Abstract
Entry into mitosis is regulated by a checkpoint at the boundary between the G2 and M phases of the cell cycle (G2/M). In many organisms, this checkpoint surveys DNA damage and cell size and is controlled by both the activation of mitotic cyclin-dependent kinases (Cdks) and the inhibition of an opposing phosphatase, protein phosphatase 2A (PP2A). Misregulation of mitotic entry can often lead to oncogenesis or cell death. Recent research has focused on discovering the signaling pathways that feed into the core checkpoint control mechanisms dependent on Cdk and PP2A. Herein, we review the conserved mechanisms of the G2/M transition, including recently discovered upstream signaling pathways that link cell growth and DNA replication to cell cycle progression. Critical consideration of the human, frog and yeast models of mitotic entry frame unresolved and emerging questions in this field, providing a prediction of signaling molecules and pathways yet to be discovered.
Keywords: G2/M, mitotic entry, G2/M checkpoint, protein phosphatase 2A, cyclin-dependent kinase, Zds proteins, Greatwall kinase
Introduction
Cell division in eukaryotes is a highly conserved process that punctuates the life of an actively dividing cell. In the 1950s, Howard and Pelc, studying the periods of cell division and non-division in Vicia faba (fava bean), established the concept of a cell cycle divided into four sequential phases of unequal length: G1 (gap phase 1), S (DNA synthesis), G2 (gap phase 2) and M (mitosis).1 Later studies with other cell types defined a quiescent phase, called G0, outside the four-step life cycle of actively dividing cells.2-5 Except under very specific experimental conditions,6,7 the cell is driven “forward” from one phase of the cell cycle to the next (G1 to S to G2 to M),8 with a circuit from G1 to G1 constituting one cell cycle. The studies by Howard and Pelc, as well as subsequent studies in the field, recognized G1 as the primary cellular growth phase, S as the phase in which genome duplication and the initial steps of mitotic spindle formation occur, G2 as an additional growth phase and M as the phase in which both mitosis and cytokinesis occur. Correct timing of the transitions between cell cycle phases is critical for proper cell division. For example, if the genome does not fully replicate or is physically broken prior to chromosomal segregation, the resulting cells would not contain equivalent copies of the genome. To prevent precocious progression of the cell cycle and its ensuing detrimental outcomes, such as aberrant cell proliferation or death, checkpoints operate throughout the cell cycle, most often at the border between cell cycle phases.9,10 A checkpoint is a point in the cell cycle at which cell cycle progression arrests until the previous stage of growth or division has been completed with fidelity, or until certain requirements for cell division are met. For example, in both yeast and mammalian cells, before progression into S phase, there is a nutrient-sensing cell growth checkpoint (for a review, see ref. 11). The checkpoint that is the focus of this review lies at the G2/M border and regulates entry into M phase. Other checkpoints include those that monitor spindle position, chromosomal separation and mitotic exit.12,13 The actual pre-conditions (e.g., cell size, nutrient availability, DNA integrity) that allow a cell to move through a checkpoint and into the next phase of the cell cycle without arrest (i.e., without activating the checkpoint) varies from checkpoint to checkpoint, from organism to organism and from somatic to embryonic cells. However, the core molecular mechanisms of checkpoint control remain highly conserved.
From a molecular viewpoint, oscillation of the activity of cyclin-dependent kinases (Cdks), when they are in complex with adaptor molecules known as cyclins, is the minimal engine of the cell cycle that temporally orders the phases of the cell cycle.7 Without Cdk activity, the cell cycle does not progress.14 In addition to kinase activation, cyclins confer substrate specificity (for a review see ref. 15). The activity of these Cdk/cyclin complexes, which phosphorylate serine/threonine residues, is regulated by inhibitory proteins and by post-translational modifications (e.g., phosphorylation).15 Prior to the biochemical purification and cloning of Cdks and their associated cyclins in the late 1980s and early 1990s, several types of Cdk-cyclin complexes were first identified physiologically as factors required for entry into specific phases of the cell cycle (e.g., S phase-promoting complex and M phase-promoting complex, also known as maturation-promoting factor or MPF).8,16,17 Because specific classes of cyclins are expressed only during certain phases of the cell cycle, specific Cdk-cyclin complexes form in each phase of the cell cycle and prepare the cell for the next cell cycle phase through the phosphorylation of specific substrates.18 Thus, the cyclical expression of individual cyclins, in conjunction with the activation, degradation or inhibition of Cdk/cyclin complex regulators, creates a self-organized, hysteretic, temporal pattern.19-21 Under certain experimental conditions, these mechanisms of regulation are dispensable for cell cycle progression.7 Quality control within the cell cycle is enforced by the aforementioned checkpoints.
One of the major checkpoints in cell division lies at the G2/M boundary and controls entry into mitosis. At its core, this cell cycle checkpoint is a phospho-regulated switch. Phosphorylation of specific Cdk residues within M phase-specific Cdk/cyclin complexes can either inhibit or promote the activity of these complexes toward their substrates, thereby preventing or activating entry into mitosis.18 As an additional block to mitosis, mitotic Cdk substrates are kept dephosphorylated by the activity of protein phosphatase 2A (PP2A), which contributes to ordering cell cycle phase transitions.22-25 Thus, a balance between the active levels of a mitosis-activating kinase and a mitosis-inhibiting phosphatase is thought to ensure that mitotic events do not occur precociously. Fine-tuning the balance between kinase and phosphatase activities is important for maintaining the fidelity of cell division. Cells that fail to accurately balance these opposing effects face catastrophe.26,27 In certain cancer cells, the G2/M checkpoint is especially important.28 When cancer cells have a defective G1 checkpoint, they rely strongly on the G2/M checkpoint to prevent cell death during cellular replication.29 As such, drugs that force cancer cells to ignore the G2/M checkpoint are an exciting new therapeutic treatment for selectively inducing cell death in cancer cells.
G2/M Checkpoint in S. pombe and Vertebrates
In the fission yeast Schizosaccharomyces pombe, cell size is coordinated with cell cycle progression (as opposed to Xenopus oocytes, which do not have a cell size checkpoint).30 Regulation occurs principally at two control points, one in G1 and another in G2 just prior to mitosis, with deviations in cell size at these points indicative of misregulated cell cycle progression. A striking spectrum of cell size phenotypes, coupled with a forward genetic approach, aided the identification of many genes that regulate progression from G2 to M, including cdc2+, wee1+ and cdc25+.30-33 The products of these S. pombe genes and their homologs in S. cerevisiae and vertebrates constitute the conserved core mechanism of the G2/M checkpoint, consisting of a Cdk (Cdc2), which is phospho-regulated by an inhibitory kinase (Wee1) and an activating phosphatase (Cdc25). Both Wee1 and Cdc25, with their opposing kinase and phosphatase activities, respectively, are dose-dependent regulators of the phosphorylation state of Cdc2 (Cdk).31,33-36 In turn, Cdc2 (Cdk) and PP2A have opposing kinase and phosphatase activities, respectively, that regulate the phosphorylation state of Cdc2 (Cdk)/cyclin complex substrates. As an additional level of regulatory feedback, PP2A also dephosphorylates Cdc25.37
In addition to monitoring DNA integrity,38,39 the Wee1-dependent G2/M checkpoint in S. pombe couples cell size to mitotic entry.30,40,41 While this coupling has been known since the 1970s, the mechanism has only been elucidated recently. Fission yeast are rod shaped and divide following growth at the ends of the rod, with cellular scission occurring in the middle of the rod at the midzone. Prior to mitosis, a gradient of Pom1 kinase forms from each end to the midzone.42,43 Pom1 regulates an inhibitory kinase of Wee1, known as Cdr1/Nim1 (Hsl1p in S. cerevisiae). As the cell grows in size, the ends extend away from the midzone, decreasing the concentration of Pom1 at the midzone, which relieves the inhibition of Nim1. The result is the degradation of Wee1, following phosphorylation by Nim1, and subsequent entry into mitosis.42,43 The extent to which a Pom1-like mechanism is conserved has yet to be determined. However, there is some evidence that suggests conservation across species. A Pom1 homolog, called Yak1p, exists in S. cerevisiae and functions as an inhibitor of the cAMP-protein kinase A (PKA) stress response pathway.44 If Yak1p regulates Hsl1p, it may serve to link the G2/M checkpoint to cell stress responses in S. cerevisiae.
