Abstract
The PI3K/AKT pathway is hyperactivated in prostate cancer but its effective therapeutic targeting has proven difficult. In particular, the antitumor activity of AKT inhibitors is attenuated by upregulation of receptor tyrosine kinases (RTKs) through an uncharacterized feedback mechanism. In this report, we show that RNAi-mediated silencing or pharmacological inhibition of Pim-1 activity curtails AKT inhibitor-induced upregulation of RTKs in prostate cancer cells. Although Pim kinases have been implicated in cap-dependent translational control, we find that in the context of AKT inhibition the expression of RTKs is controlled by Pim-1 in a cap-independent manner, by controlling internal ribosome entry. Combination of Pim and AKT inhibitors resulted in synergistic inhibition of prostate tumor growth in vitro and in vivo. Together, our results show that Pim-1 mediates resistance to AKT inhibition, and suggest its targeting to improve the efficacy of AKT inhibitors in anticancer therapy.
Keywords: IRES, RTK, Pim-1, AKT, Prostate Cancer
Introduction
The PI3K/AKT pathway is commonly activated in human cancer and controls cellular processes that contribute to the initiation and maintenance of cancer (1). It is activated in 40% of primary and 70% of metastatic prostate cancers secondary to mutations or deletions in PTEN (1–3). Activation of the pathway can be associated with mutations in the PI3K catalytic subunit P110 alpha and regulatory subunit (1); mutations in each of the three AKT isoforms (1, 4); and activation of receptor tyrosine kinases (RTKs) by mutation (e.g., EGFR), or gene amplification (e.g., HER2), which can result in activation of downstream PI3K/AKT (1, 5). Multiple small molecules inhibitors have been developed to target PI3K/mTOR or AKT (6) but the efficacy of these drugs is compromised by the stimulation of compensatory signaling pathways that have the potential to enhance tumor growth (7–9). There is accumulating evidence that inhibition of the PI3K/AKT pathway can lead to adaptive resistance due to upregulation and activation of RTKs (7–9). The mechanism underlying the AKT inhibition-induced upregulation of some of these RTKs, including HER3, INSR, and IGF1R, has been shown to in part involve FOXO transcription factors (7); however, these transcription factors do not appear to be involved in the AKT inhibition-induced upregulation of other RTKs, including MET, HER2, and RET (7).
The Pim family of serine/threonine kinases regulates cell survival pathways and has been implicated in the progression of several human cancers, including prostate cancer (10). Clinically, the expression of the Pim kinases is elevated in human prostate cancer (10), in which the PI3K/AKT pathway is activated, and the levels of Pim correlate with survival of patients with certain subtypes of human lymphoma (11), suggesting that the Pim kinases could play an important role in regulating tumor growth and, potentially, patient survival. As the Pim kinases have overlapping activity with AKT with both regulating apoptosis, cell-cycle progression, and cellular metabolism (12–13) and AKT and the Pim kinases share substrates in common (12–13), it has been suggested that Pim could play an important role in the activation of AKT (14). Reciprocal regulation of AKT and Pim-1 levels is suggested by the report that forced expression of nuclear-targeted AKT induces Pim-1 and either expression of a dominant-negative Pim-1 or genetic deletion of the enzyme increased AKT expression and phospho-AKT levels in cardiomyocytes (14).
Here, we demonstrate that inhibition of AKT leads to transcriptional induction of the Pim-1 protein kinase, and in turn, Pim-1 regulates the expression of RTKs. The anticancer activity of small molecule AKT and Pim kinase inhibitors has been investigated.
MATERIALS AND METHODS
Reagents and Antibodies
GSK690693 was provided by GlaxoSmithKline for in vitro and in vivo studies.
MK2206, PP242, AZD8055, BEZ235 were purchased from Selleck Biochemicals.
Antibodies are listed in the Supplementary Data.
Plasmids
The 5′-UTR of human Met (15) was amplified by PCR using genomic DNA extracted from PC3-LN4 cells as template with the following two primers: 5′-ATACTAGTGCTGCAGCGGCCGCGGTGGCTGA-3′ and 5′-AACCATGGCCCAACCTCCAGGATGTCGGCGCA-3′. The PCR product was sequenced and cloned into the EcoRI and NcoI sites of the plasmid of pRF to create pR-MET-F.
Immunoblotting
Cells were harvested in lysis buffer A consisting of 50 mM Tris pH 7.4, 150 mM NaCl, 1% NP-40, 5 mM EDTA. Protein concentrations were determined by DC Protein Assay (BioRad, Hercules, CA).
Cell Culture and transfections
Cell lines were grown in RPMI (PC3-LN4, DU145, 22RV1, VCAP, and BT474) or DMEM (HeLa, MEFs) in 5% CO2. DU145, 22RV1, VCAP, BT474, and HeLa cells were supplied by American Type Culture Collection (ATCC) and passaged in our laboratory for fewer than 6 months after receipt. PC3-LN4 cells were described before (16). The mouse embryo fibroblasts (MEFs) which were triple knock-out (TKO) for all Pim genes were previously described (17). Cells were transfected with lipofectamine 2000 reagent according to manufacturer’s instructions.
Real-time PCR analyses
SYBR Green reactions were done using a BioRad iQ5 real-time quantitative PCR system. For data analysis, raw counts were normalized to the housekeeping gene averaged for the same time point and condition (ΔCt). Counts are reported as fold change relative to the untreated control (2−ΔΔCt). All primers were designed and synthesized by Integrated DNA Technologies (IDT). Primers are listed in the Supplementary Data.
Luciferase Assays
Firefly luciferase and Renilla luciferase activities were measured in a luminometer (Model TD 20/20; Turner Designs) using the reagents provided with the dual luciferase reporter kit (Promega).
Soft-agar colony formation assays
The soft-agar assay was performed on 6-well plates in duplicate. For each well, 5,000 cells were mixed in growth medium containing 0.7% agarose and GSK690693 or SMI-4a. Cells were then layered over 1% agarose in regular medium. Medium containing GSK690693 or SMI-4a was added to each well every four days. The assays were terminated after 21 days and colonies were stained with crystal violet and counted under a microscope.
