Abstract
Diabetes mellitus affects virtually every organ system in the body and the degree of organ involvement depends on the duration and severity of the disease, and other co-morbidities. Gastrointestinal (GI) involvement can present with esophageal dysmotility, gastro-esophageal reflux disease (GERD), gastroparesis, enteropathy, non alcoholic fatty liver disease (NAFLD) and glycogenic hepatopathy. Severity of GERD is inversely related to glycemic control and management is with prokinetics and proton pump inhibitors. Diabetic gastroparesis manifests as early satiety, bloating, vomiting, abdominal pain and erratic glycemic control. Gastric emptying scintigraphy is considered the gold standard test for diagnosis. Management includes dietary modifications, maintaining euglycemia, prokinetics, endoscopic and surgical treatments. Diabetic enteropathy is also common and management involves glycemic control and symptomatic measures. NAFLD is considered a hepatic manifestation of metabolic syndrome and treatment is mainly lifestyle measures, with diabetes and dyslipidemia management when coexistent. Glycogenic hepatopathy is a manifestation of poorly controlled type 1 diabetes and is managed by prompt insulin treatment. Though GI complications of diabetes are relatively common, awareness about its manifestations and treatment options are low among physicians. Optimal management of GI complications is important for appropriate metabolic control of diabetes and improvement in quality of life of the patient. This review is an update on the GI complications of diabetes, their pathophysiology, diagnostic evaluation and management.
Keywords: Gastrointestinal complications, Diabetes mellitus, Esophageal complications, Nonalcoholic fatty liver disease, Diabetic gastroparesis, Diabetic enteropathy, Glycogenic hepatopathy
Core tip: Although relatively common, gastrointestinal (GI) complications of diabetes mellitus are under-recognized by most physicians. Early identification and prompt management of GI complications are of paramount importance as they are associated with significant morbidity. Common GI complications are esophageal dysmotility, gastro-esophageal reflux disease, gastroparesis, enteropathy, non alcoholic fatty liver disease (NAFLD) and glycogenic hepatopathy. Damage to the myenteric neurons due to longstanding diabetes causes esophageal, gastric and enteric disease. NAFLD is a hepatic manifestation of metabolic syndrome and is commonly seen in type 2 diabetes while glycogenic hepatopathy is due to poor glycemic control in type 1 diabetes. Clinical manifestations, pathogenesis, diagnostic evaluation and management of GI complications of diabetes are discussed in this article.
INTRODUCTION
The prevalence of diabetes mellitus has now reached epidemic proportions in both developed and developing countries, affecting more than 366 million people worldwide[1]. This number is likely to increase in the coming years as a result of an ageing global population, urbanization, rising prevalence of obesity and sedentary lifestyles. Diabetes affects virtually every organ system in the body and the duration and severity of the disease may have a direct impact on organ involvement. Though gastrointestinal (GI) complications are common in longstanding diabetes, the awareness of these complications is low among physicians. Early identification and appropriate management of GI complications are important for improving both diabetic care and quality of life of the affected patient. This review aims to outline the GI complications of diabetes and the latest management options.
ESOPHAGEAL COMPLICATIONS
The thoracic esophagus and lower esophageal sphincter (LES) are composed of smooth muscle fibres innervated by myenteric plexus, and these autonomic nerves can be affected by diabetic neuropathy in patients with longstanding diabetes. Autonomic neuropathy and structural remodeling of the esophageal musculature in diabetes results in abnormal peristalsis, spontaneous contractions and reduced LES tone[2]. Morphological and biomechanical properties of the esophagus have been found to be altered significantly in animal models of diabetes[3]. The prevalence of esophageal dysmotility in diabetes has been reported to be as high as 63%[4]. The same study also found that there was no difference in dysmotility between patients with type 1 and type 2 diabetes or between genders and there was a strong association with retinopathy. Patients with dysmotility had longer duration of diabetes compared with those without dysmotility. Although the prevalence of esophageal dysmotility is high among patients with diabetes, only a minority present with the classical symptoms of dysphagia and heartburn[5].
The prevalence of gastroesophageal reflux symptoms in diabetes could be as high as 41%[6]. Erosive esophagitis (EE) was more frequent (66.7%) in diabetic patients with neuropathy than those without neuropathy (33.3%); also asymptomatic EE was significantly more frequent in the same group. In patients with type 2 diabetes, peripheral neuropathy is an independent risk factor for EE; however patients may be asymptomatic and a gastroscopy may be recommended in these patients[7]. Circulating levels of adiponectin, a potential anti-inflammatory adipocytokine is inversely related to visceral fat accumulation and it has been shown that the prevalence of gastroesophageal reflux disease is higher in type 2 diabetic patients with metabolic syndrome and low levels of serum adiponectin[8].
Diagnosis of reflux and dysmotility has relied on esophageal pH monitoring and conventional manometry for many years. The use of the wireless Bravo pH capsule, which allows catheter-free monitoring and impedance-pH measurement, a catheter-based technique which allows detection of acid and non-acid reflux have been major developments in the diagnostic field recently[9]. Two new procedures are available to assess esophageal motility: high resolution manometry which uses many pressure sensors and provides spatiotemporal plots of esophageal pressure changes; and impedance manometry, a test that directly measures bolus transit and provides conventional manometric data[9].
Gastroesophageal reflux disease was found to be inversely related to glycemic control and better glycemic control may improve esophageal dysmotility and reflux[10]. Management of reflux disease involves prokinetic drugs, such as metoclopramide and proton pump inhibitors. A two-week course of erythromycin has been shown to reduce mean esophageal transit time and gastric emptying time in type 2 diabetics[11]. Patients are also advised to drink fluids immediately after taking medications to avoid pill-induced esophagitis.
GASTROPARESIS
Gastroparesis, one of the commonest GI complications of diabetes mellitus, produces symptoms of gastric retention in the absence of physical obstruction[12]. The incidence of gastroparesis in a population with diabetes is reportedly low (5.2% over 10 years in type 1 and 1% in type 2 diabetes), but greater than in the general population (0.2%)[13]. Delayed gastric emptying can be demonstrated in 27%-65% of patients with type 1 diabetes and about 30% of patients with type 2 diabetes[14]. The incidence of gastroparesis is higher in women[15]. A recent study has reported obesity as a significant independent predictor of symptoms suggestive of gastroparesis in patients with type 2 diabetes mellitus (T2DM) and neuropathy[16].
Pathogenesis
The pathogenesis of diabetic gastroparesis is multifactorial and currently poorly understood. Delayed gastric emptying may be the first indication of gastroparesis in diabetes[15]. Elevated glycated hemoglobin level, duration of diabetes in excess of 10 years and the presence of macro- and microvascular complications are all accepted risk factors for the development of diabetic gastroparesis. Delayed gastric emptying contributes to poor glycemic control and may be the first indication that the patient is developing gastroparesis. Loss of the normal Migrating Motor Complexes, blunted antral contractions, spasm of the pylorus and small intestine and poor meal accommodation in the stomach are all demonstrable in diabetes[12]. Other factors that may have a role in the pathogenesis includes impaired inhibitory nitric oxide containing nerves, absent or dysmorphic interstitial cells of Cajal, smooth muscle fibrosis and abnormal macrophage- containing immune infiltrates[17,18]. Bezoar formation can contribute to the development of gastroparesis in some individuals. Endoscopic biopsies from diabetic gastroparesis demonstrate abnormal mucosal nerve density and morphology, reflecting possible potential for endoscopic diagnosis of enteric neuropathy[19]. Neurohumoral factors including glucagon-like peptide-1 (GLP-1) can play a role in gastroparesis and the use of GLP-1 agonists Exenatide and Liraglutide can lead to symptoms of gastroparesis. A recent study showed that deficiency of apolipoprotein E can be a risk factor in diabetic gastroparesis in an animal model[20]. Extrinsic factors such as medications as well as concomitant disorders such as anxiety and depression may result in increased reporting of symptoms.
Clinical features
Symptoms of gastroparesis include nausea, vomiting, early satiety, postprandial fullness, bloating and upper abdominal pain. Worsening glycemic control along with frequent hypoglycemic episodes or unexplained alternating hyper- and hypoglycemia due to a mismatch between insulin action and carbohydrate absorption should prompt the clinician to evaluate the patient for diabetic gastroparesis. About 53% of patients may experience weight loss but 18%-24% may experience weight gain[14]. More than half of affected individuals present with acute onset of symptoms and the others insidiously. One third of cases have chronic symptoms with periodic exacerbations and one third have chronic worsening symptoms[14]. Epigastric distention and succussion splash may be observed in some patients but physical examination may not be always helpful.
Evaluation
A technical review from the American Gastroenterological Association recommends performing an initial evaluation consisting of careful history taking and physical examination, followed by complete blood count, thyroid stimulating hormone test, metabolic panel and optional amylase and pregnancy test[21]. History taking should particularly focus on macro- and micro-vascular complications of diabetes, although gastroparesis may occur in their absence. Additionally rumination syndrome should be excluded. Physical examination should focus on looking for evidence of peripheral and autonomic neuropathy, epigastric distension and the presence of succussion splash one hour post mealtimes. This is followed by upper GI endoscopy to rule out mechanical obstruction. Alternatively, an upper GI series with small bowel follow-through or small bowel magnetic resonance imaging can be performed. In the presence of significant abdominal pain, an abdominal ultrasound scan should be carried out to rule out biliary colic[16]. Presence of food in the stomach at endoscopy following a 12-h fast, in the absence of gastric outlet obstruction, is strongly suggestive of gastroparesis.
