Abstract
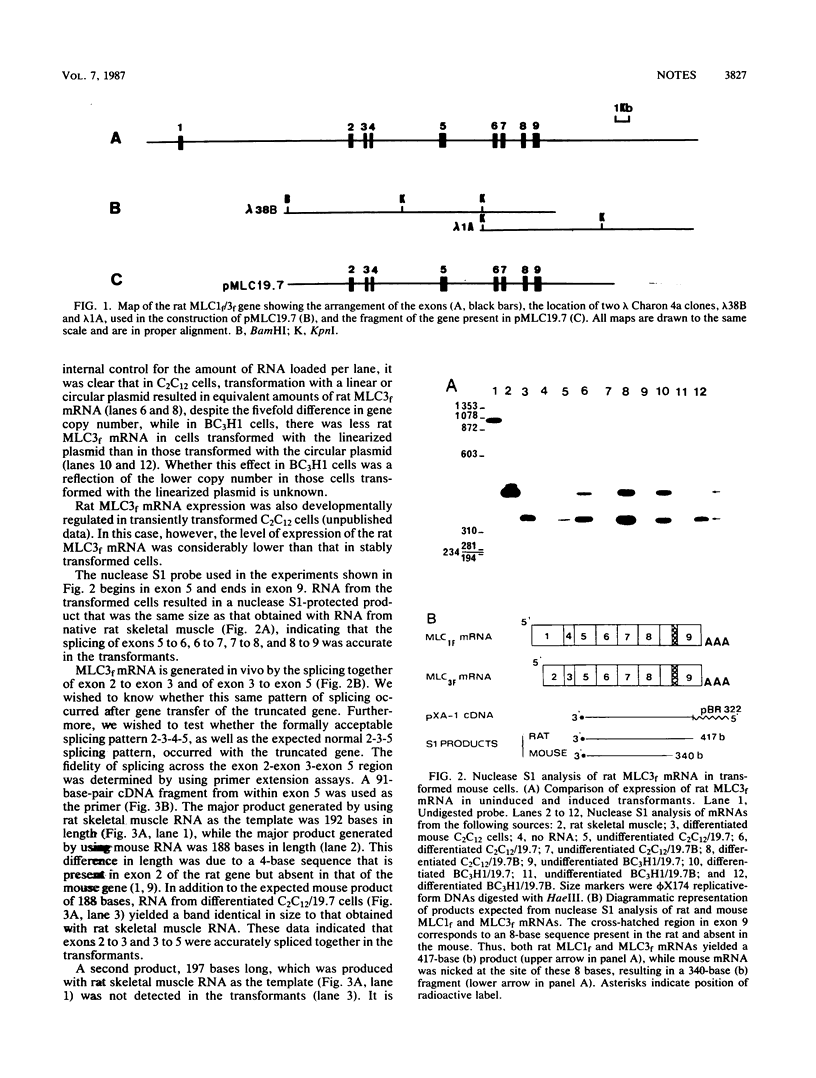
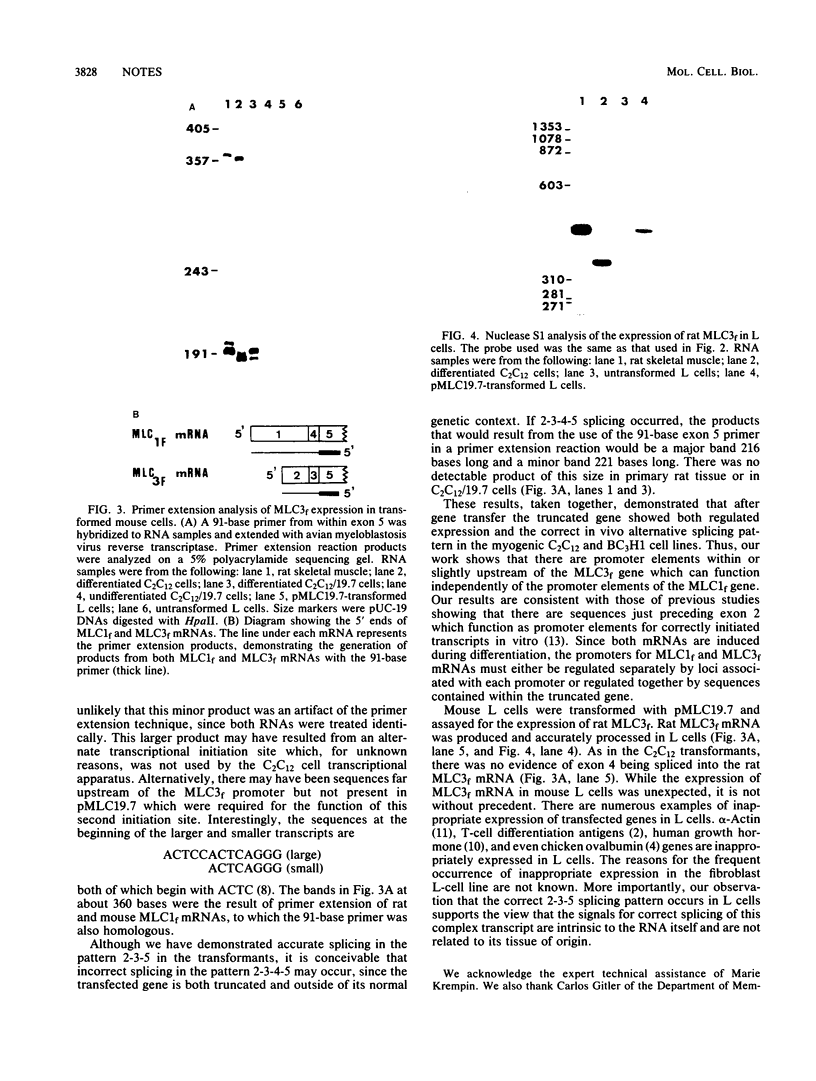
Fast skeletal muscle myosin light-chain I (MLC1f) and myosin light-chain 3 (MLC3f) mRNAs are both derived from a single rat MLC1/3f gene. MLC1f mRNA begins at the first exon of the gene, while MLC3f mRNA begins with exon 2, 10 kilobases downstream. Both mRNAs require alternate splicing of internal exons for accurate expression. We showed that a truncated rat MLC1f/3f gene lacking exon 1 and the first 6.3 kilobases of the intron separating exons 1 and 2 produced rat MLC3f mRNA in a developmentally regulated manner after introduction into myogenic mouse cells, thus demonstrating in vivo the presence of a functional promoter associated with exon 2. Correctly spliced mRNA was produced after transfer of this truncated gene into both myogenic and nonmyogenic cells, indicating that the pattern of splicing of this complex transcript was due to a structural features of the RNA and was independent of cell type.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Garfinkel L. I., Periasamy M., Nadal-Ginard B. Cloning and characterization of cDNA sequences corresponding to myosin light chains 1, 2, and 3, troponin-C, troponin-T, alpha-tropomyosin, and alpha-actin. J Biol Chem. 1982 Sep 25;257(18):11078–11086. [PubMed] [Google Scholar]
- Kavathas P., Herzenberg L. A. Stable transformation of mouse L cells for human membrane T-cell differentiation antigens, HLA and beta 2-microglobulin: selection by fluorescence-activated cell sorting. Proc Natl Acad Sci U S A. 1983 Jan;80(2):524–528. doi: 10.1073/pnas.80.2.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. K., Wold B. J. Stable reduction of thymidine kinase activity in cells expressing high levels of anti-sense RNA. Cell. 1985 Aug;42(1):129–138. doi: 10.1016/s0092-8674(85)80108-2. [DOI] [PubMed] [Google Scholar]
- Lai E. C., Woo S. L., Bordelon-Riser M. E., Fraser T. H., O'Malley B. W. Ovalbumin is synthesized in mouse cells transformed with the natural chicken ovalbumin gene. Proc Natl Acad Sci U S A. 1980 Jan;77(1):244–248. doi: 10.1073/pnas.77.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levis R., Hazelrigg T., Rubin G. M. Effects of genomic position on the expression of transduced copies of the white gene of Drosophila. Science. 1985 Aug 9;229(4713):558–561. doi: 10.1126/science.2992080. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Ong E. S., Rosenfeld M. G., Verma I. M., Evans R. M. Infectious and selectable retrovirus containing an inducible rat growth hormone minigene. Science. 1984 Sep 7;225(4666):993–998. doi: 10.1126/science.6089340. [DOI] [PubMed] [Google Scholar]
