Abstract
Adipose tissue is a heterogeneous organ with remarkable variations in fat cell metabolism depending on the anatomical location. However, the pattern and distribution of bioactive lipid mediators between different fat depots and their relationships in complex diseases have not been investigated. Using LC-MS/MS-based metabolo-lipidomics, here we report that human subcutaneous (SC) adipose tissues possess a range of specialized proresolving mediators (SPM) including resolvin (Rv) D1, RvD2, protectin (PD) 1, lipoxin (LX) A4, and the monohydroxy biosynthetic pathway markers of RvD1 and PD1 (17-HDHA), RvE1 (18-HEPE), and maresin 1 (14-HDHA). The “classic” eicosanoids prostaglandin (PG) E2, PGD2, PGF2α, leukotriene (LT) B4, 5-hydroxyeicosatetraenoic acid (5-HETE), 12-HETE, and 15-HETE were also identified in SC fat. SC fat from patients with peripheral vascular disease (PVD) exhibited a marked deficit in PD1 and 17-HDHA levels. Compared with SC, perivascular adipose tissue displayed higher SPM levels, suggesting an enhanced resolution capacity in this fat depot. In addition, augmented levels of eicosanoids and SPM were observed in SC fat surrounding foot wounds. Notably, the profile of SC PGF2α differed significantly when patients were grouped by body mass index (BMI). In the case of peri-wound SC fat, BMI negatively correlated with PGE2. In this tissue, proresolving mediators RvD2 and LXA4 were identified in lower levels than the proinflammatory LTB4. Collectively, these findings demonstrate a diverse distribution of bioactive lipid mediators depending on the localization of human fat depots and uncover a specific SPM pattern closely associated with PVD.
Keywords: anti-inflammatory and proresolving mediators, vascular disease
inflammation plays a vital role in host defense against invasive pathogens and tissue and wound repair. However, excessive or unresolved inflammation becomes chronic, leading to tissue injury and destruction or incomplete healing with fibrosis and scarring (27). Since unresolved inflammation is detrimental to the host, higher organisms have evolved protective mechanisms to ensure resolution of the inflammatory response in a specific time-limited manner (28). Among the mechanisms that facilitate resolution, lipid mediators (LM) derived from the metabolism of essential long-chain polyunsaturated fatty acids are the most efficient to stop inflammation in a highly coordinated, active process (27, 28). Indeed, lipoxins (LX) generated in human tissues via transcellular biosynthesis from the omega-6 fatty acid arachidonic acid (AA), together with the resolvin, protectin, and maresin families derived from the omega-3 fatty acids eicosapentaenoic (EPA) and docosahexaenoic (DHA) acids, are widely recognized as the most potent and together constitute a new genus of specialized proresolving mediators (SPM) (27, 28). These SPM are capable of resolving acute inflammatory responses with minimal damage to the surrounding tissue in a variety of inflammatory scenarios (reviewed in Ref. 31), including recent observations in amyotrophic lateral sclerosis, autoimmune Sjögren's syndrome, atopic dermatitis, and infantile eczema (16, 18, 22, 40). A key point in the resolution process is that the same proinflammatory factors initiating the inflammatory response [i.e., prostaglandins (PGs) PGE2 and PGD2] also signal the end by stimulating the local production of SPM that actively stimulate resolution mechanisms (17).
Adipose tissue is currently recognized as an endocrine tissue with an important homeostatic role in glucose and lipid metabolism (10, 36). A wealth of evidence indicates that most vascular and metabolic disorders are linked to the presence of persistent unresolved inflammation in this tissue (7). The presence of unresolved inflammation appears to be the consequence of a deregulated balance between the heightened biosynthesis of proinflammatory lipid- and peptide-derived chemical mediators [i.e., leukotriene (LT) B4, interleukin (IL)-6, tumor necrosis factor-α (TNF-α), and monocyte chemotactic protein-1 (MCP-1)] and reduced levels of anti-inflammatory molecules such as adiponectin (8, 15). Notably, a deficit in the biosynthesis of SPM has recently been uncovered in adipose tissues from an experimental animal model of obesity and insulin resistance (5). These SPM have been shown to improve insulin sensitivity in obese-diabetic mice and to attenuate age-associated adiposity (4, 14, 35). Here, we used liquid-chromatography-tandem mass spectrometry (LC-MS/MS)-based metabolo-lipidomics to address this question in humans. We analyzed different adipose tissues obtained from selected anatomic locations to identify unique signature profiles and relationship(s) between pro- and anti-inflammatory lipid mediators identified within different fat depots to assess their potential pathological implications in these clinically relevant settings. In particular, we examined the profiles of lipid mediators in human adipose tissue from patients with peripheral vascular disease (PVD) progressing to clinical need for major lower extremity amputation and compared them with those obtained from control subjects undergoing elective orthopedic procedures.1
MATERIALS AND METHODS
Sample collection.
After approval by the Partners Human Research Committee institutional review board (protocol nos. 2009-P-000740/9 and 2010-P-001343/9), 14 patients with PVD undergoing major (above knee or below knee) lower extremity amputation were included in the study. Samples were deidentified. Control subcutaneous (SC) adipose tissue samples were obtained from 12 subjects without history of peripheral vascular disease undergoing elective hip or knee replacement. Harvested adipose tissue from the amputation specimens include SC fat from the proximal portion of the amputation stump (n = 13), perivascular (PV) fat (n = 14) surrounding one of the major named leg arteries (i.e., popliteal, anterior tibial, posterior tibial, peroneal arteries), as well as peri-wound (n = 9) and non-wound (n = 7) SC foot fat from patients with concomitant open foot ulcers. Samples from the control group were limited to SC lower extremity fat. All fat samples were harvested with sharp dissection intraoperatively as soon as tissues were exposed. They were immediately snap-frozen in liquid nitrogen and stored at −80°C. Demographic and clinical data were collected from patients' electronic medical records. LM profiling was performed with these deidentified materials in accordance with Partners Human Research Committee protocol no. 1999-P-001279 for discarded materials.
Targeted lipid mediator-metabolo-lipidomics.