The diagnosis of gastroparesis is made by gastric emptying scintigraphy using 99mTc sulphur colloid bound to solid food[21]. This noninvasive, quantitative method is considered the gold standard test for diagnosing gastroparesis. The patient ingests a technetium-labeled egg meal and gastric emptying is then measured by scintiscanning at 15-min intervals for 4 h. However this test lacks standardization. A newer four image simplified scanning method has also shown comparable results[22]. The American Neurogastroenterology and Motility Society recommended a test meal of two slices of bread with jam plus two large eggs labeled with technetium-99m sulphur colloid and scintigraphy carried out at 0, 1, 2 and 4 h post prandially. A diagnosis of gastroparesis can be made if there is > 90% retention at 1 h, > 60% at 2 h and > 10% at 4 h[23].
An alternative method for gastric emptying study uses an indigestible wireless motility capsule (WMC), which senses intraluminal pH, temperatures and pressures as it traverses the gastrointestinal tract. The capsule wirelessly transmits the data to a receiver worn by the patient until it is excreted. WMC gastric emptying times greater than 5 h are said to be delayed, and this correlates with scintigraphic measurements[24]. Non-radioactive 13C-breath tests quantify exhaled 13CO2 after duodenal assimilation of a standardized substrate (octanoate, spirulina platensis) and are an alternative to scintigraphy[25]. The main advantage of these newer technologies is the lack of radiation exposure; however their general availability is limited.
Selected patients can be offered additional testing to exclude other contributions to the symptoms. Antroduodenal manometry excludes small bowel dysmotility, found in 17%-85% of gastroparetics[12]. Electrogastrography (EGG) can be used to detect rhythm disruptions and blunted postprandial responses. However a recent study has demonstrated the relative insensitivity of clinical EGG methodologies[26].
Treatment
Gastroparesis treatments include general measures, dietary modifications, medications that enhance emptying or lessen vomiting, non-medication interventions, psychological therapies and consideration of more invasive surgical treatment[12]. A grading system for assessing severity and guiding the management of gastroparesis has been suggested (Table 1)[27].
Table 1.
Classification of severity of gastroparesis
| Grade 1: Mild | Symptoms easily controlled |
| Regular diet/minor dietary modifications helps to maintain normal nutritional status | |
| Grade 2: Compensated | Moderate symptoms that are reasonably controlled with prokinetics and anti-emetics |
| Maintenance of nutrition with diet/lifestyle changes | |
| Hospitalizations-infrequent | |
| Grade 3: Gastric failure | Refractory symptoms |
| Inadequate nutrition | |
| Needing hospitalization for therapy and nutritional supplementation (either enteral or parenteral) | |
| May need surgical or endoscopic intervention or gastric "pacemaker" |
Originated from Abell et al[27].
General approaches and dietary modifications
General approaches to management of gastroparesis include ensuring good hydration, correcting electrolyte imbalances, management of glycemic control and symptom reduction with pharmacotherapeutic agents. Any medications that can delay gastric emptying should be discontinued if possible. Dietary modifications include increasing liquid-based meals (as the rate of emptying liquid from the stomach is usually the same in diabetic gastroparesis), reducing fat and non-digestible fibre intake, avoiding large meals with high calorie contents and ensuring small frequent meals spread throughout the day.
Maintaining euglycemia has been one of the main principles of managing diabetic gastroparesis. Prolonged postprandial hyperglycemia has been observed in patients with diabetic gastroparesis compared to those with normal gastric emptying[28]. Another study observed a reduction of 1.8% in hemoglobin A1C after initiating insulin pump therapy[29]. This eventually reduced the number and length of hospitalizations for diabetic gastroparetics.
Prokinetics
Prokinetics are medications that augment gastrointestinal motility. In general these increase gastric motility and enhance stomach emptying. Medications commonly used in treatment are shown in Table 2.
Table 2.
Drugs useful in treatment of diabetic gastroparesis
| Drug/drug group | Mechanism of action | Common side effects | Efficacy |
| Metoclopramide 10 mg 4 times/d | Anti-emetic, reduces nausea and post-prandial fullness, increases gastro-esophageal sphincter tone and improves antro-pyloro-duodenal coordination | Tardive dyskinesia, drowsiness, irritability, extrapyramidal symptoms and dystonic reactions | Symptom control in 1/3 to 2/3 of patients |
| Domperidone 10 -20 mg 3 times/d | Similar to metoclopramide with fewer CNS side effects due to a predominant peripheral mechanism of action | May prolong QTc interval in ECG; in turn may provoke cardiac arrhythmia | Effective in up to 60% of cases; tachyphylaxis develops in a few weeks requiring discontinuation |
| Erythromycin 50-250 mg thrice daily | Motilin receptor agonist. Reduces gastric emptying time | Nausea and vomiting at high doses | Modest symptom control Intravenous form can be useful in refractory vomiting |
| Promethazine, prochlorperazine and chlorpromazine | Mechanism of antiemesis poorly understood | Drowsiness, liver injury and extrapyramidal effects | Marginal improvement of symptoms Intramuscular chlorpromazine is very effective in refractory vomiting |
| Ondansetron | Central serotonin receptor (5-HT3) antagonist Inhibits vagus nerve | Extrapyramidal effect | Modest efficacy |
Originated from Hasler[12]. CNS: Central nervous system; ECG: Electrocardiogram; QTc: Corrected QT interval.
Mosapride is a selective 5-HT4 agonist that accelerates gastric emptying. Orally administered mosapride citrate has been associated with significantly increased food intake in ob/ob obese mice, with a tendency to decrease fasting blood glucose and fructosamine concentrations compared with controls[30]. A recent study reported symptom reductions in interferon induced gastroparesis in hepatitis C patients, treated with mosapride[31]. Other agents with gastric stimulating effects in gastroparesis include the new 5-HT4 agonists prucalopride, velusetrag, naronapride and the acetycholinesterase inhibitor acotiamide, although their benefits are yet to be proven[12].
Ghrelin is peptide hormone secreted by the gastric fundic mucosa and pancreas. It is the first identified circulating hormone that controls hunger. One important physiological action of ghrelin is regulation of gastric motility[32]. Intravenous use of the ghrelin agonist TZP-101 was reported to reduce nausea and vomiting in patients with diabetic gastroparesis when compared to placebo[33]. Another study with the oral ghrelin analog TZP-102 also reported overall and individual reduction in the symptoms of diabetic gastroparetics[34].
Some published case reports have also claimed efficacy for the dopamine antagonist thiethylpeazine, the neurokinin NK1 antagonist aprepitant and the antidepressant mirtazapine. A retrospective study reported decreased symptoms in 88% of diabetics with tricyclic antidepressants. The herbal extract STW5 (iberogast) is also reported to be beneficial in functional dyspepsia and gastroparesis[12].
Endoscopic and surgical treatments
Mearin et al[35] proposed pyloric spasmodic contractions as one of the factors delaying gastric emptying. Endoscopic pyloric injections of botulinum toxin have been tried in the management of gastroparesis. This neurotoxin inhibits the release of acetylcholine at the neuromuscular junction, causing paralysis of the pylorus. Improved symptoms and accelerated gastric emptying persisting up to 3-6 mo were reported with pyloric botulinum toxin injections, especially in women and those with idiopathic gastroparesis[36]. It was also observed to be more beneficial in older men with vomiting[37]. However, small underpowered placebo-controlled trials did not show superior responses for botulinum toxins vs placebo.
Gastric electrical stimulator implantations have also been shown to have benefits extending for more than 10 years and giving up to 80% reductions in nausea and vomiting. Additionally, there are reported improvements in nutritional and metabolic status, quality of life and health care utilizations[38]. Despite this, most studies show no effect on measured gastric emptying. One recent study showed improved symptoms in gastroparetics with gastric stimulators due to reduced gastric retention in diabetic patients[39]. Other newer technology in this field includes use of miniature wireless gastric stimulators inserted during endoscopy[40]. More studies are needed to ascertain the efficacy compared to other procedures.
Surgical treatments are rarely performed and are mainly reserved for patients with refractory gastroparetic symptoms who have failed to improve with other measures. A recent study demonstrated about 83% symptom reduction in gastroparetics after Heineke-Mikulicz pyloroplasty[41]. Completion gastrectomy was shown to give long-term symptom relief in some patients with post surgical gastroparesis, but data on patients with diabetic gastroparesis are limited. The possible benefits of pancreatic transplants for diabetic gastroparesis have not been proved[12].
Other measures include jejunostomy feeding and total parenteral nutrition. Jejunostomy feeding improves overall health and shows trends towards reduced healthcare utilization in diabetic gastroparesis[42]. The role of venting percutaneous gastrostomy in refractory idiopathic gastroparesis is controversial. One study reported symptom improvement as well as improvement in nutritional and functional status in patients with idiopathic gastroparesis[43]. Total parenteral nutrition can reverse rapid weight loss and ensure adequate sustenance and is usually used in patients with associated intestinal dysmotility[12].