Cycling Xenopus egg extracts and Xenopus oocytes, which have been used to study cell cycle progression biochemically, have been integral experimental models for the elucidation of the G2/M checkpoint.45,46 Utilization of Xenopus cycling egg extracts showed that in addition to the destruction of Wee1,47 a protein that maintains the Cdc2/Cdk in an inhibited state by phosphorylation of T15,43 bypass of the G2/M checkpoint and entry into mitosis also requires the removal of this inhibitory phosphate.33 The Xenopus model was therefore the first to show that the Cdc25 phosphatase dephosphorylates T15.34,49 In cycling Xenopus extracts, Cdc25 itself becomes hyper-phosphorylated with the approach of mitosis.37 Cdc25 hyper-phosphorylation leads to increased enzymatic activity in vitro, with the hyper-phosphorylated form of Cdc25 in Xenopus and S. pombe acting as a mitotic activator,37,50 consistent with the observation that deletion of cdc25+ in S. pombe51 causes a delay at G2/M that results in elongated cells.31
G2/M Checkpoint in S. cerevisiae
As in S. pombe and vertebrates, Cdk/cyclin complexes in the budding yeast S. cerevisiae promote progression through the cell cycle. In S. cerevisiae, a single Cdk, Cdc28p, which is orthologous to S. pombe Cdc2, is essential for cell cycle progression. Although S. cerevisiae possesses other Cdks, such as Pho85p, only the deletion of CDC28 is lethal.15 Clns and Clbs are the two main classes of cyclins in S. cerevisiae, with Clns 1–3 serving in G1 and Clbs 1–6 serving in S, G2 or M phase (for a review, see ref. 18). Cdc28p/Clb2p is the key Cdk/cyclin complex that regulates G2 to M progression. Between the end of S phase and the beginning of mitosis, a checkpoint regulated by the Swe1p (Saccharomyces wee1+ homolog) kinase can inhibit Clb2/Cdc28p and thus mitotic entry.52
Study of the core mechanism of G2/M checkpoint regulation in S. cerevisiae has focused mainly on the phospho-regulation of the Cdc28p/Clb2p complex. When phosphorylated on residue T19 (functionally equivalent to T15 in S. pombe), Cdc28p has significantly reduced kinase activity, resulting in a delay in entry into mitosis.53 The Swe1p kinase is responsible for this inhibitory phosphorylation.52 Both Swe1p and Mih1p (mitotic inducer homolog), which is a homolog of S. pombe Cdc25,51 shuttle in and out of the nucleus, regulating both nuclear and cytoplasmic pools of Cdc28p.54 At G2/M, Swe1p translocates from the nucleus to the bud neck, where it localizes to septin rings, dependent upon the activity of the Nim1p-like kinase Hsl1p and the prior localization of Hsl1p and Hsl7p (an arginine methyltransferase) to septin rings.55-58 After Swe1p localizes to the mother-bud neck, it is sequentially phosphorylated by the Polo kinase Cdc5p59 and the p21-activated kinase Cla4p.60,61 This hyper-phosphorylated form of Swe1p becomes ubiquitinated and is thereby targeted for degradation by the Skp, Cullin, F-box-containing (SCF) ubiquitin ligase complex.62 Without Swe1p, no inhibitory phosphorylation of Cdc28p occurs. Efficient Swe1p degradation relies on hyper-phosphorylation and is necessary for entry into mitosis. However, phosphorylation of Swe1p is not just inhibitory. In a feedback loop, Cdc28p/Clb2p phosphorylates Swe1p,59 further activating Swe1p kinase activity,63 while at the same time priming Swe1p for degradation by the Cdc5p- and Cla4p-dependent mechanism just described.60,64,65 The function of this dual regulatory effect, in which Cdc28p both activates and inhibits its own inhibitor, is still not fully understood. It is possible that this mechanism allows for inhibition of mitosis until the cell reaches a critical level of Cdc28/Clb2, allowing for an “all or nothing” switch for entry into mitosis.63
Similar to the mechanism described in S. pombe, after degradation of Swe1p, activation of the Cdc28p/Clb2p complex requires the removal of the inhibitory phosphate on T19 through the activity of Mih1p phosphatase.49,51,53,66 In contrast to vertebrate cells and S. pombe, Mih1p in S. cerevisiae is hyper-phosphorylated in G1 and becomes dephosphorylated as cells approach mitosis.67 Dephosphorylation of Mih1p requires PP2ACdc55p.67 This appearance of a dephosphorylated form of Mih1p as cells approach mitosis has led some researchers to hypothesize that, in contrast to the situation in vertebrates and S. pombe,37,68 dephosphorylated Mih1p is more active toward Cdc28p.67 However, formal biochemical proof of this idea is lacking.
Although the existence of a cell size checkpoint in G1 is well established in S. cerevisiae,69,70 controversy remains as to whether the G2/M checkpoint regulated by Swe1p and Mih1p in S. cerevisiae monitors cell size. In contrast to the deletion of wee1+ in S. pombe, which results in a significant reduction in cell size,30 deletion of SWE1 in S. cerevisiae yields only a small, though statistically significant, reduction in cell size.41,71 In some strain backgrounds, SWE1 deletion does not have an obvious effect on cell size, morphology or cell cycle progression in logarithmically growing cultures in the lab.53 Likewise, deletion of MIH1 does not lead to obviously larger cells,51 in contrast to the elongated cells that result from CDC25 deletion in S. pombe.30,31 Although deletion of SWE1 does not significantly affect cell division, overexpression of Swe1p delays nuclear division, with cells pausing with short spindles.53 This delay, if prolonged, results in a distinct hyper-elongated bud with reiterative constrictions along the length of the bud, indicative of arrest in G2. In addition, failure to form a bud delays nuclear division in a manner dependent upon the inhibitory phosphorylation of Cdc28p.72 These findings led to the hypothesis that the Swe1p-dependent G2/M checkpoint serves as a “morphogenesis checkpoint” that detects incorrectly formed buds and delays mitosis to allow for proper bud growth or size before nuclear division. Seeming to support this “morphogenesis checkpoint” hypothesis were the observations that SWE1 deletion rescued certain genetic manipulations that slow bud growth or cause aberrant bud morphologies.26,73-77 However, it was later shown that nuclear division continues in a strain with a defective myosin (myo2–16ts), which arrests at the small-budded stage when shifted to a non-permissive temperature during S phase.78 This result showed that the Swe1p-dependent G2/M checkpoint does not appear to monitor bud geometry.
Does the Swe1p-dependent G2/M checkpoint in budding yeast monitor DNA integrity as it does in fission yeast and vertebrate cells?38,39 The answer is no. Induction of DNA damage by irradiation causes S. cerevisiae cells to arrest in a SWE1-independent fashion, indicating that, in contrast to fission yeast and vertebrate cells, the Swe1p-dependent checkpoint in budding yeast does not directly monitor DNA damage.79,80 DNA damage-induced arrest in budding yeast is controlled instead by the Rad9p/Rad53p/Mec1p pathway, which limits G1/S transition and anaphase progression.10,81,82
What the G2/M Swe1p-dependent checkpoint actually monitors in S. cerevisiae remains unknown, though new ideas of its physiological significance are emerging. Wild type cells have an absolute requirement for polymerized actin for progression through G2/M. Treatment of cells with the drug latrunculin, which leads to actin depolymerization, or the presence of specific mutations in ACT1, the only actin-encoding gene in S. cerevisiae, causes activation of the G2/M checkpoint.78 Deletion of SWE1 results in an inability to halt mitotic progression upon disruption of the actin cytoskeleton.78,83 This observation led to the hypothesis that the G2/M Swe1p-dependent checkpoint is an actin-sensing checkpoint that monitors the integrity of the actin cytoskeleton.78 A similar cytoskeleton-checkpoint link exists in mammals. Margolis et al.84 showed that keratin filaments act as a sink for a 14-3-3 protein, an inhibitor of the Cdc25p phosphatase (see refs. 85–87 and refs. therein), allowing for coordination between intermediate filament networks and mitotic progression. However, there are no known keratin genes in S. cerevisiae, and the 14-3-3 protein homologs (BMH1 and BMH2) have no obvious links to the S. cerevisiae Swe1p-dependent G2/M checkpoint. Therefore, evidence that the Swe1p-dependent G2/M checkpoint directly senses actin is lacking. An interesting modified version of the “actin-sensing” hypothesis is that progression through the Swe1p checkpoint merely requires an actin-dependent process, such as polarized exocytosis, rather than the checkpoint acting as an intrinsic actin sensor. An intriguing recent study by Kellogg and colleagues,88 discussed below, supports this hypothesis.
Protein Phosphatase 2A (PP2A) and the G2/M transition
PP2A catalyzes a large portion of the dephosphorylation events in the cell, including those that regulate the G2/M checkpoint. PP2A is a highly abundant and ubiquitous heterotrimeric serine/threonine phosphatase (reviewed in ref. 89). The catalytic (C) subunit of this phosphatase is constitutively associated with the structural (A) subunit. Localization and substrate specificity is accomplished through binding to various regulatory B subunits.90 In mammalian cells, there are at least 16 regulatory subunits comprising four classes: B, B′, B,′′ and B′′′ (PP2A structure reviewed in refs. 91 and 92). PP2A gained notoriety when it was discovered that suppression of PP2A, by treating mice with okadaic acid in a two-stage carcinogenesis experiment, led to an increase in tumors.93,94 PP2A regulates the G2/M checkpoint by modulating the phosphorylation state, and thus the activity, of Cdc25 and its homologs.37,49,95 In addition, PP2A has been show to regulate the phosphorylation state of Swe1.96 For these activities, PP2A utilizes the regulatory B55δ or 56 subunits in Xenopus,23,84 the B subunit Cdc55p in S. cerevisiae67,96 and most likely Pab1 in S. pombe97 and B55 in humans.90 Because PP2A regulates a checkpoint that is suppressed in many cancers, it is a target of several antitumor treatments (reviewed in ref. 29).
