Abstract
Probably due to caffeine-induced gastric acid secretion, negative effects of coffee upon various upper-gastrointestinal diseases have been precariously accepted, despite the inadequate epidemiological evidence. Our aim is to evaluate the effect of coffee consumption on four major acid-related diseases: gastric ulcer (GU), duodenal ulcer (DU), reflux esophagitis (RE), and non-erosive reflux disease (NERD) based on the large-scale multivariate analysis. Of the 9,517 healthy adults, GU, DU, and RE were diagnosed by endoscopy, and NERD was diagnosed by the symptoms of heartburn and regurgitation without esophageal erosion. Associations between coffee consumption and the four disorders were evaluated, together with age, gender, body mass index (BMI), Helicobacter pylori (HP) infection status, pepsinogen I/II ratio, smoking, and alcohol. We further performed meta-analysis using the random effects model to redefine the relationship between coffee intake and peptic ulcer disease. The eligible 8,013 study subjects comprised of 5,451 coffee drinkers and 2,562 non-coffee drinkers. By univariate analysis, age, BMI, pepsinogen I/II ratio, smoking, and alcohol showed significant associations with coffee consumption. By multiple logistic regression analysis, positively correlated factors with significance were HP infection, current smoking, BMI, and pepsinogen I/II ratio for GU; HP infection, pepsinogen I/II ratio, and current smoking for DU; HP non-infection, male, BMI, pepsinogen I/II ratio, smoking, age, and alcohol for RE; younger age, smoking, and female for NERD. The meta-analyses could detect any association of coffee consumption with neither GU nor DU. In conclusion, there are no significant relationship between coffee consumption and the four major acid-related upper gastrointestinal disorders.
Introduction
Coffee is one of the most widely consumed beverages in the world; in particular, Japan is one of the biggest coffee markets in Asia [1]. Coffee consumption has been reported to be associated with several diseases including peptic ulcer (PU) and gastroesophageal reflux disease (GERD), both of which are very common esophago-gastro-duodenal disorders worldwide [2]. PU is comprised of gastric ulcer (GU) and duodenal ulcer (DU), and GERD is comprised of reflux esophagitis (RE) and non-erosive reflux disease (NERD); these four are the most frequent upper gastrointestinal disorders considered to be acid-related [3]. It is generally thought that coffee intake should influence on these disorders probably due to gastric acid secretion induced by coffee containing caffeine [4]. However, results of many previous reports were still controversial: some studies denoted that PU has no association with coffee consumption [5]–[15], other studies reported the correlation between PU and coffee intake [9], [16]. For GERD, non-epidemiological studies have reported that coffee causes a relaxation of the lower esophageal sphincter [17], [18], which could increase the risk of both RE and NERD. Two epidemiological studies also implied that coffee consumption might affect the risk of GERD [19], [20], but the numbers of studies investigating the relation of coffee with GERD are at present very small. Totally, the effects of coffee consumption upon these four upper gastrointestinal disorders are still disputable matters.
To evaluate the effect of coffee consumption on four upper gastrointestinal disorders precisely, effects of many causative factors such as Helicobacter pylori (HP) infection, obesity, smoking, alcohol drinking, etc. should be taken into consideration [21]–[29]. Among the most important is thought to be HP infection, which is an evident risk factor for peptic ulcer diseases [30], and also an apparent preventive marker for reflux esophagitis [31]. From the standpoint of confounding variables, effects of coffee consumption upon the four upper gastrointestinal disorders should be carefully evaluated, as some reports denoted that coffee intake presents considerable association with HP infection, obesity, smoking, or alcohol drinking [32]–[34]. As the subjects of our present study mostly composed of Japanese, who are known to be very high prevalence of HP infection [35] and also known to be considerably high rate of smokers [36], a detailed investigation considering the effects of these confounding factors should be conducted.
Materials and Methods
Study Population
Study participants were 9,517 adults who received a medical checkup at Kameda Medical Center Makuhari from October 2010 to September 2011. In this study, all the participants were asked to respond to the Frequency Scale for the Symptoms of GERD (FSSG) [37] and also respond to the detailed questionnaire below-mentioned. They also underwent a variety of examinations such as upper gastrointestinal endoscopy, abdominal ultrasonography, blood chemistry tests, chest X-ray, physical examinations, and so on. The gender breakdown of participants was 5,675 men (51.5±8.8 years old, range 20 to 82 years) and 3,842 women (50.3±8.7 years old, range 20 to 87 years). This study was approved by the ethics committees of the University of Tokyo, and written informed consent was obtained from each subject according to the Declaration of Helsinki.
Diagnoses of the Four Acid-related Upper Gastrointestinal Disorders
Gastric ulcer (GU) and duodenal ulcer (DU) were diagnosed by endoscopy. In the present study, only active ulcers were considered as GU or DU respectively. Peptic ulcer (PU) was defined as the presence of GU and/or DU. Reflux esophagitis (RE) was also diagnosed by endoscopy, according to the modified Los Angeles (LA) classification [38]. Non-erosive reflux disease (NERD) was defined as the presence of heartburn and/or acid regurgitation among the subjects with no esophageal mucosal break [39]. To evaluate the symptoms of heartburn and acid regurgitation, two questions in the above-mentioned FSSG were used [37].
Evaluation of Serum Anti-Helicobacter Pylori Antibody and Serum Pepsinogen Levels
Serum anti-Helicobacter pylori antibody was measured using a commercial EIA kit (E-plate “EIKEN” H. pylori antibody, EIKEN Chemical Co Ltd, Tokyo, Japan). According to the manufacture’s instruction, the antibody titer above 10 U/ml was considered as HP-positive. Serum pepsinogen I and II were measured using a commercial LAR kit (LZ test “EIKEN” pepsinogen I and pepsinogen II, EIKEN Chemical Co Ltd).
