Abstract
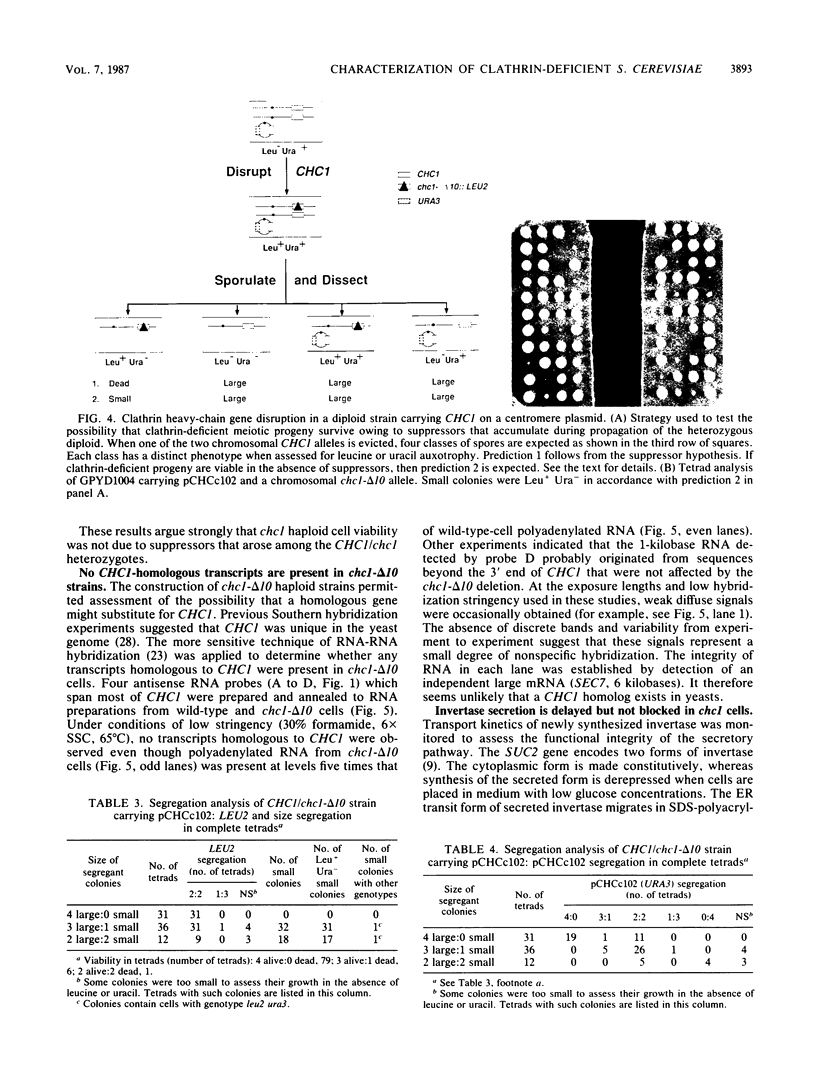
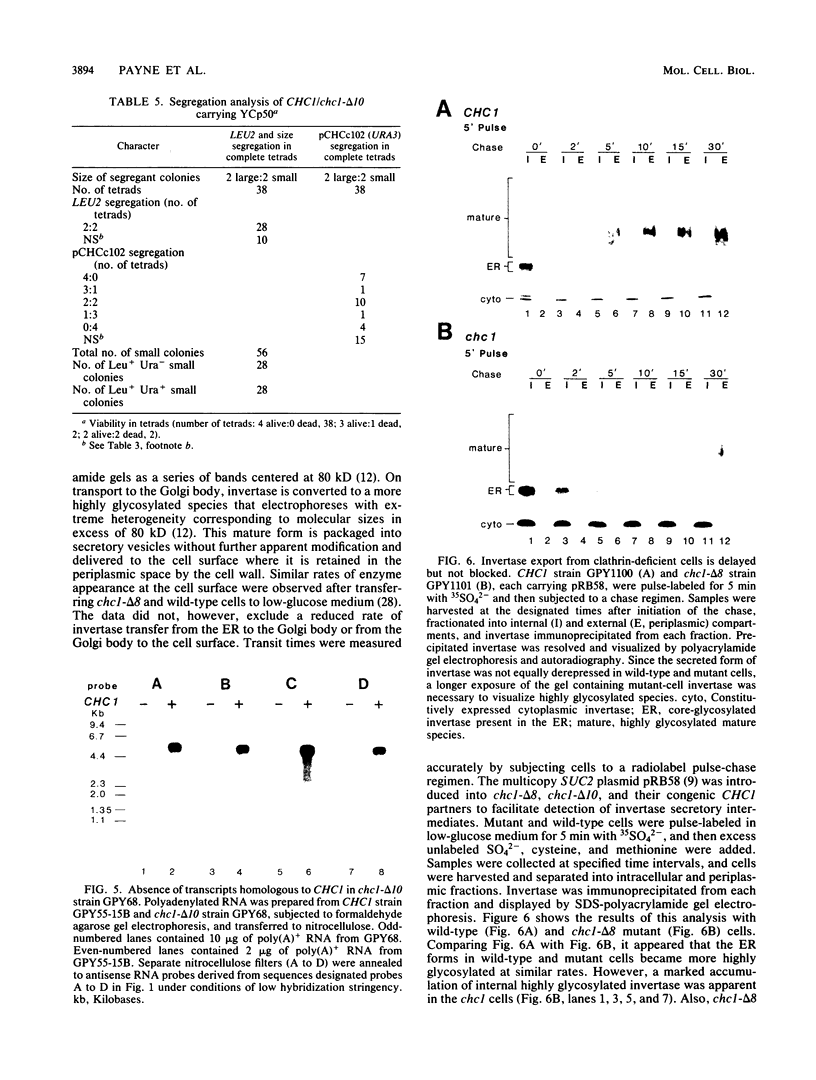
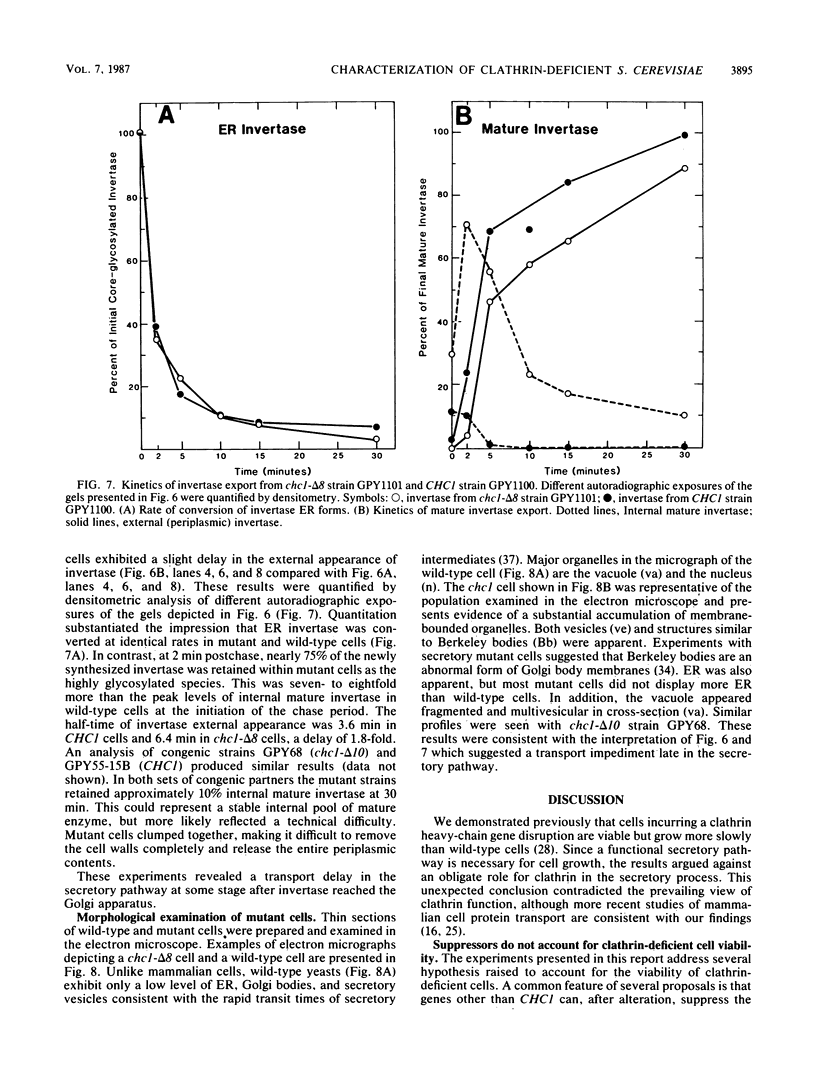
Clathrin is important but not essential for yeast cell growth and protein secretion. Diploid Saccharomyces cerevisiae cells heterozygous for a clathrin heavy-chain gene (CHC1) disruption give rise to viable, slow-growing, clathrin heavy-chain-deficient meiotic progeny (G. Payne and R. Schekman, Science 230:1009-1014, 1985). The possibility that extragenic suppressors account for growth of clathrin-deficient cells was examined by deletion of CHC1 from haploid cell genomes by single-step gene transplacement and independently by introduction of a centromere plasmid carrying the complete CHC1 gene into diploid cells before eviction of a chromosomal CHC1 locus and subsequent tetrad analysis. Both approaches yielded clathrin-deficient haploid strains. In mutants missing at least 95% of the CHC1 coding domain, transcripts related to CHC1 were not detected. The time course of invertase modification and secretion was measured to assess secretory pathway functions in the viable clathrin-deficient cells. Core-glycosylated invertase was converted to the mature, highly glycosylated form at equivalent rates in mutant and wild-type cells. Export of mature invertase from mutant cells was delayed but not prevented. Abnormal vacuoles, accumulated vesicles, and Golgi body-derived structures were visualized in mutant cells by electron microscopy. We conclude that extragenic suppressors do not account for the viability of clathrin-deficient cells and, furthermore, that many standard laboratory strains can sustain a CHC1 disruption. Clathrin does not appear to mediate protein transfer from the endoplasmic reticulum to the Golgi body but may function at a later stage of the secretory pathway.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benson R. J., Porter-Jordan K., Buoniconti P., Fine R. E. Biochemical and cytochemical evidence indicates that coated vesicles in chick embryo myotubes contain newly synthesized acetylcholinesterase. J Cell Biol. 1985 Nov;101(5 Pt 1):1930–1940. doi: 10.1083/jcb.101.5.