | 2002 | IV | Continuous | 1 | 1 | 0 |
| P57 | 12 | 79 | 1995 | IV | Continuous | 1 | 1 | 0 |
Abbreviations: See previous tables. Ig = immunoglobulins, IV = intravenous route of injection with a monthly frequency, SC = subcutaneous route of injection with a weekly frequency.
Dysmorphia and Abnormalities of Connective Tissue and Skeleton
Dysmorphia was observed in 95% of patients, with a high and prominent forehead in 85% (51/60), prognathism or retrognathism in 53% (32/60), enlargement of the interalar distance in 85% (51/60), thickening of the soft tissues of the ear or nose in 73% (44/60), and high arched palate in 53% (32/60) of patients (see Table 1). Dysmorphia clearly tended to increase with age. The retention of deciduous teeth was observed in 65% (34/52) of informative patients over the age of 8 years. Abnormal bone fractures had occurred in 42% of patients (25/60; 56 fractures were registered) with a median of 2 fractures per patient (range, 1-10). Half the patients had undergone bone mineral density explorations, and osteopenia was observed in 59% (17/29) of these patients. Joint hyperextensibility was found in 50% (30/60) of patients, including genu valgum, varum or recurvatum and/or varus foot; episodes of unusual joint dislocation and scoliosis were found in 38% (23/60) of patients. Some other skeletal abnormalities were reported, including craniosynostosis (n = 2), retracted tendons (n = 2), spondylolisthesis L5-S1 (n = 1), and spina bifida occulta (n = 1). We also noted neurologic features, consisting of syringomyelia in Patient 11 treated by surgery at age 5 years and a posterior fossa arachnoid cyst in Patient 35 treated by surgery at age 26 years.
Neoplasia
Four of the 60 (7%) patients (Patients 12, 20, 25, 26) had a history of hematologic malignancy (Table 8). All 4 had suffered from non-Hodgkin lymphoma (NHL), with a mean age at onset of 21 years (range, 8-34 yr): 2 cases of Burkitt lymphoma (1 disseminated and 1 localized), 1 case of disseminated anaplastic lymphoma kinase (ALK)-negative anaplastic NHL, and 1 case of localized diffuse large B-cell lymphoma (DLBCL). No association with EBV was observed. All patients were successfully treated by chemotherapy, with a CHOP (prednisone + vincristine + cyclophosphamide + adriamycine)-type regimen used in 3 patients (Patients 12, 20, 26). Patient 26 also received high-dose methotrexate and cytarabine, followed by autologous stem cell transplantation. Burkitt lymphoma in Patient 25 was treated by an intensified version of the LMB protocol. No serious side effects were observed during treatment, with no exacerbation of infection, fatal sepsis, or unusual toxic side effects in particular. None of these patients underwent radiotherapy, and all remained in complete remission after a median follow-up period of 4 years (range, 1.5-13.5 yr).
Table 8. Non-Hodgkin Lymphoma in the French Cohort of STAT3-Deficient Patients.
| Pt | Age at NHL Diagnosis (yr) | STAT3 Mutation | NHL Type | Immunohistochemistry | Disease Stage | Site Involved | Treatment | Response/Follow-Up |
|---|---|---|---|---|---|---|---|---|
| P12 | 34 | K642E | DLBCL* | CD20+, CD10+,CD5-, Bcl2+, Bcl6+, LMP- | I | Single right inguinal lymph node (8×4×3cm) | R-CHOP 14 × 6 | CR/3 yr |
| P20 | 17 | R382W | Burkitt-like NHL | CD20+, CD10+, Bcl2-, Bcl6+, Ki67 100%, LMP- | I | Single right inguinal lymph node | R-CHOP × 6 (intrathecal CT × 4) | CR/6 yr |
| P25 | 8 | R382W | Burkitt NHL | CD20+, CD30-, Ki67 100%, LMP-, c-myc rearranged | IV | Intraabdominal tumor, mesenteric lymph nodes, bone marrow | French LMB protocol (COP, COPADM, CYM) | CR/14 yr |
| P26 | 21 | V713M | Anaplastic NHL | CD30+, CD15-, ALK-, EMA-, TiA1+, LMP- | IV | Lymph nodes above and below diaphragm, liver, bone marrow | CEEP × 2, MTX + Aracytine × 1, intensification by BEAM with ASCT | CR/2 yr |
Abbreviations: See previous tables. ALK = anaplastic lymphoma kinase, ASCT = autologous stem cell transplantation, BEAM = carmustine + etoposide + cytarabine + melphalan, CEEP = prednisone + Eldisine + cyclophosphamide + epirubicin, CHOP = prednisone + vincristine + cyclophosphamide + Adriamycin, CR = complete remission, CT = chemotherapy, DLBCL = diffuse large B-cell lymphoma, LMB protocol = Lymphome Malin B (French protocol), LMP = latent membrane protein, MTX = methotrexate.
___.[AQ][AQ: Pt 12: DLBCL has an asterisk--add footnote or delete asterisk.]
Other Clinical Features
Cardiovascular manifestations consisted of hypertension complicated with left ventricular hypertrophy in Patient 18, a case of anoxic brain hemorrhagic necrosis consecutive to septic shock (Patient 10), and a history of incidental deep venous thrombosis in 3 patients, with leg phlebitis (Patients 3, 40, 43; 6 episodes) and pulmonary embolism in 2 patients (Patients 3, 40; 3 episodes). Only 1 patient (Patient 42) had brain magnetic resonance imaging at the time of this retrospective study, and she had asymptomatic linear periventricular hyperintensities with white matter hyperintensity at age 7 years. In other patients, neurologic signs were observed including postinfectious myelitis-related spastic tetraparesis in Patient 44 and unexplained ataxia with acute exacerbations in Patient 50. Asthma and allergic manifestations were observed in 8% (5/60) and 22% (13/60) of patients, respectively, and consisted of food allergies (n = 5), respiratory allergies to agents such as pollen and dust mites (n = 4), and 1 case of iodinated contrast agent-related urticaria. Finally, allergies to antibiotics (sulfamethoxazole, penicillins) were found in 8 patients, including 3 who had had sulfamethoxazole-induced Quincke edema early in life (Patients 6, 21, 58) and 1 patient who had tetracycline-induced drug rash with hypereosinophilia and systemic symptoms (DRESS) syndrome. No particular association with autoimmune signs was observed, although specific searches were not performed systematically in all patients.
Discussion
We provide here a detailed description of the clinical, immunologic, and genetic features and outcome of 60 patients with STAT3 deficiency followed in France. We report the functional impact of various heterozygous STAT3 mutations. STAT3 deficiency is a primary immunodeficiency with heterogeneous clinical and laboratory features, and STAT3 mutation screening should be undertaken if clinical and laboratory signs are present, such as atypical eczematiform dermatitis, recurrent cutaneous cold abscesses due to bacteria, chronic mucocutaneous candidiasis, recurrent pneumonia caused by pyogenic bacteria complicated with bronchiectasis and pneumatocyst formation, the development of aspergillosis in these lung lesions, and connective tissue and/or bone abnormalities. [45,62,70] These clinical signs are associated with the following laboratory features: hyper-IgE, eosinophilia, and Th17 lymphopenia. In the current study, as previously reported, [3,65] we found a low percentage of memory B cells, contrasting with a normal total B-cell population in almost all patients. Indeed, 94.5% of the STAT3-deficient patients of this cohort displayed CD27+CD19+ lymphopenia. This evaluation is easier for laboratories to carry out in routine practice than Th17 lymphocyte counts, which requires intracellular staining due to the presence of very small T-cell populations ([lt]2%). [13,43,44,62,70] We suggest that CD19+CD27+ cell counts should be included in recommendations for the diagnosis of patients with AD-HIES, before STAT3 genetic analysis is carried out.