Although the mechanism by which PP2A regulates the Swe1p-dependent G2/M checkpoint in S. cerevisiae has yet to be fully explored, loss of the B-class PP2A regulatory subunit Cdc55p has yielded insights. In wild type S. cerevisiae, Mih1p is dephosphorylated as cells enter mitosis.67 Loss of Cdc55p causes persistent hyper-phosphorylation of Mih1p,67 suggesting that Mih1p is a PP2ACdc55p substrate. Whether hyper-phosphorylation affects Mih1p phosphatase activity (either in vitro or in vivo), targeting, or localization is not known. Loss of Cdc55p also delays Swe1p hyper-phosphorylation and degradation,98 in addition to promoting hyper-polarized bud growth reminiscent of Swe1p overexpression.26 These results led to the proposal that PP2ACdc55p is a negative regulator of Swe1p, in contrast with the fact that PP2A is a positive regulator of Wee1 (Swe1p homolog) in vertebrates.99 Inconsistent with the PP2ACdc55p -Swe1p negative regulation model in S. cerevisiae are data indicating that deletion of CDC55 suppresses the Swe1p-dependent G2/M checkpoint.100,101 Thus, the function of PP2ACdc55p in S. cerevisiae Swe1p-dependent checkpoint regulation remains debatable. However, these controversies may be explained by evidence that Cdc55p, presumably associated with other PP2A subunits, plays a more complex role in mitosis than was appreciated initially.
The Other Side of the Coin: Phosphatase Inhibition for G2 to M Progression
For decades, the central dogma of mitotic control has revolved around the regulation of the mitotic Cdks and cyclins. However, recent evidence indicates that control of a phosphatase that opposes the kinase activity of mitotic Cdk-cyclin complexes is equally important. Prior to M phase, phosphorylation of mitotic substrates is counteracted by PP2AB55δ, the activity levels of which are high in interphase and low in mitosis, as shown with cycling Xenopus extracts.23 Therefore, in addition to the activation of mitotic Cdks, PP2A inhibition is needed for mitosis to begin. Four concurrent independent studies shed light on the mechanism of PP2A inhibition at the entry into mitosis. Two of these studies were in vitro, utilizing frog egg extracts,102,103 and the other two were in vivo, utilizing S. cerevisiae.101,104
In Xenopus, PP2A inhibition at G2/M requires Greatwall (Gwl) kinase,102,103 which is required for entry into mitosis.23,25,105 Gwl kinase was first discovered in Drosophila, in which mutations of this kinase resulted in delayed G2 to M progression.106 Because Gwl kinase does not directly phosphorylate PP2A,25,105 the existence of one or more additional signaling proteins to bridge Gwl kinase to PP2A was postulated. In 2010, Mochida et al.103 and Gharbi-Ayachi et al.102 showed that cAMP-regulated phosphoprotein-19 (Arpp19) and α-endosulfine (Ensa), two proteins with high sequence homology, inhibited PP2A complexes containing a B55δ regulatory subunit,102,103 and that this inhibition required phosphorylation of Arpp19 and Ensa by Gwl kinase.102,103 It is inconclusive, however, whether both Arpp19 and Ensa inhibit PP2A upon mitotic entry. Mochida et al.103 reported that Ensa-depleted Xenopus extracts never enter mitosis, whereas Gharbi-Ayachi et al.102 reported that only the depletion of Arpp19, but not Ensa, completely inhibited entry into mitosis. Nonetheless, these elegant and labor-intensive studies linked an upstream signaling event in vertebrates to the regulation of mitotic entry by PP2A. Moreover, the relevance to disease is direct; depletion of Arpp19 in HeLa cells leads to a 50% decrease in the number of mitotic cells.102
A similar mechanism of PP2A regulation at G2/M appears to exist in budding yeast, though it does not employ an obvious homolog of Ensa or Arpp19. In S. cerevisiae, Zds2p and its paralog, Zds1p, are inhibitors of PP2ACdc55p.101 The Zds proteins, which may have a role analogous to Arpp19 and possibly Ensa, were discovered in S. cerevisiae as regulators of calcium tolerance,107 Cdc42p-mediated polarized cell growth108 and cell cycle progression.109 In all, the ZDS genes were identified in 20 independent studies (summarized in ref. 110), hence the name zillion different screens (ZDS). Deletion of both ZDS1 and ZDS2, but neither alone, results in elongated buds with multiple constrictions along the bud and a G2 delay, reminiscent of the morphology of SWE1-overexpressing cells.108,109 Suppression of this phenotype by loss of Swe1p suggested that the Zds proteins regulate the Swe1p-dependent G2/M checkpoint.111 In deciphering the mechanism of this regulation, Yasutis et al.,101 relying on a genetic approach that focused on Zds2p, and Wicky et al.,104 relying on a more biochemical approach that focused on Zds1p, identified Cdc55p, a regulatory subunit of PP2A, as a Zds-interacting protein.101,104 These data are consistent with high-throughput studies (see refs. in ref. 110) that showed protein-protein interactions between the Zds proteins and Cdc55p as well as with reports of co-immunopurification of Cdc55p and Zds1p.104,112 Two observations position PP2ACdc55p downstream of the Zds proteins in the G2/M checkpoint: (1) accelerated entry into mitosis upon Zds1p or Zds2p overexpression is Cdc55p-dependent, and (2) deletion of CDC55 suppresses the G2 arrest phenotype (long buds) of zds1∆ zds2∆ cells.101 These observations indicate that the Zds proteins inhibit Cdc55p at G2/M, allowing the transition into mitosis. In contrast to Arpp19 and Ensa,102,103 data to show that Zds proteins inhibit the phosphatase activity of PP2ACdc55p in vitro are lacking, though an experiment by Queralt and Uhlmann112 showed that PP2ACdc55p isolated from cells that overexpressed Zds1p was less active than PP2ACdc55p isolated from wild-type cells.
There are several interesting data that contradict the model of Zds proteins serving as negative regulators of PP2ACdc55p in the G2/M transition. In vivo, the Zds proteins are strictly required for the PP2ACdc55p-dependent dephosphorylation of the Mih1p phosphatase, the Cdc25 homolog that opposes the phosphorylation of Cdk1p-cyclin complexes by Swe1p (Wee1).104 Thus, the Zds proteins can promote PP2ACdc55p activity. Furthermore, work by Rossio and Yoshida113 showed that the Zds proteins, which localize to the cytoplasm and bud tip,101,108,113 are necessary for the translocation of Cdc55p from the nucleus to the cytoplasm at G2/M. A mutant form of Cdc55p containing a nuclear export signal rescued the G2/M transition delay caused by deletion of the ZDS genes, whereas sequestration of Cdc55p in the nucleus delayed entry into mitosis. These observations provide a model in which the Zds proteins promote Cdc55p function in the cytoplasm, thereby inhibiting Swe1p and, in turn, Cdc28p phosphorylation.113 Inconsistent with this model, however, is the observation by Harvey et al.95 that hyperphosphorylation of Swe1p, which promotes mitosis, is inhibited by PP2ACdc55p. This same study also showed that PP2ACdc55p inhibits the initial activating phosphorylation of Swe1p. Taking these data together, it appears that PP2ACdc55p has two opposing functions at G2/M, perhaps regulated by localization, which resolves the conflict between what are viewed as mutually exclusive models for the regulation of PP2ACdc55p by the Zds proteins.