ENTEROPATHY
Small intestinal and colorectal dysfunctions are common in patients with longstanding diabetes, especially in those with gastroparesis[44]. Diabetes-related enteropathy may present with diarrhea, constipation or fecal incontinence. The mechanism of development of enteropathy is similar to that of upper GI involvement in diabetes[45]. Advanced glycation end products (AGEs) cause damage to cellular DNA and tissues in diabetes. AGEs and their receptors are increased in the ganglia, crypt and brush border of diabetic jejunum and ileum as well as in the ganglia of diabetic colon in animal models[46]. Damage to the myenteric nerve plexus due to autonomic neuropathy and fibrosis of the intestinal muscular layers result in stasis of the intestinal contents. Reduced bowel motility results in constipation that may sometimes lead to overflow incontinence. Small intestinal bacterial overgrowth (SIBO), which can result in diarrhea, is usually a consequence of intestinal stasis.
Constipation alternating with diarrhea is one of the most common symptoms of diabetic enteropathy. The diarrhea is typically painless, may be associated with fecal incontinence and occurs during the day but more often at night[47]. Characteristically, it is seen in patients with poorly controlled diabetes who have peripheral and autonomic neuropathy[48]. Other causes of diarrhea in diabetics include pancreatic insufficiency, bile salt malabsorption, steatorrhea and drugs (Metformin). These should be excluded by appropriate investigations before making a diagnosis of diabetic enteropathy.
Constipation is a common problem affecting up to 60% of patients with long-standing diabetes mellitus[49]. Severe constipation leading to megacolon or colonic intestinal pseudo-obstruction occurs rarely. Stercoral ulcer, perforation and overflow diarrhea are encountered infrequently.
Fecal incontinence, particularly nocturnal, due to internal and external sphincter dysfunction secondary to autonomic neuropathy is a troublesome symptom. Acute hyperglycemia has been shown to inhibit external anal sphincter function and decrease rectal compliance, potentially increasing the risk of fecal incontinence[50].
Patients should undergo endoscopic examination, ultrasound or computed tomography to exclude other diagnosis. Although aspiration and direct culture of jejunal contents are regarded by many as the gold standards for the diagnosis of SIBO[51], these methods have several limitations, including the potential for contamination by oropharyngeal bacteria during intubation, and the fact that bacterial overgrowth may be patchy and may be missed by a single aspiration. Non-invasive diagnostic tests for SIBO are largely based on excretion of hydrogen in exhaled breath, following metabolism of carbohydrate by luminal bacteria. These tests have a specificity of 80%, but lack sensitivity (40%) and have their own limitations[52]. A radio opaque marker test is useful for excluding possible slow transit constipation. Tests for fecal incontinence include endoanal ultrasound and anorectal manometry.
Treatment of diabetic diarrhea mainly involves symptom relief, correction of fluid and electrolyte deficits, improvement of nutrition and glycemic control, and management of underlying causes[53]. Anti-diarrheal agents should be used with caution as there is a risk of toxic megacolon. Rifaximin is a minimally absorbed oral antimicrobial agent that is concentrated in the gastrointestinal tract, has broad spectrum in vitro activity against gram-positive and gram-negative aerobic and anaerobic bacteria, and has low risk of inducing bacterial resistance[54,55]. It has been shown to eradicate bacterial overgrowth in up to 84% of patients[56]. Other antibiotics used to treat this condition include amoxicillin-clavulanic acid, doxycycline, ciprofloxacin, metronidazole, neomycin and norfloxacin. There are anecdotal reports of successful treatment with somatostatin analogues of otherwise intractable secretory diarrhea in diabetic patients with autonomic neuropathy[57,58].
Loperamide may prove useful in fecal incontinence. Constipation may be treated with prompt hydration, regular exercise and increased intake of dietary fibre. Lactulose and osmotic laxatives may be necessary in more severe cases. Newer drugs for treatment of chronic constipation include prucalopride, a selective 5-HT4 receptor agonist that enhances colonic transit and lubiprostone, which stimulates colonic water and electrolyte secretion through activation of type 2 chloride channels in enterocytes. They may prove useful in the future for treatment of chronic constipation in diabetes mellitus due to autonomic neuropathy and slow transit.
NONALCOHOLIC FATTY LIVER DISEASE
The definition of nonalcoholic fatty liver disease (NAFLD) requires that there is evidence of hepatic steatosis, either by imaging or by histology, and that there are no causes for secondary hepatic fat accumulation such as significant alcohol consumption, use of steatogenic medication or hereditary disorders[59]. NAFLD is considered to be the hepatic manifestation of metabolic syndrome[60]. Metabolic syndrome encompasses the clinical tetrad of hyperinsulinemia with insulin resistance, visceral obesity, dyslipidemia and hypertension. In the majority of patients, NAFLD is associated with metabolic risk factors such as obesity (60%-95%), diabetes mellitus (28%-55%) and dyslipidemia (27%-92%) and, less clearly, with raised arterial pressure[61]. Histologically, NAFLD is further subdivided into nonalcoholic fatty liver (NAFL) and nonalcoholic steatohepatitis (NASH).
Data from various studies indicate that the prevalence of NAFLD in the general population ranges from 6.3% to 33%. NAFLD is now the most common cause of chronic liver disease in North America, and it is estimated that 30% of the population of the United States has NAFLD[62]. In an ultrasonographic study, 69% of patients with T2DM had NAFLD[63]. Another study showed a prevalence of 62.3% (127 of the 204 diabetes patients had a fatty infiltration on ultrasound) and 87% of these patients with fatty infiltration who consented to biopsy had histological confirmation of the condition[64].
Clinical features, course and prognosis
Although the majority of patients with NAFLD are asymptomatic, some may present with nonspecific symptoms such as malaise and right upper quadrant pain. Clinical disease in NAFLD ranges from mild elevation of liver enzymes to severe liver disease with fibrosis and nodular degeneration. A recent study identified that approximately 30% of NAFLD cases with isolated steatosis will progress to NASH and, of these, approximately 20% will develop cirrhosis. About 40% of these cirrhotic patients develop decompensated liver disease[65].
Patients with simple fatty change had no increase in mortality, whereas patients with NASH had reduced survival and more cases died from cardiovascular disease (15.5% vs 7.5%) than liver related disease (2.8% vs 0.2%)[66]. Another long term study, conducted in Minnesota United States, of 420 patients in the community with NAFLD showed higher mortality in patients with impaired fasting glucose and cirrhosis, when compared with the general population. Liver-related mortality was also higher in this group than in the general population (13% vs < 1%)[67].
Hepatocellular carcinoma (HCC) is a well recognized complication of cirrhosis due to NAFLD[68-71]. Diabetes, obesity and cirrhosis-associated carcinogenic factors may have roles in the development of HCC in patients with NAFLD[68,70,72]. Presence of diabetes, elevated body mass index and liver fibrosis were identified as risk factors for progression to HCC among NAFLD cases[73]. Recent evidence from animal models shows that metabolic syndrome itself is high risk state for the development of NASH and HCC[74].
Pathogenesis
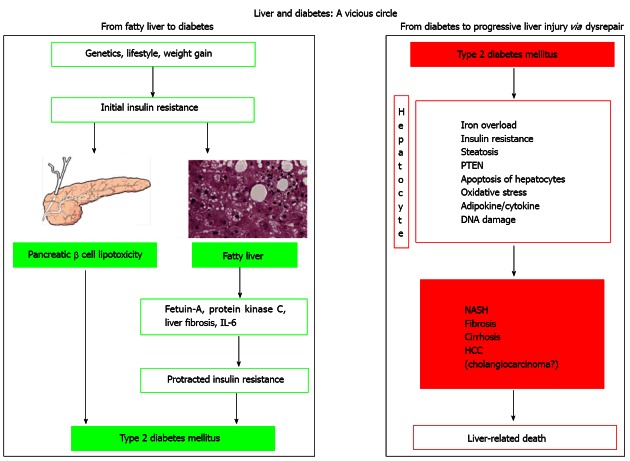
The development of NAFLD involves complex mechanisms and the relationship between T2DM and NAFLD is depicted in the Figure 1. Obesity, insulin resistance and metabolic syndrome are linked to the development of NAFLD[75]. It is now postulated that a combination of “multi hits” leads to development of steatohepatitis. This concept has replaced the earlier two hit hypothesis[76,77]. There is strong association between NASH, insulin resistance and increased level of free fatty acids in the liver[78,79]. Several factors including tumor necrosis factor alpha, oxidative stress, adiponectin, leptin, apoptosis and genetic factors are believed to have a role in the pathogenesis of NAFLD and NASH.
Figure 1.
Molecular mechanisms involved in the vicious circle linking fatty liver to diabetes and diabetes to progressive liver injury. Left: The first part of the journey, leading from initial insulin resistance to fatty liver and eventually to the development of type 2 diabetes mellitus (T2DM) in those predisposed individuals in whom pancreatic lipotoxicity occurs; Right: The mechanism that (triggered by long-lasting/decompensated T2DM) may be conducive to progressive liver disease including primary liver cancer in predisposed individuals. HCC: Hepatocellular carcinoma; IL: Interleukin; NASH: Non-alcoholic steatohepatitis; PTEN: Phosphatase and tensin homolog. Reproduced from Loria et al[121].