The results obtained for the current series of patients enlarges the spectrum of heterozygous STAT3 mutations. We identified 13 new mutations and confirmed the existence of 11 previously reported mutations, including the 4 recurrent mutations R382W, R382Q, V463del, and V637M (found in 17%, 15.5%, 12%, and 8.6% of patients from this cohort, respectively). These heterozygous STAT3 mutations---R382W, R382Q, V463del, and V637M---have been found in STAT3-deficient patients described in previous studies (21%, 11%, 7%, and 14.5% of patients, respectively, accounting for more than 53% of the mutations identified to date). We have confirmed that these mutations are located at mutation hotspots. [28,29,33,47,55,60,62,70,71] Exons 13, 16, and 21---in which these mutations are located---could thus be sequenced first, in cases of suspected STAT3 deficiency. An impairment of STAT3 phosphorylation and STAT3 translocation to the nucleus and DNA-binding were found in EBV-B cell lines from 9 patients bearing 5 previously identified heterozygous mutations (R382W, R382Q, T412S, Vdel463, and V637M) and from 7 patients bearing 6 previously unidentified heterozygous mutations (N472D, S614G, T708N, K709E, V713M, and T714I). One of these 6 new mutations, K709E, was considered by the Polyphen2 algorithm to have a benign impact in STAT3 functions. However, we showed that this mutation has an important impact on STAT3 phosphorylation and STAT3 nuclear translocation or accumulation in functional experiments. These results indicate that the Polyphen2 algorithm provides an indication, but additional functional assays are required to determine the true impact of each of the new mutations on STAT3 phosphorylation and nuclear translocation.
The 3 hallmarks of the clinical infectious phenotype of STAT3-deficient patients are recurrent “cold” staphylococcal skin abscesses, recurrent bacterial pneumonia, and chronic mucocutaneous candidiasis. All patients in the current cohort had developed staphylococcal skin infections. This frequency of staphylococcal skin infections is similar to that reported for other large cohorts. [29,60,62,70] The blood cells of STAT3-deficient patients display defective responses to IL-6, [47] because STAT3 plays a major role in the signaling pathway downstream from IL-6Rs. [45] Evidence suggesting a specific role of IL-6 in the pathogenesis of staphylococcal disease has been provided by the description of neutralizing circulating anti-IL-6 autoantibodies in a child with recurrent staphylococcal skin disease. [59]
Up to 95% of the patients in the current cohort had recurrent bacterial pneumonia. A similar frequency has been reported for other large cohorts of STAT3-deficient patients (Table 9). [29,60,62,70] After these infections, the major complication is pneumatocele, which was found in up to half the patients in our cohort. Evidence suggestive of a specific role for STAT3 in the pathogenesis of lung infections was provided by the demonstration of STAT3 involvement in the production of antibacterial factors by keratinocytes and bronchial epithelial cells. [46]
Table 9. Frequency of Laboratory and Clinical Signs in Large Series of STAT3-Deficient Patients, Present and Previous Reports.
| Source (First Author, ref.) (%) | ||||
|---|---|---|---|---|
|
| ||||
| Schimke62 (n=48) | Woellner70 (n=64) | Jiao29 (n=12) | French Cohort (PR) (n=60) | |
| NIH score >40 | 96 | 76.7 | NA | 82 |
| Hypereosinophilia | 93 | 70.7 | NA | 80 |
| IgE | 96 | NA | 100 | 96 |
| B memory lymphopenia | NA | NA | NA | 94.5 |
| Th17 lymphopenia | 100 | 53 | NA | 93 |
| Neonatal rash | 74 | 64.9 | 45 | 48 |
| Dermatitis | 98 | 90.6 | 100 | 92 |
| Skin abscesses | 85 | 90.6 | 100 | 100 |
| Pneumonia | 94 | 95.3 | 100 | 90 |
| Pneumatocele | 48 | 74.6 | 45 | 52 |
| Candidiasis | 56 | 43.1 | 64 | 85 |
| Other severe infections | 89 | 56.7 | nd | 43 |
| Retention of deciduous teeth | 79 | 80 | 27 | 65 |
| Bone fractures | 60 | 45.8 | 18 | 42 |
| Dysmorphism | 90 | 90.6 | 100 | 95 |
| Large interalar distance | 22 | 51.9 | NA | 85 |
| Cathedral palate | 22 | 54.7 | NA | 53 |
| Hyperextensibility | 60 | 52.7 | 55 | 50 |
| Scoliosis | 42 | 26 | 27 | 38 |
| Lymphoma | NA | NA | 9 | 7 |
Abbreviations: See previous tables. PR = present report.
About 85% of the patients in this cohort developed chronic candidiasis infection. In the other large cohorts of STAT3-deficient patients previously studied, chronic mucocutaneous candidiasis was reported in 43%, 56% and 64% of patients (see Table 9). [29,62,70] Based on the finding that mouse IL-17-producing T cells play a role in immunity to both systemic and mucosal C. albicans infection, [16] several groups have shown that IL-17-producing T-cell lymphopenia occurs in STAT3-deficient patients. [13,43,44,46,60] Evidence for a specific role of IL-17-producing T cells in the pathogenesis of mucosal C. albicans infection was recently provided by the description, in patients with isolated chronic mucocutaneous candidiasis, of autosomal recessive IL-17RA deficiency or AD-IL-17F deficiency, indicating that human IL-17A and IL-17F are essential for protective immunity to C. albicans and, to a lesser extent, Staph. aureus in mucosae, but that they are otherwise redundant. [56-58] Moreover, we recently found gain-of-function STAT1 mutations in patients with chronic mucocutaneous candidiasis. [42] Mutations in the same domain of STAT1 were found independently by another group. [67] The mechanism involves loss-of-STAT1 dephosphorylation, and the gain-of-function results in enhanced cellular responses to STAT1-dependent IL-17 inhibitors IL-27, IFN[alpha]/[beta], and IFN[gamma], as well as STAT3-dependent IL-17 inducers IL-6, IL-21, and possibly IL-23. [42] These experiments of nature neatly illustrate how the genetic dissection of infectious diseases will illuminate the pathogenesis of the various infections seen in AD-HIES patients. [1] The pathogenesis of staphylococcal disease in HIES patients, atleast the cutaneous lesions, might result from impaired IL-6 immunity. [59]
Clinical presentation tended to improve with time, probably due to correct diagnosis and more appropriate care, except in patients with progressive pulmonary insufficiency and chronic bronchial suppuration. [20] Preventive treatment included antibiotic prophylaxis, mostly with cotrimoxazole. [66] However, antibiotics specifically targeting Staph. aureus, such as cloxacillin, are a good alternative (C. Aguilar, personal communication). The documentation of bacterial infections also facilitated the adaptation of antibiotic treatment to the microbiologic ecology of each patient. Such antibiotic prophylaxis was clearly of benefit for skin infections and external otitis. However, it was difficult to evaluate the effect of antibiotic prophylaxis, because almost all the patients in our cohort had been on such prophylaxis since childhood.
