Abstract
Few studies have examined the relative degree of brain volume loss in both the hippocampi and subcortical structures in unilateral temporal lobe epilepsy (TLE), and their association with clinical seizure correlates. In this study, quantitative MRI volumes were measured in the hippocampus, thalamus, caudate, putamen, and corpus callosum in 48 unilateral TLE patients (26 rights, and 22 lefts) and compared to 29 healthy controls. The ipsilateral hippocampus, corpus callosum, and bilateral thalami showed the greatest volume loss, reflected by large to moderate effect size differences compared to controls. Bilaterally, the putamen showed the next highest volume reduction. The contralateral hippocampus and bilateral caudate nuclei showed the least volume reduction, characterized by small effect sizes. Furthermore, clinical seizure characteristics (e.g, duration of epilepsy) showed different patterns of association with the volume reductions observed across these structures. Findings suggest that distinct neurodevelopmental features may play a role in the volume abnormality observed in these regions.
Keywords: Temporal lobe epilepsy, Subcortical, Quantitative magnetic resonance imaging
1. Introduction
Quantitative MRI studies have characterized the nature, extent, and clinical seizure correlates of hippocampal atrophy in unilateral temporal lobe epilepsy (TLE) [1–3]. The typical pattern is asymmetric hippocampal volume loss with greater abnormality in the ipsilateral hippocampus compared to the contralateral hippocampus. In addition, both disease course features (e.g., duration) and neurodevelopmental features (e.g., history of complex febrile seizures in childhood) have been implicated in hippocampal atrophy [4–7].
Quantitative MRI studies have confirmed that TLE is also associated with brain volume abnormalities in structures outside the hippocampus. Brain volume loss has been reported for adjacent mesial temporal lobe structures including the amygdala and parahippocampal region, as well as more distal structures within the ipsilateral temporal lobe [8, 9]. More recent investigations have provided evidence that regions outside of the temporal lobe, including the thalamus, striatum, and corpus callosum, are also affected [10–14].
To date, there has been limited examination of multiple subcortical structures and the hippocampus in the same study sample. Across studies there is considerable heterogeneity in the composition of TLE groups studied, which may account for the differences in findings reported in the literature. For example, findings are mixed as to the extent of damage evident in the thalamus (ipsilateral or bilateral) and basal ganglia, and there are also differences reported concerning the relationship of potential etiologic factors to subcortical brain volume abnormalities [11, 13, 14].
The purpose of this study was to examine MR volume integrity of the hippocampus and multiple subcortical structures in the same cohort of subjects. There are several advantages to examining multiple structures in a single cohort of TLE patients. First, it ensures that the subject characteristics of the TLE group, clinical seizure characteristics, and MR measurement characteristics will be the same for all the structures examined. Second, it facilitates quantification of the relative extent of volume abnormality across different structures. Third, it provides an opportunity to examine the influence of both course features and early neurodevelopmental features across multiple structures within the same cohort. Recent studies employing this approach have focused on the medial temporal lobe region and have helped to delineate the relative impact of atrophy evident within different parts of this region and its associated clinical seizure correlates [15].
This study examined the hippocampus, thalamus, caudate, putamen, and corpus callosum. Selection of regions of interest (ROIs) was guided by previous findings indicating atrophy in these regions, and evidence that these structures are considered important in seizure initiation, modulation, and/or propagation [16]. Specifically, we used quantitative MRI to: 1) measure and compare MR volumes of the hippocampus, thalamus, corpus callosum, caudate, and putamen; (2) examine the association of age of onset, duration of epilepsy, number of antiepileptic drugs (AEDs), and lifetime number of secondarily generalized seizures with brain volume in these structures; and 3) provide a systematic presentation of the relative volume abnormality (effect size and percent difference) evident in these structures.
2. Methods
2.1 Participants
The study sample consisted of a total of 77 subjects between the ages of 14 and 60 years of age; 48 subjects (26 right and 22 left) with unilateral chronic temporal lobe epilepsy and 29 healthy controls. The Institutional Review Boards at the University of Wisconsin Hospital and Rosalind Franklin University of Medicine and Science approved the study and informed consent was obtained from each study participant. Comprehensive assessment of seizure history, clinical semiology, and neuroimaging findings (e.g., PET) were used for diagnosis of TLE. For all patients, findings from video-EEG telemetry with scalp recordings confirmed evidence of unilateral temporal lobe onset of spontaneous seizures. Patients with independent left and right temporal lobe onset were excluded. All scans were reviewed by a neuroradiologist. Patients with MRI evidence of lesions (e.g., tumor, vascular malformation) other than hippocampal sclerosis were also excluded. TLE patients were typically interviewed in the presence of a family member regarding details of their epilepsy history and clinical course. Available medical records concerning previous epilepsy-related hospitalizations and records from treating physicians were reviewed.
Healthy controls were either friends or family members, primarily spouses, of the TLE patients. Inclusion of friends and spouses helped rule out shared genetic factors that may contribute to cognitive and neural development. They were also between the ages of 14 and 60 years, with no current substance abuse, medical conditions, no history of loss of consciousness longer than five minutes, and no history of developmental learning disorder.
2.2 MRI acquisition and postprocessing
Images were obtained on a 1.5 Tesla GE Signa MRI scanner. Sequences acquired for each subject included: 1) T1-weighted, three-dimensional SPGR acquired with the following parameters: TE = 5, TR = 24, flip angle = 40, NEX = 2, FOV =26, slice thickness = 1.5 mm, slice plane = coronal, matrix = 256×192; 2) Proton Density (PD), and 3) T2-weighted images acquired with the following parameters: TE = 36 msec (for PD) or 96 msec (for T2), TR = 3000 msec, NEX = 1, FOV = 26, slice thickness = 3.0 mm, slice plane = coronal, matrix = 256×192, and echo train length = 8.
