Telomerase reverse transcriptase (TERT) activities are frequently upregulated in human cancers, which is thought to be an important mechanism contributing to human tumorigenesis.1,2 Mutations in the TERT promoter were recently reported in melanomas.3,4 Two of them, 1,295,228 C > T and 1,295,250 C > T (termed C228T and C250T, respectively), were particularly common. These mutations were found to be absent in benign tumors and normal human population. Here, we investigated these mutations in bladder cancer and glioblastoma. Use of primary bladder cancer and glioblastoma tissues was based on institutional review board-approved protocols. Genomic DNA from tumor tissues was isolated using standard procedures of protease K digestion, phenol-chloroform extraction and ethanol precipitation. A fragment of the TERT promoter was amplified by polymerase chain reaction (PCR) using primers 5′ AGTGGATTCGCGGGCACAGA 3′(sense) and 5′ CAGCGCTGCCTGAAACTC 3′(antisense), resulting in a PCR product of 235 bp, which contained the sites of C228T and C250T mutations. Amplification PCR was performed with an initial denaturation at 95°C for 3 min, followed by 10 cycles of 95°C denaturation for 30 sec, 55°C annealing for 30 sec and 68°C elongation for 1 min. This was followed by 30 cycles of the same settings except for the elongation for additional 5 sec in each cycle. Quality of PCR products was confirmed by gel electrophoresis. Sequencing PCR was performed using a Big Dye terminator v3.1 cycle sequencing ready reaction kit (Applied Biosystems) and an ABI PRISM 3730 automated next generation genetic analyzer (Applied Biosystems). Mutations were confirmed by repeating amplification PCR and using both primers in the Big Dye sequencing.
As summarized in Table 1, we found highly prevalent TERT promoter mutations in bladder cancer, bladder cancer cell lines and glioblastoma. C228T was far more common than C250T in all cases. Specifically, we found C228T in 81% (42/52) of bladder cancer samples and C250T in 4% (2/52) of samples. The two mutations were mutually exclusive in bladder cancer and were collectively found in 85% (44/52) of samples. C228T was found in 88% (7/8) of bladder cancer cell lines, and no C250T was found in these cell clines. We found C228T in 65% (48/74) of glioblastoma samples and C250T in 19% (14/74) of samples. The two mutations were also mutually exclusive in glioblastomas and were collectively found in 84% (62/74) of samples.
Table 1. TERT promoter mutations in bladder cancer and glioblastoma.
| Tumor types | TERT promoter mutations | ||
|---|---|---|---|
| |
1,295,228 C>T (C228T) n/N (%) |
1,295,250 C>T (C250T) n/N (%) |
Overall n/N (%) |
|
Bladder cancer |
42/52 (80.77) |
2/52 (3.85) |
44/52 (84.62) |
|
Bladder cancer cell lines |
7/8 (87.50) |
0/8 (0.00) |
7/8 (87.50) |
| Glioblastoma | 48/74 (64.86) | 14/74 (18.92) | 62/74 (83.78) |
During this study, a report came online also showing common TERT promoter mutations in bladder cancer and glioblastoma,5 consistent with our findings. TERT promoter mutations were examined in a small number (21 cases) of bladder cancer samples in that study.5 Our study on a large number of cases demonstrated a high prevalence of TERT promoter mutations in bladder cancer, establishing the common occurrence of these mutations in bladder cancer. This prevalence of 85% is unusually high for somatic mutations in any human cancer. We also found a higher prevalence of TERT promoter mutations in glioblastoma than that in the recent report,5 which, like the prevalence of TERT promoter mutations in bladder cancer, is also unusually high for somatic mutations in any human cancer. Our finding of the high prevalence of TERT promoter mutations in bladder cancer and glioblastoma helps establish the common occurrence of this genetic alteration in the two cancers. Given the high prevalence, it is safe to assume that TERT promoter mutations play an important role in the tumorigenesis and pathogenesis of bladder cancer and glioblastoma.