Evaluation
According to guidelines from the American Association for the Study of Liver Diseases (AASLD), the diagnosis of NAFLD requires that there is hepatic steatosis by imaging or histology, there is no significant alcohol overconsumption, there are no competing etiologies for hepatic steatosis, and there are no co-existing causes for chronic liver disease[59].
The following conditions should be excluded: history of alcohol intake > 20 g/d, nutritional causes (e.g., total parenteral nutrition and rapid weight loss), metabolic disorders (glycogen storage disorders), chronic hepatitis C (particularly genotype 3), other causes of chronic liver diseases (autoimmune liver disease, Wilson’s disease and hemochromatosis) and endocrine disorders such as polycystic ovary syndrome, hypopituitarism and hypothyroidism. Drug-induced steatosis can be caused by a number of agents including glucocorticoids, synthetic estrogens, amiodarone, methotrexate and highly active antiretroviral drugs. NAFL is considered benign whereas NASH can progress to cirrhosis, liver failure, and liver cancer.
Liver biopsy is considered the most reliable approach for identifying the presence of steatohepatitis and fibrosis in patients with NAFLD, but its limitations include cost, sampling error, and procedure-related morbidity and mortality. Features of the metabolic syndrome can predict the presence of steatohepatitis in patients with NAFLD. Hence, liver biopsy is recommended in patients with NAFLD who have the metabolic syndrome[80-84]. There has been increasing interest in developing non-invasive methods to identify fibrosis in patients with NAFLD. NAFLD Fibrosis Score is a clinically useful tool for identifying NAFLD patients with higher likelihood of having bridging fibrosis and/or cirrhosis. In a meta-analysis of 13 studies consisting of 3064 patients, it was shown that NAFLD Fibrosis Score has a 90% sensitivity and 60% specificity to exclude advanced fibrosis and 67% sensitivity and 97% specificity to identify the presence of advanced fibrosis[83]. The NAFLD Fibrosis Score is based on six variables [age, body mass index (BMI), hyperglycemia, platelet count, albumin, aspartate aminotransferase/alanine aminotransferase ratio] and it is calculated using the published formula (http://nafldscore.com).
A novel biomarker that has been investigated for the presence of steatohepatitis in patients with NAFLD is circulating levels of cytokeratin-18 fragments[85,86]. This has a sensitivity of 78% and specificity of 87% for identifying steatohepatitis in patients with NAFLD. Transient elastography (TE), which measures liver stiffness non-invasively, showed high sensitivity and specificity for identifying fibrosis in NAFLD in a recent meta-analysis[85]. However, TE has a high failure rate in individuals with a higher BMI. There is some evidence that the Enhanced Liver Fibrosis test which uses the fibrosis markers hyaluronic acid, amino-terminal propeptide-of-type-III-collagen and tissue-inhibitor of matrix-metalloproteinase-1, compares favourably with the use of TE[87].
Management
The management of patients with NAFLD consists of treating liver disease and the associated metabolic co-morbidities such as obesity, hyperlipidemia, insulin resistance and T2DM.
Lifestyle modification and weight reduction: Modifications in diet and lifestyle along with weight reduction and exercise are the cornerstones of treatment of NAFLD, as it is a disease related to excess weight and sedentary lifestyle. Many studies have shown that lifestyle modification can reduce aminotransferase levels and improve hepatic steatosis when measured either by ultrasound[88-91] or MR imaging and spectroscopy[92-95]. A randomized study of 31 obese persons with NASH who underwent intensive lifestyle changes (diet, behaviour modification and 200 min a week of moderate physical activity for 48 wk) vs structured basic education alone showed improvement in steatosis, necrosis and inflammation in the obese group and participants with 7% weight loss had significant improvement in steatosis, lobular inflammation, ballooning, and NAFLD Activity Score[96].
Insulin sensitizing agents: Insulin resistance plays a key role in the pathogenesis of NAFLD. The two main classes of insulin-sensitizing drugs used in the management of patients with NAFLD/NASH are biguanides (metformin) and the thiazolidinediones (pioglitazone).
Metformin increases insulin sensitivity by decreasing hepatic gluconeogenesis and decreasing triglyceride production[97]. Early small, open-label studies showed a reduction in insulin resistance and serum levels of aminotransferases[98-100] but no significant improvement in liver histology[99,100]. A recent meta-analysis examining effects of medical treatment and/or lifestyle intervention did not show significant benefit of metformin in NAFLD[101]. Metformin showed no effect on liver histology and is not recommended as a specific treatment for liver disease in adults with NASH.
Pioglitazone has been available for over a decade for the treatment of T2DM. It acts by promoting peripheral and hepatic insulin sensitivity and increasing circulating levels of adiponectin[102]. A recent meta-analysis showed that pioglitazone improved histological disease activity, glucose, lipid and inflammatory variables and delayed fibrosis progression in patients with NAFLD[101]. The current recommendation by AASLD is that Pioglitazone can be used to treat steatohepatitis in patients with biopsy-proven NASH, although the long term safety and efficacy of pioglitazone in patients with NASH is unknown.
Vitamin E: The antioxidants vitamin E and betaine were investigated as potential therapeutic agents in NASH[103,104]. When administered for 2 years vitamin E improved liver histology, but increased insulin resistance and plasma triacylglycerols[101]. Therefore, the current recommendation by the AASLD is that vitamin E (α-tocopherol), administered at a daily dose of 800 IU/d should be considered as first-line pharmacotherapy for non-diabetic adults with biopsy-proven NASH. However, vitamin E is not recommended to treat NASH in diabetic patients, NAFLD without liver biopsy, NASH cirrhosis or cryptogenic cirrhosis due to lack of supporting evidence.
Incretin mimetics: Incretins are a group of gastrointestinal hormones released after food intake that enhance insulin release from pancreatic beta cells. The most studied among these hormones is GLP-1. The role of the GLP-1 analogues exenatide and liraglutide in the management of T2DM in obesity is well established. These drugs may emerge as new options in management of NAFLD because of similar mechanisms in its pathogenesis.
Dipeptidyl-peptidase IV (DPP4) inhibitors were introduced as an alternative means to increase GLP-1 activity. There is increased serum DPP4 activity in patients with NASH, and this has a positive correlation with the histological grade and degree of liver steatosis[105] DPP4 inhibitors are already established oral treatments for type 2 diabetes[106], and data from experimental studies suggest that they may also reduce liver inflammation and steatosis[107]. Incretin mimetics may, in the future, represent a novel therapeutic option for slowing the progression of NAFLD.
Omega-3 fatty acids: So far, there is no clear evidence for the use of omega-3 fatty acids for the specific treatment NAFLD and NASH[108]. A large multicenter study of omega-3 fatty acid (eicosapentanoic acid) for treatment of NASH is ongoing in the United States.
Other agents: Orlistat, Sibutramine and Rimonabant (a cannabinoid receptor antagonist) have all been investigated for their potential as weight loss medications in NAFLD/NASH, although Sibutramine and Rimonabant have been withdrawn due to their side effects[109]. A single large multicenter randomized controlled trial showed that ursodeoxycholic acid offers no histological benefit over placebo in patients with NASH[110]. Recent data from animal models showed that consumption of hydrogen-rich water may be an effective treatment for NASH by reducing hepatic oxidative stress, apoptosis, inflammation, and hepatocarcinogenesis[111].
Bariatric surgery: AASLD recommends that foregut bariatric surgery is not contraindicated in otherwise eligible obese individuals with NAFLD or NASH. In a study of 381 adult obese patients by Mathurin et al[112] there was a significant improvement in the prevalence and severity of steatosis and ballooning at 1 and 5 years following bariatric surgery. A recently published Cochrane review concluded that lack of randomized clinical trials or quasi-randomized clinical studies precludes definitive assessment of the benefits and harms of bariatric surgery as a therapeutic approach for patients with NASH[113].
GLYCOGENIC HEPATOPATHY
Glycogenic hepatopathy is defined as pathological overloading of hepatocytes with glycogen leading to hepatic enlargement and/or derangement of liver enzymes and is usually seen in patients with longstanding poorly-controlled type 1 diabetes mellitus (T1DM)[114]. Glycogen accumulation in the liver was first described in 1930 as a component of Mauriac’s Syndrome. This syndrome was characterized by unstable diabetes, hepatomegaly, hyperlipidemia, dwarfism, cushingoid features and delayed sexual maturity. It is now recognized that glycogen accumulation within hepatocytes can be present without all the findings described in Mauriac’s Syndrome. Inadequate control of T1DM results in concomitant presence of insulin and excess glucose that increases glycogen storage in the liver. Insulin activates the enzyme glycogen synthase phosphatase which dephosphorylates and activates glycogen synthase, another enzyme that is required for the conversion of glucose-1-phosphate to glycogen[115]. This results in increased glycogen storage in the liver and blocks glycogenolysis. The histological picture is characterized by pale appearance of the hepatocytes with compression of the sinusoids, glycogenated nuclei and giant mitochondria. Steatosis may be present, usually mild, or absent. Glycogen accumulation, the hallmark of this condition is demonstrated by PAS-diastase staining[114].
