Summary
Biology is replete with examples of regeneration, the process that allows animals to replace or repair cells, tissues and organs. As on land, vertebrates in aquatic environments experience the occurrence of injury with varying frequency and to different degrees. Studies demonstrate that ray-finned fishes possess a very high capacity to regenerate different tissues and organs when they are adults. Among fishes that exhibit robust regenerative capacities are the neotropical electric fishes of South America (Teleostei: Gymnotiformes). Specifically, adult gymnotiform electric fishes can regenerate injured brain and spinal cord tissues and restore amputated body parts repeatedly. We have begun to identify some aspects of the cellular and molecular mechanisms of tail regeneration in the weakly electric fish Sternopygus macrurus (long-tailed knifefish) with a focus on regeneration of skeletal muscle and the muscle-derived electric organ. Application of in vivo microinjection techniques and generation of myogenic stem cell markers are beginning to overcome some of the challenges owing to the limitations of working with non-genetic animal models with extensive regenerative capacity. This review highlights some aspects of tail regeneration in S. macrurus and discusses the advantages of using gymnotiform electric fishes to investigate the cellular and molecular mechanisms that produce new cells during regeneration in adult vertebrates.
Key words: epimorphic regeneration, electric fish, satellite cells, muscle regeneration, tail regeneration, electric organ, Sternopygus, dedifferentiation
Introduction
Biology is replete with examples of regeneration, the process that allows animals to replace or repair cells, tissues and organs. Regeneration provides obvious benefits to survival and function and is known to occur widely among animal phyla (Bely, 2010; Sánchez Alvarado and Tsonis, 2006). Some animals perform this remarkable feat almost flawlessly. However, the ability to fully regenerate complex tissues decreases as one moves from the basal groups in the animal kingdom to those more highly derived groups (Goss, 1969). For example, invertebrates including jellyfish, planarians, segmented worms, mollusks and starfish can fully regenerate multiple tissues and even entire body parts (Brusca and Brusca, 1990; Ferretti and Geraudie, 1998; Brockes and Kumar, 2002). Among vertebrates, amphibians and fishes generally express a robust regeneration response to limb loss, whereas reptiles, birds and mammals have a more limited capacity to regenerate complex body structures (Brockes and Kumar, 2008; Galis et al., 2003). The evolutionary loss of regenerative ability in some groups, to varying degrees, remains an unsolved problem that attracts many biologists (Bely and Nyberg, 2010). And, not surprisingly, the limited capacity of humans to regenerate and restore tissues and organs fuels enormous interest from a medical perspective in elucidating the mechanisms responsible for the extensive regenerative capacities in vertebrates.
In general, studies exploring regeneration show that vertebrate species that can restore amputated limb structures during early development exhibit little to no regenerative capacity as adults. For example, all adult frogs either do not regenerate their limbs at all or they regenerate a spike of cartilage surrounded by skin – an ‘outgrowth’, but hardly a limb – whereas all immature frogs or ‘tadpoles’ easily regenerate their limbs (Muneoka et al., 1986). Progressive decline in the regenerative response to limb amputation with increasing age has also been demonstrated in rodents (Deuchar, 1976; Lee and Chan, 1991; Wanek et al., 1989). In mammals, skeletal muscle is considered a highly regenerative tissue as it can repair itself after injury or disease. Similar to limb regeneration, aging skeletal muscle shows a diminished ability to repair itself and an increased susceptibility to contraction-induced injury (Alnaqeeb and Goldspink, 1987; Brooks and Faulkner, 1994). Failure to repair a damaged region of muscle fiber can result in severe cell atrophy and even cell death. This is in stark contrast to the inexhaustible regeneration of skeletal muscle tissues and even entire body parts exhibited by some adult non-mammalian species (Unguez and Zakon, 1998; Kirschbaum and Meunier, 1988; Sánchez Alvarado and Tsonis, 2006).
Regeneration processes are grouped according to the presence or absence of cell division during the replacement of lost tissues (Morgan, 1901). Morphallaxis occurs in the absence of cell proliferation, and epimorphosis requires cell proliferation. Epimorphosis is further categorized according to whether a regeneration blastema forms. A blastema is a specialized proliferative zone of mesenchymal cells that forms beneath the epidermis at the wound site. Blastema formation is a key process in epimorphic regeneration in both invertebrates and vertebrates (Thouveny and Tassava, 1998). The widespread distribution of this feature across animal taxa supports the hypothesis that a program for epimorphic regeneration may have been conserved through evolution (Brusca and Brusca, 1990; Sanchez-Alvarado, 2000). To date, the general notion is that a blastema is formed primarily from the re-entry of mature cells near the injury site into the cell cycle, i.e. dedifferentiation, to contribute to the restoration of lost tissue. In fact, the idea that a greater potential for epimorphic regeneration is associated with a corresponding potential for cell dedifferentiation remains broadly accepted (Brockes and Kumar, 2002; Nye et al., 2003; Tsonis, 1991). Support for this idea is based largely on the vast body of evidence from experiments in urodele amphibians such as the newt and axolotls, which are able to regenerate limbs, tail, jaws, ocular tissues including retina and lens, and some portions of the heart. Specifically, in vivo cell tracing experiments in urodele amphibians have shown that mature cells including muscle fibers can dedifferentiate, proliferate and enter the blastema (Stocum, 1995; Echeverri et al., 2001; Brockes and Kumar, 2002; Nye et al., 2003; Nechiporuk and Keating, 2002; Tanaka and Reddien, 2011). Similarly, the robust regenerative capacity of zebrafish to regrow amputated fins, heart and neural tissue such as the spinal cord and retina is suggested to depend on dedifferentiation of cells near the amputation site by studies using transgenesis, loss- and gain-of-function technology, and cell labeling methodology (Tanaka and Reddien, 2011; Singh et al., 2012; Poss et al., 2003; Poss, 2010).
Although cell dedifferentiation might be favored under certain conditions, it is not possible to exclude the contribution of alternative modes of regeneration. For example, generation of reliable cell markers have led to recent findings that resident, tissue-specific stem cells also respond to injury and contribute to blastema formation in some vertebrate species (Li et al., 2007; Morrison et al., 2006; Poss et al., 2003; Singh et al., 2012; Weber et al., 2012). Hence, the extent to which cell dedifferentiation versus stem cell activation occurs in response to injury in vertebrates warrants further investigation. Interestingly, multiple regeneration responses may come into play following injury or disease in the same animal. In mammals, as in zebrafish, liver regeneration is accomplished largely by hepatocyte dedifferentiation (Kan et al., 2009; Fausto et al., 2006; Michalopoulos, 2007). However, in mammals, skeletal muscle regeneration depends on a myogenic stem cell-dependent process without cell dedifferentiation (Zammit et al., 2006). These findings suggest that programs of tissue regeneration might be broadly conserved across these two vertebrate groups, but that their expression is regulated by tissue-specific constraints.
Vertebrate teleost fish: regenerative capabilities
Adult bony fishes, unlike cartilaginous fishes, can completely regenerate fins and segments of liver and heart tissues (Table 1). They can also regenerate almost any part of their central nervous system including parts of the retina, optic nerve, sections of the brainstem and many, if not all, spinal cord axons (Table 1). Unfortunately, the majority of fish species that possess a high regenerative capacity are not yet amenable to genetic molecular approaches. Consequently, studies on these fish species are limited in the extent to which they can address molecular and cellular mechanisms of regeneration.
Table 1.
Regeneration in adult teleost fishes

Regeneration in adult zebrafish
Research in fish regeneration biology has focused largely on the zebrafish, as it possesses a large regenerative capacity and is an ideal model system for investigation using cellular, molecular and genetic approaches and (Poss et al., 2003; Akimenko et al., 2003). Studies on zebrafish regeneration are contributing extensively to our current understanding of regeneration mechanisms to replace different cell and tissue types. Indeed, the ability to carry out loss- and gain-of-function experiments in combination with genetic screens and in vivo cell tracing is increasing the identification of regulatory factors and progenitor cells involved in zebrafish regeneration (Tanaka and Reddien, 2011; Sánchez Alvarado and Tsonis, 2006; Singh et al., 2012). Even so, the ability of adult zebrafish to fully repair and restore all damaged or lost tissues, organs and limbs is limited. For example, a zebrafish cannot regenerate its tail if amputation occurs proximal to the caudal fin (Fig. 1). In contrast, many South American gymnotiform electric fish species can repeatedly regenerate their tails following amputation during adulthood.
