Abstract
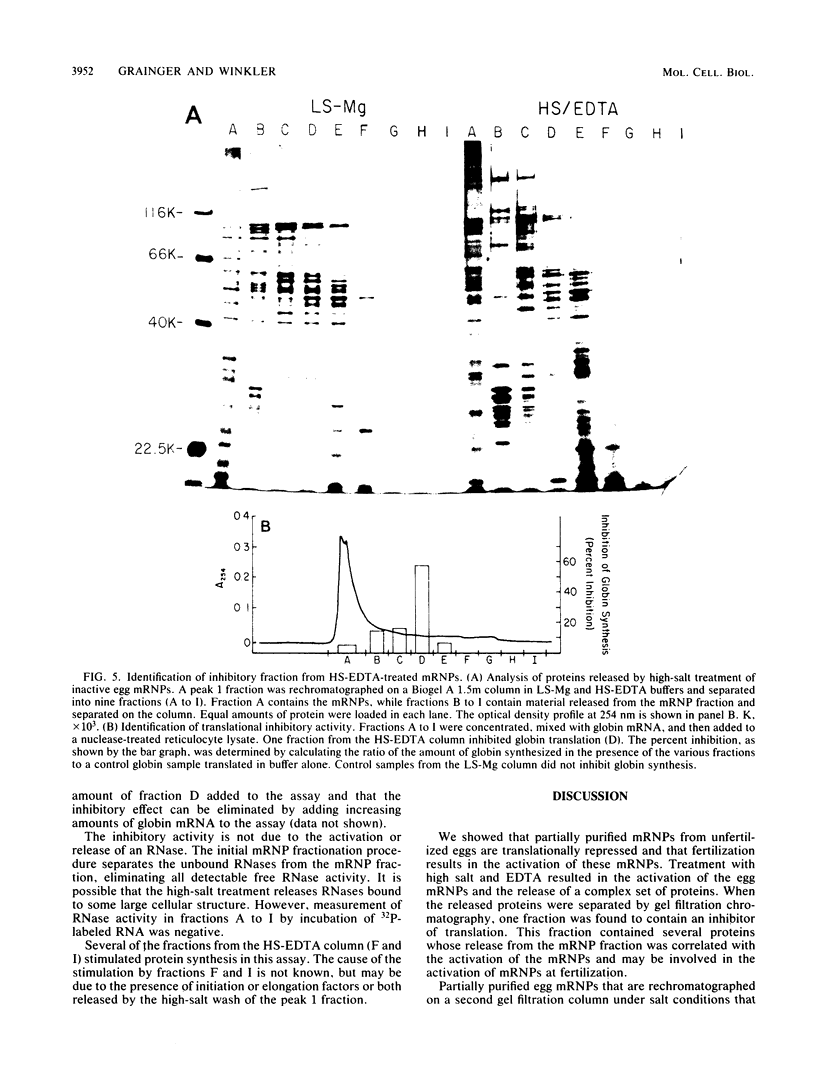
Fertilization of sea urchin eggs results in a large increase in the rate of protein synthesis which is mediated by the translation of stored maternal mRNA. The masked message hypothesis suggests that messenger ribonucleoprotein particles (mRNPs) from unfertilized eggs are translationally inactive and that fertilization results in alterations of the mRNPs such that they become translationally active. Previous workers have isolated egg mRNPs by sucrose gradient centrifugation and have assayed their translational activity in heterologous cell-free systems. The conflicting results they obtained are probably due to the sensitivity of mRNPs to artifactual activation and inactivation. Previously, we demonstrated that unfractionated mRNPs in a sea urchin cell-free translation system were translationally inactive. Now, using large-pore gel filtration chromatography, we partially purified egg mRNPs while retaining their translationally repressed state. Polysomal mRNPs from fertilized eggs isolated under the same conditions were translationally active. The changes in the pattern of proteins synthesized by fractionated unfertilized and fertilized mRNPs in vitro were similar to those changes observed in vivo. Treatment of egg mRNPs with buffers containing high salt and EDTA, followed by rechromatography, resulted in the activation of the mRNPs and the release of an inhibitor of translation from the mRNPs. Analysis of the inhibitory fraction on one-dimensional sodium dodecyl sulfate gels indicated that this fraction contains a complex set of proteins, several of which were released from high-salt-EDTA-activated mRNPs and not from inactive low-salt control mRNPs. One of the released proteins may be responsible for the repression of egg mRNPs in vitro and be involved in the unmasking of mRNPs at fertilization.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann J. E., Lodish H. F. A kinetic model of protein synthesis. Application to hemoglobin synthesis and translational control. J Biol Chem. 1979 Dec 10;254(23):11927–11937. [PubMed] [Google Scholar]
- Brandhorst B. P. Two-dimensional gel patterns of protein synthesis before and after fertilization of sea urchin eggs. Dev Biol. 1976 Sep;52(2):310–317. doi: 10.1016/0012-1606(76)90248-7. [DOI] [PubMed] [Google Scholar]
- Calzone F. J., Angerer R. C., Gorovsky M. A. Regulation of protein synthesis in Tetrahymena: isolation and characterization of polysomes by gel filtration and precipitation at pH 5.3. Nucleic Acids Res. 1982 Mar 25;10(6):2145–2161. doi: 10.1093/nar/10.6.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colin A. M., Hille M. B. Injected mRNA does not increase protein synthesis in unfertilized, fertilized, or ammonia-activated sea urchin eggs. Dev Biol. 1986 May;115(1):184–192. doi: 10.1016/0012-1606(86)90239-3. [DOI] [PubMed] [Google Scholar]
