Abstract
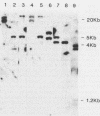
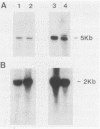
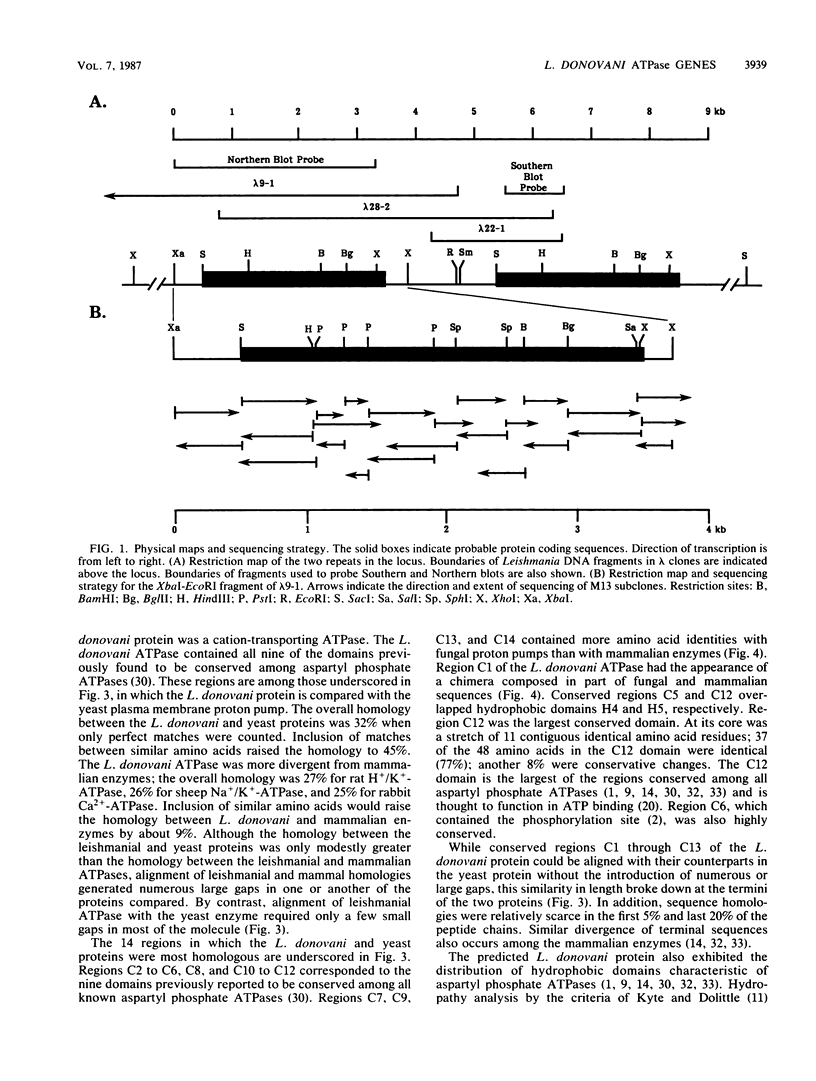
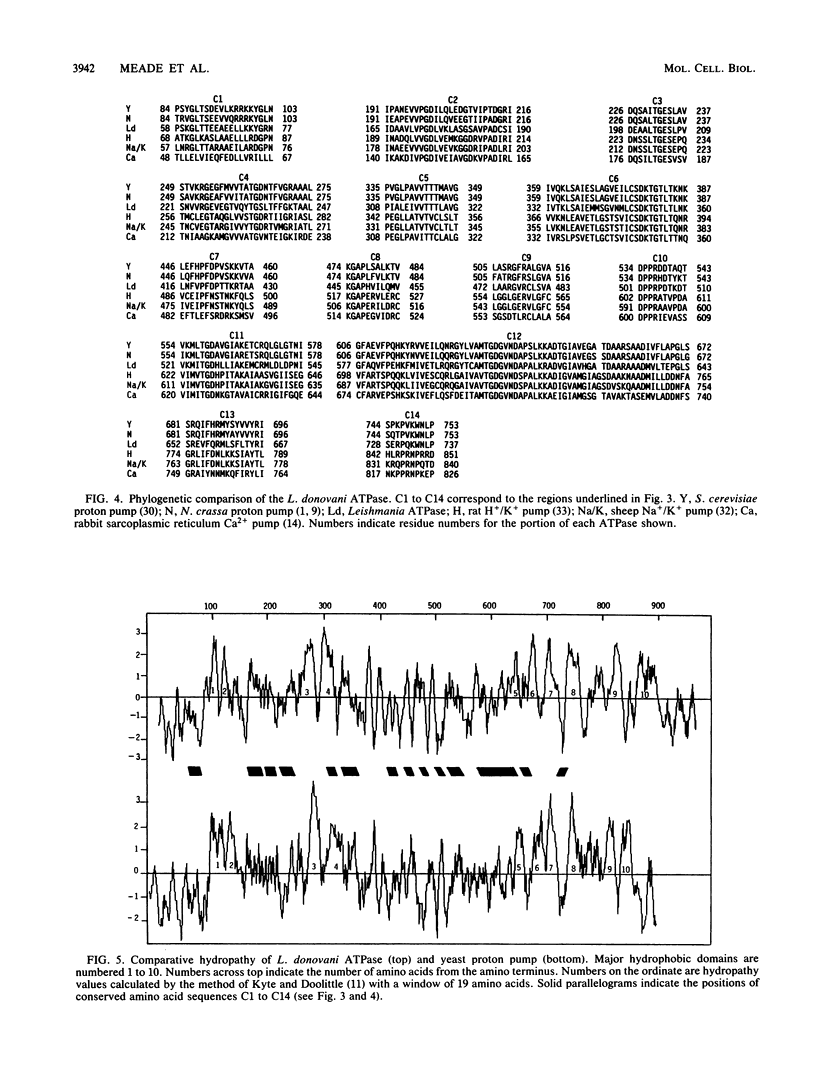
An oligonucleotide probe was used to clone a cation-transporting ATPase gene from the genome of Leishmania donovani. The nucleotide sequence of the gene contained a 2,922-base-pair open reading frame that was predicted to encode a 107,406-dalton protein composed of 974 amino acids. The predicted L. donovani protein contained all the structural and functional domains expected to be present in a cation-transporting ATPase of the aspartyl phosphate class. The nucleotide sequence encoding the ATPase gene was duplicated in tandem in the parasite genome. Partial sequenation of the second member of the tandem repeat, which lay 2 kilobase pairs downstream of the ATPase gene, indicated that it was either identical to the first gene or very closely related to it. RNA homologous to either the ATPase gene or its adjacent relative was 5 kilobases in size and was approximately equally abundant in both promastigote and amastigote forms of the organism.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addison R. Primary structure of the Neurospora plasma membrane H+-ATPase deduced from the gene sequence. Homology to Na+/K+-, Ca2+-, and K+-ATPase. J Biol Chem. 1986 Nov 15;261(32):14896–14901. [PubMed] [Google Scholar]
- Allen G., Green N. M. A 31-residue tryptic peptide from the active site of the [Ca++]-transporting adenosine triphosphatase of rabbit sarcoplasmic reticulum. FEBS Lett. 1976 Mar 15;63(1):188–192. doi: 10.1016/0014-5793(76)80223-2. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berens R. L., Brun R., Krassner S. M. A simple monophasic medium for axenic culture of hemoflagellates. J Parasitol. 1976 Jun;62(3):360–365. [PubMed] [Google Scholar]
- Beverley S. M., Ellenberger T. E., Cordingley J. S. Primary structure of the gene encoding the bifunctional dihydrofolate reductase-thymidylate synthase of Leishmania major. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2584–2588. doi: 10.1073/pnas.83.8.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd D., Jain S. K., Crampton J., Barrett K. J., Drysdale J. Isolation and characterization of a cDNA clone for human ferritin heavy chain. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4751–4755. doi: 10.1073/pnas.81.15.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Hager K. M., Mandala S. M., Davenport J. W., Speicher D. W., Benz E. J., Jr, Slayman C. W. Amino acid sequence of the plasma membrane ATPase of Neurospora crassa: deduction from genomic and cDNA sequences. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7693–7697. doi: 10.1073/pnas.83.20.7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Landfear S. M., Miller S. I., Wirth D. F. Transcriptional mapping of Leishmania enrietti tubulin mRNAs. Mol Biochem Parasitol. 1986 Dec;21(3):235–245. doi: 10.1016/0166-6851(86)90129-5. [DOI] [PubMed] [Google Scholar]
