Abstract
Purpose of review
The overarching goal of this review is to compare and contrast the cognitive-behavioral features of fragile X syndrome (FraX) and Williams syndrome and to review the putative neural and molecular underpinnings of these features. Information is presented in a framework that provides guiding principles for conceptualizing gene-brain-behavior associations in neurodevelopmental disorders.
Recent findings
Abnormalities, in particular cognitive-behavioral domains with similarities in underlying neurodevelopmental correlates, occur in both FraX and Williams syndrome including aberrant frontostriatal pathways leading to executive function deficits, and magnocellular/dorsal visual stream, superior parietal lobe, inferior parietal lobe, and postcentral gyrus abnormalities contributing to deficits in visuospatial function. Compelling cognitive–behavioral and neurodevelopmental contrasts also exist in these two disorders, for example, aberrant amygdala and fusiform cortex structure and function occurring in the context of contrasting social behavioral phenotypes, and temporal cortical and cerebellar abnormalities potentially underlying differences in language function. Abnormal dendritic development is a shared neurodevelopmental morphologic feature between FraX and Williams syndrome. Commonalities in molecular machinery and processes across FraX and Williams syndrome occur as well – microRNAs involved in translational regulation of major synaptic proteins; scaffolding proteins in excitatory synapses; and proteins involved in axonal development.
Summary
Although the genetic variations leading to FraX and Williams syndrome are different, important similarities and contrasts in the phenotype, neurocircuitry, molecular machinery, and cellular processes in these two disorders allow for a unique approach to conceptualizing gene–brain–behavior links occurring in neurodevelopmental disorders.
Keywords: fragile X syndrome, genetic, neuroimaging, Williams syndrome
INTRODUCTION
Recent developments in molecular biology and neuroimaging have allowed for an unprecedented opportunity to explore the pathophysiology of neurodevelopmental disorders with greatly improved level of mechanistic detail. In this article, we will compare and contrast the phenotypes, neurocircuitry and molecular mechanisms associated with two specific neurodevelopmental conditions: fragile X syndrome (FraX) and Williams syndrome. FraX is the most common inherited cause of intellectual disability [1] and autism [2]. Mutations of the responsible gene (FMR1) in the X-chromosome are associated with significantly reduced levels of FMR1 protein (FMRP). Absent or attenuated levels of FMRP, such as that observed in individuals with full mutations, are associated with intellectual disability [2–4], sensorimotor aberrations [5,6▪, 7], cognitive difficulties [6▪, 8,9], and behavioral impairments, including a particularly high prevalence of autistic behavior [10]. Williams syndrome is a more rare neurogenetic disorder, caused by deletion of a region on chromosome 7 consisting of approximately 26–28 genes including CYLN2, GTF2I, and LIMK1 As is the case for FraX, the Williams syndrome cognitive–behavioral profile includes intellectual disability, attention deficits, and aberrant social behavior [11,12]. Although the genetic alterations of the two disorders are different, similar key domains of cognitive–behavioral function are affected, and similar molecular processes may be involved in the pathophysiology of the disorders. In this review, we will compare and contrast the deficits in social behaviors, executive function, visuospatial functioning and language processing and ability, as well as the associated putative neurocircuitry for these domains. We will also discuss the recent developments of the molecular mechanisms that may be shared by FraX and Williams syndrome. This review will conclude with a discussion of new insights and ideas regarding future directions for conceptualizing neurodevelopmental and neurogenetic disorders.
NEUROCIRCUITRY AND COGNITIVE-BEHAVIORAL PHENOTYPES IN FRAGILE X SYNDROME AND WILLIAMS SYNDROME
One of the most remarkable contrasts between FraX and Williams syndrome relates to their respective social phenotypes (Table 1). Social abilities observed in these disorders tend to fall along a continuum with increased social avoidance in FraX [13] and elevated social approach in Williams syndrome [14]. In particular, FraX is characterized by social withdrawal, poor eye contact [15], gaze aversion [16], and increased social anxiety [15], whereas Williams syndrome is characterized by social disinhibition [17,18], increased approachability [19], increased attention and fixation on social cues such as faces and eyes [20] (especially happy faces [21]), dissociation between social and physical threat [22], and increased generalized anxiety [23]. Despite these distinct differences, individuals with both FraX and Williams syndrome show difficulty in maintaining appropriate social interactions in terms of relating effectively to peers as well as making and maintaining social bonds.
Table 1.
Cognitive/behavioral phenotypes and putative neural correlates of fragile X syndrome and Williams syndrome
| Cognitive/behavioral features |
Putative neural correlate |
|||||
|---|---|---|---|---|---|---|
| Fragile X syndrome | Williams syndrome | Fragile X syndrome | Williams syndrome | |||
| Social | Similarities: | Similarities: | ||||
| Deficits in recognizing subtle social cues and impaired effectiveness in social interaction with peers. | Amygdala and fusiform cortex are affected, albeit in different directions. | |||||
| Contrasts: | Contrasts: | |||||
| Avoidance of social interactions [13]. Poor eye contact [15]; gaze aversion [16]. hcreased social anxiety [15]. | Hypersociability [17,18]; approachable [19]; greater attention bias towards happy faces than angry faces [21]. Difficulty in disengaging eye contact in children and adolescents [20]. Ieased phobic like-fears and general anxiety [23]. | Smaller amygdala [24▪,25]. Greater sensitization in the left amygdala with successive exposure to direct gaze [16]. Reduced response to facial expressions within the fusiform cortex [32]. | Larger amygdala [26,27]. Reduced amygdala response to fearful facial expressions [28,29]. Abnormally greater response to facial expressions within fusiform cortex face area [31▪]. Right lateral OFC activation in response to positive faces [33]; right medial OFC activation in response to negative faces [33]. | |||
| Response inhibition | Similarities: | Similarities: | ||||
| Both FraX [34,35] and WS [36] show deficits in response inhibition. | Dysfunction of frontostriatal circuitry (FXS [38]; WS[39]), albeit in opposite directions. | |||||
| Contrasts: | Contrasts: | |||||
| Response inhibition is dysfunctional in male [35] and female [34] adolescents. Deficits in disengaging attention and set-shifting in toddlers [37]. | Difficulty inhibiting inappropriately high appetitive drive towards social interaction [36]. Deficits in selective attention in toddlers [37]. | Reduced volume of frontal lobe [30▪]. Dramatically increased caudate nucleus volume [25,40,41,42▪▪,43]. Ineased density of white matter tracts in left ventral frontostriatal pathway [38]. Significant correlation between FMRP and neural responses in right VLPFC and both left and right striatum [34]. | Disproportionately large frontal areas [44]. Reduced volume of caudate nucleus [45]. | |||
| Visuospatial | Similarities: | Similarities: | ||||
| Impairments in visuospatial working memory tasks (FraX [8]; WS [46]) and visuomotor coordination (FraX [2]; WS [47,48]). | Deficits in the magnocellular/dorsal pathway functioning (FraX [49,50]; WS [51,52]). Decreased activation in SPL during visuospatial working memory task (FraX [53]; WS [54]). | |||||
| Contrasts: | Contrasts: | |||||
| Deficits in lower level visual processing have been rarely documented, except for aberrant saccade behavior [55], which may confound assessment of visuospatial functioning. | Deficits in lower level visualprocessing (strabismus [56], reduced visual acuity [57], reduced stereopsis [58], and impairment in making simple saccades [59]) may confound assessment of visuospatial functioning. | Deficits on tasks with stimuli targeting the magnocellular pathway [49,50], Increased size of IPL [25,30▪]. Reduced IPL activation during visuospatial working memory tasks [53]. White matter connectivity in postcentral gyrus is decreased [60]. | DTI reveals that white matter tracts within the magnocellular pathway such as the SLF exhibit an abnormal structural integrity [51,52]. Visuomotor abilities are associated with gray matter density of left IPL [61]. Greater gray matter density within the postcentral gyrus [62]. | |||
| Language | Similarities: | Similarities: | ||||
| Impairments in auditory working memory (FraX [8]; WS [63]) | Abnormalities in size of STG and cerebellum, although the deviations from normal are in opposite directions. | |||||
| Contrasts: | Contrasts: | |||||
| Atypical speech, limited expressive communication. | Unusually loquacious and highly expressive [64]. | Small STG volume [32]. Reduced volume of cerebellar vermis [65]. | Asymmetric STG [66] and disproportionately larger STG volume [45]. Enlarged cerebellar vermis [67]. | |||
DTI, diffusion tensor imaging; FraX, fragile X syndrome; IFC, inferior prefrontal cortex; IPL, inferior parietal lobe; OFC, orbitofrontal cortex; SLF, superior longitudinal fasciculus; SPL, superior parietal lobe; STG, superior temporal gyrus; VLPFC, ventral lateral prefrontal cortex; WS, Williams syndrome.
These contrasting social phenotypes can potentially be linked to aberrations in brain regions involved in social behavior: theamygdala (emotional and social salience) and the fusiform cortex (face processing). Remarkably, while persons with FraX exhibit abnormally small amygdala volumes [24▪, 25], individuals with Williams syndrome exhibit abnormally large volumes [26,27]. Similarly, while FraX is associated with greater sensitization in the left amygdala with successive exposure to direct gaze [16], Williams syndrome is associated with reduced amygdala response to fearful facial expressions [28,29]. FraX is associated with a relatively reduced response to facial expressions within the fusiform gyrus [30▪], whereas Williams syndrome is associated with an abnormally greater response (specifically, greater volume of the functionally defined fusiform face area) to facial expressions [31▪].
