Abstract
Mutations in the human gene encoding connexin 26 (Cx26 or GJB2) cause either nonsyndromic deafness or syndromic deafness associated with skin diseases. That distinct clinical disorders can be caused by different mutations within the same gene suggests that different channel activities influence the ear and skin. Here we use three different expression systems to examine the functional characteristics of two Cx26 mutations causing either mild (Cx26-D50A) or lethal (Cx26-A88V) keratitis-ichthyosis-deafness (KID) syndrome. In either cRNA-injected Xenopus oocytes, transfected HeLa cells, or transfected primary human keratinocytes, we show that both Cx26-D50A and Cx26-A88V form active hemichannels that significantly increase membrane current flow compared with wild-type Cx26. This increased membrane current accelerated cell death in low extracellular calcium solutions and was not due to increased mutant protein expression. Elevated mutant hemichannel currents could be blocked by increased extracellular calcium concentration. These results show that these two mutations exhibit a shared gain of functional activity and support the hypothesis that increased hemichannel activity is a common feature of human Cx26 mutations responsible for KID syndrome.
Keywords: connexin, channel, deafness, skin disease, GJB2, mutation
gap junctions contain clusters of intercellular channels that allow the exchange of ions, second messengers, and small metabolites between adjacent cells (3). Gap junction channels are formed by oligomers of connexin proteins, which also have the ability to form functioning “hemichannels” in nonjunctional plasma membranes (4, 19). Mutations in connexin genes have been linked to several human diseases (33, 47). For example, mutations in connexin 26 (Cx26 or GJB2) are a major cause of nonsyndromic deafness and can also result in syndromic deafness associated with skin disorders like palmoplantar keratoderma, keratitis-ichthyosis-deafness (KID) syndrome, or Vohwinkel syndrome (34, 39). There is abundant experimental evidence to suggest that either total, or partial, loss of function mutations in Cx26 is responsible for nonsyndromic deafness (22, 45, 50). Since nonsyndromic deafness linked to Cx26 is predominantly a loss of function disorder, it has been proposed that syndromic Cx26 mutations must show an alteration, or gain, of function to cause skin disease in addition to hearing loss (23, 28, 45).
Many connexins can form functional hemichannels in addition to gap junction channels (8, 11, 24), suggesting that some disease-linked connexin mutations may result in abnormal hemichannel activity that could contribute to the observed pathology. This idea has been supported by studies of connexin mutations linked to skin disorders such as KID syndrome. The first characterized KID mutation was Cx26-A40V, which caused a severe form of the disease including the follicular occlusion triad (31). Injection of Cx26-A40V cRNA into Xenopus oocytes resulted in the induction of large membrane currents followed by cell death, suggesting that the mutation increased hemichannel activity (31). Subsequent evaluation of additional KID syndrome mutations has indicated that altered hemichannel activity may be a general pathological mechanism for this syndromic Cx26 disorder (9, 13, 21, 37, 41).
Here we report the functional characteristics of two additional Cx26 mutations linked to KID syndrome, Cx26-D50A and Cx26-A88V.1 Cx26-D50A was identified in one child with profound deafness, corneal abnormalities, hyperkeratosis, and Dandy-Walker malformation (6). Cx26-A88V has been described in two pediatric patients with the lethal form of KID syndrome. These children had congenital deafness, alopecia, severe hyperkeratosis, and recurrent skin infections that eventually lead to septicemia and death in early childhood (15, 20). Using two different electrophysiological assays, we show that both Cx26-D50A and Cx26-A88V form active hemichannels that significantly increase membrane current flow compared with wild-type Cx26. Expression of either mutant accelerates cell death in low extracellular calcium solutions, and the elevated hemichannel currents can be attenuated by increased extracellular calcium concentration. These results suggest that these two mutations exhibit a shared gain of functional activity and further support the observation that increased hemichannel activity is a common feature of human Cx26 mutations responsible for KID syndrome.
MATERIALS AND METHODS
Molecular cloning.
Human wild-type Cx26 was cloned into the pCS2+ vector (43) for functional studies in Xenopus laevis oocytes as previously described (27). Mutant Cx26-D50A and Cx26-A88V were prepared by site-directed mutagenesis using the gene splicing by overlap extension method (16) using wild-type human Cx26 as a template. Following amplification, Cx26-D50A and Cx26-A88V were first cloned into pBlueScript II (Agilent Technologies, Santa Clara, CA) and sequenced on both strands before subcloning into the pCS2+ vector for Xenopus expression or the pIRES2-EGFP2 vector (Clontech Laboratories, Mountain View, CA) for mammalian cell transfection.
In vitro transcription and oocyte microinjection.
Human Cx26, Cx26-D50A, and Cx26-A88V plasmid DNAs were linearized using NotI and transcribed using the SP6 mMessage mMachine RNA protocol (Ambion, Austin, TX). Adult Xenopus females were anesthetized with ethyl 3-aminobenzoate methanesulfonate, and ovarian lobes were surgically removed and digested for 15 min at 37°C with constant shaking in a solution containing 7.5 mg/ml collagenase B and 5 mg/ml hyaluronidase in modified Barth's medium (MB) without Ca2+. Stage V-VI oocytes were collected, washed with MB, and injected with 10 ng of antisense Xenopus Cx38 oligonucleotide to eliminate this endogenous connexin (1, 2). Antisense-treated oocytes were then injected with wild-type Cx26, Cx26-D50A, or Cx26-A88V cRNA transcripts or H2O as a negative control. Oocytes were cultured in MB without calcium or MB with elevated Ca2+ (4 mM CaCl2) before electrophysiological recording or microscopic imaging on an SZX16 dissecting microscope (Olympus America, Center Valley, PA). Our Xenopus surgery protocol was approved by the Stony Brook University Institutional Animal Care and Use Committee.
Human cell transfection.