Samples were taken for solid extraction procedures optimized for maximum recovery of lipid mediator from human tissues. Briefly, 2 volumes of cold methanol were added to the frozen adipose tissue samples and held at −80°C for 12 h for protein precipitation. Samples were then centrifuged and supernatants were collected. After removal of the organic solvent under a stream of nitrogen, samples were suspended in methanol and rapidly acidified to pH 3.5 with HCl. Acidified samples were then loaded into C-18 solid phase extraction cartridges [Sep-Pak Vac 6 ml (500 mg) C18 cartridges, Waters, Milford, MA], rapidly neutralized, and eluted with hexane (fatty acids) and methyl formate (prostaglandins, leukotrienes, HETEs, and SPM). Next, solvents were removed under a stream of nitrogen and residues were suspended in mobile phase for LC-MS/MS analyses. LC-MS/MS-based metabolo-lipidomics was performed using linear ion trap triple quadrupole mass spectrometer MS (3200 QTRAP, Applied Biosystems, Foster City, CA) equipped with two HPLC pumps (LC-20AD, Shimadzu, Kyoto, Japan) coupled to an Eclipse Plus C18 reverse phase column (4.6 mmX50 mmX1.8 μm, Agilent Technologies, Palo Alto, CA). The mobile phase consisted of MeOH:H2O/acetic acid at a ratio of 60:40:0.01 (vol:vol:vol) and ramped to 80:20:0.01 after 10 min and to 100:0:0.01 after 12 min. Instrument control and data acquisition were performed using Analyst 1.5 software (Applied Biosystems). Ion pairs from reported multiple reaction monitoring (MRM) methods (41) were used for identification, profiling, and quantification of bioactive lipid mediators and pathway markers. Quantification was performed using standard calibration curves for each, and recoveries were calculated using deuterated internal standards (d4-LTB4, d4-PGE2, and d8–5-HETE) to cover each chromatographic region.
Adipokine analyses.
Proteins were isolated from the samples in ice-cold Dulbecco's phosphate-buffered saline with Protease Inhibitor Cocktail (Roche Applied Science, Indianapolis, IN), homogenized, and centrifuged (2,000 g, 5 min) to remove gross debris. The homogenates were next centrifuged once more (10,000 g, 10 min). The supernatant was then collected for quantitative protein analysis via multiple antigen flow microparticle bead assay Luminex (Luminex, Austin, TX) for key biologic mediators [i.e., IL-6, IL-8, IL-10, leptin, TNF-α, MCP-1, adiponectin, resistin, and plasminogen activator inhibitor-1 (PAI-1)]. Quantities of the biologic mediators were normalized to total protein as determined via a Bradford protein assay. Data acquisition and analysis were conducted using StarStation software (version 2.3; Applied Cytometry Systems, Dinnington, UK).
Histology.
Adipose tissue was fixed in 3.3% formalin, paraffin-embedded, sectioned, and stained at the Research Pathology Core of the Dana-Farber/Harvard Cancer Center (Boston, MA).
Data analysis.
To compare continuous variables between groups (e.g., diabetes, hyperlipidemia, statin use, and other categories), the Mann-Whitney U-test for nonparametric data was conducted. For comparing paired continuous variables, the Wilcoxon signed-rank test for nonparametric data was used. To assess the monotonic relationship between two continuous variables, the Spearman's rank correlation coefficient (Spearman's rho, rs) for nonparametric data was employed.
RESULTS
Demographic and clinical characteristics of the patient cohorts are listed in Table 1. There were no statistically significant differences in age, race/ethnicity, and sex between the two cohorts of the study. As typical of major amputation patients, this represented a medically ill patient population and had a greater incidence of diabetes mellitus, coronary artery disease (CAD), and renal disease (Table 1). In addition, this cohort took beta blockers at higher frequency and showed a lower overall BMI.
Table 1.
Demographic and clinical characteristics
| Variable | Amputation Cohort (n = 14) | Control Cohort (n = 12) | P |
|---|---|---|---|
| Age, yr | 65.0 (26.0–88.0) | 57.0 (22.0–78.0) | 0.26 |
| Race/ethnicity | 0.67 | ||
| Caucasian | 9 (64.3) | 9 (75.0) | |
| African-American | 3 (21.4) | 3 (25.0) | |
| Hispanic | 2 (14.3) | 0 (0.0) | |
| Female | 3 (21.4) | 4 (33.3) | |
| BMI, kg/m2 | 23.0 (19.2–38.5) | 31.1 (23.7–50.9) | < 0.01 |
| BMI category, kg/m2 | < 0.01 | ||
| <18.5 | 0 (0.0) | 0 (0.0) | |
| 18.5–24.9 | 11 (78.6) | 1 (8.3) | |
| 25.0–29.9 | 1 (7.1) | 3 (25.0) | |
| 30.0–34.9 | 1 (7.1) | 6 (50.0) | |
| 35.0–39.9 | 1 (7.1) | 0 (0.0) | |
| ≥40.0 | 0 (0.0) | 2 (16.7) | |
| Diabetes mellitus | 11 (78.6) | 1 (8.3) | < 0.01 |
| Hypertension | 13 (92.9) | 8 (66.7) | 0.15 |
| CHF | 5 (35.7) | 0 (0.0) | 0.04 |
| CVA | 3 (21.4) | 0 (0.0) | 0.22 |
| CAD | 10 (71.4) | 0 (0.0) | < 0.01 |
| Renal disease | 7 (50.0) | 0 (0.0) | < 0.01 |
| Pulmonary disease | 6 (42.9) | 0 (0.0) | 0.02 |
| Smoking history | 8 (57.1) | 3 (25.0) | 0.10 |
| Hyperlipidemia | 10 (71.4) | 6 (50.0) | 0.42 |
| Total cholesterol, mg/dl | 139.0 (112.0–195.0) | 174.0 (129.0–199.0) | 0.27 |
| LDL, mg/dl | 79.0 (54.0–114.0) | 88.2 (63.0–124.0) | 0.47 |
| HDL, mg/dl | 39.0 (28.0–66.0) | 57.0 (40.0–66.0) | 0.08 |
| Triglycerides, mg/dl | 104.0 (76.0–173.0) | 123.5 (78.0–247.0) | 0.38 |
| Glucose, mg/dl | 117.5 (94.0–334.0) | 92.5 (71.0–160.0) | 0.01 |
| HbA1c, % | 6.95 (5.1–10.8) | 5.85 (5.4–6.3) | 0.31 |
| HbA1c (diabetics only), % | 7.0 (5.1–10.8) | 6.3 (6.3–6.3) | 0.56 |
| Creatinine, mg/dl | 1.4 (0.7–7.8) | 1.0 (0.7–1.4) | 0.06 |
| Aspirin | 10 (71.4) | 9 (75.0) | 0.99 |
| Warfarin | 4 (28.6) | 5 (41.7) | 0.68 |
| Calcium channel blocker | 4 (28.6) | 1 (8.3) | 0.33 |
| Beta blocker | 12 (85.7) | 1 (8.3) | < 0.01 |
| ACE inhibitor | 4 (28.6) | 4 (33.3) | 0.99 |
| ARB | 2 (14.3) | 3 (25.0) | 0.63 |
| Statin | 11 (78.6) | 5 (41.7) | 0.10 |
| Metformin | 2 (14.3) | 1 (8.3) | 0.99 |
| Oral steroid | 2 (14.3) | 1 (8.3) | 0.99 |
Data are reported as no. (%) or median (range). BMI, body mass index; CHF, congestive heart failure; CVA, cerebrovascular accident; CAD, coronary artery disease; LDL, low-density lipoprotein; HDL, high-density lipoprotein; HbA1c, hemoglobin A1c; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker.
