Abstract
PrLZ/PC-1 is a newly-identified, prostate-specific and androgen-inducible gene. Our previous study demonstrated that PrLZ can enhance the proliferation and invasive capability of LNCaP cells, contributing to the development of prostate cancer (PCa). However, its potential role in androgen-independent processes remains elusive. In this study, we showed that PrLZ enhanced in vitro growth and colony formation of PCa cells upon androgen deprivation as well as tumorigenicity in castrated nude mice. In addition, PrLZ stabilized mitochondrial transmembrane potential, prevented release of cytochrome c from mitochondria to cytoplasm, and inhibited intrinsic apoptosis induced by androgen depletion. Mechanistically, PrLZ elevated the phosphorylation of Akt and Stat3 and upregulated Bcl-2 expression. Our data indicate that PrLZ protects PCa cells from apoptosis and promotes tumor progression following androgen deprivation. Because of its prostate specificity and the involvement in PCa progression, PrLZ appears to be a novel and attractive therapeutic target for advanced prostate malignancy.
Keywords: PrLZ, Apoptosis, Stat3, Bcl-2, Prostate cancer
Introduction
Prostate cancer (PCa) is the most common malignancy and the second leading cause of cancer death among males in the United States (1). Growth of PCa in the early stage is androgen dependent, and tumor cells undergo apoptosis upon androgen depletion, forming the basis for androgen ablation therapy (2). However, almost all patients will relapse with hormone-refractory disease due to the outgrowth of androgen-independent cancer cells (2, 3). In this stage, the ratio of proliferation to apoptosis is unbalanced and cells are more resistant to apoptosis (4), leading to failure of androgen ablation therapy and leaving patients with fewer therapeutic options. Until now, the detailed apoptosis-regulating mechanisms in PCa cells resistant to androgen ablation therapy are still not clear.
The established LNCaP/C4-2 cell models mimic the clinical progression of PCa (5). LNCaP is androgen-dependent, non-metastatic, and weakly tumorigenic, whereas its lineage-derived subline C4-2, obtained from tumor-stoma interaction, possesses the capabilities of androgen-independent growth and distant organ metastasis (6). These two cell lines constitute an ideal experimental model system to explore the genetic and/or epigenetic differences between androgen-dependent and androgen-independent PCa cells.
PrLZ, also known as PC-1, was first identified from 1,500 arrayed genes using cDNA differential expression microarray in LNCaP and C4-2 cells (7). Compared to the low expression in LNCaP cells, PrLZ was distinctively upregulated in C4-2 cells. It localizes to human chromosome 8q21.1, one of the most amplified regions in PCa (8, 9). PrLZ belongs to the tumor protein D52 (TPD52) family, which is mainly associated with the proliferation and progression of tumors (10). Unlike the extensive expression of TPD52 in many tumor tissues and cell lines, multiple tissue expression assays showed that PrLZ was predominantly expressed in prostate, with only minimal expression in the gastrointestinal tract and other secretory glandular tissues (7). Immunohistochemical stains revealed that PrLZ was more highly expressed in high-grade prostatic intraepithelial neoplasia (PIN) and PCa than in normal prostate or benign prostatic hyperplasia (BPH) (7). Notably, intense staining of PrLZ was limited to malignant cells, but not neighboring unaffected or normal cells (7), indicating its direct relationship to carcinogenesis and tumor progression in PCa.
Our previous research demonstrated that PrLZ expression was associated with the proliferation and invasion of PCa cells (11, 12), which is consistent with other reports (13, 14). To elucidate the potential role and mechanism of PrLZ in the progression from androgen-dependent to androgen-independent PCa, we established the gain-of-function LNCaP cell model using PrLZ expression vector and the loss-of-function C4-2 cell model using PrLZ-specific microRNA (miRNA) vector. Our data suggested that PrLZ enhanced the in vitro and in vivo growth and tumorigenicity of PCa cells in response to androgen deprivation. PrLZ also protected androgen-sensitive PCa cells from intrinsic apoptosis following the deprivation of androgen via enhancement of Akt and Stat3 phosphorylation and upregulation of Bcl-2. Taken together, PrLZ appears to be a critical factor in promoting androgen-independent survival and progression of PCa.
Materials and methods
Cells and reagents
Human PCa LNCaP and C4-2 cells were previously reported (5) and maintained in RPMI-1640 (GIBCO, Grand Island, NY) supplemented with 10% (v/v) fetal bovine serum (FBS, Sijiqing, Hangzhou, China) at 37°C with 5% CO2 in a humidified incubator. To deplete androgen, cells were cultured in phenol red-free RPMI 1640 with 10% charcoal/dextran-treated FBS (C/D FBS, Hyclone). Stattic (Stat3 inhibitor V) and LY 294002 (PI3K inhibitor) was purchased from Calbiochem (San Diego, CA) and Cell Signaling Technology (Danvers, MA) respectively. Antibodies for Akt, phosphorylated Akt (Ser473), cleaved caspase-9, caspase-3, and PARP were purchased from Cell Signaling Technology (Danvers, MA). Antibodies for Stat3, phosphorylated Stat3 (Tyr705), Bcl-2, Bcl-xL, Mcl-1, cytochrome c, and VEGF were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Establishment of stable PrLZ-overexpressing, PrLZ-knockdown cells and transient Stat3 and Bcl-2 siRNA transfection
Stable PrLZ-overexpressing sublines (i.e, LNCaP/PrLZ) and empty vector control (i.e., LNCaP/EV) subline were established as previousely described (11). PrLZ-specific miRNA-expressing plasmid pcDNA6.2-GW/EmGFP-miPrLZ and siRNA for Stat3 or Bcl-2 were designed and synthesized by Invitrogen (Shanghai, China). Sequence of miRNA or siRNA used in this study were as follows: miRNA for PrLZ forward 5'-TGC TGT TTG CAA GTT CTC TTC TTA GCG TTT TGG CCA CTG ACT GAC GCT AAG AAG AAC TTG CAAA-3' and reverse 5'-CCT GTT TGC AAG TTC TTC TTA GCG TCA GTC AGT GGC CAA AAC GCT AAG AAG AGA ACT TGC AAAC-3'; siRNA for Stat3 sense 5'-GAA GCA GCA GAU GGA GCTT-3' and antisense 5'-GCU CCA UCU GCU GCU UCTT-3'; siRNA for Bcl-2 sense 5'-UGA CUG AGU ACC UGA ACC GTT-3' and antisense 5'-CGG UUC AGG UAC UCA GUC ATC-3'. Transfection using Lipofectamine 2000 reagent (Invitrogen) was carried out according to the manufacturer’s protocol. For obtaining stable transfectants, transfected cells were selected in complete growth medium containing 3.0 µg/mL Blasticidin (Invitrogen). All clones selected were further verified by Western blot.