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein M., Hoffmann W., Ammerer G., Schekman R. Characterization of a gene product (Sec53p) required for protein assembly in the yeast endoplasmic reticulum. J Cell Biol. 1985 Dec;101(6):2374–2382. doi: 10.1083/jcb.101.6.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botstein D., Falco S. C., Stewart S. E., Brennan M., Scherer S., Stinchcomb D. T., Struhl K., Davis R. W. Sterile host yeasts (SHY): a eukaryotic system of biological containment for recombinant DNA experiments. Gene. 1979 Dec;8(1):17–24. doi: 10.1016/0378-1119(79)90004-0. [DOI] [PubMed] [Google Scholar]
- Brill S. J., DiNardo S., Voelkel-Meiman K., Sternglanz R. Need for DNA topoisomerase activity as a swivel for DNA replication for transcription of ribosomal RNA. 1987 Mar 26-Apr 1Nature. 326(6111):414–416. doi: 10.1038/326414a0. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Byers B., Goetsch L. Behavior of spindles and spindle plaques in the cell cycle and conjugation of Saccharomyces cerevisiae. J Bacteriol. 1975 Oct;124(1):511–523. doi: 10.1128/jb.124.1.511-523.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carle G. F., Olson M. V. An electrophoretic karyotype for yeast. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3756–3760. doi: 10.1073/pnas.82.11.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson M., Botstein D. Two differentially regulated mRNAs with different 5' ends encode secreted with intracellular forms of yeast invertase. Cell. 1982 Jan;28(1):145–154. doi: 10.1016/0092-8674(82)90384-1. [DOI] [PubMed] [Google Scholar]
- Chan R. K., Otte C. A. Isolation and genetic analysis of Saccharomyces cerevisiae mutants supersensitive to G1 arrest by a factor and alpha factor pheromones. Mol Cell Biol. 1982 Jan;2(1):11–20. doi: 10.1128/mcb.2.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Esmon B., Novick P., Schekman R. Compartmentalized assembly of oligosaccharides on exported glycoproteins in yeast. Cell. 1981 Aug;25(2):451–460. doi: 10.1016/0092-8674(81)90063-5. [DOI] [PubMed] [Google Scholar]