Like many neurodevelopmental disorders, deficits in executive function are common to both FraX and Williams syndrome (Table 1). For example, response inhibition, a core feature of executive function that consists of the ability to inhibit an inappropriate or maladaptive behavior, is aberrant in both FraX [34,35] and Williams syndrome [36]. In one of the few studies comparing visual attention in FraX and Williams syndrome, Scerif et al. [37] showed that, on the same task, toddlers with FraX made more preservative errors, whereas toddlers with Williams syndrome confused distractors and targets. This suggests that early manifestations of inhibitory deficits seem to affect disengaging and set-shifting abilities in FraX and selective attention in Williams syndrome. Efficient inhibition abilities are essential for ‘higher-order’ executive functioning abilities including impulsivity control, executive working memory, and organization of thoughts and behavior to reach a goal (planning, self-correcting, verifying, and adapting).
The frontal lobe and frontostriatal circuits play an important role in executive function. In FraX, executive functioning deficits may be associated with reduced volume of frontal lobe [30▪], aberrant maturation of the prefrontal cortex [68▪], reduced activation in the left orbitofrontal gyrus [69], and atypical frontostriatal circuitry [38,60]. FMRP deficiencies may lead to aberrant development of white matter within the frontostriatal pathway [38,60]. Dramatically increased caudate nucleus volume has been documented in FraX [25,40,41, 42▪▪,43], often in association with decreased frontal lobe volume [30▪]. In Williams syndrome, inhibition/executive function deficits may be associated with reduced volume of the caudate nucleus [45], disproportionately large frontal areas [44], aberrant orbitofrontal activation [33], as well as dysfunction of the frontostriatal pathway [39].
Executive function also contributes to visuospatial and visuoconstructive processing, that is, the ability to process, manipulate, and organize visual information in space. Individuals with both disorders show impairments in visuospatial processing (FraX [8]; Williams syndrome, [46]) and visuomotor coordination (FraX [2]; Williams syndrome [47,48]). Boys with FraX and young adult men with Williams syndrome show deficits in tasks requiring manipulations of spatial information in memory, while the ability to remember spatial locations is spared in Williams syndrome (FraX [8]; Williams syndrome [63]).
Several regions within the parietal-temporal-occipital cortex are implicated in visual processing. Visual-perceptual processing primarily involves the parvocellular/ventral pathway (the ‘what’ stream of processing), whereas visuospatial processing is principally based in the magnocellular/dorsal pathway (the ‘where’ stream of processing). Structures relevant for visuospatial processing include the superior parietal lobe (SPL), inferior parietal lobe (IPL), and postcentral gyrus (PCG). There is evidence of functional abnormalities in the magnocellular/ dorsal pathway of individuals with FraX [49,50] and persons with Williams syndrome exhibit abnormal structural integrity in white matter tracts [specifically the superior longitudinal fasciculus (SLF)] of this pathway [51,52]. Further, reduced activation in the SPL during visuospatial working memory tasks was observed in both FraX [53] and Williams syndrome [54]. Although increased size [25,30▪] and reduced activation of the IPL during visuospatial working memory has been reported in FraX [53], this structure appears to be unaffected in Williams syndrome [61,70]. Finally, decreased white matter connectivity in the PCG was reported in FraX [60], whereas greater gray matter density occurs within the PCG in Williams syndrome [62].
In the language domain, persons with both FraX [8] and Williams syndrome [63] exhibit deficits in verbal working memory. However, while individuals with Williams syndrome show excessive paralinguistic language content (e.g. high level of emotional content/hooks) [64], those with FraX show delayed development in many domains of language (vocabulary, morphosyntax, and pragmatics) [71]. Interestingly, volumes of superior temporal gyrus are small in FraX [32], while disproportionately large [26,27,45] and asymmetric [66] in Williams syndrome. Phonological memory, which entails maintaining and manipulating linguistic information, is impaired in FraX [72–74] and has not been studied extensively in Williams syndrome, although auditory working memory is abnormal in Williams syndrome [75]. Phonological memory is thought to require functional participation of the cerebellar vermis and caudate and their connections to the frontal lobe [76]. Individuals with FraX and Williams syndrome exhibit abnormal structure of the cerebellar vermis. A decrease in size of the cerebellar fastigial nucleus is found in Fmrl -knockout mice [77], which is consistent with reduced size of the posterior cerebellar vermis in humans with FraX [65]. Decreased number of Purkinje cells in the cerebellar vermis have also been documented in postmortem histologic analysis of tissue in FraX [78▪]. In contrast, the cerebellar vermis is significantly enlarged in Williams syndrome [67].
Taken together, we have described specific cognitive-behavioral abnormalities that are common to both FraX and Williams syndrome, and provided information as to the neurocircuitry potentially associated with such deficits – frontostriatal pathways for response inhibition/executive function; and magnocellular/dorsal pathway, SPL, IPL, and PCGfor visuospatial function. We have also described prominent contrasts in cognitive–behavioral function, oftenwith correspondingcontrasts in putativeneural correlates – for example, amygdala and fusiform cortex for social behavior; STG and cerebellar vermis for language. This critical analysis provides a basis for understanding the relationship between brain structure and human behavior, and may provide opportunities to use neuroimaging findings as biomarkers for specific cognitive–behavioral problems in neurodevelopmental disorders in general.
ADVANCES IN MOLECULAR BIOLOGY OF FRAGILE X SYNDROME AND WILLIAMS SYNDROME
Studying cellular and molecular mechanisms in FraX and Williams syndrome complements our understanding of the neural correlates of cognitive – behavioral profiles in these disorders. The common defective mechanism in many neurodevelopmental disorders appears to reside at the synapse. FMRP serves as an mRNA-binding protein regulating synaptic plasticity, dendritic pruning, and axonal development (Table 2). In Fmrl -knockout mice, loss of FMRP results in dysregulation of glutamatergic signaling maturation and alters the timing of the critical period for synaptic plasticity in the somatosensory cortex [96] and hippocampus [97]. These synaptic processes are believed to be critical in brain development and cognitive–behavioral functions. Emerging evidence supports the contention that microRNAs are involved in the translational regulation of major synaptic proteins in both FraX and Williams syndrome [80▪,98]. In hippocampal neurons of Fmr1-knockout mice, microRNA 125b (miR-125b) and FMRP regulate the expression of an important subunit of N-methyl d-aspartate receptor (NMDAR) [80▪], which controls a type of long-term potentiation (LTP) important for memory and learning. In Williams syndrome, miR-134 inhibits the translation of LIMK1 [98], which is important in synaptic plasticity [99]. It is likely that microRNAs are important in the pathogenesis of other neurodevelopmental disorders as well.
Table 2.
Partial list of FMRP target mRNAs and associated molecules in fragile X syndrome or Fmr1 knock-out mice
| Category of function | Molecule | mRNA | Location of altered expression studied | Expression | References |
|---|---|---|---|---|---|
| Transcriptional regulator | MeCP2 | Mecp2 | Cerebellum; hippocampus, cortex, diencephalon, brainstem |
↑mRNA; ↓mRNA | [79] |
| Translational regulator | miRNA-125b | – | Hippocampus | ↑miRNA | [80▪] |
| Translational regulator | miRNA-132 | – | Hippocampus | ↑miRNA | [80▪] |
| mGlu receptor | mGluR1/2 | GαluR1/2 | Hippocampus, cortex, dentate gyrus | ↑mRNA, ↑protein | [81] |
| NMDA receptor component | NR1 | Grin 1 | Prefrontal cortex; PSD of neocortex, hippocampus | ↓protein; ↑protein | [82▪,83] |
| NMDA receptor component | NR2A | Grin2A | Prefrontal cortex | ↓protein | [82▪] |
| NMDA receptor component | NR2B | Grin2B | Prefrontal cortex; PSD of neocortex, hippocampus | ↓protein; ↑protein | [82▪,83] |
| GABA receptor component | GABAAR δ subunit | Gaba-Aδ | Cortex, subiculum, hippocampus | ↓mRNA, ↓protein | [84,85] |
| Glucocorticoid receptor α | GRα | NR3CI | CA1 of hippocampus | ↓protein | [86] |
| Signal transduction (Gq) | RGS5 | Rgs5 | – | ↑mRNA | [84] |
| Scaffolding protein | PSD-95 | Psd95 | Hippocampus, prefrontal cortex | ↓mRNA, ↓protein | [81,82▪,83,87] |
| Scaffolding protein | SAPAP3 | Sapap3/4 | Prefrontal cortex | ↓mRNA, ↓protein | [82▪, 83,84] |
| Scaffolding protein | Shankl | Shank 1 | Neocortex, hippocampus | ↑mRNA | [83] |
| Scaffolding protein | APP | App | Cortex | ↑mRNA, ↓protein | [88] |
| Plasticity-related protein | Arc/Arg3.1 | Arc | Hippocampus, cortex | ↓mRNA, ↓protein | [82▪, 89] |
| Scaffolding protein | Gephyrin | Gphn | Cortex | ↓mRNA | [90] |
| Cell adhesion | Neuroligin 1 | Neuroligin 1 | Hippocampus | ↑protein | [91] |
| Cell adhesion | Neuroligin 2 | Neuroligin 2 | Hippocampus | ↑protein | [91] |
| GABA metabolism | GABAT | Abat | Cortex | ↓mRNA, ↓protein | [85] |
| GABA metabolism | SSADH | ALDH5A1 | Cortex, cerebellum | ↓mRNA, ↓protein | [85] |
| GABA and glutamate metabolism | GAD | Gad | Cortex, cerebellum; amygdala; whole forebrain, cortex |
↓mRNA; ↓protein; ↑protein | [92] |
| GABA transporter | GAT | Slc6a12 | Whole forebrain, cortex, cerebellum | ↓mRNA, ↓protein | [85] |
| Neurotransmitter | GABA | – | Amygdala | ↓release | [92] |
| Microtubule dynamics, neurite extension |
MAP1B | Map 1b | – | – | [93] |
| Component of Big potassium channel |
BK channel a subunit | KCNMA1 | Cortex | ↓protein | [94] |
| Calcium signaling | CaMK llα | CamKllα | Hippocampus | ↑mRNA, ↑protein | [81] |
| Axonal development | Sema3A and Sema3F | Sema3A and Sema3F | – | – | [95] |
APP, amyloid precursor protein; Arc, activity-regulated cytoskeleton-associated protein; CaMK llα, calcium/calmodulin-dependent protein kinases llα t; GABA-T, GABA transaminase; GAD, glutamate decarboxylase; MAPI B, microtubule-associated protein 1B; MeCP2, methyl CpG-binding protein 2; mGluR1/2, metabotropic glutamate receptor type 1/2; PI3K, phosphatidyl inositol 3-kinases; RGS5, regulator of G-protein signaling 5; Sema, semaphorin; SSADH, succinic semialdehyde dehydrogenase.
