gap junctions provide a pathway for intercellular communication primarily by directly interconnecting the cytoplasm of adjacent cells (3). This pathway consists of arrays of channels, composed of proteins known as connexins. Connexin channels create a direct conduit enabling diffusion of cytoplasmic molecules, ions, and water between cells. Gap junctions thus coordinate signaling and metabolism between otherwise disconnected cells within a tissue.
A complete gap junction channel consists of two hexamers, one on each cell, docked to each other and organized into plaques (Figure 1). There are two dozen human connexin genes. Different tissues express different connexins, which, in turn, produce channels with different composition, permeability, and regulation. In this way, gap junctional communication is controlled. Conversely, misregulation of intercellular communication, due to mutations that cause a loss or alteration of connexin function, has the capacity to cause human disease (3).
Fig. 1.
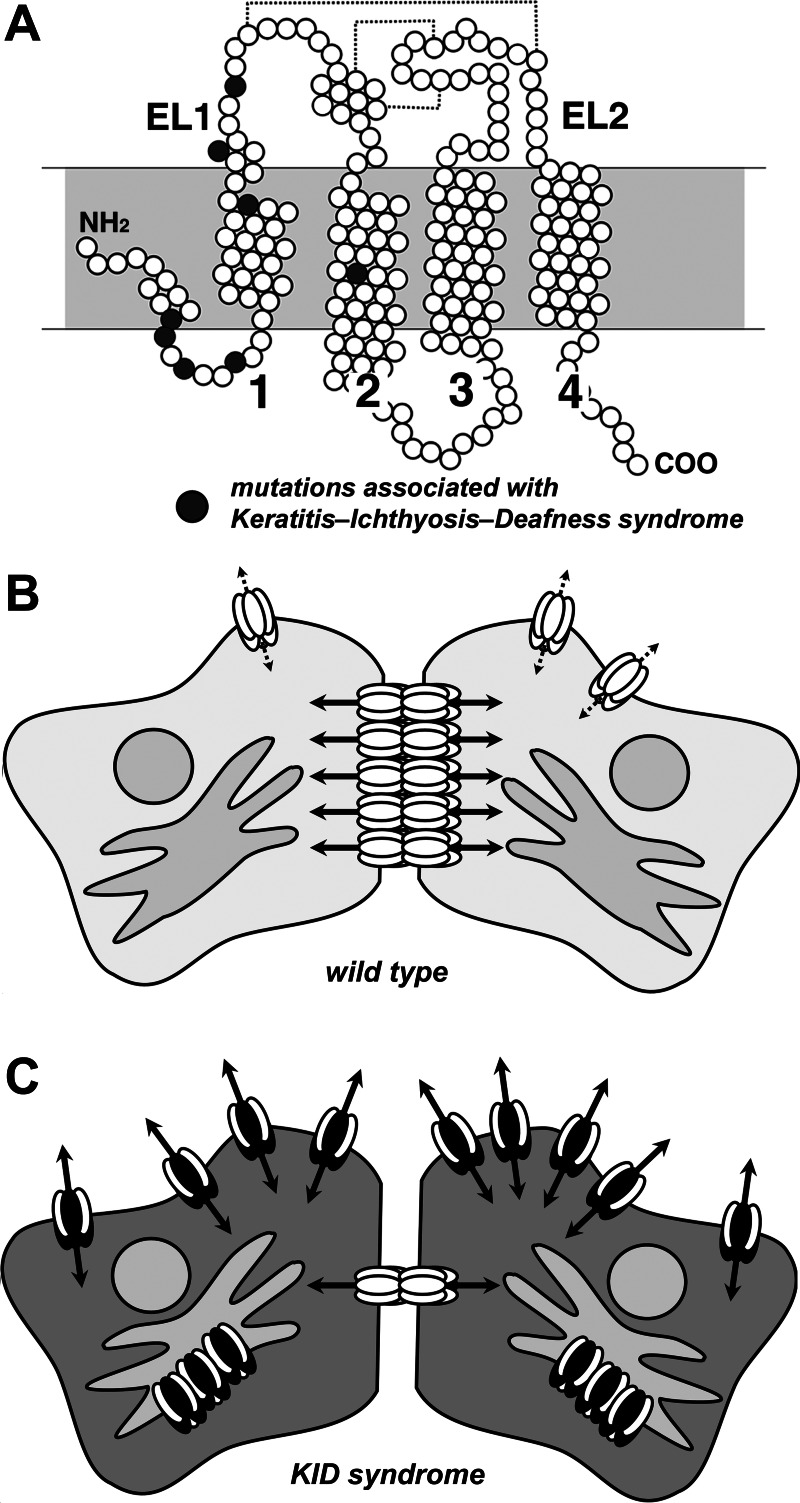
Effects of mutant connexin 26 (Cx26) on gap junction hemichannels. A: structure of Cx26. Shown is a diagram depicting individual amino acids of Cx26 as circles. Filled circles represent amino acid positions mutated in keratitis-ichthyosis-deafness (KID) syndrome. Indicated are the first and second extracellular loop domains (EL1 and EL2) as well as the four transmembrane domains. Dashed lines represent disulfide bonds between EL1 and EL2. B: cells expressing wild-type Cx26 (white ovals) primarily form gap junction channels (solid arrows) and have minimal hemichannel activity (dashed arrows). Here only Cx26 is shown for simplicity; cells in the cochlea or skin express other connexins in addition to Cx26. C: cells expressing mutant Cx26 (dark ovals) interact with wild-type Cx26 to inhibit trafficking and formation of gap junction channels. Instead Cx26 hemichannels show increased activity that impairs cell function.