Cell Proliferation Measurement
Cells were plated in 96-well plates at 3000 cells/well in 100 μl of 10% FBS-containing medium. After 24 hr incubation, the medium was replaced with 0.2% FBS medium with GSK690693, SMI-4a or DMSO for 72 hrs. Cell viability was measured using a MTT assay. The absorbance was read at 590 nm with a reference filter of 620 nm.
In vitro transcription and RNA transfection
The mRNAs were purified with MEGA clear kit (Ambion), quantified spectrophotometrically and their qualities were verified on a denaturing agarose gel. RNA transfection was performed with TransIT®-mRNA Transfection Kit (Mirus) according to the manufacturer’s suggestion. An aliquot of 1 μg of capped mRNAs and 2 μl of TransIT mRNA reagent together with 1 μl of mRNA boost reagent were used to transfect 80% confluent cells grown in 12-well plates. At 16 h after transfection cells were harvested and lysed for luciferase assay.
Animal Experiments
4–6 week old nu/nu nude male mice were obtained from Charles River Laboratories and maintained in pressurized ventilated caging. All studies were performed in compliance with institutional guidelines under an IACUC approved protocol (MUSC#3081). For efficacy studies, mice with well-established tumors were selected and randomized fourteen days post-implantation (Size > 150mm3); PC3-LN4 xenograft tumors were established in nude mice by subcutaneously injecting 5 × 106 cells suspended in PBS into the right flank. Mice were treated with vehicle, GSK690693, or SMI-4a, or GSK690693 + SMI-4a at the indicated doses. GSK690693 was dissolved in 30% propylene glycol, 5% Tween-80, 65% of 5% dextrose in water (pH4–5), and administered intraperitoneally daily while SMI-4a was dissolved in the same solvent and administered by oral gavage twice daily. Tumor dimensions were measured with a caliper and tumor volumes calculated (tumor volume (mm3) = (length × width2)/2.).
Statistical Analysis
The results of quantitative studies are reported as mean ±SD or mean ±SEM (for animal experiments). Differences were analyzed by Student’s t test. P values of < 0.05 were regarded as significant.
RESULTS
AKT inhibition induces Pim-1 expression in prostate cancer cells
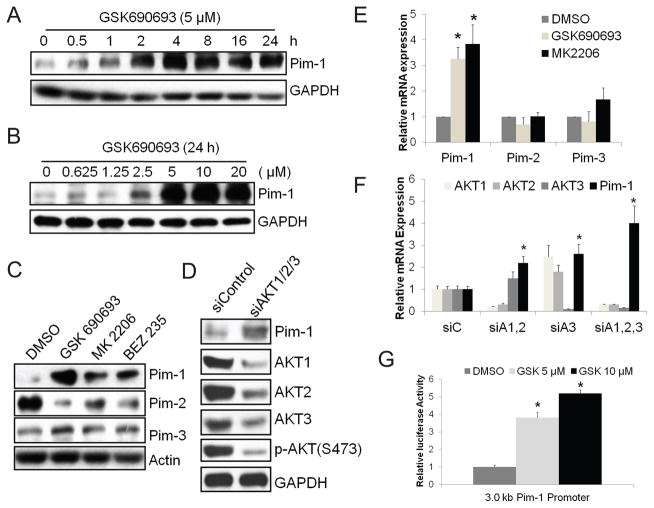
Treatment of the prostate cancer PC3-LN4 cells with the pan-AKT inhibitor GSK690693 markedly increased the levels of Pim-1 protein in a time and concentration-dependent fashion (Fig. 1A and B) but had a minimal effect on the expression of Pim-3 protein and reduced the levels of Pim-2 (Fig. 1C). Similar results were obtained using another AKT inhibitor, MK2206 and a PI3K/mTOR dual inhibitor, BEZ235 (Fig. 1C). The induction of Pim-1 was also observed with GSK690693 treatment of human prostate cancer cell lines DU145, 22RV1, and VCAP (Supplementary Fig. S1A). The effect of GSK690693 on Pim-1 was not secondary to an off-target effect as knockdown in PC3-LN4 cells of all three AKTs with small interfering RNAs (siRNAs) increased the levels of Pim-1 protein (Fig. 1D). Treatment of PC3-LN4 cells with GSK690693 or MK2206 resulted in elevations in the level of Pim-1 mRNA, but not Pim-2 or Pim-3 (Fig. 1E). Similarly, treatment of PC3-LN4 cells with siRNAs directed at AKT1, AKT2, and AKT3 also resulted in the elevation of Pim-1 mRNA (Fig. 1F). To further determine whether GSK690693 regulates the transcription of the Pim-1 gene, a 3.0 kb promoter fragment of the Pim-1 promoter was cloned upstream of a luciferase reporter. Addition of GSK690693 increased the activity of this promoter in PC3-LN4 cells (Fig. 1G).
Fig. 1.
AKT inhibition induces expression of Pim-1. PC3-LN4 cells were treated with (A) 5 μM GSK690693 for the times indicated, (B) increasing doses of GSK690693 as indicated for 24 h, (C) 5 μM GSK690693, 2 μM MK2206, or 0.5 μM BEZ235 for 24 h, and (D) siRNAs against AKT1, AKT2, and AKT3 or a negative control siRNA for 72 h. Whole cell lysates were subjected to immunoblot analyses with the indicated antibodies. (E) Cells as in (C) were harvested and total RNA was isolated. Real-time qPCR analyses were performed with Pim-1, Pim-2, Pim-3-specific primers. Results were normalized to the expression of β-actin. (F) PC3-LN4 cells were treated with siRNAs against AKT1,2 (siA1,2), AKT3 (siA3), AKT1,2,3 (siA1,2,3), or a nontargeting control siRNA (siC) for 72 h and then RNA isolated, and real-time qPCR with indicated primers performed. (G) PC3-LN4 cells were transfected with a luciferase reporter containing a 3.0 kb human Pim-1 promoter. After 24 h, cells were treated with DMSO or two different doses of GSK690693 (GSK) as indicated for additional 24 h before harvesting for luciferase assays. Results were normalized to Renilla luciferase activity by a co-transfected plasmid carrying this enzyme. Data in E, F, and G are mean ±SD of three independent experiments. *p<0.05 compared to the corresponding negative control.