- Landfear S. M., Wirth D. F. Control of tubulin gene expression in the parasitic protozoan Leishmania enriettii. Nature. 1984 Jun 21;309(5970):716–717. doi: 10.1038/309716a0. [DOI] [PubMed] [Google Scholar]
- MacLennan D. H., Brandl C. J., Korczak B., Green N. M. Amino-acid sequence of a Ca2+ + Mg2+-dependent ATPase from rabbit muscle sarcoplasmic reticulum, deduced from its complementary DNA sequence. Nature. 1985 Aug 22;316(6030):696–700. doi: 10.1038/316696a0. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade J. C., Glaser T. A., Bonventre P. F., Mukkada A. J. Enzymes of carbohydrate metabolism in Leishmania donovani amastigotes. J Protozool. 1984 Feb;31(1):156–161. doi: 10.1111/j.1550-7408.1984.tb04307.x. [DOI] [PubMed] [Google Scholar]
- Michels P. A., Poliszczak A., Osinga K. A., Misset O., Van Beeumen J., Wierenga R. K., Borst P., Opperdoes F. R. Two tandemly linked identical genes code for the glycosomal glyceraldehyde-phosphate dehydrogenase in Trypanosoma brucei. EMBO J. 1986 May;5(5):1049–1056. doi: 10.1002/j.1460-2075.1986.tb04321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchinson C., Wilderspin A. F., Trinnaman B. J., Green N. M. Identification of a labelled peptide after stoicheiometric reaction of fluorescein isothiocyanate with the Ca2+ -dependent adenosine triphosphatase of sarcoplasmic reticulum. FEBS Lett. 1982 Sep 6;146(1):87–92. doi: 10.1016/0014-5793(82)80710-2. [DOI] [PubMed] [Google Scholar]
- Morales N. M., Schaefer F. W., 3rd, Keller S. J., Meyer R. R. Effects of ethidium bromide and several acridine dyes on the kinetoplast DNA of Leishmania tropica. J Protozool. 1972 Nov;19(4):667–672. doi: 10.1111/j.1550-7408.1972.tb03557.x. [DOI] [PubMed] [Google Scholar]
- Mukkada A. J., Meade J. C., Glaser T. A., Bonventre P. F. Enhanced metabolism of Leishmania donovani amastigotes at acid pH: an adaptation for intracellular growth. Science. 1985 Sep 13;229(4718):1099–1101. doi: 10.1126/science.4035350. [DOI] [PubMed] [Google Scholar]
- Noda M., Ikeda T., Suzuki H., Takeshima H., Takahashi T., Kuno M., Numa S. Expression of functional sodium channels from cloned cDNA. 1986 Aug 28-Sep 3Nature. 322(6082):826–828. doi: 10.1038/322826a0. [DOI] [PubMed] [Google Scholar]