Questionnaires
The Frequency Scale for the Symptoms of GERD (FSSG) is a widely used questionnaire for diagnosis of GERD and also for evaluating the effectiveness of digestive drug treatment [37]. Along with FSSG, a detailed questionnaire investigating symptoms related to the upper gastrointestinal disorders, the medical history, lifestyle factors, coffee consumption, etc., was given to all the participants. We analyzed answers for six questions as follows: i) “How often do you drink alcohol in a week?”; ii) “Do you have a habit of smoking?”; iii) “Have you ever undergone an eradication therapy for Helicobacter pylori?”; iv) “Do you have a history of gastric surgery?”; v) “Are you taking proton pump inhibitors (PPIs) or histamine H2-receptor antagonists (H2RAs)?”; and vi) “How much coffee do you drink?”. The answers for question i) were selected from five classifications (never, seldom, sometimes, often, and always), which were further categorized into two groups as nominal variables: rarely drinking group (never or seldom) and usually drinking group (sometimes, often, or always). The answers for question ii) were categorized into two groups as nominal variables: current or past habitual smoking (smoker group), and lifelong nonsmoking (nonsmoker group). The answer for iii), iv), and v) were “yes” or “no”. The answers for question vi) were categorized into three groups as ordinal variables: drinking less than a cup of coffee per day, 1–2 cups of coffee per day, and 3 or more cups of coffee per day.
Meta-Analysis
The meta-analysis was conducted according to the PRISMA guidelines (Figure S1). Previous studies used in our meta-analysis were selected based on the inclusion criteria as follows: case-control or cohort design, registered in PubMED, CiNii (Scholarly and Academic Information Navigator) or Ichushi Web (NPO Japan Medical Abstracts Society) databases, statistically evaluating the association between coffee consumption and some ulcer disease (GU, DU, or PU), and describing the disease frequencies corresponding to all categories of coffee intake. The data sources were searched from September 2011 to September 2012. We excluded studies showing the results of significance but lacking the data on disease frequencies, because we cannot calculate the odds ratio in meta-analysis [7], [23], [40]–[42]. We adopted random effects meta-analysis method, because we assume that the analyzed datasets have a distribution with some central value and some degree of variability. All the results were presented graphically in forest plots, in which the diamonds at the bottom represent the pooled odds ratios of overall studies with the 95% confidence interval. In the forest plots, vertical lines (1) representing no effect were also demonstrated, which made us easy to grasp significance of odds ratios for all analyzed studies (shown as gray boxes) and overall pooled one (shown as a diamond). Major risks of bias in our meta-analyses were different designs for respective studies and a small number of eligible reports. We therefore performed a test for heterogeneity using a Cochran’s Q-statistics and I2 statistics.
Statistical Analysis
The association of candidate background factors with the four major upper-gastrointestinal acid-related diseases was evaluated by univariate and multivariate analyses using the JMP® 9 program (SAS Institute Inc., Cary, NC, USA). After subjects with missing values were omitted, subjects with prior gastric surgery, taking PPIs and/or H2RAs, and having past history of HP eradication were further excluded from the study population, since such background factors might adversely affect accurate analysis. In the present study, we used eight factors as explanatory variables: age, body mass index (BMI), gender, drinking habit, smoking habit, Helicobacter pylori infection status, ratio of pepsinogen I/pepsinogen II (PG I/II ratio), and coffee consumption.
We categorized age into five groups to apply a univariate analysis: <40, 40–49, 50–59, 60–69, and ≥70. BMI and PG I/II ratio were respectively categorized into three groups: <18.5 (underweight), 18.5–24.9 (normal range), and ≥25.0 (overweight) for BMI; <2.0, 2.0–2.9, and ≥3.0 for PG I/II ratio. Based on the above-mentioned criteria, smoking, alcohol drinking, and HP infection status were divided into two groups: smoker and nonsmoker; drinking and rarely drinking; HP-positive and HP-negative.
Univariate analyses were done using Pearson's chi-square test, Student's t-test, and Welch's t-test to evaluate association between coffee consumption and other background factors. In addition, multiple logistic regression analysis was applied for evaluating the relationship between the above four esophago-gastro-duodenal diseases and eight background factors respectively. Specifically, we applied firth's penalized-likelihood method to deal with issues of separability, small event sizes, and bias of the parameter estimates for GU and DU. Age, BMI, and PG I/II ratio were evaluated as continuous variables, whereas smoking, alcohol drinking, HP infection status, and coffee consumption were analyzed as ordinal or nominal variables. A p-value of less than 0.05 was considered significant.