Communication deficient HeLa cells were plated on 22-mm2 coverslips, grown to 50% confluence, and transiently transfected with wild-type Cx26, Cx26-D50A, or Cx26-A88V in pIRES2-EGFP2 using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) as previously described (29, 30) with the exception that calcium concentrations in the tissue culture media were elevated to a final concentration of 4 mM with supplemental CaCl2. Primary human keratinocytes were obtained from the Living Skin Bank (Stony Brook University, Stony Brook, NY) and cultured in KGM-Gold keratinocyte growth medium (Lonza, Walkersville, MD). Keratinocytes were plated on glass coverslips and transiently transfected with Cx26-D50A, or Cx26-A88V in pIRES2-EGFP2 using Lipofectamine 2000 as described for HeLa cells.
Electrophysiological recording of hemichannel currents.
Macroscopic recordings of hemichannel currents were obtained from single Xenopus oocytes 24 h after cRNA injection using a GeneClamp 500 amplifier controlled by a PC-compatible computer through a Digidata 1320 interface (Axon Instruments, Foster City, CA). pClamp 8.0 software (Axon Instruments) was used to program stimulus and data collection paradigms. To obtain hemichannel current-voltage (I-V) curves, cells were initially clamped at −40 mV and subjected to 5-s depolarizing voltage steps ranging from −30 to +60 mV in 10-mV increments (21). Electrophysiological measurements in transfected HeLa cells or primary human keratinocytes were carried out using whole cell patch clamp at room temperature as previously described (29, 30). At the beginning of each experiment, cells were clamped at 0 mV and then stepped for 2-s intervals to different voltages (−110 to +110 mV in 20-mV increments). To test the effect of added calcium on hemichannel currents, dishes were perfused with media supplemented with 2 mM CaCl2 after the first series of current recordings. Hemichannel currents were then recorded again within 2–4 min of the media exchange.
Preparation of oocyte samples for Western blot analysis.
Oocytes were collected in 1 ml of buffer containing 5 mM Tris pH 8.0, 5 mM EDTA, and protease inhibitors and were lysed by mechanical passage through a series of needles of diminishing size (46). Extracts were centrifuged at 1,000 g at 4°C for 5 min to remove yolk granules. The supernatant was then centrifuged at 100,000 g at 4°C for 30 min. Membrane pellets were resuspended in SDS sample buffer (2 ul per oocyte), separated on 15% SDS gels, and transferred to nitrocellulose membranes. Blots were blocked with 5% BSA in 1× PBS for 1 h and probed with a polyclonal Cx26 antibody at a 1:1,000 dilution (Invitrogen) followed by incubation with a horseradish peroxidase-conjugated anti-rabbit secondary antibody (Jackson ImmunoResearch, West Grove, PA). As a loading control, blots were stripped and reprobed with a monoclonal β-actin antibody (Abcam, Cambridge, MA) followed by incubation with horseradish peroxidase-conjugated anti-mouse secondary antibody (GE Healthcare Biosciences, Pittsburgh, PA).
Immunofluorescent staining of transfected cells.
Transiently transfected HeLa cells or human keratinocytes were fixed with 1% paraformaldehyde in PBS and blocked with 5% BSA in PBS with 0.1% Tx-100 and 0.02% NaN3. Cells were stained with a polyclonal Cx26 antibody followed by incubation with a Cy3 conjugated goat anti-rabbit secondary antibody (Jackson ImmunoResearch). Keratinocytes were also stained with rhodamine-conjugated wheat germ agglutinin (Vector Laboratories, Burlingame, CA). Cells were viewed and photographed on a BX51 microscope using a DP72 digital camera (Olympus America).
RESULTS
Cx26-D50A and Cx26-A88V show increased hemichannel currents in single Xenopus oocytes.
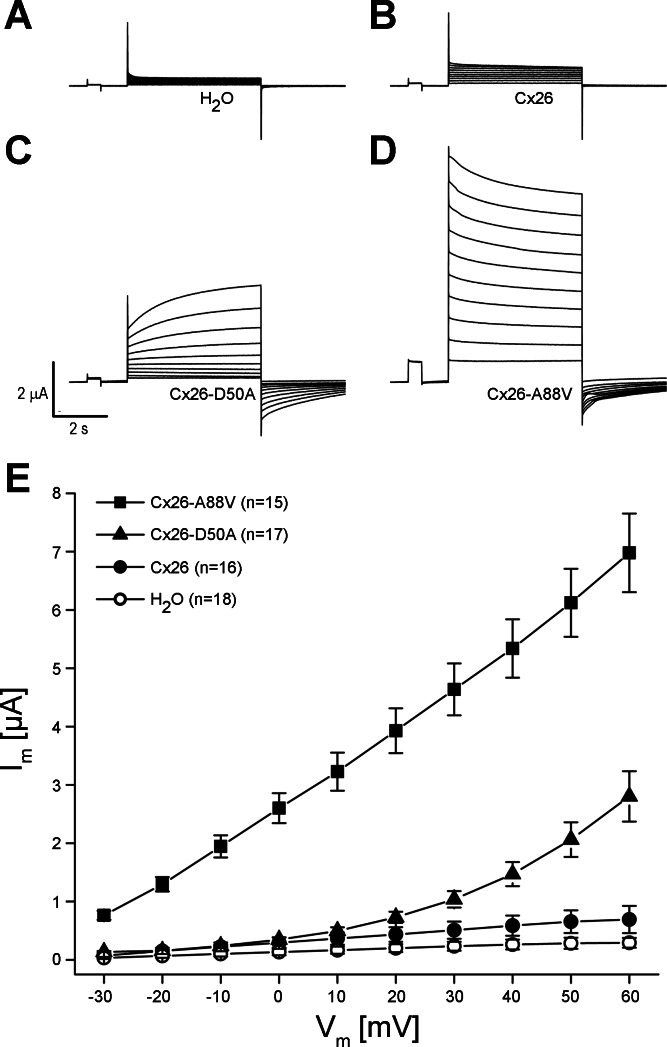
The KID mutations Cx26-D50A and Cx26-A88V are single amino acid substitutions in the first extracellular loop and second transmembrane domains of Cx26, respectively. To assess the functional consequences of these mutations, wild-type Cx26, Cx26-D50A, and Cx26-A88V were all expressed in Xenopus oocytes. Single cells were subjected to depolarizing voltage pulses, and the resulting membrane currents were recorded. Control oocytes injected with H2O showed negligible current flow for voltage steps between −30 and +60 mV (Fig. 1A). The low intrinsic hemichannel activity of wild-type human Cx26-injected cells has been previously reported (13, 14, 21, 36, 37) and was characterized by outward currents that increased with greater depolarization (Fig. 1B). Both of the KID mutants showed a large increase in the magnitude of these outward currents (Fig. 1, C and D) compared with the either H2O or wild-type Cx26-injected cells.