Synapse formation is essential for neurotransmission. Glutamatergic pathways control the majority of excitatory neurotransmission and GABAergic pathways represent the major form of inhibitory neurotransmission. Aberrations of these systems can deviate the balance between overall excitatory and inhibitory synaptic activity [100]. In addition to this excitation/inhibition imbalance, these pathways serve other important functions in learning, memory and behavior [e.g. long-term depression (LTD), LTP, and dendritic pruning]. mGluR5-dependent LTD is a well established form of synaptic plasticity and putative molecular mechanism involved in FraX [101]. Activation of mG1uR1/5 leads to cascades of signaling events driving the activation of protein synthesis involved in the internalization of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs), a process involving an assembly of Arc/ Arg3.1 and other proteins [102]. FMRP binds to the mRNA of Arc/Arg3.1 and other synaptic proteins, and is an essential part of cellular survival mechanisms through the modulation of signaling pathways [103]. mGluR5 is associated with scaffolding proteins [83] and cell adhesion proteins [91,104]. FMRP co-expresses with and silences the translation of mRNAs encoding postsynaptic density (PSD) components, including PSD-95 [82▪,83]. Impairment of PSD-95 may be involved in hippocampal-dependent learning defects [87], which are common in individuals with FraX [105]. Interestingly, knocking out the mRNA for PSD-95 results in decrease in Cyln2, which is a candidate gene for motor and cognitive deficits in Williams syndrome [106▪]. Various mGluR antagonists have been developed for the potential treatment of symptoms of FraX (see Table 3 and Table 4 [107–110,114,115▪▪,120,121]).
Table 3.
Fragile X syndrome treatments targeting specific pathways in animals
| Compound | Target | Animal model | Effects in animal model | References |
|---|---|---|---|---|
| JNJ1 6259685 | mGluR1 antagonist | Fmr 1-knockout mice | Decreased marble burying; decreased audiogenic seizures; no effect on prepulse inhibition and motor coordination. |
[107] |
| 2-Methyl-6-(phenylethynyl)- pyridine (MPEP) |
mGluR5 antagonist | Fmr 1-knockout mice | Decreased marble burying; improved motor learning; completely abolished the manifestation of audiogenic seizures; no effect on prepulse inhibition. |
[107] |
| Balb/c and Swiss Webster mice | Impaired some measures of sociability in both tested species, while reduced the intensity of some spontaneous measures of stereotypic behaviors emerging during free social interaction in Swiss Webster mice. |
[108] | ||
| 2-Chloro-4-((2,5-dimethyl-1- (4-(trifluoromethoxy)phenyl)- 1 H-imidazol-4-yl)ethynyl) pyridine (CTEP) |
mGluR5 antagonist | Sprague-Dawley rats and NMRI mice |
Active in the stress-induced hyperthermia procedure in mice and the Vogel conflict drinking test in rats with minimal effective doses of 0.1 and 0.3 mg/kg, respectively. Long half-life of approximately 18 h and high oral bioavailability. |
[109] |
| AFQ056 | mGluR5 antagonist | Fmr 1-knockout mice | Rescued a deficit in inhibition of the startle response after a prepulse. |
[110] |
| Dicyclomine | M1 antagonist (Gq-coupled) |
Fmr 1-knockout mice | Decreased marble burying; decreased audiogenic seizures. |
[111] |
| Tropicamide | M4 antagonist (Gi-coupled) |
Fmr 1-knockout mice | Decreased marble burying, increased activity in the open-field assay, improved performance in the passive avoidance assay, reduced audiogenic seizures. |
[112] |
| Gaboxadol (THIP) | GABAA receptor agonist |
Fmr 1-knockout mice | Reduced hyperactivity and prepulse inhibition. No effect on cued fear startle response. |
[113] |
Table 4.
Recent clinical trials for treatments targeting specific molecular mechanisms of fragile X syndrome
| Compound | Target and effect | Study, patients | Treatment outcomes | References |
|---|---|---|---|---|
| Acamprosate | mGluR5 | Open-label trial with three male patients with FraX. |
Improved linguistic communication and global clinical benefit. |
[114] |
| AFQ056 | mGluR5 | Randomized, double-blind, two-treatment, two-period, crossover study of 30 male patients with FraX. |
Did not improve primary outcome measure, ABC-C score. However, patients with full FMR1 promoter methylation and no detectable FMR1 messenger RNA improved. No response was found in patients with partial promoter methylation. |
[115▪▪] |
| Memantine | NMDA antagonist | Open-label trial with six patients with FraX. |
Symptom-specific rating scales showed no statistically significant improvement. However, four of six patients showed global clinical benefit on ratings with the CGI-I. |
[116] |
| Donepezil | Enhanced cholinergic neurotransmission |
Open-label trial 12 adolescent and young adult patients with FraX for 6 weeks. |
Increased CNT scores and decreased ABC Total, Hyperactivity, and Irritability scores as well as decreased CBCL/ABCL Attention Problems scores. |
[117] |
| Riluzole | Agonist of GABAA receptors, synaptic and extrasynaptic; inhibitor of GABA transporters (GAT) |
Six-week open-label prospective pilot study of riluzolein with six adults with FraX. |
No significant clinical response was detected. |
[118] |
| Valproic acid | Histone deacetylase inhibitor | Open-label study with 10 boys with FraX and ADHD. |
Reduced ADHD symptoms as measured by CPRS. |
[119] |
ABC, Aberrant Behavior Checklist; ABCL, Adult Behavior Checklist; CBCL, Child Behavior Checklist; CGI-I, Clinical Global Impression – Improvement; CNT, Contingency Naming Task; CPRS, Conner′s Parent Rating Scale – Revised.
In addition to glutamatergic mechanisms, growing evidence supports the putative role of the GABAergic circuit dysfunction in FraX [122] and Williams syndrome [123▪]. Recent evidence suggests that the GABAergic system undergoes complex patterns of changes during brain development in FraX [85]. On the basis of the premise that individuals with FraX exhibit decreased GABAergic activity, GABAA receptor agonists have been tested in animals (Table 3; [113]) with human trials likely to occur in the near future. Interestingly, GTF2I, a candidate gene for Williams syndrome, is a regulator of Dlx, which is involved in the differentiation of GABAergic projection neurons [123▪]. An important class of protein involved in the migration of GABAergic neurons, semaphorin [95], is associated with both FMR1 [124] and GTF2I [125]. Collectively, modulation of glutamatergic and GABAergic pathways presents opportunities for pharmacologic interventions in both of these disorders.
In summary, we have identified three levels of molecular machinery common to FraX and Williams syndrome: microRNAs involved in translational regulation of major synaptic proteins; scaffolding proteins in excitatory synapses; and proteins contributing to axonal development. Abnormal dendritic pruning is a shared morphologic feature between FraX [126,127] and Williams syndrome [106▪] as well.