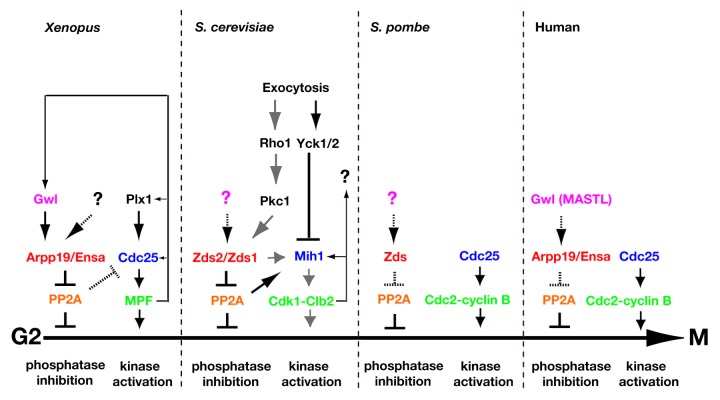
Although the S. cerevisiae and Xenopus models of PP2A-dependent cell cycle regulation contain non-homologous signaling proteins at some signaling steps, the overall architecture of these regulatory pathways shows commonalities (Fig. 1). First, in both species, an upstream inhibitor of PP2A promotes entry into mitosis in the absence of a phosphatase (Mih1p in S. cerevisiae; Cdc25 in Xenopus) that dephosphorylates mitotic Cdk-cyclin. This observation indicates that regulation of mitotic entry by Zds proteins or Arpp19/Ensa is through PP2A and not by direct regulation of mitotic Cdk-cyclins.101,102 However, it remains possible that the Zds proteins, and possibly Arpp19/Ensa, partially contribute directly to mitotic Cdk-cyclin regulation because, as observed in S. cerevisiae, overexpression of ZDS1 and ZDS2 in mih1∆ cells induced mitotic entry, though at a rate much slower than measured in cells with wild-type MIH1.101 Wicky and colleagues104 also noted that Zds1p and Zds2p regulate the dephosphorylation of Mih1p by PP2ACdc55p. A second commonality between Xenopus and S. cerevisiae is that the upstream inhibitor of PP2A is activated by phosphorylation in a cell cycle-dependent manner.104 In Xenopus, the activating kinase is Gwl.102,103 In S. cerevisiae, Protein Kinase C (Pkc1p) activates the Zds proteins.88 It appears, therefore, that Gwl and Pkc1p are functional homologs with respect to PP2A inhibitor activation. As a third commonality, the PP2A inhibitors in each mechanism (i.e., Arpp19/Ensa or Zds) appear to be signaling nodes that integrate multiple upstream signaling pathways. This idea is supported by the observation that both Arpp19 and Ensa contain target consensus sequences for both Cdk and protein kinase A,103 in addition to a target consensus sequence for Gwl kinase.102,103 While much research has focused on the regulation of mitotic Cdk-cyclin pairs, the future lies in determining the signaling pathways that regulate the core checkpoint kinase, Cdk and its opposing phosphatase, PP2A. These studies represent the first exciting steps in this direction.
Figure 1. Cross-species comparison of opposing phosphatase and kinase signaling pathways that regulate G2 to M progression, with predictions of proteins yet to be identified (question marks) or pathways yet to be validated (hatched arrows and T-bars). Shown are PP2A-dependent signaling pathways known to inhibit entry into M phase as well as the Cdk (green)-dependent pathways that promote entry into M phase. Not shown is Wee1 kinase (and similar kinases), which inactivates Cdk directly. Functional homologs are shown in the same color. Solid arrows and T-bars denote experimentally validated signaling events. For S. cerevisiae, gray and black arrows denote pathways discovered independently.
Cell Growth Lies Upstream of Zds Proteins
The signals that link cell growth to cell cycle progression are especially significant and have been a mystery for quite some time. In this regard, a recent study made a major stride in delineating a signaling pathway between a process that supports cell growth and one that allows entry into mitosis.88 Kellogg and colleagues88 found that inactivation of the Swe1p-dependent G2/M checkpoint and entry into mitosis depends upon exocytosis (Fig. 1). In their work, mutational block of exocytosis, but not endocytosis, prevented Pkc1p-dependent phosphorylation of Zds proteins, the subsequent activation of the Mih1p phosphatase and, in turn, the dephosphorylation (activation) of Cdk1p. (To explain why the mutation used by Kellogg and colleagues88 to block exocytosis triggered a Swe1-dependent arrest, while that used by Lew and colleagues78,114 did not, requires further research). Pkc1p, a known effector of Rho1p, depends upon Rho1p for activation.115,116 Prior to this biochemical study,88 genetic interactions revealed a link between PKC1 and the ZDS genes.117,118 What sensor signal Rho1p is actually transmitting is not known; however, genetics may again provide a clue. ZDS1 was identified as a multi-copy suppressor of a temperature-sensitive allele of FKS1, which encodes glucan synthase, an enzyme required for cell wall formation.119 In this pathway, Rho1p is not responding to cell size, because cell size does not appear to be coupled to the Swe1p-dependent G2/M checkpoint in S. cerevisiae. It is more likely that Rho1p is transmitting a cell wall integrity signal. In this model, when the cell wall lacks integrity, Rho1p is not activated, preventing activation of Pkc1p and the Zds proteins, as well as inhibition of the G2/M checkpoint. Consistent with this model, disruption of Zds and Zds-like proteins in the yeasts S. pombe and Cryptococcus neoformans increases cell wall thickness or mass concomitant with a reduction in cell wall integrity, implicating Zds proteins in a signaling pathway(s) that reports cell wall integrity.120,121 Drawing firm conclusions about the relationship between cell wall integrity and checkpoint control will be challenging because extended activation of the Swe1p-dependent G2/M checkpoint results in decreased cell wall integrity along the lateral bud cortex,122 making it unclear whether reduced cell wall integrity activates the checkpoint or is the result of checkpoint activation.
Perspectives
Since the discovery of cell cycle mutants in yeast and MPF in frog extracts over four decades ago,16,30,123 we have learned much about the mechanics of the cell cycle. Milestones include the biochemical purification of MPF,124 the landmark discovery that Cdks and their regulatory proteins are functionally conserved across species from yeast to vertebrates,32,125-127 and the discovery of transcriptional, translational and post-translational regulatory mechanisms of cell cycle progression.128-131 More recently, we have gained an appreciation that cell cycle progression relies not only on a core mechanism consisting of the activation of a master kinase but also on the concomitant inhibition of an opposing phosphatase. Together, this research has allowed us to formulate the first system-level models of cell cycle progression and checkpoint control.
Much work still lies ahead to continue the development of a model of cell cycle progression that utilizes insights gained from the four traditional model organisms of cell cycle research (Xenopus, S. cerevisiae, S. pombe and human cells) as well as from Drosophila, C. elegans andplants. Currently, the Xenopus and S. cerevisiae models are more developed with respect to delineation of signaling pathways upstream of Cdk and PP2A than those of other model organisms, but in unique ways. The Xenopus model has provided rich data that describe both the cell-signaling events that lead to the inhibition of a phosphatase that opposes Cdks and the positive feedforward regulation of Cdk-cyclin complexes. The S. cerevisiae model lags slightly on both points. The S. cerevisiae model, however, excels in connecting an upstream cellular process, i.e., exocytosis, to the G2/M checkpoint. Whether cell types that divide without growth (e.g., Xenopus oocytes) share a similar connection between intracellular trafficking and checkpoint control remains to be seen. Regardless of which cellular process is generating a signal to the G2/M checkpoint, the goal remains in each model to link these upstream signals to the core checkpoint mechanism, which consists of a master Cdk and an opposing phosphatase. This elucidation is especially important for cancer research, where it is now appreciated that cancer cells that have mutations that render them almost entirely dependent on the G2/M checkpoint for division control are much like yeast cells.29 A priori, it is not possible to assign greater value to one model organism for this G2/M research than another, because what appears important is the architecture of the pathways that feed into the core checkpoint mechanism, not the identity of the proteins per se. For example, at G2/M, Ensa and Arpp19 in vertebrates are likely functional homologs of the Zds proteins in fungi, even though they share no sequence similarity. Notably, phosphorylation of the bona fide Ensa/Arpp19 sequence homologs, Igo1p and Igo2p, in S. cerevisiae by the Gwl-like kinase Rim15p does not influence mitotic entry.132 The activity of Igo1p and Igo2p appears centered instead on entry into G0, when, in the absence of nutrients, Igo1p (and perhaps Igo2p) binds to and inhibits PP2ACdc55.133 Thus the value of different models of cell cycle progression lies not only in revealing similarities in the regulation of G2/M progression, but also in revealing the variance and plasticity possible.134
Irrespective of the model used, the challenges in building a systems level view of cell cycle progression will be to determine the following: (1) the stress signaling pathways that feed into the checkpoint mechanism and feedback regulation; (2) the physiological relevance of the specific phospho-states of proteins; (3) the binding constants of interacting proteins and (4) how these pathways are spatially regulated, considering the nucleocytoplasmic shuttling of checkpoint proteins.54
Acknowledgments
The authors thank R. Smindak and D. Schafer for helpful suggestions.