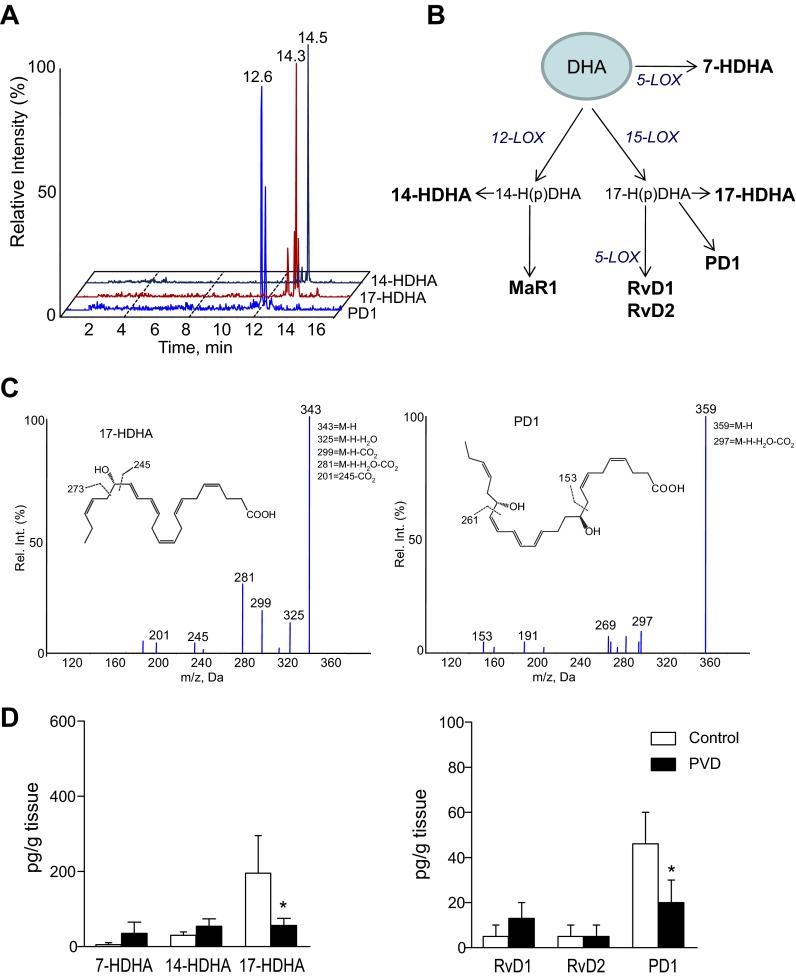
By means of LC-MS/MS-based metabolo-lipidomics, we first sought evidence for endogenous levels of SPM in adipose tissue from control subjects. In profiles obtained from human SC adipose tissue we identified RvD1 (7S,8R,17S-trihydroxy-4Z,9E,11E,13Z,15E,19Z-docosahexaenoic acid), RvD2 (7S,16R,17S-trihydroxy-4Z,8E,10Z,12E,14E,19Z-docosahexaenoic acid), and PD1 (10R,17S-dihydroxy-4Z,7Z,11E,13E,15Z,19Z-docosahexaenoic acid). In these samples, we also identified 17S-hydroxydocosahexaenoic acid (17-HDHA: 17S-hydroxy-4Z,7Z,10Z,13Z,15E,19Z-docosahexaenoic acid) and 14S-hydroxydocosahexaenoic acid (14-HDHA: 14S-hydroxy-4Z,7Z,10Z,12E,16Z,19Z-docosahexaenoic acid), the monohydroxy markers for RvD1, PD1, and maresin 1 (MaR1) biosynthesis pathways (29, 30), respectively, as well as 7S-hydroxydocosahexaenoic acid (7-HDHA: 7S-hydroxy-4Z,8E,10Z,13Z,16Z,19Z-docosahexaenoic acid). Representative chromatograms of selected MRM are shown in Fig. 1A. Figure 1B shows the DHA metabolome indicating the human biosynthetic pathways for the active mediators. Representative tandem mass spectra for 17-HDHA and PD1 obtained from human adipose tissue are each shown in Fig. 1C. The monohydroxy markers for RvE1 [18-hydroxyeicosapentaenoic acid (18-HEPE: 18R-hydroxy-5Z,8Z,11Z,14Z,16E-eicosapentaenoic acid)] and 15-epi-LXA5 (15-HEPE: 15-hydroxy-5Z,8Z,11Z,13E,17Z-eicosapentaenoic acid) (26) were also identified in these tissues (data not shown).
Fig. 1.
Specialized proresolving mediators (SPM) in human subcutaneous adipose tissue. LC-MS/MS-based lipid mediator metabolo-lipidomics. A: multiple reaction monitoring (MRM) chromatograms. B: schematic illustration of the docosahexaenoic acid (DHA) metabolome. C and D: representative tandem mass spectra of 17S-hydroxydocosahexaenoic acid (17-HDHA) and protectin D1 (PD1) with diagnostic ions (C) and quantitation of SPM (D) in subcutaneous adipose tissue from patients with peripheral vascular disease (PVD) and controls. Rv, resolvin. Results represent means ± SE of 12–14 different individuals. *P < 0.01 vs. control.
We next compared the profile of SPM obtained in SC adipose from control subjects with that of SC fat from patients with PVD. As shown in Fig. 1D, levels of 17-HDHA and PD1 were significantly decreased in patients with PVD. The presence of 15-HEPE was also decreased in these samples (data not shown). This reduced and/or impaired SPM level in SC fat from patients with PVD was coincident with increased MCP-1, resistin, PAI-1, and IL-10 levels (data not shown). Multiple linear regression analysis did not identify any relationship between identified adipose-tissue lipid mediator levels and adipokines, including PAI-1 and resistin (data not shown).