MR scans were acquired at the University of Wisconsin Hospital and were processed using a semi-automated software package, i.e., Brain Research: Analysis of Images, Networks, and Systems (BRAINS2) [17]. The T1-weighted images were spatially normalized so that the anterior-posterior axis of the brain was realigned parallel to the anterior commissure - posterior commissure (ACPC) line, and the interhemispheric fissure was aligned on the other two axes. A six-point linear transformation was used to warp the standard Talairach atlas space onto the resampled image. Images from the three pulse sequences were then coregistered using a local adaptation of automated image registration software. Following alignment of the image sets, the PD and T2 images were resampled into 1 mm cubic voxels, following which an automated algorithm classified each voxel as gray matter, white matter, CSF, blood, or “other.” Neuroimaging analyses were conducted by raters blinded to group status, cognitive functioning, and both clinical and sociodemographic characteristics of the subjects. ROIs included the hippocampus, thalamus, caudate, putamen, and corpus callosum. Figures 1a–1c illustrate prototypical tracings of these ROIs.
Figure 1.
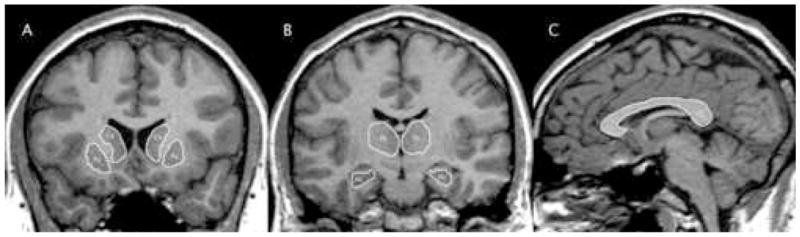
MRI regions of interest. A. Caudate (Ca) and putamen (Pu). B. Thalamus (Th) and hippocampus (Hc). C. Corpus callosum (CC).
2.3 Hippocampus tracing guidelines
An automated neural network application was used to trace the hippocampus using guidelines established and psychometrically validated by the University of Iowa, with manual correction of the traces by a qualified technician [18]. Guide traces were performed in the sagittal view, while the neural net was edited in the coronal view. These tracings included the pes or head of the hippocampus, the body, and the tail. Within the hippocampus, the subiculum, Ammon’s horn, and dentate gyrus were included. The white matter structures of the alveus, fimbria, and the fornix were excluded. Within the sagittal orientation, the alveus and the uncal recess marked the anterior border. The tail of the hippocampus ended at the atrium of the lateral ventricle, serving as the posterior border. The white matter of the parahippocampal gyrus defined the ventral border. The temporal horn of the lateral ventricle served as the dorsal border except in the tail of the hippocampus, where the pulvinar of the thalamus was the border. Interrater reliability for tracing of the hippocampus ranged from .73 – .83 [18]. Reliability below .90 was largely attributable to the fact that the MRI resolution in this study made distinguishing some borders difficult. Additionally, the hippocampal neural net was dependent upon manual guide traces, while all other neural nets were independent of user interface.
2.4 Thalamus tracing guidelines
An automated neural network and additional guidelines established at the University of Iowa Image Processing Lab were used to guide the thalamus trace [19]. All images were traced in the coronal plane using a color-enhanced T1 with reference to the segmented image and unenhanced T1. The thalamus was traced rostral to caudal with the most anterior portion of the thalamus determined by the neural net and the presence of the anterior commissure. The genu and posterior limb of the internal capsule served as the lateral border. The CSF of the third ventricle served as the medial border. The left and right portions of the thalami were traced separately, excluding the massa intermedia when present. The superior border was determined by the lateral ventricles throughout, and the fornix in more posterior slices. The traces extended caudally and included both the lateral and medial geniculate bodies. The thalamus protrudes caudally until coming into contact with either the atrium of the lateral ventricle, the tail of the hippocampus, or both structures. In our lab, we have achieved an inter-rater reliability of .98 in tracing the thalamus.
2.5 Caudate tracing guidelines
An automated neural network and additional guidelines established at the University of Iowa Image Processing Lab were used to guide the caudate trace in the coronal view [20]. Tracings included the head and body of the caudate nucleus. The tail was excluded due to poor visibility on MR scans. The nucleus accumbens was excluded by comparing the voxel intensities of the two structures and by referring to a color enhanced T1 image. The medial border was defined by the lateral ventricles, while the lateral border was defined by the internal capsule. Striatal cell bridges between the caudate and putamen in the rostral portion were included in the caudate trace, but excluded from the putamen trace. Inter-rater reliability of .99 was achieved.
2.6 Putamen tracing guidelines
An automated neural network and additional guidelines established at the University of Iowa Image Processing Lab were used to guide the putamen trace in the coronal view [21]. The anterior limb of the internal capsule separated the anterior portion of the putamen from the caudate. As previously noted, any tissue connecting the caudate and putamen was included as part of the caudate. The nucleus accumbens was also separated from the putamen trace using voxel intensity comparison. The internal capsule defined the medial border and the external capsule defined the lateral boundary throughout. Inter-rater reliability of .98 was achieved.
