Oncogene-induced senescence (OIS) is a protective mechanism through which normal cells defend themselves against transformation.1 Oncogenic stress (induced by mutation or proto-oncogene overexpression) activates this tumor-suppressive pathway, wherein cells enter a stage of irreversible cell-cycle arrest, thereby restricting uncontrolled cellular proliferation and malignant growth. OIS has been described both in vitro in primary cells, as well as in vivo in pre-malignant lesions, for multiple oncogenes and in various cellular contexts.
We recently reported a unique form of OIS activated by overexpression of the oncogenic splicing factor SRSF1.2 SRSF1 encodes a multi-functional protein with regulatory roles in many aspects of RNA biogenesis and function, including constitutive and alternative splicing, mRNA export and translation.3 Aberrant SRSF1 activity is deleterious for the cell: SRSF1 depletion triggers genomic instability, cell-cycle arrest and apoptosis,4 whereas its overexpression leads to oncogenic transformation.5 Consequently, SRSF1 expression is strictly regulated. SRSF1 autoregulates its expression through multiple post-transcriptional and translational mechanisms.3 Our recent finding that SRSF1 keeps a check on the oncogenic outcome of its overexpression by activating a novel OIS pathway represents yet another mechanism of SRSF1 autoregulation.
Interestingly, senescence induction by SRSF1 is tightly coupled to the ribosomal-stress-response pathway, which was previously shown to stabilize the critical cell-cycle regulator and tumor-suppressor protein p53, upon ribosomal perturbation.6 Ribosome biogenesis and function are critical regulators of cell growth and proliferation, and are highly sensitive to nutrient and growth-factor availability, as well as oncogenic burden. Aberrant ribosome assembly or function triggers formation of a complex of ribosomal proteins, including RPL5 and RPL11, with the E3-ubiquitin ligase MDM2. Sequestration of MDM2 in this nucleoplasmic complex inhibits ubiquitylation of the primary MDM2 substrate, p53, promoting its stability.
We demonstrated that SRSF1 interacts with both RPL5 and MDM2, and this interaction is promoted by inducers of ribosomal stress, indicating a role of SRSF1 in the ribosomal-stress pathway. Consistent with the established RPL5-MDM2 function, SRSF1 overexpression decreases p53’s ubiquitylation and increases its stability at the protein level, without affecting TP53 transcription, mRNA splicing or mRNA stability. Furthermore, upon overexpression in primary human and murine cells, SRSF1 limits its own oncogenic activity by recruiting the RPL5-MDM2 complex to promptly activate a tumor-suppressive barrier, i.e., p53-mediated premature cellular senescence.
Our results provide new insights into the mechanisms of both ribosomal stress and OIS. Previous reports on the RP-MDM2 complex described ternary and quaternary complexes comprising RPL5, MDM2 and other ribosomal proteins, primarily RPL11 and RPL23.6 The RPL5-MDM2 interaction was reported to be strengthened in the presence of RPL11. Because SRSF1 depletion destabilizes the RPL5-MDM2 interaction, SRSF1 apparently plays a similar role as RPL11. SRSF1 might replace RPL11 in one of the complexes, perhaps in response to particular stress signals. It will be interesting to investigate whether the different complexes are redundant or activate stress responses varying in magnitude or precise outcome. Furthermore, considering that SRSF1 recruits the RP-MDM2 complex to limit its own aberrant activity, this may be a generic mechanism that other oncogenic SR proteins perhaps also adopt to limit the consequences of their own overexpression.
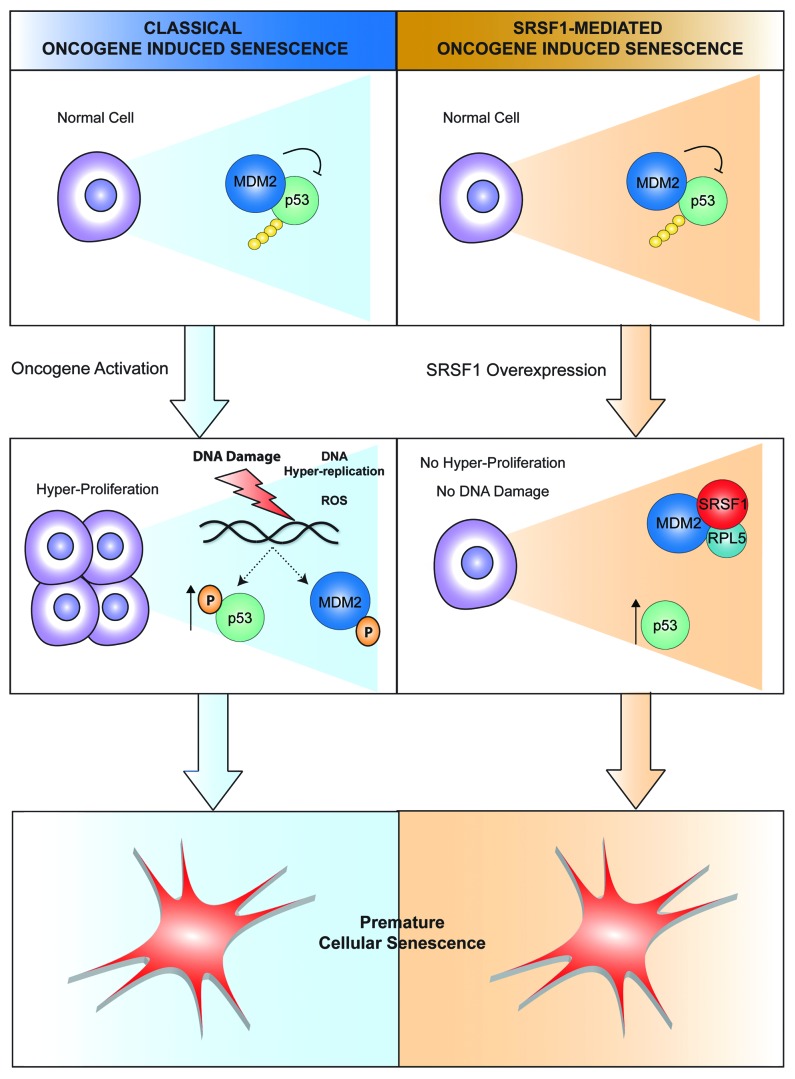