Results
Study Subjects
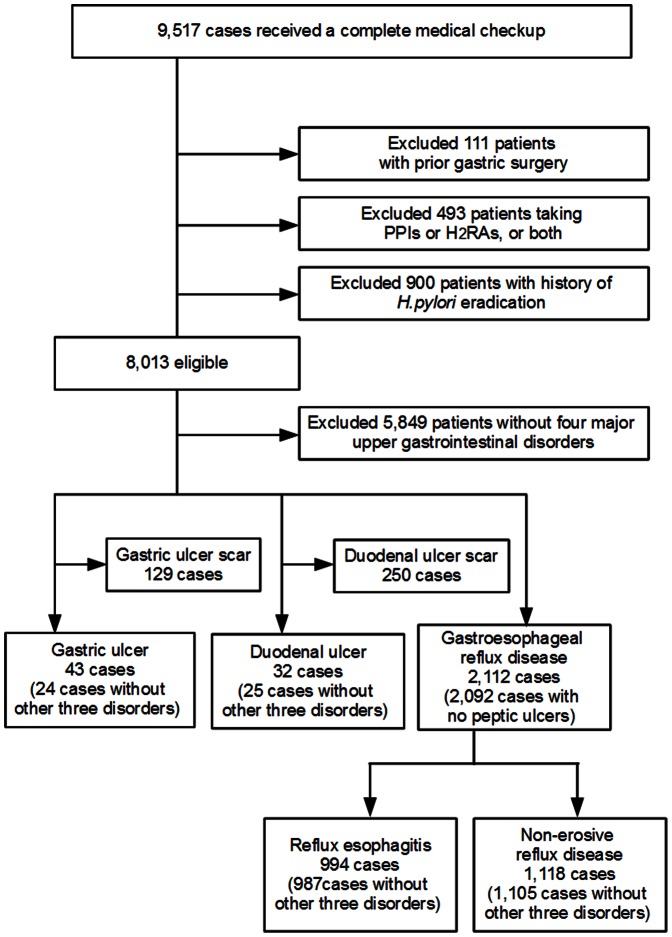
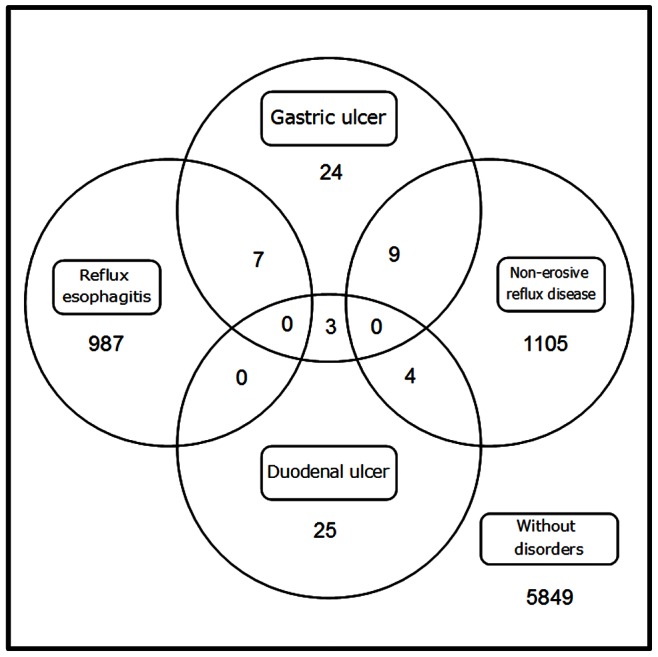
Of the 9,517 subjects who attended this study, we excluded 1,504 subjects due to a history of gastric surgery (111), intake of PPI and/or H2RAs (493), and a history of H. pylori eradication (900). Among the eligible 8,013 study subjects (4,670 men and 3,343 women, 50.4±8.8 years old, range 20 to 84 years), 5,849 had neither PU nor GERD (Figure 1). Among the residual 2,164 subjects, 43 subjects (0.5%) had GU; 32 subjects (0.4%) had DU; 994 subjects (12.4%) had RE; and 1,118 subjects (14.0%) had NERD (Figure 1). Distribution of subjects with these four disorders is represented by venn diagram (Figure 2), in which one glance is enough to grasp the much higher prevalence of GERD (RE and NERD) in comparison with PU (GU and DU).
Figure 1. Study recruitment flowchart.
Of the 9,517 healthy adults, we excluded subjects with prior gastric surgery (111), taking PPIs and/or H2RAs (493), and having history of Helicobacter pylori eradication (900). Among the eligible 8,013 subjects, numbers of subjects with GU, DU, RE, NERD, and other subjects free from the four major upper gastrointestinal disorders are shown.
Figure 2. A venn diagram showing numbers of the four acid-related upper gastrointestinal disorders in our cohort.
Coffee Consumption and Seven Background Factors
Characteristics of our study population are shown in Table 1, which is categorized based on the presence of coffee intake. Between the coffee drinkers (one or more cups of coffee per day) and non-drinkers (less than a cup of coffee per day), age, BMI, PG I/II ratio, smoking, and alcohol drinking showed statistically significant difference, whereas gender and HP infection status did not (Table 1). From our results, coffee drinkers tend to be younger, smoke, drink alcohol, and present a higher level of PG I/II ratio.
Table 1. Characteristics of the study population and univariate analysis of risk factors for coffee.
| Drinker | Non-drinker | |||
| N = 5,451 | N = 2,562 | |||
| N (%) | N (%) | p-value | ||
| Age | ||||
| <40 | 626 (67.6) | 300 (32.4) | <0.001* | † |
| 40–49 | 1,937 (72.7) | 727 (27.3) | ||
| 50–59 | 2,265 (69.0) | 1,017 (31.0) | ||
| 60–69 | 583 (57.1) | 438 (42.9) | ||
| 70≤ | 40 (33.3) | 80 (66.7) | ||
| Mean(±SD) | 49.8 (±8.2) | 51.5 (±9.7) | <0.001* | ‡ |
| Sex | ||||
| female | 3,194 (67.5) | 1,476 (32.5) | 0.405 | † |
| male | 2,257 (67.5) | 1,086 (32.5) | ||
| BMI | ||||
| <18.5 | 302 (63.7) | 172 (36.3) | 0.020* | † |
| 18.5–24.9 | 3,921 (68.9) | 1,772 (31.1) | ||
| 25≤ | 1,228 (66.6) | 618 (33.4) | ||
| Mean(±SD) | 22.9 (±3.2) | 23.1 (±3.5) | 0.059 | ‡ |
| PG-I/PG-II | ||||
| <2 | 312 (62.3) | 189 (37.7) | 0.003* | † |
| 2–2.9 | 588 (65.8) | 306 (34.2) | ||
| 3≤ | 4,553 (68.8) | 2,065 (31.2) | ||
| Mean(±SD) | 5.4 (±2.0) | 5.2 (±2.1) | 0.007* | ¶ |
| Smoking | ||||
| nonsmoker | 2,695 (64.4) | 1,487 (35.6) | <0.001* | † |
| former smoker | 1,567 (67.8) | 744 (32.2) | ||
| current smoker | 1,189 (78.2) | 331 (21.8) | ||
| Alcohol | ||||
| rarely drinking | 2,028 (65.2) | 1,081 (34.8) | <0.001* | † |
| usually drinking | 3,423 (69.8) | 1,481 (30.2) | ||
| H. pylori | ||||
| positive | 1,681 (67.1) | 823 (32.9) | 0.247 | † |
| negative | 3,770 (68.4) | 1,739 (31.6) |
A p-value less than 0.05 was considered statistically significant.