MTT assay
Cell growth rate was determined by 3-(3,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) proliferation assay as in our previous study (15). Briefly, after cells were seeded in 96-well plates in medium containing C/D FBS, MTT (final concentration 0.5 mg/mL, Sigma, St. Louis, MO) was added, incubated for 4 h, and DMSO was then added to solubilize the formazan crystals. The absorbance (O.D.) was measured at 590nm using the Microplate Autoreader (Bio-Tek Instruments, Vermont, USA). Independent experiments were repeated in triplicate.
Colony formation assay
A total of 1000 cells per well of single-cell suspension were seeded in 6-well plates. After 24 h, medium was replaced with fresh medium containing C/D FBS and plates were incubated at 37°C with 5% CO2 in a humidified incubator for 14 days; fresh medium was added every 4 days. The plates were then washed with ice-cold PBS, fixed with 4% paraformaldehyde, stained in crystal violet solution for 15 min at room temperature and washed with distilled water until no color was evident in the rinse. Plates were dried in air and the colony numbers were counted.
Apoptosis and mitochondrial transmembrane potential assay
Cells were plated in medium containing C/D FBS for 72 h and then harvested and washed by PBS. Apoptosis kit (Invitrogen) containing Annexin V-FITC and propidium iodide (PI) were used to determine cell apoptosis. Mitochondrial transmembrane potential (ΔΨm) were determined by JC-1 staining. Data were collected by flow cytometric analysis using FACS Calibur (Becton Dickinson, San Jose, CA). For each assay, three independent experiments were performed.
Western blot analysis
The whole cell lysate was prepared using RIPA buffer containing proteinase inhibitors. The cytosol/mitochondria fractionation was prepared using a mitochondria extraction kit (Runtai Biotech Ltd. Co., Tianjin, China). An equal amount of lysates (30 µg) was separated by 12% SDS-PAGE and transferred to nitrocellulose membranes. The membranes were initially blocked with 5% skim milk in TBS for 1 h at room temperature, followed by incubation with primary antibodies at 4°C overnight. After washing with TBST buffer, membranes were incubated with secondary antibodies coupled with horseradish peroxidase for 1 h at room temperature, and protein signal was then detected using the ECL chemiluminescent detection system (Amersham, Piscataway, NJ). GAPDH was used as a loading control.
Xenograft animal model
6- to 8-week-old nude athymic BALB/c male mice were used to determine the in vivo tumor take and tumor growth rate. Cells (5×106) were suspended in 200 µL serum-free RPMI 1640 containing Matrigel (1:1, v/v, BD Biosciences) and injected subcutaneously (s.c.) into both flanks of mice using a 27-gauge insulin syringe. Mice bearing tumors were castrated 4 weeks after cell injection. Tumor volumes were measured weekly for 8 weeks. Then the primary tumors were removed, fixed in 4% paraformaldehyde, embedded in paraffin, sectioned and stained with H&E. Immunohistochemical staining was carried out as described previously (16), and results were quantified using software of Image-Pro 6.3.
Statistical analysis
All data analyses were done by software of SPSS13.0 for Windows. P<0.05 was regarded as the threshold value for statistical significance.
Results
Establishment of PrLZ-overexpressing and -knockdown PCa cell lines
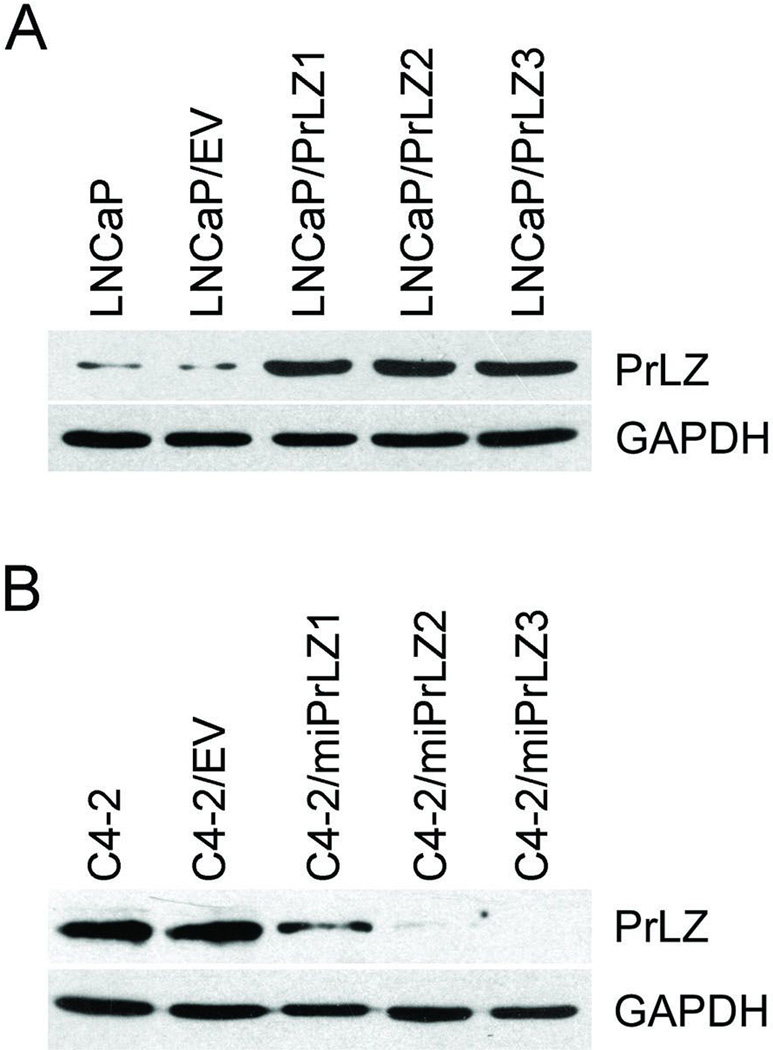
Stable PrLZ-overexpressing (i.e., LNCaP/PrLZ) cells were established and reported in our previous studies (11). Three LNCaP/PrLZ sublines, LNCaP/PrLZ1, LNCaP/PrLZ2, and LNCaP/PrLZ3, exhibited elevated PrLZ protein expression (Fig. 1A); two of them were randomly chosen for future study. To establish PrLZ knockdown in C4-2 cells, PrLZ-specific miRNA-expressing vector or control vector was transfected into C4-2 cells, followed by selection with Blasticidin (3 µg/mL) for 4 weeks and then individual Blasticidin-resistant clones were isolated. Three clones with decreased expression of PrLZ determined by Western blot were designated as C4-2/miPrLZ1, C4-2/miPrLZ2, and C4-2/miPrLZ3 (Fig. 1B); two of them were chosen for future study. The empty vector-transfected control cells were designated as C4-2/EV.