INTEGRATION OF NEUROIMAGING AND MOLECULAR MECHANISMS: AN EXAMPLE
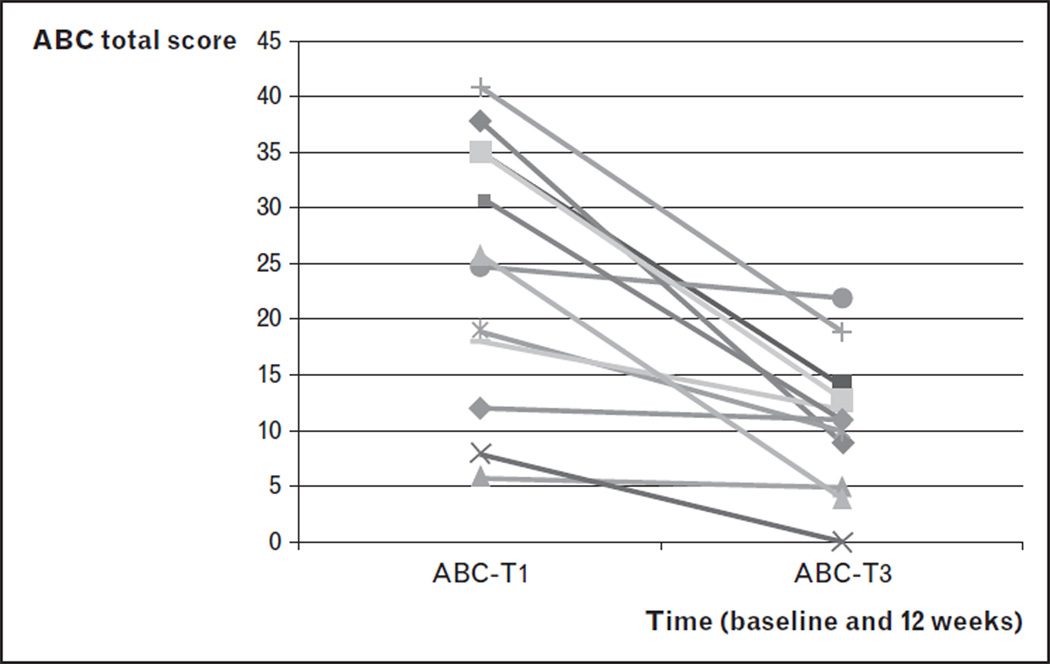
As shown above, significant deficits in executive function in FraX are associated with frontostriatal dysfunction. In addition, our group has shown significantly less activation in the hippocampus and basal forebrain in women with FraX during functional MRI [105]. These brain regions are known to have particularly high levels of FMR1 transcription during fetal development and are involved in cholinergic neurotransmission. Evidence of cholinergic dysfunction in the dorsolateral prefrontal cortex of men with FraX was also suggested from a 1H magnetic resonance spectroscopy study [117]. These data led us to hypothesize that deficits in executive function in FraX may be associated with cholinergic dysfunction in the frontostriatal pathway. Other lines of evidence also support this hypothesis. In Fmr1 -knockout mice, the α subunit of the large conductance Ca2+-activated potassium (BK) channel was among the proteins most reduced in the animal [94]. BK channels play an important role in cholinergic interneurons of the striatum [128], a brain region shown to be abnormal in FraX through multimodal imaging studies performed by our group [25]. Interestingly, BK channels are also important regulators of the hypothalamic–pituitary–adrenal axis, a system that regulates hormonal response to stress, a function that appears abnormal in both individuals with FraX and the mouse model of this disorder [129]. Finally, mutations in the gene coding for the a subunit of the BK channel have been implicated in the pathogenesis of non-FraX autism [130]. Taken together, we hypothesize that correcting a deficit in neurotransmission within cholinergic striatal interneurons and striatal–cortical ‘tone’ (by using an acetylcholinesterase inhibitor such as donepezil) might be helpful in treating executive function-related symptoms (hyperactivity, impairments in cognitive and behavioral inhibition) in FraX. In an open-label, pilot trial of donepezil, five patients with FraX showed significantly improved cognitive–behavioral function [117]. Results of this open-label study were extended to 12 patients with significant benefits of donepezil shown in Fig. 1 and Table 4. (An NIMH funded, double-blind, placebo-controlled trial of donepezil is currently ongoing at our center.)
FIGURE 1.
Change in Aberrant Behavior Checklist (ABC) total score by subject in an open-label trial of donepezil for the treatment of behavioral problems in fragile X syndrome.
Although initial results from our open donepezil trial suggest that increased cholinergic neurotransmission may be beneficial to individuals with FraX, antagonism of cholinergic Ml [111] and M4 [112] receptors in Fmrl-knockout mice have been associated with decreased perseverative behaviors (Table 3). This conflicting information is puzzling, but may be related to interspecies differences, receptor subtype specificities, and complex interactions among Gq-coupled G-protein coupled receptors (GPCRs) [131–135]. More research in this area is clearly needed to resolve this issue and to address the impact of treatments targeting the cholinergic pathway for potential cognitive enhancement and reduction of inappropriate behaviors in FraX.
FUTURE DIRECTIONS
Advances in our understanding of neurocircuitry and molecular processes involved in neurogenetic conditions have allowed us to gain insight into the pathogenesis of cognitive–behavioral profiles of these disorders and to design pharmacologic interventions. As outlined above, we have illustrated this strategy through the example of cholinergic dysfunction in the frontostriatal pathway in FraX. In this review article, we have summarized commonalities and differences between FraX and Williams syndrome (Table 1 and Table 5). Executive function deficits associated with aberrant frontostriatal pathway are observed in these known neurogenetic disorders, as well as other neurodevelopmental disorders such as velocardiofacial syndrome [136,137] and idiopathic autism [24▪]. This observation supports the examination of cholinergic function in these syndromes, as modulating the cholinergic pathway may be helpful in treating symptoms related to deficits in executive functions and other behaviors.
Table 5.
Commonalities in molecular mechanisms between Fragile X syndrome and Williams syndrome
| Fragile X syndrome | Williams syndrome | |
|---|---|---|
| Gene mutation | FMR 1 at Xq27.3 | Microdeletion at 7q1 1.23. Candidate genes involved: CL1P2, CYLN2, ELN, GTF21, GTF21RD1, and L1MK1. |
| Translational regulation | miRNA-125b inhibits translation of NR2A in Fmr 1-knockout mice [80▪]. |
miRNA-134 inhibits translation of Limk1 [96]. |
| Scaffolding proteins | In the Fmr 1-knockout mice, FMRP co-expresses with mRNAs encoding PSD-95, and also silences the translation of its mRNAs [83]. |
Dlg4 encodes PSD-95. Dlg4 −/− mice showed altered forebrain expression of various synaptic genes, including Cyln2, which regulates cytoskeletal dynamics and is a candidate gene for WS [106▪]. |
| Dendritic spine development |
Overexpression of miR-1 25b induces long narrow spines in hippocampal neurons of Fmr 1-knockout mice, which correlated with a reduction in mEPSC amplitude [80▪]. In contrast, overexpressing miR-1 32 increased dendritic protrusion width and increased mEPSC amplitude [80▪]. |
D/g4−/− mice had subtle dysmorphology of amygdale dendritic spines [106▪]. |
| Axonal development | Sema3F is a target of FMRP [124]. |
GTF2I, a candidate gene of WS, regulates Dlx gene expression [123▪]. Dlx homeobox genes promote cortical interneuron migration from the basal forebrain by direct repression of the class 3 semaphorin receptor neuropilin-2 [125]. |
WS, Williams syndrome.
In terms of developing a more accurate picture of the pathophysiology of cognitive–behavioral phenotypes in neurodevelopmental disorders, further defining neurocircuitry by linking molecular biomarkers with neuroimaging (e.g. positron emission tomography with functional MRI) will likely lead to new insights, thus setting the stage for improved circuit-based and molecular-based treatment strategies for specific symptoms. Longitudinal studies of both FraX and Williams syndrome during early development are also necessary to better understand the complex relationships among cognitive–behavioral profiles, brain development, and molecular mechanisms, particularly with respect to establishing cause. Such information has critical implications for early behavioral and pharmaceutical interventions.
CONCLUSION
The genetic variations leading to FraX and Williams syndrome are different, yet important similarities and contrasts in phenotype, neurocircuitry, molecular machinery, and cellular processes occur in these disorders. This permits a unique approach to conceptualizing gene–brain–behavior links in FraX and Williams syndrome. This approach could be applied to other neurodevelopmental disorders, such as the many currently unknown conditions comprising the category of idiopathic autism.
KEY POINTS.
Contrasting cognitive-behavioral and social profiles in FraX and Williams syndrome and their putative neural correlates include amygdala and fusiform cortex for social behavior, superior temporal gyrus and cerebellar vermis contributing to language.
Similarities in cognitive-behavioral features and underlying neurocircuitry in FraX and Williams syndrome include frontostriatal pathways for executive function, and magnocellular/dorsal pathway, superior parietal lobe, inferior parietal lobe, and postcentral gyrus for visuospatial function.
Research focused on gene-brain-behavior relationships in FraX has resulted in improved circuit-based and molecular-based understanding and experimental treatments of major target symptoms. Similar insights are expected to result from additional investigation of Williams syndrome.
Acknowledgements
A.L.R. has received funding in the form of research grants from the National Institutes of Health during the time this article was written, including MH050047 and HD049653. The information presented in this study was supported by the NIH grants MH050047 and HD049653 to A.L.R.
Footnotes
Conflicts of interest There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 208–209).
- 1.Freund LS. Reiss AL Cognitive profiles associated with the fra(X) syndrome in males and females. Am J Med Genet. 1991;38:542–547. doi: 10.1002/ajmg.1320380409. [DOI] [PubMed] [Google Scholar]
- 2.Reiss AL, Dant CC. The behavioral neurogenetics of Fragile X syndrome: analyzing gene-brain-behavior relationships in child developmental psy-chopathologies. Dev Psychopathol. 2003;15:927–968. doi: 10.1017/s0954579403000464. [DOI] [PubMed] [Google Scholar]
- 3.Hall SS, Burns DD, Lightbody AA, Reiss AL. Longitudinal changes in intellectual development in children with Fragile X syndrome. J Abnorm Child Psychol. 2008;36:927–939. doi: 10.1007/s10802-008-9223-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lodish MB, Stratakis CA. Endocrine tumours in neurofibromatosis type 1, tuberous sclerosis and related syndromes. Best Pract Res Clin Endocrinol Metab. 2010;24:439–449. doi: 10.1016/j.beem.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuhas J, Cordeiro L, Tassone F, et al. Brief report: Sensorimotor gating in idiopathic autism and autism associated with Fragile X syndrome. J Autism Dev Disord. 2011;41:248–253. doi: 10.1007/s10803-010-1040-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Roberts JE, Hatton DD, Long AC, et al. Visual attention and autistic behavior in infants with Fragile X syndrome. J Autism Dev Disord. 2011 doi: 10.1007/s10803-011-1316-8. [Epub ahead of print]▪ This is the first longitudinal study of gaze direction and heart rate in infants with FraX. The authors suggest that infants with FraX may be less physiologically reactive and have difficulty modulating arousal, given the findings of atypical visual attention and increased heart rate.