The disease is under-recognized and usually presents with abdominal pain, nausea, vomiting and abnormalities in liver function tests. While hepatic dysfunction is usually due to NAFLD in T2DM, liver dysfunction in T1DM usually results from glycogenic hepatopathy. It cannot be distinguished from NAFLD clinically or by ultrasound and confirmation requires a liver biopsy. The disorder should be suspected when liver dysfunction occurs in patients with T1DM, especially when viral, autoimmune and metabolic liver diseases are excluded by laboratory investigations. The hallmark of this condition is its reversibility with improved glycemic control. Unlike hepatic steatosis, glycogen overload is not known to progress to fibrosis distinct from fatty liver disease[116]. Prompt improvement with optimal diabetes control by insulin treatment within 4 wk is usually seen in these patients[117,118].
HEPATOGENOUS DIABETES
Up to 79% of cirrhotic subjects can have abnormalities of glucose metabolism[119]. T2DM is usually associated with metabolic syndrome that can lead to NAFLD and cirrhosis. The term “hepatogenous diabetes” (HD) is used to describe diabetes developing in patients with cirrhosis[119]. Numerous factors, including reduced insulin clearance, peripheral hyperinsulinemia and down-regulation of insulin receptors, lead to development of diabetes in cirrhosis[120]. HD is clinically different from T2DM in that it is less frequently associated with microangiopathy and patients suffer from complications of cirrhosis more frequently. However, HD is not yet recognized by the American Diabetes Association and the World Health Organization.
Footnotes
P- Reviewers Cui WP, Gangula PRR S- Editor Gou SX L- Editor Hughes D E- Editor Li JY
References
- 1.5th Edition of the Diabetes Atlas released on World Diabetes Day. Brussels, Belgium: International Diabetes Federation, 2011. (Accessed on 6th April 2013.) Available from: http: //www.idf.org/diabetesatlas/news/fifth-edition-release.
- 2.Frokjaer JB, Andersen SD, Ejskjaer N, Funch-Jensen P, Drewes AM, Gregersen H. Impaired contractility and remodeling of the upper gastrointestinal tract in diabetes mellitus type-1. World J Gastroenterol. 2007;13:4881–4890. doi: 10.3748/wjg.v13.i36.4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang J, Zhao J, Liao D, Gregersen H. Biomechanical properties of the layered oesophagus and its remodelling in experimental type-1 diabetes. J Biomech. 2006;39:894–904. doi: 10.1016/j.jbiomech.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 4.Gustafsson RJ, Littorin B, Berntorp K, Frid A, Thorsson O, Olsson R, Ekberg O, Ohlsson B. Esophageal dysmotility is more common than gastroparesis in diabetes mellitus and is associated with retinopathy. Rev Diabet Stud. 2011;8:268–275. doi: 10.1900/RDS.2011.8.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lluch I, Ascaso JF, Mora F, Minguez M, Peña A, Hernandez A, Benages A. Gastroesophageal reflux in diabetes mellitus. Am J Gastroenterol. 1999;94:919–924. doi: 10.1111/j.1572-0241.1999.987_j.x. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Pitchumoni CS, Chandrarana K, Shah N. Increased prevalence of symptoms of gastroesophageal reflux diseases in type 2 diabetics with neuropathy. World J Gastroenterol. 2008;14:709–712. doi: 10.3748/wjg.14.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee SD, Keum B, Chun HJ, Bak YT. Gastroesophageal Reflux Disease in Type II Diabetes Mellitus With or Without Peripheral Neuropathy. J Neurogastroenterol Motil. 2011;17:274–278. doi: 10.5056/jnm.2011.17.3.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirata A, Kishida K, Nakatsuji H, Inoue K, Hiuge-Shimizu A, Funahashi T, Shimomura I. High prevalence of gastroesophageal reflux symptoms in type 2 diabetics with hypoadiponectinemia and metabolic syndrome. Nutr Metab (Lond) 2012;9:4. doi: 10.1186/1743-7075-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson JA, Vela MF. New esophageal function testing (impedance, Bravo pH monitoring, and high-resolution manometry): clinical relevance. Curr Gastroenterol Rep. 2008;10:222–230. doi: 10.1007/s11894-008-0047-2. [DOI] [PubMed] [Google Scholar]
- 10.Lauffer A, Forcelini CM, Ruas LO, Madalosso CA, Fornari F. Gastroesophageal reflux disease is inversely related with glycemic control in morbidly obese patients. Obes Surg. 2011;21:864–870. doi: 10.1007/s11695-011-0372-7. [DOI] [PubMed] [Google Scholar]
- 11.Chang CT, Shiau YC, Lin CC, Li TC, Lee CC, Kao CH. Improvement of esophageal and gastric motility after 2-week treatment of oral erythromycin in patients with non-insulin-dependent diabetes mellitus. J Diabetes Complications. 2003;17:141–144. doi: 10.1016/s1056-8727(02)00168-x. [DOI] [PubMed] [Google Scholar]
- 12.Hasler WL. Gastroparesis. Curr Opin Gastroenterol. 2012;28:621–628. doi: 10.1097/MOG.0b013e328358d619. [DOI] [PubMed] [Google Scholar]
- 13.Choung RS, Locke GR, Schleck CD, Zinsmeister AR, Melton LJ, Talley NJ. Risk of gastroparesis in subjects with type 1 and 2 diabetes in the general population. Am J Gastroenterol. 2012;107:82–88. doi: 10.1038/ajg.2011.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parkman HP, Yates K, Hasler WL, Nguyen L, Pasricha PJ, Snape WJ, Farrugia G, Koch KL, Calles J, Abell TL, et al. Similarities and differences between diabetic and idiopathic gastroparesis. Clin Gastroenterol Hepatol. 2011;9:1056–1064; quiz e133-134. doi: 10.1016/j.cgh.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rayner CK, Samsom M, Jones KL, Horowitz M. Relationships of upper gastrointestinal motor and sensory function with glycemic control. Diabetes Care. 2001;24:371–381. doi: 10.2337/diacare.24.2.371. [DOI] [PubMed] [Google Scholar]
- 16.Boaz M, Kislov J, Dickman R, Wainstein J. Obesity and symptoms suggestive of gastroparesis in patients with type 2 diabetes and neuropathy. J Diabetes Complications. 2001;25:325–328. doi: 10.1016/j.jdiacomp.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Ordög T, Takayama I, Cheung WK, Ward SM, Sanders KM. Remodeling of networks of interstitial cells of Cajal in a murine model of diabetic gastroparesis. Diabetes. 2000;49:1731–1739. doi: 10.2337/diabetes.49.10.1731. [DOI] [PubMed] [Google Scholar]
- 18.Grover M, Farrugia G, Lurken MS, Bernard CE, Faussone-Pellegrini MS, Smyrk TC, Parkman HP, Abell TL, Snape WJ, Hasler WL, et al. Cellular changes in diabetic and idiopathic gastroparesis. Gastroenterology. 2011;140:1575–1585.e8. doi: 10.1053/j.gastro.2011.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Selim MM, Wendelschafer-Crabb G, Redmon JB, Khoruts A, Hodges JS, Koch K, Walk D, Kennedy WR. Gastric mucosal nerve density: a biomarker for diabetic autonomic neuropathy? Neurology. 2010;75:973–981. doi: 10.1212/WNL.0b013e3181f25f19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ravella K, Yang H, Gangula PR. Impairment of gastric nitrergic and NRF2 system in apolipoprotein E knockout mice. Dig Dis Sci. 2012;57:1504–1509. doi: 10.1007/s10620-012-2070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parkman HP, Hasler WL, Fisher RS. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127:1592–1622. doi: 10.1053/j.gastro.2004.09.055. [DOI] [PubMed] [Google Scholar]
- 22.Tougas G, Chen Y, Coates G, Paterson W, Dallaire C, Paré P, Boivin M, Watier A, Daniels S, Diamant N. Standardization of a simplified scintigraphic methodology for the assessment of gastric emptying in a multicenter setting. Am J Gastroenterol. 2000;95:78–86. doi: 10.1111/j.1572-0241.2000.01703.x. [DOI] [PubMed] [Google Scholar]
- 23.Abell TL, Camilleri M, Donohoe K, Hasler WL, Lin HC, Maurer AH, McCallum RW, Nowak T, Nusynowitz ML, Parkman HP, et al. Consensus recommendations for gastric emptying scintigraphy: a joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. Am J Gastroenterol. 2008;103:753–763. doi: 10.1111/j.1572-0241.2007.01636.x. [DOI] [PubMed] [Google Scholar]