Figure 1.
Determination of PrLZ expression in stable transfected cells. A, The expression of PrLZ in parental LNCaP, control LNCaP/EV, and PrLZ-overexpressing LNCaP/PrLZ cells was determined using Western blot with GAPDH as an internal control. B, The expression of PrLZ in parental C4-2, control C4-2/EV, and PrLZ miRNA-targeting C4-2/miPrLZ cells was determined using Western blot with GAPDH as an internal control.
PrLZ promotes in vitro and in vivo growth in PCa cells following androgen depletion
Previously, we demonstrated that PrLZ enhanced the in vitro growth capability of LNCaP cells (11). In this study, we further characterized the effect of PrLZ on the in vitro growth of PCa cells under androgen deprivation conditions (i.e., medium containing C/D FBS). As shown in Fig. 2A, LNCaP/PrLZ cells exhibited an accelerated growth profile compared to parental LNCaP or control LNCaP/EV cells, which displayed no significant growth over six days of androgen depletion. In contrast, both C4-2 and C4-2/EV cells were able to grow under androgen deprivation conditions, whereas PrLZ-knockdown C4-2/miPrLZ cells displayed dramatically decreased growth rates (Fig. 2B).
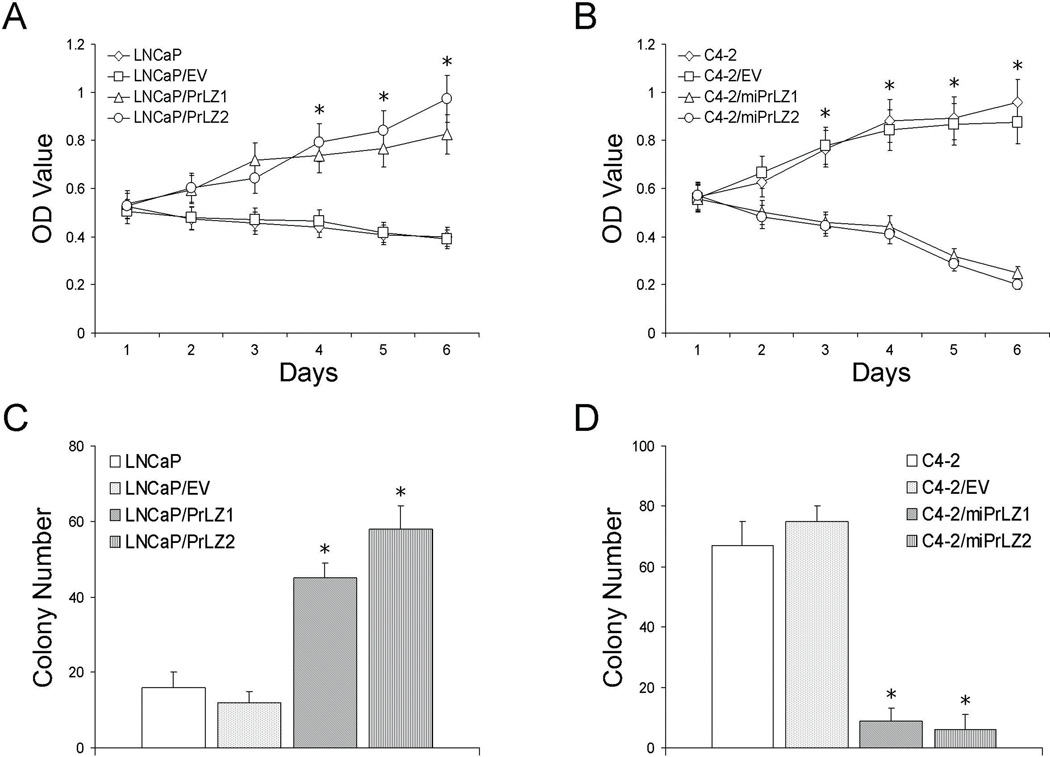
Figure 2.
Effect of PrLZ on in vitro growth and colony formation under androgen deprivation. A and B, MTT assays. Cells were cultured in phenol red-free RPMI 1640 containing 10% C/D FBS and results were evaluated as described in Materials and Methods. *P<0.05, compared to parental or control cells. C and D, colony formation assays. The colony-forming capability in response to androgen deprivation was determined as detailed in Materials and Methods. *P<0.05, compared to parental or control cells.