- 7.Van der Molen MJ, Van der Molen MW, Ridderinkhof KR, et al. Auditory and visual cortical activity during selective attention in Fragile X syndrome: a cascade of processing deficiencies. Clin Neurophysiol. 2011 doi: 10.1016/j.clinph.2011.08.023. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Baker S, Hooper S, Skinner M, et al. Working memory subsystems and task complexity in young boys with Fragile X syndrome. J Intellect Disabil Res. 2011;55:19–29. doi: 10.1111/j.1365-2788.2010.01343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heilman KJ, Harden ER, Zageris DM, et al. Autonomic regulation in Fragile X syndrome. Dev Psychobiol. 2011;53:785–795. doi: 10.1002/dev.20551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gabis LV, Baruch YK, Jokel A, Raz R. Psychiatric and autistic comorbidity in Fragile X syndrome across ages. J Child Neurol. 2011;26:940–948. doi: 10.1177/0883073810395937. [DOI] [PubMed] [Google Scholar]
- 11.Morris CA. The behavioral phenotype of Williams syndrome: a recognizable pattern of neurodevelopment. Am J Med Genet C Semin Med Genet. 2010;l54C:427–431. doi: 10.1002/ajmg.c.30286. [DOI] [PubMed] [Google Scholar]
- 12.Plesa Skwerer D, Ammerman E, Andre MC, et al. A multimeasure approach to investigating affective appraisal of social information in Williams syndrome. J Neurodev Disord. 2011;3:325–334. doi: 10.1007/s11689-011-9100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siegel MS, Smith WE. Psychiatric features in children with genetic syndromes: toward functional phenotypes. Pediatr Clin North Am. 2011;58:833–864. doi: 10.1016/j.pcl.2011.06.010. x. [DOI] [PubMed] [Google Scholar]
- 14.Jarvinen-Pasley A, Bellugi U, Reilly J, et al. Defining the social phenotype in Williams syndrome: a model for linking gene, the brain, and behavior. Dev Psychopathol. 2008;20:1–35. doi: 10.1017/S0954579408000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feinstein C, Singh S. Social phenotypes in neurogenetic syndromes. Child Adolesc Psychiatr Clin North Am. 2007;16:631–647. doi: 10.1016/j.chc.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Watson C, Hoeft F, Garrett AS, et al. Aberrant brain activation during gaze processing in boys with Fragile X syndrome. Arch Gen Psychiatry. 2008;65:1315–1323. doi: 10.1001/archpsyc.65.11.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Capitao L, Sampaio A, Fernandez M, et al. Williams syndrome hypersocia-bility: a neuropsychological study of the amygdala and prefrontal cortex hypotheses. Res Dev Disabil. 2011;32:1169–1179. doi: 10.1016/j.ridd.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Dodd HF, Porter MA, Peters GL, Rapee RM. Social approach in preschool children with Williams syndrome: the role of the face. J Intellect Disabil Res. 2010;54:194–203. doi: 10.1111/j.1365-2788.2009.01241.x. [DOI] [PubMed] [Google Scholar]
- 19.Jawaid A, Riby DM, Egridere S, et al. Approachability in Williams syndrome. Neuropsychologia. 2010;48:1521–1523. doi: 10.1016/j.neuropsychologia.2009.12.031. [DOI] [PubMed] [Google Scholar]
- 20.Riby DM, Jones N, Brown PH, et al. Attention to faces in Williams syndrome. J Autism Dev Disord. 2011;41:1228–1239. doi: 10.1007/s10803-010-1141-5. [DOI] [PubMed] [Google Scholar]
- 21.Dodd HF, Porter MA. I see happy people: attention bias towards happy but not angry facial expressions in Williams syndrome. Cogn Neuropsychiatry. 2010;15:549–567. doi: 10.1080/13546801003737157. [DOI] [PubMed] [Google Scholar]
- 22.Dodd HF, Porter MA. Interpretation of ambiguous situations: evidence for a dissociation between social and physical threat in Williams syndrome. J Autism Dev Disord. 2011;41:266–274. doi: 10.1007/s10803-010-1048-1. [DOI] [PubMed] [Google Scholar]
- 23.Dodd HF, Schniering CA, Porter MA. Beyond behaviour: is social anxiety low in Williams syndrome? J Autism Dev Disord. 2009;12:1673–1681. doi: 10.1007/s10803-009-0806-4. [DOI] [PubMed] [Google Scholar]
- 24. Hoeft F, Walter E, Lightbody AA, et al. Neuroanatomical differences in toddler boys with Fragile X syndrome and idiopathic autism. Arch Gen Psychiatry. 2011;68:295–305. doi: 10.1001/archgenpsychiatry.2010.153.▪ Few studies have documented different patterns of brain structures between FraX and idiopathic autism (iAUT) in early childhood. Findings include greater frontal and temporal grey and white matter regions, amygdala, insula, and dorsal cingulum in iAUT than in FraX.
- 25.Gothelf D, Furfaro JA, Hoeft F, et al. Neuroanatomy of Fragile X syndrome is associated with aberrant behavior and the Fragile X mental retardation protein (FMRP) Ann Neurol. 2008;63:40–51. doi: 10.1002/ana.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reiss AL, Eckert MA, Rose FE, et al. An experiment of nature: brain anatomy parallels cognition and behavior in Williams syndrome. J Neurosci. 2004;24:5009–5015. doi: 10.1523/JNEUROSCI.5272-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Capitao L, Sampaio A, Sampaio C, et al. MRI amygdala volume in Williams Syndrome. Res Dev Disabil. 2011;32:2767–2772. doi: 10.1016/j.ridd.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 28.Haas BW, Mills D, Yam A, et al. Genetic influences on sociability: heightened amygdala reactivity and event-related responses to positive social stimuli in Williams syndrome. J Neurosci. 2009;29:1132–1139. doi: 10.1523/JNEUROSCI.5324-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyer-Lindenberg A, Hariri AR, Munoz KE, et al. Neural correlates of genetically abnormal social cognition in Williams syndrome. Nat Neurosci. 2005;8:991–993. doi: 10.1038/nn1494. [DOI] [PubMed] [Google Scholar]
- 30. Hallahan BP, Craig MC, Toal F, et al. In vivo brain anatomy of adult males with Fragile X syndrome: an MRI study. Neuroimage. 2011;54:16–24. doi: 10.1016/j.neuroimage.2010.08.015.▪ This study replicates previous findings of increase caudate nucleus size and parietal lobes alongside a decrease in volume of the left frontal lobe.
- 31. Golarai G, Hong S, Haas BW, et al. The fusiform face area is enlarged in Williams syndrome. J Neurosci. 2010;30:6700–6712. doi: 10.1523/JNEUROSCI.4268-09.2010.▪ This study used structural and functional neuroimaging to demonstrate that the volume of the functionally defined fusiform face area is larger in Williams syndrome as compared to controls.
- 32.Garrett AS, Menon V, MacKenzie K, Reiss AL. Here’s looking at you, kid: neural systems underlying face and gaze processing in Fragile X syndrome. Arch Gen Psychiatry. 2004;61:281–288. doi: 10.1001/archpsyc.61.3.281. [DOI] [PubMed] [Google Scholar]
- 33.Mimura M, Hoeft F, Kato M, et al. A preliminary study of orbitofrontal activation and hypersociability in Williams Syndrome. J Neurodev Disord. 2010;2:93–98. doi: 10.1007/s11689-009-9041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Menon V, Leroux J, White CD, Reiss AL. Frontostriatal deficits in Fragile X syndrome: relation to FMR1 gene expression. Proc Natl Acad Sci USA. 2004;101:3615–3620. doi: 10.1073/pnas.0304544101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoeft F, Hernandez A, Parthasarathy S, et al. Fronto-striata I dysfunction and potential compensatory mechanisms in male adolescents with Fragile X syndrome. Hum Brain Mapp. 2007;28:543–554. doi: 10.1002/hbm.20406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frigerio E, Burt DM, Gagliardi C, et al. Is everybody always my friend? Perception of approachability in Williams syndrome. Neuropsychologia. 2006;44:254–259. doi: 10.1016/j.neuropsychologia.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 37.Scerif G, Cornish K, Wilding J, et al. Visual search in typically developing toddlers and toddlers with Fragile X or Williams syndrome. Dev Sci. 2004;7:116–130. doi: 10.1111/j.1467-7687.2004.00327.x. [DOI] [PubMed] [Google Scholar]
- 38.Haas BW, Barnea-Goraly N, Lightbody AA, et al. Early white-matter abnormalities of the ventral frontostriatal pathway in Fragile X syndrome. Dev Med Child Neurol. 2009;51:593–599. doi: 10.1111/j.1469-8749.2009.03295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mobbs D, Eckert MA, Mills D, et al. Frontostriatal dysfunction during response inhibition in Williams syndrome. Biol Psychiatry. 2007;62:256–261. doi: 10.1016/j.biopsych.2006.05.041. [DOI] [PubMed] [Google Scholar]
- 40.Bray S, Hirt M, Jo B, et al. Aberrant frontal lobe maturation in adolescents with Fragile X syndrome is related to delayed cognitive maturation. Biol Psychiatry. 2011;70:852–858. doi: 10.1016/j.biopsych.2011.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoeft F, Lightbody AA, Hazlett HC, et al. Morphometric spatial patterns differentiating boys with Fragile X syndrome, typically developing boys, and developmentally delayed boys aged 1 to 3 years. Arch Gen Psychiatry. 2008;65:1087–1097. doi: 10.1001/archpsyc.65.9.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hoeft F, Carter JC, Lightbody AA, et al. Region-specific alterations in brain development in one- to three-year-old boys with Fragile X syndrome. Proc Natl Acad Sci USA. 2010;107:9335–9339. doi: 10.1073/pnas.1002762107.▪▪ This is the first longitudinal neuroimaging study of FraX in early childhood. The findings indicate that structural abnormalities of different brain regions in FraX evolve differently over time reflecting time-dependent effects of FMRP deficiency and provide insight into their neuropathologic underpinnings.