- 24.Lee A, Wilding G, Kuo B. Variable abnormal physiological motility in the proximal upper gastrointestinal tract in gastroparesis. Neurogastroenterol Motil. 2012;24:652–657, e276. doi: 10.1111/j.1365-2982.2012.01905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perri F, Bellini M, Portincasa P, Parodi A, Bonazzi P, Marzio L, Galeazzi F, Usai P, Citrino A, Usai-Satta P. (13)C-octanoic acid breath test (OBT) with a new test meal (EXPIROGer): Toward standardization for testing gastric emptying of solids. Dig Liver Dis. 2010;42:549–553. doi: 10.1016/j.dld.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 26.O’Grady G, Angeli TR, Du P, Lahr C, Lammers WJ, Windsor JA, Abell TL, Farrugia G, Pullan AJ, Cheng LK. Abnormal initiation and conduction of slow-wave activity in gastroparesis, defined by high-resolution electrical mapping. Gastroenterology. 2012;143:589–598.e1-3. doi: 10.1053/j.gastro.2012.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abell TL, Bernstein RK, Cutts T, Farrugia G, Forster J, Hasler WL, McCallum RW, Olden KW, Parkman HP, Parrish CR, et al. Treatment of gastroparesis: a multidisciplinary clinical review. Neurogastroenterol Motil. 2006;18:263–283. doi: 10.1111/j.1365-2982.2006.00760.x. [DOI] [PubMed] [Google Scholar]
- 28.Ramzan Z, Duffy F, Gomez J, Fisher RS, Parkman HP. Continuous glucose monitoring in gastroparesis. Dig Dis Sci. 2011;56:2646–2655. doi: 10.1007/s10620-011-1810-z. [DOI] [PubMed] [Google Scholar]
- 29.Sharma D, Morrison G, Joseph F, Purewal TS, Weston PJ. The role of continuous subcutaneous insulin infusion therapy in patients with diabetic gastroparesis. Diabetologia. 2011;54:2768–2770. doi: 10.1007/s00125-011-2282-6. [DOI] [PubMed] [Google Scholar]
- 30.Asakawa A, Ueno N, Katagi M, Ijuin Y, Morita Y, Mizuno S, Inui T, Sakamaki R, Shinfuku N, Uemoto M. Mosapride improves food intake, while not worsening glycemic control and obesity, in ob/ob obese mice with decreased gastric emptying. J Diabetes Complications. 2006;20:56–58. doi: 10.1016/j.jdiacomp.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 31.Kawamura E, Enomoto M, Kotani K, Hagihara A, Fujii H, Kobayashi S, Iwai S, Morikawa H, Kawabe J, Tominaga K, et al. Effect of mosapride citrate on gastric emptying in interferon-induced gastroparesis. Dig Dis Sci. 2012;57:1510–1516. doi: 10.1007/s10620-012-2085-8. [DOI] [PubMed] [Google Scholar]
- 32.Mondal A, Xie Z, Miyano Y, Tsutsui C, Sakata I, Kawamoto Y, Aizawa S, Tanaka T, Oda S, Sakai T. Coordination of motilin and ghrelin regulates the migrating motor complex of gastrointestinal motility in Suncus murinus. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1207–G1215. doi: 10.1152/ajpgi.00379.2011. [DOI] [PubMed] [Google Scholar]
- 33.Wo JM, Ejskjaer N, Hellström PM, Malik RA, Pezzullo JC, Shaughnessy L, Charlton P, Kosutic G, McCallum RW. Randomised clinical trial: ghrelin agonist TZP-101 relieves gastroparesis associated with severe nausea and vomiting--randomised clinical study subset data. Aliment Pharmacol Ther. 2011;33:679–688. doi: 10.1111/j.1365-2036.2010.04567.x. [DOI] [PubMed] [Google Scholar]
- 34.Camilleri M. Clinical practice. Diabetic gastroparesis. N Engl J Med. 2007;356:820–829. doi: 10.1056/NEJMcp062614. [DOI] [PubMed] [Google Scholar]
- 35.Mearin F, Camilleri M, Malagelada JR. Pyloric dysfunction in diabetics with recurrent nausea and vomiting. Gastroenterology. 1986;90:1919–1925. doi: 10.1016/0016-5085(86)90262-3. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez L, Rosen R, Manfredi M, Nurko S. Endoscopic intrapyloric injection of botulinum toxin A in the treatment of children with gastroparesis: a retrospective, open-label study. Gastrointest Endosc. 2012;75:302–309. doi: 10.1016/j.gie.2011.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coleski R, Anderson MA, Hasler WL. Factors associated with symptom response to pyloric injection of botulinum toxin in a large series of gastroparesis patients. Dig Dis Sci. 2009;54:2634–2642. doi: 10.1007/s10620-008-0660-9. [DOI] [PubMed] [Google Scholar]
- 38.McCallum RW, Lin Z, Forster J, Roeser K, Hou Q, Sarosiek I. Gastric electrical stimulation improves outcomes of patients with gastroparesis for up to 10 years. Clin Gastroenterol Hepatol. 2011;9:314–319.e1. doi: 10.1016/j.cgh.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 39.Hou Q, Lin Z, Mayo MS, Sarosiek I, Gajewski BJ, McCallum RW. Is symptom relief associated with reduction in gastric retention after gastric electrical stimulation treatment in patients with gastroparesis? A sensitivity analysis with logistic regression models. Neurogastroenterol Motil. 2012;24:639–645, e274. doi: 10.1111/j.1365-2982.2012.01917.x. [DOI] [PubMed] [Google Scholar]
- 40.Deb S, Tang SJ, Abell TL, Rao S, Huang WD, To SD, Lahr C, Chiao JC. An endoscopic wireless gastrostimulator (with video) Gastrointest Endosc. 2012;75:411–415, 415.e1. doi: 10.1016/j.gie.2011.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hibbard ML, Dunst CM, Swanström LL. Laparoscopic and endoscopic pyloroplasty for gastroparesis results in sustained symptom improvement. J Gastrointest Surg. 2011;15:1513–1519. doi: 10.1007/s11605-011-1607-6. [DOI] [PubMed] [Google Scholar]
- 42.Fontana RJ, Barnett JL. Jejunostomy tube placement in refractory diabetic gastroparesis: a retrospective review. Am J Gastroenterol. 1996;91:2174–2178. [PubMed] [Google Scholar]
- 43.Kim CH, Nelson DK. Venting percutaneous gastrostomy in the treatment of refractory idiopathic gastroparesis. Gastrointest Endosc. 1998;47:67–70. doi: 10.1016/s0016-5107(98)70301-3. [DOI] [PubMed] [Google Scholar]
- 44.Phillips LK, Rayner CK, Jones KL, Horowitz M. An update on autonomic neuropathy affecting the gastrointestinal tract. Curr Diab Rep. 2006;6:417–423. doi: 10.1007/s11892-006-0073-0. [DOI] [PubMed] [Google Scholar]
- 45.Chandrasekharan B, Anitha M, Blatt R, Shahnavaz N, Kooby D, Staley C, Mwangi S, Jones DP, Sitaraman SV, Srinivasan S. Colonic motor dysfunction in human diabetes is associated with enteric neuronal loss and increased oxidative stress. Neurogastroenterol Motil. 2011;23:131–138, e26. doi: 10.1111/j.1365-2982.2010.01611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen P, Zhao J, Gregersen H. Up-regulated expression of advanced glycation end-products and their receptor in the small intestine and colon of diabetic rats. Dig Dis Sci. 2012;57:48–57. doi: 10.1007/s10620-011-1951-0. [DOI] [PubMed] [Google Scholar]
- 47.Lee TH, Lee JS. Ramosetron might be useful for treating diabetic diarrhea with a rapid small bowel transit time. Korean J Intern Med. 2013;28:106–107. doi: 10.3904/kjim.2013.28.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lysy J, Israeli E, Goldin E. The prevalence of chronic diarrhea among diabetic patients. Am J Gastroenterol. 1999;94:2165–2170. doi: 10.1111/j.1572-0241.1999.01289.x. [DOI] [PubMed] [Google Scholar]
- 49.Ohlsson B, Melander O, Thorsson O, Olsson R, Ekberg O, Sundkvist G. Oesophageal dysmotility, delayed gastric emptying and autonomic neuropathy correlate to disturbed glucose homeostasis. Diabetologia. 2006;49:2010–2014. doi: 10.1007/s00125-006-0354-9. [DOI] [PubMed] [Google Scholar]
- 50.Russo A, Botten R, Kong MF, Chapman IM, Fraser RJ, Horowitz M, Sun WM. Effects of acute hyperglycaemia on anorectal motor and sensory function in diabetes mellitus. Diabet Med. 2004;21:176–182. doi: 10.1111/j.1464-5491.2004.01106.x. [DOI] [PubMed] [Google Scholar]
- 51.Corazza GR, Menozzi MG, Strocchi A, Rasciti L, Vaira D, Lecchini R, Avanzini P, Chezzi C, Gasbarrini G. The diagnosis of small bowel bacterial overgrowth. Reliability of jejunal culture and inadequacy of breath hydrogen testing. Gastroenterology. 1990;98:302–309. doi: 10.1016/0016-5085(90)90818-l. [DOI] [PubMed] [Google Scholar]
- 52.Ghoshal UC. How to interpret hydrogen breath tests. J Neurogastroenterol Motil. 2011;17:312–317. doi: 10.5056/jnm.2011.17.3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Virally-Monod M, Tielmans D, Kevorkian JP, Bouhnik Y, Flourie B, Porokhov B, Ajzenberg C, Warnet A, Guillausseau PJ. Chronic diarrhoea and diabetes mellitus: prevalence of small intestinal bacterial overgrowth. Diabetes Metab. 1998;24:530–536. [PubMed] [Google Scholar]