Next, we determined the colony formation capacity of PCa cells with or without exogenous PrLZ expression under androgen deprivation condition. We found that the parental androgen-dependent LNCaP and control LNCaP/EV cells formed a similar number of colonies, which was significantly lower than that formed by the LNCaP/PrLZ cells (P < 0.05, Fig. 2C). On the contrary, both C4-2 and C4-2/EV cells formed significantly more colonies than C4-2/miPrLZ cells under androgen deprivation condition (P < 0.05, Fig. 2D).
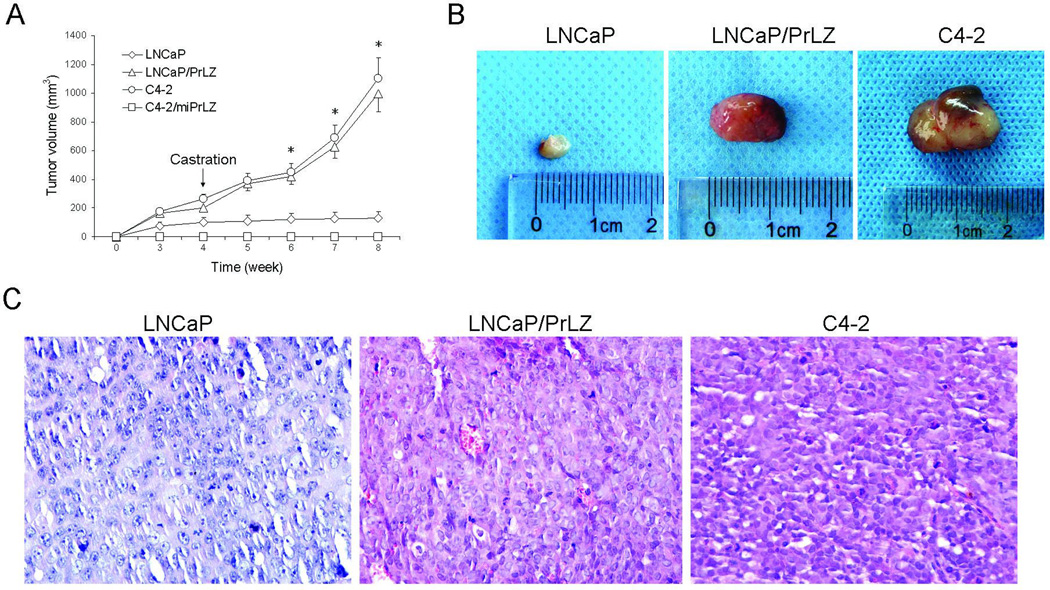
In addition, we also determined in vivo tumor growth and tumor take of these cells in nude mice. Similar to our previous work (11), data from s.c. model indicated that ectopic PrLZ expression facilitated tumor take rate and accelerated tumor growth in LNCaP/PrLZ cells; detectable tumors were observed within as few as 10 days after injection. In contrast, parental LNCaP cells could not form tumors until at least 3 weeks after injection. In addition, the growth rate of parental LNCaP cells was much slower than LNCaP/PrLZ cells (P<0.001, Fig. 3A and B). Similar results were observed in PrLZ-positive C4-2 cells, however, C4-2/miPrLZ cells didn’t form any detectable tumors. As we expected, when all the mice were castrated 4 weeks after cell injection, the parental LNCaP tumor displayed growth retardation whereas LNCaP/PrLZ and C4-2 tumors maintained a rapid growth rate in the xenograft mice model (Fig. 3A and B). Histological analysis indicated that tumors derived from LNCaP/PrLZ cells exhibited more blood vessels compared to parental LNCaP cells (Fig. 3C).
Figure 3.
Effect of PrLZ on in vivo tumor growth in castrated nude mice. A, tumor growth assay. LNCaP, LNCaP/PrLZ, C4-2, or C4-2/miPrLZ cells were injected s.c. into both flank of male nude mice. Mice were castrated 4 weeks after cell injection. Tumor volume was measured weekly. *P<0.001, compared to LNCaP cells. B, representative tumors isolated from LNCaP-, LNCaP/PrLZ- and C4-2-injected mice. C, histology from LNCaP, LNCaP/PrLZ, and C4-2 tumor are stained with HE and photographed under light microscropy (40×).