- 43.Hazlett HC, Poe MD, Lightbody AA, et al. Teasing apart the heterogeneity of autism: Same behavior, different brains in toddlers with Fragile X syndrome and autism. J Neurodev Disord. 2009;1:81–90. doi: 10.1007/s11689-009-9009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Campbell LE, Daly E, Toal F, et al. Brain structural differences associated with the behavioural phenotype in children with Williams syndrome. Brain Res. 2009;1258:96–107. doi: 10.1016/j.brainres.2008.11.101. [DOI] [PubMed] [Google Scholar]
- 45.Chiang MC, Reiss AL, Lee AD, et al. 3D pattern of brain abnormalities in Williams syndrome visualized using tensor-based morphometry. Neuroimage. 2007;36:1096–1109. doi: 10.1016/j.neuroimage.2007.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vicari S, Bellucci S, Carlesimo GA. Visual and spatial long-term memory: differential pattern of impairments in Williams and Down syndromes. Dev Med Child Neurol. 2005;47:305–311. doi: 10.1017/s0012162205000599. [DOI] [PubMed] [Google Scholar]
- 47.Hocking DR, Rinehart NJ, McGinley JL, et al. Gait adaptation during obstacle crossing reveals impairments in the visual control of locomotion in Williams syndrome. Neuroscience. 2011;197:320–329. doi: 10.1016/j.neuroscience.2011.08.075. [DOI] [PubMed] [Google Scholar]
- 48.Hocking DR, Rinehart NJ, McGinley JL, et al. A kinematic analysis of visually-guided movement in Williams syndrome. J Neurol Sci. 2011;301:51–58. doi: 10.1016/j.jns.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 49.Kogan CS, Bertone A, Cornish K, et al. Integrative cortical dysfunction and pervasive motion perception deficit in Fragile X syndrome. Neurology. 2004;63:1634–1639. doi: 10.1212/01.wnl.0000142987.44035.3b. [DOI] [PubMed] [Google Scholar]
- 50.Kogan CS, Boutet I, Cornish K, et al. Differential impact of the FMR1 gene on visual processing in Fragile X syndrome. Brain. 2004;127(Pt 3):591–601. doi: 10.1093/brain/awh069. [DOI] [PubMed] [Google Scholar]
- 51.Hoeft F, Barnea-Goraly N, Haas BW, et al. More is not always better: increased fractional anisotropy of superior longitudinal fasciculus associated with poor visuospatial abilities in Williams syndrome. J Neurosci. 2007;27:11960–11965. doi: 10.1523/JNEUROSCI.3591-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marenco S, Siuta MA, Kippenhan JS, et al. Genetic contributions to white matter architecture revealed by diffusion tensor imaging in Williams syndrome. Proc Natl Acad Sci USA. 2007;104:15117–15122. doi: 10.1073/pnas.0704311104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kwon H, Menon V, Eliez S, et al. Functional neuroanatomy of visuospatial working memory in Fragile X syndrome: relation to behavioral and molecular measures. Am J Psychiatry. 2001;158:1040–1051. doi: 10.1176/appi.ajp.158.7.1040. [DOI] [PubMed] [Google Scholar]
- 54.Meyer-Lindenberg A, Kohn P, Mervis CB, et al. Neural basis of genetically determined visuospatial construction deficit in Williams syndrome. Neuron. 2004;43:623–631. doi: 10.1016/j.neuron.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 55.Lasker AG, Mazzocco MM, Zee DS. Ocular motor indicators of executive dysfunction in Fragile X and Turner syndromes. Brain Cogn. 2007;63:203–220. doi: 10.1016/j.bandc.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 56.Winter M, Pankau R, Amm M, et al. The spectrum of ocular features in the Williams-Beuren syndrome. Clin Genet. 1996;49:28–31. doi: 10.1111/j.1399-0004.1996.tb04320.x. [DOI] [PubMed] [Google Scholar]
- 57.Atkinson J, Anker S, Braddick O, et al. Visual and visuospatial development in young children with Williams syndrome. Dev Med Child Neurol. 2001;43:330–337. doi: 10.1017/s0012162201000615. [DOI] [PubMed] [Google Scholar]
- 58.Van der Geest JN, Lagers-van Haselen GC, van Hagen JM, et al. Visual depth processing in Williams-Beuren syndrome. Exp Brain Res. 2005;166:200–209. doi: 10.1007/s00221-005-2355-1. [DOI] [PubMed] [Google Scholar]
- 59.Van der Geest JN, Lagers-van Haselen GC, van Hagen JM, et al. Saccade dysmetria in Williams-Beuren syndrome. Neuropsychologia. 2004;42:569–576. doi: 10.1016/j.neuropsychologia.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 60.Barnea-Goraly N, Eliez S, Hedeus M, et al. White matter tract alterations in Fragile X syndrome: preliminary evidence from diffusion tensor imaging. Am J Med Genet B Neuropsychiatr Genet. 2003;118B:81–88. doi: 10.1002/ajmg.b.10035. [DOI] [PubMed] [Google Scholar]
- 61.Menghini D, Di Paola M, Federico F, et al. Relationship between brain abnormalities and cognitive profile in Williams syndrome. Behav Genet. 2011;41:394–402. doi: 10.1007/s10519-010-9419-0. [DOI] [PubMed] [Google Scholar]
- 62.Reiss AL, Eckert MA, Rose FE, et al. An experiment of nature: brain anatomy parallels cognition and behavior in Williams syndrome. J Neurosci. 2004;24:5009–5015. doi: 10.1523/JNEUROSCI.5272-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rhodes SM, Riby DM, Fraser E, Campbell LE. The extent of working memory deficits associated with Williams syndrome: Exploration of verbal and spatial domains and executively controlled processes. Brain Cogn. 2011;77:208–214. doi: 10.1016/j.bandc.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 64.Fishman I, Yam A, Bellugi U, Mills D. Language and sociability: insights from Williams syndrome. J Neurodev Disord. 2011;3:185–192. doi: 10.1007/s11689-011-9086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mostofsky SH, Mazzocco MM, Aakalu G, et al. Decreased cerebellar posterior vermis size in Fragile X syndrome: correlation with neurocognitive performance. Neurology. 1998;50:121–130. doi: 10.1212/wnl.50.1.121. [DOI] [PubMed] [Google Scholar]
- 66.Sampaio A, Sousa N, Fernandez M, et al. MRI assessment of superior temporal gyrus in Williams syndrome. Cogn Behav Neurol. 2008;21:150–156. doi: 10.1097/WNN.0b013e31817720e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schmitt JE, Eliez S, Warsofsky IS, et al. Enlarged cerebellar vermis in Williams syndrome. J Psychiatr Res. 2001;35:225–229. doi: 10.1016/s0022-3956(01)00024-3. [DOI] [PubMed] [Google Scholar]
- 68. Bray S, Hirt M, Jo B, et al. Aberrant frontal lobe maturation in adolescents with Fragile X syndrome is related to delayed cognitive maturation. Biol Psychiatry. 2011;70:852–858. doi: 10.1016/j.biopsych.2011.05.038.▪ The results indicate that aberrant maturation of the frontal lobe may contribute to intellectual delays throughout development in FraX, more than abnormalities of other structures such as the caudate nucleus.
- 69.Tamm L, Menon V, Johnston CK, Hessl DR, Reiss AL. fMRI study of cognitive interference processing in females with Fragile X syndrome. J Cogn Neurosci. 2002;14:160–171. doi: 10.1162/089892902317236812. [DOI] [PubMed] [Google Scholar]
- 70.Eckert MA, Hu D, Eliez S, et al. Evidence for superior parietal impairment in Williams syndrome. Neurology. 2005;64:152–153. doi: 10.1212/01.WNL.0000148598.63153.8A. [DOI] [PubMed] [Google Scholar]
- 71.Abbeduto L, Brady N, Kover ST. Language development and Fragile X syndrome: profiles, syndrome-specificity, and within-syndrome differences. Ment Retard Dev Disabil Res Rev. 2007;13:36–46. doi: 10.1002/mrdd.20142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pierpont El, Richmond EK, Abbeduto L, et al. Contributions of phonological and verbal working memory to language development in adolescents with Fragile X syndrome. J Neurodev Dis. 2011;3:335–347. doi: 10.1007/s11689-011-9095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baker S, Hooper S, Skinner M, et al. Working memory subsystems and task complexity in young boys with Fragile X syndrome. J Intellect Disabil Res. 2011;55:19–29. doi: 10.1111/j.1365-2788.2010.01343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Munir F, Cornish KM, Wilding J. Nature of the working memory deficit in Fragile-X syndrome. Brain Cogn. 2000;44:387–401. doi: 10.1006/brcg.1999.1200. [DOI] [PubMed] [Google Scholar]
- 75.Menghini D, Addona F, Costanzo F, Vicari S. Executive functions in individuals with Williams syndrome. J Intellect Disabil Res. 2010;54:418–432. doi: 10.1111/j.1365-2788.2010.01287.x. [DOI] [PubMed] [Google Scholar]
- 76.Wager TD, Smith EE. Neuroimaging studies of working memory: a metaanalysis. Cogn Affect Behav Neurosci. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- 77.Ellegood J, Pacey LK, Hampson DR, et al. Anatomical phenotyping in a mouse model of Fragile X syndrome with magnetic resonance imaging. Neuroimage. 2010;53:1023–1029. doi: 10.1016/j.neuroimage.2010.03.038. [DOI] [PubMed] [Google Scholar]
- 78.Greco CM, Navarro CS, Hunsaker MR, et al. Neuropathologic features in the hippocampus and cerebellum of three older men with Fragile X syndrome. Mol Autism. 2011;2:2. doi: 10.1186/2040-2392-2-2.▪ There are currently very few postmortem studies of brain tissue of individuals with FraX (most are of patients with Fragile X tremor-ataxia syndrome). Thickening of hippocampal CA1, irregularities of the dentate gyrus, and decreased number of Purkinje cells in the cerebellar vermis were found postmortem, which may indicate impaired neuronal migration.