Fig. 1.

Tail amputation in an adult zebrafish Danio rerio. (A) An adult control fish. Dotted line shows site of tail amputation immediately proximal to anal fin. (B) The same zebrafish 2 weeks after amputation. Note the blastema formed at the base of the amputation plane that appears as a visible swelling at the end of the tail. Photo credit: Michael McDowell.
Regeneration in adult gymnotiform electric fish
There are more than 700 vertebrate species known that are capable of generating electricity and all of them are fishes representing 11 independent lineages (Albert and Crampton, 2006). Of these, approximately 173 species are gymnotiforms, or South American knifefishes, and in all but one group, the electric organ derives from striated muscle cells that suppress many muscle phenotypic properties (Bennett, 1971). Gymnotiforms have been used to study the formation of sensory and motor specializations (Fox and Richardson, 1978; Fox and Richardson, 1979; Kirschbaum and Schwassmann, 2008; Denizot et al., 1998; Denizot et al., 2007; Vischer, 1989a; Vischer, 1989b; Zakon, 1984; Lannoo et al., 1990), animal communication (Zakon et al., 2008; Carr and Friedman, 1999; Hopkins, 1988), speciation (Arnergard et al., 2010; Feulner et al., 2009; Lovejoy et al., 2010), evolution of neural networks (Alves-Gomes, 2001; Bass, 1986; Zakon et al., 2008; Kawasaki, 2009; Arnegard et al., 2010), physiology of membrane excitability and synaptic plasticity (Bell et al., 2005; Gómez et al., 2005; Lewis and Maler, 2004; Markham et al., 2009), and have been a source of material for the isolation of many molecules involved in these biological processes. Not as well known is the considerable regeneration capacity of South American gymnotiform electric fishes. Weakly electric fish are among the most highly regenerative species known (Table 1; PubMed has an incomplete listing of 63 beginning in 1969 – it does not include many publications outside of the USA). All gymnotiforms that have been tested can regenerate their tails following amputation. Specifically, they can replace lost dermis, spinal cord, electroreceptors, skeleton, blood vessels, fins, skeletal muscle and the muscle-derived electric organ in a near-perfect replication of tissue pattern and without scar formation (Table 1). Some gymnotiforms can replace all tissues lost after repeated tail amputations, suggesting an inexhaustible regeneration capacity in the adult (Unguez and Zakon, 1998).
Skeletal muscle and electric organ regeneration in the long-tailed knifefish, Sternopygus macrurus
We have studied tail regeneration in the adult gymnotiform electric fish Sternopygus macrurus (Unguez and Zakon, 1998; Unguez and Zakon, 2002; Weber et al., 2012). We use this model system because skeletal muscle cells of electric fish express a unique ability to undergo changes in their biochemical and morphological properties and give rise to electrocytes, the current-producing cells of the electric organ (Bennett, 1971; Fox and Richardson, 1978; Fox and Richardson, 1979), and we have begun to determine how the developmental program for striated muscle has been altered to produce an electrogenic, non-contractile cell that retains a partial muscle phenotype (Unguez and Zakon, 1998; Zakon and Unguez, 1999; Unguez and Zakon, 2002; Kim et al., 2004; Kim et al., 2008; Cuellar et al., 2006). We have exploited the inexhaustible regenerative capacity of adult S. macrurus to study the effects of neural input on the differentiation of muscle fibers and their phenotypic conversion into electrocytes. Recently, we have also begun to address some aspects of the cellular and molecular mechanisms underlying the regeneration of complex tail structures such as skeletal muscle and the electric organ in this teleost fish.
Tail regeneration in gymnotiforms has been most studied in S. macrurus (Fig. 2) (Patterson and Zakon, 1993; Patterson and Zakon, 1996; Patterson and Zakon, 1997; Unguez and Zakon, 1998; Unguez and Zakon, 2002; Weber et al., 2012). Like all gymnotiforms studied to date, tail regeneration in S. macrurus begins by epidermal cells covering the wound followed by formation of a blastema (Fig. 2). Specifically, when the tip of the tail is amputated, epidermal cells at the wound margin rapidly proliferate and cover the wound within 24 h (Fig. 2B). After 6–8 days, a blastema appears as a small swelling at the end of the tail (Fig. 2C). At this stage, the blastema is generally less than 4 mm in length. Over the following 6–8 days, this swelling elongates to an average 7 mm in length (Fig. 2D), and after 20–22 days, the regenerated tail is covered by pigmented epithelium that is indistinguishable from the tail of uncut fish (Fig. 2A).
Fig. 2.
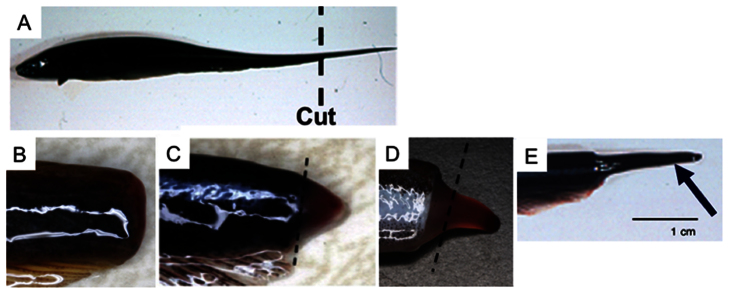
Tail regeneration in an adult South American gymnotiform Sternopygus macrurus after amputation. (A) An adult control fish. (B) A tail 24 h after amputation. Note the wound epithelium with no scar formation. (C) A 1-week blastema formed at the base of the amputation plane (dashed line) that appears as a visible swelling at the end of the tail. Cell differentiation proceeds from proximal to distal regions of the blastema. (D) A 2-week blastema formed at the base of the amputation plane (dashed line) that appears as a visible swelling at the end of the tail. (E) A 3-week regenerated tail showing the epidermal layer that is indistinguishable from that of control fish. Panels A and E are reproduced from Unguez and Zakon (Unguez and Zakon, 2002). Panels C and D are reproduced from Kim et al. (Kim et al., 2008).
Activation of myogenic stem cells in response to tail amputation in adult S. macrurus
A study by Patterson and Zakon in 1993 tested the contribution of local reserve stem cells in the tail to the regeneration blastema (Patterson and Zakon, 1993). Using light microscopy observations and 5′-bromodeoxyuridine (BrdU) incorporation studies, the authors concluded that existing stem cells associated with muscle fibers and electrocytes proliferated and contributed to the regeneration blastema. Although it was demonstrated that myogenic stem cells were activated following tail amputation, a more rigorous identification of proliferative cell types and their degree of contribution to the blastema was warranted. Hence, we began to study the cellular mechanisms underlying the repetitive regeneration of myogenic tissues in S. macrurus using ultrastructural analysis, immunolabeling detection of the adult muscle stem cells called satellite cells, and in vivo microinjection studies of high molecular weight cell lineage tracers into single muscle fibers or electrocytes.
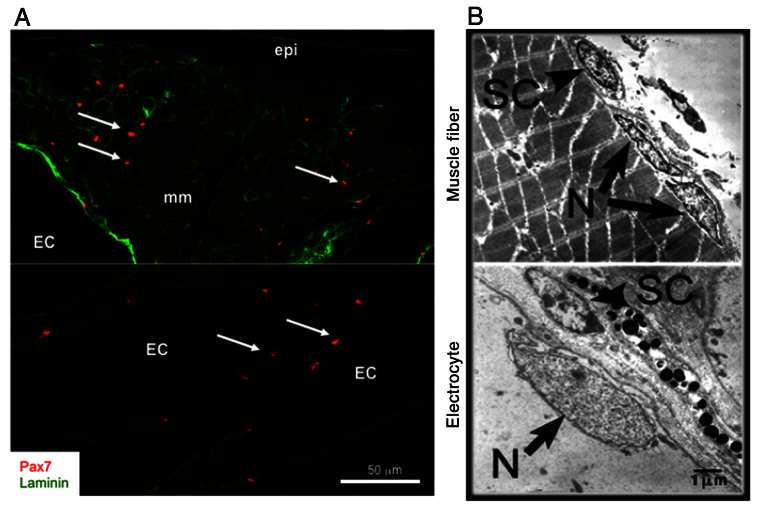
First, our ultrastructural studies verified the anatomical presence of myogenic satellite cells in both muscle and the electric organ (Fig. 3). Second, because Pax7 is a commonly used marker for identifying quiescent satellite cells in mammals (Seale et al., 2000; Zammit et al., 2004), we carried out molecular and immunolabeling studies of Pax7 expression in S. macrurus tissues. These studies showed that Pax7-positive cells were associated with electrocytes and muscle fibers of intact adult tails (Fig. 3). By combining nuclear Pax7 expression with ultrastructural analysis of satellite cells, we concluded that Pax7 is a reliable satellite cell marker for S. macrurus. By virtue of their nuclear Pax7 expression, morphology and location, these cells associated with muscle fibers and electrocytes were considered to be homologs of satellite cells observed in mammals (Schultz et al., 2006; Ishido et al., 2009), salamanders (Morrison et al., 2006), frogs (Chen et al., 2006) and other teleosts (Steinbacher et al., 2007). Moreover, these data provided the first example of Pax7-positive muscle progenitor cells localized within a non-contractile electrogenic tissue, representing an exception to the tissue specificity of these myogenic stem cells to striated muscle fibers in vertebrates.