Fig. 1.
Connexin (Cx)26-D50A and Cx26-A88V induce large hemichannel currents in Xenopus oocytes. Single cells were clamped at a holding potential of −40 mV and subjected to voltage pulses ranging from −30 to +60 mV in 10-mV steps. A: H2O-injected cells displayed negligible membrane currents. Wild-type Cx26 (B)-, Cx26-D50A (C)-, and Cx26-A88V (D)-expressing oocytes exhibited hemichannel currents with both of the keratitis-ichthyosis-deafness (KID) syndrome mutation hemichannels displaying much larger currents than wild-type. E: steady-state currents from each pulse were plotted as a function of membrane voltage. Steady-state currents in H2O-injected control cells (□) were negligible at all membrane voltages. Cx26 currents (●) were similar to those observed in control cells at lower voltages but increased at higher membrane voltage. Cx26-D50A (▲)- or Cx26-A88V (■)-expressing cells exhibited significantly increased steady-state currents compared with either control or Cx26 oocytes. Data are the means ± SE.
Mean steady-state currents were plotted as a function of the membrane potential to quantify differences in the recorded hemichannel activity (Fig. 1E). Control cells injected with H2O showed negligible currents at all tested voltages. In comparison, wild-type Cx26-injected cells displayed larger outward currents that increased at greater depolarizing voltages. At all voltages tested wild-type cells showed a maximum current 2.0–2.5 times greater than control cells (P < 0.05, one-way ANOVA). The Cx26-D50A-expressing cells produced significantly larger outward currents than wild-type Cx26-injected cells at membrane potentials greater than or equal to +20 mV. At +60 mV, Cx26-D50A produced currents that were 4 times larger than wild-type and 10 times larger than control cells, differences that were statistically significant (P < 0.05). In contrast, Cx26-A88V-expressing oocytes displayed currents that were 10 times larger than wild-type Cx26 at all tested potentials (P < 0.05). This significantly increased membrane current suggested the presence of increased hemichannel activity.
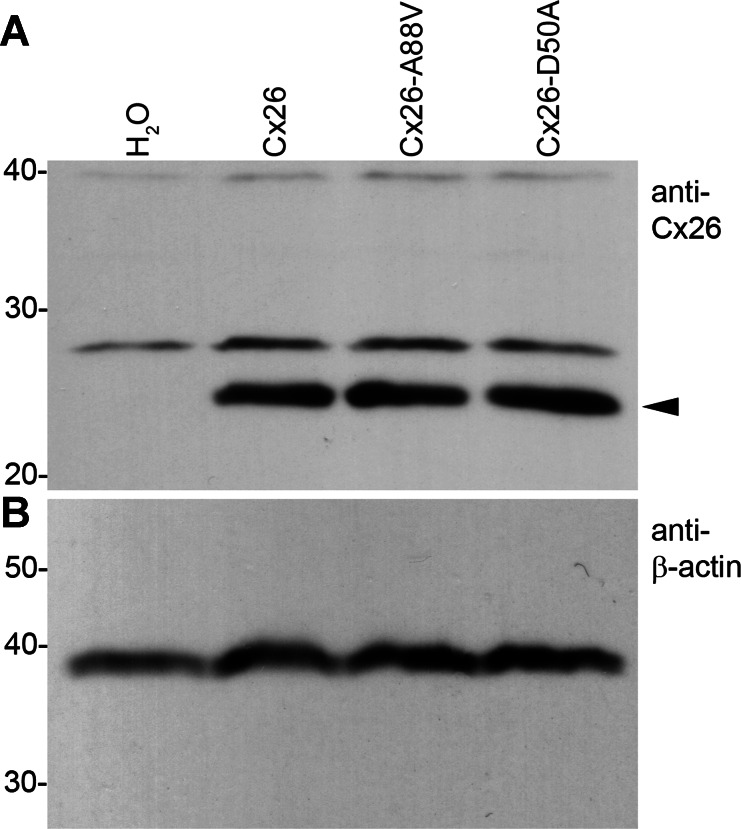
These two KID-associated mutations may contribute to epidermal pathology through an increase in hemichannel activity. However, wild-type Cx26 also induced low levels of hemichannel currents, and differences in current magnitude could have resulted from different levels of protein expression. The levels of Cx26 protein expressed in oocytes following injection of wild-type or mutant cRNA were examined by Western blot analysis (Fig. 2). Antibodies for Cx26 failed to detect specific protein expression in H2O-injected cells but readily detected expression of wild-type Cx26, Cx26-A88V, and Cx26 D50A at qualitatively similar levels (Fig. 2A, arrowhead). To control for equal sample loading, Western blots were stripped and reprobed with an antibody to β-actin, which was present in all four samples at similar levels (Fig. 2B). These data suggest that the significant increase in hemichannel current magnitude in Cx26-D50A- and Cx26-A88V-injected cells was not simply due to an increased expression of mutant protein.