- Darnbrough C., Legon S., Hunt T., Jackson R. J. Initiation of protein synthesis: evidence for messenger RNA-independent binding of methionyl-transfer RNA to the 40 S ribosomal subunit. J Mol Biol. 1973 May 25;76(3):379–403. doi: 10.1016/0022-2836(73)90511-1. [DOI] [PubMed] [Google Scholar]
- DeLeon D. V., Cox K. H., Angerer L. M., Angerer R. C. Most early-variant histone mRNA is contained in the pronucleus of sea urchin eggs. Dev Biol. 1983 Nov;100(1):197–206. doi: 10.1016/0012-1606(83)90211-7. [DOI] [PubMed] [Google Scholar]
- Epel D., Steinhardt R., Humphreys T., Mazia D. An analysis of the partial metabolic derepression of sea urchin eggs by ammonia: the existence of independent pathways. Dev Biol. 1974 Oct;40(2):245–255. doi: 10.1016/0012-1606(74)90127-4. [DOI] [PubMed] [Google Scholar]
- Evans T., Rosenthal E. T., Youngblom J., Distel D., Hunt T. Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell. 1983 Jun;33(2):389–396. doi: 10.1016/0092-8674(83)90420-8. [DOI] [PubMed] [Google Scholar]
- Grainger J. L., Winkler M. M., Shen S. S., Steinhardt R. A. Intracellular pH controls protein synthesis rate in the sea urchine egg and early embryo. Dev Biol. 1979 Feb;68(2):396–406. doi: 10.1016/0012-1606(79)90213-6. [DOI] [PubMed] [Google Scholar]
- Grainger J. L., von Brunn A., Winkler M. M. Transient synthesis of a specific set of proteins during the rapid cleavage phase of sea urchin development. Dev Biol. 1986 Apr;114(2):403–415. doi: 10.1016/0012-1606(86)90205-8. [DOI] [PubMed] [Google Scholar]
- Görlach M., Hilse K. Stoichiometric association of cap-binding protein I with translated polysomal globin mRNP. EMBO J. 1986 Oct;5(10):2629–2635. doi: 10.1002/j.1460-2075.1986.tb04544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HULTIN T. The effect of puromycin on protein metabolism and cell division in fertilized sea urchin eggs. Experientia. 1961 Sep 15;17:410–411. doi: 10.1007/BF02157974. [DOI] [PubMed] [Google Scholar]
- Ilan J., Ilan J. Translation of maternal messenger ribonucleoprotein particles from sea urchin in a cell-free system from unfertilized eggs and product analysis. Dev Biol. 1978 Oct;66(2):375–385. doi: 10.1016/0012-1606(78)90246-4. [DOI] [PubMed] [Google Scholar]
- Jackson R. J., Hunt T. Preparation and use of nuclease-treated rabbit reticulocyte lysates for the translation of eukaryotic messenger RNA. Methods Enzymol. 1983;96:50–74. doi: 10.1016/s0076-6879(83)96008-1. [DOI] [PubMed] [Google Scholar]