Fig. 3.
Myogenic stem cells in adult skeletal muscle and the electric organ of S. macrurus. Pax7 labels myogenic satellite cells in adult skeletal muscle and the electric organ. (A) Pax7-positive cells (red) co-label with laminin (green) in tissue cryosections (20 mm thick). Arrows point to localization of Pax7. Abbreviations: EC, electrocyte; mm, muscle fiber; epi, epidermis. (B) Electron micrograph cross-sections of adult intact tail show that satellite cells (SC) unlike other nuclei (N) are found between the plasma membrane (arrow) and basal lamina in both electrocytes and muscle fibers. Reproduced from Weber et al. (Weber et al., 2012).
We then studied the response of Pax7-positive cells following amputation, blastema formation, and regeneration of muscle fibers and electrocytes over a 2 week period. Upon tail amputation, we detected an increase in the number of Pax7-positive cells that associated with intact electrocytes and muscle fibers near the amputation site (Fig. 4). This increase in the number of Pax7-positive cells continued up to 14 days after tail amputation, at which time their density was markedly higher (Fig. 5) than that found in control tissues (Fig. 3). Within the regeneration blastema, Pax7-positive cells were found exclusively in regions where muscle fibers and electrocytes form de novo (Fig. 6A), as demonstrated by the co-labeling of myogenic markers for sarcomeric myosin heavy chain expression (Fig. 6B). Further, these myogenic precursor cells, at least based on their compartmentalization in the regeneration blastema, did not seem to contribute to tissues other than skeletal muscle and the electric organ. For these reasons, our results suggest that muscle and electrocyte regeneration in adult S. macrurus is similar to the satellite-dependent muscle repair process common in vertebrates, including mammals, frogs, birds, fish and some salamanders (Hawke and Garry, 2001; Zammit et al., 2004; Morrison et al., 2006; Morrison et al., 2010; Feldman et al., 1993).
Fig. 4.
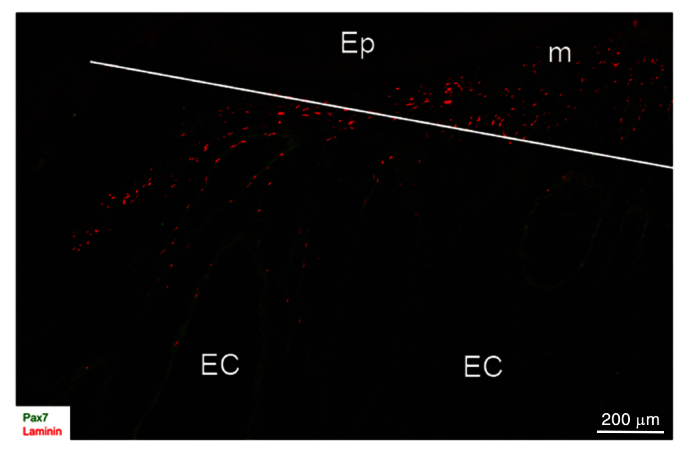
Activation of Pax7-positive cells in S. macrurus near amputation site. Portions of a longitudinal cryosection (20 mm thick) from 7-day blastema co-labeled with anti-Pax7 (red) and anti-laminin (green) antibodies. White line shows the site of tail amputation. Abbreviations: EC, electrocyte; Ep, epithelium; m, muscle fiber.
Fig. 5.
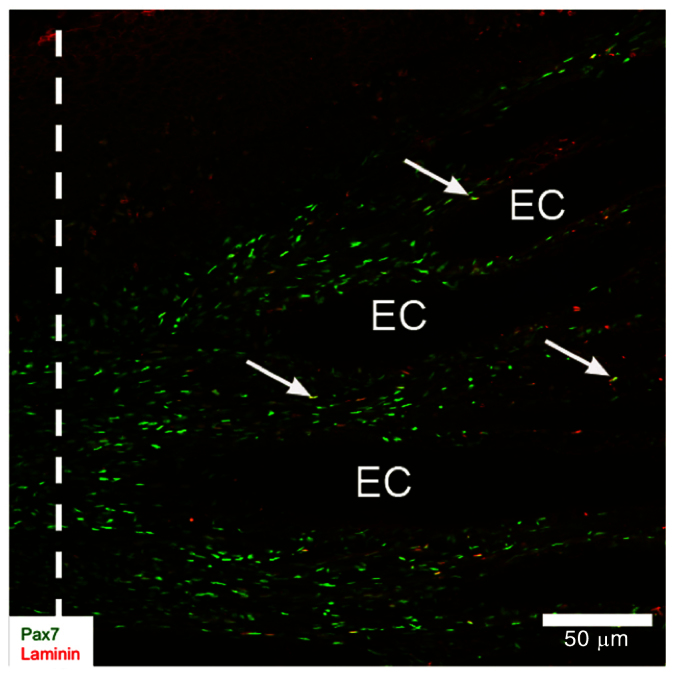
Activation of Pax7-positive cells near intact electrocytes (EC). Portions of longitudinal cryosection (20 mm thick) from 14-day blastema co-labeled with anti-Pax7 (green) and anti-BrdU (red) antibodies. White dashed line shows the site of tail amputation. Arrows point to cells that were co-labeled with Pax7 and BrdU. Reproduced from Weber et al. (Weber et al., 2012).
Fig. 6.
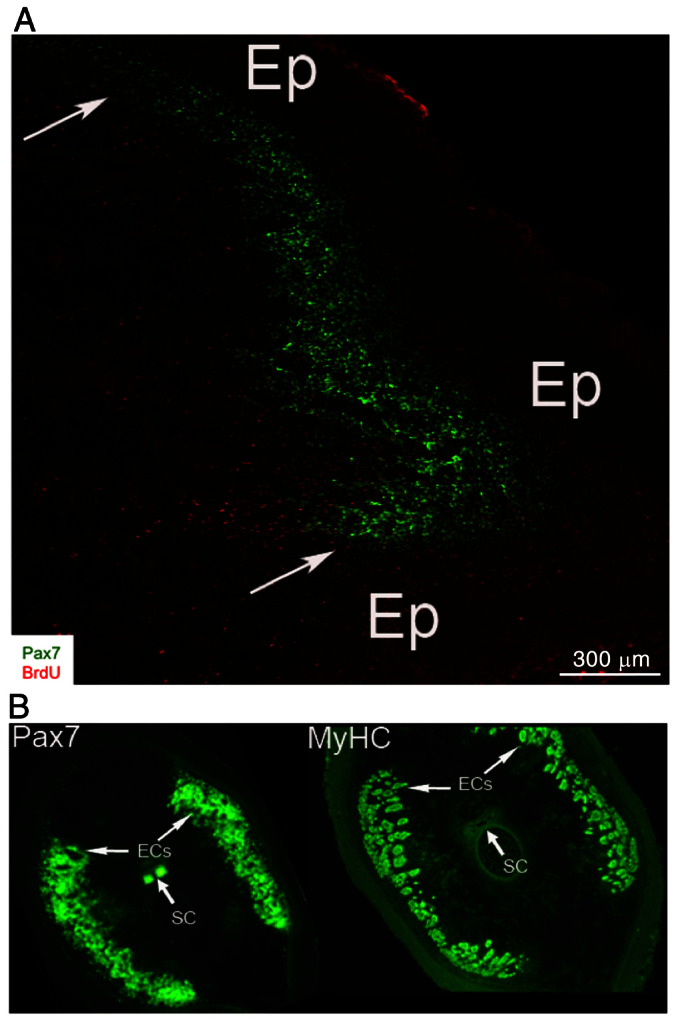
Distribution of Pax7-positive cells in regions that give rise to muscle fibers and electrocytes. (A) Spatial distribution of Pax7-positive cells in regeneration blastema. Confocal images of longitudinal cryosection (20 mm thick) from 14-day blastema immunolabeled with anti-Pax7 and BrdU antibodies. Arrowheads point to Pax7-positive cells adjacent to the epithelium (Ep). Reproduced from Weber et al. (Weber et al., 2012). (B) Pax7-positive cells are localized in blastema regions that give rise to muscle, electric organ and dorsal spinal cord. Serial cross-sections taken from distal half of 14-day blastemas immunolabeled with antibodies against Pax7 (green) and myosin heavy chain (MyHC). MyHC is present in all muscle fibers, which are small cells located between epithelium and developing electrocytes (ECs), which are larger cells more medially located (arrows). Co-labeling by anti-Pax7 and anti-MyHC antibodies was detected in peripheral regions of the blastema underneath the epithelium. Pax7 labeling was also detected in dorsal spinal cord (SC). Reproduced from Weber et al. (Weber et al., 2012).