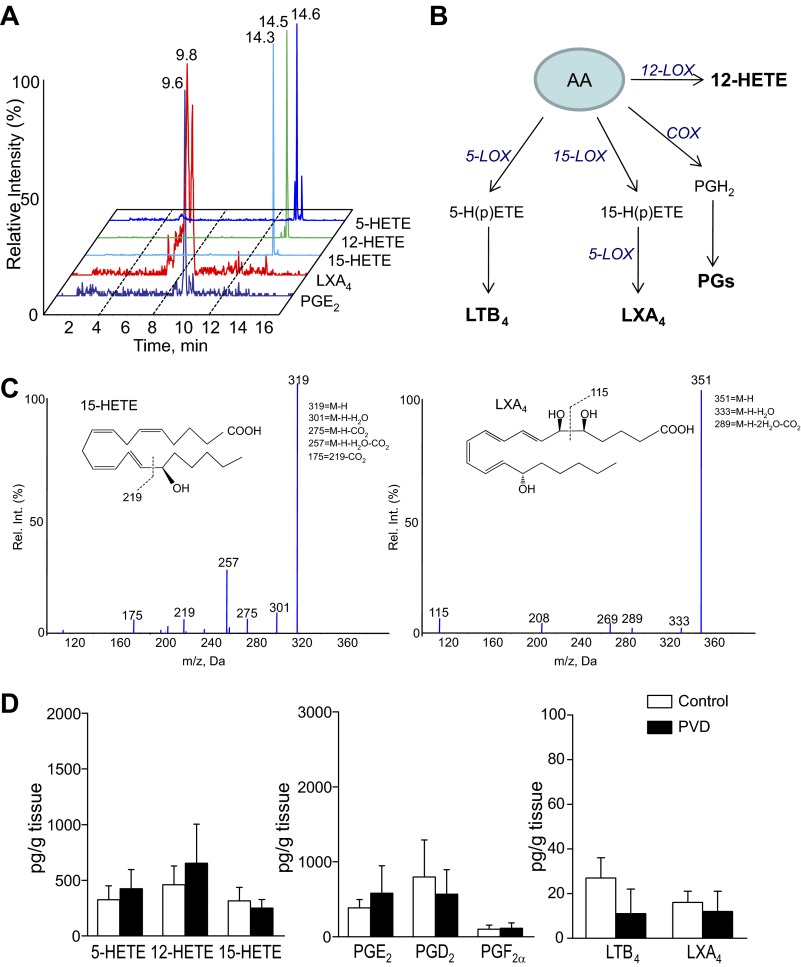
In addition to SPM, we also identified in human SC fat from control subjects significant levels of cyclooxygenase (COX) (PGE2, PGD2, and PGF2α) and lipoxygenase (LOX) [LTB4, 5-hydroxyeicosatetraenoic acid (5-HETE), 12-HETE and 15-HETE] products biosynthesized from endogenous sources of AA. LXA4 (5S,6R,15S-trihydroxy-eicosa-7E,9E,11Z,13E-tetraenoic acid) produced during the interaction between LOX-LOX pathways (29) was also present in these fat tissues. Representative chromatograms for these are shown in Fig. 2A, and representative tandem mass spectra for 15-HETE and LXA4 are each shown in Fig. 2C. Figure 2B shows the AA metabolome with targeted active mediators and pathway markers in the MRM targeted LC-MS/MS profiles. Compared with controls, similar levels of AA-derived COX and LOX products were observed in SC adipose tissue from patients with PVD (Fig. 2D).
Fig. 2.
LC-MS/MS based metabolo-lipidomics of lipid mediators derived from arachidonic acid (AA) in human subcutaneous adipose tissue. A: representative MRM chromatograms of targeted monohydroxy AA pathway markers and prostaglandins (PGs). B: schematic representation of the AA metabolome. C and D: representative tandem mass spectra of 15-hydroxyeicosatetraenoic acid (15-HETE) and lipoxin (LX) A4 (LXA4) with diagnostic ions (C) and their quantitation (D) in subcutaneous adipose tissue from patients with peripheral vascular disease and controls. Results represent means ± SE of 12–14 different individuals.
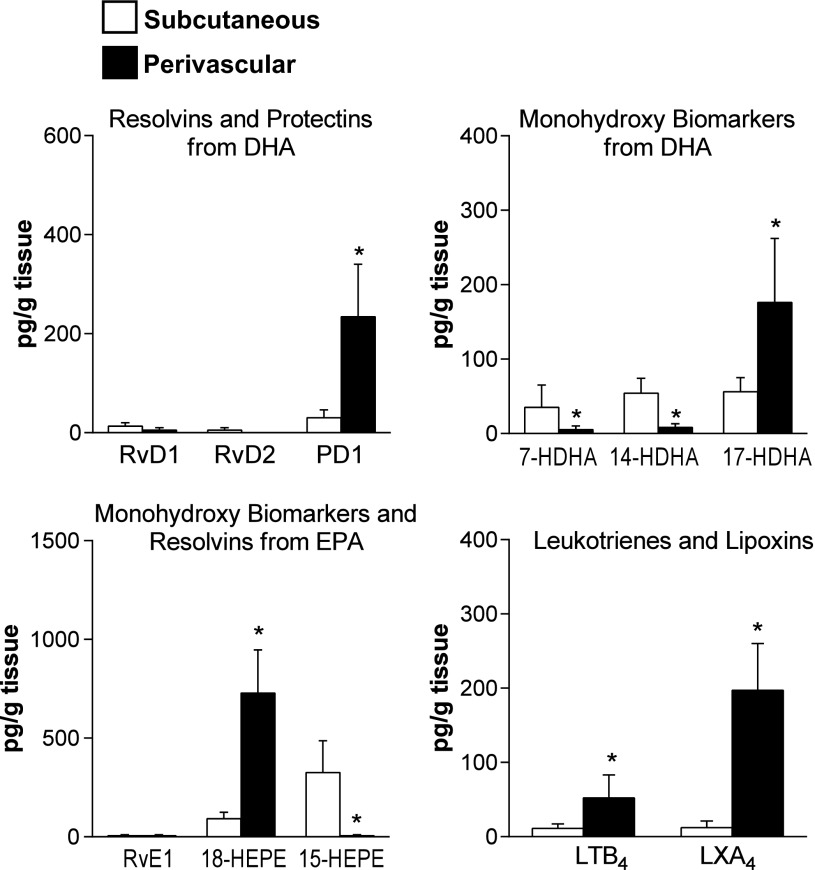
To examine whether the distribution of adipose tissue-derived lipid mediators varies depending on the anatomic localization, we compared the LC-MS/MS profiles in SC and PV fat depots obtained from patients with PVD. As shown in Fig. 3, PD1, 17-HDHA, 18-HEPE, and LXA4 were more abundant, while levels of 7-HDHA, 14-HDHA, and 15-HEPE were diminished in PV versus SC adipose. LTB4 levels were also increased (Fig. 3), while no changes in PGs and 5-, 12- and 15-HETE (data not shown) were observed. By using signed rank test, 18-HEPE, the RvE1 marker, was identified as a distinct signature variable in PV versus SC fat depots (n = 9). These results recognize a diverse profile of lipid mediators depending on the regional localization of adipose tissue within patients with PVD.
Fig. 3.
Profile of lipid mediators in adipose tissue from subcutaneous and perivascular depots in patients with peripheral vascular disease. Metabolo-lipidomics of resolvins and protectins from DHA, monohydroxy pathway markers from DHA, monohydroxy biomarkers and resolvins from EPA, and leukotrienes and lipoxins from AA was performed by LC-MS/MS. Results represent means ± SE of 13 different individuals. *P < 0.01 vs. subcutaneous.