Pearson's chi-square test;
Welch's t test;
Student's t-test.
Prevalence of the four upper-gastrointestional disorders are next shown in Table 2, in which the study subjects were classified into three categories based on the coffee consumption per day. In our study cohort, almost 30% of study subjects have one or more acid-related upper gastrointestinal disorders. By the univariate analysis, we could detect no noticeable association between the degree of coffee consumption and all the four disorders (Table 2).
Table 2. The presence or absence of disorders with coffee consumption (in cups/day).
| without disorders | Gastric ulcer | Duodenal ulcer | Reflux esophagitis | Non-erosive reflux disease | ||||||
| Coffee consumption per day | No of subjects | N (%) | p-value | N (%) | p-value | N (%) | p-value | N (%) | p-value | N (%) |
| <1/day | 2,562 | 1,848 (31.6) | 0.229 | 14 (32.6) | 0.093 | 12 (37.5) | 0.360 | 339 (34.1) | 0.174 | 358 (32.0) |
| 1–2/day | 2,978 | 2,206 (37.7) | 10 (23.2) | 8 (25.0) | 345 (34.7) | 414 (37.0) | ||||
| ≥3/day | 2,473 | 1,795 (30.7) | 19 (44.2) | 12 (37.5) | 310 (31.2) | 346 (31.0) | ||||
| Total | 8,013 | 5,849 | 43 | 32 | 994 | 1,118 |
Include overlapping disorders of Gastric ulcer, Duodenal ulcer, Reflux esophagitis and Non-erosive reflux disease.
Cochran–Armitage test for trend.
Peptic Ulcer
For gastric ulcer, we compared GU patients (n = 43) with GU-free subjects (n = 7,970). By multiple logistic regression analysis (Table 3), BMI, PG I/II ratio, smoking, and HP infection status showed a significant association with GU. Judging from the value of standardized coefficients (β), positively correlated factors of gastric ulcer in order of significance are HP infection (β = 0.746; OR = 18.55), current smoking (β = 0.275; OR = 3.57), higher BMI (β = 0.253; OR = 1.15), and higher PG I/II ratio (β = 0.248; OR = 1.24). Coffee consumption as well as age, sex, and alcohol drinking did not show significant association with GU.
Table 3. Summary of the estimate of Peptic Ulcer Diseases in multiple logistic regression analysis.
| Gastric ulcer (N = 8,013) | Duodenal ulcer (N = 8,013) | |||||
| Standardized Coefficient | Odds Ratio (95% CI) | p-value | Standardized Coefficient | Odds Ratio (95% CI) | p-value | |
| Age | 0.173 | 1.04 (0.99–1.08) | 0.066 | −0.024 | 1.00 (0.95–1.04) | 0.811 |
| Sex | ||||||
| female | reference | reference | ||||
| male | −0.031 | 0.89 (0.40–2.14) | 0.780 | 0.152 | 1.75 (0.65–5.23) | 0.252 |
| BMI | 0.253 | 1.15 (1.06–1.24) | <0.001* | −0.101 | 0.95 (0.83–1.06) | 0.332 |
| PG-I/PG-II | 0.248 | 1.24 (1.01–1.50) | 0.022* | 0.420 | 1.45 (1.17–1.74) | <0.001* |
| Smoking | ||||||
| nonsmoker | reference | reference | ||||
| former smoker | 0.187 | 2.12 (0.89–5.31) | 0.083 | −0.053 | 0.81 (0.28–2.26) | 0.669 |
| Smoker | 0.275 | 3.57 (1.49–8.98) | 0.003* | 0.184 | 2.35 (0.98–5.94) | 0.048* |
| Alcohol | ||||||
| rarely drinking | reference | reference | ||||
| usually drinking | −0.018 | 0.94 (0.48–1.90) | 0.841 | 0.016 | 1.03 (0.79–1.37) | 0.336 |
| Coffee | ||||||
| <1/day | reference | reference | ||||
| 1–2/day | −0.104 | 0.68 (0.29–1.52) | 0.329 | −0.166 | 0.54 (0.21–1.28) | 0.145 |
| ≥3/day | 0.059 | 1.26 (0.62–2.61) | 0.505 | −0.059 | 0.79 (0.35–1.80) | 0.557 |
| H. pylori | ||||||
| negative | reference | reference | ||||
| positive | 0.746 | 18.55 (6.89–53.72) | <0.001* | 0.924 | 37.23 (12.39–127.30) | <0.001* |
: A p-value less than 0.05 was considered statistically significant.
For duodenal ulcer, we compared DU subjects (n = 32) with DU-free subjects (n = 7,981). By multiple logistic regression analysis (Table 3), PG I/II ratio, smoking, and HP infection status showed a significant association with DU. Judging from the value of standardized coefficients (β), positively correlated factors of duodenal ulcer in order of significance are HP infection (β = 0.924; OR = 37.23), higher PG I/II ratio (β = 0.420; OR = 1.45), and current smoking (β = 0.184; OR = 2.35). Coffee consumption as well as age, sex, BMI, and alcohol drinking did not show significant association with DU.