A positive impact of prophylaxis for bacterial infections was also observed with IgG treatment in this study. The rationale for IgG treatment was based on the recurrence of pyogenic bacterial infections with a particular lung and ENT tropism and the occurrence of memory B-cell lymphopenia with an accelerated decline of specific antibody titers after initially normal primary Antibody responses and the lower than normal secondary Antibody responses observed in STAT3-deficient patients in the present report and previous studies. [3,4,63,65] The duration of follow-up from the initiation of IgG treatment in the current cohort is short, but a decrease in the frequency of bacterial pneumonia was observed, with the occurrence of atleast 1 episode of bacterial pneumonia in 27.8% of years without IgG treatment and 9.3% of years with IgG treatment. These encouraging results are preliminary and require confirmation and validation in prospective studies to evaluate the precise impact of IgG treatment on the prevention of lung abnormalities. An in vivo study of the kinetics of antibody production in STAT3-deficient patients is also now required.
Antifungal prophylaxis should be offered to patients with structural airway abnormalities. The mechanism responsible for invasive fungal infections in STAT3-deficient patients is probably related to local epithelial impairment due to structural injuries following recurrent bacterial pneumonia, rather than myeloid dysfunction. [22,46,68] The prevention and treatment of these destructive pulmonary lesions by prophylactic surgery to exclude pneumatocele formation may constitute a valuable way of preventing filamentous fungal infections in STAT3-deficient patients.
Characteristic facial features in STAT3-deficient patients include dysmorphia, with a prominent forehead, broad nasal bridge, and abnormally large interalar distance. [22] STAT3-deficient patients also have bone abnormalities, including recurrent pathologic fractures with osteoporosis, delayed shedding of deciduous teeth, hyperextensibility of joints, scoliosis, and craniosynostosis. [23] The percentages of patients with dysmorphia, delayed shedding of the deciduous teeth, and skeletal features in the current cohort were similar to those reported for other cohorts of STAT3-deficient patients. [29,60,62,70] It is noteworthy that in St[uml]uve-Wiedemann syndrome, a severe autosomal recessive condition caused by a null mutation in the leukemia inhibitory factor receptor gene (LIFR), [11,31] LIFR-deficient patients had several clinical features in common with STAT3-deficient patients, including osteoporosis, spontaneous fractures, joint hyperextensibility, and scoliosis. Several studies in mice have confirmed the role of LIFR in the control of osteoclastogenesis, [7] and STAT3 has been implicated in the signaling pathway of LIFR, which is associated with gp130. [25] The skeletal features reported in STAT3-deficient patients probably result from impairment of the LIFR signaling pathway. Other features, such as the retention of primary teeth, result from abnormal IL-11 responses, as suggested by the recent identification of IL-11R deficiency in patients with cranial dysostosis and dental anomalies (49). It is probable that the genetic dissection of the various phenotypes associated with the HIES will proceed along these lines, not only for the infectious and tumoral features (IL-17F, IL-17RA), but also for the developmental features (LIFR, IL11R).
However, the noninfectious features of STAT3-deficient patients were of less importance than the frequency and severity of infections, with the exception of lymphoma. We confirm the association of STAT3 deficiency and lymphoma in this series, as already reported. [35,37] All the lymphoma cases reported in this cohort were unrelated to EBV. [69] The 22 previously reported cases included 6 of T-cell origin; 12 of B-cell origin, NHL; and 4 cases of Hodgkin lymphoma. Half were nodal and most were unrelated to EBV. [35] The median age at presentation of lymphoma was 21 years in the current cohort (range, 8-34 yr), and 19 years in previous studies (range, 4-69 yr). [35] All patients with lymphoma in the current report were alive at the end of follow-up, with persistent complete remission of the lymphoma, whereas 9 of the patients described in previous studies died. Gain-of-function somatic mutations in the STAT3 gene are known to be associated with carcinoma and hematologic malignancies including multiple myeloma, leukemia, and lymphoma. [15,34,72] By contrast, no such association has been reported for loss-of-function mutations of the STAT3 gene. As STAT3 is an oncogene, the occurrence of lymphomas in these patients is paradoxical and unexplained. Further investigations are required to improve our understanding of the mechanism of tumorigenesis in patients with germline loss-of-function STAT3 mutations.
In conclusion, recurrent bacterial skin and/or respiratory tract infections were consistently observed in the STAT3-deficient patients of the current cohort. Staph. aureus was the most commonly isolated pathogen by far, documented in all informative patients and in 72% (250/345 episodes) of all documented bacterial episodes. Most patients experienced their first infection very early in life, with neonatal infection reported in 39% of patients and infections during the first year of life in 81% (48/59) of patients. Fungal infections were also frequent: 85% of patients had chronic mucocutaneous candidiasis, and 22% had aspergillosis infection and/or colonization. STAT3-deficient patients clearly benefit from stringent clinical follow-up, with appropriate continuous antibiotic prophylaxis, IgG therapy in patients with recurrent lung infections, antifungal prophylaxis in patients with lung abnormalities, and good compliance with treatment and follow-up, which should be monitored with particular attention.
Supplementary Material
Supplementary Figure 1. STAT3 phosphorylation in EBV-B cells from patients and controls, as assessed by intracellular flow cytometry. Induction of phospho-STAT3 in EBV-B cell lines from healthy controls (squares); 8 STAT3-deficient patients with DNA-binding domain (DBD) mutations (R382W [lsqb]Patient 18[rsqb], R382Q [lsqb]Patient 22[rsqb], T412S [lsqb]Patient 36[rsqb], Vdel463 [lsqb]Patients 14, 15, 16, 44[rsqb], N472D [lsqb]Patient 46]) (triangles); 2 STAT3-deficient patients with SH2 domain mutations (S614G [lsqb]Patient 27[rsqb], V637M [lsqb]Patient 1]) (diamonds); and 3 STAT3-deficient patients with TA domain mutations (T708N [lsqb]Patient 5[rsqb], K709E [lsqb]Patient 50[rsqb], V713M [lsqb]Patient 26]) (filled circles), in response to IL-6 (a), IL-10 (b), IL-21 (c), and IFN-[alpha] (d). Scatter plots (with median line) showing the mean percentages of phospho-STAT3-positive cells obtained following cytokine stimulation, for each subject for whom atleast 2 independent experiments were carried out. The gray circle designates the patient (Patient 50) with heterozygous K709E STAT3 mutation.