Fig. 2.
Wild-type and mutant connexins are equivalently expressed in Xenopus oocytes. A: equal amounts of membrane extracts were first probed with an antibody that recognized Cx26. H2O-injected controls did not express Cx26 as expected. Wild-type Cx26, Cx26-D50A, and Cx26-A88V were readily detected in lanes corresponding to each injection condition with similar band intensities. B: to confirm equal sample loading, blots were stripped and reprobed with an antibody against β-actin, which was present at comparable levels in all lanes.
Oocytes expressing KID mutations display extracellular Ca2+ dependent cell death.
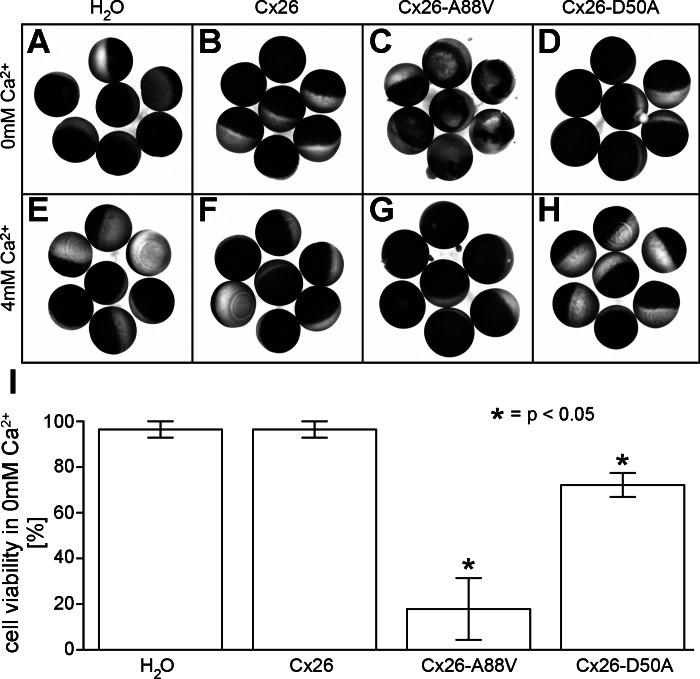
It has been previously demonstrated that other dominant Cx26 mutations associated with KID syndrome produced abnormal hemichannel currents in cells, leading to accelerated death (13, 21, 31, 41, 42). Previous studies have also shown that increased extracellular calcium reduced hemichannel activity and delayed cell death (11, 13, 21, 32, 37, 41). To test if expression of Cx26-D50A and Cx26-A88V affected cell viability in a calcium-dependent manner, oocytes were cultured in MB medium in the presence or absence of 4 mM Ca2+ (Fig. 3). H2O and wild-type Cx26-injected cells remained healthy when incubated in MB medium without calcium (Fig. 3, A and B) for 72 h. In contrast, cells expressing Cx26-A88V displayed gross pigment disorganization and leakage of the ooplasm into the culture medium within 72 h of injection (Fig. 3C). Cx26-D50A-expressing oocytes displayed a phenotype in calcium free medium similar to Cx26-A88V, although the extent of cell death was reduced (Fig. 3D). H2O and wild-type Cx26-injected cells incubated in MB media containing 4 mM Ca2+ were indistinguishable from those cultured in the absence of calcium (Fig. 3, E and F). In contrast, the appearances of Cx26-A88V- and Cx26-D50A-injected oocytes were dramatically improved by an increase in the extracellular calcium concentration (Fig. 3, G and H), although the Cx26-A88V-injected cells began to show initial signs of ooplasm leakage by 72 h. The extent of oocyte death in MB without added Ca2+ was quantified and compared using data from four independent experiments (Fig. 3I). For H2O and wild-type Cx26-injected cells, <5% died within 72 h. In contrast, 85% of Cx26-A88V- and 27% of Cx26-D50A-expressing oocytes died, differences that were statistically significant (P < 0.05) compared with H2O and wild-type Cx26-injected cells. These data suggested that elevation of the extracellular calcium concentration suppressed activity of the mutant hemichannels and prolonged cell viability.
Fig. 3.
Cx26-D50A and Cx26-A88V hemichannels cause cell death that can be rescued by elevated extracellular Ca2+. Control, wild-type Cx26-, Cx26-D50A-, and Cx26-A88V-injected cells were incubated in the presence or absence of 4 mM extracellular calcium. H2O (A)- and wild-type Cx26 (B)-injected cells survived in Ca2+ free media, while Cx26-A88V-injected cells displayed increased pigment disorganization and blebbing (C). Cx26-D50A-expressing oocytes also showed increased blebbing in the absence of calcium (D). Incubation of oocytes in 4 mM Ca2+ (E-H) dramatically improved the appearance of Cx26-D50A- and Cx26-A88V-injected cells. Quantification of the extent of oocyte death in modified Barth's medium without added Ca2+ (I) showed that 85% of Cx26-A88V- and 27% of Cx26-D50A-expressing oocytes died within 72 h (n = 26 oocytes/condition). Data are the means ± SE.
Cx26-D50A and Cx26-A88V show increased hemichannel currents in transfected HeLa cells.