Gastroesophageal Reflux Disease (GERD)
For reflux esophagitis, the subjects with RE (n = 994) were compared with GERD-free subjects (n = 5,901). By multiple logistic regression analysis (Table 4), age, gender, BMI, PG I/II ratio, smoking, alcohol drinking, and HP infection showed a significant association with RE. Judging from the value of standardized coefficients (β), positively correlated factors of reflux esophagitis in order of significance are HP non-infection (β = 0.482; OR = 1/0.35 = 2.86), male gender (β = 0.426; OR = 2.37), higher BMI (β = 0.399; OR = 1.13), higher PG I/II ratio (β = 0.220; OR = 1.11), current smoking (β = 0.214; OR = 1.62), alcohol drinking (β = 0.143; OR = 1.34), and former smoking (β = 0.109; OR = 1.24). Among the examined variables, only coffee consumption did not show significant association with RE.
Table 4. Summary of the estimate of GERD syndrome in multiple logistic regression analysis.
| Reflux esophagitis (N = 6,895) | Non-erosive reflux disease (N = 7,019) | |||||
| Standardized Coefficient | Odds Ratio (95% CI) | p-value | Standardized Coefficient | Odds Ratio (95% CI) | p-value | |
| Age | 0.159 | 1.02 (1.01–1.03) | <0.001* | −0.154 | 0.98 (0.97–0.99) | <0.001* |
| Sex | ||||||
| female | reference | reference | ||||
| male | 0.426 | 2.37 (1.95–2.90) | <0.001* | −0.125 | 0.78 (0.66–0.91) | 0.002* |
| BMI | 0.399 | 1.13 (1.11–1.15) | <0.001* | 0.073 | 1.02 (1.00–1.04) | 0.035* |
| PG-I/PG-II | 0.220 | 1.11 (1.06–1.17) | <0.001* | −0.031 | 0.99 (0.94–1.03) | 0.521 |
| Smoking | ||||||
| nonsmoker | reference | reference | ||||
| former smoker | 0.109 | 1.24 (1.04–1.49) | 0.019* | 0.086 | 1.19 (0.63–1.32) | 0.048* |
| smoker | 0.214 | 1.62 (1.33–1.98) | <0.001* | 0.139 | 1.36 (1.12–1.64) | 0.002* |
| Alcohol | ||||||
| rarely drinking | reference | reference | ||||
| usually drinking | 0.143 | 1.34 (1.14–1.58) | <0.001* | 0.059 | 1.13 (0.98–1.30) | 0.093 |
| Coffee | ||||||
| <1/day | reference | reference | ||||
| 1–2/day | −0.062 | 0.88 (0.74–1.04) | 0.133 | −0.036 | 0.93 (0.79–1.08) | 0.336 |
| ≥3/day | −0.081 | 0.84 (0.70–1.01) | 0.057 | −0.032 | 0.93 (0.79–1.10) | 0.408 |
| H. pylori | ||||||
| Negative | reference | reference | ||||
| positive | −0.482 | 0.35 (0.28–0.45) | <0.001* | 0.065 | 1.15 (0.94–1.40) | 0.158 |
: A p-value less than 0.05 was considered statistically significant.
For non-erosive reflux disease (NERD), the subjects with NERD (n = 1,118) were compared with GERD-free subjects (n = 5,901). By multiple logistic regression analysis (Table 4), age, gender, BMI, and smoking showed a significant association with NERD. Judging from the value of standardized coefficients (β), positively correlated factors of NERD in order of significance are younger age (β = 0.154; OR = 1/0.98 = 1.02), current smoking (β = 0.139; OR = 1.36), female gender (β = 0.125; OR = 1/0.78 = 1.28), former smoking (β = 0.086; OR = 1.19), and higher BMI (β = 0.073; OR = 1.02). Coffee consumption as well as PG I/II ratio, alcohol drinking, and HP infection status did not show significant association with NERD.
Meta-Analysis
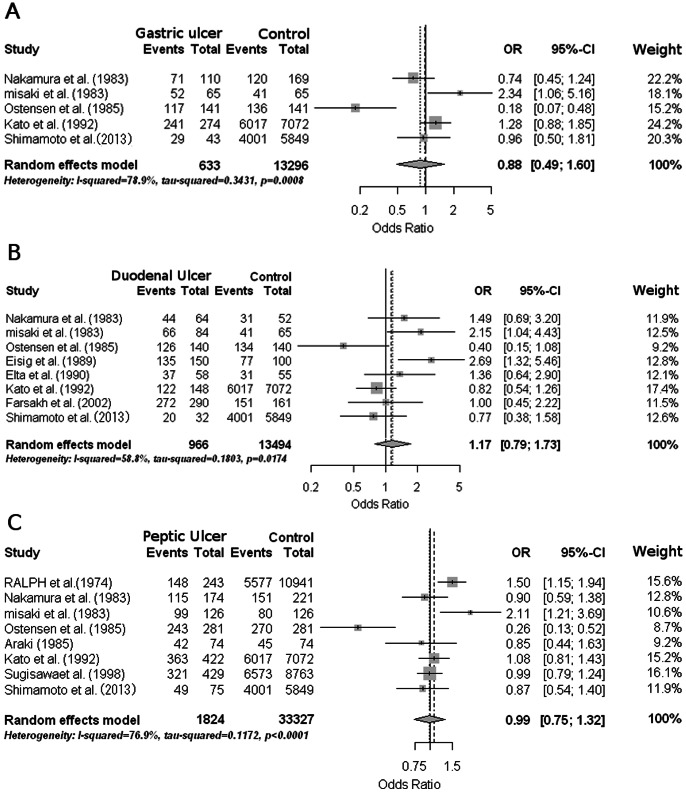
To get an overview of the accumulated reports concerning association between coffee consumption and the four upper gastrointestinal disorders, we performed meta-analyses using the random effects model. For peptic ulcer diseases, we found six case-control and four cohort studies fulfilling the inclusion criteria for meta-analysis (Table S1). We have found other five studies evaluating the association of coffee with peptic ulcer diseases (Table S2), which did not meet the criteria and could not be used in our meta-analysis. A total of 10 papers met the inclusion criteria and were included in the meta-analysis, together with our present study (Figure 3). For GERD (RE and NERD), we could not perform meta-analysis, because a number of studies meeting the criteria was too small.