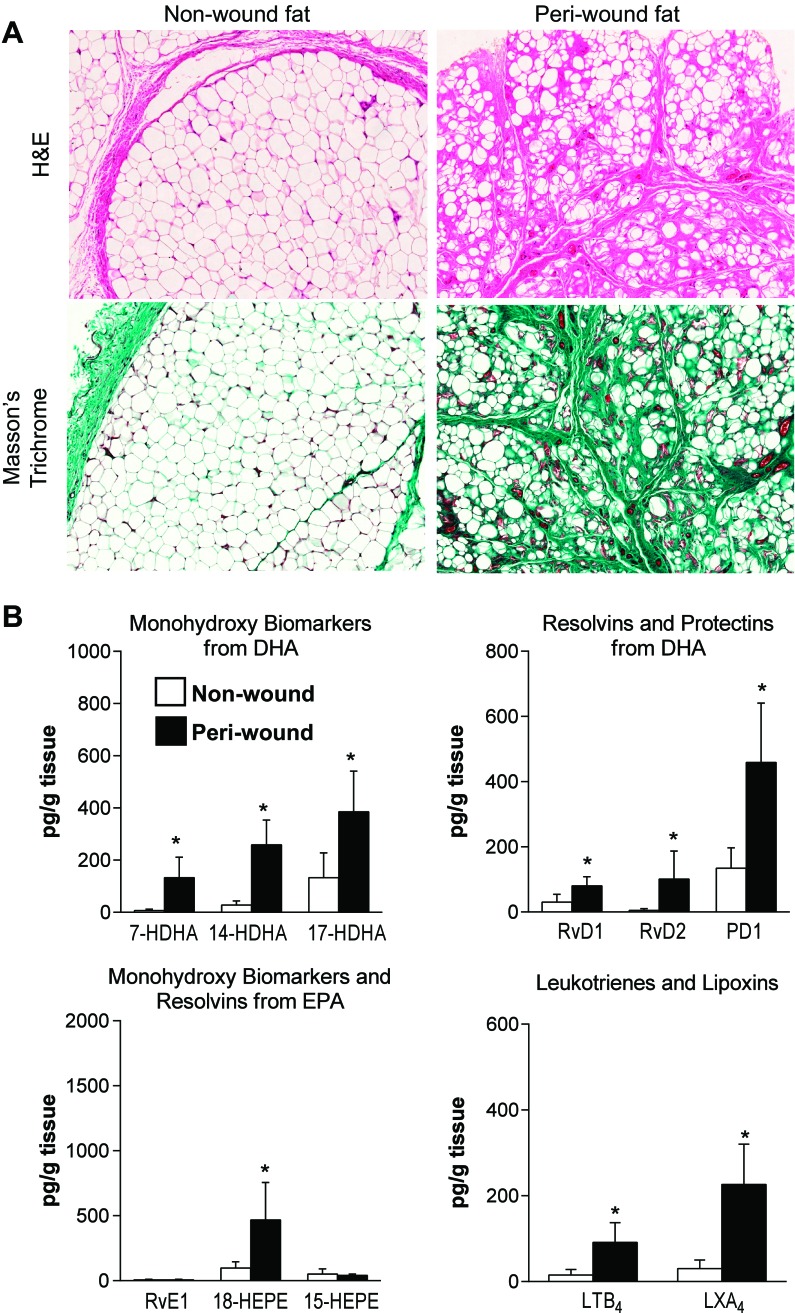
We next compared the profile of lipid mediators in SC fat surrounding foot wounds from PVD patients with concomitant open foot ulcers with that of non-wound SC foot fat. Representative photomicrographs of sections stained with hematoxylin-eosin and Masson's trichrome are shown in Fig. 4A. While the peri-wound SC fat samples demonstrated overt fibrosis, non-wound fat showed virtually normal adipose tissue (Fig. 4A). Importantly, production of SPM was significantly increased in peri-wound SC fat (Fig. 4B). Peri-wound fat also carried significantly higher amounts of LTB4 (Fig. 4B) and PGE2, PGD2 and 5-LOX, 12-LOX and 15-LOX products from arachidonic acid (data not shown). Linear regression analysis revealed a correlation between active PAI-1 and PGF2α (n = pairs; Beta = 1.00) in these samples.
Fig. 4.
Profile of lipid mediators in adipose tissue from subcutaneous peri-wound and non-wound fat from patients with peripheral vascular disease. A: hematoxylin/eosin (H&E) and Masson's trichrome-stained adipose tissue sections. B: LC-MS/MS-based lipid mediator metabolo-lipidomics of monohydroxy pathway markers from DHA, resolvins and protectins from DHA, monohydroxy pathway markers and resolvins from EPA, and leukotrienes and lipoxins from AA. Results represent means ± SE of 7–9 different individuals. *P < 0.01 vs. non-wound.
Patients with PVD and controls were analyzed with BMI as a continuous variable and also categorically. In the overall cohort, leptin positively correlated with BMI (rs = 0.71, P < 0.0001), and the relationship of BMI with IL-8 (rs = −0.42, P = 0.03) and resistin (rs = −0.040, P = 0.048) was negative (Tables 2 and 3). The profile of SC PGF2α differed significantly in the overall cohort when subjects were grouped by BMI and was approximately fivefold higher when BMI was over 35 (P = 0.005) (Table 2). In the vascular disease cohort, BMI was not significantly associated with any specific lipid mediator and differences in SPM levels in SC fat were not directly related to BMI or beta blocker treatment. Control patients (no confounding PVD) exhibited significant positive relationships between BMI and 18-HEPE (rs = 0.76, P = 0.007) and 14-HDHA (rs = 0.66, P = 0.03) (Table 3). In peri-wound fat, BMI negatively correlated with PGE2 (rs = −0.88, P = 0.004) (Table 3). Finally, deficient LXA4 and RvD2 levels accompanied by augmented levels of LTB4 was a hallmark of peri-wound SC fat from patients with a BMI higher than 25 (data not shown).
Table 2.
Analysis of association between each lipid mediator/adipokine and BMI category
| <18.5 | 18.5–24.9 | 25.0–29.9 | 30.0–34.9 | 35.0–39.9 | ≥40.0 | P | |
|---|---|---|---|---|---|---|---|
| Overall cohort | |||||||
| Subcutaneous | |||||||
| PGF2α, pg/g | NA | 62.4 | 13.9 | 0 | 229.6 | 208.4 | 0.005 |
| Leptin, pg/g | NA | 410,348 | 1,336,042 | 2,076,126 | 446,183 | 4,000,150 | 0.015 |
| Control cohort | |||||||
| Subcutaneous | |||||||
| PGF2α, pg/g | NA | 193.5 | 27.8 | 0 | NA | 208.4 | 0.03 |
Median values are reported. NA, not applicable.
Table 3.