- 54.Jiang ZD, DuPont HL. Rifaximin: in vitro and in vivo antibacterial activity--a review. Chemotherapy. 2005;51 Suppl 1:67–72. doi: 10.1159/000081991. [DOI] [PubMed] [Google Scholar]
- 55.Gerard L, Garey KW, DuPont HL. Rifaximin: a nonabsorbable rifamycin antibiotic for use in nonsystemic gastrointestinal infections. Expert Rev Anti Infect Ther. 2005;3:201–211. doi: 10.1586/14787210.3.2.201. [DOI] [PubMed] [Google Scholar]
- 56.Pimentel M. Review of rifaximin as treatment for SIBO and IBS. Expert Opin Investig Drugs. 2009;18:349–358. doi: 10.1517/13543780902780175. [DOI] [PubMed] [Google Scholar]
- 57.Meyer C, O’Neal DN, Connell W, Alford F, Ward G, Jenkins AJ. Octreotide treatment of severe diabetic diarrhoea. Intern Med J. 2003;33:617–618. doi: 10.1111/j.1445-5994.2003.00494.x. [DOI] [PubMed] [Google Scholar]
- 58.Corbould A, Campbell J. Efficacy of octreotide but not long-acting somatostatin analogue for severe refractory diabetic diarrhoea. Diabet Med. 2009;26:828–829. doi: 10.1111/j.1464-5491.2009.02766.x. [DOI] [PubMed] [Google Scholar]
- 59.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 60.Ratziu V, Bellentani S, Cortez-Pinto H, Day C, Marchesini G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol. 2010;53:372–384. doi: 10.1016/j.jhep.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 61.Falck-Ytter Y, Younossi ZM, Marchesini G, McCullough AJ. Clinical features and natural history of nonalcoholic steatosis syndromes. Semin Liver Dis. 2001;21:17–26. doi: 10.1055/s-2001-12926. [DOI] [PubMed] [Google Scholar]
- 62.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202–1219. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 63.Setji TL, Holland ND, Sanders LL, Pereira KC, Diehl AM, Brown AJ. Nonalcoholic steatohepatitis and nonalcoholic Fatty liver disease in young women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:1741–1747. doi: 10.1210/jc.2005-2774. [DOI] [PubMed] [Google Scholar]
- 64.Ebert EC. Gastrointestinal complications of diabetes mellitus. Dis Mon. 2005;51:620–663. doi: 10.1016/j.disamonth.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 65.McCullough AJ. The clinical features, diagnosis and natural history of nonalcoholic fatty liver disease. Clin Liver Dis. 2004;8:521–533, viii. doi: 10.1016/j.cld.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 66.Ekstedt M, Franzén LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 67.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 68.Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, Musso A, De Paolis P, Capussotti L, Salizzoni M, et al. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134–140. doi: 10.1053/gast.2002.34168. [DOI] [PubMed] [Google Scholar]
- 69.Marrero JA, Fontana RJ, Su GL, Conjeevaram HS, Emick DM, Lok AS. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2002;36:1349–1354. doi: 10.1053/jhep.2002.36939. [DOI] [PubMed] [Google Scholar]
- 70.Ratziu V, Bonyhay L, Di Martino V, Charlotte F, Cavallaro L, Sayegh-Tainturier MH, Giral P, Grimaldi A, Opolon P, Poynard T. Survival, liver failure, and hepatocellular carcinoma in obesity-related cryptogenic cirrhosis. Hepatology. 2002;35:1485–1493. doi: 10.1053/jhep.2002.33324. [DOI] [PubMed] [Google Scholar]
- 71.Hibbard ML, Dunst CM, Swanström LL. Laparoscopic and endoscopic pyloroplasty for gastroparesis results in sustained symptom improvement. J Gastrointest Surg. 2011;15:1513–1519. doi: 10.1007/s11605-011-1607-6. [DOI] [PubMed] [Google Scholar]
- 72.Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Diabetes increases the risk of hepatocellular carcinoma in the United States: a population based case control study. Gut. 2005;54:533–539. doi: 10.1136/gut.2004.052167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Adams LA, Sanderson S, Lindor KD, Angulo P. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol. 2005;42:132–138. doi: 10.1016/j.jhep.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 74.Nishida T, Tsuneyama K, Fujimoto M, Nomoto K, Hayashi S, Miwa S, Nakajima T, Nakanishi Y, Sasaki Y, Suzuki W, et al. Spontaneous onset of nonalcoholic steatohepatitis and hepatocellular carcinoma in a mouse model of metabolic syndrome. Lab Invest. 2013;93:230–241. doi: 10.1038/labinvest.2012.155. [DOI] [PubMed] [Google Scholar]
- 75.Marchesini G, Brizi M, Morselli-Labate AM, Bianchi G, Bugianesi E, McCullough AJ, Forlani G, Melchionda N. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107:450–455. doi: 10.1016/s0002-9343(99)00271-5. [DOI] [PubMed] [Google Scholar]
- 76.Day CP. Pathogenesis of steatohepatitis. Best Pract Res Clin Gastroenterol. 2002;16:663–678. doi: 10.1053/bega.2002.0333. [DOI] [PubMed] [Google Scholar]
- 77.Chitturi S, Abeygunasekera S, Farrell GC, Holmes-Walker J, Hui JM, Fung C, Karim R, Lin R, Samarasinghe D, Liddle C, et al. NASH and insulin resistance: Insulin hypersecretion and specific association with the insulin resistance syndrome. Hepatology. 2002;35:373–379. doi: 10.1053/jhep.2002.30692. [DOI] [PubMed] [Google Scholar]
- 78.James O, Day C. Non-alcoholic steatohepatitis: another disease of affluence. Lancet. 1999;353:1634–1636. doi: 10.1016/S0140-6736(99)00163-4. [DOI] [PubMed] [Google Scholar]
- 79.Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, Mascianà R, Forgione A, Gabrieli ML, Perotti G, et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877–1887. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 80.Vuppalanchi R, Chalasani N. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: Selected practical issues in their evaluation and management. Hepatology. 2009;49:306–317. doi: 10.1002/hep.22603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kowdley KV. The role of iron in nonalcoholic fatty liver disease: the story continues. Gastroenterology. 2010;138:817–819. doi: 10.1053/j.gastro.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 82.Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, Natale S, Vanni E, Villanova N, Melchionda N, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 83.Kang H, Greenson JK, Omo JT, Chao C, Peterman D, Anderson L, Foess-Wood L, Sherbondy MA, Conjeevaram HS. Metabolic syndrome is associated with greater histologic severity, higher carbohydrate, and lower fat diet in patients with NAFLD. Am J Gastroenterol. 2006;101:2247–2253. doi: 10.1111/j.1572-0241.2006.00719.x. [DOI] [PubMed] [Google Scholar]
- 84.Ryan MC, Wilson AM, Slavin J, Best JD, Jenkins AJ, Desmond PV. Associations between liver histology and severity of the metabolic syndrome in subjects with nonalcoholic fatty liver disease. Diabetes Care. 2005;28:1222–1224. doi: 10.2337/diacare.28.5.1222. [DOI] [PubMed] [Google Scholar]
- 85.Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617–649. doi: 10.3109/07853890.2010.518623. [DOI] [PubMed] [Google Scholar]
- 86.Wieckowska A, McCullough AJ, Feldstein AE. Noninvasive diagnosis and monitoring of nonalcoholic steatohepatitis: present and future. Hepatology. 2007;46:582–589. doi: 10.1002/hep.21768. [DOI] [PubMed] [Google Scholar]
- 87.Friedrich-Rust M, Rosenberg W, Parkes J, Herrmann E, Zeuzem S, Sarrazin C. Comparison of ELF, FibroTest and FibroScan for the non-invasive assessment of liver fibrosis. BMC Gastroenterol. 2010;10:103. doi: 10.1186/1471-230X-10-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kugelmas M, Hill DB, Vivian B, Marsano L, McClain CJ. Cytokines and NASH: a pilot study of the effects of lifestyle modification and vitamin E. Hepatology. 2003;38:413–419. doi: 10.1053/jhep.2003.50316. [DOI] [PubMed] [Google Scholar]
- 89.Sreenivasa Baba C, Alexander G, Kalyani B, Pandey R, Rastogi S, Pandey A, Choudhuri G. Effect of exercise and dietary modification on serum aminotransferase levels in patients with nonalcoholic steatohepatitis. J Gastroenterol Hepatol. 2006;21:191–198. doi: 10.1111/j.1440-1746.2005.04233.x. [DOI] [PubMed] [Google Scholar]