2.7 Corpus callosum tracing guidelines
Manual trace of the corpus callosum began in the midsagittal plane. Using a previously established tracing protocol [12], three co-registered image sets were used to delineate the corpus callosum. Boundary identification was based on a pre-manipulated trimodal image (a composite of the T1, T2, and PD images) as the primary image set, with reference to the continuously segmented image and a discretely segmented image in areas of poorly defined borders (such as the confluence of the fornix and the ventral callosum). Additionally, voxel signal intensities were used to separate white matter voxels from neighboring voxels of cerebral spinal fluid, blood vessel wall, and gray matter. In addition to the midsagittal slice, two additional slices extending laterally from the midsagittal view were traced. The anterior and posterior extreme of the corpus callosum trace were kept consistent when extending the trace to parasagittal slices. Although the lateral extent of the corpus callosum extends beyond the central five sagittal slices, these regions were excluded from the trace due to difficulty in boundary determination. Interrater reliability of .98 was achieved.
2.8 Statistical analyses
All statistical analyses were conducted using raw MR volumes adjusted for intracranial volume (ICV). Multivariate analysis of covariance (MANCOVA; ICV used as covariate) was used to compare MR volumes between epilepsy and control groups. Follow-up analyses of covariance (ANCOVA) identified specific group differences. To permit formal statistical comparison of ipsilateral and contralateral regions, controls were assigned “ipsilateral” and “contralateral” volume values by calculating an average of each individual’s left and right hemisphere regions. There was not a significant difference between the left and right hemisphere volumes for the controls in the structures of interest. Effect sizes for all three groups are presented as Cohen’s d and were defined as small (d < .20), medium (d = .21–.50), and large (d > .51) according to standard conventions [22]. In addition, the percent difference between both epilepsy groups and controls was calculated.
The relationship between clinical seizure variables and structural volumes was examined with partial correlations (controlled for ICV). Alpha levels were set at .01 to account for multiple comparisons. Clinical seizure variables included current number of AEDs, epilepsy duration, age of onset of epilepsy, and lifetime number of secondarily generalized seizures. Because of the inherent difficulty in establishing a reliable count of the lifetime number of generalized seizures, an ordinal scale was devised to estimate lifetime generalized seizures (1 = none, 2 = 1–10 seizures, 3 = 11–50, 4 = 50–99, 5 = 100+).
3. Results
3.1 Patient and control information
Table 1 displays the mean age and years of education for the three groups along with clinical seizure characteristics for the TLE groups. There were no significant group differences in age (F [2, 75] = .09, p = .76) or years of education (F [2, 75] = .05, p = .83). There were also no significant group differences between the left and right TLE groups for age of onset, duration of epilepsy, number of AED medications, or lifetime number of secondarily generalized seizures (all p’s > .05). Independent samples t-tests showed that the two TLE groups significantly differed from the control group on measures of verbal and visual memory (WMS-III) and Full Scale IQ (WAIS-III) (all p’s < .01), but the two TLE groups did not differ from one another on these measures (all p’s > .05). In all instances the epilepsy group had lower scores.
Table 1.
Demographic, clinical, and neuropsychological characteristics
| Control (n = 29) | Right TLE (n = 26) | Left TLE (n = 22) | |
|---|---|---|---|
| Chronological Age (yrs) | 36.55 (12.44) | 38.96 (12.63) | 33.09 (12.55) |
| Gender (M/F) | 11/18 | 8/18 | 6/16 |
| Education (yrs) | 13.54 (2.22) | 13.15 (2.41) | 12.14 (2.15) |
| Age of Onset (yrs) | NA | 13.55 (9.19) | 12.06 (5.87) |
| Duration of Epilepsy (yrs) | NA | 24.90 (14.07) | 20.75 (12.19) |
| # of AEDs | NA | 1.92 (.63) | 1.71 (.72) |
| # of Generalized Seizuresa | NA | 1.80 (1.32) | 1.90 (1.61) |
| Full Scale IQ (SS)b | 106.11 (14.13) | 93.56 (17.69) | 88.71 (13.88) |
| Verbal Memory (SS)b | 113.96 (7.87) | 98.8 (14.53) | 91.95 (18.28) |
| Visual Memory (SS)b | 108.18 (14.72) | 90.92 (13.53) | 85.00 (15.72) |
Data are mean (SD).
Denotes a scaled variable.
SS = Standard Score; mean = 100, SD = 15
3.2 Comparison of MRI volumes
Table 2 provides the mean ICV-adjusted volumes for the five brain structures under study for the three groups. Both groups showed a similar pattern of volume loss across all structures. Therefore, subsequent analyses combined the two TLE groups according to ipsilateral and contralateral brain volumes. A one-way MANCOVA (ICV as covariate) yielded a significant main effect of group (Wilks Lambda F [9, 66] = 4.09, p < .001). Follow-up one-way ANCOVAs (ICV as covariate) indicated significant group differences for the ipsilateral hippocampus (F [1, 74] = 23.05, p < .001), ipsilateral thalamus (F [1, 74] = 16.58, p < .001), contralateral thalamus (F [1, 74] = 11.03, p = .001), and corpus callosum (F [1, 74] = 10.98, p = .001). In each instance, smaller volumes were observed in the TLE group compared to controls. There were no significant differences between the TLE group and controls for volumes in the contralateral hippocampus, ipsilateral and contralateral caudate nuclei, or ipsilateral and contralateral putamen (p’s > .05).
Table 2.