Monotonic correlation between lipid mediators/adipokines and BMI as a continuous variable
| Spearman's Correlation Coefficient (rho) | P | |
|---|---|---|
| Overall cohort | ||
| Subcutaneous | ||
| IL-8 (n = 25), pg/g | −0.43 | 0.03 |
| Leptin (n = 25), pg/g | 0.71 | <0.0001 |
| Resistin (n = 25), pg/g | −0.40 | 0.048 |
| Control cohort | ||
| Subcutaneous | ||
| 18-HEPE (n = 11), pg/g | 0.76 | 0.007 |
| 14-HDHA (n = 11), pg/g | 0.66 | 0.03 |
| Vascular disease cohort | ||
| Perivascular | ||
| Leptin (n = 14), pg/g | 0.65 | 0.01 |
| Wound | ||
| PGE2 (n = 9), pg/g | −0.88 | 0.004 |
| Adiponectin (n = 10), pg/g | −0.72 | 0.02 |
| Subcutaneous | ||
| Leptin (n = 13), pg/g | 0.67 | 0.01 |
| Foot | ||
| LTB4 (n = 6), pg/g | 0.85 | 0.03 |
| TNF-α (n = 5), pg/g | −1.0 | <0.0001 |
DISCUSSION
In the present report we described the results of the first study that addresses the identification and distribution of local chemical lipid mediators of inflammation resolution in different anatomically localized fat depots. Specifically, we focused on identifying by means of a LC-MS/MS-based metabolo-lipidomic approach the signature profile of established SPM derived from polyunsaturated fatty acids of the omega-6 and omega-3 series, namely lipoxins, resolvins, protectins, and maresins (e.g., LXA4, RvD1, RvD2, PD1, and RvE1) as well as their intermediate and pathway markers 17-HDHA and 18-HEPE. The study was performed prospectively in adipose tissues collected from patients with PVD undergoing major lower extremity amputation and a control group of patients that underwent total hip or knee replacement. By utilizing these real-world patient cohorts, we obtained direct evidence of major phenotypic differences in the profile of adipose tissue lipid mediators among different fat depots and among different stages of disease.
Adipose tissue fulfills a wide range of functions including metabolic and blood pressure regulation and others (37). Adipose tissue localizes focally throughout the body, and it can be categorized into three gross anatomic depots: visceral, SC, and PV (34). While adipose tissue was historically considered a homogenous organ, fundamental differences among fat depots have been reported (24, 37). Although visceral adipose is now recognized to be central to metabolic diseases and to have a more negative impact on health than other fat depots (37), these data cannot be appropriately extrapolated to reflect the status of all adipose tissue depots. Therefore, the characteristics of SC and PV adipose depots in terms of inflammatory/anti-inflammatory lipid mediator production are a potential missing link in the role of adipose tissue in PVD. In our metabolo-lipidomic analysis, we found that SC adipose from patients with PVD had deficient levels of PD1 and its intermediate precursor 17-HpDHA marker, 17-HDHA, a specialized proresolving mediator with potent protective actions in vascular inflammation (20). This deficit in anti-inflammatory and proresolving mechanisms is consistent with our recent study demonstrating an enhanced inflammatory status in SC adipose tissue from PVD patients (19a). Unexpectedly, BMI did not appear to have a dominant effect on the levels of adipose tissue-derived lipid mediators in PVD patients. In these patients, only PGF2α and PGE2 levels differed significantly when patients were grouped by BMI. Further studies are needed to ascertain the pathological relevance of this observation and whether this finding is specific of PVD patients.
A key feature of the present study was that lipid mediators were determined in parallel with adipokine concentrations in SC fat from patients with PVD and controls. Since PVD patients showed increased inflammatory adipokine (i.e., MCP-1, resistin, and PAI-1) in parallel with reduced anti-inflammatory SPM levels, we suspect that an imbalance in the formation of anti-inflammatory mediators is a determinant of adipokine secretion in vascular disease. Indeed, the ability of SPM including RvD1, RvE1, and PD1 to regulate adiponectin secretion has been established earlier in murine adipose tissues (5, 11, 14). Moreover, RvD1 significantly reduces adipose leptin, TNF-α, and IL-1β secretion (5). Both RvD1 and LXA4 inhibit IL-6 secretion while inducing the anti-inflammatory cytokine IL-10 (4, 5). The existing link between SPM and adipokines is illustrated in Fig. 5. The fact that we did not identify any inverse correlations between adipokine and individual SPM levels suggests that they possess separate signaling pathways.
Fig. 5.
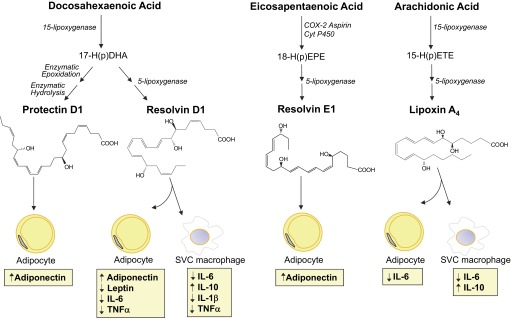
Functional relationship between SPM and adipose tissue-derived adipokines. During the process of resolution, long-chain polyunsaturated fatty acids (i.e., eicosapentaenoic acid, docosahexaenoic acid, and arachidonic acid) are converted into potent anti-inflammatory and proresolving SPM including protectin D1, resolvin D1, resolvin E1, and lipoxin A4. In the inflamed adipose tissue, these SPM are able to regulate the secretion of adipokines by adipocytes and macrophages from the stromal vascular cell (SVC) fraction. The biosynthetic pathways are illustrated here in abbreviated fashion. For complete details on biosynthesis and stereochemical assignments, see recent reviews (29, 32). IL, interleukin; TNF-α, tumor necrosis factor-α; COX-2, cyclooxygenase-2; Cyt P450, cytochrome P450; 17-HDHA, 17S-hydroxy(peroxy)docosahexaenoic acid; 18-HEPE, 18-hydroxyeicosapentaenoic acid; 15-HETE, 15-hydroxyeicosatetraenoic acid.
A major finding of our study was that we obtained results supporting the presence of an enhanced biosynthetic capacity of adipose tissue-derived SPM in PV fat. Perivascular adipose tissue surrounding systemic vessels holds particular relevance to vascular biology in view of its tissue mass, anatomic proximity, and emerging role in vascular pathologies (23). In our cohort of PVD patients, we found that PV adipose tissue displayed higher SPM levels than SC, suggesting activation of resolution circuits in this localization. Whether this signature is specific for patients with PVD is unknown, mainly because the availability of PV fat samples is a major limitation in extending this observation to healthy control subjects. The finding of increased biosynthesis of mediators of resolution in PV fat is in line with our recent study showing a specific adipokine signature in an amputation PVD patient cohort, with the PV adipose displaying relative less inflammation than SC adipose (19b). It is important to note that apart from LTB4, a potent leukocyte chemoattractant (25), the formation of inflammatory prostanoids in the PV and SC depots is essentially similar.
Lipid mediators such as the eicosanoids (prostaglandins and leukotrienes) are in general proinflammatory autacoids (25), as are the proresolving mediators (29). In this regard, the SPM autacoids are made locally, act in their surrounding tissue milieu, and are metabolically inactivated. Both RvD1 and RvD2 are further metabolically converted by adipose tissues to oxo-Rv products (5). Some of the oxo-Rv products are inactive while others retain their proresolving actions. In the present studies, we identified LM within fat tissues. Hence, the temporal metabolic flux in these metabolic pathways may have been altered in fat tissues in that the LM such as Rv, MaR1, and PD1 may be trapped within the adipose, giving rise to either a proresolving or proinflammatory tissue status depending on the levels of individual LM. Unfortunately, our patient study design did not allow the assessment of the dynamic changes in lipid mediator generation and measurements were made at only one time point. It is likely that there are temporal changes in both proinflammatory and proresolving lipid mediators in human adipose as demonstrated in temporal analyses of self-limited resolving exudates in mouse peritonitis (1). Analyses of temporal relationships between the families of lipid mediators in human adipose remain of interest. Nonetheless, their documentation herein provides a critical initial step.