Upregulation of Pim-1 is required for AKT inhibitor-associated induction of RTKs
Consistent with previous reports of upregulation of these RTKs in response to AKT inhibition (7), treatment of PC3-LN4 cells with GSK690693 increased the protein levels of multiple RTKs, including MET, EPHA2, RON, EGFR, HER2, HER3, INSR, and IGF1R (Supplementary Fig. S1B). Additionally, we observed increased ERK phosphorylation resulting from treatment with GSK690693 (Supplementary Fig. S1B). This is in keeping with previous finding that PI3K inhibition leads to MAPK pathway activation (18–19).
GSK690693 also completely blocked the phosphorylation of two well-known AKT substrates, GSK3β and PRAS40, demonstrating the effectiveness of this compound (Supplementary Fig. S1C) and caused the paradoxical hyperphosphorylation of AKT at its two regulatory sites (Thr308 and Ser473) (Supplementary Fig. S1C), a common property of ATP-competitive AKT inhibitors (20).
To determine if Pim-1 plays an important regulatory role in the ability of AKT inhibitors to modulate RTKs, we first determined the effects of Pim-directed siRNAs and small molecule inhibitors. The use of siRNA directed at Pim-1 demonstrated that a forced reduction in Pim-1 levels markedly reduced the ability of GSK690693 to elevate the protein levels of multiple RTKs, including MET and EPHA2, HER3, HER2, INSR, and IGF1R, as well as the phosphorylation of ERK (Fig. 2A). The addition of SMI-4a, a small molecule Pim kinase inhibitor (21) reduced GSK690693-induced upregulation of RTK protein levels in PC3-LN4 (Fig. 2B), DU145, 22RV1, and VCAP cells (Supplementary Fig. S2)
Fig. 2.
Pim-1 is required for elevated expression of RTKs induced by AKT inhibition. Immunoblot analyses were carried out with the indicated antibodies in (A) PC3-LN4 cells were treated with two different siRNAs (1 & 2) against Pim-1 as well as a nontargeting control siRNA (two left lanes) for 48 h followed by the addition of GSK690693 (5 μM) for an additional 24 h, (B) PC3-LN4 cells were treated with GSK690693 (GSK, 5 μM), or SMI-4a (4a, 10 μM), or the combination of the two compounds for 24 h, (C) Wild-type (WT), Pim kinase triple-knockout (TKO) murine embryonic fibroblast cells were treated with 5 μM GSK690693 for 24 h, (D) PC3-LN4 cells were treated with three different Pim inhibitors, SMI-4a (4a, 10 μM), SMI-16a (16a, 10 μM), or K00135 (K, 5 μM) for 24 h, (E) PC3-LN4 cells were transfected with a nontargeting control siRNA, siRNA against Pim-1, an empty vector, or a Pim-1 expressing plasmid for 72 h.
The results of phospho-RTK antibody array (RPPA) analysis revealed that treatment of PC3-LN4 cells with GSK690693 increased the tyrosine phosphorylation of a number of RTKs tested in the assay, i.e., MET, EPHA2, HER2, INSR, and EGFR (Supplementary Fig. S3). The lack of complete correlation in these assays may arise from the differing specificity of the antibodies used in the RPPA analysis. This change in RTK phosphorylation is consistent with the AKT-inhibitor-induced increases in the protein levels of the RTKs; however, it cannot be ruled out that GSK690693 stimulates RTK phosphorylation through an alternative mechanism (7). Treatment with SMI-4a blocked the GSK690693-induced RTK-phosphorylation (Supplementary Fig. S3), demonstrating that the inhibition of Pim reverses the activity of this AKT inhibitor. To further evaluate the role of Pim-1 in regulating AKT-inhibitor induced upregulation of RTKs, MEFs were treated with GSK690693. In wild-type cells, but not in the Pim kinase-deficient (TKO) cells, GSK690693 treatment of the cells increased the levels of the RTKs tested, i.e., MET, HER3, IGF1R, and EPHA2 protein, as well as the phosphorylation of ERK (Fig. 2C). We treated PC3-LN4 (Fig. 2D) and VCAP (Supplementary Fig. S4) cells with three different Pim kinase inhibitors, SMI-4a, SMI-16a (21), and K00135 (22), to test whether Pim-1 activity affects the baseline level of RTK proteins in tumor cells. Treatment decreased the protein levels of the RTKs e.g., MET, EPHA2, and HER3, in both cell lines. Similarly, siRNA targeting of Pim-1 decreased the levels of MET, HER3, HER2 and EGFR protein in PC3-LN4 cells (Fig. 2E). Conversely, overexpression of human Pim-1 in PC3-LN4 increased the levels of the RTKs, MET, HER3, EPHA2, HER2 and EGFR (Fig. 2E).
AKT inhibition increases cap-independent translation
AKT protein kinase activity controls protein synthesis by regulating the multistep process of mRNA translation at multiple stages from ribosome biogenesis to translation initiation and elongation (23). Although GSK690693 treatment of prostate cancer cells did not modify phosphorylation of 4E-BP1, this compound increased phosphorylation of eIF2α and eliminated phosphorylation of ribosomal protein S6 (Fig. 3A). To further define the role of cap-dependent translation in the mechanism of action of this agent, GSK690693 was combined with two potent inhibitors of mTORC1/mTORC2 and thus cap-dependent translation, PP242 and AZD8055 (24–25). These inhibitors in combination with GSK690693 resulted in reduced phosphorylation of 4E-BP1, and increased eIF2α phosphorylation compared to GSK690693 alone (Fig. 3A), suggesting inhibition of 5′-cap dependent translation. We measured the binding of eIF4G and 4E-BP1 to the 5′ mRNA cap by using m7GTP-sepharose. The structure of these beads mimics the 5′ mRNA cap and precipitates cap-interacting proteins. In agreement with the effect on phosphorylation of 4E-BP1, PP242 or AZD8055 in combination with GSK690693 strongly reduced eIF4G, and increased 4E-BP1, binding to m7GTP-sepharose, while GSK690693 alone did not have a significant effect (Supplementary Fig. S5A). However, the treatment of prostate cancer cells with these mTORC1 inhibitors did not reduce the GSK690693-induced elevation of MET, EPHA2, HER3, and IGF1R (Fig. 3A). A recent study (26) utilizing Torin 1, an ATP competitive mTOR inhibitor demonstrated that Torin 1-resistant mRNAs are enriched for RTKs such as MET, IGF1R, and INSR, indicating that the translation initiation of these mRNAs do not depend on mTOR activity (27). We found that treatment of PC3-LN4 cells with PP242 or AZD8055 indeed did not inhibit the expression of MET, EPHA2, HER3, IGF1R, or INSR (Supplementary Fig. S5B). Additionally, the expression of Bcl-2 whose translation under cellular stress (28) has been shown to be controlled by a cap-independent mechanism was not suppressed by treatment with mTOR inhibitors while, proteins known to be sensitive to mTOR inhibiton, YB-1, HSP90, RPS7 (26, 29) were reduced (Supplementary Fig. S5B). Reduced eIF4G and increased 4EBP1 binding to m7GTP-sepharose, and increased eIF2α phosphorylation (Supplementary Fig. S5C) confirmed that cap-dependent translation was efficiently inhibited. Together these data suggest that upregulation of RTKs is not controlled by cap-dependent mechanisms.