No evidence for myogenic cell dedifferentiation after tail amputation in adult S. macrurus
As in previous urodele regeneration experiments, we also performed in vivo microinjection studies using high molecular weight lineage tracers to test whether the source of proliferating cells proximal to the amputation site were mature cells that underwent dedifferentiation. In these experiments, intact electrocytes and muscle fibers were labeled with single-cell dextran injections prior to tail amputation. Tails analyzed up to 14 days post-amputation showed an intact morphology of these myogenic cells with no fragmentation into smaller cellular components (Fig. 7), suggesting that skeletal muscle or electric organ dedifferentiation did not occur. In a separate group of fish, serial longitudinal cryosections were immunolabeled with laminin and morphological analysis did not reveal any evidence of electrocyte or muscle fiber fragmentation (Weber et al., 2012). Although these data do not negate the possibility that some cell dedifferentiation does take place, they do not support cell dedifferentiation as the essential mechanism underlying the robust regeneration capacity of myogenic tissues in S. macrurus. Instead, together with our Pax7 analyses, our studies provide support for the occurrence of a stem cell activation mechanism for epimorphic regeneration in this vertebrate species.
Fig. 7.
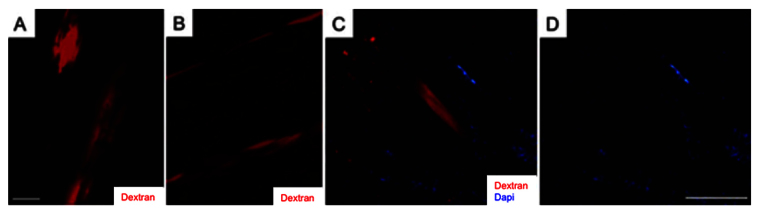
Intracellular tracer dye injections into single muscle fibers and electrocytes at the level of the distal-most ventral fin reveal no cell fragmentation. Fluororuby (red) was injected into single electrocytes (A) and muscle fibers (B) and visualized 7 days after injection by retracting the overlying skin of the fish. Tail sections at the level of the distal-most region of the ventral fin were processed for immunolabeling and viewed under a fluorescent microscope. (C) Region of a cryosection containing muscle fibers injected with Fluororuby Dextran (red) and counterstained with DAPI (blue). (D) Same image as C showing only DAPI labeling. Scale bars: (A) 20 mm; (D) 10 mm. Reproduced from Weber et al. (Weber et al., 2012).
Conclusions and future directions
Regeneration is not merely a curious coincidence, it highlights a biologically relevant feature that has been selected for in both terrestrial and aquatic environments. Gymnotiform fishes, like urodele amphibians, possess a large capacity as adults to fully regenerate injured or amputated body parts that contain multiple tissue types, including excised portions of spinal cord and skeletal muscle. Data from our Pax7 expression studies in combination with intracellular dye injection experiments are significant because they reveal that in the adult S. macrurus, restoration of skeletal muscle and the muscle-derived electric organ relies on the activation of myogenic stem cells for the renewal of both myogenic tissues. These data are consistent with the emergent concept in vertebrate regeneration that different tissues are sources of distinct progenitor cell populations to the regeneration blastema, and these progenitor cells subsequently restore the original tissue (Tanaka and Reddien, 2011). In conclusion, cell dedifferentiation is likely one regeneration strategy in adult S. macrurus, but it is not a requirement in the regeneration of skeletal muscle and the muscle-derived electric organ. Similarly, the incidence of tissue-specific stem cells contributing to the blastema in adults has been reported in limb regeneration in urodele amphibians (Morrison et al., 2006) and antler regeneration in deer (Li et al., 2007). These reports emphasize the significance of investigating a broad range of species in order to expand our knowledge on the different strategies used to restore lost tissues and assess the extent to which the identified cellular and molecular mechanisms that produce new cells are conserved across different groups. One could imagine that inclusion of a wider range of species in regeneration studies may generate novel information useful to modify our current model of epimorphic regeneration in vertebrates.
Our studies using S. macrurus also underline the significance of determining whether the Pax7-positive cells in muscle and the electric organ form a single multipotent class of cells or whether they represent discrete satellite cells with different tissue-specific fates and replication capacities following injury. For example, it will be interesting to determine whether Pax7-positive cells in adult myogenic tissues reflect their cell type of origin during the recapitulation of tissue organization after tail amputation, as has been observed with satellite cell populations that give rise to either slow or fast muscle fibers in birds and rodents (Feldman and Stockdale, 1991; Rosenblatt et al., 1996). Differences in replication capacities between satellite cells associated with muscle fibers versus cells of the electric organ, if present, would also be consistent with findings from mammalian satellite cell studies (Collins et al., 2007; Zammit et al., 2006; Zammit, 2008; Kuang et al., 2007). One way to explore this is to replicate differentiation of muscle fibers and electrocytes in culture. Our ability to isolate satellite cells from muscle fibers or electrocytes will allow us to directly test the potential of these myogenic cells under suitable differentiation conditions (Shadd et al., 2008). Future studies can help identify specific environmental cues that may influence the proliferative and differentiation potentials of satellite cells associated with muscle and the electric organ.
The effectiveness of satellite cells in restoring damaged muscle in vertebrates is regulated by the interplay between differences in the inherent potential of the satellite cells themselves and the external cues from the surrounding environment (Dhawan and Rando, 2005; Collins et al., 2007). In this regard, studies have reported that muscle regeneration in adult mammals can initially proceed to some extent in the absence of innervation, but growth and terminal differentiation of regenerated fibers and continued regeneration is impeded in denervated muscles beyond a week (Mussini et al., 1987; Sesodia and Cullen, 1991). Whether this ineffective muscle regeneration is due to changes in neural factors that inhibit the regenerative ability of otherwise competent satellite cells is not known. Relatively little work has been carried out to date on the potential influences of neuronal factors (electrical activity or activity-independent) on satellite cells and early events in muscle regeneration. Addressing these issues in a model system such as S. macrurus, where satellite cells associate with muscle fibers and electrocytes, which differ significantly in nerve-dependent activation patterns (Mills et al., 1992), would provide important insight into the roles that defined environmental cues play in the satellite cell biological phenotype.
The South American gymnotiform fishes are monophyletic but comprise a considerably diversified group (Albert and Crampton, 2006). Although closely related, different gymnotiform species exhibit diversity in their general morphology and anatomy, size and shape of their individual electrocytes, and shape and duration of their electric organ discharge signals (Bennett, 1971; Albert and Crampton, 2006). It is important to note that adults also vary considerably in the extent to which they restore the complexity and structural organization of lost tissues after tail amputation. For example, the glass knifefish Eigenmannia virescens regenerates its tail following amputation much like S. macrurus, i.e. a regeneration blastema forms, grows and restores the different tissues of the tail in a structural organization resembling that of an uncut tail (Figs 2, 8). Interestingly, a comparable tail amputation in the adult black ghost knifefish, Apteronotus albifrons, proceeds with formation of a regeneration blastema, but its growth and differentiation results in a stunted tail with an anal fin-like structure at the distal end (Fig. 8). To what extent are similar mechanisms to regenerate myogenic tissue at work? And what factors determine the type and shape of tissues that comprise the tail regenerate in A. albifrons? Experimental approaches (intracellular dye microinjections) and availability of useful markers (cell type markers) that can be applied across close relatives will be essential in comparing molecular factors and cellular processes underlying regeneration among gymnotiforms. In sum, our findings in S. macrurus regeneration after tail amputation not only raise important questions regarding the extent to which a myogenic stem cell-dependent regeneration process is conserved and active in vertebrate species other than urodele amphibians and zebrafish, but also warrant further investigations to determine why and how regenerative abilities differ among the closely related species of gymnotiform electric fish.