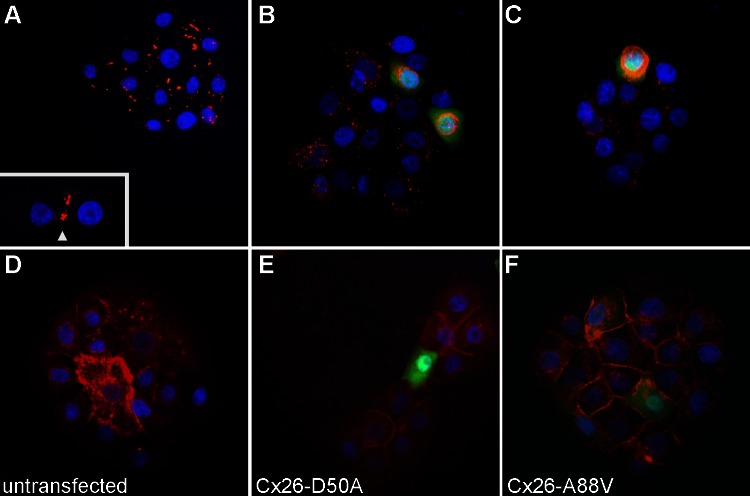
The majority of Cx26 KID mutations tested have been shown to form active hemichannels in the Xenopus oocyte expression system (23); however, only Cx26-G45E and Cx26-A40V have been documented by electrophysiological methods to also form active hemichannels in mammalian cells (23, 29). To test if the expression of Cx26-A88V and Cx26-D50A significantly altered whole cell membrane currents in mammalian cells, we transfected HeLa cells with wild-type Cx26, Cx26-A88V, or Cx26-D50A DNAs in the pIRES2-EGFP2 vector. To confirm wild-type and mutant protein expression, transfected cells were fixed and stained with an antibody against Cx26 (Fig. 4). Wild-type-transfected cells displayed a strong Cx26 labeling that concentrated at cell-to-cell appositions and correlated with the expression of GFP (Fig. 4A), whereas untransfected control HeLa cells showed no signal for Cx26, or GFP (Fig. 4B). Both Cx26-A88V and Cx26-D50A also displayed a clear Cx26 labeling that correlated with GFP expression, although the Cx26 signal was not predominantly found at cellular interfaces (Fig. 4, C and D).
Fig. 4.
Expression of wild-type Cx26, Cx26-D50A, and Cx26-A88V in transfected HeLa cells. A: wild-type Cx26-transfected cells (blue DAPI stain) displayed a strong Cx26 (red) labeling that concentrated at cell-to-cell interfaces and correlated with GFP (green) fluorescence. B: untransfected HeLa cells failed to express either GFP or Cx26. Both Cx26-A88V (C) and Cx26-D50A (D) also displayed a clear Cx26 labeling that correlated with GFP expression, although the Cx26 signal was not focused at cellular appositions.