Supplementary Figure 2. STAT3 nuclear translocation in EBV-B cells from patients and controls, as assessed by EMSA ELISA. The values shown represent ratios of absorbance at 450 nm, for nuclear samples extracted from cytokine-stimulated or nonstimulated cells. STAT3 nuclear translocation from control EBV-B cell lines (squares); 8 STAT3-deficient patients with DNA-binding domain (DBD) mutations (R382W [lsqb]Patient 18[rsqb], R382Q [lsqb]Patient 22[rsqb], T412S [lsqb]Patient 36[rsqb], Vdel463 [lsqb]Patients 14, 15, 16, 44[rsqb], N472D [lsqb]Patient 46]) (triangles); 3 STAT3-deficient patients with SH2 domain mutations (S614G [lsqb]Patient 27[rsqb], V637M [lsqb]Patients 1, 34]) (diamonds); and 5 STAT3-deficient patients with TA domain mutations (T708N [lsqb]Patient 5[rsqb], K709E [lsqb]Patients 31, 50[rsqb], V713M [lsqb]Patient 26[rsqb], T714I [lsqb]Patient 13]) (filled circles), in response to IL-6 (a), IL-10 (b), IL-21 (c), and IFN-[alpha] (d). Each dot represents a mean value obtained for a single subject for whom atleast 3 independent experiments were performed. The gray circle designates the patient (Patient 50) with heterozygous K709E STAT3 mutation.
Supplementary Figure 3. Defective expression of the STAT3 target gene SOCS3. SOCS3 gene induction in EBV-B cell lines from healthy controls (squares); 8 STAT3-deficient patients with heterozygous DNA-binding domain (DBD) mutations (R382W [lsqb]Patient 18[rsqb], R382Q [lsqb]Patient 22[rsqb], T412S [lsqb]Patient 36[rsqb], Vdel463 [lsqb]Patients 14, 15, 16, 44[rsqb], N472D [lsqb]Patient 46]) (triangles); 4 STAT3-deficient patients with heterozygous SH2 domain mutations (S614G [lsqb]Patient 27[rsqb], V637M [lsqb]Patients 1, 34[rsqb], S668Y [lsqb]Patient 21]) (diamonds); and 5 STAT3-deficient patients with heterozygous TA domain mutations (T708N [lsqb]Patient 5[rsqb], K709E [lsqb]Patients 31, 50[rsqb], V713M [lsqb]Patient 26[rsqb], T714I [lsqb]Patient 13]) (filled circles), in response to IL-6 (a), IL-10 (b), IL-21 (c), and IFN-[alpha] (d), as evaluated by quantitative RT-PCR. The gray circle designates the patient (Patient 50) with heterozygous K709E STAT3 mutation.
Acknowledgments
We thank the patients and their families for their trust and cooperation. We thank Maya Chrahieb, Martine Courat, Michele N'Guyen, and Yelena Nemirovskaya for technical and secretarial assistance. We thank Alexandre Bolze and Dr. Laurent Abel from Laboratory of Human Genetics of Infectious Diseases, Dr. Lucas Peltier from CEDI, and Dr. Pauline Brosselin from CEREDIH.
Financial support: The Laboratory of Human Genetics of Infectious Diseases was supported by the March of Dimes, the Dana Foundation, and INSERM. The St Giles Laboratory of Human Genetics of Infectious Diseases was supported by grants from The Rockefeller University Center for Clinical and Translational Science grant number 5UL1RR024143-03 and The Rockefeller University. IM was supported by the Fondation pourla Recherche M edicale. JLC was an International Scholar of the Howard Hughes Medical Institute from 2005 to 2008.
Abbreviations
- AD
autosomal dominant
- CEREDIH
Centre de Reference des Deficits Immunitaires Hereditaires
- DRESS
drug rash with hypereosinophilia and systemic symptoms
- EBV-B cells
Epstein-Barr virus-transformed B lymphocytes
- EMSA ELISA
electrophoretic mobility shift assay/enzyme-linked immunosorbent assay
- ENT
ear, nose, and throat
- EORTC/MSG
European Organization for Research and Treatment of Cancer/Mycoses Study Group
- HIES
hyper-immunoglobulin E syndrome
- HSV
herpes simplex virus
- IFN
interferon
- Ig
immunoglobulin
- IL
interleukin
- LIF
leukemia inhibitory factor
- LIFR
leukemia inhibitory factor receptor
- NHL
non-Hodgkin lymphoma
- NIH
National Institutes of Health, PCR, polymerase chain reaction
- RSV
respiratory syncytial virus
- RT
reverse transcription
- SD
standard deviation
- SH2
Src homology 2
- SOCS
suppressor of cytokine signaling
- STAT3
signal transducer and activator of transcription 3
- TA
transactivation
Footnotes
Conflicts of interest: The authors have no conflict of interest to disclose.
References
- 1.Alcais A, Quintana-Murci L, Thaler DS, Schurr E, Abel L, Casanova JL. Life-threatening infectious diseases of childhood: single-gene inborn errors of immunity? Ann N Y Acad Sci. 2010;1214:18–33. doi: 10.1111/j.1749-6632.2010.05834.x. [DOI] [PubMed] [Google Scholar]
- 2.Anolik R, Elmariah S, Lehrhoff S, Votava HJ, Martiniuk FT, Levis W. Hyperimmunoglobulin E syndrome with a novel STAT3 mutation. Dermatol Online J. 2009;15:16. [PubMed] [Google Scholar]
- 3.Avery DT, Deenick EK, Ma CS, Suryani S, Simpson N, Chew GY, Chan TD, Palendira U, Bustamante J, Boisson-Dupuis S, Choo S, Bleasel KE, Peake J, King C, French MA, Engelhard D, Al-Hajjar S, Al-Muhsen S, Magdorf K, Roesler J, Arkwright PD, Hissaria P, Riminton DS, Wong M, Brink R, Fulcher DA, Casanova JL, Cook MC, Tangye SG. B cell-intrinsic signaling through IL-21 receptor and STAT3 is required for establishing long-lived antibody responses in humans. J Exp Med. 2010;207:155–171. doi: 10.1084/jem.20091706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avery DT, Ma CS, Bryant VL, Santner-Nanan B, Nanan R, Wong M, Fulcher DA, Cook MC, Tangye SG. STAT3 is required for IL-21-induced secretion of IgE from human naive B cells. Blood. 2008;112:1784–1793. doi: 10.1182/blood-2008-02-142745. [DOI] [PubMed] [Google Scholar]
- 5.Belohradsky BH, Daumling S, Kiess W, Griscelli C. The hyper-IgE-syndrome (Buckley- or Job-syndrome) Ergeb Inn Med Kinderheilkd. 1987;55:1–39. [PubMed] [Google Scholar]
- 6.Borges WG, Hensley T, Carey JC, Petrak BA, Hill HR. The face of Job. J Pediatr. 1998;133:303–305. doi: 10.1016/s0022-3476(98)70243-4. [DOI] [PubMed] [Google Scholar]
