Acetyl-CoA carboxylase (ACACA) is the rate-limiting enzyme in the biogenesis of long-chain fatty acids. Previous studies in yeast have shown that disruption of the ACACA gene impairs nuclear division, with ACACA-defective yeast cells developing large undivided nuclei and showing abnormally shortened mitotic spindles that lastly result in aberrant mitosis and cell cycle arrest at G2/M;1,2 these findings suggested that ACACA might have an essential role in the progression of cell division. Moreover, the addition of a mixture of long-chain fatty acids, the final product of the endogenous fatty acid synthesis, failed to overcome the cell cycle arrest, additionally suggesting that the role of ACACA in cell division may be independent of its well-recognized biosynthetic function or, alternatively, an strict coupling between lipogenesis and cell cycle progression. More recent studies have described that mitotic mammalian cells exhibit a significant increase in the serine 79-phosphorylated form of ACACA (phospho-ACACASer79) compared with cells in interphase.3 It should be noted that, although the acute control of ACACA enzymatic activity is the product of integrated changes in substrate supply and allosteric ligands, the phosphorylation of multiple serine residues by other proteins is the ACACA's primary short-term regulatory mechanism. Among these proteins, it is well-known that the cellular fuel gauge and master metabolic regulator AMP-activated protein kinase (AMPK) phosphorylates ACACA on serine-79 to cause the inhibition of the ACACA enzymatic activity. Importantly, the mitosis-related enhancement of phospho-ACACASer79 is attenuated in the presence of compound C, an AMPK inhibitor, thus implying that AMPK may phosphorylate ACACA when cells enter mitosis.3
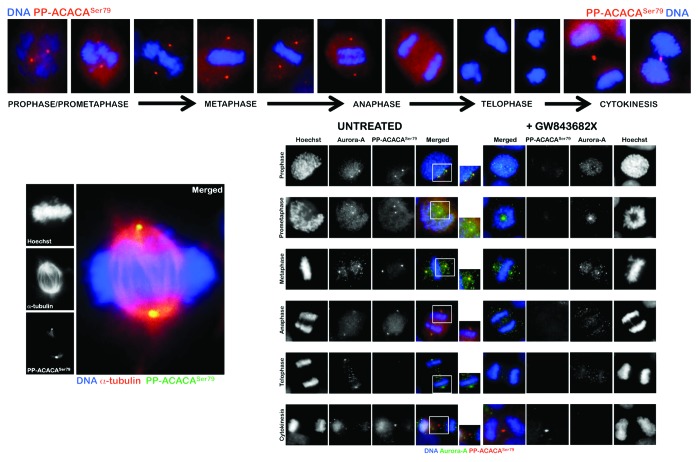
Our group has previously demonstrated that the activated form of the α-catalytic subunit of AMPK (phospho-AMPKαThr172) displays a highly dynamic localization during the different phases of cell division. Threonine172-phosphorylated AMPKα transiently associates with several mitotic structures, including centrosomes, spindle poles, the central spindle midzone and the midbody throughout all of the mitotic stages and cytokinesis;4,5 other studies have further identified a network of proteins involved in mitosis that are substrates of AMPK.6 Indeed, it has been unambiguously confirmed that threonine172-phosphorylated AMPKα localizes to the mitotic spindle poles and increases when cells enter mitosis;7 the mitotic AMPK activity appears to be essential for normal spindle orientation, and when it is defective, mitosis does not proceed efficiently. In this scenario, we envisioned that the mitosis-associated phosphorylated status of ACACA, a downstream target of AMPK, could also be explained in terms of a previously unrecognized ability of phospho-ACACA to directly associate with the mitotic/cytokinetic apparatus during cell division. Using an automated-confocal imaging system for high-resolution images and 3D reconstructions, we have recently explored the spatio-temporal dynamic distribution of phospho-ACACASer79 during mitosis and cytokinesis. Interestingly, phospho-ACACASer79 was found to display a distinct punctuate staining during chromosome condensation from prophase to metaphase, then almost disappearing from early anaphase to late telophase during chromatid separation and, finally, reappearing at the constriction ring until the end of the furrowing process through to completion of cytokinesis (Fig. 1, upper). Because subcellular areas corresponding to the localizations and patterns of centrosomes appeared to be stained with phospho-ACACASer79 (Fig. 1, lower-left), we sought to confirm a centrosomal-like localization of phosho-ACACASer79 by performing co-localization analyses with the mitotic kinase Aurora A, a specific marker for centrosomes (Fig. 1, lower-right). There was a notable co-localization of Aurora A and phospho-ACACASer79 in the duplicated centrosomes in cells in prophase, which suggested an early localization of phospho-ACACASer79 in the centrosome at the onset of the mitotic process. As the cells progressed through mitosis, the staining and overlapping of Aurora A with phospho-ACACASer79 continued to be observed at the spindle poles. Phospho-ACACASer79 remained associated to some extent with Aurora A at the spindle poles during anaphase when chromatids are pulled apart and start migrating towards the poles. Phospho-ACACASer79 abandoned its Aurora A-like centrosomal localization during anaphase-telophase transition, and there was no longer co-localization during telophase and cytokinesis. Although the spatio-temporal dynamics of mitotic phospho-ACACASer79 notably recapitulated that of phospho-AMPKαThr172 during early mitosis,4,5 it should be noted that a key feature of the mitotic behaviour of phospho-AMPKαThr172 relates to its compaction to the midzone of the central spindle/nascent midbody during late anaphase/early telophase transition. In late telophase, loss of staining of phospho-AMPKαThr172 and of co-localization with the centrosomal marker Aurora A occurs at the poles and phospho-AMPKαThr172 become further concentrated at the junction between the two daughter cells, thus suggesting a similar but not identical subcellular re-localization of phospho-AMPKαThr172 to that occupied by bona fide chromosomal passenger proteins (CPPs). When phospho-ACACASer79 abandons its Aurora A-like centrosomal localization during late metaphase transition, however, the mitotic staining of phospho-ACACASer79 largely disappears to become reactivated exclusively at the cleavage furrow during cytokinesis (Fig. 1 upper, lower-left). At the completion of telophase, a phospho-AMPKαThr172-like staining of phospho-ACACASer79 as a doublet-like structure can be observed on either side of the midbody within the intercellular cytokinetic bridge.