Glossary
Abbreviations:
- Arrp
cAMP-regulated phosphoprotein-19
- Cdk
cyclin-dependent kinase
- Ensa
α-endosulfine
- Gwl
Greatwall kinase
- MPF
maturation promoting factor
- PKA
protein kinase A
- PKC
protein kinase C
- PP2A
protein phosphatase 2A
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/24637
References
- 1.Howard A, Pelc SR. Synthesis of deoxyribonucleic acid in normal and irrradiated cells and its relation to chromosome breakage. Heredity. 1952;6(Suppl.):261–73. [Google Scholar]
- 2.Mendelsohn ML. Autoradiographic analysis of cell proliferation in spontaneous breast cancer of C3H mouse. III. The growth fraction. J Natl Cancer Inst. 1962;28:1015–29. [PubMed] [Google Scholar]
- 3.Pardee AB. A restriction point for control of normal animal cell proliferation. Proc Natl Acad Sci USA. 1974;71:1286–90. doi: 10.1073/pnas.71.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zetterberg A, Larsson O. Kinetic analysis of regulatory events in G1 leading to proliferation or quiescence of Swiss 3T3 cells. Proc Natl Acad Sci USA. 1985;82:5365–9. doi: 10.1073/pnas.82.16.5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schneider C, King RM, Philipson L. Genes specifically expressed at growth arrest of mammalian cells. Cell. 1988;54:787–93. doi: 10.1016/S0092-8674(88)91065-3. [DOI] [PubMed] [Google Scholar]
- 6.Potapova TA, Daum JR, Pittman BD, Hudson JR, Jones TN, Satinover DL, et al. The reversibility of mitotic exit in vertebrate cells. Nature. 2006;440:954–8. doi: 10.1038/nature04652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coudreuse D, Nurse P. Driving the cell cycle with a minimal CDK control network. Nature. 2010;468:1074–9. doi: 10.1038/nature09543. [DOI] [PubMed] [Google Scholar]
- 8.Rao PN, Johnson RT. Mammalian cell fusion: studies on the regulation of DNA synthesis and mitosis. Nature. 1970;225:159–64. doi: 10.1038/225159a0. [DOI] [PubMed] [Google Scholar]
- 9.Hartwell LH, Weinert TA. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989;246:629–34. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- 10.Weinert T, Hartwell L. Control of G2 delay by the rad9 gene of Saccharomyces cerevisiae. J Cell Sci Suppl. 1989;12(Suppl.):145–8. doi: 10.1242/jcs.1989.supplement_12.12. [DOI] [PubMed] [Google Scholar]
- 11.Foster DA, Yellen P, Xu L, Saqcena M. Regulation of G1 cell cycle progression: Distinguishing the restriction point from a nutrient-sensing cell growth checkpoint(s) Genes Cancer. 2010;1:1124–31. doi: 10.1177/1947601910392989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lew DJ, Burke DJ. The spindle assembly and spindle position checkpoints. Annu Rev Genet. 2003;37:251–82. doi: 10.1146/annurev.genet.37.042203.120656. [DOI] [PubMed] [Google Scholar]
- 13.Queralt E, Uhlmann F. Cdk-counteracting phosphatases unlock mitotic exit. Curr Opin Cell Biol. 2008;20:661–8. doi: 10.1016/j.ceb.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bishop AC, Ubersax JA, Petsch DT, Matheos DP, Gray NS, Blethrow J, et al. A chemical switch for inhibitor-sensitive alleles of any protein kinase. Nature. 2000;407:395–401. doi: 10.1038/35030148. [DOI] [PubMed] [Google Scholar]
- 15.Mendenhall MD, Hodge AE. Regulation of Cdc28 cyclin-dependent protein kinase activity during the cell cycle of the yeast Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 1998;62:1191–243. doi: 10.1128/mmbr.62.4.1191-1243.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masui Y, Markert CL. Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J Exp Zool. 1971;177:129–45. doi: 10.1002/jez.1401770202. [DOI] [PubMed] [Google Scholar]
- 17.Rossow PW, Riddle VG, Pardee AB. Synthesis of labile, serum-dependent protein in early G1 controls animal cell growth. Proc Natl Acad Sci USA. 1979;76:4446–50. doi: 10.1073/pnas.76.9.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bloom J, Cross FR. Multiple levels of cyclin specificity in cell-cycle control. Nat Rev Mol Cell Biol. 2007;8:149–60. doi: 10.1038/nrm2105. [DOI] [PubMed] [Google Scholar]
- 19.Pomerening JR, Sontag ED, Ferrell JE., Jr. Building a cell cycle oscillator: hysteresis and bistability in the activation of Cdc2. Nat Cell Biol. 2003;5:346–51. doi: 10.1038/ncb954. [DOI] [PubMed] [Google Scholar]
- 20.Sha W, Moore J, Chen K, Lassaletta AD, Yi CS, Tyson JJ, et al. Hysteresis drives cell-cycle transitions in Xenopus laevis egg extracts. Proc Natl Acad Sci USA. 2003;100:975–80. doi: 10.1073/pnas.0235349100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Novak B, Tyson JJ, Gyorffy B, Csikasz-Nagy A. Irreversible cell-cycle transitions are due to systems-level feedback. Nat Cell Biol. 2007;9:724–8. doi: 10.1038/ncb0707-724. [DOI] [PubMed] [Google Scholar]
- 22.Krasinska L, Domingo-Sananes MR, Kapuy O, Parisis N, Harker B, Moorhead G, et al. Protein phosphatase 2A controls the order and dynamics of cell-cycle transitions. Mol Cell. 2011;44:437–50. doi: 10.1016/j.molcel.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Mochida S, Ikeo S, Gannon J, Hunt T. Regulated activity of PP2A-B55 delta is crucial for controlling entry into and exit from mitosis in Xenopus egg extracts. EMBO J. 2009;28:2777–85. doi: 10.1038/emboj.2009.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lorca T, Bernis C, Vigneron S, Burgess A, Brioudes E, Labbé J-C, et al. Constant regulation of both the MPF amplification loop and the Greatwall-PP2A pathway is required for metaphase II arrest and correct entry into the first embryonic cell cycle. J Cell Sci. 2010;123:2281–91. doi: 10.1242/jcs.064527. [DOI] [PubMed] [Google Scholar]
- 25.Vigneron S, Brioudes E, Burgess A, Labbé JC, Lorca T, Castro A. Greatwall maintains mitosis through regulation of PP2A. EMBO J. 2009;28:2786–93. doi: 10.1038/emboj.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Burke DJ. Cdc55p, the B-type regulatory subunit of protein phosphatase 2A, has multiple functions in mitosis and is required for the kinetochore/spindle checkpoint in Saccharomyces cerevisiae. Mol Cell Biol. 1997;17:620–6. doi: 10.1128/mcb.17.2.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burgess A, Vigneron S, Brioudes E, Labbé JC, Lorca T, Castro A. Loss of human Greatwall results in G2 arrest and multiple mitotic defects due to deregulation of the cyclin B-Cdc2/PP2A balance. Proc Natl Acad Sci USA. 2010;107:12564–9. doi: 10.1073/pnas.0914191107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuntz K, O’Connell MJ. The G(2) DNA damage checkpoint: could this ancient regulator be the Achilles heel of cancer? Cancer Biol Ther. 2009;8:1433–9. doi: 10.4161/cbt.8.15.9081. [DOI] [PubMed] [Google Scholar]
- 29.Kawabe T. G2 checkpoint abrogators as anticancer drugs. Mol Cancer Ther. 2004;3:513–9. [PubMed] [Google Scholar]
- 30.Nurse P. Genetic control of cell size at cell division in yeast. Nature. 1975;256:547–51. doi: 10.1038/256547a0. [DOI] [PubMed] [Google Scholar]
- 31.Russell P, Nurse P. cdc25+ functions as an inducer in the mitotic control of fission yeast. Cell. 1986;45:145–53. doi: 10.1016/0092-8674(86)90546-5. [DOI] [PubMed] [Google Scholar]
- 32.Lee MG, Nurse P. Complementation used to clone a human homologue of the fission yeast cell cycle control gene cdc2. Nature. 1987;327:31–5. doi: 10.1038/327031a0. [DOI] [PubMed] [Google Scholar]
- 33.Russell P, Nurse P. Negative regulation of mitosis by wee1+, a gene encoding a protein kinase homolog. Cell. 1987;49:559–67. doi: 10.1016/0092-8674(87)90458-2. [DOI] [PubMed] [Google Scholar]
- 34.Kumagai A, Dunphy WG. The cdc25 protein controls tyrosine dephosphorylation of the cdc2 protein in a cell-free system. Cell. 1991;64:903–14. doi: 10.1016/0092-8674(91)90315-P. [DOI] [PubMed] [Google Scholar]