- Osinga K. A., Swinkels B. W., Gibson W. C., Borst P., Veeneman G. H., Van Boom J. H., Michels P. A., Opperdoes F. R. Topogenesis of microbody enzymes: a sequence comparison of the genes for the glycosomal (microbody) and cytosolic phosphoglycerate kinases of Trypanosoma brucei. EMBO J. 1985 Dec 30;4(13B):3811–3817. doi: 10.1002/j.1460-2075.1985.tb04152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queen C., Korn L. J. A comprehensive sequence analysis program for the IBM personal computer. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):581–599. doi: 10.1093/nar/12.1part2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sather S., Agabian N. A 5' spliced leader is added in trans to both alpha- and beta-tubulin transcripts in Trypanosoma brucei. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5695–5699. doi: 10.1073/pnas.82.17.5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano R., Kielland-Brandt M. C., Fink G. R. Yeast plasma membrane ATPase is essential for growth and has homology with (Na+ + K+), K+- and Ca2+-ATPases. Nature. 1986 Feb 20;319(6055):689–693. doi: 10.1038/319689a0. [DOI] [PubMed] [Google Scholar]
- Shull G. E., Lingrel J. B. Molecular cloning of the rat stomach (H+ + K+)-ATPase. J Biol Chem. 1986 Dec 25;261(36):16788–16791. [PubMed] [Google Scholar]
- Shull G. E., Schwartz A., Lingrel J. B. Amino-acid sequence of the catalytic subunit of the (Na+ + K+)ATPase deduced from a complementary DNA. Nature. 1985 Aug 22;316(6030):691–695. doi: 10.1038/316691a0. [DOI] [PubMed] [Google Scholar]
- Swinkels B. W., Gibson W. C., Osinga K. A., Kramer R., Veeneman G. H., van Boom J. H., Borst P. Characterization of the gene for the microbody (glycosomal) triosephosphate isomerase of Trypanosoma brucei. EMBO J. 1986 Jun;5(6):1291–1298. doi: 10.1002/j.1460-2075.1986.tb04358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschudi C., Young A. S., Ruben L., Patton C. L., Richards F. F. Calmodulin genes in trypanosomes are tandemly repeated and produce multiple mRNAs with a common 5' leader sequence. Proc Natl Acad Sci U S A. 1985 Jun;82(12):3998–4002. doi: 10.1073/pnas.82.12.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walder J. A., Eder P. S., Engman D. M., Brentano S. T., Walder R. Y., Knutzon D. S., Dorfman D. M., Donelson J. E. The 35-nucleotide spliced leader sequence is common to all trypanosome messenger RNA's. Science. 1986 Aug 1;233(4763):569–571. doi: 10.1126/science.3523758. [DOI] [PubMed] [Google Scholar]
- Zilberstein D., Dwyer D. M., Matthaei S., Horuk R. Identification and biochemical characterization of the plasma membrane glucose transporter of Leishmania donovani. J Biol Chem. 1986 Nov 15;261(32):15053–15057. [PubMed] [Google Scholar]
- Zilberstein D., Dwyer D. M. Protonmotive force-driven active transport of D-glucose and L-proline in the protozoan parasite Leishmania donovani. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1716–1720. doi: 10.1073/pnas.82.6.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]