Fig. 3.
AKT inhibition increases cap-independent translation. (A) PC3-LN4 cells were treated with GSK690693 (5 μM) alone, or in combination with PP242 (2 μM) or AZD8055 (1 μM) for 24 h, and immunoblotting performed. (B) Dicistronic luciferase plasmids containing viral (CrPV and HCV) or cellular (HIF1α, VEGF, and Myc) IRESs were transfected into PC3-LN4 cells. GSK690693 (5 μM) was added 6 h after transfection for additional 24 h and luciferase activities were determined. Data are mean ±SD of four independent experiments. *p<0.05 compared to the corresponding DMSO control. (C) PC3-LN4 cells were treated with increasing doses of GSK690693 as indicated for 24 h and lysates examined by Western blotting.
Under conditions of decreased cap-dependent translation, the internal ribosome entry site (IRES)-mediated translation can play a larger role in regulating protein synthesis (30). Recently, it has been shown that inhibition of PI3K/mTOR leads to increased IRES-mediated translation (8). Inhibition of AKT by GSK690693 resulted in increased IRES activity measured by ratio of firefly to Renilla luciferase activities in constructs containing either cellular (HIF1α, Myc, and VEGF (31)) or viral (cricket paralysis virus, CrPV, and hepatitis C virus, HCV (32)) IRES sequences (Fig. 3B). In agreement with these findings, GSK690693 induced expression of Bcl-2, Myc, VEGF, and HIF1α all of which can be translated in a cap-independent manner under cellular stress (28, 31, 33–34), further suggesting the possibility that cap-independent translation is upregulated (Fig. 3C).
Pim-1 regulates RTK expression through cap-independent translation
Expression of human Pim-1 in PC3-LN4 cells did not affect the levels of RTK mRNAs (Supplementary Fig. S6A and B) or the half-life of the RTKs (Supplementary Fig. S6C), suggesting that Pim-1 may control the levels of these proteins through a translational mechanism. Plus, GSK690693 increased cap-independent translation (Fig. 3). Taken together, we speculated that the upregulation of the RTKs induced by AKT inhibitors could be controlled, at least in part, by a cap-independent mechanism. We first determined whether the MET 5′ untranslated region (UTR) contains an IRES that could be stimulated by either GSK690693 or Pim-1. The MET 5′UTR is relatively long (408 nt) and is GC-rich (15), which are two common properties of IRES-containing 5′UTRs. The 5′UTR of MET was cloned and inserted in front of firefly luciferase in the dicistronic vector pRF (35). The presence of the MET 5′UTR sequence increased the expression of downstream firefly luciferase relative to Renilla by 38-fold compared to the vector control (Fig. 4A), suggesting that it could function as an IRES. In comparison, the IRESs of encephalomyocarditis virus (EMCV), HIF1α, and VEGF produced 18-, 9-, and 13-fold increases, respectively. In PC3-LN4 cells transfected with the pRF vector containing the MET IRES, overexpression of Pim-1 or treatment of GSK690693 resulted in an increase in ratio of firefly to Renilla luciferase activities as compared to control treatment (Fig. 4A). Knockdown of Pim-1 suppressed GSK690603-induced MET IRES activities (Supplementary Fig. S7). Collectively, these results indicated that Pim-1 can potentially regulate translation of MET in a cap-independent fashion.
Fig. 4.
Pim-1 regulates RTK translation by controlling IRES activity. (A) Dicistronic plasmids pRF, pR-EMCV-F, pR-HIF-F, pR-VEGF-F, and pR-MET-F were transfected into PC3-LN4 cells. A Pim-1 expressing plasmid was cotransfected with pR-MET-F as indicated. GSK690693 (GSK, 5 μM) was added 6 h after transfection and luciferase activities were determined 24 h after transfection. Data are mean ±SD of four independent experiments. *p<0.05 compared to the MET. (B) Capped, polyadenylated dicistronic mRNAs were transfected into PC3-LN4 cells. The ratios of firefly/Renilla activities are shown relative to the ratio for RF, which was given a value of 1. (C) A dicistronic plasmid containing IGF1R IRES was transfected into PC3-LN4 cells with or without either a Pim-1 expressing plasmid or siRNA targeted at Pim-1. GSK690693 (GSK, 5 μM) was added 6 h after transfection and at 48 h luciferase activities were determined. (D) Dicistronic luciferase plasmids containing viral (CrPV and HCV) or cellular (HIF1α and Myc) IRESs were transfected into PC3-LN4 cells together with siRNA against Pim-1 or a nontargeting control siRNA, and luciferase activities were determined 48h after transfection. (E) PC3-LN4 cells were treated with two different siRNAs (1 & 2) against Pim-1 as well as a nontargeting control siRNA (two left lanes) for 48h followed by adding GSK690693 (5 μM) for additional 24h as indicated. Whole cell lysates were subjected to immunoblot analyses with the indicated antibodies. Data in B, C, D, and E reflect the mean ±SD of four independent experiments. *p<0.05 compared to corresponding negative controls.