With the high prevalence, testing of TERT promoter mutations for the diagnosis of bladder cancer and glioblastoma would be expected to have a high sensitivity. As these mutations are only found in cancers,3-5 testing of TERT promoter mutations are likely to be also highly specific for bladder cancer and glioblastoma. Both the C228T and C250T mutations create an 11-base nucleotide stretch 5′-CCCCTTCCGGG-3′, which contains a consensus binding site, GGAA (in reverse complement), for ETS transcription factors. This has strong functional implications for these mutations; it is expected that these TERT promoter mutations would lead to upregulation of TERT through the action of ETS transcript factors in bladder cancer and glioblastoma. Indeed, the two mutations were demonstrated to confer upon the TERT promoter increased transcriptional activity.3,4 As such, these TERT mutations may be novel prognostic factors and therapeutic targets for bladder cancer and glioblastoma. Thus, the discovery of these TERT promoter mutations has strong clinical implications for the development of novel diagnostic, prognostic and therapeutic strategies for bladder cancer and glioblastoma as well as other cancers that may harbor these mutations. (Fig. 1)
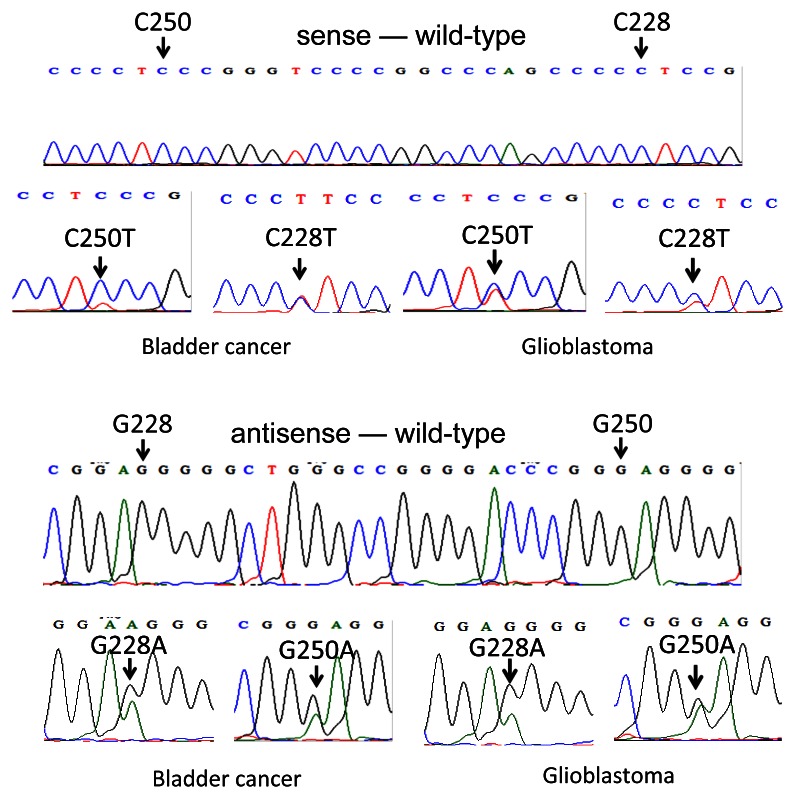
Figure 1.TERT promoter mutations in bladder cancer and glioblastoma. Shown are representative electropherograms of the wild-type TERT promoter and two TERT promoter mutations as indicated for bladder cancer and glioblastoma. The upper portion of the figure shows electropherograms of the sense sequences of the wild-type DNA and the nucleotide changes of the two mutations in the two cancers. The lower portion of the figure shows the electropherograms of the antisense sequences of the wild-type DNA and the nucleotide changes of the two mutations in the two cancers.
Acknowledgments
This study was supported by United States National Institutes of Health grant R01CA134225 to M. Xing.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/24662
References
- 1.Smekalova EM, Shubernetskaya OS, Zvereva MI, Gromenko EV, Rubtsova MP, Dontsova OA. Telomerase RNA biosynthesis and processing. Biochemistry (Mosc) 2012;77:1120–8. doi: 10.1134/S0006297912100045. [DOI] [PubMed] [Google Scholar]
- 2.Mocellin S, Pooley KA, Nitti D. Telomerase and the search for the end of cancer. Trends Mol Med. 2013;19:125–33. doi: 10.1016/j.molmed.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Huang FW, Hodis E, Xu MJ, Kryukov GV, Chin L, Garraway LA. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339:957–9. doi: 10.1126/science.1229259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horn S, Figl A, Rachakonda PS, Fischer C, Sucker A, Gast A, et al. TERT promoter mutations in familial and sporadic melanoma. Science. 2013;339:959–61. doi: 10.1126/science.1230062. [DOI] [PubMed] [Google Scholar]
- 5.Killela PJ, Reitman ZJ, Jiao Y, Bettegowda C, Agrawal N, Diaz LA, Jr., et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci USA. 2013;110:6021–6. doi: 10.1073/pnas.1303607110. [DOI] [PMC free article] [PubMed] [Google Scholar]