Figure 1. Spatio-temporal dynamics of phospho-ACACASer79 during mitosis and cytokinesis. After fixation and permeabilization of asynchronously growing PC-9 lung carcinoma cells in 96-well clear-bottom imaging tissue culture plates (Becton Dickinson Biosciences) optimized for automated imaging applications, cells were stained with antibodies against phospho-ACACASer79 (PP-ACACASer79), α-tubulin, Aurora A and/or with Hoechst 33258 for DNA counterstaining, as specified. The figures show representative portions of images containing dividing cells that were captured with a 20× objective (NA 075 Olympus) in different channels for Alexa Fluor® 488 (pseudo-colored red), Alexa Fluor® 594 (pseudo-colored green) and Hoechst 33258 (pseudo-colored blue) on a BD Pathway™ 855 Bioimager System (Becton Dickinson Biosciences). Merged images were obtained according to the Recommended Assay Procedure using BD Attovision™ software. The rectangular regions (white line) are enlarged and shown as high magnification insets in the right panels. The following antibodies were used in this study: rabbit polyclonal anti-phospho-Acetyl-CoA Carboxylase (Ser79) Antibody#3661 (Cell Signaling Technology®) and mouse mAb anti-Aurora A (purified mouse anti-IAK1; BD Transduction Laboratories™, Cat. No. 610939).
To evaluate whether mitosis-specific activators might permit mitotic apparatus-bound phospho-ACACASer79 to operate independently of cellular energy status, we took advantage of our recently discovered link between Polo-like kinase 1 (PLK1) activity and the mitotic phosphorylation of AMPKα.5 PLK1 and phospho-AMPKαThr172 exhibit a major spatio-temporal mitotic overlap at centrosomes from prophase until anaphase and at the midbody during telophase and cytokinesis; importantly, short-term treatment with GW843682X (compound 1), a thiophene benzimidazole ATP-competitive inhibitor of PLK1, is sufficient to largely prevent the localization of phospho-AMPKαThr172 to several mitotic and cytokinetic structures independently of glucose availability. When we explored whether the activation status of PLK1 might impact on the mitotic dynamics of phospho-ACACASer79, we observed that treatment with GW843682X fully abolished centrosomal but not cytokinetic activation of phospho-ACACASer79 (Fig. 1 lower-left), thus mimicking the causal link between PLK1 activity and the mitotic phosphorylation of AMPKα solely during early mitosis.