Mean volumes (ICV adjusted)*
| Control | Right TLE | Left TLE | |
|---|---|---|---|
| Left Hippocampus | 1.90 (.06) | 1.84 (.38) | 1.48 (.38) |
| Right Hippocampus | 1.82 (.06) | 1.41 (.30) | 1.77 (.30) |
| Left Thalamus | 6.46 (.64) | 5.93 (.65) | 5.78 (.65) |
| Right Thalamus | 6.64 (.73) | 5.93 (.74) | 6.20 (.74) |
| Left Caudate | 3.12 (.48) | 3.09 (.49) | 3.05 (.48) |
| Right Caudate | 3.02 (.48) | 2.94 (.49) | 3.03 (.48) |
| Left Putamen | 4.00 (.62) | 3.62 (.63) | 3.81 (.63) |
| Right Putamen | 4.01 (.58) | 3.83 (.58) | 3.87 (.58) |
| Corpus Callosum | 2.82 (.48) | 2.39 (.49) | 2.48 (.49) |
Data are mean (SD).
Units are expressed as cubic centimeters.
Table 3 shows the effect sizes (Cohen’s d) for the volume differences from controls of all brain structures calculated separately for the right and left TLE groups, and in the combined TLE group. Additionally, the percent difference between each TLE group and the controls is also in Table 3. The largest effect sizes were evident for the ipsilateral hippocampus, ipsilateral thalamus, contralateral thalamus, and corpus callosum. Effect sizes were in the moderate range for the putamen, and low range for the contralateral hippocampus and both ipsilateral and contralateral caudate nuclei.
Table 3.
Effect sizes and percent difference between controls and TLE (ICV adjusted)
| Right TLE | Left TLE | Combined TLE | ||||
|---|---|---|---|---|---|---|
| d | % difference | d | % difference | d | % difference | |
| Ipsilateral Hippocampus | 1.36 | −22.64 | 1.11 | −22.20 | 1.26 | −22.38 |
| Contralateral Hippocampus | .14 | −2.85 | .17 | −2.75 | .15 | −2.75 |
| Ipsilateral Thalamus | .97 | −10.70 | 1.02 | −10.21 | .96 | −10.56 |
| Contralateral Thalamus | .78 | −7.89 | .59 | −6.57 | .72 | −7.13 |
| Ipilateral Caudate | .17 | −2.68 | .15 | −2.31 | .17 | −2.71 |
| Contralateral Caudate | .06 | −.090 | −.02 | 0.33 | .01 | −.07 |
| Ipsilateral Putamen | .31 | −4.54 | .31 | −4.80 | .35 | −4.92 |
| Contralateral Putamen | .61 | −9.50 | .25 | −3.59 | .41 | −6.37 |
| Corpus Callosum | .88 | −15.26 | .70 | −12.03 | .79 | −13.67 |
3.3 Clinical seizure characteristics and volumes
Table 4 shows the partial correlations (controlled for ICV) between ipsilateral and contralateral MRI volumes and clinical seizure variables for the combined right and left TLE groups. The ipsilateral hippocampus was the only structure to show a significant association with age of seizure onset, with earlier onset associated with smaller volume. The ipsilateral hippocampus was also associated with longer duration of epilepsy, increased number of AED medications, and increased lifetime number of generalized seizures. Longer duration of epilepsy was also significantly correlated with ipsilateral and contralateral thalami, ipsilateral caudate nuclei, and ipsilateral and contralateral putamen. An increased lifetime number of generalized seizures were associated with smaller ipsilateral thalamic and corpus callosum volumes. There was no significant association between any of the clinical seizure variables and contralateral hippocampal volume.
Table 4.
Correlations between MR volumes and clinical seizure variables
| Age of Onset | Duration of Epilepsy | # AEDs | Generalized Seizures | |
|---|---|---|---|---|
| Ipsilateral Hippocampus | .38b | −.56a | −.40b | −.54a |
| Contralateral Hippocampus | −.13 | .33 | −.16 | −.12 |
| Ipsilateral Thalamus | .04 | −.64a | −.16 | −.45b |
| Contralateral Thalamus | −.20 | −.58a | −.35 | −.32 |
| Ipilateral Caudate | .10 | −.51a | −.29 | −.06 |
| Contralateral Caudate | .08 | −.35 | −.24 | .02 |
| Ipsilateral Putamen | −.13 | −.50a | −.27 | −.32 |
| Contralateral Putamen | −.01 | −.56a | −.30 | −.30 |
| Corpus Callosum | −.05 | −.17 | −.22 | −.49b |
p ≤ .001
p ≤ .01
A subgroup of 28 TLE patients with a positive history for an initial precipitating incident (IPI+) (e.g., complex febrile seizure, infectious disease) was compared to a group of 13 TLE patients without a history of an initial precipitating incident (IPI−). Within the IPI+, the hippocampus was the only structure in which the ipsilateral side showed a significantly reduced volume compared to the contralateral side (t [27] = 4.78, p < .001). The IPI+ group also showed reduced ipsilateral hippocampal volume compared to the IPI− group. A similar pattern was not observed for any of the subcortical structures examined. Figure 2 shows hippocampal volumes for IPI+ and IPI− groups.
Figure 2.