The results showing enhanced levels of lipid mediators with both pro- and anti-inflammatory properties in peri-wound foot fat from patients with PVD and concomitant open foot ulcers are intriguing. Fibrosis reflects postinflammatory scarring of the tissue as the tissue capacity for repair is overwhelmed and normal functional parenchyma is replaced by extracellular matrix (39). Matrix accumulation in this context arises in response to paracrine and autocrine mediators, resulting in fibroblast activation and accumulation and transition of epithelial cells, endothelial cells, and pericytes to a more mesenchymal phenotype (39). While proinflammatory lipid mediators such as LTs and PGs activate fibrogenic responses in solid organs including skin, lung, liver and kidneys (9), administration of SPM analogs or mimetics of these endogenously produced biotemplates has been shown to exert both anti-inflammatory and antifibrotic activities (3, 6). Therefore, the observed overproduction of SPM by peri-wound foot fat is suggestive of a parallel counterregulatory mechanism of the exacerbated induction of proinflammatory and profibrogenic pathways. Given the strong chemotaxic effect of LTB4 and specific high-affinity binding to BLT1 receptors on human neutrophils, which also binds RvE1 and RvE2, LTB4 may override the anti-inflammatory actions of SPM in peri-wound SC fat tissue at a given time point during inflammation (38). An alternative explanation is that SPM, which also restore tissue homeostasis (12) and divert organ fibrosis (6), can promote the healing process by stimulating wound closure in skin connective tissue and their actions on resolving macrophages (33). Indeed, recent findings support a role for SPM in promoting wound healing in diabetes (13, 21) and in preventing secondary thrombosis and necrosis in a mouse burn wound model (2).
In summary, this investigation establishes the first phenotypic differences in the capacity and levels of specific proresolving mediators between adipose tissue from patients with end-stage PVD and a control patient cohort. In addition, this study identifies a remarkable structural heterogeneity among different human fat depots and among different clinical stages of the disease. The relevance of our present findings is highlighted by the recent identification and presence of biologically active concentrations of these proresolving mediators of self-limited resolution of inflammation in serum and plasma in healthy subjects with omega-3 fatty acid supplementation (19).
GRANTS
This work was supported in part by National Institutes of Health (Grants P01-GM-095467 and R01-GM-038765 to C. N. Serhan and T32 HL-007734 to B. T. Nguyen), the American Heart Association (Grant 12GRNT951000 to C. K. Ozaki), and Spanish Health, Science and Innovation Ministries (Grants SAF2012/32789 and BA10/00036 to J. Clària).
DISCLOSURES
C. N. Serhan is an inventor on patents [resolvins] assigned to Brigham and Women's Hospital and licensed to Resolvyx Pharmaceuticals. C. N. Serhan is a scientific founder of Resolvyx Pharmaceuticals and owns equity in the company. C. N. Serhan's interests were reviewed and are managed by the Brigham and Women's Hospital and Partners HealthCare in accordance with their conflict of interest policies. The other authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS
J.C., B.T.N., and C.K.O. performed experiments; J.C., A.M., C.K.O., and C.N.S. analyzed data; J.C. and C.K.O. drafted manuscript; J.C., A.M., C.K.O., and C.N.S. edited and revised manuscript; J.C., C.N.S. and C.K.O. interpreted results of experiments and prepared figures; C.N.S. conceived and designed the research; C.N.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank M. H. Small for expert assistance with manuscript preparation.
J. Clària was on sabbatical from Department of Biochemistry and Molecular Genetics and Department of Physiological Sciences I, Hospital Clínic, University of Barcelona, Barcelona E-08036, Spain (jclaria@clinic.ub.es).
Footnotes
This article is the topic of an Editorial Focus by János G. Filep (8a).
REFERENCES
- 1. Bannenberg GL, Chiang N, Ariel A, Arita M, Tjonahen E, Gotlinger KH, Hong S, Serhan CN. Molecular circuits of resolution: formation and actions of resolvins and protectins. J Immunol 174: 4345–4355, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Bohr S, Patel SJ, Sarin D, Irimia D, Yarmush ML, Berthiaume F. Resolvin D2 prevents secondary thrombosis and necrosis in a mouse burn wound model. Wound Repair Regen 21: 35–43, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Börgeson E, Docherty NG, Murphy M, Rodgers K, Ryan A, O'Sullivan TP, Guiry PJ, Goldschmeding R, Higgins DF, Godson C. Lipoxin A4 and benzo-lipoxin A4 attenuate experimental renal fibrosis. FASEB J 25: 2967–2979, 2011 [DOI] [PubMed] [Google Scholar]
- 4. Börgeson E, McGillicuddy FC, Harford KA, Corrigan N, Higgins DF, Maderna P, Roche HM, Godson C. Lipoxin A4 attenuates adipose inflammation. FASEB J 26: 4287–4294, 2012 [DOI] [PubMed] [Google Scholar]
- 5. Clària J, Dalli J, Yacoubian S, Gao F, Serhan CN. Resolvin D1 and resolvin D2 govern local inflammatory tone in obese fat. J Immunol 189: 2597–2605, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Duffield JS, Hong S, Vaidya VS, Lu Y, Fredman G, Serhan CN, Bonventre JV. Resolvin D series and protectin D1 mitigate acute kidney injury. J Immunol 177: 5902–5911, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Elks CM, Francis J. Central adiposity, systemic inflammation, and the metabolic syndrome. Curr Hypertens Rep 12: 99–104, 2010 [DOI] [PubMed] [Google Scholar]
- 8. Ferrante AW., Jr Obesity-induced inflammation: a metabolic dialogue in the language of inflammation. J Intern Med 262: 408–414, 2007 [DOI] [PubMed] [Google Scholar]
- 8a. Filep JG. Resolution pathways in inflammation: the devil in the adipose tissues and in the details. Focus on “Diversity of lipid mediators in human adipose tissue depots.” Am J Physiol Cell Physiol (March 6, 2013). doi: 10.1152/ajpcell.00063.2013 [DOI] [PubMed] [Google Scholar]
- 9. Flamand N, Mancuso P, Serezani CH, Brock TG. Leukotrienes: mediators that have been typecast as villains. Cell Mol Life Sci 64: 2657–2670, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol 316: 129–139, 2010 [DOI] [PubMed] [Google Scholar]