Figure 3. Forest plots of the odds ratios and 95% confidential intervals for upper gastrointestinal peptic ulcer.
Forest plots of the odds ratios and 95% confidential intervals for gastric ulcer (A), duodenal ulcer (B), and peptic ulcer (C) relating coffee intake. The gray box represents the odds ratio estimates in each study, and the horizontal line indicates the 95% confidential intervals for each study. Diamonds at the bottom represent the pooled odds ratio estimates. Weights are from random effect meta-analysis.
Meta-analysis was executed using a random effects model, because a test of heterogeneity was statistically significant. As shown in Figure 3A, the meta-analysis of two case-control and three cohort studies showed no significant association between coffee consumption and GU (pooled odds ratio, 0.88; 95% CI, 0.49 to 1.60; p for heterogeneity, 0.0008; I2, 78.9%). The meta-analysis of five case-control and three cohort studies also detected no significant association between coffee consumption and DU (Figure 3B; pooled odds ratio 1.17; 95% CI, 0.79 to 1.73; p for heterogeneity, 0.0174; I2, 58.8%). For PU, we analyzed three case-control and five cohort studies (Figure 3C), which again denied significant association with coffee consumption (pooled odds ratio 0.99; 95% CI, 0.75 to 1.32; p for heterogeneity, <0.0001; I2, 76.9%). To sum up, the meta-analyses could not detect a significant association of coffee consumption with the upper gastrointestinal ulcer diseases.
Publication bias of each meta-analysis was further assessed by a funnel plot and funnel plot regression, in which p-values less than 0.1 were considered statistically significant. In all meta-analyses we performed, significance tests of the asymmetry were not significant (GU: p = 0.582, DU: p = 0.146, PU: p = 0.396). We thence concluded that publication bias can be considered as nonsignificant.
Discussion
All the four upper gastrointestinal disorders examined in the present study have been considered as acid-related diseases [3]. Therefore, it is easy to conceive that coffee containing caffeine stimulates the gastric acid production [4], [43]–[45], and consequentially increases the risk of these disorders. Especially for PU, it has been repeatedly reported that the coffee is a risk factor for both gastric and duodenal ulcer [9], [16]. However, multivariate analysis of the healthy subjects (Table 3) could not detect significant association between coffee intake and upper gastroduodenal ulcer diseases. The meta-analysis including our present study was further conducted, which denied the meaningful association between them (Figure 3). We speculated that some preventive effects of coffee intake might outweigh the risks of increased gastric acid secretion: relaxing effect, antioxidant effect, phytochemical effect, and so on [46]–[48].
For GERD, we also could not detect a significant association between coffee intake and the incidence of GERD (both RE and NERD), although some past study have reported that coffee intake may predispose to GERD syndrome [19]. Besides the stimulating effect upon gastric acrid production, it was also reported that coffee intake relaxes the lower esophageal sphincter [49], which might cause the chronic gastric acid reflux. Excessive secretion of gastric acid can damage not only the gastroduodenal but also esophageal mucosa, but our multivariate analysis of the healthy subjects (Table 3) did not detect significant association between coffee consumption and GERD (both RE and NERD). At present, epidemiological studies concerning coffee intake and GERD have been very few. Many studies like ours should be accumulated in the future, which will make it possible to perform the reliable meta-analysis.
One of limitations of our present study was of course a cross-sectional design, which should be precisely validated in the future prospective study. We are following the present large-scale cohort to validate our present conclusion in the upcoming trial. Another limitation of our study was lacking more detailed information of coffee, such as kinds of coffee beans, use of milk or sugar, regular coffee or not, the time of coffee drinking, etc. These minute data concerning coffee intake will be added to the future study, which will make our next research more accurate and polished to verify our present conclusion.
Supporting Information
Flow diagram of the meta-analysis literature search results.
(DOC)
Summary characteristics of cohort or case-control studies were included from the meta-analysis that compare the relationship of coffee and peptic ulcer.
(XLS)
Summary characteristics of cohort or case-control studies were excluded from the meta-analysis that compare the association of coffee and peptic ulcer.
(XLS)
Acknowledgments
We thank Mr. Minoru Okada, Mr. Masanori Fujiwara, and Mr. Koichi Yamashita (Kameda Medical Center Makuhari, Chiba-shi, Chiba, Japan) for great assistance with establishment and maintenance of the study database.
Funding Statement
This work was supported in part by a research grant from the Tokyo Society of Medical Sciences, and was also supported in part by a research grant from the Daiwa Security Health Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.List of countries by coffee consumption per capita, (Wikipedia, Available: http://en.wikipedia.org/wiki/List_of_countries_by_coffee_consumption_per_capita, Accessed 2012 September 5.