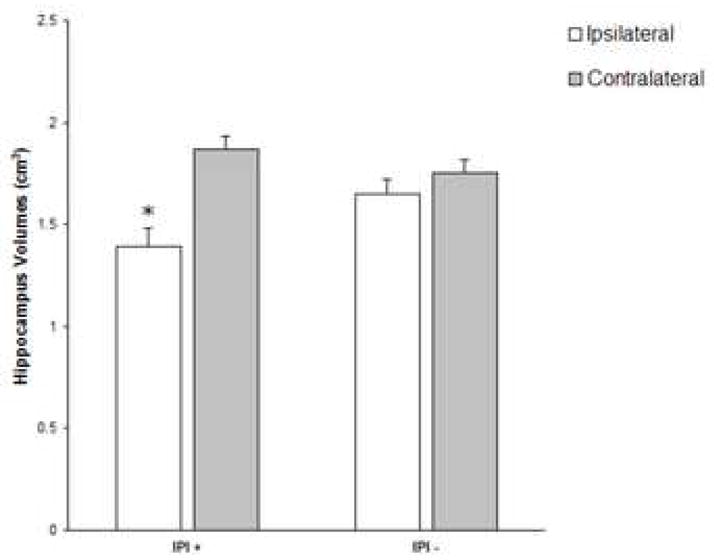
Hippocampal volumes with (IPI+) and without (IPI−) a history of initial precipitating incident.
* Indicates significant difference from contralateral hippocampus.
4. Discussion
This study examined quantitative MRI volumes of subcortical structures in a single cohort of patients with video-EEG ictal verification of unilateral TLE. Although volume reduction was apparent across all the structures, they were not affected to the same degree. In addition, there were different associations observed between MR volume and clinical seizure correlates across these structures. Details of these findings and their implications for understanding the nature and course of MRI volume reductions in TLE are discussed below.
4.1 Hippocampus
As expected, the ipsilateral hippocampus showed the greatest effect size for volume reduction compared to controls. Additionally, a marked asymmetry of volume reduction between the ipsilateral (22% volume loss) and contralateral (3% volume loss) hippocampi was observed. Indeed, the volume difference in the contralateral hippocampus was not significantly different between the TLE group and controls. Ipsilateral hippocampal volume reduction with a relatively intact contralateral volume is the MRI signature of unilateral TLE [1, 2, 5].
Ipsilateral hippocampal volume, but not contralateral hippocampal volume, was significantly correlated with both seizure onset-related factors and duration of epilepsy [7, 15]. Ipsilateral hippocampal volume was significantly reduced compared to contralateral hippocampal volume only in a subgroup of patients with a positive history of a precipitating event. This suggests that early neurodevelopmental factors (e.g., complex febrile seizures, infectious processes) have an adverse impact on the development of the hippocampus [23, 24]. It is possible that the early insult to the hippocampus permits the development of cellular changes in the contralateral hippocampus which inhibit the spread of seizure activity [25]. None of the other subcortical structures examined showed a similar pattern of volume asymmetry with an initial precipitating incident.
In addition, increased duration of epilepsy also negatively impacted ipsilateral hippocampus volume. These findings are consistent with the notion of a dual impact on the ipsilateral hippocampus [26]. In contrast, contralateral hippocampal volume was not significantly related to either of these variables and appears to maintain an independent course of development. Recent longitudinal findings are consistent with these findings of asymmetric involvement of the ipsilateral hippocampus in continued volume loss [27–29]. Other course related variables such as increased number of lifetime generalized seizures and increased number of current AED medications were also associated with reduced ipsilateral hippocampal volume.
4.2 Corpus callosum
The corpus callosum also showed a large effect size difference in volumes loss (14%) compared to controls, and volume reduction was significantly associated with increased lifetime number of secondarily generalized seizures. There has been minimal investigation of volume integrity of the corpus callosum in unilateral TLE. Previous studies have examined heterogeneous samples of epilepsy subjects and have produced mixed results [30–32]. In addition, none of these studies examined corpus callosum volume in relation to other brain structures. Consistent with the current findings, significant corpus callosum volume reduction was reported for TLE patients with early onset compared to late onset patients and controls [12], and the current findings confirm this result in a TLE group with ictal EEG determined unilateral temporal lobe epilepsy.
EEG research has shown that the corpus callosum facilitates bilateral hemisphere seizure susceptibility and/or interhemispheric transmission of epileptiform discharges [33]. Although the hippocampus does not directly project to the corpus callosum, the corpus callosum is important in the spread of epileptiform discharges to contralateral regions. This is consistent with the significant association observed between number of generalized seizures and corpus callosum volume abnormality. In addition, the large effect size for corpus callosum volume atrophy is consistent with recent findings of extensive bilateral whole brain white matter volume atrophy in unilateral TLE [7, 34].
4.3 Thalamus
Both the ipsilateral (11% volume loss) and contralateral (7% volume loss) thalami showed a moderate effect size for volume reduction, although the effect was stronger for the ipsilateral thalamus. This is consistent with the known extensive anatomic interconnectivity between the hippocampus and thalamus [35], and the significant role of the thalamus in seizure initiation, modulation, and propagation [16, 36, 37]. Here we found significant bilateral volume loss compared to the control group with greater ipsilateral than contralateral volume loss. A recent voxel-based morphometry study indicated that anterior thalamic nuclei that show the strongest connectivity to the hippocampus are most affected [14], and this may account for the divergent findings.
The precise mechanism associated with bilateral involvement of the thalamus in unilateral TLE remains unclear. We did find that duration of epilepsy was significantly correlated with volume reduction for both the ipsilateral and contalateral thalami and that increased number of generalized seizures was also associated with reduced ipsilateral thalamic volume. These findings are consistent with previous reports of significant bilateral thalamic volume abnormality in TLE patients. [38, 39].