- 35.Gould KL, Moreno S, Owen DJ, Sazer S, Nurse P. Phosphorylation at Thr167 is required for Schizosaccharomyces pombe p34cdc2 function. EMBO J. 1991;10:3297–309. doi: 10.1002/j.1460-2075.1991.tb04894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Den Haese GJ, Walworth N, Carr AM, Gould KL. The Wee1 protein kinase regulates T14 phosphorylation of fission yeast Cdc2. Mol Biol Cell. 1995;6:371–85. doi: 10.1091/mbc.6.4.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumagai A, Dunphy WG. Regulation of the cdc25 protein during the cell cycle in Xenopus extracts. Cell. 1992;70:139–51. doi: 10.1016/0092-8674(92)90540-S. [DOI] [PubMed] [Google Scholar]
- 38.Enoch T, Nurse P. Mutation of fission yeast cell cycle control genes abolishes dependence of mitosis on DNA replication. Cell. 1990;60:665–73. doi: 10.1016/0092-8674(90)90669-6. [DOI] [PubMed] [Google Scholar]
- 39.Dasso M, Newport JW. Completion of DNA replication is monitored by a feedback system that controls the initiation of mitosis in vitro: studies in Xenopus. Cell. 1990;61:811–23. doi: 10.1016/0092-8674(90)90191-G. [DOI] [PubMed] [Google Scholar]
- 40.Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990;344:503–8. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- 41.Bähler J, Nurse P. Fission yeast Pom1p kinase activity is cell cycle regulated and essential for cellular symmetry during growth and division. EMBO J. 2001;20:1064–73. doi: 10.1093/emboj/20.5.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin SG, Berthelot-Grosjean M. Polar gradients of the DYRK-family kinase Pom1 couple cell length with the cell cycle. Nature. 2009;459:852–6. doi: 10.1038/nature08054. [DOI] [PubMed] [Google Scholar]
- 43.Moseley JB, Mayeux A, Paoletti A, Nurse P. A spatial gradient coordinates cell size and mitotic entry in fission yeast. Nature. 2009;459:857–60. doi: 10.1038/nature08074. [DOI] [PubMed] [Google Scholar]
- 44.Aranda S, Laguna A, de la Luna S. DYRK family of protein kinases: evolutionary relationships, biochemical properties, and functional roles. FASEB J. 2011;25:449–62. doi: 10.1096/fj.10-165837. [DOI] [PubMed] [Google Scholar]
- 45.Li M, Yin S, Yuan J, Wei L, Ai JS, Hou Y, et al. Cdc25A promotes G2/M transition in oocytes. Cell Cycle. 2008;7:1301–2. doi: 10.4161/cc.7.9.5958. [DOI] [PubMed] [Google Scholar]
- 46.Philpott A, Yew PR. The Xenopus cell cycle: an overview. Mol Biotechnol. 2008;39:9–19. doi: 10.1007/s12033-008-9033-z. [DOI] [PubMed] [Google Scholar]
- 47.Michael WM, Newport J. Coupling of mitosis to the completion of S phase through Cdc34-mediated degradation of Wee1. Science. 1998;282:1886–9. doi: 10.1126/science.282.5395.1886. [DOI] [PubMed] [Google Scholar]
- 48.Gould KL, Nurse P. Tyrosine phosphorylation of the fission yeast cdc2+ protein kinase regulates entry into mitosis. Nature. 1989;342:39–45. doi: 10.1038/342039a0. [DOI] [PubMed] [Google Scholar]
- 49.Dunphy WG, Kumagai A. The cdc25 protein contains an intrinsic phosphatase activity. Cell. 1991;67:189–96. doi: 10.1016/0092-8674(91)90582-J. [DOI] [PubMed] [Google Scholar]
- 50.Furnari B, Blasina A, Boddy MN, McGowan CH, Russell P. Cdc25 inhibited in vivo and in vitro by checkpoint kinases Cds1 and Chk1. Mol Biol Cell. 1999;10:833–45. doi: 10.1091/mbc.10.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Russell P, Moreno S, Reed SI. Conservation of mitotic controls in fission and budding yeasts. Cell. 1989;57:295–303. doi: 10.1016/0092-8674(89)90967-7. [DOI] [PubMed] [Google Scholar]
- 52.Sia RA, Herald HA, Lew DJ. Cdc28 tyrosine phosphorylation and the morphogenesis checkpoint in budding yeast. Mol Biol Cell. 1996;7:1657–66. doi: 10.1091/mbc.7.11.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Booher RN, Deshaies RJ, Kirschner MW. Properties of Saccharomyces cerevisiae wee1 and its differential regulation of p34CDC28 in response to G1 and G2 cyclins. EMBO J. 1993;12:3417–26. doi: 10.1002/j.1460-2075.1993.tb06016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Keaton MA, Szkotnicki L, Marquitz AR, Harrison J, Zyla TR, Lew DJ. Nucleocytoplasmic trafficking of G2/M regulators in yeast. Mol Biol Cell. 2008;19:4006–18. doi: 10.1091/mbc.E08-03-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shulewitz MJ, Inouye CJ, Thorner J. Hsl7 localizes to a septin ring and serves as an adapter in a regulatory pathway that relieves tyrosine phosphorylation of Cdc28 protein kinase in Saccharomyces cerevisiae. Mol Cell Biol. 1999;19:7123–37. doi: 10.1128/mcb.19.10.7123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Longtine MS, Theesfeld CL, McMillan JN, Weaver E, Pringle JR, Lew DJ. Septin-dependent assembly of a cell cycle-regulatory module in Saccharomyces cerevisiae. Mol Cell Biol. 2000;20:4049–61. doi: 10.1128/MCB.20.11.4049-4061.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cid VJ, Shulewitz MJ, McDonald KL, Thorner J. Dynamic localization of the Swe1 regulator Hsl7 during the Saccharomyces cerevisiae cell cycle. Mol Biol Cell. 2001;12:1645–69. doi: 10.1091/mbc.12.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crutchley J, King KM, Keaton MA, Szkotnicki L, Orlando DA, Zyla TR, et al. Molecular dissection of the checkpoint kinase Hsl1p. Mol Biol Cell. 2009;20:1926–36. doi: 10.1091/mbc.E08-08-0848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Asano S, Park JE, Sakchaisri K, Yu LR, Song S, Supavilai P, et al. Concerted mechanism of Swe1/Wee1 regulation by multiple kinases in budding yeast. EMBO J. 2005;24:2194–204. doi: 10.1038/sj.emboj.7600683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sakchaisri K, Asano S, Yu LR, Shulewitz MJ, Park CJ, Park JE, et al. Coupling morphogenesis to mitotic entry. Proc Natl Acad Sci USA. 2004;101:4124–9. doi: 10.1073/pnas.0400641101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee KS, Asano S, Park J-E, Sakchaisri K, Erikson RL. Monitoring the cell cycle by multi-kinase-dependent regulation of Swe1/Wee1 in budding yeast. Cell Cycle. 2005;4:1346–9. doi: 10.4161/cc.4.10.2049. [DOI] [PubMed] [Google Scholar]
- 62.Kaiser P, Sia RAL, Bardes EGS, Lew DJ, Reed SI. Cdc34 and the F-box protein Met30 are required for degradation of the Cdk-inhibitory kinase Swe1. Genes Dev. 1998;12:2587–97. doi: 10.1101/gad.12.16.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harvey SL, Charlet A, Haas W, Gygi SP, Kellogg DR. Cdk1-dependent regulation of the mitotic inhibitor Wee1. Cell. 2005;122:407–20. doi: 10.1016/j.cell.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 64.Park JE, Park CJ, Sakchaisri K, Karpova T, Asano S, McNally J, et al. Novel functional dissection of the localization-specific roles of budding yeast polo kinase Cdc5p. Mol Cell Biol. 2004;24:9873–86. doi: 10.1128/MCB.24.22.9873-9886.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Simpson-Lavy KJ, Brandeis M, Brandeis M. Clb2 and the APC/C(Cdh1) regulate Swe1 stability. Cell Cycle. 2010;9:3046–53. doi: 10.4161/cc.9.15.12457. [DOI] [PubMed] [Google Scholar]
- 66.Gautier J, Solomon MJ, Booher RN, Bazan JF, Kirschner MW. cdc25 is a specific tyrosine phosphatase that directly activates p34cdc2. Cell. 1991;67:197–211. doi: 10.1016/0092-8674(91)90583-K. [DOI] [PubMed] [Google Scholar]
- 67.Pal G, Paraz MTZ, Kellogg DR. Regulation of Mih1/Cdc25 by protein phosphatase 2A and casein kinase 1. J Cell Biol. 2008;180:931–45. doi: 10.1083/jcb.200711014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kovelman R, Russell P. Stockpiling of Cdc25 during a DNA replication checkpoint arrest in Schizosaccharomyces pombe. Mol Cell Biol. 1996;16:86–93. doi: 10.1128/mcb.16.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fantes PA, Grant WD, Pritchard RH, Sudbery PE, Wheals AE. The regulation of cell size and the control of mitosis. J Theor Biol. 1975;50:213–44. doi: 10.1016/0022-5193(75)90034-X. [DOI] [PubMed] [Google Scholar]
- 70.Johnston GC, Pringle JR, Hartwell LH. Coordination of growth with cell division in the yeast Saccharomyces cerevisiae. Exp Cell Res. 1977;105:79–98. doi: 10.1016/0014-4827(77)90154-9. [DOI] [PubMed] [Google Scholar]