To determine whether the MET 5′UTR is sufficient to drive translation by acting as an IRES and to rule out the possibility of a cryptic promoter in the 5′UTR of MET, we in vitro transcribed the pRF vector containing the MET IRES yielding a capped dicistronic mRNA, and then transfected this mRNA directly into PC3-LN4 cells. Insertion of the MET or VEGF 5′UTR resulted in a 7or 5-fold increase in the firefly/Renilla ratio, respectively. In comparison, when the pRF vector containing the viral EMCV IRES was transcribed, and transduced into these cells, the firefly/Renilla ratio increased by 114-fold (Fig. 4B). Thus, in comparison to a viral IRES, both the MET and VEGF sequences have relatively weak IRES activities. Besides MET, other RTKs including IGF1R have been reported to have IRES elements in their 5′UTRs (36). As shown in Fig. 4C, the IRES activity of the 5′UTR of IGF1R was increased on treatment of the cells with GSK690693 or Pim-1 overexpression and, conversely, was decreased on knockdown of endogenous Pim-1 protein levels. Furthermore, knockdown of Pim-1 suppressed GSK690603-induced IGF1R IRES activities (Supplementary Fig. S7). It is possible that this mechanism is important for the control of other RTKs since in general these genes have long 5′UTRs. Additionally, knockdown of Pim-1 in PC3-LN4 cells led to a reduction of IRES activities of viral, CrPV and HCV, and cellular, HIF1α and Myc, IRESs (Fig. 4D). This data suggests that Pim-1 could be a more general regulator of IRES-mediated translation. This concept is further supported by our finding that the upregulation of proteins whose translation can be controlled by an IRES-mediated mechanism under cellular stress, Bcl-2, Myc, VEGF, and HIF1α, is stimulated by GSK690693 and requires Pim-1 expression (Fig. 4E).
Ribosomal stress abrogates AKT inhibition-induced up regulation of RTK expression
Pim-1 has been shown to physically interact with ribosomal protein S19 and to co-sediment with ribosomes (37–38). Knockdown of ribosomal protein S19 or S6 abolished upregulation of MET, EPHA2, HER3, and IGF1R induced by GSK690693 without affecting Pim-1 induction (Fig. 5A). Consistent with findings from other laboratories (38–40), reduced protein expression of ribosomal protein S6 was seen when S19 was decreased by siRNA and vice versa (Fig. 5A). To test the effect of ribosomal stress on RTK upregulation independently of ribosomal protein knockdowns, low concentrations of actinomycin D (ActD) were used to inhibit RNA polymerase I, and thus induce ribosomal stress (41–42). Similar to S19 and S6 knockdowns, ActD treatment blocked upregulation of MET, EPHA2, HER3, and IGF1R induced by GSK690693 (Fig. 5B). ActD treatment also inhibited upregulation of MET, EPHA2, and HER3 resulting from direct Pim-1 overexpression in PC3-LN4 cells (Fig. 5C). Ribosomal stress did not appear to affect global translation as the expression of Src and ERK1/2 proteins was not altered (Fig. 5 A and B). These data suggest Pim-1 may work through intact ribosomes to control RTK expression.
Fig. 5.

Ribosomal stress abrogates RTK upregulation induced by GSK690693. (A) PC3-LN4 cells were treated for 48 h with siRNAs against Pim-1, ribosomal protein S19, S6 as well as a nontargeting control siRNA (two left lanes) followed by adding GSK690693 (5 μM) for an additional 24 h. (B) PC3-LN4 cells were treated with increasing dose of actinomycin D (ActD) with and without 5 μM GSK690693 for 24 h. (C) PC3-LN4 cells were transfected with a Pim-1 expressing plasmid or a control vector. ActD (5 nM) was added 24h after transfection for an additional 16 h. Whole cell lysates were subjected to immunoblot analyses with the indicated antibodies.
Combination treatment with an AKT and a Pim inhibitor synergistically blocksprostate tumor growth in vitro and in vivo
As a preliminary test of whether combined inhibition of AKT and Pim kinases might provide synergistic antitumor efficacy, we tested the effects of the inhibitors on the proliferation of PC3-LN4 cells in vitro. Treatment of PC3-LN4 cells with the Pim inhibitor SMI-4a in combination with the AKT inhibitor GSK690693 resulted in a synergistic enhancement of the inhibition of proliferation as demonstrated by combination index of less than 0.5 (Fig. 6A and data not shown), and a markedly greater reduction in both the numbers and the size of colonies seen in a soft-agar colony formation assay (Fig. 6B). GSK690693 and SMI-4a blocked the proliferation of DU145 in a similar fashion (Supplementary Fig. S8).
Fig. 6.
Combined inhibition of AKT and Pim demonstrates synergistic antitumor activity. (A) PC3-LN4 cells were treated with increasing doses of GSK690693 and SMI-4a (4a) as indicated in media containing 0.2% serum for 72 h followed by a MTT assay. Similar results were obtained from three independent experiments. One representative experiment is shown. (B) PC3-LN4 cells were plated in 10% serum and 0.7% agarose-containing medium with 10 μM of GSK690693 or SMI-4a alone or in combination. Colonies were stained with crystal violet and counted after 21 days and the data are mean ±SD of three independent experiments. Bar, 200 μM. (C) Nu/Nu mice bearing PC3-LN4 tumors were randomized into four groups: vehicle, GSK690693 (30 mg/kg i.p. daily), SMI-4a (60 mg/kg oral twice/day), and the combination. Tumor size was measured every three days. The results are presented as the mean tumor volume ±SEM (n = 6 mice/group). **P < 0.02 for the GSK + SMI-4a group vs all other treatment groups. (D) Immunoblot analyses of tumors in (C) with the indicated antibodies. Tumors were harvested on Day 36 6 h after the last dose of therapy. The numbers above each lane represent individual tumors in that treatment group. (E) A model for the feedback upregulation of RTK expression mediated by Pim-1 kinase.