- 11. González-Périz A, Horrillo R, Ferré N, Gronert K, Dong B, Moran-Salvador E, Titos E, Martínez-Clemente M, López-Parra M, Arroyo V, Clària J. Obesity-induced insulin resistance and hepatic steatosis are alleviated by omega-3 fatty acids. A role for resolvins and protectins. FASEB J 23: 1946–1957, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hasturk H, Kantarci A, Goguet-Surmenian E, Blackwood A, Andry C, Serhan CN, Van Dyke TE. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J Immunol 179: 7021–7029, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Hellmann J, Tang Y, Spite M. Proresolving lipid mediators and diabetic wound healing. Curr Opin Endocrinol Diabetes Obes 19: 104–108, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hellmann J, Tang Y, Kosuri M, Bhatnagar A, Spite M. Resolvin D1 decreases adipose tissue macrophage accumulation and improves insulin sensitivity in obese-diabetic mice. FASEB J 25: 2399–2407, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Horrillo R, González-Périz A, Martínez-Clemente M, López-Parra M, Ferré N, Titos E, Morán-Salvador E, Deulofeu R, Arroyo V, Clària J. 5-Lipoxygenase activating protein signals adipose tissue inflammation and lipid dysfunction in experimental obesity. J Immunol 184: 3978–3987, 2010 [DOI] [PubMed] [Google Scholar]
- 16. Kim TH, Kim GD, Jin YH, Park YS, Park CS. Omega-3 fatty acid-derived mediator, resolvin E1, ameliorates 2,4-dinitrofluorobenzene-induced atopic dermatitis in NC/Nga mice. Int Immunopharmacol 14: 384–391, 2012 [DOI] [PubMed] [Google Scholar]
- 17. Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol 2: 612–619, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Liu G, Fiala M, Mizwicki MT, Sayre J, Magpantay L, Siani A, Mahanian M, Chattopadhyay M, La Cava A, Wiedau-Pazos M. Neuronal phagocytosis by inflammatory macrophages in ALS spinal cord: inhibition of inflammation by resolvin D1. Am J Neurodegener Dis 1: 60–74, 2012 [PMC free article] [PubMed] [Google Scholar]
- 19. Mas E, Croft KD, Zahra P, Barden A, Mori TA. Resolvins D1, D2, and other mediators of self-limited resolution of inflammation in human blood following n-3 fatty acid supplementation. Clin Chem 58: 1476–1484, 2012 [DOI] [PubMed] [Google Scholar]
- 19a. Mauro CR, Nguyen B, Yu P, Tao M, Gao I, Seidman MA, Nguyen LL, Ozaki CK. Inflammatory “adiposopathy” in major amputation patients. Ann Vasc Surg 27: 346–35, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19b. Mauro CR, Ilonzo G, Nguyen B, Yu P, Tao M, Gao I, Seidman MA, Nguyen LL, Ozaki CK. Attenuated adiposopathy in perivascular adipose tissue compared to subcutaneous human adipose tissue. Am J Surg. doi: 10.1016/j.amjsurg.2012.07.032.[Epubaheadofprint] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Merched AJ, Ko K, Gotlinger KH, Serhan CN, Chan L. Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J 22: 3595–3606, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Norling LV, Spite M, Yang R, Flower RJ, Perretti M, Serhan CN. Humanized nano-proresolving medicines mimic inflammation-resolution and enhance wound healing. J Immunol 186: 5543–5547, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Odusanwo O, Chinthamani S, McCall A, Duffey ME, Baker OJ. Resolvin D1 prevents TNF-α-mediated disruption of salivary epithelial formation. Am J Physiol Cell Physiol 302: C1331–C1345, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rajsheker S, Manka D, Blomkalns AL, Chatterjee TK, Stoll LL, Weintraub NL. Crosstalk between perivascular adipose tissue and blood vessels. Curr Opin Pharmacol 10: 191–196, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sackmann-Sala L, Berryman DE, Munn RD, Lubbers ER, Kopchick JJ. Heterogeneity among white adipose tissue depots in male C57BL/6J mice. Obesity 20: 101–111, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Samuelsson B. Role of basic science in the development of new medicines: examples from the eicosanoid field. J Biol Chem 287: 10070–10080, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med 192: 1197–1204, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol 8: 349–361, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Serhan CN, Brain SD, Buckley CD, Gilroy DW, Haslett C, O'Neill LAJ, Perretti M, Rossi AG, Wallace JL. Resolution of inflammation: state of the art, definitions and terms. FASEB J 21: 325–332, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Serhan CN. Resolution phases of inflammation: novel endogenous anti-inflammatory and pro-resolving lipid mediators and pathways. Annu Rev Immunol 25: 101–137, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, Oh SF, Spite M. Maresins: novel macrophage mediators with potent anti-inflammatory and pro-resolving actions. J Exp Med 206: 15–23, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Serhan CN. Novel lipid mediators and resolution mechanisms in acute inflammation: to resolve or not? Am J Pathol 177: 1576–1591, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Serhan CN, Petasis NA. Resolvins and protectins in inflammation resolution. Chem Rev 111: 5922–5943, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tang Y, Zhang MJ, Hellmann J, Kosuri M, Bhatnagar A, Spite M. Proresolution therapy for the treatment of delayed healing of diabetic wounds. Diabetes 62: 618–627, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thalmann S, Meier CA. Local adipose tissue depots as cardiovascular risk factors. Cardiovasc Res 75: 690–701, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Titos E, Rius B, González-Périz A, López-Vicario C, Morán-Salvador E, Martínez-Clemente M, Arroyo V, Clària J. Resolvin D1 and its precursor docosahexaenoic acid promote resolution of adipose tissue inflammation by eliciting macrophage polarization toward an M2-like phenotype. J Immunol 187: 5408–5418, 2011 [DOI] [PubMed] [Google Scholar]
- 36. Trayhurn P. Endocrine and signalling role of adipose tissue: new perspectives on fat. Acta Physiol Scand 184: 285–293, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Wajchenberg BL, Giannella-Neto D, da Silva ME, Santos RF. Depot-specific hormonal characteristics of subcutaneous and visceral adipose tissue and their relation to the metabolic syndrome. Horm Metab Res 34: 616–621, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Wan M, Godson C, Guiry PJ, Agerberth B, Haeggström JZ. Leukotriene B4/antimicrobial peptide LL-37 proinflammatory circuits are mediated by BLT1 and FPR2/ALX and are counterregulated by lipoxin A4 and resolvin E1. FASEB J 25: 1697–1705, 2011 [DOI] [PubMed] [Google Scholar]
- 39. Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol 214: 199–210, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wu SH, Chen XQ, Liu B, Wu HJ, Dong L. Efficacy and safety of 15(R/S)-methyl-lipoxin A4 in topical treatment of infantile eczema. Br J Dermatol 168: 172–178, 2013 [DOI] [PubMed] [Google Scholar]
- 41. Yang R, Chiang N, Oh SF, Serhan CN. Metabolomics-lipidomics of eicosanoids and docosanoids generated by phagocytes. Curr Protoc Immunol 95: 14.26.1–14.26.26, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]