- 79.Zhang A, Shen CH, Ma SY, et al. Altered expression of autism-associated genes in the brain of Fragile X mouse model. Biochem Biophys Res Commun. 2009;379:920–923. doi: 10.1016/j.bbrc.2008.12.172. [DOI] [PubMed] [Google Scholar]
- 80. Edbauer D, Neilson JR, Foster KA, et al. Regulation of synaptic structure and function by FMRP-associated microRNAs miR-1 25b and miR-132. Neuron. 2010;65:373–384. doi: 10.1016/j.neuron.2010.01.005.▪ This study demonstrated that miR-1 25b and miR-132, as well as several other miRNAs, are associated with FMRP levels, dendritic spine morphology and synaptic physiology in hippocampal neurons. The authors found that NMDA receptor subunit NR2A is a target of miR-1 25b and showed that NR2A mRNA is specifically associated with FMRP in the mouse brain.
- 81.Muddashetty RS, Kelic S, Gross C, et al. Dysregulated metabotropic glutamate receptor-dependent translation of AMPA receptor and postsynaptic density-95 mRNAs at synapses in a mouse model of Fragile X syndrome. J Neurosci. 2007;27:5338–5348. doi: 10.1523/JNEUROSCI.0937-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Krueger DD, Osterweil EK, Chen SP, et al. Cognitive dysfunction and prefrontal synaptic abnormalities in a mouse model of Fragile X syndrome. Proc Natl Acad Sci USA. 2011;108:2587–2592. doi: 10.1073/pnas.1013855108.▪ This study demonstrated a robust mouse model for the PFC-associated molecular, cellular, and behavioral abnormalities in FraX. The authors found that levels of proteins involved in synaptic function (NR1, NR2A, and NR2B), scaffolding proteins (PSD-95 and SAPAP3),and the plasticity-related gene Arc are decreased in the prefrontal cortex of Fmr 1-knockout mice and are partly correlated with behavioral performance.
- 83.Schutt J, Falley K, Richter D, et al. Fragile X mental retardation protein regulates the levels of scaffold proteins and glutamate receptors in postsynaptic densities. J Biol Chem. 2009;284:25479–25487. doi: 10.1074/jbc.M109.042663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dictenberg JB, Swanger SA, Antar LN, et al. A direct role for FMRP in activity-dependent dendritic mRNA transport links filopodial-spine morphogenesis to Fragile X syndrome. Dev Cell. 2008;14:926–939. doi: 10.1016/j.devcel.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Adusei DC, Pacey LK, Chen D, Hampson DR. Early developmental alterations in GABAergic protein expression in Fragile X knockout mice. Neuropharmacology. 2010;59:167–171. doi: 10.1016/j.neuropharm.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 86.Miyashiro KY, Beckel-Mitchener A, Purk TP, et al. RNA cargoes associating with FMRP reveal deficits in cellular functioning in Fmr1 null mice. Neuron. 2003;37:417–431. doi: 10.1016/s0896-6273(03)00034-5. [DOI] [PubMed] [Google Scholar]
- 87.Zhu ZW, Xu O, Zhao ZY, et al. Spatiotemporal expression of PSD-95 in Fmr1 knockout mice brain. Neuropathology. 2011;1:223–229. doi: 10.1111/j.1440-1789.2010.01165.x. [DOI] [PubMed] [Google Scholar]
- 88.Westmark CJ, Malter JS. FMRP mediates mGluR5-dependent translation of amyloid precursor protein. PLoS Biol. 2007;5:e52. doi: 10.1371/journal.pbio.0050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Park S, Park JM, Kim S, et al. Elongation factor 2 and Fragile X mental retardation protein control the dynamic translation of Arc/Arg3.1 essential for mGluR-LTD. Neuron. 2008;59:70–83. doi: 10.1016/j.neuron.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.D’Hulst C, Heulens I, Brouwer JR, et al. Expression of the GABAergic system in animal models for Fragile X syndrome and Fragile X associated tremor/ataxia syndrome (FXTAS) Brain Res. 2009;1253:176–183. doi: 10.1016/j.brainres.2008.11.075. [DOI] [PubMed] [Google Scholar]
- 91.Dahlhaus R, El-Husseini A. Altered neuroligin expression is involved in social deficits in a mouse model of the Fragile X syndrome. Behav Brain Res. 2010;208:96–105. doi: 10.1016/j.bbr.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 92.Olmos-Serrano JL, Paluszkiewicz SM, Martin BS, et al. Defective GABAergic neurotransmission and pharmacological rescue of neuronal hyperexcitability in the amygdala in a mouse model of Fragile X syndrome. J Neurosci. 2010;30:9929–9938. doi: 10.1523/JNEUROSCI.1714-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Menon L, Mader SA, Mihailescu MR. Fragile X mental retardation protein interactions with the microtubule associated protein 1B RNA. RNA. 2008;14:1644–1655. doi: 10.1261/rna.1100708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liao L, Park SK, Xu T, et al. Quantitative proteomic analysis of primary neurons reveals diverse changes in synaptic protein content in fmr1 knockout mice. Proc Natl Acad Sci USA. 2008;105:15281–15286. doi: 10.1073/pnas.0804678105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tamamaki N, Fujimori K, Nojyo Y, et al. Evidence that Sema3A and Sema3F regulate the migration of GABAergic neurons in the developing neocortex. J Comp Neurol. 2003;455:238–248. doi: 10.1002/cne.10476. [DOI] [PubMed] [Google Scholar]
- 96.Harlow EG, Till SM, Russell TA, et al. Critical period plasticity is disrupted in the barrel cortex of FMR1 knockout mice. Neuron. 2010;65:385–398. doi: 10.1016/j.neuron.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Deng PY, Sojka D, Klyachko VA. Abnormal presynaptic short-term plasticity and information processing in a mouse model of Fragile X syndrome. J Neurosci. 2011;31:10971–10982. doi: 10.1523/JNEUROSCI.2021-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schratt GM, Tuebing F, Nigh EA, et al. A brain-specific microRNA regulates dendritic spine development. Nature. 2006;439:283–289. doi: 10.1038/nature04367. [DOI] [PubMed] [Google Scholar]
- 99.Yang EJ, Yoon JH, Min DS, Chung KC. LIM kinase 1 activates cAMP-responsive element-binding protein during the neuronal differentiation of immortalized hippocampal progenitor cells. J Biol Chem. 2004;279:8903–8910. doi: 10.1074/jbc.M311913200. [DOI] [PubMed] [Google Scholar]
- 100.Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of Fragile X mental retardation. Proc Natl Acad Sci US A. 2002;99:7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chowdhury S, Shepherd JD, Okuno H, et al. Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron. 2006;52:445–459. doi: 10.1016/j.neuron.2006.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jeon SJ, Seo JE, Yang SI, et al. Cellular stress-induced up-regulation of FMRP promotes cell survival by modulating PI3K-Akt phosphorylation cascades. J Biomed Sci. 2011;18:17. doi: 10.1186/1423-0127-18-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dahlhaus R, Hines RM, Eadie BD, et al. Overexpression of the cell adhesion protein neuroligin-1 induces learning deficits and impairs synaptic plasticity by altering the ratio of excitation to inhibition in the hippocampus. Hippocampus. 2010;20:305–322. doi: 10.1002/hipo.20630. [DOI] [PubMed] [Google Scholar]
- 105.Greicius MD, Boyett-Anderson JM, Menon V, Reiss AL. Reduced basal forebrain and hippocampal activation during memory encoding in girls with Fragile X syndrome. Neuroreport. 2004;15:1579–1583. doi: 10.1097/01.wnr.0000134472.44362.be. [DOI] [PubMed] [Google Scholar]
- 106. Feyder M, Karlsson RM, Mathur P, et al. Association of mouse Dlg4 (PSD-95) ▪ gene deletion and human DLG4 gene variation with phenotypes relevant to autism spectrum disorders and Williams’ syndrome. Am J Psychiatry. 2010;167:1508–1517. doi: 10.1176/appi.ajp.2010.10040484.▪ This study used multiple methods, including histology on mice and neuroimag-ing in humans, to demonstrate a relationship between CYLYN expression, DLG4 variation and alterations in neuronal micro and macro structure. The authors demonstrated autistic-like behaviors in mice with Dlg4 (PSD-95) gene deletion, subtle dysmorphology of amygdala dendritic spines, and altered fore-brain expression of various synaptic genes, including Cyln2, a candidate gene for Williams’ syndrome. They also showed a significant association between variations in two human DLG4 SNPs and anatomic and functional abnormalities consistent Williams syndrome.