- 7.Bozec A, Bakiri L, Hoebertz A, Eferl R, Schilling AF, Komnenovic V, Scheuch H, Priemel M, Stewart CL, Amling M, Wagner EF. Osteoclast size is controlled by Fra-2 through LIF/LIF-receptor signalling and hypoxia. Nature. 2008;454:221–225. doi: 10.1038/nature07019. [DOI] [PubMed] [Google Scholar]
- 8.Buckley RH, Becker WG. Abnormalities in the regulation of human IgE synthesis. Immunol Rev. 1978;41:288–314. doi: 10.1111/j.1600-065x.1978.tb01469.x. [DOI] [PubMed] [Google Scholar]
- 9.Buckley RH, Wray BB, Belmaker EZ. Extreme hyperimmunoglobulinemia E and undue susceptibility to infection. Pediatrics. 1972;49:59–70. [PubMed] [Google Scholar]
- 10.Cohen-Solal M, Prieur AM, Prin L, Denne MA, Launay JM, Graulet AM, Brazier M, Griscelli C, de Vernejoul MC. Cytokine-mediated bone resorption in patients with the hyperimmunoglobulin E syndrome. Clin Immunol Immunopathol. 1995;76:75–81. doi: 10.1006/clin.1995.1090. [DOI] [PubMed] [Google Scholar]
- 11.Dagoneau N, Scheffer D, Huber C, Al-Gazali LI, Di Rocco M, Godard A, Martinovic J, Raas-Rothschild A, Sigaudy S, Unger S, Nicole S, Fontaine B, Taupin JL, Moreau JF, Superti-Furga A, Le Merrer M, Bonaventure J, Munnich A, Legeai-Mallet L, Cormier-Daire V. Null leukemia inhibitory factor receptor (LIFR) mutations in Stuve-Wiedemann/Schwartz-Jampel type 2 syndrome. Am J Hum Genet. 2004;74:298–305. doi: 10.1086/381715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis SD, Schaller J, Wedgwood RJ. Job's syndrome. Recurrent, “cold”, staphylococcal abscesses. Lancet. 1966;1:1013–1015. doi: 10.1016/s0140-6736(66)90119-x. [DOI] [PubMed] [Google Scholar]
- 13.de Beaucoudrey L, Puel A, Filipe-Santos O, Cobat A, Ghandil P, Chrabieh M, Feinberg J, von Bernuth H, Samarina A, Janniere L, Fieschi C, Stephan JL, Boileau C, Lyonnet S, Jondeau G, Cormier-Daire V, Le Merrer M, Hoarau C, Lebranchu Y, Lortholary O, Chandesris MO, Tron F, Gambineri E, Bianchi L, Rodriguez-Gallego C, Zitnik SE, Vasconcelos J, Guedes M, Vitor AB, Marodi L, Chapel H, Reid B, Roifman C, Nadal D, Reichenbach J, Caragol I, Garty BZ, Dogu F, Camcioglu Y, Gulle S, Sanal O, Fischer A, Abel L, Stockinger B, Picard C, Casanova JL. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J Exp Med. 2008;205:1543–1550. doi: 10.1084/jem.20080321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler CC, Kullberg BJ, Marr KA, Munoz P, Odds FC, Perfect JR, Restrepo A, Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR, Zaoutis T, Bennett JE. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding BB, Yu JJ, Yu RY, Mendez LM, Shaknovich R, Zhang Y, Cattoretti G, Ye BH. Constitutively activated STAT3 promotes cell proliferation and survival in the activated B-cell subtype of diffuse large B-cell lymphomas. Blood. 2008;111:1515–1523. doi: 10.1182/blood-2007-04-087734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 17.Emmendorffer A, Nakamura M, Rothe G, Spiekermann K, Lohmann-Matthes ML, Roesler J. Evaluation of flow cytometric methods for diagnosis of chronic granulomatous disease variants under routine laboratory conditions. Cytometry. 1994;18:147–155. doi: 10.1002/cyto.990180306. [DOI] [PubMed] [Google Scholar]
- 18.Erlewyn-Lajeunesse MD. Hyperimmunoglobulin-E syndrome with recurrent infection: a review of current opinion and treatment. Pediatr Allergy Immunol. 2000;11:133–141. doi: 10.1034/j.1399-3038.2000.00091.x. [DOI] [PubMed] [Google Scholar]
- 19.Freeman AF, Holland SM. The hyper-IgE syndromes. Immunol Allergy Clin North Am. 2008;28:277–291. viii. doi: 10.1016/j.iac.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freeman AF, Kleiner DE, Nadiminti H, Davis J, Quezado M, Anderson V, Puck JM, Holland SM. Causes of death in hyper-IgE syndrome. J Allergy Clin Immunol. 2007;119:1234–1240. doi: 10.1016/j.jaci.2006.12.666. [DOI] [PubMed] [Google Scholar]
- 21.Gahr M, Allgeier B, Speer CP. Hyper-IgE syndrome. Monatsschr Kinderheilkd. 1987;135:329–335. [PubMed] [Google Scholar]
- 22.Grimbacher B, Holland SM, Gallin JI, Greenberg F, Hill SC, Malech HL, Miller JA, O'Connell AC, Puck JM. Hyper-IgE syndrome with recurrent infections---an autosomal dominant multisystem disorder. N Engl J Med. 1999;340:692–702. doi: 10.1056/NEJM199903043400904. [DOI] [PubMed] [Google Scholar]
- 23.Grimbacher B, Holland SM, Puck JM. Hyper-IgE syndromes. Immunol Rev. 2005;203:244–250. doi: 10.1111/j.0105-2896.2005.00228.x. [DOI] [PubMed] [Google Scholar]
- 24.Grimbacher B, Schaffer AA, Holland SM, Davis J, Gallin JI, Malech HL, Atkinson TP, Belohradsky BH, Buckley RH, Cossu F, Espanol T, Garty BZ, Matamoros N, Myers LA, Nelson RP, Ochs HD, Renner ED, Wellinghausen N, Puck JM. Genetic linkage of hyper-IgE syndrome to chromosome 4. Am J Hum Genet. 1999;65:735–744. doi: 10.1086/302547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heinrich PC, Behrmann I, Muller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998;334(Pt 2):297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill HR, Ochs HD, Quie PG, Clark RA, Pabst HF, Klebanoff SJ, Wedgwood RJ. Defect in neutrophil granulocyte chemotaxis in Job's syndrome of recurrent “cold” staphylococcal abscesses. Lancet. 1974;2:617–619. doi: 10.1016/s0140-6736(74)91942-4. [DOI] [PubMed] [Google Scholar]
- 27.Hoger PH, Boltshauser E, Hitzig WH. Craniosynostosis in hyper-IgE-syndrome. Eur J Pediatr. 1985;144:414–417. doi: 10.1007/BF00441793. [DOI] [PubMed] [Google Scholar]
- 28.Holland SM, DeLeo FR, Elloumi HZ, Hsu AP, Uzel G, Brodsky N, Freeman AF, Demidowich A, Davis J, Turner ML, Anderson VL, Darnell DN, Welch PA, Kuhns DB, Frucht DM, Malech HL, Gallin JI, Kobayashi SD, Whitney AR, Voyich JM, Musser JM, Woellner C, Schaffer AA, Puck JM, Grimbacher B. STAT3 mutations in the hyper-IgE syndrome. N Engl J Med. 2007;357:1608–1619. doi: 10.1056/NEJMoa073687. [DOI] [PubMed] [Google Scholar]