Fig. 8.
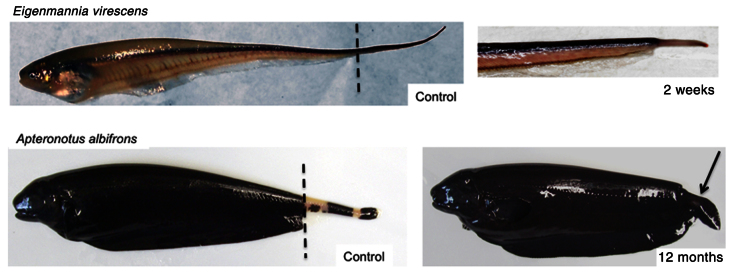
Tail regeneration in adult gymnotiforms Eigenmannia virescens and Apteronotus albifrons following amputation. Left panels: adult control Eigenmannia virescens (glass knifefish) and Apteronotus albifrons (black ghost knifefish). In both gymnotiforms, regeneration proceeds through the formation of a blastema at the base of the amputation plane (dashed line) and cell differentiation occurs from proximal to distal regions of the blastema. Right panels: regenerated tails in Eigenmannia virescens and Apteronotus albifrons 2 weeks and 12 months after tail cut, respectively. The 2-week regeneration blastema in E. virescens (top right panel) parallels that observed in S. macrurus and its subsequent regeneration results in the restoration of all tissues and structures in proportions comparable to that of an intact adult tail. In A. albifrons, regeneration after amputation proceeds with blastema formation (not shown) and differentiation of anal-like fin structure (arrow) with stunted tail segment. Photo credit: Vincent Gutschick.
Supplementary Material
Acknowledgements
Live fish photography by Vince Gutschick and Michael McDowell. Dr Laura Burrus provided insightful comments on the manuscript.
Footnotes
Competing interests
No competing interests declared.
Funding
This work was supported by National Institutes of Health grants S06-GM008136, RR16480 and U56-CA96286 [all to G.A.U.]. Deposited in PMC for release after 12 months.
References
- Akimenko M. A., Marí-Beffa M., Becerra J., Géraudie J. (2003). Old questions, new tools, and some answers to the mystery of fin regeneration. Dev. Dyn. 226, 190-201 [DOI] [PubMed] [Google Scholar]
- Albert J. S., Crampton W. G. R. (2006). Electroreception and electrogenesis. In The Physiology of Fishes, 3rd edn (Evans D. H., Claiborne J. B.), pp. 431-472 Boca Raton, FL: Taylor and Francis; [Google Scholar]
- Alnaqeeb M. A., Goldspink G. (1987). Changes in fibre type, number and diameter in developing and ageing skeletal muscle. J. Anat. 153, 31-45 [PMC free article] [PubMed] [Google Scholar]
- Alonso M., Tabata Y. A., Rigolino M. G., Tsukamoto R. Y. (2000). Effect of induced triploidy on fin regeneration of juvenile rainbow trout, Oncorhynchus mykiss. J. Exp. Zool. 287, 493-502 [DOI] [PubMed] [Google Scholar]
- Alves-Gomes J. A. (2001). The evolution of electroreception and bioelectrogenesis in teleost fish: a phylogenetic perspective. J. Fish Biol. 58, 1489-1511 [Google Scholar]
- Anderson M. J., Waxman S. G. (1981). Morphology of regenerated spinal cord in Sternarchus albifrons. Cell Tissue Res. 219, 1-8 [DOI] [PubMed] [Google Scholar]
- Anderson M. J., Waxman S. G. (1983a). Regeneration of spinal neurons in inframammalian vertebrates: morphological and developmental aspects. J. Hirnforsch. 24, 371-398 [PubMed] [Google Scholar]
- Anderson M. J., Waxman S. G. (1983b). Caudal spinal cord of the teleost Sternarchus albifrons resembles regenerating cord. Anat. Rec. 205, 85-92 [DOI] [PubMed] [Google Scholar]
- Anderson M. J., Waxman S. G., Tadlock C. H. (1984). Cell death of asynaptic neurons in regenerating spinal cord. Dev. Biol. 103, 443-455 [DOI] [PubMed] [Google Scholar]
- Anderson M. J., Fong H. L., Waxman S. G. (1985). Retrograde labeling of regenerated electromotor neurons with HRP in a teleost fish, Sternarchus albifrons: relation to cell death. Cell Tissue Res. 241, 237-240 [DOI] [PubMed] [Google Scholar]
- Arnegard M. E., Zwickl D. J., Lu Y., Zakon H. H. (2010). Old gene duplication facilitates origin and diversification of an innovative communication system – twice. Proc. Natl. Acad. Sci. USA 107, 22172-22177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillet-Derbin C. (1969). [Regeneration of the electric organ in Gymnotus carapo (Pisces)] [In French]. Arch. Anat. Microsc. Morphol. Exp. 58, 387-392 [PubMed] [Google Scholar]
- Baillet-Derbin C. (1978). Cytodifferentiation of the regenerating electrocyte in an electric fish. Biol Cell 33, 15-24 [Google Scholar]
- Bass A. H. (1986). A hormone-sensitive communication system in an electric fish. J. Neurobiol. 17, 131-155 [DOI] [PubMed] [Google Scholar]
- Becker T., Wullimann M. F., Becker C. G., Bernhardt R. R., Schachner M. (1997). Axonal regrowth after spinal cord transection in adult zebrafish. J. Comp. Neurol. 377, 577-595 [DOI] [PubMed] [Google Scholar]
- Bell C. C., Meek J., Yang J. Y. (2005). Immunocytochemical identification of cell types in the mormyrid electrosensory lobe. J. Comp. Neurol. 483, 124-142 [DOI] [PubMed] [Google Scholar]
- Bely A. E. (2010). Evolutionary loss of animal regeneration: pattern and process. Integr. Comp. Biol. 50, 515-527 [DOI] [PubMed] [Google Scholar]
- Bely A. E., Nyberg K. G. (2010). Evolution of animal regeneration: re-emergence of a field. Trends Ecol. Evol. 25, 161-170 [DOI] [PubMed] [Google Scholar]
- Bennett M. V. L. (1971). Electric Organs. In Fish Physiology, Vol. 5 (ed. Hoar W. S., Randall D. J.), pp. 347-491 New York: Academic Press; [Google Scholar]
- Bensouilah M., Denizot J. P. (1994). Formation of new sensory cells in deafferented tuberous organs of the gymnotid fish Eigenmannia virescens. J. Neurosci. Res. 39, 545-555 [DOI] [PubMed] [Google Scholar]
- Bernhardt R. R., Tongiorgi E., Anzini P., Schachner M. (1996). Increased expression of specific recognition molecules by retinal ganglion cells and by optic pathway glia accompanies the successful regeneration of retinal axons in adult zebrafish. J. Comp. Neurol. 376, 253-264 [DOI] [PubMed] [Google Scholar]
- Böckelmann P. K., Ochandio B. S., Bechara I. J. (2010). Histological study of the dynamics in epidermis regeneration of the carp tail fin (Cyprinus carpio, Linnaeus, 1758). Rev. Bras. Biol. 70, 217–223 [DOI] [PubMed] [Google Scholar]
- Brockes J. P., Kumar A. (2002). Plasticity and reprogramming of differentiated cells in amphibian regeneration. Nat. Rev. Mol. Cell Biol. 3, 566-574 [DOI] [PubMed] [Google Scholar]
- Brockes J. P., Kumar A. (2008). Comparative aspects of animal regeneration. Annu. Rev. Cell Dev. Biol. 24, 525-549 [DOI] [PubMed] [Google Scholar]
- Brooks S. V., Faulkner J. A. (1994). Skeletal muscle weakness in old age: underlying mechanisms. Med. Sci. Sports Exerc. 26, 432-439 [PubMed] [Google Scholar]
- Brusca R. C., Brusca G. J. (1990). Invertebrates. Sunderland, MS: Sinauer Associates, Inc. [Google Scholar]
- Camargo A. A. P., Carvalho R. F., Dal-Pai V., Pellizzon C. H., Dal-Pai-Silva M. (2004). Morphological aspects of muscle regeneration in the Nile tilapia (Oreochromis niloticus). J. Submicrosc. Cytol. Pathol. 36, 319-326 [PubMed] [Google Scholar]
- Carr C. E., Friedman M. A. (1999). Evolution of time coding systems. Neural Comput. 11, 1-20 [DOI] [PubMed] [Google Scholar]
- Chen Y., Lin G., Slack J. M. W. (2006). Control of muscle regeneration in the Xenopus tadpole tail by Pax7. Development 133, 2303-2313 [DOI] [PubMed] [Google Scholar]
- Collins C. A., Zammit P. S., Ruiz A. P., Morgan J. E., Partridge T. A. (2007). A population of myogenic stem cells that survives skeletal muscle aging. Stem Cells 25, 885-894 [DOI] [PubMed] [Google Scholar]