PrLZ protects PCa cells from apoptosis in response to androgen deprivation
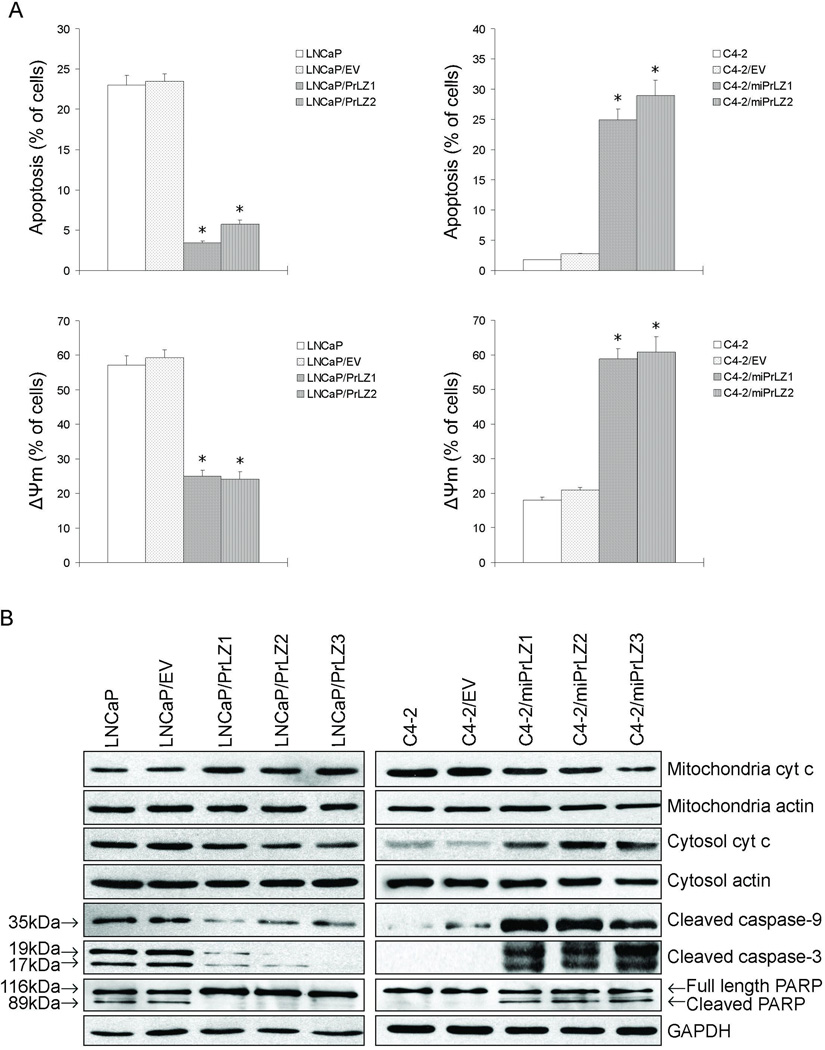
Androgen deprivation can cause apoptosis in both normal and malignant prostate cells (17). To investigate the role of PrLZ in apoptosis, cells were exposed to medium containing C/D FBS for 72 h then the degree of apoptosis was determined. Our results showed that the percentage of apoptotic cells was significantly lower in LNCaP/PrLZ and C4-2 cells compared to parental LNCaP and C4-2/miPrLZ cells (P < 0.01; Fig. 4A, upper panels). These data indicated that PrLZ is able to inhibit cell apoptosis under androgen deprivation condition. Since the loss of mitochondrial transmembrane potential (ΔΨm) plays an important role in triggering apoptotic pathways, we measured the effect of PrLZ on ΔΨm. Following androgen deprivation for 72 h, consistent with the apoptosis data (Fig. 4A), a significant loss of ΔΨm was observed in LNCaP and C4-2/miPrLZ cells compared to PrLZ-overexpressing LNCaP/PrLZ and parental C4-2 cells respectively (Fig. 4A, lower panels). These results indicated that PrLZ can stabilize mitochondria membrane in apoptotic events in response to androgen deprivation.
Figure 4.
Effect of PrLZ on apoptosis in response to androgen deprivation. A, cells were stained with Annexin V-FITC/PI or JC-1 and analyzed by flow cytometry after 72 h androgen withdrawal. Data presented here were obtained from three independent experiments (*P<0.01). B, molecular changes after androgen depletion were detected by Western blot with various targets.
We further explored the effect of PrLZ on the apoptosis cascade under androgen deprivation condition. As shown in Fig. 4B, mitochondrial cytochrome c levels in LNCaP, LNCaP/EV and C4-2/miPrLZ cells decreased in response to androgen deprivation, whereas cytosolic cytochrome c levels became elevated, indicating that there was a increased release of cytochrome c from mitochondria to cytosol in these cells. Also, data (Fig. 4B) from Western blot revealed that elevated expression of cleaved caspase-9, caspase-3 and PARP was detected in LNCaP, LNCaP/EV and C4-2/miPrLZ cells following androgen deprivation. Only minimal levels of these cleaved proteins were detectable in LNCaP/PrLZ, parental C4-2 or control C4-2/EV cells, suggesting that PrLZ probably antagonizes the intrinsic apoptosis.
PrLZ elicits the phosphorylation of Akt and Stat3 and upregulates the expression of Bcl-2, Bcl-xL, Mcl-1, and VEGF
Both Akt and signal transducer and activator of transcription 3 (Stat3) have been demonstrated to play a critical role in the malignancy, castration-resistance, and metastases of PCa (18–20). Therefore, we further determined the effect of PrLZ on their expression and activation/phosphorylation under androgen deprivation conditions. As shown in Fig. 5A, PrLZ elicited the phosphorylation of Akt (Ser473) and Stat3 (Tyr705) in LNCaP/PrLZ cells, while C4-2/miPrLZ cells displayed a decreased level compared to parental C4-2 cells. In addition, Bcl-2, Bcl-xL, and Mcl-1, downstream targets of Stat3 and potent anti-apoptotic proteins, were also examined. As expected, their levels were increased in LNCaP/PrLZ and C4-2 cells relative to those in LNCaP and C4-2/miPrLZ cells respectively (Fig. 5A).
Figure 5.
Effect of PrLZ on Akt and Stat3 phosphorylation and Bcl-2, Bcl-xL, Mcl-1, and VEGF expression. A, phosphorylated Akt (Ser473), total Akt, phosphorylated Stat3 (Tyr705), total Stat3, Bcl-2, Bcl-xL, and Mcl-1 were determined by Western blot in PCa cells. B, PrLZ, Stat3, Bcl-2, and VEGF were detected by immunohistochemical staining in tumors derived from LNCaP, LNCaP/PrLZ, and C4-2 cells. C, immunohistochemical staining was quantified by using software of ImagePro 6.3 (*P<0.05).
In addition, immunohistochemistry was applied to determine the expression pattern of Stat3 and Bcl-2 in tumor specimens from nude mice. The results demonstrated that the nuclear expression of Stat3 (activated Stat3) and Bcl-2 expression were significantly elevated in LNCaP/PrLZ and C4-2 tumors, whereas tumors derived from parental LNCaP cells exhibited cytoplasmic expression of Stat3 (inactivated Stat3) and low staining of Bcl-2 (Fig. 5B). Furthermore, vascular endothelial growth factor (VEGF), a hallmark of angiogenesis, also elevated in tumors derived from LNCaP/PrLZ and C4-2 cells (Fig. 5B). In addition, quantified analysis showed that parental LNCaP cells exhibited lower expression of nuclear Stat3, Bcl-2, and VEGF compared to LNCaP/PrLZ and C4-2 cells (Fig. 5C).