- 29.Jiao H, Toth B, Erdos M, Fransson I, Rakoczi E, Balogh I, Magyarics Z, Derfalvi B, Csorba G, Szaflarska A, Megarbane A, Akatcherian C, Dbaibo G, Rajnavolgyi E, Hammarstrom L, Kere J, Lefranc G, Marodi L. Novel and recurrent STAT3 mutations in hyper-IgE syndrome patients from different ethnic groups. Mol Immunol. 2008;46:202–206. doi: 10.1016/j.molimm.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Josephs SH, Buckley RH. Serum IgD concentrations in normal infants, children, and adults and in patients with elevated IgE. J Pediatr. 1980;96:417–420. doi: 10.1016/s0022-3476(80)80684-6. [DOI] [PubMed] [Google Scholar]
- 31.Jung C, Dagoneau N, Baujat G, Le Merrer M, David A, Di Rocco M, Hamel B, Megarbane A, Superti-Furga A, Unger S, Munnich A, Cormier-Daire V. Stuve-Wiedemann syndrome: long-term follow-up and genetic heterogeneity. Clin Genet. 2010;77:266–272. doi: 10.1111/j.1399-0004.2009.01314.x. [DOI] [PubMed] [Google Scholar]
- 32.Kanis JA, Melton LJ, III, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9:1137–1141. doi: 10.1002/jbmr.5650090802. [DOI] [PubMed] [Google Scholar]
- 33.Kim HJ, Kim JH, Shin YK, Lee SI, Ahn KM. A novel mutation in the linker domain of the signal transducer and activator of transcription 3 gene, p.Lys531Glu, in hyper-IgE syndrome. J Allergy Clin Immunol. 2009;123:956–958. doi: 10.1016/j.jaci.2009.01.068. [DOI] [PubMed] [Google Scholar]
- 34.Kube D, Holtick U, Vockerodt M, Ahmadi T, Haier B, Behrmann I, Heinrich PC, Diehl V, Tesch H. STAT3 is constitutively activated in Hodgkin cell lines. Blood. 2001;98:762–770. doi: 10.1182/blood.v98.3.762. [DOI] [PubMed] [Google Scholar]
- 35.Kumanovics A, Perkins SL, Gilbert H, Cessna MH, Augustine NH, Hill HR. Diffuse large B cell lymphoma in hyper-IgE syndrome due to STAT3 mutation. J Clin Immunol. 2010;30:886–893. doi: 10.1007/s10875-010-9452-z. [DOI] [PubMed] [Google Scholar]
- 36.Lee WI, Huang JL, Lin SJ, Yeh KW, Chen LC, Hsieh MY, Huang YC, Kuo HC, Yang KD, Yu HR, Jaing TH, Yang CH. Clinical aspects and genetic analysis of Taiwanese patients with the phenotype of hyper-immunoglobulin E recurrent infection syndromes (HIES) J Clin Immunol. 2011;31:272–280. doi: 10.1007/s10875-010-9479-1. [DOI] [PubMed] [Google Scholar]
- 37.Leonard GD, Posadas E, Herrmann PC, Anderson VL, Jaffe ES, Holland SM, Wilson WH. Non-Hodgkin's lymphoma in Job's syndrome: a case report and literature review. Leuk Lymphoma. 2004;45:2521–2525. doi: 10.1080/10428190400004463. [DOI] [PubMed] [Google Scholar]
- 38.Leung DY, Geha RS. Clinical and immunologic aspects of the hyperimmunoglobulin E syndrome. Hematol Oncol Clin North Am. 1988;2:81–100. [PubMed] [Google Scholar]
- 39.Leung DY, Key L, Steinberg JJ, Young MC, Von Deck M, Wilkinson R, Geha RS. Increased in vitro bone resorption by monocytes in the hyper-immunoglobulin E syndrome. J Immunol. 1988;140:84–88. [PubMed] [Google Scholar]
- 40.Levy DE, Darnell JE., Jr STATs: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 41.Liu JY, Li Q, Chen TT, Guo X, Ge J, Yuan LX. Destructive pulmonary staphylococcal infection in a boy with hyper-IgE syndrome: a novel mutation in the signal transducer and activator of transcription 3 (STAT3) gene (p.Y657S) Eur J Pediatr. 2011;170:661–666. doi: 10.1007/s00431-010-1349-6. [DOI] [PubMed] [Google Scholar]
- 42.Liu L, Okada S, Kong XF, Kreins AY, Cypowyj S, Abhyankar A, Toubiana J, Itan Y, Audry M, Nitschke P, Masson C, Toth B, Flatot J, Migaud M, Chrabieh M, Kochetkov T, Bolze A, Borghesi A, Toulon A, Hiller J, Eyerich S, Eyerich K, Gulacsy V, Chernyshova L, Chernyshov V, Bondarenko A, Maria Cortes Grimaldo R, Blancas-Galicia L, Madrigal Beas IM, Roesler J, Magdorf K, Engelhard D, Thumerelle C, Burgel PR, Hoernes M, Drexel B, Seger R, Kusuma T, Jansson AF, Sawalle-Belohradsky J, Belohradsky B, Jouanguy E, Bustamante J, Bue M, Karin N, Wildbaum G, Bodemer C, Lortholary O, Fischer A, Blanche S, Al-Muhsen S, Reichenbach J, Kobayashi M, Rosales FE, Lozano CT, Kilic SS, Oleastro M, Etzioni A, Traidl-Hoffmann C, Renner ED, Abel L, Picard C, Marodi L, Boisson-Dupuis S, Puel A, Casanova JL. Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J Exp Med. 2011;208:1635–1648. doi: 10.1084/jem.20110958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma CS, Chew GY, Simpson N, Priyadarshi A, Wong M, Grimbacher B, Fulcher DA, Tangye SG, Cook MC. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J Exp Med. 2008;205:1551–1557. doi: 10.1084/jem.20080218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, Kanno Y, Spalding C, Elloumi HZ, Paulson ML, Davis J, Hsu A, Asher AI, O'Shea J, Holland SM, Paul WE, Douek DC. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452:773–776. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Minegishi Y. Hyper-IgE syndrome. Curr Opin Immunol. 2009;21:487–492. doi: 10.1016/j.coi.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 46.Minegishi Y, Saito M, Nagasawa M, Takada H, Hara T, Tsuchiya S, Agematsu K, Yamada M, Kawamura N, Ariga T, Tsuge I, Karasuyama H. Molecular explanation for the contradiction between systemic Th17 defect and localized bacterial infection in hyper-IgE syndrome. J Exp Med. 2009;206:1291–1301. doi: 10.1084/jem.20082767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Minegishi Y, Saito M, Tsuchiya S, Tsuge I, Takada H, Hara T, Kawamura N, Ariga T, Pasic S, Stojkovic O, Metin A, Karasuyama H. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature. 2007;448:1058–1062. doi: 10.1038/nature06096. [DOI] [PubMed] [Google Scholar]
- 48.Murray PJ. The JAK-STAT signaling pathway: input and output integration. J Immunol. 2007;178:2623–2629. doi: 10.4049/jimmunol.178.5.2623. [DOI] [PubMed] [Google Scholar]