- 90.Hickman IJ, Jonsson JR, Prins JB, Ash S, Purdie DM, Clouston AD, Powell EE. Modest weight loss and physical activity in overweight patients with chronic liver disease results in sustained improvements in alanine aminotransferase, fasting insulin, and quality of life. Gut. 2004;53:413–419. doi: 10.1136/gut.2003.027581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Suzuki A, Lindor K, St Saver J, Lymp J, Mendes F, Muto A, Okada T, Angulo P. Effect of changes on body weight and lifestyle in nonalcoholic fatty liver disease. J Hepatol. 2005;43:1060–1066. doi: 10.1016/j.jhep.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 92.Larson-Meyer DE, Heilbronn LK, Redman LM, Newcomer BR, Frisard MI, Anton S, Smith SR, Alfonso A, Ravussin E. Effect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care. 2006;29:1337–1344. doi: 10.2337/dc05-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cowin GJ, Jonsson JR, Bauer JD, Ash S, Ali A, Osland EJ, Purdie DM, Clouston AD, Powell EE, Galloway GJ. Magnetic resonance imaging and spectroscopy for monitoring liver steatosis. J Magn Reson Imaging. 2008;28:937–945. doi: 10.1002/jmri.21542. [DOI] [PubMed] [Google Scholar]
- 94.Viljanen AP, Iozzo P, Borra R, Kankaanpää M, Karmi A, Lautamäki R, Järvisalo M, Parkkola R, Rönnemaa T, Guiducci L, et al. Effect of weight loss on liver free fatty acid uptake and hepatic insulin resistance. J Clin Endocrinol Metab. 2009;94:50–55. doi: 10.1210/jc.2008-1689. [DOI] [PubMed] [Google Scholar]
- 95.Lazo M, Solga SF, Horska A, Bonekamp S, Diehl AM, Brancati FL, Wagenknecht LE, Pi-Sunyer FX, Kahn SE, Clark JM. Effect of a 12-month intensive lifestyle intervention on hepatic steatosis in adults with type 2 diabetes. Diabetes Care. 2010;33:2156–2163. doi: 10.2337/dc10-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR, Fava JL, Wing RR. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51:121–129. doi: 10.1002/hep.23276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Torres DM, Harrison SA. Diagnosis and therapy of nonalcoholic steatohepatitis. Gastroenterology. 2008;134:1682–1698. doi: 10.1053/j.gastro.2008.02.077. [DOI] [PubMed] [Google Scholar]
- 98.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Zoli M, Melchionda N. Metformin in non-alcoholic steatohepatitis. Lancet. 2001;358:893–894. doi: 10.1016/s0140-6736(01)06042-1. [DOI] [PubMed] [Google Scholar]
- 99.Uygun A, Kadayifci A, Isik AT, Ozgurtas T, Deveci S, Tuzun A, Yesilova Z, Gulsen M, Dagalp K. Metformin in the treatment of patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2004;19:537–544. doi: 10.1111/j.1365-2036.2004.01888.x. [DOI] [PubMed] [Google Scholar]
- 100.Nair S, Diehl AM, Wiseman M, Farr GH, Perrillo RP. Metformin in the treatment of non-alcoholic steatohepatitis: a pilot open label trial. Aliment Pharmacol Ther. 2004;20:23–28. doi: 10.1111/j.1365-2036.2004.02025.x. [DOI] [PubMed] [Google Scholar]
- 101.Musso G, Cassader M, Rosina F, Gambino R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetologia. 2012;55:885–904. doi: 10.1007/s00125-011-2446-4. [DOI] [PubMed] [Google Scholar]
- 102.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hasegawa T, Yoneda M, Nakamura K, Makino I, Terano A. Plasma transforming growth factor-beta1 level and efficacy of alpha-tocopherol in patients with non-alcoholic steatohepatitis: a pilot study. Aliment Pharmacol Ther. 2001;15:1667–1672. doi: 10.1046/j.1365-2036.2001.01083.x. [DOI] [PubMed] [Google Scholar]
- 104.Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2003;98:2485–2490. doi: 10.1111/j.1572-0241.2003.08699.x. [DOI] [PubMed] [Google Scholar]
- 105.Balaban YH, Korkusuz P, Simsek H, Gokcan H, Gedikoglu G, Pinar A, Hascelik G, Asan E, Hamaloglu E, Tatar G. Dipeptidyl peptidase IV (DDP IV) in NASH patients. Ann Hepatol. 2007;6:242–250. [PubMed] [Google Scholar]
- 106.Ahrén B. GLP-1-based therapy of type 2 diabetes: GLP-1 mimetics and DPP-IV inhibitors. Curr Diab Rep. 2007;7:340–347. doi: 10.1007/s11892-007-0056-9. [DOI] [PubMed] [Google Scholar]
- 107.Yilmaz Y, Atug O, Yonal O, Duman D, Ozdogan O, Imeryuz N, Kalayci C. Dipeptidyl peptidase IV inhibitors: therapeutic potential in nonalcoholic fatty liver disease. Med Sci Monit. 2009;15:HY1–HY5. [PubMed] [Google Scholar]
- 108.Masterton GS, Plevris JN, Hayes PC. Review article: omega-3 fatty acids - a promising novel therapy for non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2010;31:679–692. doi: 10.1111/j.1365-2036.2010.04230.x. [DOI] [PubMed] [Google Scholar]
- 109.Rucker D, Padwal R, Li SK, Curioni C, Lau DC. Long term pharmacotherapy for obesity and overweight: updated meta-analysis. BMJ. 2007;335:1194–1199. doi: 10.1136/bmj.39385.413113.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lindor KD, Kowdley KV, Heathcote EJ, Harrison ME, Jorgensen R, Angulo P, Lymp JF, Burgart L, Colin P. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 2004;39:770–778. doi: 10.1002/hep.20092. [DOI] [PubMed] [Google Scholar]
- 111.Kawai D, Takaki A, Nakatsuka A, Wada J, Tamaki N, Yasunaka T, Koike K, Tsuzaki R, Matsumoto K, Miyake Y, et al. Hydrogen-rich water prevents progression of nonalcoholic steatohepatitis and accompanying hepatocarcinogenesis in mice. Hepatology. 2012;56:912–921. doi: 10.1002/hep.25782. [DOI] [PubMed] [Google Scholar]
- 112.Mathurin P, Hollebecque A, Arnalsteen L, Buob D, Leteurtre E, Caiazzo R, Pigeyre M, Verkindt H, Dharancy S, Louvet A, et al. Prospective study of the long-term effects of bariatric surgery on liver injury in patients without advanced disease. Gastroenterology. 2009;137:532–540. doi: 10.1053/j.gastro.2009.04.052. [DOI] [PubMed] [Google Scholar]
- 113.Chavez-Tapia NC, Tellez-Avila FI, Barrientos-Gutierrez T, Mendez-Sanchez N, Lizardi-Cervera J, Uribe M. Bariatric surgery for non-alcoholic steatohepatitis in obese patients. Cochrane Database Syst Rev. 2010;(1):CD007340. doi: 10.1002/14651858.CD007340.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Torbenson M, Chen YY, Brunt E, Cummings OW, Gottfried M, Jakate S, Liu YC, Yeh MM, Ferrell L. Glycogenic hepatopathy: an underrecognized hepatic complication of diabetes mellitus. Am J Surg Pathol. 2006;30:508–513. doi: 10.1097/00000478-200604000-00012. [DOI] [PubMed] [Google Scholar]
- 115.Rogal SS, Ukomadu C, Levy BD, Loscalzo J. Clinical problem-solving. A sweet source of abdominal pain. N Engl J Med. 2011;364:1762–1767. doi: 10.1056/NEJMcps0905921. [DOI] [PubMed] [Google Scholar]
- 116.Saxena P, Turner I, McIndoe R. Education and Imaging. Hepatobiliary and pancreatic: Glycogenic hepatopathy: a reversible condition. J Gastroenterol Hepatol. 2010;25:646. doi: 10.1111/j.1440-1746.2010.06178.x. [DOI] [PubMed] [Google Scholar]
- 117.Abaci A, Bekem O, Unuvar T, Ozer E, Bober E, Arslan N, Ozturk Y, Buyukgebiz A. Hepatic glycogenosis: a rare cause of hepatomegaly in Type 1 diabetes mellitus. J Diabetes Complications. 2008;22:325–328. doi: 10.1016/j.jdiacomp.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 118.Munns CF, McCrossin RB, Thomsett MJ, Batch J. Hepatic glycogenosis: reversible hepatomegaly in type 1 diabetes. J Paediatr Child Health. 2000;36:449–452. doi: 10.1046/j.1440-1754.2000.00547.x. [DOI] [PubMed] [Google Scholar]
- 119.García-Compeán D, Jáquez-Quintana JO, Lavalle-González FJ, Reyes-Cabello E, González-González JA, Muñoz-Espinosa LE, Vázquez-Elizondo G, Villarreal-Pérez JZ, Maldonado-Garza HJ. The prevalence and clinical characteristics of glucose metabolism disorders in patients with liver cirrhosis. A prospective study. Ann Hepatol. 2012;11:240–248. [PubMed] [Google Scholar]
- 120.Holstein A, Hinze S, Thiessen E, Plaschke A, Egberts EH. Clinical implications of hepatogenous diabetes in liver cirrhosis. J Gastroenterol Hepatol. 2002;17:677–681. doi: 10.1046/j.1440-1746.2002.02755.x. [DOI] [PubMed] [Google Scholar]
- 121.Loria P, Lonardo A, Anania F. Liver and diabetes. A vicious circle. Hepatol Res. 2013;43:51–64. doi: 10.1111/j.1872-034X.2012.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]