The hallmarks of SRSF1-induced senescence are distinct from most OIS pathways described to date (Fig. 1). Classical OIS, as described for other oncogenes, such as H-ras V12, is primarily a DNA-damage response induced by hyper-proliferation and oxidative stress.1 SRSF1-induced senescence, on the other hand, proceeds rapidly in the absence of hyper-proliferation or DNA damage. Furthermore, we did not observe induction of the cell-cycle regulators Rb or ARF/p14, which play critical roles in regulating Ras-induced senescence and MYC-induced apoptosis. Although SRSF1-induced senescence shares common features with PTEN-loss-induced cellular senescence and the related Akt-induced senescence,7 unlike the latter it does not require mTOR for p53 activation. Thus, we have identified a new OIS mechanism that relies on cross-talk between spliceosomal and ribosomal components.
Figure 1. SRSF1-induced senescence is mechanistically distinct from classical oncogene-induced senescence.
Our results indicate that p53 inactivation is likely a pre-requisite for SRSF1-driven tumorigenesis. About 50% of human tumors bear missense mutations in TP53. In addition, tumors can also harbor lesions in regulators of p53 expression, such as MDM2 amplification or ARF deletion. An intriguing scenario is that SRSF1 itself may acquire mutations, so as to prevent its association with MDM2. SRSF1-overexpressing cells might also escape OIS by accumulating oncogenic mutations in TP53,8 in which case SRSF1-mediated stabilization of mutant p53 would show oncogenic cooperation, leading to a more aggressive phenotype. Thus, though our findings emphasize the potential for regression of SRSF1-dependent tumors by anti-cancer therapies aimed at reactivating the p53-tumor suppressor pathway, they also reinforce the need for molecular characterization of tumors so as to adopt suitable therapies.
In summary, our recent publication highlights a novel OIS mechanism that identifies the regulators of the ribosomal-stress response as key players in this tumor-protective pathway. Whether this is unique to SRSF1 activation, or is a conserved function of the RPL-MDM2 complexes remains to be explored. However, it is clear that SRSF1 not only functions as a mediator of ribosomal stress, but also utilizes this mechanism to add another layer to its autoregulation. Furthermore, our study implicates spliceosomal and ribosomal components in non-canonical roles as regulators of a pathway critical for maintenance of cellular homeostasis, further emphasizing the inherent complexity of these key cellular processes.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/24749
References
- 1.Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes Dev. 2010;24:2463–79. doi: 10.1101/gad.1971610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fregoso OI, Das S, Akerman M, Krainer AR. Splicing-Factor Oncoprotein SRSF1 Stabilizes p53 via RPL5 and Induces Cellular Senescence. Mol Cell. 2013;50:56–66. doi: 10.1016/j.molcel.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Twyffels L, Gueydan C, Kruys V. Shuttling SR proteins: more than splicing factors. FEBS J. 2011;278:3246–55. doi: 10.1111/j.1742-4658.2011.08274.x. [DOI] [PubMed] [Google Scholar]
- 4.Li X, Wang J, Manley JL. Loss of splicing factor ASF/SF2 induces G2 cell cycle arrest and apoptosis, but inhibits internucleosomal DNA fragmentation. Genes Dev. 2005;19:2705–14. doi: 10.1101/gad.1359305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karni R, de Stanchina E, Lowe SW, Sinha R, Mu D, Krainer AR. The gene encoding the splicing factor SF2/ASF is a proto-oncogene. Nat Struct Mol Biol. 2007;14:185–93. doi: 10.1038/nsmb1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dai MS, Zeng SX, Jin Y, Sun X-X, David L, Lu H. Ribosomal protein L23 activates p53 by inhibiting MDM2 function in response to ribosomal perturbation but not to translation inhibition. Mol Cell Biol. 2004;24:7654–68. doi: 10.1128/MCB.24.17.7654-7668.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Astle MV, Hannan KM, Ng PY, Lee RS, George AJ, Hsu AK, et al. AKT induces senescence in human cells via mTORC1 and p53 in the absence of DNA damage: implications for targeting mTOR during malignancy. Oncogene. 2012;31:1949–62. doi: 10.1038/onc.2011.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freed-Pastor WA, Prives C. Mutant p53: one name, many proteins. Genes Dev. 2012;26:1268–86. doi: 10.1101/gad.190678.112. [DOI] [PMC free article] [PubMed] [Google Scholar]