- 49.Nieminen P, Morgan NV, Fenwick AL, Parmanen S, Veistinen L, Mikkola ML, van der Spek PJ, Giraud A, Judd L, Arte S, Brueton LA, Wall SA, Mathijssen IM, Maher ER, Wilkie AO, Kreiborg S, Thesleff I. Inactivation of IL11 signaling causes craniosynostosis, delayed tooth eruption, and supernumerary teeth. Am J Hum Genet. 2011;89:67–81. doi: 10.1016/j.ajhg.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O'Connell AC, Puck JM, Grimbacher B, Facchetti F, Majorana A, Gallin JI, Malech HL, Holland SM. Delayed eruption of permanent teeth in hyperimmunoglobulinemia E recurrent infection syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:177–185. doi: 10.1067/moe.2000.103129. [DOI] [PubMed] [Google Scholar]
- 51.O'Shea JJ, Murray PJ. Cytokine signaling modules in inflammatory responses. Immunity. 2008;28:477–487. doi: 10.1016/j.immuni.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Papanastasiou AD, Mantagos S, Papanastasiou DA, Zarkadis IK. A novel mutation in the signal transducer and activator of transcription 3 (STAT3) gene, in hyper-IgE syndrome. Mol Immunol. 2010;47:1629–1634. doi: 10.1016/j.molimm.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 53.Paulson ML, Freeman AF, Holland SM. Hyper IgE syndrome: an update on clinical aspects and the role of signal transducer and activator of transcription 3. Curr Opin Allergy Clin Immunol. 2008;8:527–533. doi: 10.1097/ACI.0b013e3283184210. [DOI] [PubMed] [Google Scholar]; 53a Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Picard C, von Bernuth H, Ghandil P, Chrabieh M, Levy O, Arkwright PD, McDonald D, Geha RS, Takada H, Krause JC, Creech CB, Ku CL, Ehl S, Marodi L, Al-Muhsen S, Al-Hajjar S, Al-Ghonaium A, Day-Good NK, Holland SM, Gallin JI, Chapel H, Speert DP, Rodriguez-Gallego C, Colino E, Garty BZ, Roifman C, Hara T, Yoshikawa H, Nonoyama S, Domachowske J, Issekutz AC, Tang M, Smart J, Zitnik SE, Hoarau C, Kumararatne DS, Thrasher AJ, Davies EG, Bethune C, Sirvent N, de Ricaud D, Camcioglu Y, Vasconcelos J, Guedes M, Vitor AB, Rodrigo C, Almazan F, Mendez M, Arostegui JI, Alsina L, Fortuny C, Reichenbach J, Verbsky JW, Bossuyt X, Doffinger R, Abel L, Puel A, Casanova JL. Clinical Features and Outcome of Patients With IRAK-4 and MyD88 Deficiency. Medicine (Baltimore) 2010;89:403–425. doi: 10.1097/MD.0b013e3181fd8ec3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Powers AE, Bender JM, Kumanovics A, Ampofo K, Augustine N, Pavia AT, Hill HR. Coccidioides immitis meningitis in a patient with hyperimmunoglobulin E syndrome due to a novel mutation in signal transducer and activator of transcription. Pediatr Infect Dis J. 2009;28:664–666. doi: 10.1097/INF.0b013e31819866ec. [DOI] [PubMed] [Google Scholar]
- 56.Puel A, Cypowyj S, Bustamante J, Wright JF, Liu L, Lim HK, Migaud M, Israel L, Chrabieh M, Audry M, Gumbleton M, Toulon A, Bodemer C, El-Baghdadi J, Whitters M, Paradis T, Brooks J, Collins M, Wolfman NM, Al-Muhsen S, Galicchio M, Abel L, Picard C, Casanova JL. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332:65–68. doi: 10.1126/science.1200439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Puel A, Doffinger R, Natividad A, Chrabieh M, Barcenas-Morales G, Picard C, Cobat A, Ouachee-Chardin M, Toulon A, Bustamante J, Al-Muhsen S, Al-Owain M, Arkwright PD, Costigan C, McConnell V, Cant AJ, Abinun M, Polak M, Bougneres PF, Kumararatne D, Marodi L, Nahum A, Roifman C, Blanche S, Fischer A, Bodemer C, Abel L, Lilic D, Casanova JL. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J Exp Med. 2010;207:291–297. doi: 10.1084/jem.20091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Puel A, Picard C, Cypowyj S, Lilic D, Abel L, Casanova JL. Inborn errors of mucocutaneous immunity to Candida albicans in humans: a role for IL-17 cytokines? Curr Opin Immunol. 2010;22:467–474. doi: 10.1016/j.coi.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Puel A, Picard C, Lorrot M, Pons C, Chrabieh M, Lorenzo L, Mamani-Matsuda M, Jouanguy E, Gendrel D, Casanova JL. Recurrent staphylococcal cellulitis and subcutaneous abscesses in a child with autoantibodies against IL-6. J Immunol. 2008;180:647–654. doi: 10.4049/jimmunol.180.1.647. [DOI] [PubMed] [Google Scholar]
- 60.Renner ED, Rylaarsdam S, Anover-Sombke S, Rack AL, Reichenbach J, Carey JC, Zhu Q, Jansson AF, Barboza J, Schimke LF, Leppert MF, Getz MM, Seger RA, Hill HR, Belohradsky BH, Torgerson TR, Ochs HD. Novel signal transducer and activator of transcription 3 (STAT3) mutations, reduced T(H)17 cell numbers, and variably defective STAT3 phosphorylation in hyper-IgE syndrome. J Allergy Clin Immunol. 2008;122:181–187. doi: 10.1016/j.jaci.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Renner ED, Torgerson TR, Rylaarsdam S, Anover-Sombke S, Golob K, LaFlam T, Zhu Q, Ochs HD. STAT3 mutation in the original patient with Job's syndrome. N Engl J Med. 2007;357:1667–1668. doi: 10.1056/NEJMc076367. [DOI] [PubMed] [Google Scholar]
- 62.Schimke LF, Sawalle-Belohradsky J, Roesler J, Wollenberg A, Rack A, Borte M, Rieber N, Cremer R, Maass E, Dopfer R, Reichenbach J, Wahn V, Hoenig M, Jansson AF, Roesen-Wolff A, Schaub B, Seger R, Hill HR, Ochs HD, Torgerson TR, Belohradsky BH, Renner ED. Diagnostic approach to the hyper-IgE syndromes: immunologic and clinical key findings to differentiate hyper-IgE syndromes from atopic dermatitis. J Allergy Clin Immunol. 2010;126:611–617.e1. doi: 10.1016/j.jaci.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 63.Sheerin KA, Buckley RH. Antibody responses to protein, polysaccharide, and phi X174 antigens in the hyperimmunoglobulinemia E (hyper-IgE) syndrome. J Allergy Clin Immunol. 1991;87:803–811. doi: 10.1016/0091-6749(91)90126-9. [DOI] [PubMed] [Google Scholar]
- 64.Smithwick EM, Finelt M, Pahwa S, Good RA, Naspitz CK, Mendes NF, Kopersztyk S, Spira TJ, Nahmias AJ. Cranial synostosis in Job's syndrome. Lancet. 1978;1:826. doi: 10.1016/s0140-6736(78)93028-3. [DOI] [PubMed] [Google Scholar]