Inactivation of Akt inhibits Stat3 phosphorylation in LNCaP/PrLZ and C4-2 cells
Previous findings indicated that crosstalk existed between JAK/Stat and PI3K/Akt signaling pathways (21–23). In this study, cells were treated with LY 294002 (an inhibitor of PI3K) or Stattic (a Stat3 specific inhibitor). As shown in Fig. 6A, both inhibitors abolished Akt or Stat3 phosphorylation respectively. Notably, LY 294002 treatment not only reduced Akt activation, but also decreased Stat3 phosphorylation. However, when cells were exposed to Stattic, no changes in Akt phosphorylation were observed. These data indicate that Akt is an upstream regulator for Stat3.
Figure 6.
Analyses of the pathways associated with anti-apoptotic function of PrLZ. A, cells were treated with either LY 294002 (30 µM) or Stattic (20 µM) for 1 h and cell lysates were subjected to Western blot for determining total or phosphorylated Akt and Stat3 levels. B and C, LNCaP/PrLZ and C4-2 cells were treated with siRNA specific to Stat3 or Bcl-2 or Stattic, underwent androgen withdrawal for 72 h, and then were analyzed for apoptosis and mitochondrial transmembrane potential (ΔΨm) by flow cytometry (*P<0.01; **P<0.05). D, Western blot analysis of apoptosis-associated proteins.
Stat3/Bcl-2 signaling pathway mediated the anti-apoptotic function of PrLZ
To further investigate the significance of Stat3 signaling in the anti-apoptotic function of PrLZ following androgen deprivation in PCa cells, we targeted Stat3 with either siRNA or Stattic. Notably, knockdown or inhibition of Stat3 led to apoptosis as well as loss of ΔΨm in PrLZ-overexpression LNCaP/PrLZ cells and C4-2 cells with high endogenous PrLZ expression following androgen withdrawal (Fig. 6B). In addition, Bcl-2 protein was also inhibited by knocking down Stat3 (Fig. 6C). Furthermore, when knocking down Bcl-2 in LNCaP/PrLZ and C4-2 cells, both increased apoptosis and loss of ΔΨm were observed regardless of the presence of PrLZ and Stat3 phosphorylation (Fig. 6B). These alterations were concomitant with elevation of cleaved caspase-9, caspase-3 and PARP detected by Western blot (Fig. 6C).
Discussion
PrLZ is a newly identified, prostate-specific and androgen-inducible gene (7). Since it is up-regulated in androgen-resistant C4-2 cells and overexpressed in human PCa, we hypothesized that PrLZ may play a role in the outgrowth of androgen-independent PCa. To test this hypothesis, we determined the effect of PrLZ on the growth capability of PCa cells under androgen deprivation in both androgen-sensitive LNCaP cells and androgen-independent C4-2 cells. Several lines of evidence from our study demonstrated that PrLZ is critical for the outgrowth of androgen-independent PCa cells. First, PrLZ overexpression promoted the growth of LNCaP cells in the absence of androgen, whereas PrLZ-knockdown cells exhibited a dramatically reduced growth in C4-2 cells. Second, the colony formation assay demonstrated that cell transformation capability was enhanced with PrLZ overexpression in LNCaP/PrLZ cells but reduced in C4-2/miPrLZ cells. Third, consistent results were obtained from a xenograft animal model. Comparing with parental LNCaP cells, LNCaP/PrLZ cells exhibited higher tumor take rate and accelerated tumor growth under androgen deprivation condition. Similarly, C4-2/miPrLZ cells failed to form any tumors compared with C4-2 cells. These in vitro and in vivo experiments clearly indicate that PrLZ is sufficient to drive androgen-independent growth in PCa cells and is indispensable for the survival of androgen-independent C4-2 cells. Our data also suggest that PrLZ may play a critical role in regulating PCa progression during androgen-dependent to androgen-independent conversion.
Accumulating data have demonstrated that either JAK/Stat or PI3K/Akt signaling pathway are critical for cancer cell survival and metastasis (18–20, 24, 25). Upon Stat activation, Stat proteins are phosphorylated forms either homodimers or heterodimers then translocates into nucleus where they can bind to their specific promoter sequences and activate gene transcription (26). Stat3, one of the key members of this family, has been demonstrated to play a crucial role in pro-survival activities of PCa cells (27, 28). In addition, a recent study indicates that PrLZ overexpression can enhance Akt phosphorylation underlying the progression of PCa (14). In this study, we demonstrate that PrLZ could increase Stat3 phosphorylation mediated by activation of Akt, which is further demonstrated by rescue experiments demonstrating that inhibition of PI3K by specific inhibitor could abolish both Akt and Stat3 phosphorylation in PrLZ-expressing cells, whereas inhibition of Stat3 activation could not change Akt phosphorylation. Based on these data, we conclude that Akt is an upstream regulator for Stat3 in PrLZ mediated anti-apoptotic effects. In addition, it has been reported that interleukin-6 (IL-6) or epidermal growth factor (EGF) increases Stat3 phosphorylation and regulate LNCaP cells growth (29, 36–39). We examined whether elevated IL-6 or EGF can be detected in PrLZ-expressing cells. However, we failed to demonstrate that PrLZ mediated Stat3 activation goes through IL-6 or EGF regulation loop in these cells (Supplementary Fig. S1).