To test the activity of these agents in vivo, PC3-LN4 cells were injected into mice and treated with GSK690693 alone, SMI-4a alone, or both drugs in combination on a daily basis for 21 days starting at 15 days after tumor implantation. When used alone, treatment these drugs caused a modest inhibition of tumor growth whereas the combined treatment resulted in a markedly greater inhibition of tumor growth (Fig. 6C). As shown in Fig. 6D, immunoblot analysis of lysates of tumors harvested at the termination of the experiment on day 36 had upregulated levels of MET, EPHA2, and HER3 protein in mice treated with GSK690693 as compared with the tumors from mice treated with vehicle (Fig. 6D). Interestingly, the levels of Pim-1 were increased in the combined therapy, and could suggest an in vivo interaction between these agents cannot be ruled out. This upregulation of the RTKs was significantly reduced in the tumors from mice treated with a combination of GSK690693 and SMI-4a (Fig. 6D).
DISCUSSION
The results of these experiments provide insights into the mechanisms underlying the compensatory interplay between AKT and Pim-1 in the regulation of prostate cancer cell behavior influenced by the expression of RTKs. They suggest a model in which reduction in AKT activity is associated with an increase in the levels of Pim-1 protein kinase that occurs through a transcriptional mechanism. This increase in Pim-1 kinase is associated, in turn, with promotion of the expression of RTKs through a cap-independent mechanism. Down-regulation of Pim-1 blocks the feedback elevation in RTKs associated with inhibition of AKT (Fig. 6E). Likewise inhibitors of Pim synergize with small molecule AKT inhibitors to block the growth of prostate cancer cells.
The control of Pim-1 protein levels is complex and has been shown to involve the ubiquitin proteasome pathway and translational mechanisms (43). In the current study, we demonstrate that inhibition of AKT can increase the levels of Pim-1 through a transcriptional mechanism; however, it is possible that additional alternative mechanisms could also play a role in increasing Pim-1 protein levels. The induction of Pim-1 by AKT inhibition coincides with suppression of total protein synthesis (Supplementary Fig. S9) and is not inhibited by further treatment with mTORC inhibitors (Fig. 3A), suggesting that Pim-1 protein levels could also be regulated in a cap-independent manner. The Pim-1 5′UTR may contain an IRES that could also be regulated by specific cellular growth conditions (44), although the existence of this IRES is controversial (57).
It has been demonstrated previously that inhibition of AKT regulates the transcription of RTKs by modulating the activity of Foxo transcription factors (7); however, in the same study no change was seen in the level of HER2, RET, or MET mRNAs, suggesting that the levels of specific RTKs might be controlled by other mechanisms. Cap-dependent translation plays a role in both PI3K/AKT and Pim-2 enhancement of the synthesis of specific proteins (45). It should be noted, however, that molecules that blocked mTORC1 activity could not inhibit the Pim-2 protein kinase and an agent that blocked eIF4A function, which is known to take part in IRES mediated transcription, was required. Additionally, small molecule mTORC inhibitors can decrease the translation of many mRNAs, e.g., 5′-terminal oligopyrimidine tracts (5′TOP) mRNAs, while increasing the level of translation of RTKs (26), again suggesting that these RTK mRNAs may be translated in a cap-independent fashion. Moreover, further inhibition of cap-dependent translation with the mTORC1/2 inhibitors, PP242 and AZD8055, had no effect on the ability of GSK690693 or Pim-1 to induce RTKs (Fig. 3A), suggesting that in the experimental conditions used in these studies the mechanism by which this agent controls RTK levels is not cap-dependent.
Our results are consistent with the hypothesis put forward by Muranen and colleagues that inhibition of PI3K/mTOR could lead to enhanced cap-independent translation (8). Cloning of the Met 5′ UTR into a dicistronic luciferase vector demonstrated that it can function as an IRES element, although weakly in comparison to viral sequences, and its activity is enhanced by GSK690693 and Pim-1 overexpression (Fig. 4A and B). Further supporting evidence of the ability of GSK690693 and Pim-1 to regulate the activity of the IRES is the observation that the IGF1R IRES (46) is stimulated by these agents and that Pim-1 knockdown decreases the activity of this element (Fig. 4C). Our data further suggest that Pim-1 may be essential for full IRES activity of additional viral and cellular IRES elements, including HCV, CrPV, HIF1α and Myc (Fig. 4D), suggesting a general role of Pim in the control of cap-independent translation.
It has been suggested previously that because they are both survival kinases, AKT and Pim protein kinases could be important pharmacologic targets to inhibit tumor growth (12). Our experiments demonstrate a high degree of synergism between small molecule inhibitors of AKT and Pim in their ability to kill prostate cancer cells both in tissue culture and in a xenograft model (Fig. 6). Analysis of the tumors from treated animals showed that AKT inhibitor treatment elevates RTKs in the tumor cells grown in vivo and that simultaneous treatment with a Pim inhibitor down-regulates this effect (Fig. 6D). Because both these kinase pathways are highly activated in human prostate cancer, dual inhibitor treatment of these tumors could be a particularly attractive chemotherapeutic strategy.
Supplementary Material
Acknowledgments
We thank members of the Kraft laboratory, including Dr. Zanna Beharry for critical reading of the manuscript, Dr. Jin Song for helping to carry out experiments, and Dr. Marina Zemskova for providing the luciferase construct containing the human Pim-1 promoter. We are grateful to GlaxoSmithKline for supplying GSK690693. Dicistronic plasmids were kindly provided by Drs. Scott Blume, University of Alabama at Birmingham, Birmingham, AL (IGF1R); Gregory Goodall, Institute of Medical and Veterinary Science, Adelaide SA 5000, Australia (HIF, c-Myc, and VEGF); Gregg Johannes, Drexel University, Philadelphia, PA (EMCV); and Robert Gemmill, Medical University of South Carolina, Charleston, SC (CrPV and HCV).
Financial support: This work is supported by NIH Grant 1K01DK085196 (to B.C.), DOD W81XWH-09-1-0300 (to A.S.K.), DOD W81XWH-10-1-0249 (to A.S.K.), NIH/NCRR Grant UL1RR029882, and in part by pilot research funding, Hollings Cancer Center’s Cancer Center Support Grant P30 CA138313 at the Medical University of South Carolina.