4.4 Putamen
Moderate effect sizes for volume loss in both the ipsilateral (5%) and contralateral putamen (6%) were observed, and volume loss was associated with increased duration of epilepsy. Dreifuss et al. (2001) also reported bilateral putamen volume loss in a sample of TLE subjects. However, in contrast to the current study findings, there was not a significant association with increased duration of epilepsy. One possible explanation for the different findings may lie in the nature of the subject sample studied. They included a substantial number of TLE patients with identifiable structural lesions (33%) other than hippocampal sclerosis which could attenuate duration effects associated with long-standing non-lesional epilepsy. The current study included both left and right TLE subjects without a visible MR structural lesion and both groups were similar in age and comparable in other demographic characteristics to controls.
4.5 Caudate
We observed very small and statistically non-significant volume abnormalities in both the ipsilateral (3%) and contralateral (1%) caudate nuclei. In contrast, Dreifuss et al (2001) did find significant bilateral caudate volume abnormalities in a TLE sample. However, there are important differences between studies in the manner in which the caudate nucleus was traced. Their study included the caudate tail in the tracing, which we did not because it is very difficult to distinguish in the coronal view. A recent study that also excluded the tail of the caudate found no significant caudate volume difference between TLE patients and controls [40]. It may be that the tail of the caudate is most likely to develop volume reduction in unilateral TLE. Once again, duration of epilepsy was significantly associated with volume reduction in the caudate.
4.6 Limitations
There are several limitations that should be considered when evaluating the current findings. First, the cause of volume abnormalities cannot be determined from a cross sectional study such as this one. Volume loss may represent consequences of early cerebral injury, structural differences secondary to recurrent abnormal electrical activity, and/or other factors associated with duration of epilepsy. Ultimately, longitudinal studies (ideally beginning in childhood) are necessary to answer the important questions concerning the causes and course of extrahippocampal volume abnormality. A second issue concerns the effect of AED medications on MR volumes. In the current study, AED medication regimen (i.e., number of AEDs) at time of testing was not a strong predictor of volume in these structures. However, use of current AEDs is obviously a crude index of their potential impact. We did not have available the lifetime medication history of these subjects. Nevertheless, there is considerable variability across brain structures in the extent of volume abnormality, which argues against a generalized impact of AED medications. Finally, we did not examine all the potential subcortical structures that may be affected in unilateral TLE. For example, there are reports of cerebellar volume loss in TLE patients [41].
In summary, subcortical volumes differed in the extent of observed volume loss in this sample of unilateral TLE patients. Within the constraints of a cross-sectional design, the pattern of volumetric abnormalities observed here, and the associated impact of clinical seizure variables, suggests a different neurodevelopmental course of volume abnormality for these subcortical structures. Along with the ipsilateral hippocampus, particularly affected were the thalamus and corpus callosum. In addition, clinical seizure correlates associated with disease progression were consistently associated with both the hippocampus and subcortical volume abnormalities. However, only the hippocampus showed a significant relationship with seizure onset-related factors (IPI history and age of onset). Further investigation of the implication of volume abnormalities in these subcortical structures for cognitive status and seizure management would be of considerable interest.
Acknowledgments
This work was supported by 2RO1 NINDS 37738 and MO1 RR 03186 (GCRC). This investigation was completed with the help of Drs. Brian Bell and Jana Jones and Michelle Szomi who was responsible for recruiting subjects and Kevin Dabbs for coordinating the MR scan analyses. We sincerely thank Drs. Paul Rutecki, Fred Edeleman, Raj Sheth, Jack Jones, Brian Beinlich, and Kevin Ruggles for referring their patients to this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jack CR. MRI-based hippocampal volume measurements in epilepsy. Epilepsia. 1994;35(Suppl 6):S21–29. doi: 10.1111/j.1528-1157.1994.tb05986.x. [DOI] [PubMed] [Google Scholar]