- 71.Harvey SL, Kellogg DR. Conservation of mechanisms controlling entry into mitosis: budding yeast wee1 delays entry into mitosis and is required for cell size control. Curr Biol. 2003;13:264–75. doi: 10.1016/S0960-9822(03)00049-6. [DOI] [PubMed] [Google Scholar]
- 72.Lew DJ, Reed SI. A cell cycle checkpoint monitors cell morphogenesis in budding yeast. J Cell Biol. 1995;129:739–49. doi: 10.1083/jcb.129.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McMillan JN, Longtine MS, Sia RAL, Theesfeld CL, Bardes ESG, Pringle JR, et al. The morphogenesis checkpoint in Saccharomyces cerevisiae: cell cycle control of Swe1p degradation by Hsl1p and Hsl7p. Mol Cell Biol. 1999;19:6929–39. doi: 10.1128/mcb.19.10.6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McMillan JN, Sia RAL, Bardes ESG, Lew DJ. Phosphorylation-independent inhibition of Cdc28p by the tyrosine kinase Swe1p in the morphogenesis checkpoint. Mol Cell Biol. 1999;19:5981–90. doi: 10.1128/mcb.19.9.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sreenivasan A, Kellogg D. The elm1 kinase functions in a mitotic signaling network in budding yeast. Mol Cell Biol. 1999;19:7983–94. doi: 10.1128/mcb.19.12.7983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Harrison JC, Bardes ESG, Ohya Y, Lew DJ. A role for the Pkc1p/Mpk1p kinase cascade in the morphogenesis checkpoint. Nat Cell Biol. 2001;3:417–20. doi: 10.1038/35070104. [DOI] [PubMed] [Google Scholar]
- 77.Weiss EL, Bishop AC, Shokat KM, Drubin DG. Chemical genetic analysis of the budding-yeast p21-activated kinase Cla4p. Nat Cell Biol. 2000;2:677–85. doi: 10.1038/35036300. [DOI] [PubMed] [Google Scholar]
- 78.McNulty JJ, Lew DJ. Swe1p responds to cytoskeletal perturbation, not bud size, in S. cerevisiae. Curr Biol. 2005;15:2190–8. doi: 10.1016/j.cub.2005.11.039. [DOI] [PubMed] [Google Scholar]
- 79.Amon A, Surana U, Muroff I, Nasmyth K. Regulation of p34CDC28 tyrosine phosphorylation is not required for entry into mitosis in S. cerevisiae. Nature. 1992;355:368–71. doi: 10.1038/355368a0. [DOI] [PubMed] [Google Scholar]
- 80.Sorger PK, Murray AW. S-phase feedback control in budding yeast independent of tyrosine phosphorylation of p34cdc28. Nature. 1992;355:365–8. doi: 10.1038/355365a0. [DOI] [PubMed] [Google Scholar]
- 81.Weinert TA, Hartwell LH. Characterization of RAD9 of Saccharomyces cerevisiae and evidence that its function acts posttranslationally in cell cycle arrest after DNA damage. Mol Cell Biol. 1990;10:6554–64. doi: 10.1128/mcb.10.12.6554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Weinert TA, Hartwell LH. Cell cycle arrest of cdc mutants and specificity of the RAD9 checkpoint. Genetics. 1993;134:63–80. doi: 10.1093/genetics/134.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McMillan JN, Sia RAL, Lew DJ. A morphogenesis checkpoint monitors the actin cytoskeleton in yeast. J Cell Biol. 1998;142:1487–99. doi: 10.1083/jcb.142.6.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Margolis SS, Perry JA, Forester CM, Nutt LK, Guo Y, Jardim MJ, et al. Role for the PP2A/B56δ phosphatase in regulating 14-3-3 release from Cdc25 to control mitosis. Cell. 2006;127:759–73. doi: 10.1016/j.cell.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kumagai A, Guo Z, Emami KH, Wang SX, Dunphy WG. The Xenopus Chk1 protein kinase mediates a caffeine-sensitive pathway of checkpoint control in cell-free extracts. J Cell Biol. 1998;142:1559–69. doi: 10.1083/jcb.142.6.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kumagai A, Yakowec PS, Dunphy WG. 14-3-3 proteins act as negative regulators of the mitotic inducer Cdc25 in Xenopus egg extracts. Mol Biol Cell. 1998;9:345–54. doi: 10.1091/mbc.9.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gardino AK, Yaffe MB. 14-3-3 proteins as signaling integration points for cell cycle control and apoptosis. Semin Cell Dev Biol. 2011;22:688–95. doi: 10.1016/j.semcdb.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Anastasia SD, Nguyen DL, Thai V, Meloy M, MacDonough T, Kellogg DR. A link between mitotic entry and membrane growth suggests a novel model for cell size control. J Cell Biol. 2012;197:89–104. doi: 10.1083/jcb.201108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eichhorn PJA, Creyghton MP, Bernards R. Protein phosphatase 2A regulatory subunits and cancer. Biochim Biophys Acta. 2009;1795:1–15. doi: 10.1016/j.bbcan.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 90.Slupe AM, Merrill RA, Strack S. Determinants for substrate specificity of protein phosphatase 2A. Enzyme Res. 2011;2011:398751. doi: 10.4061/2011/398751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Janssens V, Longin S, Goris J. PP2A holoenzyme assembly: in cauda venenum (the sting is in the tail) Trends Biochem Sci. 2008;33:113–21. doi: 10.1016/j.tibs.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 92.Shi Y. Assembly and structure of protein phosphatase 2A. Sci China C Life Sci. 2009;52:135–46. doi: 10.1007/s11427-009-0018-3. [DOI] [PubMed] [Google Scholar]
- 93.Bialojan C, Takai A. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochem J. 1988;256:283–90. doi: 10.1042/bj2560283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Suganuma M, Fujiki H, Suguri H, Yoshizawa S, Hirota M, Nakayasu M, et al. Okadaic acid: an additional non-phorbol-12-tetradecanoate-13-acetate-type tumor promoter. Proc Natl Acad Sci USA. 1988;85:1768–71. doi: 10.1073/pnas.85.6.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kumagai A, Dunphy WG. Regulation of Xenopus Cdc25 protein. Methods Enzymol. 1997;283:564–71. doi: 10.1016/S0076-6879(97)83044-3. [DOI] [PubMed] [Google Scholar]
- 96.Harvey SL, Enciso G, Dephoure N, Gygi SP, Gunawardena J, Kellogg DR. A phosphatase threshold sets the level of Cdk1 activity in early mitosis in budding yeast. Mol Biol Cell. 2011;22:3595–608. doi: 10.1091/mbc.E11-04-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kinoshita K, Nemoto T, Nabeshima K, Kondoh H, Niwa H, Yanagida M. The regulatory subunits of fission yeast protein phosphatase 2A (PP2A) affect cell morphogenesis, cell wall synthesis and cytokinesis. Genes Cells. 1996;1:29–45. doi: 10.1046/j.1365-2443.1996.02002.x. [DOI] [PubMed] [Google Scholar]
- 98.Yang H, Jiang W, Gentry M, Hallberg RL. Loss of a protein phosphatase 2A regulatory subunit (Cdc55p) elicits improper regulation of Swe1p degradation. Mol Cell Biol. 2000;20:8143–56. doi: 10.1128/MCB.20.21.8143-8156.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mueller PR, Coleman TR, Dunphy WG. Cell cycle regulation of a Xenopus Wee1-like kinase. Mol Biol Cell. 1995;6:119–34. doi: 10.1091/mbc.6.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chiroli E, Rossio V, Lucchini G, Piatti S. The budding yeast PP2ACdc55 protein phosphatase prevents the onset of anaphase in response to morphogenetic defects. J Cell Biol. 2007;177:599–611. doi: 10.1083/jcb.200609088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yasutis K, Vignali M, Ryder M, Tameire F, Dighe SA, Fields S, et al. Zds2p regulates Swe1p-dependent polarized cell growth in Saccharomyces cerevisiae via a novel Cdc55p interaction domain. Mol Biol Cell. 2010;21:4373–86. doi: 10.1091/mbc.E10-04-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gharbi-Ayachi A, Labbé JC, Burgess A, Vigneron S, Strub JM, Brioudes E, et al. The substrate of Greatwall kinase, Arpp19, controls mitosis by inhibiting protein phosphatase 2A. Science. 2010;330:1673–7. doi: 10.1126/science.1197048. [DOI] [PubMed] [Google Scholar]
- 103.Mochida S, Maslen SL, Skehel M, Hunt T. Greatwall phosphorylates an inhibitor of protein phosphatase 2A that is essential for mitosis. Science. 2010;330:1670–3. doi: 10.1126/science.1195689. [DOI] [PubMed] [Google Scholar]