- Cuellar H., Kim J. A., Unguez G. A. (2006). Evidence of post-transcriptional regulation in the maintenance of a partial muscle phenotype by electrogenic cells of S. macrurus. FASEB J. 20, 2540 [DOI] [PubMed] [Google Scholar]
- Cuervo R., Hernández-Martínez R., Chimal-Monroy J., Merchant-Larios H., Covarrubias L. (2012). Full regeneration of the tribasal Polypterus fin. Proc. Natl. Acad. Sci. USA 109, 3838-3843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denizot J. P., Kirschbaum F., Schugardt C., Bensouilah M. (1998). Larval electroreceptors indicate a larval electric system in mormyrids. Neurosci. Lett. 241, 103-106 [DOI] [PubMed] [Google Scholar]
- Denizot J.-P., Bensouilah M., Roesler R., Schugardt C., Kirschbaum F. (2007). Larval electroreceptors in the epidermis of mormyrid fish: II. The promormyromast. J. Comp. Neurol. 501, 810-823 [DOI] [PubMed] [Google Scholar]
- Deuchar E. (1976). Regeneration of amputated limb-buds in early rat embryos. J. Embryol. Exp. Morphol. 35, 345-354 [PubMed] [Google Scholar]
- Dhawan J., Rando T. A. (2005). Stem cells in postnatal myogenesis: molecular mechanisms of satellite cell quiescence, activation and replenishment. Trends Cell Biol. 15, 666-673 [DOI] [PubMed] [Google Scholar]
- Dufourcq P., Roussigné M., Blader P., Rosa F., Peyrieras N., Vriz S. (2006). Mechano-sensory organ regeneration in adults: the zebrafish lateral line as a model. Mol. Cell. Neurosci. 33, 180-187 [DOI] [PubMed] [Google Scholar]
- Echeverri K., Clarke J. D., Tanaka E. M. (2001). In vivo imaging indicates muscle fiber dedifferentiation is a major contributor to the regenerating tail blastema. Dev. Biol. 236, 151-164 [DOI] [PubMed] [Google Scholar]
- Fausto N., Campbell J. S., Riehle K. J. (2006). Liver regeneration. Hepatology 43 Suppl. 1, S45-S53 [DOI] [PubMed] [Google Scholar]
- Feldman J. L., DiMario J. X., Stockdale F. E. (1993). Developmental appearance of adult myoblasts (satellite cells): studies of adult myoblasts in culture and adult myoblast transfer into embryonic avian limbs. Prog. Clin. Biol. Res. 383B, 563-574 [PubMed] [Google Scholar]
- Feldman J. L., Stockdale F. E. (1991). Skeletal muscle satellite cell diversity: satellite cells form fibers of different types in cell culture. Dev. Biol. 143, 320-334 [DOI] [PubMed] [Google Scholar]
- Ferguson H. W., Kongtorp R. T., Taksdal T., Graham D., Falk K. (2005). An outbreak of disease resembling heart and skeletal muscle inflammation in Scottish farmed salmon, Salmo salar L., with observations on myocardial regeneration. J. Fish Dis. 28, 119-123 [DOI] [PubMed] [Google Scholar]
- Ferretti P., Geraudie J. (1998). Cellular and Molecular Basis of Regeneration: From Invertebrates to Humans. Chichester: John Wiley and Sons; [Google Scholar]
- Feulner P. G., Plath M., Engelmann J., Kirschbaum F., Tiedemann R. (2009). Magic trait electric organ discharge (EOD): Dual function of electric signals promotes speciation in African weakly electric fish. Commun. Integr. Biol. 2, 329-331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox G. Q., Richardson G. P. (1978). The developmental morphology of Torpedo marmorata: electric organ – myogenic phase. J. Comp. Neurol. 179, 677-697 [DOI] [PubMed] [Google Scholar]
- Fox G. Q., Richardson G. P. (1979). The developmental morphology of Torpedo marmorata: electric organ – electrogenic phase. J. Comp. Neurol. 185, 293-315 [DOI] [PubMed] [Google Scholar]
- Fritzsch B., Zakon H. H., Sanchez D. Y. (1990). Time course of structural changes in regenerating electroreceptors of a weakly electric fish. J. Comp. Neurol. 300, 386-404 [DOI] [PubMed] [Google Scholar]
- Galis F., Wagner G. P., Jockusch E. L. (2003). Why is limb regeneration possible in amphibians but not in reptiles, birds, and mammals? Evol. Dev. 5, 208-220 [DOI] [PubMed] [Google Scholar]
- Goldshmit Y., Sztal T. E., Jusuf P. R., Hall T. E., Nguyen-Chi M., Currie P. D. (2012). Fgf-dependent glial cell bridges facilitate spinal cord regeneration in zebrafish. Neuroscience 32, 7477–7492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez L., Kanneworff M., Budelli R., Grant K. (2005). Dendritic spike back propagation in the electrosensory lobe of Gnathonemus petersii. J. Exp. Biol. 208, 141-155 [DOI] [PubMed] [Google Scholar]
- Goss R. J. (1969). Principles of Regeneration. New York, NY: Academic Press; [Google Scholar]
- Hawke T. J., Garry D. J. (2001). Myogenic satellite cells: physiology to molecular biology. J. Appl. Physiol. 91, 534-551 [DOI] [PubMed] [Google Scholar]
- Hopkins C. D. (1988). Neuroethology of electric communication. Annu. Rev. Neurosci. 11, 497-535 [DOI] [PubMed] [Google Scholar]
- Ishido M., Uda M., Kasuga N., Masuhara M. (2009). The expression patterns of Pax7 in satellite cells during overload-induced rat adult skeletal muscle hypertrophy. Acta Physiol. 195, 459-469 [DOI] [PubMed] [Google Scholar]
- Jørgensen J. M. (1991). Regeneration of lateral line and inner ear vestibular cells. Ciba Found. Symp. 160, 151-163, discussion 163-170 [DOI] [PubMed] [Google Scholar]
- Kan N. G., Junghans D., Izpisua Belmonte J. C. I. (2009). Compensatory growth mechanisms regulated by BMP and FGF signaling mediate liver regeneration in zebrafish after partial hepatectomy. FASEB J. 23, 3516-3525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki M. (2009). Evolution of time-coding systems in weakly electric fishes. Zool. Sci. 26, 587-599 [DOI] [PubMed] [Google Scholar]
- Kemp N. E., Park J. H. (1970). Regeneration of lepidotrichia and actinotrichia in the tailfin of the teleost Tilapia mossambica. Dev. Biol. 22, 321-342 [DOI] [PubMed] [Google Scholar]
- Khalil S. H., Aziz F. K. (1989). Regeneration of the caudal and pectoral fins of a bony fish, Gambusia (Haplochilus) schoelleri. Folia Morphol. 37, 208-212 [PubMed] [Google Scholar]
- Kim J. A., Jonsson C. B., Calderone T., Unguez G. A. (2004). Transcription of MyoD and myogenin in the non-contractile electrogenic cells of the weakly electric fish, Sternopygus macrurus. Dev. Genes Evol. 214, 380-392 [DOI] [PubMed] [Google Scholar]
- Kim J. A., Laney C., Curry J., Unguez G. A. (2008). Expression of myogenic regulatory factors in the muscle-derived electric organ of Sternopygus macrurus. J. Exp. Biol. 211, 2172-2184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum F., Meunier F. J. (1988). South American gymnotiform fishes as model animals for regeneration experiments? Monogr. Dev. Biol. 21, 112-123 [PubMed] [Google Scholar]
- Kirschbaum F., Schwassmann H. O. (2008). Ontogeny and evolution of electric organs in gymnotiform fish. J. Physiol. Paris 102, 347-356 [DOI] [PubMed] [Google Scholar]
- Kuang S., Kuroda K., Le Grand F., Rudnicki M. A. (2007). Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell 129, 999-1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannoo M. J., Vischer H. A., Maler L. (1990). Development of the electrosensory nervous system of Eigenmannia (Gymnotiformes): II. The electrosensory lateral line lobe, midbrain, and cerebellum. J. Comp. Neurol. 294, 37-58 [DOI] [PubMed] [Google Scholar]