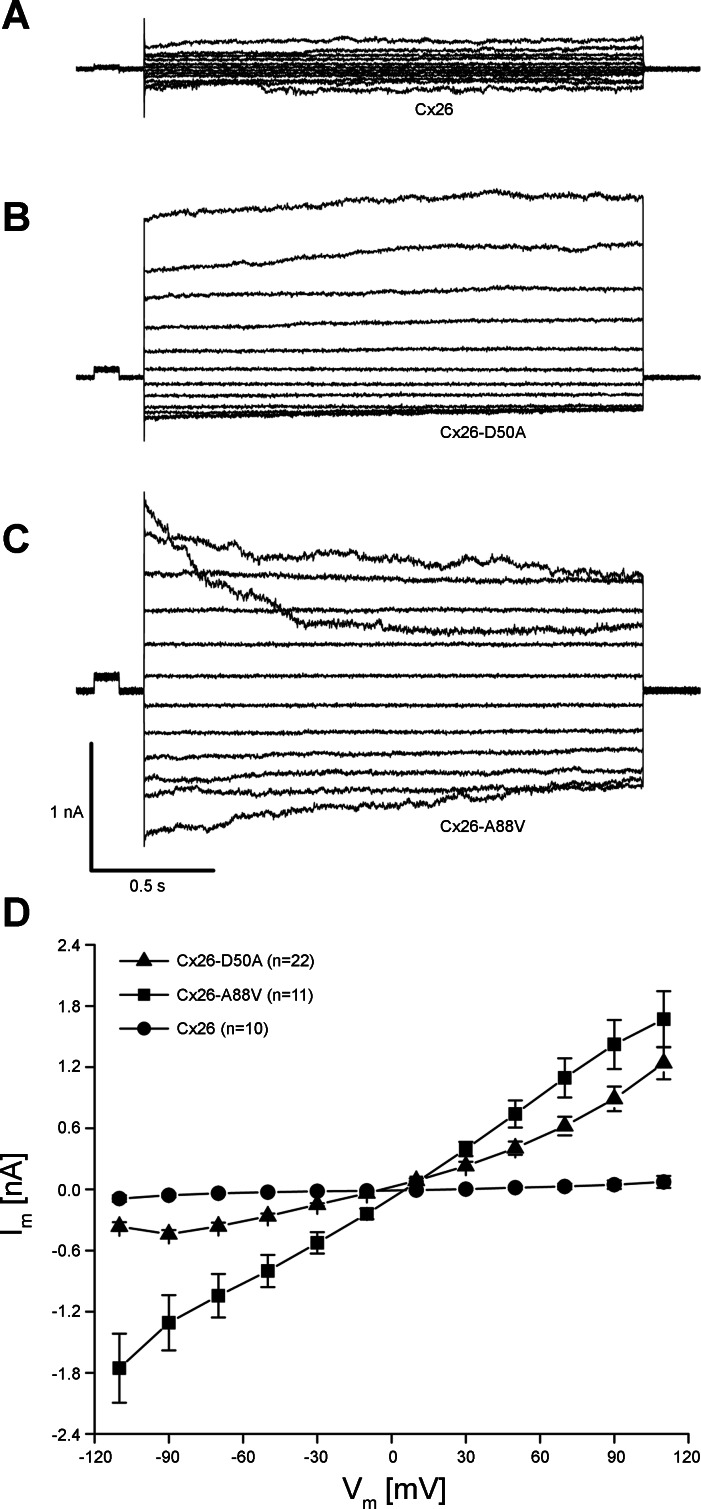
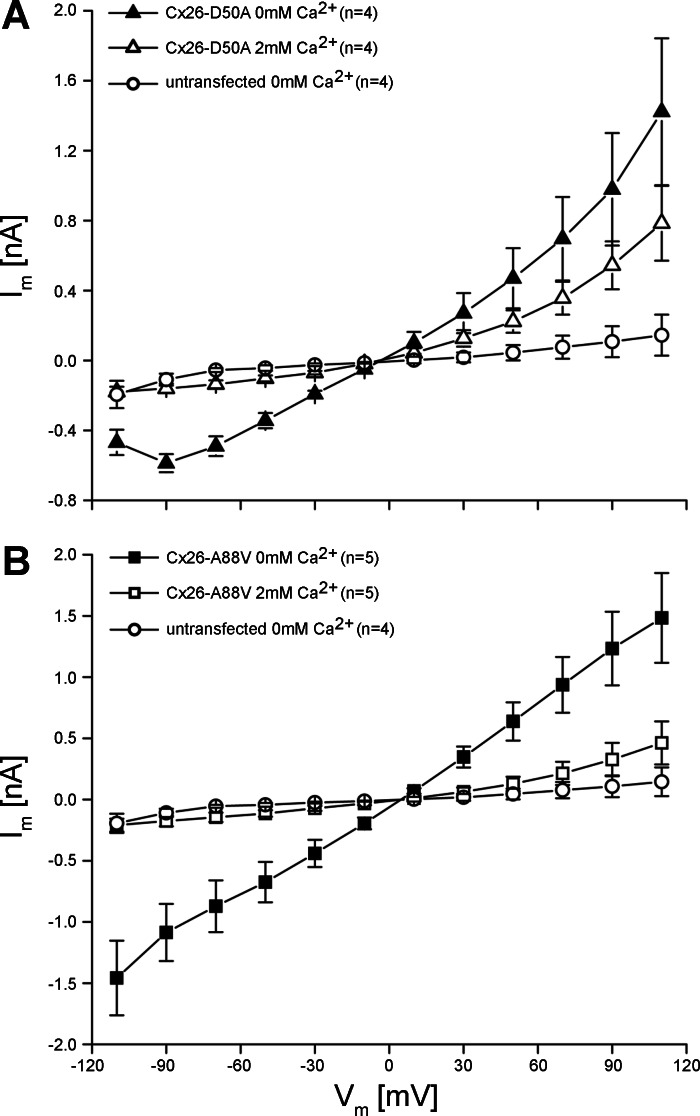
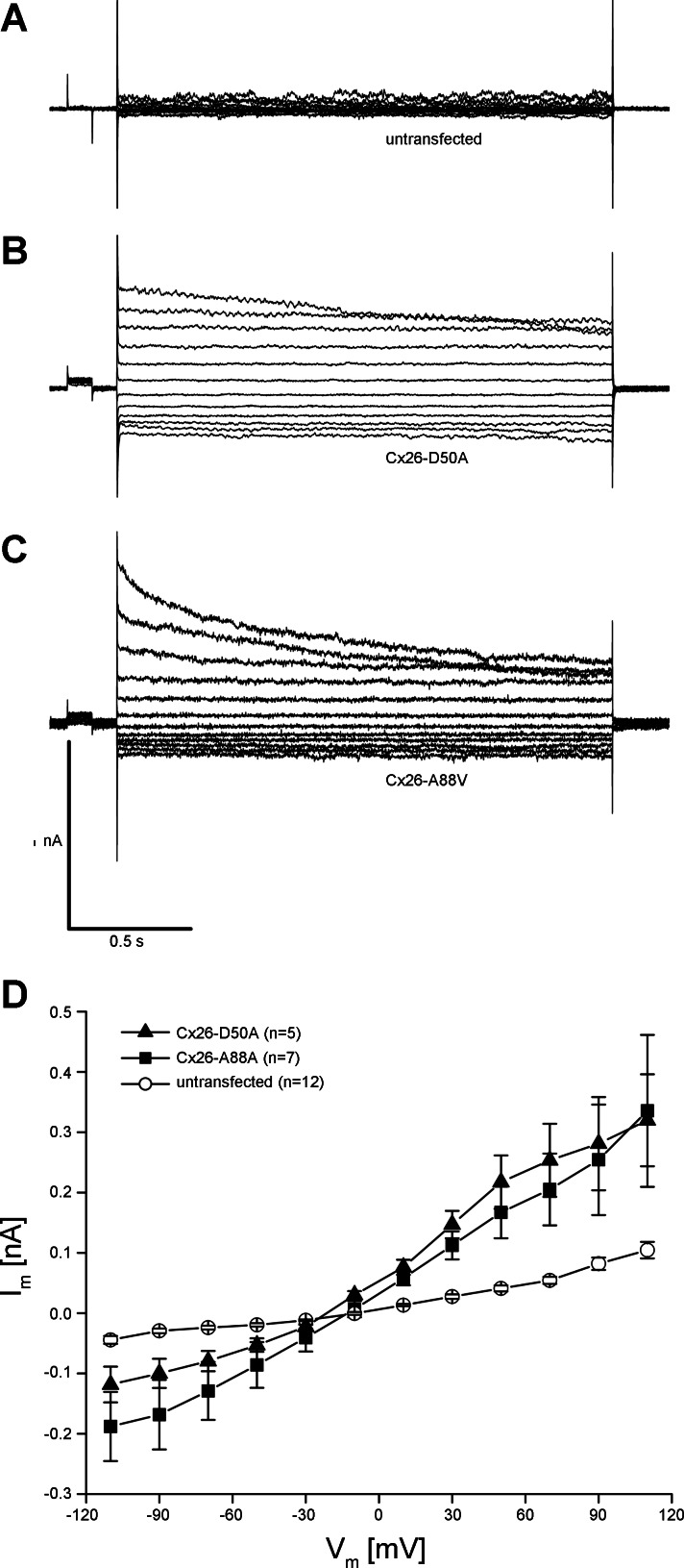
To measure hemichannel activity, membrane currents were recorded using whole cell patch-clamp electrophysiology (Fig. 5). Wild-type Cx26-transfected cells displayed modest membrane currents when stepped to membrane potentials between −110 and +110 mV (Fig. 5A). In contrast, HeLa cells expressing either Cx26-D50A or Cx26-A88V exhibited much larger whole cell membrane currents at both hyperpolarizing and depolarizing potentials (Fig. 5, B and C). Plotting the whole cell current as a function of membrane potential (Fig. 5D) showed that at all membrane voltages greater than ±10 mV, membrane currents mediated by both Cx26-D50A and Cx26-A88V were significantly larger (P < 0.05, one-way ANOVA) than those recorded in wild-type Cx26-transfected cells. However, there was a pronounced difference in the gating polarity between the two KID mutations. Cx26-A88V hemichannels were equally active at both positive and negative membrane potentials and were increased ∼20 times compared with wild-type Cx26 at either polarity. In contrast, Cx26-D50A hemichannel activity was 17 times greater than wild-type Cx26 at positive voltages but only 4 times greater than wild-type at negative membrane potentials. Thus expression of Cx26-D50A and Cx26-A88V resulted in significantly increased membrane currents in transfected HeLa cells consistent with the elevated hemichannel activity observed in Xenopus oocytes (Fig. 1).