- 2. El-Serag HB (2007) Time trends of gastroesophageal reflux disease: a systematic review. Clin Gastroenterol Hepatol 5: 17–26. [DOI] [PubMed] [Google Scholar]
- 3. Schubert ML, Peura DA (2008) Control of gastric acid secretion in health and disease. Gastroenterology 134: 1842–1860. [DOI] [PubMed] [Google Scholar]
- 4. Cohen S, Booth GH (1975) Gastric acid secretion and lower-esophageal-sphincter pressure in response to coffee and caffeine. N Engl J Med 293: 897–899. [DOI] [PubMed] [Google Scholar]
- 5.Boekema PJ, Samsom M, van Berge Henegouwen GP, Smout AJ (1999) Coffee and gastrointestinal function: facts and fiction. A review. Scand J Gastroenterol Suppl 230: 35–39. [DOI] [PubMed]
- 6. Kaltenbach T, Crockett S, Gerson LB (2006) Are lifestyle measures effective in patients with gastroesophageal reflux disease? An evidence-based approach. Arch Intern Med 166: 965–971. [DOI] [PubMed] [Google Scholar]
- 7. Aldoori WH, Giovannucci EL, Stampfer MJ, Rimm EB, Wing AL, et al. (1997) A prospective study of alcohol, smoking, caffeine, and the risk of duodenal ulcer in men. Epidemiology 8: 420–424. [DOI] [PubMed] [Google Scholar]
- 8. Kato I, Nomura AM, Stemmermann GN, Chyou PH (1992) A prospective study of gastric and duodenal ulcer and its relation to smoking, alcohol, and diet. Am J Epidemiol 135: 521–530. [DOI] [PubMed] [Google Scholar]
- 9. Eisig JN, Zaterka S, Massuda HK, Bettarello A (1989) Coffee drinking in patients with duodenal ulcer and a control population. Scand J Gastroenterol 24: 796–798. [DOI] [PubMed] [Google Scholar]
- 10. Elta GH, Behler EM, Colturi TJ (1990) Comparison of coffee intake and coffee-induced symptoms in patients with duodenal ulcer, nonulcer dyspepsia, and normal controls. Am J Gastroenterol 85: 1339–1342. [PubMed] [Google Scholar]
- 11. Abu Farsakh NA (2002) Risk factors for duodenal ulcer disease. Saudi Med J 23: 168–172. [PubMed] [Google Scholar]
- 12. Ostensen H, Gudmundsen TE, Ostensen M, Burhol PG, Bonnevie O (1985) Smoking, alcohol, coffee, and familial factors: any associations with peptic ulcer disease? A clinically and radiologically prospective study. Scand J Gastroenterol 20: 1227–1235. [DOI] [PubMed] [Google Scholar]
- 13. Araki S (1985) The factors affecting gastric and duodenal ulcers in Japanese factory workers. A case-control study. Sangyo Igaku 27: 242–247. [DOI] [PubMed] [Google Scholar]
- 14. Nakamura T, Kamakami T, Ohkuni A, Ko S, Itoh Y, et al. (1983) [Effects of smoking, alcohol and coffee drinking on the course of peptic ulcer]. Nihon Shokakibyo Gakkai Zasshi 80: 2493–2503. [PubMed] [Google Scholar]
- 15. Atsuko S, Tetsunojo U (1998) Onset of Peptic Ulcer and Its Relation to Work-Related Factors and Life Events : A Prospective Study. Journal of occupational health 40: 22–31.9513261 [Google Scholar]
- 16. Misaki F, Hayashi K, Watanabe Y, Kawai K (1983) [An epidemiological study on risk factors in peptic ulcer]. Nihon Shokakibyo Gakkai Zasshi 80: 2504–2511. [PubMed] [Google Scholar]
- 17. Van Deventer G, Kamemoto E, Kuznicki JT, Heckert DC, Schulte MC (1992) Lower esophageal sphincter pressure, acid secretion, and blood gastrin after coffee consumption. Dig Dis Sci 37: 558–569. [DOI] [PubMed] [Google Scholar]
- 18. Thomas FB, Steinbaugh JT, Fromkes JJ, Mekhjian HS, Caldwell JH (1980) Inhibitory effect of coffee on lower esophageal sphincter pressure. Gastroenterology 79: 1262–1266. [PubMed] [Google Scholar]
- 19. Wendl B, Pfeiffer A, Pehl C, Schmidt T, Kaess H (1994) Effect of decaffeination of coffee or tea on gastro-oesophageal reflux. Aliment Pharmacol Ther 8: 283–287. [DOI] [PubMed] [Google Scholar]
- 20. Pehl C, Pfeiffer A, Wendl B, Kaess H (1997) The effect of decaffeination of coffee on gastro-oesophageal reflux in patients with reflux disease. Aliment Pharmacol Ther 11: 483–486. [DOI] [PubMed] [Google Scholar]
- 21. Eslick GD, Talley NJ (2009) Gastroesophageal reflux disease (GERD): risk factors, and impact on quality of life-a population-based study. J Clin Gastroenterol 43: 111–117. [DOI] [PubMed] [Google Scholar]
- 22. Wang JY, Liu SB, Chen SY, Dobson A (1996) Risk factors for peptic ulcer in Shanghai. Int J Epidemiol 25: 638–643. [DOI] [PubMed] [Google Scholar]
- 23. Rosenstock S, Jorgensen T, Bonnevie O, Andersen L (2003) Risk factors for peptic ulcer disease: a population based prospective cohort study comprising 2416 Danish adults. Gut 52: 186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Meucci G, Di Battista R, Abbiati C, Benassi R, Bierti L, et al. (2000) Prevalence and risk factors of Helicobacter pylori-negative peptic ulcer: a multicenter study. J Clin Gastroenterol 31: 42–47. [DOI] [PubMed] [Google Scholar]
- 25.Yaghoobi M, Farrokhyar F, Yuan Y, Hunt RH (2010) Is there an increased risk of GERD after Helicobacter pylori eradication?: a meta-analysis. Am J Gastroenterol 105: 1007–1013; quiz 1006, 1014. [DOI] [PubMed]
- 26. Malfertheiner P, Chan FK, McColl KE (2009) Peptic ulcer disease. Lancet 374: 1449–1461. [DOI] [PubMed] [Google Scholar]