Our results reveal for the first time that the Serine 79-phosphorylated and metabolically inactive form of the ACACA is relocated at centrosomes when cells enter mitosis and the nuclear envelop breakdown. Furthermore, we have observed that inhibition of PLK1 activity abrogates mitotic activation of P-ACCSer79 as it does with phospho-AMPKαThr172 at the centrosomes, thus suggesting that the tight relationship between the phosphorylation and activation status of the phospho-AMPKα (active)/phospho-ACACA (inactive) tandem is spatially and temporally conserved, through direct or indirect mechanisms, during the mitotic process. Multi-faceted PLK1 directs centrosome maturation and spindle formation, regulates the assembly of the bipolar spindle by promoting chromosome attachments to spindle microtubules. On the other hand, PLK1 is a well-recognized positive regulator of cytokinesis that localizes at the midbody during telophase and cytokinesis. Because PLK1 inhibition efficiently suppresses the occurrence of phospho-AMPKαThr178 and phospho-ACACASer79 at the centrosomes, and given that the position of the cytokinesis furrow is specified by the position of the mitotic spindle, forthcoming studies should evaluate a new role of the AMPK → ACACA axis in regulating mitotic spindle orientation and/or ensuring that cytokinesis occurs at the proper place and time. Our findings also raise an intriguing scenario when considering that the protein encoded by BRCA1, the first susceptibility human gene associated with breast and ovarian cancer, combines not only with other tumor suppressors, DNA damage sensors and signal transducers to form a large multi-subunit protein complex known as the BRCA1-associated genome surveillance complex (BASC), but also with the Ser79-phosphorylated form of ACACA, preventing its dephosphorylation and inhibiting lipogenesis.8 Of note, BRCA1 is a DNA damage response protein that functions not only in the nucleus to stimulate DNA repair but also at the centrosome to inhibit centrosome overduplication in response to DNA damage.9 Because lipid biosynthesis is closely coordinated with cell cycle progression (e.g., phospholipid biosynthesis is greatest during G1 and S phases in preparation for cell division) and the loss or mutation of BRCA1 causes centrosome amplification and abnormal mitotic spindle assembly in cancer cells, forthcoming studies should be designated to unambiguously establish whether BRCA1’s ability to stabilize the phosphorylated form of ACACA might causally contribute to centrosome and/or mitotic spindle structural integrity, thus linking an ever-growing family of “metabo-mitotic sensors” with BRCA1-regulated genomic instability.
Acknowledgements
This work was financially supported by the Instituto de Salud Carlos III (Ministerio de Sanidad y Consumo, Fondo de Investigación Sanitaria (FIS), Spain, grants CP05-00090, PI06-0778 and RD06-0020-0028), the Fundación Científica de la Asociación Española Contra el Cáncer (AECC, Spain) and the Ministerio de Ciencia e Innovación (SAF2009-11579, Plan Nacional de I+D+ I, MICINN). Alejandro Vazquez-Martin received a Sara Borrell post-doctoral contract (CD08/00283, Ministerio de Sanidad y Consumo, Fondo de Investigación Sanitaria -FIS-). Sílvia Cufí received a research fellowship (Formación de Personal Investigador, FPI) from the Ministerio de Ciencia e Innovación (MICINN).
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/24700
References
- 1.Saitoh S, Takahashi K, Nabeshima K, Yamashita Y, Nakaseko Y, Hirata A, et al. Aberrant mitosis in fission yeast mutants defective in fatty acid synthetase and acetyl CoA carboxylase. J Cell Biol. 1996;134:949–61. doi: 10.1083/jcb.134.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Feel W, DeMar JC, Wakil SJ. A Saccharomyces cerevisiae mutant strain defective in acetyl-CoA carboxylase arrests at the G2/M phase of the cell cycle. Proc Natl Acad Sci USA. 2003;100:3095–100. doi: 10.1073/pnas.0538069100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mao L, Li N, Guo Y, Xu X, Gao L, Xu Y, et al. AMPK phosphorylates GBF1 for mitotic Golgi disassembly. J Cell Sci. 2013 doi: 10.1242/jcs.121954. [DOI] [PubMed] [Google Scholar]
- 4.Vazquez-Martin A, Oliveras-Ferraros C, Menendez JA. The active form of the metabolic sensor: AMP-activated protein kinase (AMPK) directly binds the mitotic apparatus and travels from centrosomes to the spindle midzone during mitosis and cytokinesis. Cell Cycle. 2009;8:2385–98. doi: 10.4161/cc.8.15.9082. [DOI] [PubMed] [Google Scholar]
- 5.Vazquez-Martin A, Oliveras-Ferraros C, Cufí S, Menendez JA. Polo-like kinase 1 regulates activation of AMP-activated protein kinase (AMPK) at the mitotic apparatus. Cell Cycle. 2011;10:1295–302. doi: 10.4161/cc.10.8.15342. [DOI] [PubMed] [Google Scholar]
- 6.Banko MR, Allen JJ, Schaffer BE, Wilker EW, Tsou P, White JL, et al. Chemical genetic screen for AMPKα2 substrates uncovers a network of proteins involved in mitosis. Mol Cell. 2011;44:878–92. doi: 10.1016/j.molcel.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thaiparambil JT, Eggers CM, Marcus AI. AMPK regulates mitotic spindle orientation through phosphorylation of myosin regulatory light chain. Mol Cell Biol. 2012;32:3203–17. doi: 10.1128/MCB.00418-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magnard C, Bachelier R, Vincent A, Jaquinod M, Kieffer S, Lenoir GM, et al. BRCA1 interacts with acetyl-CoA carboxylase through its tandem of BRCT domains. Oncogene. 2002;21:6729–39. doi: 10.1038/sj.onc.1205915. [DOI] [PubMed] [Google Scholar]
- 9.Tarapore P, Hanashiro K, Fukasawa K. Analysis of centrosome localization of BRCA1 and its activity in suppressing centrosomal aster formation. Cell Cycle. 2012;11:2931–46. doi: 10.4161/cc.21396. [DOI] [PMC free article] [PubMed] [Google Scholar]