- Lannoo M. J., Maler L., Hawkes R. (1993). Collateral sprouting in the electrosensory lateral line lobe of weakly electric teleosts (Gymnotiformes) following ricin ablation. J. Comp. Neurol. 333, 246-256 [DOI] [PubMed] [Google Scholar]
- Lee K. K., Chan W. Y. (1991). A study on the regenerative potential of partially excised mouse embryonic fore-limb bud. Anat. Embryol. 184, 153-157 [DOI] [PubMed] [Google Scholar]
- Lee Y., Grill S., Sanchez A., Murphy-Ryan M., Poss K. D. (2005). Fgf signaling instructs position-dependent growth rate during zebrafish fin regeneration. Development 132, 5173-5183 [DOI] [PubMed] [Google Scholar]
- Lewis J. E., Maler L. (2004). Synaptic dynamics on different time scales in a parallel fiber feedback pathway of the weakly electric fish. J. Neurophysiol. 91, 1064-1070 [DOI] [PubMed] [Google Scholar]
- Li C., Mackintosh C. G., Martin S. K., Clark D. E. (2007). Identification of key tissue type for antler regeneration through pedicle periosteum deletion. Cell Tissue Res. 328, 65-75 [DOI] [PubMed] [Google Scholar]
- Liu Q., Azodi E., Kerstetter A. E., Wilson A. L. (2004). Cadherin-2 and cadherin-4 in developing, adult and regenerating zebrafish cerebellum. Brain Res. Dev. Brain Res. 150, 63-71 [DOI] [PubMed] [Google Scholar]
- Lovejoy N. R., Lester K., Crampton W. G. R., Marques F. P. L., Albert J. S. (2010). Phylogeny, biogeography, and electric signal evolution of neotropical knifefishes of the genus Gymnotus (Osteichthyes: Gymnotidae). Mol. Phylogenet. Evol. 54, 278-290 [DOI] [PubMed] [Google Scholar]
- Mark R. F., Marotte L. R., Johnstone J. R. (1970). Reinnervated eye muscles do not respond to impulses in foreign nerves. Science 170, 193-194 [DOI] [PubMed] [Google Scholar]
- Markham M. R., McAnelly M. L., Stoddard P. K., Zakon H. H. (2009). Circadian and social cues regulate ion channel trafficking. PLoS Biol. 7, e1000203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalopoulos G. K. (2007). Liver regeneration. J. Cell. Physiol. 213, 286-300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills A., Zakon H. H., Marchaterre M. A., Bass A. H. (1992). Electric organ morphology of Sternopygus macrurus, a wave-type, weakly electric fish with a sexually dimorphic EOD. J. Neurobiol. 23, 920-932 [DOI] [PubMed] [Google Scholar]
- Misof B. Y., Wagner G. P. (1992). Regeneration in Salaria pavo (Blenniidae, Teleostei). Histogenesis of the regenerating pectoral fin suggests different mechanisms for morphogenesis and structural maintenance. Anat. Embryol. 186, 153-165 [DOI] [PubMed] [Google Scholar]
- Morgan T. H. (1901). Regeneration. New York, NY: The Macmillan Company; [Google Scholar]
- Morrison J. I., Lööf S., He P., Simon A. (2006). Salamander limb regeneration involves the activation of a multipotent skeletal muscle satellite cell population. J. Cell Biol. 172, 433-440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison J. I., Borg P., Simon A. (2010). Plasticity and recovery of skeletal muscle satellite cells during limb regeneration. FASEB J. 24, 750-756 [DOI] [PubMed] [Google Scholar]
- Muneoka K., Holler-Dinsmore G., Bryant S. V. (1986). Intrinsic control of regenerative loss in Xenopus laevis limbs. J. Exp. Zool. 240, 47-54 [DOI] [PubMed] [Google Scholar]
- Mussini I., Favaro G., Carraro U. (1987). Maturation, dystrophic changes and the continuous production of fibers in skeletal muscle regenerating in the absence of nerve. J. Neuropathol. Exp. Neurol. 46, 315-331 [DOI] [PubMed] [Google Scholar]
- Nechiporuk A., Keating M. T. (2002). A proliferation gradient between proximal and msxb-expressing distal blastema directs zebrafish fin regeneration. Development 129, 2607-2617 [DOI] [PubMed] [Google Scholar]
- Nye H. L., Cameron J. A., Chernoff E. A., Stocum D. L. (2003). Regeneration of the urodele limb: a review. Dev. Dyn. 226, 280-294 [DOI] [PubMed] [Google Scholar]
- Ogai K., Hisano S., Mawatari K., Sugitani K., Koriyama Y., Nakashima H., Kato S. (2012). Upregulation of anti-apoptotic factors in upper motor neurons after spinal cord injury in adult zebrafish. Neurochem. Int. 61, 1202-1211 [DOI] [PubMed] [Google Scholar]
- Patterson J. M., Zakon H. H. (1993). Bromodeoxyuridine labeling reveals a class of satellite-like cells within the electric organ. J. Neurobiol. 24, 660-674 [DOI] [PubMed] [Google Scholar]
- Patterson J. M., Zakon H. H. (1996). Differential expression of proteins in muscle and electric organ, a muscle derivative. J. Comp. Neurol. 370, 367-376 [DOI] [PubMed] [Google Scholar]
- Patterson J. M., Zakon H. H. (1997). Transdifferentiation of muscle to electric organ: regulation of muscle-specific proteins is independent of patterned nerve activity. Dev. Biol. 186, 115-126 [DOI] [PubMed] [Google Scholar]
- Poss K. D. (2010). Advances in understanding tissue regenerative capacity and mechanisms in animals. Nat. Rev. Genet. 11, 710-722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poss K. D., Wilson L. G., Keating M. T. (2002). Heart regeneration in zebrafish. Science 298, 2188-2190 [DOI] [PubMed] [Google Scholar]
- Poss K. D., Keating M. T., Nechiporuk A. (2003). Tales of regeneration in zebrafish. Dev. Dyn. 226, 202-210 [DOI] [PubMed] [Google Scholar]
- Ramachandran R., Reifler A., Parent J. M., Goldman D. (2010). Conditional gene expression and lineage tracing of tuba1a expressing cells during zebrafish development and retina regeneration. J. Comp. Neurol. 518, 4196-4212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt J. D., Parry D. J., Partridge T. A. (1996). Phenotype of adult mouse muscle myoblasts reflects their fiber type of origin. Differentiation 60, 39-45 [DOI] [PubMed] [Google Scholar]
- Rowlerson A., Radaelli G., Mascarello F., Veggetti A. (1997). Regeneration of skeletal muscle in two teleost fish: Sparus aurata and Brachydanio rerio. Cell Tissue Res. 289, 311-322 [DOI] [PubMed] [Google Scholar]
- Sánchez Alvarado A. (2000). Regeneration in the metazoans: why does it happen? Bioessays 22, 578-590 [DOI] [PubMed] [Google Scholar]
- Sánchez Alvarado A., Tsonis P. A. (2006). Bridging the regeneration gap: genetic insights from diverse animal models. Nat. Rev. Genet. 7, 873-884 [DOI] [PubMed] [Google Scholar]
- Santamaría J. A., Marí-Beffa M., Santos-Ruiz L., Becerra J. (1996). Incorporation of bromodeoxyuridine in regenerating fin tissue of the goldfish Carassius auratus. J. Exp. Zool. 275, 300-307 [DOI] [PubMed] [Google Scholar]
- Scherer S. S. (1986). Reinnervation of the extraocular muscles in goldfish is nonselective. Neuroscience 6, 764–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. T., Shashoua V. E. (1988). Antibodies to ependymin block the sharpening of the regenerating retinotectal projection in goldfish. Brain Res. 446, 269-284 [DOI] [PubMed] [Google Scholar]
- Schultz E., Chamberlain C., McCormick K. M., Mozdziak P. E. (2006). Satellite cells express distinct patterns of myogenic proteins in immature skeletal muscle. Dev. Dyn. 235, 3230–3239 [DOI] [PubMed] [Google Scholar]