It is known that Bcl-2, Bcl-xL, Mcl-1, and VEGF, are all the downstream target genes of Stat3 (29, 30). Both Bcl-2 and Bcl-xL localize on the outer mitochondrial membrane (33) and are able to stabilize the mitochondrial membrane potential, maintain mitochondrial permeability, and prevent the release of cytochrome c from mitochondrial to cytoplasm upon repressed apoptosis (34, 35). In PrLZ-overexpressed PCa cells, we have observed a stabilization of mitochondrial transmembrane potential along with the induction of Bcl-2, Bcl-xL, and Mcl-1 in the condition of androgen deletion. In contrast, both loss of mitochondrial transmembrane potential and decreased expression of Bcl-2, Bcl-xL, and Mcl-1 were observed in PrLZ knocked-down PCa cells in the condition of androgen deletion. In addition, when Stat3 was knocked down using siRNA or inactivated by inhibitor, Bcl-2 was downregulated accordingly. Also, increased apoptosis and loss of mitochondrial transmembrane potential were observed. Taken together, these data provide supporting evidence that PrLZ play the survival roles in PCa cells upon androgen-independent progression (31, 32).
In summary, our data demonstrated that overexpression of PrLZ could protect PCa cells from apoptosis induced by androgen deprivation, which is mediated by the activation of Stat3/Bcl-2 signaling pathway. Thus, PrLZ is a potential factor in the outgrowth of androgen-independent PCa. Considering its prostate specificity and upregulation in androgen-independent PCa, PrLZ could be an attractive therapeutic target for PCa therapy.
Supplementary Material
Acknowledgements
We thank Karen Wolf at Rochester of University for kindly editing this manuscript.
Grant support: This study was supported by the National Natural Science Foundation of China (NSFC No.30500502).
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.Jemal A, Murray T, Ward E, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 2.Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. N Engl J Med. 2003;349:366–381. doi: 10.1056/NEJMra021562. [DOI] [PubMed] [Google Scholar]
- 3.Pienta KJ, Bradley D. Mechanisms Underlying the Development of Androgen-Independent Prostate Cancer. Clin Cancer Res. 2006;12:1665–1671. doi: 10.1158/1078-0432.CCR-06-0067. [DOI] [PubMed] [Google Scholar]
- 4.Denmeade SR, Lin XS, Isaacsh JT. Role of programmed (apoptotic) cell death during the progression and therapy for prostate cancer. Prostate. 1996;28:251–265. doi: 10.1002/(SICI)1097-0045(199604)28:4<251::AID-PROS6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 5.Thalmann GN, Anezinis PE, Chang SM, et al. Androgen-independent cancer progression and bone metastasis in the LNCaP model of human prostate cancer. Cancer Res. 1994;54:2577–2581. [PubMed] [Google Scholar]
- 6.Wu HC, Hsieh JT, Gleave ME, Brown NM, Pathak S, Chung LW. Derivation of androgen-independent human LNCaP prostatic cancer cell sublines: role of bone stromal cells. Int J Cancer. 1994;57:406–412. doi: 10.1002/ijc.2910570319. [DOI] [PubMed] [Google Scholar]
- 7.Wang R, Xu J, Saramäki O, et al. PrLZ, a novel prostate-specific and androgen-responsive gene of the TPD52 family, amplified in chromosome 8q21.1 and overexpressed in human prostate cancer. Cancer Res. 2004;64:1589–1594. doi: 10.1158/0008-5472.can-03-3331. [DOI] [PubMed] [Google Scholar]
- 8.Macoska JA, Trybus TM, Sakr WA, et al. Fluorescence in situ hybridization analysis of 8p allelic loss and chromosome 8 instability in human prostate cancer. Cancer Res. 1994;54:3824–3830. [PubMed] [Google Scholar]
- 9.Visakorpi T, Kallioniemi AH, Syvänen AC, et al. Genetic changes in primary and recurrent prostate cancer by comparative genomic hybridization. Cancer Res. 1995;55:342–347. [PubMed] [Google Scholar]
- 10.Boutros R, Fanayan S, Shehata M, Byrne JA. The tumor protein D52 family: many pieces, many puzzles. Biochem Biophys Res Commun. 2004;325:1115–1121. doi: 10.1016/j.bbrc.2004.10.112. [DOI] [PubMed] [Google Scholar]
- 11.Li L, Zhang D, Zhang L, et al. PrLZ expression is associated with the progression of prostate cancer LNCaP cells. Mol Carcinog. 2009;48:432–440. doi: 10.1002/mc.20481. [DOI] [PubMed] [Google Scholar]
- 12.Zhang D, Li L, Zhang LL, Xue Y, Wang XY, He DL. Impact of PrLZ overexpression on invasion of prostate cancer LNCaP cells in vitro. Ai Zheng. 2009;28:483–486. [PubMed] [Google Scholar]
- 13.Wang R, Xu J, Mabjeesh N, et al. PrLZ is expressed in normal prostate development and in human prostate cancer progression. Clin Cancer Res. 2007;13:6040–6048. doi: 10.1158/1078-0432.CCR-07-0640. [DOI] [PubMed] [Google Scholar]
- 14.Zhang H, Wang J, Pang B, et al. PC-1/PrLZ contributes to malignant progression in prostate cancer. Caner Res. 2007;67:8906–8913. doi: 10.1158/0008-5472.CAN-06-4214. [DOI] [PubMed] [Google Scholar]
- 15.Li L, He D, He H, et al. Overexpression of PML induced apoptosis in bladder cancer cell by caspase dependent pathway. Cancer Lett. 2006;236:259–268. doi: 10.1016/j.canlet.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 16.Zhu G, Zhau HE, He H, et al. Sonic and desert hedgehog signaling in human fetal prostate development. Prostate. 2007;67:674–684. doi: 10.1002/pros.20563. [DOI] [PubMed] [Google Scholar]