Connexin 26 (Cx26) is critical for hearing; several mutations that inhibit Cx26 channel function impair cochlear potassium homeostasis and cause deafness (10). However, Cx26 mutations are implicated in a spectrum of diseases ranging from nonsyndromic hearing loss to diseases affecting multiple organ systems. In this issue of American Journal of Physiology-Cell Physiology, Mhaske et al. (5) characterized two Cx26 mutations (D50A and A88V) associated with keratitis-ichthyosis-deafness (KID) syndrome, which is a disease that disrupts the eye and skin barriers as well as causes deafness. They found that instead of simply inhibiting channel formation, these mutations produced forms of Cx26 that formed high conductance hemichannels at the cell surface. “Gain of function” Cx26 mutations thus create channels that have more generalized toxicity than mutations that lead to nonfunctional Cx26, which, more typically, are limited to affecting cochlear function. Other KID-associated Cx26 mutations have a comparable effect on hemichannel permeability, suggesting a unifying mechanism linking this particular deafness associated mutation with skin disease (10). In each case, KID-associated mutations in Cx26 affected conductance but had less of an effect on spontaneous open probability. In other words, the channels still required a stimulus to open, but channels containing mutant Cx26 were more permeable than wild-type Cx26. Despite comparable open probabilities, the high permeability of hemichannels composed of mutant Cx26 facilitates leakage of critical metabolites and leads to cell death.
Importantly, KID mutants of Cx26 exhibit a similar effect when transfected into normal human keratinocytes and had a dominant negative effect on wild-type Cx26 (5). Specifically, the D50A and A88V Cx26 mutations inhibited the trafficking and assembly of wild-type Cx26 into gap junctions. This property further exacerbates the pathology in cells as mutant Cx26 both impairs beneficial intercellular communication as well as induces aberrant hemichannel activity (Fig. 1). The results of this study (5) also suggest that heteromeric hemichannels containing both mutant and wild-type Cx26 have the ability to form high conductance hemichannels, although this remains to be rigorously demonstrated. Alternatively, in cells expressing mutated Cx26, plasma membrane hemichannels may be strictly composed of mutant Cx26 and deficient in wild-type Cx26. In either case, the mutant form of Cx26 functionally dominates over wild-type Cx26. The high conductivity of these Cx26 mutants indicates that a lack of Cx26 activity is not sufficient to cause the accompanying skin disease and that other connexins have the capacity to compensate for loss of Cx26-mediated gap junctional communication in the skin of patients with inactivating mutations in Cx26 that are not associated with keratitis.
As shown in Fig. 1, Cx26 mutations associated with KID are concentrated in the NH2-terminal and first transmembrane domain (TM1), although A88V is in the second transmembrane domain (TM2). Based on the high-resolution structure of Cx26, all of these domains are structural elements of the aqueous pore of the channel (4). In particular, the NH2-terminal domain folds into the cytoplasmic aspect of the pore to form a funnel that narrows the cytoplasmic entrance of the channel and regulates permeability by lining the channel entrance with charged α-helices (6). However, the structure of the NH2-terminal funnel also promotes formation of an open channel in addition to restricting permeability. A likely mechanism for KID mutations is to increase hemichannel permeability by affecting folding of the NH2-terminal domain or how it interacts with the entrance to the pore. Other pore-lining elements in TM1 and TM2 also have the potential to alter permeability by changing the charge or bulk of side chains exposed to the aqueous part of the connexin channel. By contrast, there is a lack of KID-related mutations in elements that regulate hemichannel opening, especially the extracellular loop domains. Thus hemichannel opening of both wild-type and mutant Cx26 is likely regulated in a comparable manner, despite the significant differences in channel permeability.