Fig. 5.
Cx26-D50A and Cx26-A88V induce hemichannel currents in HeLa cells. A: HeLa cells transfected with wild-type Cx26 had modest membrane currents when stepped to membrane potentials between −100 and +110 mV. B: HeLa cells expressing Cx26-D50A produced much larger whole cell currents, particularly at depolarizing membrane voltages. C: Cx26-A88V-transfected HeLa cells had large currents at both hyperpolarizing and depolarizing potentials. D: quantification of the current-voltage (I-V) relationships in wild-type Cx26-, Cx26-D50A-, and Cx26-A88V-transfected HeLa cells. Initial currents from each pulse were plotted as a function of membrane voltage. Currents recorded in wild-type Cx26-expressing cells (●) in were significantly smaller than those recorded in Cx26-A88V (■)-transfected cells at all tested voltages (P < 0.05, ANOVA). Cells expressing Cx26-D50A (▲) also exhibited significantly increased membrane currents (P < 0.05) compared with wild-type Cx26, with a greater increase in current at positive membrane voltages. Data are the means ± SE.
Cx26-D50A and Cx26-A88V hemichannel activity can be suppressed by extracellular Ca2+.
Oocytes expressing the Cx26-D50A and Cx26-A88V mutations that showed increased hemichannel activity also experienced accelerated cell death in the absence of elevated extracellular Ca2+, suggesting that the addition of extracellular Ca2+ blocked the hemichannel activity and delayed death, as had been previously documented for other KID mutations (13, 21). To assess this possibility, hemichannel currents were first recorded in medium without added calcium, and then medium containing 2 mM Ca2+ was introduced by perfusion and the membrane currents were recorded a second time. Steady-state current values in the presence and absence of calcium were then plotted as a function of voltage for a series of cells expressing each mutation (Fig. 6). Cx26-D50A-transfected HeLa cells showed a complete inhibition of hemichannel activity following the addition of 2 mM Ca2+ at negative membrane potentials, where whole cell currents became indistinguishable from those recorded in untransfected control cells. At positive voltages, inhibition was less complete and varied from 54% at +30 mV to 45% at +110 mV (Fig. 6A). Cx26-A88V hemichannels were also fully inhibited at negative voltages and showed a more robust inhibition at positive membrane potentials compared with Cx26-D50A (Fig. 6B). At +110 mV, 2 mM Ca2+ was able to inhibit 70% of the hemichannel current recorded in the absence of calcium. The addition of 2 mM Ca2+ produced a significant decrease in current flow at all tested voltages for both KID mutations and shifted the threshold of voltage activation to more positive potentials.
Fig. 6.
Increased extracellular Ca2+ inhibits Cx26-D50A and Cx26-A88V hemichannel currents. A: mean steady-state currents observed in Cx26-D50A-transfected HeLa cells in the absence of Ca2+ (▲) were significantly reduced (△, P < 0.05) after perfusion of media supplemented with 2 mM Ca2+. At negative membrane voltages, the inhibition was complete and current levels matched those of untransfected control HeLa cells (○). B: Cx26-A88V hemichannels also displayed large membrane currents that were suppressed by perfusion with 2 mM Ca2+. Data are the means ± SE.
Cx26-D50A and Cx26-A88V increase hemichannel activity in primary human keratinocytes.