- 17.Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: Directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23:8253–8261. doi: 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- 18.Abdulghani J, Gu L, Dagvadorj A, et al. Stat3 promotes metastatic progression of prostate cancer. Am J Pathol. 2008;172:1717–1728. doi: 10.2353/ajpath.2008.071054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SO, Lou W, Qureshi KM, Mehraein-Ghomi F, Trump DL, Gao AC. RNA interference targeting Stat3 inhibits growth and induces apoptosis of human prostate cancer cells. Prostate. 2004;60:303–309. doi: 10.1002/pros.20072. [DOI] [PubMed] [Google Scholar]
- 20.Sarker D, Reid AH, Yap TA, de Bono JS. Targeting the PI3K/AKT pathway for the treatment of prostate cancer. Clin Cancer Res. 2009;15:4799–4805. doi: 10.1158/1078-0432.CCR-08-0125. [DOI] [PubMed] [Google Scholar]
- 21.Mao W, Iwai C, Liu J, Sheu SS, Fu M, Liang CS. Darbepoetin alfa exerts a cardioprotective effect in autoimmune cardiomyopathy via reduction of ER stress and activation of the PI3K/Akt and STAT3 pathways. J Mol Cell Cardiol. 2008;45:250–260. doi: 10.1016/j.yjmcc.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X, Shan P, Alam J, Fu XY, Lee PJ. Carbon monoxide differentially modulates STAT1 and STAT3 and inhibits apoptosis via a PI3K/Akt and p38 kinase dependent STAT3 pathway during anoxiareoxygenation injury. J Biol Chem. 2005;280:8714–8721. doi: 10.1074/jbc.M408092200. [DOI] [PubMed] [Google Scholar]
- 23.Ponnusamy M, Pang M, Annamaraju, et al. Transglutaminase-1 protects renal epithelial cells from hydrogen peroxide-induced apoptosis through activation of STAT3 and AKT signaling pathways. Am J Physiol Renal Physiol. 2009;297:F1361–F1370. doi: 10.1152/ajprenal.00251.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang S. Regulation of metastases by signal transducer and activator of transcription 3 signaling pathway: clinical implications. Clin Caner Res. 2007;13:1362–1366. doi: 10.1158/1078-0432.CCR-06-2313. [DOI] [PubMed] [Google Scholar]
- 25.Goswami A, Ranganathan P, Rangnekar VM. The phosphoinositide 3-kinase/Akt1/Par-4 axis: a cancer-selective therapeutic target. Cancer Res. 2006;66:2889–2892. doi: 10.1158/0008-5472.CAN-05-4458. [DOI] [PubMed] [Google Scholar]
- 26.Darnell JE., Jr STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 27.Mora LB, Buettner R, Seigne J, et al. Constitutive activation of Stat3 in human prostate tumors and cell lines: direct inhibition of Stat3 signaling induces apoptosis of prostate cancer cells. Cancer Res. 2002;62:6659–6666. [PubMed] [Google Scholar]
- 28.DeMiguel F, Lee SO, Lou W, et al. Stat3 enhances the growth of LNCaP human prostate cancer cells in intact and castrated male nude mice. Prostate. 2002;52:123–129. doi: 10.1002/pros.10110. [DOI] [PubMed] [Google Scholar]
- 29.Kim DJ, Chan KS, Sano S, Digiovanni J. Signal transducer and activator of transcription 3 (Stat3) in epithelial carcinogenesis. Mol Carcinog. 2007;46:725–731. doi: 10.1002/mc.20342. [DOI] [PubMed] [Google Scholar]
- 30.Niu G, Wright KL, Huang M, et al. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21:2000–2008. doi: 10.1038/sj.onc.1205260. [DOI] [PubMed] [Google Scholar]
- 31.Lin YT, Fukuchi J, Hiipakka RA, Kokontis JM, Xiang JL. Up-regulation of Bcl-2 is required for the progression of prostate cancer cells from an androgen-dependent to an androgen-independent growth stage. Cell Res. 2007;17:531–536. doi: 10.1038/cr.2007.12. [DOI] [PubMed] [Google Scholar]
- 32.Castilla C, Congregado B, Chinchón D, Torrubia FJ, Japón MA, Sáez C. Bcl-xL is overexpressed in hormone-resistant prostate cancer and promotes survival of LNCaP cells via interaction with proapoptotic Bak. Endocrinology. 2006;147:4960–4967. doi: 10.1210/en.2006-0502. [DOI] [PubMed] [Google Scholar]
- 33.Krajewski S, Tanaka S, Takayama S, Schibler MJ, Fenton W, Reed JC. Investigation of the subcellular distribution of the bcl-2 oncoprotein: Residence in the nuclear envelope, endoplasmic reticulum, and outer mitochondrial membranes. Cancer Res. 1993;53:4701–4714. [PubMed] [Google Scholar]
- 34.Yang J, Liu X, Bhalla K, et al. Prevention of apoptosis by Bcl-2: Release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 35.Vander Heiden MG, Chandel NS, Williamson EK, Schumacker PT, Thompson CB. BclXL regulates the membrane potential and volume homeostasis of mitochondria. Cell. 1997;91:627–637. doi: 10.1016/s0092-8674(00)80450-x. [DOI] [PubMed] [Google Scholar]
- 36.Degeorges A, Tatoud R, Fauvel-Lafeve F, et al. Stromal cells from human benign prostate hyperplasia produce a growth-inhibitory factor for LNCaP prostate cancer cells, identified as interleukin-6. Int J Cancer. 1996;68:207–214. doi: 10.1002/(SICI)1097-0215(19961009)68:2<207::AID-IJC12>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 37.Spiotto MT, Chung TD. STAT3 mediates IL-6-induced growth inhibition in the human prostate cancer cell line LNCaP. Prostate. 2000;42:88–98. doi: 10.1002/(sici)1097-0045(20000201)42:2<88::aid-pros2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 38.Lou W, Ni Z, Dyer K, Tweardy DJ, Gao AC. Interleukin-6 induces prostate cancer cell growth accompanied by activation of stat3 signaling pathway. Prostate. 2000;42:239–242. doi: 10.1002/(sici)1097-0045(20000215)42:3<239::aid-pros10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 39.Song L, Turkson J, Karras JG, Jove R, Haura EB. Activation of Stat3 by receptor tyrosine kinases and cytokines regulates survival in human non-small cell carcinoma cells. Oncogene. 2003;22:4150–4165 . doi: 10.1038/sj.onc.1206479. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.