Although Cx26 mutants associated with KID exhibit deleterious hemichannel activity, there are several examples in which connexin hemichannels have beneficial physiological roles. For instance, connexin hemichannels can act as high conductance plasma membrane channels, most frequently by providing a pathway for stimulated ATP secretion as part of an overall purinergic receptor signaling response (9). However, conclusive evidence for connexin hemichannels is frequently obscured by other classes of high conductance channels, including pannexins (7) and calcium homeostasis modulator (CALHM) channels (8). Of particular relevance to hearing, regulated ATP secretion by connexin hemichannels was found to be preserved in pannexin-1-deficient mice but impaired in Cx26- or Cx30-deficient mice (2). Although this could potentially implicate both Cx26 and Cx30 in ATP secretion, cochlear Cx26 expression is also decreased in Cx30-deficient knockout mice. When Cx26 expression is maintained, decreased Cx30, in and of itself, has no effect on hearing (1). These findings underscore a physiological role for normal Cx26 hemichannels in cochlear intercellular signaling.
In order for a large conductance plasma membrane channel to be physiologically beneficial, it needs to be tightly regulated. One mode of regulating these channels is to have a low open probability, where the channels are predominantly closed. In KID, Cx26 open probability is unchanged; however, when mutant Cx26 hemichannels do open, permeability is higher than wild-type Cx26 and causes toxic leakage of cell metabolites. Thus therapeutic approaches will have a greater chance of success by aiming to decrease hemichannel permeability as opposed to keeping these channels closed. In particular, the skin pathology of KID may be amenable to topical application of as yet undiscovered agents with the capacity to specifically attenuate mutant Cx26 hemichannels.
GRANTS
This work was supported by National Institutes of Health Grants HL-083120 and AA-013757 and Emory University Research Committee.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.K. prepared figures; M.K. drafted manuscript; M.K. edited and revised manuscript; M.K. approved final version of manuscript.
REFERENCES
- 1. Ahmad S, Tang W, Chang Q, Qu Y, Hibshman J, Li Y, Sohl G, Willecke K, Chen P, Lin X. Restoration of connexin26 protein level in the cochlea completely rescues hearing in a mouse model of human connexin30-linked deafness. Proc Natl Acad Sci USA 104: 1337–1341, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anselmi F, Hernandez VH, Crispino G, Seydel A, Ortolano S, Roper SD, Kessaris N, Richardson W, Rickheit G, Filippov MA, Monyer H, Mammano F. ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc Natl Acad Sci USA 105: 18770–18775, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goodenough DA, Paul DL. Gap junctions. Cold Spring Harb Perspect Biol 1: a002576, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maeda S, Nakagawa S, Suga M, Yamashita E, Oshima A, Fujiyoshi Y, Tsukihara T. Structure of the connexin 26 gap junction channel at 3.5 A resolution. Nature 458: 597–602, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Mhaske PV, Levit NA, Li L, Wang HZ, Lee JR, Shuja Z, Brink PR, White TW. The human Cx26-D50A and Cx26-A88V mutations causing keratitis-ichthyosis-deafness syndrome display increased hemichannel activity. Am J Physiol Cell Physiol (February 27, 2013). doi: 10.1152/ajpcell.00374.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oshima A, Tani K, Toloue MM, Hiroaki Y, Smock A, Inukai S, Cone A, Nicholson BJ, Sosinsky GE, Fujiyoshi Y. Asymmetric configurations and N-terminal rearrangements in connexin26 gap junction channels. J Mol Biol 405: 724–735, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Penuela S, Gehi R, Laird DW. The biochemistry and function of pannexin channels. Biochim Biophys Acta 1828: 15–22, 2013 [DOI] [PubMed] [Google Scholar]
- 8. Siebert AP, Ma Z, Grevet JD, Demuro A, Parker I, Foskett JK. Structural and functional similarities of calcium homeostasis modulator 1 (CALHM1) ion channel with connexins, pannexins, and innexins. J Biol Chem 288: 6140–6153, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang N, De Bock M, Decrock E, Bol M, Gadicherla A, Vinken M, Rogiers V, Bukauskas FF, Bultynck G, Leybaert L. Paracrine signaling through plasma membrane hemichannels. Biochim Biophys Acta 1828: 35–50, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xu J, Nicholson BJ. The role of connexins in ear and skin physiology–functional insights from disease-associated mutations. Biochim Biophys Acta 1828: 167–178, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]