- Scott S. A. (1977). Maintained function of foreign and appropriate junctions on reinnervated goldfish extraocular muscles. J. Physiol. 268, 87-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P., Sabourin L. A., Girgis-Gabardo A., Mansouri A., Gruss P., Rudnicki M. A. (2000). Pax7 is required for the specification of myogenic satellite cells. Cell 102, 777-786 [DOI] [PubMed] [Google Scholar]
- Seger C., Hargrave M., Wang X., Chai R. J., Elworthy S., Ingham P. W. (2011). Analysis of Pax7 expressing myogenic cells in zebrafish muscle development, injury, and models of disease. Dev. Dyn. 240, 2440–2451 [DOI] [PubMed] [Google Scholar]
- Sesodia S., Cullen M. J. (1991). The effect of denervation on the morphology of regenerating rat soleus muscles. Acta Neuropathol. 82, 21-32 [DOI] [PubMed] [Google Scholar]
- Shadd V., Archer E., Escobedo N., Kim H. J., Unguez G. A. (2008). Comparative analysis of satellite cells from blastema and adult tissues of the electric fish S. macrurus. Dev. Biol. 319, 557 [Google Scholar]
- Singh S. P., Holdway J. E., Poss K. D. (2012). Regeneration of amputated zebrafish fin rays from de novo osteoblasts. Dev. Cell 22, 879-886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sîrbulescu R. F., Zupanc G. K. H. (2010). Effect of temperature on spinal cord regeneration in the weakly electric fish, Apteronotus leptorhynchus. J. Comp. Physiol. A 196, 359-368 [DOI] [PubMed] [Google Scholar]
- Sîrbulescu R. F., Ilieş I., Zupanc G. K. H. (2009). Structural and functional regeneration after spinal cord injury in the weakly electric teleost fish, Apteronotus leptorhynchus. J. Comp. Physiol. A 195, 699-714 [DOI] [PubMed] [Google Scholar]
- Srivastava C. B. (1978). Differentiation of electric organ from muscle precursor in the regenerating tail of a weakly electric teleost: a morphogenetic approach. Indian J. Exp. Biol. 16, 762-767 [PubMed] [Google Scholar]
- Steinbacher P., Haslett J. R., Obermayer A., Marschallinger J., Bauer H. C., Sänger A. M., Stoiber W. (2007). MyoD and Myogenin expression during myogenic phases in brown trout: a precocious onset of mosaic hyperplasia is a prerequisite for fast somatic growth. Dev. Dyn. 236, 1106-1114 [DOI] [PubMed] [Google Scholar]
- Stocum D. L. (1995). Wound Repair, Regeneration, and Artificial Tissues. Austin, TX: R. G. Landes Co. [Google Scholar]
- Tanaka E. M., Reddien P. W. (2011). The cellular basis for animal regeneration. Dev. Cell 21, 172-185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thouveny Y., Tassava R. A. (1998). Regeneration throught phylogenesis. In Cellular and Molecular Basis of Regeneration: From Invertebrates to Humans (ed. Ferretti P., Gúraudie J.), pp. 9-43, Chichester: John Wiley and Sons; [Google Scholar]
- Thummel R., Burket C. T., Hyde D. R. (2006). Two different transgenes to study gene silencing and re-expression during zebrafish caudal fin and retinal regeneration. Sci. World J. 6 Suppl. 1, 65-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unguez G. A., Zakon H. H. (1998). Phenotypic conversion of distinct muscle fiber populations to electrocytes in a weakly electric fish. J. Comp. Neurol. 399, 20-34 [PubMed] [Google Scholar]
- Unguez G. A., Zakon H. H. (2002). Skeletal muscle transformation into electric organ in S. macrurus depends on innervation. J. Neurobiol. 53, 391-402 [DOI] [PubMed] [Google Scholar]
- van Raamsdonk W., Smit-Onel M. J., Maslam S., Velzing E., de Heus R. (1998). Changes in the synaptology of spinal motoneurons in zebrafish following spinal cord transection. Acta Histochem. 100, 133-148 [DOI] [PubMed] [Google Scholar]
- Vihtelic T. S., Hyde D. R. (2000). Light-induced rod and cone cell death and regeneration in the adult albino zebrafish (Danio rerio) retina. J. Neurobiol. 44, 289-307 [DOI] [PubMed] [Google Scholar]
- Vischer H. A. (1989a). The development of lateral-line receptors in Eigenmannia (Teleostei, Gymnotiformes). I. The mechanoreceptive lateral-line system. Brain Behav. Evol. 33, 205-222 [DOI] [PubMed] [Google Scholar]
- Vischer H. A. (1989b). The development of lateral-line receptors in Eigenmannia (Teleostei, Gymnotiformes). II. The electroreceptive lateral-line system. Brain Behav. Evol. 33, 223-236 [DOI] [PubMed] [Google Scholar]
- Wanek N., Muneoka K., Bryant S. V. (1989). Evidence for regulation following amputation and tissue grafting in the developing mouse limb. J. Exp. Zool. 249, 55-61 [DOI] [PubMed] [Google Scholar]
- Waxman S. G., Anderson M. J. (1980). Regeneration of spinal electrocyte fibers in Sternarchus albifrons: development of axon-Schwann cell relationships and nodes of Ranvier. Cell Tissue Res. 208, 343-352 [DOI] [PubMed] [Google Scholar]
- Waxman S. G., Anderson M. J. (1985). Generation of electromotor neurons in Sternarchus albifrons: differences between normally growing and regenerating spinal cord. Dev. Biol. 112, 338-344 [DOI] [PubMed] [Google Scholar]
- Weber C. M., Martindale M. Q., Tapscott S. J., Unguez G. A. (2012). Activation of Pax7-positive cells in a non-contractile tissue contributes to regeneration of myogenic tissues in the electric fish S. macrurus. PLoS ONE 7, e36819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F., Li R., Parks E., Takabe W., Hsiai T. K. (2010). Electrocardiogram signals to assess zebrafish heart regeneration: implication of long QT intervals. Ann. Biomed. Eng. 38, 2346-2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakon H. H. (1984). Postembryonic changes in the peripheral electrosensory system of a weakly electric fish: addition of receptor organs with age. J. Comp. Neurol. 228, 557-570 [DOI] [PubMed] [Google Scholar]
- Zakon H. H. (1986). The emergence of tuning in newly generated tuberous electroreceptors. Neuroscience 6, 3297-3308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakon H. H., Unguez G. A. (1999). Development and regeneration of the electric organ. J. Exp. Biol. 202, 1427-1434 [DOI] [PubMed] [Google Scholar]
- Zakon H. H., Zwickl D. J., Lu Y., Hillis D. M. (2008). Molecular evolution of communication signals in electric fish. J. Exp. Biol. 211, 1814-1818 [DOI] [PubMed] [Google Scholar]
- Zammit P. S. (2008). All muscle satellite cells are equal, but are some more equal than others? J. Cell Sci. 121, 2975-2982 [DOI] [PubMed] [Google Scholar]
- Zammit P. S., Golding J. P., Nagata Y., Hudon V., Partridge T. A., Beauchamp J. R. (2004). Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? J. Cell Biol. 166, 347-357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit P. S., Partridge T. A., Yablonka-Reuveni Z. (2006). The skeletal muscle satellite cell: the stem cell that came in from the cold. J. Histochem. Cytochem. 54, 1177-1191 [DOI] [PubMed] [Google Scholar]
- Zupanc G. K. (2001). A comparative approach towards the understanding of adult neurogenesis. Brain Behav. Evol. 58, 246-249 [DOI] [PubMed] [Google Scholar]
- Zupanc G. K., Ott R. (1999). Cell proliferation after lesions in the cerebellum of adult teleost fish: time course, origin, and type of new cells produced. Exp. Neurol. 160, 78-87 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.