- 2.van Paesschen W, Revesz T, Duncan JS, King MD, Connelly A. Quantitative neuropathology and quantitative magnetic resonance imaging of the hippocampus in temporal lobe epilepsy. Ann Neurol. 1997;42(5):756–66. doi: 10.1002/ana.410420512. [DOI] [PubMed] [Google Scholar]
- 3.Watson C, Jack CR, Cendes F. Volumetric magnetic resonance imaging. Clinical applications and contributions to the understanding of temporal lobe epilepsy. Arch Neurol. 1997;54(12):1521–31. doi: 10.1001/archneur.1997.00550240071015. [DOI] [PubMed] [Google Scholar]
- 4.Kalviainen R, Salmenpera T, Partanen K, Vainio P, Riekkinen P, Sr, Pitkanen A. Recurrent seizures may cause hippocampal damage in temporal lobe epilepsy. Neurology. 1998;50(5):1377–82. doi: 10.1212/wnl.50.5.1377. [DOI] [PubMed] [Google Scholar]
- 5.Theodore W, Bhatia S, Hatta J, Fazilat S, DeCarli C, Bookheimer SY, Gaillard WD. Hippocampal atrophy, epilepsy duration, and febrile seizures in patients with partial seizures. Neurology. 1999;52(1):132–36. doi: 10.1212/wnl.52.1.132. [DOI] [PubMed] [Google Scholar]
- 6.Spencer SS. Neural networks in human epilepsy: Evidence of and implications for treatment. Epilepsia. 2002;43(3):219–27. doi: 10.1046/j.1528-1157.2002.26901.x. [DOI] [PubMed] [Google Scholar]
- 7.Seidenberg M, Kelly KG, Parrish J, Geary E, Dow C, Rutecki P, Hermann B. Ipsilateral and contralateral MRI volumetric abnormalities in chronic unilateral temporal lobe epilepsy and their clinical correlates. Epilepsia. 2005;46(3):420–30. doi: 10.1111/j.0013-9580.2005.27004.x. [DOI] [PubMed] [Google Scholar]
- 8.Moran N, Lemieux L, Kitchen N, Fish D, Shorvon S. Extrahippocampal temporal lobe atrophy in temporal lobe epilepsy and mesial temporal sclerosis. Brain. 2001;124(Pt 1):167–75. doi: 10.1093/brain/124.1.167. [DOI] [PubMed] [Google Scholar]
- 9.Bernasconi N, Bernasconi A, Caramanoz Z, Antel S, Andermann F, Arnold D. Mesial temporal damage in temporal lobe epilepsy: a volumetric MRI study of the hippocampus, amygdala and parahippocampal region. Brain. 2003;126(Pt 2):462–69. doi: 10.1093/brain/awg034. [DOI] [PubMed] [Google Scholar]
- 10.DeCarli C, Hatta J, Fazilat S, Gaillar WD, Theodore WH. Extratemporal atrophy in patients with complex partial seizures of left temporal lobe origin. Ann Neurol. 1998;43(1):41–45. doi: 10.1002/ana.410430110. [DOI] [PubMed] [Google Scholar]
- 11.Dreifuss S, Vingerhoets FJ, Lazeyras F, Andino SG, Spinelli L, Delavelle J, Seeck M. Volumetric measurements of subcortical nuclei in patients with temporal lobe epilepsy. Neurology. 2001;57(9):1636–41. doi: 10.1212/wnl.57.9.1636. [DOI] [PubMed] [Google Scholar]
- 12.Hermann B, Hansen R, Seidenberg M, Magnotta V, O’Leary D. Neurodevelopmental vulnerability of the corpus callosum to childhood onset localization-related epilepsy. NeuroImage. 2003;18(2):284–92. doi: 10.1016/s1053-8119(02)00044-7. [DOI] [PubMed] [Google Scholar]
- 13.Natsume J, Bernasconi N, Andermann F, Bernasconi A. MRI volumetry of the thalamus in temporal, extratemporal, and idiopathic generalized epilepsy. Neurology. 2003;60(8):1296–1300. doi: 10.1212/01.wnl.0000058764.34968.c2. [DOI] [PubMed] [Google Scholar]
- 14.Bonilha L, Rorden C, Castellano G, Cendes F, Li LM. Voxel-based morphometry of the thalamus in patients with refractory medial temporal lobe epilepsy. NeuroImage. 2005;25(3):1016–21. doi: 10.1016/j.neuroimage.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 15.Bernasconi N, Natsume J, Bernasconi A. Progression in temporal lobe epilepsy: differential atrophy in mesial temporal structures. Neurology. 2005;65(2):223–28. doi: 10.1212/01.wnl.0000169066.46912.fa. [DOI] [PubMed] [Google Scholar]
- 16.Norden A, Blumenfeld H. The role of subcortical structures in human epilepsy. Epilepsy Behav. 2002;3(3):219–31. doi: 10.1016/s1525-5050(02)00029-x. [DOI] [PubMed] [Google Scholar]
- 17.Magnotta V, Harris G, Andreasen N, O’Leary D, Yuh D, Heckel D. Structural MR image processing using the BRAINS2 toolbox. Comput Med Imaging Graph. 2002;26(4):251–64. doi: 10.1016/s0895-6111(02)00011-3. [DOI] [PubMed] [Google Scholar]
- 18.Pantel J, O’Leary DS, Crestinger K, Bockholt HJ, Keefe H, Magnotta VA, Andreasen NC. A new method for the in vivo volumetric measurement of the hippocampus with high neuroanatomical accuracy. Hippocampus. 2000;10(6):752–58. doi: 10.1002/1098-1063(2000)10:6<752::AID-HIPO1012>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 19.Ooteman W, Crestinger K. Thalamus tracing guidelines. [Accessed March 1, 2006]. Available at: http://www.psychiatry.uiowa.edu/mhcrc/pdf/papers/thalamus.pdf.
- 20.Westmoreland P, Crestinger K. Caudate tracing guidelines. [Accessed March 1, 2006]. Available at: http://www.psychiatry.uiowa.edu/mhcrc/pdf/papers/caudate.pdf.
- 21.Westmoreland P, Crestinger K. Putamen tracing guidelines. [Accessed March 1, 2006]. Available at: http://www.psychiatry.uiowa.edu/mhcrc/pdf/papers/putamen.pdf.