- Fine R. E., Ockleford C. D. Supramolecular cytology of coated vesicles. Int Rev Cytol. 1984;91:1–43. doi: 10.1016/s0074-7696(08)61313-3. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S., Anderson R. G., Russell D. W., Schneider W. J. Receptor-mediated endocytosis: concepts emerging from the LDL receptor system. Annu Rev Cell Biol. 1985;1:1–39. doi: 10.1146/annurev.cb.01.110185.000245. [DOI] [PubMed] [Google Scholar]
- Griffiths G., Pfeiffer S., Simons K., Matlin K. Exit of newly synthesized membrane proteins from the trans cisterna of the Golgi complex to the plasma membrane. J Cell Biol. 1985 Sep;101(3):949–964. doi: 10.1083/jcb.101.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison S. C., Kirchhausen T. Clathrin, cages, and coated vesicles. Cell. 1983 Jul;33(3):650–652. doi: 10.1016/0092-8674(83)90007-7. [DOI] [PubMed] [Google Scholar]
- Hinnen A., Hicks J. B., Fink G. R. Transformation of yeast. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1929–1933. doi: 10.1073/pnas.75.4.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. L., Campbell J. L. Cloning of Saccharomyces cerevisiae DNA replication genes: isolation of the CDC8 gene and two genes that compensate for the cdc8-1 mutation. Mol Cell Biol. 1983 Oct;3(10):1730–1737. doi: 10.1128/mcb.3.10.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L., Glick B. S., Rothman J. E. A new type of coated vesicular carrier that appears not to contain clathrin: its possible role in protein transport within the Golgi stack. Cell. 1986 Jul 18;46(2):171–184. doi: 10.1016/0092-8674(86)90734-8. [DOI] [PubMed] [Google Scholar]
- Orci L., Ravazzola M., Amherdt M., Madsen O., Vassalli J. D., Perrelet A. Direct identification of prohormone conversion site in insulin-secreting cells. Cell. 1985 Sep;42(2):671–681. doi: 10.1016/0092-8674(85)90124-2. [DOI] [PubMed] [Google Scholar]
- Payne G. S., Schekman R. A test of clathrin function in protein secretion and cell growth. Science. 1985 Nov 29;230(4729):1009–1014. doi: 10.1126/science.2865811. [DOI] [PubMed] [Google Scholar]
- Pearse B. M., Bretscher M. S. Membrane recycling by coated vesicles. Annu Rev Biochem. 1981;50:85–101. doi: 10.1146/annurev.bi.50.070181.000505. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Fine R. E. Coated vesicles transport newly synthesized membrane glycoproteins from endoplasmic reticulum to plasma membrane in two successive stages. Proc Natl Acad Sci U S A. 1980 Feb;77(2):780–784. doi: 10.1073/pnas.77.2.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E., Schmid S. L. Enzymatic recycling of clathrin from coated vesicles. Cell. 1986 Jul 4;46(1):5–9. doi: 10.1016/0092-8674(86)90852-4. [DOI] [PubMed] [Google Scholar]