- 107.Thomas AM, Bui N, Perkins JR, et al. Group I metabotropic glutamate receptor antagonists alter select behaviors in a mouse model for Fragile X syndrome. Psychopharmacology. 2011;219:47–58. doi: 10.1007/s00213-011-2375-4. [DOI] [PubMed] [Google Scholar]
- 108.Burket JA, Herndon AL, Winebarger EE, et al. Complex effects of mGluR5 antagonism on sociability and stereotypic behaviors in mice: Possible implications for the pharmacotherapy of autism spectrum disorders. Brain Res Bull. 2011;86:152–158. doi: 10.1016/j.brainresbull.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 109.Lindemann L, Jaeschke G, Michalon A, et al. CTEP: a novel, potent, long-acting and orally bioavailable metabotropic glutamate receptor 5 inhibitor. J Pharmacol Exp Ther. 2011;339:474–486. doi: 10.1124/jpet.111.185660. [DOI] [PubMed] [Google Scholar]
- 110.Levenga J, Hayashi S, de Vrij FM, et al. AFQ056, a new mGluR5 antagonist for treatment of Fragile X syndrome. Neurobiol Dis. 2011;42:311–317. doi: 10.1016/j.nbd.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 111.Veeraragavan S, Bui N, Perkins JR, et al. Modulation of behavioral phenotypes by a muscarinic M1 antagonist in a mouse model of Fragile X syndrome. Psychopharmacology. 2011;217:143–151. doi: 10.1007/s00213-011-2276-6. [DOI] [PubMed] [Google Scholar]
- 112.Veeraragavan S, Bui N, Perkins JR, et al. The modulation of Fragile X behaviors by the muscarinic M4 antagonist, tropicamide. Behav Neurosci. 2011;125:783–790. doi: 10.1037/a0025202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Olmos-Serrano JL, Corbin JG, Burns MP. The GABA(A) receptor agonist THIP ameliorates specific behavioral deficits in the mouse model of Fragile X syndrome. Dev Neurosci. 2011;33:395–403. doi: 10.1159/000332884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Erickson CA, Mullett JE, McDougle CJ. Brief report: acamprosate in Fragile X syndrome. J Autism Dev Disord. 2010;40:1412–1416. doi: 10.1007/s10803-010-0988-9. [DOI] [PubMed] [Google Scholar]
- 115.Jacquemont S, Curie A, des Portes V, et al. Epigenetic modification of the FMR1 gene in Fragile X syndrome is associated with differential response to the mGluR5 antagonist AFQ056. Sci Transl Med. 2011;3:64ra1. doi: 10.1126/scitranslmed.3001708.▪ This study showed that mGluR5 antagonist AFQ056 is effective in treating behavioral abnormalities in FraX patients who harbored fully methylated FMR1 promoters and completely silenced expression of FMRP. Interestingly, this compound did not show improvement in behavioral outcomes in patients with partially methylated FMR1 promoters.
- 116.Erickson CA, Mullett JE, McDougle CJ. Open-label memantine in Fragile X syndrome. J Autism Dev Disord. 2009;39:1629–1635. doi: 10.1007/s10803-009-0807-3. [DOI] [PubMed] [Google Scholar]
- 117.Kesler SR, Lightbody AA, Reiss AL. Cholinergic dysfunction in Fragile X syndrome and potential intervention: a preliminary 1 H MRS study. Am J Med Genet A. 2009;149A:403–407. doi: 10.1002/ajmg.a.32697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Erickson CA, Weng N, Weiler IJ, et al. Open-label riluzole in Fragile X syndrome. Brain Res. 2011;1380:264–270. doi: 10.1016/j.brainres.2010.10.108. [DOI] [PubMed] [Google Scholar]
- 119.Torrioli M, Vernacotola S, Setini C, et al. Treatment with valproic acid ameliorates ADHD symptoms in Fragile X syndrome boys. Am J Med Genet A. 2010;152A:1420–1427. doi: 10.1002/ajmg.a.33484. [DOI] [PubMed] [Google Scholar]
- 120.Su T, Fan HX, Jiang T, et al. Early continuous inhibition of group 1 mGlu signaling partially rescues dendritic spine abnormalities in the Fmr1 knockout mouse model for Fragile X syndrome. Psychopharmacology. 2011;215:291–300. doi: 10.1007/s00213-010-2130-2. [DOI] [PubMed] [Google Scholar]
- 121.Choi CH, Schoenfeld BP, Bell AJ, et al. Pharmacological reversal of synaptic plasticity deficits in the mouse model of Fragile X syndrome by group II mGluR antagonist or lithium treatment. Brain Res. 2011;1380:106–119. doi: 10.1016/j.brainres.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Paluszkiewicz SM, Martin BS, Huntsman MM. Fragile X syndrome: the GABAergic system and circuit dysfunction. Dev Neurosci. 2011;33:349–364. doi: 10.1159/000329420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Poitras L, Yu M, Lesage-Pelletier C, et al. An SNP in an ultraconserved regulatory element affects Dlx5/Dlx6 regulation in the forebrain. Development. 2010;137:3089–3097. doi: 10.1242/dev.051052.▪ This study used a mouse model and provided evidence indicating that GABAergic function is disrupted in Williams syndrome.
- 124.Menon L, Mihailescu MR. Interactions of the G quartet forming semaphorin 3F RNA with the RGG box domain of the Fragile X protein family. Nucleic Acids Res. 2007;35:5379–5392. doi: 10.1093/nar/gkm581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Le TN, Du G, Fonseca M, et al. Dlx homeobox genes promote cortical interneuron migration from the basal forebrain by direct repression of the semaphorin receptor neuropilin-2. J Biol Chem. 2007;282:19071–19081. doi: 10.1074/jbc.M607486200. [DOI] [PubMed] [Google Scholar]
- 126.Grossman AW, Aldridge GM, Lee KJ, et al. Developmental characteristics of dendritic spines in the dentate gyrus of Fmr1 knockout mice. Brain Res. 2010;1355:221–227. doi: 10.1016/j.brainres.2010.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Galvez R, Smith RL, Greenough WT. Olfactory bulb mitral cell dendritic pruning abnormalities in a mouse model of the Fragile-X mental retardation syndrome: further support for FMRP’s involvement in dendritic development. Brain Res Dev Brain Res. 2005;157:214–216. doi: 10.1016/j.devbrainres.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 128.Bennett BD, Callaway JC, Wilson CJ. Intrinsic membrane properties underlying spontaneous tonic firing in neostriatal cholinergic interneurons. J Neurosci. 2000;20:8493–8503. doi: 10.1523/JNEUROSCI.20-22-08493.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hessl D, Rivera SM, Reiss AL. The neuroanatomy and neuroendocrinology of Fragile X syndrome. Ment Retard Dev Disabil Res Rev. 2004;10:17–24. doi: 10.1002/mrdd.20004. [DOI] [PubMed] [Google Scholar]
- 130.Laumonnier F, Roger S, Guerin P, et al. Association of a functional deficit of the BKCa channel, a synaptic regulator of neuronal excitability, with autism and mental retardation. Am J Psychiatry. 2006;163:1622–1629. doi: 10.1176/ajp.2006.163.9.1622. [DOI] [PubMed] [Google Scholar]
- 131.Volk U, Pfeiffer BE, Gibson JR, Huber KM. Multiple Gq-coupled receptors converge on a common protein synthesis-dependent long-term depression that is affected in Fragile X syndrome mental retardation. J Neurosci. 2007;27:11624–11634. doi: 10.1523/JNEUROSCI.2266-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chang S, Bray SM, Li Z, et al. Identification of small molecules rescuing Fragile X syndrome phenotypes in Drosophila. Nat Chem Biol. 2008;4:256–263. doi: 10.1038/nchembio.78. [DOI] [PubMed] [Google Scholar]
- 133.D’Antuono M, Merlo D, Avoli M. Involvement of cholinergic and gabaergic systems in the Fragile X knockout mice. Neuroscience. 2003;119:9–13. doi: 10.1016/s0306-4522(03)00103-9. [DOI] [PubMed] [Google Scholar]
- 134.Pacey LK, Tharmalingam S, Hampson DR. Subchronic administration and combination metabotropic glutamate and GABAB receptor drug therapy in Fragile X syndrome. J Pharmacol Exp Ther. 2011;338:897–905. doi: 10.1124/jpet.111.183327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rives ML, Vol C, Fukazawa Y, et al. Crosstalk between GABAB and mGlu1a receptors reveals new insight into GPCR signal integration. EMBO J. 2009;28:2195–2208. doi: 10.1038/emboj.2009.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Campbell LE, Daly E, Toal F, et al. Brain and behaviour in children with 22q11.2 deletion syndrome: a volumetric and voxel-based morphometry MRI study. Brain. 2006;129(Pt 5):1218–1228. doi: 10.1093/brain/awl066. [DOI] [PubMed] [Google Scholar]
- 137.Kates WR, Burnette CP, Bessette BA, et al. Frontal and caudate alterations in velocardiofacial syndrome (deletion at chromosome 22q11.2) J Child Neurol. 2004;19:337–342. doi: 10.1177/088307380401900506. [DOI] [PubMed] [Google Scholar]