- 22.Cohen J. A power primer. Psychol Bull. 1992;112:155–59. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 23.Mathern G, Babb TL, Vickrey BG, Melendez M, Pretorius JK. The clinical-pathogenic mechanisms of hippocampal neuron loss and surgical outcomes in temporal lobe epilepsy. Brain. 1995;118(Pt 1):105–18. doi: 10.1093/brain/118.1.105. [DOI] [PubMed] [Google Scholar]
- 24.Liu R, Lemieux L, Bell GS, Sisodiya SM, Bartlett PA, Shorvon SD, Sander JW, Duncan JS. Cerebral damage in epilepsy: a population-based longitudinal quantitative MRI study. Epilepsia. 2005;46(9):1482–94. doi: 10.1111/j.1528-1167.2005.51603.x. [DOI] [PubMed] [Google Scholar]
- 25.Arabadzisz D, Antal K, Parpan F, Emri Z, Fritschy JM. Epileptogenesis and chronic seizures in a rat model of temporal lobe epilepsy are associated with distinct EEG patterns and selective neurochemical alterations in the contralateral hippocampus. Exp Neurol. 2005;194(1):76–90. doi: 10.1016/j.expneurol.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 26.Fuerst D, Shah J, Kupsky WJ, Johnson R, Shah A, Hayman-Abello B, Ergh T, Poor Q, Canady A, Watson C. Volumetric MRI, pathological, and neuropsychological progression in hippocampal sclerosis. Neurology. 2001;57(2):184–88. doi: 10.1212/wnl.57.2.184. [DOI] [PubMed] [Google Scholar]
- 27.Fuerst D, Shah J, Shah A, Watson C. Hippocampal sclerosis is a progressive disorder: a longitudinal volumetric MRI study. Ann Neurol. 2003;53(3):413–16. doi: 10.1002/ana.10509. [DOI] [PubMed] [Google Scholar]
- 28.Briellmann RS, Berkovic SF, Syngeniotis A, King MA, Jackson GD. Seizure associated hippocampal volume loss: a longitudinal magnetic resonance study of temporal lobe epilepsy. Ann Neurol. 2002;51(5):641–44. doi: 10.1002/ana.10171. [DOI] [PubMed] [Google Scholar]
- 29.Van Paesschen W, Duncan JS, Stevens JM, Connelly A. Longitudinal quantitative hippocampal magnetic resonance imaging study of adults with newly diagnosed partial seizures: one-year follow-up results. Epilepsia. 1998;39(6):633–39. doi: 10.1111/j.1528-1157.1998.tb01432.x. [DOI] [PubMed] [Google Scholar]
- 30.Conlon P, Trimble MR. A study of the corpus callosum in epilepsy using magnetic resonance imaging. Biol Psychiatry. 1988;24(7):857–60. doi: 10.1016/0006-3223(88)90267-3. [DOI] [PubMed] [Google Scholar]
- 31.O’Kusky J, Strauss E, Kosaka B, Wada J, Li D, Druhan M, Petrie J. The corpus callosum is larger with right-hemisphere cerebral speech dominance. Ann Neurol. 1988;24(3):379–83. doi: 10.1002/ana.410240305. [DOI] [PubMed] [Google Scholar]
- 32.Atkinson DS, Abou-Khalil B, Charles DP, Welch L. Midsagittal corpus callosum area, intelligence, and language dominance in epilepsy. J Neuroimaging. 1996;6(4):235–39. doi: 10.1111/jon199664235. [DOI] [PubMed] [Google Scholar]
- 33.Matsuo A, Ono T, Baba H, Ono K. Callosal role in generation of epileptiform discharges: quantitative analysis of EEGs recorded in patients undergoing corpus callosotomy. Neurophysiol Clin. 2003;114(11):2165–71. doi: 10.1016/s1388-2457(03)00234-7. [DOI] [PubMed] [Google Scholar]
- 34.Marsh L, Morrell MJ, Shear PK, Sullivan EV, Freeman H, Marie A, Lim KO, Pfefferbaum A. Cortical and hippocampal volume deficits in temporal lobe epilepsy. Epilepsia. 1997;38(5):576–87. doi: 10.1111/j.1528-1157.1997.tb01143.x. [DOI] [PubMed] [Google Scholar]
- 35.Herrero MT, Barcia C, Navarro J. Functional anatomy of thalamus and basal ganglia. Childs Nerv Syst. 2002;18(8):386–404. doi: 10.1007/s00381-002-0604-1. [DOI] [PubMed] [Google Scholar]
- 36.Bertram EH, Mangan PS, Zhang D, Scott CA, Williamson JM. The midline thalamus: alterations and a potential role in limbic epilepsy. Epilepsia. 2001;42(8):967–78. doi: 10.1046/j.1528-1157.2001.042008967.x. [DOI] [PubMed] [Google Scholar]
- 37.Guye M, Regis J, Tamura M, Wendling F, McGonigal A, Chauvel P, Bartolomei F. The role of corticothalamic coupling in human temporal lobe epilepsy. Brain. 2006;129(Pt 7):1917–28. doi: 10.1093/brain/awl151. [DOI] [PubMed] [Google Scholar]
- 38.Dlugos DJ, Jaggi J, O’Connor WM, Ding XS, Reivich M, O’Connor MJ, Sperling MR. Hippocampal cell density and subcortical metabolism in temporal lobe epilepsy. Epilepsia. 1999;40(4):408–13. doi: 10.1111/j.1528-1157.1999.tb00734.x. [DOI] [PubMed] [Google Scholar]
- 39.Mueller SG, Laxer KD, Cashdollar N, Buckley S, Paul C, Weiner MW. Voxel-based optimized morphometry (VBM) of gray and white matter in temporal lobe epilepsy (TLE) with and without mesial temporal sclerosis. Epilepsia. 2006;47(5):900–7. doi: 10.1111/j.1528-1167.2006.00512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Szabo CA, Lancaster J, Lee S, Xiong J, Cook C, Mayes BN, Fox PT. MR imaging volumetry of subcortical structures and cerebellar hemispheres in temporal lobe epilepsy. Am J Neuroradiol. 2006;27(10):2155–60. [PMC free article] [PubMed] [Google Scholar]
- 41.Sandok EK, O’Brien TJ, Jack CR, So EL. Significance of cerebellar atrophy in intractable temporal lobe epilepsy: a quantitative MRI study. Epilepsia. 2000;41(10):1315–20. doi: 10.1111/j.1528-1157.2000.tb04611.x. [DOI] [PubMed] [Google Scholar]