- 65.Speckmann C, Enders A, Woellner C, Thiel D, Rensing-Ehl A, Schlesier M, Rohr J, Jakob T, Oswald E, Kopp MV, Sanal O, Litzman J, Plebani A, Pietrogrande MC, Franco JL, Espanol T, Grimbacher B, Ehl S. Reduced memory B cells in patients with hyper IgE syndrome. Clin Immunol. 2008;129:448–454. doi: 10.1016/j.clim.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 66.Tanaka H, Ito R, Onodera N, Waga S. Efficacy of long-term sulfamethoxazole-trimethoprim therapy in a boy with hyperimmunoglobulin E syndrome. Tohoku J Exp Med. 1998;186:61–66. doi: 10.1620/tjem.186.61. [DOI] [PubMed] [Google Scholar]
- 67.van de Veerdonk FL, Plantinga TS, Hoischen A, Smeekens SP, Joosten LA, Gilissen C, Arts P, Rosentul DC, Carmichael AJ, Smits-van der Graaf CA, Kullberg BJ, van der Meer JW, Lilic D, Veltman JA, Netea MG. STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis. N Engl J Med. 2011;365:54–61. doi: 10.1056/NEJMoa1100102. [DOI] [PubMed] [Google Scholar]
- 68.Vinh DC, Sugui JA, Hsu AP, Freeman AF, Holland SM. Invasive fungal disease in autosomal-dominant hyper-IgE syndrome. J Allergy Clin Immunol. 2010;125:1389–1390. doi: 10.1016/j.jaci.2010.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wallet N, Ghez D, Delarue R, Suarez F, Rubio MT, Fischer A, Canioni D, Varet B, Hermine O. Diffuse large B-cell lymphoma in hyperimmunoglobulinemia E syndrome. Clin Lymphoma Myeloma. 2007;7:425–427. doi: 10.3816/CLM.2007.n.022. [DOI] [PubMed] [Google Scholar]
- 70.Woellner C, Gertz EM, Schaffer AA, Lagos M, Perro M, Glocker EO, Pietrogrande MC, Cossu F, Franco JL, Matamoros N, Pietrucha B, Heropolitanska-Pliszka E, Yeganeh M, Moin M, Espanol T, Ehl S, Gennery AR, Abinun M, Breborowicz A, Niehues T, Kilic SS, Junker A, Turvey SE, Plebani A, Sanchez B, Garty BZ, Pignata C, Cancrini C, Litzman J, Sanal O, Baumann U, Bacchetta R, Hsu AP, Davis JN, Hammarstrom L, Davies EG, Eren E, Arkwright PD, Moilanen JS, Viemann D, Khan S, Marodi L, Cant AJ, Freeman AF, Puck JM, Holland SM, Grimbacher B. Mutations in STAT3 and diagnostic guidelines for hyper-IgE syndrome. J Allergy Clin Immunol. 2010;125:424–432.e8. doi: 10.1016/j.jaci.2009.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xie L, Hu X, Li Y, Zhang W, Chen L. Hyper-IgE syndrome with STAT3 mutation: a case report in Mainland China. Clin Dev Immunol. 2010;2010:289873. doi: 10.1155/2010/289873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang Q, Raghunath PN, Xue L, Majewski M, Carpentieri DF, Odum N, Morris S, Skorski T, Wasik MA. Multilevel dysregulation of STAT3 activation in anaplastic lymphoma kinase-positive T/null-cell lymphoma. J Immunol. 2002;168:466–474. doi: 10.4049/jimmunol.168.1.466. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. STAT3 phosphorylation in EBV-B cells from patients and controls, as assessed by intracellular flow cytometry. Induction of phospho-STAT3 in EBV-B cell lines from healthy controls (squares); 8 STAT3-deficient patients with DNA-binding domain (DBD) mutations (R382W [lsqb]Patient 18[rsqb], R382Q [lsqb]Patient 22[rsqb], T412S [lsqb]Patient 36[rsqb], Vdel463 [lsqb]Patients 14, 15, 16, 44[rsqb], N472D [lsqb]Patient 46]) (triangles); 2 STAT3-deficient patients with SH2 domain mutations (S614G [lsqb]Patient 27[rsqb], V637M [lsqb]Patient 1]) (diamonds); and 3 STAT3-deficient patients with TA domain mutations (T708N [lsqb]Patient 5[rsqb], K709E [lsqb]Patient 50[rsqb], V713M [lsqb]Patient 26]) (filled circles), in response to IL-6 (a), IL-10 (b), IL-21 (c), and IFN-[alpha] (d). Scatter plots (with median line) showing the mean percentages of phospho-STAT3-positive cells obtained following cytokine stimulation, for each subject for whom atleast 2 independent experiments were carried out. The gray circle designates the patient (Patient 50) with heterozygous K709E STAT3 mutation.
Supplementary Figure 2. STAT3 nuclear translocation in EBV-B cells from patients and controls, as assessed by EMSA ELISA. The values shown represent ratios of absorbance at 450 nm, for nuclear samples extracted from cytokine-stimulated or nonstimulated cells. STAT3 nuclear translocation from control EBV-B cell lines (squares); 8 STAT3-deficient patients with DNA-binding domain (DBD) mutations (R382W [lsqb]Patient 18[rsqb], R382Q [lsqb]Patient 22[rsqb], T412S [lsqb]Patient 36[rsqb], Vdel463 [lsqb]Patients 14, 15, 16, 44[rsqb], N472D [lsqb]Patient 46]) (triangles); 3 STAT3-deficient patients with SH2 domain mutations (S614G [lsqb]Patient 27[rsqb], V637M [lsqb]Patients 1, 34]) (diamonds); and 5 STAT3-deficient patients with TA domain mutations (T708N [lsqb]Patient 5[rsqb], K709E [lsqb]Patients 31, 50[rsqb], V713M [lsqb]Patient 26[rsqb], T714I [lsqb]Patient 13]) (filled circles), in response to IL-6 (a), IL-10 (b), IL-21 (c), and IFN-[alpha] (d). Each dot represents a mean value obtained for a single subject for whom atleast 3 independent experiments were performed. The gray circle designates the patient (Patient 50) with heterozygous K709E STAT3 mutation.
Supplementary Figure 3. Defective expression of the STAT3 target gene SOCS3. SOCS3 gene induction in EBV-B cell lines from healthy controls (squares); 8 STAT3-deficient patients with heterozygous DNA-binding domain (DBD) mutations (R382W [lsqb]Patient 18[rsqb], R382Q [lsqb]Patient 22[rsqb], T412S [lsqb]Patient 36[rsqb], Vdel463 [lsqb]Patients 14, 15, 16, 44[rsqb], N472D [lsqb]Patient 46]) (triangles); 4 STAT3-deficient patients with heterozygous SH2 domain mutations (S614G [lsqb]Patient 27[rsqb], V637M [lsqb]Patients 1, 34[rsqb], S668Y [lsqb]Patient 21]) (diamonds); and 5 STAT3-deficient patients with heterozygous TA domain mutations (T708N [lsqb]Patient 5[rsqb], K709E [lsqb]Patients 31, 50[rsqb], V713M [lsqb]Patient 26[rsqb], T714I [lsqb]Patient 13]) (filled circles), in response to IL-6 (a), IL-10 (b), IL-21 (c), and IFN-[alpha] (d), as evaluated by quantitative RT-PCR. The gray circle designates the patient (Patient 50) with heterozygous K709E STAT3 mutation.