- 104.Wicky S, Tjandra H, Schieltz D, Yates J, 3rd, Kellogg DR. The Zds proteins control entry into mitosis and target protein phosphatase 2A to the Cdc25 phosphatase. Mol Biol Cell. 2011;22:20–32. doi: 10.1091/mbc.E10-06-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Castilho PV, Williams BC, Mochida S, Zhao Y, Goldberg ML. The M phase kinase Greatwall (Gwl) promotes inactivation of PP2A/B55δ, a phosphatase directed against CDK phosphosites. Mol Biol Cell. 2009;20:4777–89. doi: 10.1091/mbc.E09-07-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yu J, Fleming SL, Williams B, Williams EV, Li Z, Somma P, et al. Greatwall kinase: a nuclear protein required for proper chromosome condensation and mitotic progression in Drosophila. J Cell Biol. 2004;164:487–92. doi: 10.1083/jcb.200310059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tsuchiya E, Matsuzaki G, Kurano K, Fukuchi T, Tsukao A, Miyakawa T. The Saccharomyces cerevisiae SSD1 gene is involved in the tolerance to high concentration of Ca2+ with the participation of HST1/NRC1/BFR1. Gene. 1996;176:35–8. doi: 10.1016/0378-1119(96)00204-1. [DOI] [PubMed] [Google Scholar]
- 108.Bi E, Pringle JR. ZDS1 and ZDS2, genes whose products may regulate Cdc42p in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:5264–75. doi: 10.1128/mcb.16.10.5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yu Y, Jiang YW, Wellinger RJ, Carlson K, Roberts JM, Stillman DJ. Mutations in the homologous ZDS1 and ZDS2 genes affect cell cycle progression. Mol Cell Biol. 1996;16:5254–63. doi: 10.1128/mcb.16.10.5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yasutis K. Zds2p: Negative regulator of the Swe1p-dependent G2/M checkpoint. Thesis PhD - University of Virginia 2011. [Google Scholar]
- 111.Ma XJ, Lu Q, Grunstein M. A search for proteins that interact genetically with histone H3 and H4 amino termini uncovers novel regulators of the Swe1 kinase in Saccharomyces cerevisiae. Genes Dev. 1996;10:1327–40. doi: 10.1101/gad.10.11.1327. [DOI] [PubMed] [Google Scholar]
- 112.Queralt E, Uhlmann F. Separase cooperates with Zds1 and Zds2 to activate Cdc14 phosphatase in early anaphase. J Cell Biol. 2008;182:873–83. doi: 10.1083/jcb.200801054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rossio V, Yoshida S. Spatial regulation of Cdc55-PP2A by Zds1/Zds2 controls mitotic entry and mitotic exit in budding yeast. J Cell Biol. 2011;193:445–54. doi: 10.1083/jcb.201101134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.McCusker D, Denison C, Anderson S, Egelhofer TA, Yates JR, 3rd, Gygi SP, et al. Cdk1 coordinates cell-surface growth with the cell cycle. Nat Cell Biol. 2007;9:506–15. doi: 10.1038/ncb1568. [DOI] [PubMed] [Google Scholar]
- 115.Nonaka H, Tanaka K, Hirano H, Fujiwara T, Kohno H, Umikawa M, et al. A downstream target of RHO1 small GTP-binding protein is PKC1, a homolog of protein kinase C, which leads to activation of the MAP kinase cascade in Saccharomyces cerevisiae. EMBO J. 1995;14:5931–8. doi: 10.1002/j.1460-2075.1995.tb00281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kamada Y, Qadota H, Python CP, Anraku Y, Ohya Y, Levin DE. Activation of yeast protein kinase C by Rho1 GTPase. J Biol Chem. 1996;271:9193–6. doi: 10.1074/jbc.271.16.9193. [DOI] [PubMed] [Google Scholar]
- 117.Mizunuma M, Hirata D, Miyakawa T. Implication of Pkc1p protein kinase C in sustaining Cln2p level and polarized bud growth in response to calcium signaling in Saccharomyces cerevisiae. J Cell Sci. 2005;118:4219–29. doi: 10.1242/jcs.02535. [DOI] [PubMed] [Google Scholar]
- 118.Zanelli CF, Valentini SR. Pkc1 acts through Zds1 and Gic1 to suppress growth and cell polarity defects of a yeast eIF5A mutant. Genetics. 2005;171:1571–81. doi: 10.1534/genetics.105.048082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sekiya-Kawasaki M, Abe M, Saka A, Watanabe D, Kono K, Minemura-Asakawa M, et al. Dissection of upstream regulatory components of the Rho1p effector, 1,3-β-glucan synthase, in Saccharomyces cerevisiae. Genetics. 2002;162:663–76. doi: 10.1093/genetics/162.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yakura M, Ozoe F, Ishida H, Nakagawa T, Tanaka K, Matsuda H, et al. zds1, a novel gene encoding an ortholog of Zds1 and Zds2, controls sexual differentiation, cell wall integrity and cell morphology in fission yeast. Genetics. 2006;172:811–25. doi: 10.1534/genetics.105.050906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li Z, Sun Z, Li D, Pan J, Zhu X. Identification of a Zds-like gene ZDS3 as a new mediator of stress resistance, capsule formation and virulence of the human pathogenic yeast Cryptococcus neoformans. FEMS Yeast Res. 2011;11:529–39. doi: 10.1111/j.1567-1364.2011.00744.x. [DOI] [PubMed] [Google Scholar]
- 122.Schmidt M, Drgon T, Bowers B, Cabib E. Hyperpolarized growth of Saccharomyces cerevisiae cak1P212S and cla4 mutants weakens cell walls and renders cells dependent on chitin synthase 3. FEMS Yeast Res. 2008;8:362–73. doi: 10.1111/j.1567-1364.2008.00368.x. [DOI] [PubMed] [Google Scholar]
- 123.Hartwell LH, Culotti J, Reid B. Genetic control of the cell-division cycle in yeast. I. Detection of mutants. Proc Natl Acad Sci USA. 1970;66:352–9. doi: 10.1073/pnas.66.2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lohka MJ, Hayes MK, Maller JL. Purification of maturation-promoting factor, an intracellular regulator of early mitotic events. Proc Natl Acad Sci USA. 1988;85:3009–13. doi: 10.1073/pnas.85.9.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lew DJ, Dulić V, Reed SI. Isolation of three novel human cyclins by rescue of G1 cyclin (Cln) function in yeast. Cell. 1991;66:1197–206. doi: 10.1016/0092-8674(91)90042-W. [DOI] [PubMed] [Google Scholar]
- 126.Xiong Y, Connolly T, Futcher B, Beach D. Human D-type cyclin. Cell. 1991;65:691–9. doi: 10.1016/0092-8674(91)90100-D. [DOI] [PubMed] [Google Scholar]
- 127.Koff A, Cross F, Fisher A, Schumacher J, Leguellec K, Philippe M, et al. Human cyclin E, a new cyclin that interacts with two members of the CDC2 gene family. Cell. 1991;66:1217–28. doi: 10.1016/0092-8674(91)90044-Y. [DOI] [PubMed] [Google Scholar]
- 128.Evans T, Rosenthal ET, Youngblom J, Distel D, Hunt T. Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell. 1983;33:389–96. doi: 10.1016/0092-8674(83)90420-8. [DOI] [PubMed] [Google Scholar]
- 129.Murray AW, Solomon MJ, Kirschner MW. The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature. 1989;339:280–6. doi: 10.1038/339280a0. [DOI] [PubMed] [Google Scholar]
- 130.Murray AW, Kirschner MW. Cyclin synthesis drives the early embryonic cell cycle. Nature. 1989;339:275–80. doi: 10.1038/339275a0. [DOI] [PubMed] [Google Scholar]
- 131.Glotzer M, Murray AW, Kirschner MW. Cyclin is degraded by the ubiquitin pathway. Nature. 1991;349:132–8. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- 132.Talarek N, Cameroni E, Jaquenoud M, Luo X, Bontron S, Lippman S, et al. Initiation of the TORC1-regulated G0 program requires Igo1/2, which license specific mRNAs to evade degradation via the 5′-3′ mRNA decay pathway. Mol Cell. 2010;38:345–55. doi: 10.1016/j.molcel.2010.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bontron S, Jaquenoud M, Vaga S, Talarek N, Bodenmiller B, Aebersold R, et al. Yeast endosulfines control entry into quiescence and chronological life span by inhibiting protein phosphatase 2A. Cell Rep. 2013;3:16–22. doi: 10.1016/j.celrep.2012.11.025. [DOI] [PubMed] [Google Scholar]
- 134.Kim MY, Bucciarelli E, Morton DG, Williams BC, Blake-Hodek K, Pellacani C, et al. Bypassing the Greatwall-Endosulfine pathway: plasticity of a pivotal cell-cycle regulatory module in Drosophila melanogaster and Caenorhabditis elegans. Genetics. 2012;191:1181–97. doi: 10.1534/genetics.112.140574. [DOI] [PMC free article] [PubMed] [Google Scholar]