- 27.Schottker B, Adamu MA, Weck MN, Brenner H (2012) Helicobacter Pylori Infection Is Strongly Associated With Gastric and Duodenal Ulcers in a Large Prospective Study. Clin Gastroenterol Hepatol 10: 487–493 e481. [DOI] [PubMed]
- 28. Li Z, Zou D, Ma X, Chen J, Shi X, et al. (2010) Epidemiology of peptic ulcer disease: endoscopic results of the systematic investigation of gastrointestinal disease in China. Am J Gastroenterol 105: 2570–2577. [DOI] [PubMed] [Google Scholar]
- 29. Richter JE, Falk GW, Vaezi MF (1998) Helicobacter pylori and gastroesophageal reflux disease: the bug may not be all bad. Am J Gastroenterol 93: 1800–1802. [DOI] [PubMed] [Google Scholar]
- 30. Huang JQ, Sridhar S, Hunt RH (2002) Role of Helicobacter pylori infection and non-steroidal anti-inflammatory drugs in peptic-ulcer disease: a meta-analysis. Lancet 359: 14–22. [DOI] [PubMed] [Google Scholar]
- 31. Yamaji Y, Mitsushima T, Ikuma H, Okamoto M, Yoshida H, et al. (2001) Inverse background of Helicobacter pylori antibody and pepsinogen in reflux oesophagitis compared with gastric cancer: analysis of 5732 Japanese subjects. Gut 49: 335–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brenner H, Berg G, Lappus N, Kliebsch U, Bode G, et al. (1999) Alcohol consumption and Helicobacter pylori infection: results from the German National Health and Nutrition Survey. Epidemiology 10: 214–218. [PubMed] [Google Scholar]
- 33. Brenner H, Rothenbacher D, Bode G, Adler G (1997) Relation of smoking and alcohol and coffee consumption to active Helicobacter pylori infection: cross sectional study. BMJ 315: 1489–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Quartero AO, de Wit NJ (1998) Smoking, alcohol and coffee consumption, and H pylori infection. Cross sectional study shows no protective effect of alcohol. BMJ 316: 1020. [PubMed] [Google Scholar]
- 35. World gastroenterology organisation global guideline: Helicobacter pylori in developing countries. J Dig Dis 12: 319–326. [DOI] [PubMed] [Google Scholar]
- 36.OECD Health Data 2012 - Frequently Requested Data (OECD, Available: http://www.oecd.org/health/healthpoliciesanddata/oecdhealthdata2012-frequentlyrequesteddata.htm.Accessed 2012 September 27.
- 37. Yamamichi N, Mochizuki S, Asada-Hirayama I, Mikami-Matsuda R, Shimamoto T, et al. (2012) Lifestyle factors affecting gastroesophageal reflux disease symptoms: a cross-sectional study of healthy 19864 adults using FSSG scores. BMC Med 10: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lundell LR, Dent J, Bennett JR, Blum AL, Armstrong D, et al. (1999) Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut 45: 172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Klauser AG, Schindlbeck NE, Muller-Lissner SA (1990) Symptoms in gastro-oesophageal reflux disease. Lancet 335: 205–208. [DOI] [PubMed] [Google Scholar]
- 40. Watanabe Y, Kurata JH, Kawamoto K, Kawai K (1992) Epidemiological study of peptic ulcer disease among Japanese and Koreans in Japan. J Clin Gastroenterol 15: 68–74. [DOI] [PubMed] [Google Scholar]
- 41. Friedman GD, Siegelaub AB, Seltzer CC (1974) Cigarettes, alcohol, coffee and peptic ulcer. N Engl J Med 290: 469–473. [DOI] [PubMed] [Google Scholar]
- 42. Nechige R, Sakaki N, Iida Y, Aonuma K, Ogino M, et al. (1981) Back ground Factors of Peptic Ulcer. Yamaguchi medical journal 30: 455–462. [Google Scholar]
- 43. Debas HT, Cohen MM, Holubitsky IB, Harrison RC (1971) Caffeine-stimulated acid and pepsin secretion: dose-response studies. Scand J Gastroenterol 6: 453–457. [DOI] [PubMed] [Google Scholar]
- 44. Feldman EJ, Isenberg JI, Grossman MI (1981) Gastric acid and gastrin response to decaffeinated coffee and a peptone meal. JAMA 246: 248–250. [PubMed] [Google Scholar]
- 45. Roth JA, Ivy AC (1944) The effect of caffeine upon gastric secretion in the dog, cat and man. Am J Physiol 141: 454–461. [Google Scholar]
- 46. Gomez-Ruiz JA, Leake DS, Ames JM (2007) In vitro antioxidant activity of coffee compounds and their metabolites. J Agric Food Chem 55: 6962–6969. [DOI] [PubMed] [Google Scholar]
- 47. Kang NJ, Lee KW, Shin BJ, Jung SK, Hwang MK, et al. (2009) Caffeic acid, a phenolic phytochemical in coffee, directly inhibits Fyn kinase activity and UVB-induced COX-2 expression. Carcinogenesis 30: 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Seo HS, Hirano M, Shibato J, Rakwal R, Hwang IK, et al. (2008) Effects of coffee bean aroma on the rat brain stressed by sleep deprivation: a selected transcript- and 2D gel-based proteome analysis. J Agric Food Chem 56: 4665–4673. [DOI] [PubMed] [Google Scholar]
- 49. Dennish GW, Castell DO (1972) Caffeine and the lower esophageal sphincter. Am J Dig Dis 17: 993–996. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flow diagram of the meta-analysis literature search results.
(DOC)
Summary characteristics of cohort or case-control studies were included from the meta-analysis that compare the relationship of coffee and peptic ulcer.
(XLS)
Summary characteristics of cohort or case-control studies were excluded from the meta-analysis that compare the association of coffee and peptic ulcer.
(XLS)