- Nabeshima Y., Fujii-Kuriyama Y., Muramatsu M., Ogata K. Alternative transcription and two modes of splicing results in two myosin light chains from one gene. Nature. 1984 Mar 22;308(5957):333–338. doi: 10.1038/308333a0. [DOI] [PubMed] [Google Scholar]
- Periasamy M., Strehler E. E., Garfinkel L. I., Gubits R. M., Ruiz-Opazo N., Nadal-Ginard B. Fast skeletal muscle myosin light chains 1 and 3 are produced from a single gene by a combined process of differential RNA transcription and splicing. J Biol Chem. 1984 Nov 10;259(21):13595–13604. [PubMed] [Google Scholar]
- Robert B., Daubas P., Akimenko M. A., Cohen A., Garner I., Guenet J. L., Buckingham M. A single locus in the mouse encodes both myosin light chains 1 and 3, a second locus corresponds to a related pseudogene. Cell. 1984 Nov;39(1):129–140. doi: 10.1016/0092-8674(84)90198-3. [DOI] [PubMed] [Google Scholar]
- Robins D. M., Paek I., Seeburg P. H., Axel R. Regulated expression of human growth hormone genes in mouse cells. Cell. 1982 Jun;29(2):623–631. doi: 10.1016/0092-8674(82)90178-7. [DOI] [PubMed] [Google Scholar]
- Schubert D., Harris A. J., Devine C. E., Heinemann S. Characterization of a unique muscle cell line. J Cell Biol. 1974 May;61(2):398–413. doi: 10.1083/jcb.61.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler-Tuyns A., Eldridge J. D., Paterson B. M. Expression and regulation of chicken actin genes introduced into mouse myogenic and nonmyogenic cells. Proc Natl Acad Sci U S A. 1984 May;81(10):2980–2984. doi: 10.1073/pnas.81.10.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strehler E. E., Periasamy M., Strehler-Page M. A., Nadal-Ginard B. Myosin light-chain 1 and 3 gene has two structurally distinct and differentially regulated promoters evolving at different rates. Mol Cell Biol. 1985 Nov;5(11):3168–3182. doi: 10.1128/mcb.5.11.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakimoto B. T., Kalfayan L. J., Spradling A. C. Developmentally regulated expression of Drosophila chorion genes introduced at diverse chromosomal positions. J Mol Biol. 1986 Jan 5;187(1):33–45. doi: 10.1016/0022-2836(86)90404-3. [DOI] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R. Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor. Cell. 1978 Jul;14(3):725–731. doi: 10.1016/0092-8674(78)90254-4. [DOI] [PubMed] [Google Scholar]
- Yaffe D., Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977 Dec 22;270(5639):725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]