- Jenkins N. A., Kaumeyer J. F., Young E. M., Raff R. A. A test for masked message: the template activity of messenger ribonucleoprotein particles isolated from sea urchine eggs. Dev Biol. 1978 Apr;63(2):279–298. doi: 10.1016/0012-1606(78)90134-3. [DOI] [PubMed] [Google Scholar]
- Martins de Sa C., Grossi de Sa M. F., Akhayat O., Broders F., Scherrer K., Horsch A., Schmid H. P. Prosomes. Ubiquity and inter-species structural variation. J Mol Biol. 1986 Feb 20;187(4):479–493. doi: 10.1016/0022-2836(86)90328-1. [DOI] [PubMed] [Google Scholar]
- Moon R. T., Danilchik M. V., Hille M. B. An assessment of the masked message hypothesis: sea urchin egg messenger ribonucleoprotein complexes are efficient templates for in vitro protein synthesis. Dev Biol. 1982 Oct;93(2):389–403. doi: 10.1016/0012-1606(82)90126-9. [DOI] [PubMed] [Google Scholar]
- Raychaudhuri P., Maitra U. Identification of ribosome-bound eukaryotic initiation factor 2.GDP binary complex as an intermediate in polypeptide chain initiation reaction. J Biol Chem. 1986 Jun 15;261(17):7723–7728. [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Spirin A. S. "Masked" forms of mRNA. Curr Top Dev Biol. 1966;1:1–38. [PubMed] [Google Scholar]
- Standart N. M., Bray S. J., George E. L., Hunt T., Ruderman J. V. The small subunit of ribonucleotide reductase is encoded by one of the most abundant translationally regulated maternal RNAs in clam and sea urchin eggs. J Cell Biol. 1985 Jun;100(6):1968–1976. doi: 10.1083/jcb.100.6.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G., Thomas G. Translational control of mRNA expression during the early mitogenic response in Swiss mouse 3T3 cells: identification of specific proteins. J Cell Biol. 1986 Dec;103(6 Pt 1):2137–2144. doi: 10.1083/jcb.103.6.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent A., Goldenberg S., Scherrer K. Comparisons of proteins associated with duck-globin mRNA and its polyadenylated segment in polyribosomal and repressed free messenger ribonucleoprotein complexes. Eur J Biochem. 1981 Feb;114(2):179–193. doi: 10.1111/j.1432-1033.1981.tb05135.x. [DOI] [PubMed] [Google Scholar]
- Wilt F. H., Sakai H., Mazia D. Old and new protein in the formation of the mitotic apparatus in cleaving sea urchin eggs. J Mol Biol. 1967 Jul 14;27(1):1–7. doi: 10.1016/0022-2836(67)90346-4. [DOI] [PubMed] [Google Scholar]
- Winkler M. M., Nelson E. M., Lashbrook C., Hershey J. W. Multiple levels of regulation of protein synthesis at fertilization in sea urchin eggs. Dev Biol. 1985 Feb;107(2):290–300. doi: 10.1016/0012-1606(85)90312-4. [DOI] [PubMed] [Google Scholar]
- Winkler M. M., Steinhardt R. A., Grainger J. L., Minning L. Dual ionic controls for the activation of protein synthesis at fertilization. Nature. 1980 Oct 9;287(5782):558–560. doi: 10.1038/287558a0. [DOI] [PubMed] [Google Scholar]