Footnotes
The authors declare no potential conflicts of interest.
References
- 1.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 2.Yoshimoto M, Cunha IW, Coudry RA, Fonseca FP, Torres CH, Soares FA, et al. FISH analysis of 107 prostate cancers shows that PTEN genomic deletion is associated with poor clinical outcome. Br J Cancer. 2007;97:678–85. doi: 10.1038/sj.bjc.6603924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suzuki H, Freije D, Nusskern DR, Okami K, Cairns P, Sidransky D, et al. Interfocal heterogeneity of PTEN/MMAC1 gene alterations in multiple metastatic prostate cancer tissues. Cancer Res. 1998;58:204–9. [PubMed] [Google Scholar]
- 4.Bellacosa A, Kumar CC, Di Cristofano A, Testa JR. Activation of AKT kinases in cancer: implications for therapeutic targeting. Adv Cancer Res. 2005;94:29–86. doi: 10.1016/S0065-230X(05)94002-5. [DOI] [PubMed] [Google Scholar]
- 5.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–54. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 6.LoPiccolo J, Blumenthal GM, Bernstein WB, Dennis PA. Targeting the PI3K/Akt/mTOR pathway: effective combinations and clinical considerations. Drug Resist Updat. 2008;11:32–50. doi: 10.1016/j.drup.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chandarlapaty S, Sawai A, Scaltriti M, Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, et al. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell. 2011;19:58–71. doi: 10.1016/j.ccr.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muranen T, Selfors LM, Worster DT, Iwanicki MP, Song L, Morales FC, et al. Inhibition of PI3K/mTOR Leads to Adaptive Resistance in Matrix-Attached Cancer Cells. Cancer Cell. 2012;21:227–39. doi: 10.1016/j.ccr.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakrabarty A, Sanchez V, Kuba MG, Rinehart C, Arteaga CL. Feedback upregulation of HER3 (ErbB3) expression and activity attenuates antitumor effect of PI3K inhibitors. Proc Natl Acad Sci U S A. 2012;109:2718–23. doi: 10.1073/pnas.1018001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhanasekaran SM, Barrette TR, Ghosh D, Shah R, Varambally S, Kurachi K, et al. Delineation of prognostic biomarkers in prostate cancer. Nature. 2001;412:822–6. doi: 10.1038/35090585. [DOI] [PubMed] [Google Scholar]
- 11.Hsi ED, Jung SH, Lai R, Johnson JL, Cook JR, Jones D, et al. Ki67 and PIM1 expression predict outcome in mantle cell lymphoma treated with high dose therapy, stem cell transplantation and rituximab: a Cancer and Leukemia Group B 59909 correlative science study. Leuk Lymphoma. 2008;49:2081–90. doi: 10.1080/10428190802419640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amaravadi R, Thompson CB. The survival kinases Akt and Pim as potential pharmacological targets. J Clin Invest. 2005;115:2618–24. doi: 10.1172/JCI26273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sussman MA. Mitochondrial integrity: preservation through Akt/Pim-1 kinase signaling in the cardiomyocyte. Expert Rev Cardiovasc Ther. 2009;7:929–38. doi: 10.1586/erc.09.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muraski JA, Rota M, Misao Y, Fransioli J, Cottage C, Gude N, et al. Pim-1 regulates cardiomyocyte survival downstream of Akt. Nat Med. 2007;13:1467–75. doi: 10.1038/nm1671. [DOI] [PubMed] [Google Scholar]
- 15.Gambarotta G, Pistoi S, Giordano S, Comoglio PM, Santoro C. Structure and inducible regulation of the human MET promoter. J Biol Chem. 1994;269:12852–7. [PubMed] [Google Scholar]
- 16.Pettaway CA, Pathak S, Greene G, Ramirez E, Wilson MR, Killion JJ, et al. Selection of highly metastatic variants of different human prostatic carcinomas using orthotopic implantation in nude mice. Clin Cancer Res. 1996;2:1627–36. [PubMed] [Google Scholar]
- 17.Lin YW, Beharry ZM, Hill EG, Song JH, Wang W, Xia Z, et al. A small molecule inhibitor of Pim protein kinases blocks the growth of precursor T-cell lymphoblastic leukemia/lymphoma. Blood. 2010;115:824–33. doi: 10.1182/blood-2009-07-233445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serra V, Scaltriti M, Prudkin L, Eichhorn PJ, Ibrahim YH, Chandarlapaty S, et al. PI3K inhibition results in enhanced HER signaling and acquired ERK dependency in HER2-overexpressing breast cancer. Oncogene. 2011;30:2547–57. doi: 10.1038/onc.2010.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engelman JA, Chen L, Tan X, Crosby K, Guimaraes AR, Upadhyay R, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14:1351–6. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okuzumi T, Fiedler D, Zhang C, Gray DC, Aizenstein B, Hoffman R, et al. Inhibitor hijacking of Akt activation. Nat Chem Biol. 2009;5:484–93. doi: 10.1038/nchembio.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xia Z, Knaak C, Ma J, Beharry ZM, McInnes C, Wang W, et al. Synthesis and evaluation of novel inhibitors of Pim-1 and Pim-2 protein kinases. J Med Chem. 2009;52:74–86. doi: 10.1021/jm800937p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pogacic V, Bullock AN, Fedorov O, Filippakopoulos P, Gasser C, Biondi A, et al. Structural analysis identifies imidazo[1,2-b]pyridazines as PIM kinase inhibitors with in vitro antileukemic activity. Cancer Res. 2007;67:6916–24. doi: 10.1158/0008-5472.CAN-07-0320. [DOI] [PubMed] [Google Scholar]
- 23.Hsieh AC, Truitt ML, Ruggero D. Oncogenic AKTivation of translation as a therapeutic target. Br J Cancer. 2011;105:329–36. doi: 10.1038/bjc.2011.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feldman ME, Apsel B, Uotila A, Loewith R, Knight ZA, Ruggero D, et al. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 2009;7:e38. doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chresta CM, Davies BR, Hickson I, Harding T, Cosulich S, Critchlow SE, et al. AZD8055 is a potent, selective, and orally bioavailable ATP-competitive mammalian target of rapamycin kinase inhibitor with in vitro and in vivo antitumor activity. Cancer Res. 2010;70:288–98. doi: 10.1158/0008-5472.CAN-09-1751. [DOI] [PubMed] [Google Scholar]