- Rothman J. H., Hunter C. P., Valls L. A., Stevens T. H. Overproduction-induced mislocalization of a yeast vacuolar protein allows isolation of its structural gene. Proc Natl Acad Sci U S A. 1986 May;83(10):3248–3252. doi: 10.1073/pnas.83.10.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz P. J., Solomon F., Botstein D. Genetically essential and nonessential alpha-tubulin genes specify functionally interchangeable proteins. Mol Cell Biol. 1986 Nov;6(11):3722–3733. doi: 10.1128/mcb.6.11.3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer I., Emr S., Gross C., Schekman R. Invertase signal and mature sequence substitutions that delay intercompartmental transport of active enzyme. J Cell Biol. 1985 May;100(5):1664–1675. doi: 10.1083/jcb.100.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schekman R. Protein localization and membrane traffic in yeast. Annu Rev Cell Biol. 1985;1:115–143. doi: 10.1146/annurev.cb.01.110185.000555. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Ungewickell E., Branton D. The binding of clathrin triskelions to membranes from coated vesicles. Cell. 1981 Nov;26(3 Pt 1):439–446. doi: 10.1016/0092-8674(81)90213-0. [DOI] [PubMed] [Google Scholar]
- Wehland J., Willingham M. C., Gallo M. G., Pastan I. The morphologic pathway of exocytosis of the vesicular stomatitis virus G protein in cultured fibroblasts. Cell. 1982 Apr;28(4):831–841. doi: 10.1016/0092-8674(82)90062-9. [DOI] [PubMed] [Google Scholar]
- Zaremba S., Keen J. H. Assembly polypeptides from coated vesicles mediate reassembly of unique clathrin coats. J Cell Biol. 1983 Nov;97(5 Pt 1):1339–1347. doi: 10.1083/jcb.97.5.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuker C. S., Cowman A. F., Rubin G. M. Isolation and structure of a rhodopsin gene from D. melanogaster. Cell. 1985 Apr;40(4):851–858. doi: 10.1016/0092-8674(85)90344-7. [DOI] [PubMed] [Google Scholar]