- 26.Thoreen CC, Chantranupong L, Keys HR, Wang T, Gray NS, Sabatini DM. A unifying model for mTORC1-mediated regulation of mRNA translation. Nature. 2012;485:109–13. doi: 10.1038/nature11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin F, Barends S, Jaeger S, Schaeffer L, Prongidi-Fix L, Eriani G. Cap-assisted internal initiation of translation of histone H4. Mol Cell. 2011;41:197–209. doi: 10.1016/j.molcel.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 28.Sherrill KW, Byrd MP, Van Eden ME, Lloyd RE. BCL-2 translation is mediated via internal ribosome entry during cell stress. J Biol Chem. 2004;279:29066–74. doi: 10.1074/jbc.M402727200. [DOI] [PubMed] [Google Scholar]
- 29.Hsieh AC, Liu Y, Edlind MP, Ingolia NT, Janes MR, Sher A, et al. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature. 2012;485:55–61. doi: 10.1038/nature10912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Komar AA, Hatzoglou M. Cellular IRES-mediated translation: the war of ITAFs in pathophysiological states. Cell Cycle. 2011;10:229–40. doi: 10.4161/cc.10.2.14472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lang KJ, Kappel A, Goodall GJ. Hypoxia-inducible factor-1alpha mRNA contains an internal ribosome entry site that allows efficient translation during normoxia and hypoxia. Mol Biol Cell. 2002;13:1792–801. doi: 10.1091/mbc.02-02-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Landry DM, Hertz MI, Thompson SR. RPS25 is essential for translation initiation by the Dicistroviridae and hepatitis C viral IRESs. Genes Dev. 2009;23:2753–64. doi: 10.1101/gad.1832209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stein I, Itin A, Einat P, Skaliter R, Grossman Z, Keshet E. Translation of vascular endothelial growth factor mRNA by internal ribosome entry: implications for translation under hypoxia. Mol Cell Biol. 1998;18:3112–9. doi: 10.1128/mcb.18.6.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stoneley M, Chappell SA, Jopling CL, Dickens M, MacFarlane M, Willis AE. c-Myc protein synthesis is initiated from the internal ribosome entry segment during apoptosis. Mol Cell Biol. 2000;20:1162–9. doi: 10.1128/mcb.20.4.1162-1169.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stoneley M, Paulin FE, Le Quesne JP, Chappell SA, Willis AE. C-Myc 5′ untranslated region contains an internal ribosome entry segment. Oncogene. 1998;16:423–8. doi: 10.1038/sj.onc.1201763. [DOI] [PubMed] [Google Scholar]
- 36.Giraud S, Greco A, Brink M, Diaz JJ, Delafontaine P. Translation initiation of the insulin-like growth factor I receptor mRNA is mediated by an internal ribosome entry site. J Biol Chem. 2001;276:5668–75. doi: 10.1074/jbc.M005928200. [DOI] [PubMed] [Google Scholar]
- 37.Chiocchetti A, Gibello L, Carando A, Aspesi A, Secco P, Garelli E, et al. Interactions between RPS19, mutated in Diamond-Blackfan anemia, and the PIM-1 oncoprotein. Haematologica. 2005;90:1453–62. [PubMed] [Google Scholar]
- 38.Iadevaia V, Caldarola S, Biondini L, Gismondi A, Karlsson S, Dianzani I, et al. PIM1 kinase is destabilized by ribosomal stress causing inhibition of cell cycle progression. Oncogene. 2010;29:5490–9. doi: 10.1038/onc.2010.279. [DOI] [PubMed] [Google Scholar]
- 39.Robledo S, Idol RA, Crimmins DL, Ladenson JH, Mason PJ, Bessler M. The role of human ribosomal proteins in the maturation of rRNA and ribosome production. RNA. 2008;14:1918–29. doi: 10.1261/rna.1132008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Badhai J, Frojmark AS, Razzaghian HR, Davey E, Schuster J, Dahl N. Posttranscriptional down-regulation of small ribosomal subunit proteins correlates with reduction of 18S rRNA in RPS19 deficiency. FEBS Lett. 2009;583:2049–53. doi: 10.1016/j.febslet.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lohrum MA, Ludwig RL, Kubbutat MH, Hanlon M, Vousden KH. Regulation of HDM2 activity by the ribosomal protein L11. Cancer Cell. 2003;3:577–87. doi: 10.1016/s1535-6108(03)00134-x. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y, Wolf GW, Bhat K, Jin A, Allio T, Burkhart WA, et al. Ribosomal protein L11 negatively regulates oncoprotein MDM2 and mediates a p53-dependent ribosomal-stress checkpoint pathway. Mol Cell Biol. 2003;23:8902–12. doi: 10.1128/MCB.23.23.8902-8912.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nawijn MC, Alendar A, Berns A. For better or for worse: the role of Pim oncogenes in tumorigenesis. Nat Rev Cancer. 2011;11:23–34. doi: 10.1038/nrc2986. [DOI] [PubMed] [Google Scholar]
- 44.Johannes G, Carter MS, Eisen MB, Brown PO, Sarnow P. Identification of eukaryotic mRNAs that are translated at reduced cap binding complex eIF4F concentrations using a cDNA microarray. Proc Natl Acad Sci U S A. 1999;96:13118–23. doi: 10.1073/pnas.96.23.13118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schatz JH, Oricchio E, Wolfe AL, Jiang M, Linkov I, Maragulia J, et al. Targeting cap-dependent translation blocks converging survival signals by AKT and PIM kinases in lymphoma. J Exp Med. 2011;208:1799–807. doi: 10.1084/jem.20110846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meng Z, King PH, Nabors LB, Jackson NL, Chen CY, Emanuel PD, et al. The ELAV RNA-stability factor HuR binds the 5′-untranslated region of the human IGF-IR transcript and differentially represses cap-dependent and IRES-mediated translation. Nucleic Acids Res. 2005;33:2962–79. doi: 10.1093/nar/gki603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.