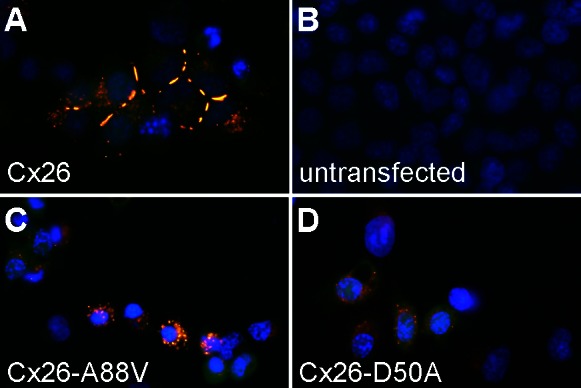
KID syndrome is a dominant disorder, requiring only one of the two GJB2 alleles to acquire an activating mutation to produce skin disease (35). If elevated hemichannel activity contributes to KID pathology, then it should still be detectable in cells that coexpress mutant and wild-type forms of Cx26. To test if the expression of Cx26-D50A and Cx26-A88V significantly altered whole cell membrane currents in human keratinocytes endogenously expressing wild-type Cx26, we transfected primary cultures of keratinocytes with Cx26-D50A or Cx26-A88V DNAs in the pIRES2-EGFP2 vector. To confirm wild-type and mutant protein expression, keratinocytes were fixed and stained with an antibody against Cx26 (Fig. 7). Untransfected human keratinocytes displayed a strong Cx26 labeling that concentrated at cell-to-cell appositions (Fig. 7A). Cells transfected with Cx26-D50A or Cx26-A88V showed an increased Cx26 labeling that correlated with GFP expression, although the Cx26 signal was no longer predominantly found at cellular interfaces (Fig. 7, B and C). To test for changes in cell morphology or the extent of cell to cell contact in mutant-transfected cells, keratinocytes were also stained with rhodamine-conjugated wheat germ agglutinin to highlight cell borders. Untransfected primary human cells grown in small three-dimensional clusters showed numerous intimate cell contacts (Fig. 7D). Transfection of these clustered cells with Cx26-D50A (Fig. 7E) or Cx26-A88V (Fig. 7F) did not noticeable alter either keratinocyte morphology or the extent of cell contact with neighboring cells.
Fig. 7.
Expression of Cx26-D50A and Cx26-A88V in transfected human keratinocytes. Untransfected primary human keratinocytes (A, blue DAPI stain) displayed a strong endogenous wild-type Cx26 (red) labeling that concentrated at cell-to-cell interfaces, which was most clearly seen in isolated pairs of cells (arrowhead in inset). Keratinocytes transfected with either Cx26-D50A (B) or Cx26-A88V (C) displayed an increased Cx26 labeling that correlated with GFP expression (green), although the Cx26 signal was no longer focused at cellular appositions. Staining of cell borders with rhodamine-conjugated wheat germ agglutinin (D-F), revealed that the expression of Cx26-D50A (E) or Cx26-A88V (F) did not markedly alter cell morphology.
To measure hemichannel activity in keratinocytes coexpressing wild-type and mutant Cx26, membrane currents were recorded using whole cell patch-clamp electrophysiology (Fig. 8). Primary human keratinocytes displayed modest membrane currents when stepped to membrane potentials between −110 and +110 mV (Fig. 8A). In contrast, keratinocytes transfected with either Cx26-D50A or Cx26-A88V exhibited much larger whole cell membrane currents at both hyperpolarizing and depolarizing potentials (Fig. 8, B and C). Plotting the whole cell current as a function of membrane potential (Fig. 8D) showed that at all membrane voltages greater than ±10 mV membrane currents mediated by both Cx26-D50A and Cx26-A88V were larger than those recorded in untransfected keratinocytes expressing endogenous wild-type Cx26. Thus expression of Cx26-D50A and Cx26-A88V in primary human keratinocytes resulted in significantly increased membrane currents consistent with the elevated hemichannel activity observed in both Xenopus oocytes and HeLa cells.
Fig. 8.
Cx26-D50A and Cx26-A88V induce hemichannel currents in human keratinocytes. A: untransfected primary keratinocytes had modest membrane currents when stepped to membrane potentials between −100 and +110 mV. B: keratinocytes expressing Cx26-D50A produced much larger whole cell currents. C: Cx26-A88V-transfected keratinocytes also had large membrane currents. D: quantification of the current voltage relationships. Initial currents from each pulse were plotted as a function of membrane voltage. Currents recorded in untransfected keratinocytes (○) were significantly smaller than those recorded in Cx26-A88V (■)-transfected cells at all tested voltages (P < 0.05, ANOVA). Cells expressing Cx26-D50A (▲) also exhibited significantly increased membrane currents (P < 0.05) compared with untransfected control cells. Data are the means ± SE.
DISCUSSION
Mutations in Cx26 are the most common cause of nonsyndromic genetic hearing loss, with ∼200 distinct sequence changes identified to date (7, 10). Cx26 mutations have also been linked to syndromic deafness associated with skin disease, although the incidence of these disorders and the number of identified mutations are much lower (22, 34, 39). The fact that distinct pathologies can arise from different mutations within the same gene has suggested that unique channel activities contribute to deafness and skin disease (17, 28, 45, 49). It has been previously shown by several laboratories that functional expression of the KID mutations Cx26-G12R, Cx26-N14K, Cx26-A40V, Cx26-G45E, and Cx26-D50N all resulted in enhanced hemichannel activity (9, 13, 21, 23, 31, 37, 41). Here we show that two additional KID-causing mutations, Cx26-D50A and Cx26-A88V, also produced greatly increased hemichannel activity in both cRNA-injected Xenopus oocytes and transiently transfected mammalian cells. This hemichannel activity was correlated with an increase in cell death and could be attenuated by elevated extracellular calcium. These findings are consistent with the hypothesis that aberrant hemichannel activity is a common feature of KID syndrome mutations and may contribute to the observed epidermal pathology in these patients.
KID syndrome is a rare disorder characterized by vascularizing keratitis, deafness, hair follicle defects, and erythrokeratoderma (35, 40, 44). KID patients have recurrent cutaneous infections that lead to lethal septicemia in severe cases where individuals carry the Cx26-G45E or Cx26-A88V mutations (15, 18, 20, 38). Some cases of KID syndrome also show additional features comprising the follicular occlusion triad (dissecting folliculitis, hidradenitis suppurativa, and cystic acne) or display an increased incidence of squamous cell carcinoma (26, 31, 44). In all of our in vitro assays, the magnitude of hemichannel current produced by Cx26-D50A was less than that of Cx26-A88V, particularly at negative membrane potentials. The disease progression in the Cx26-D50A patient was also less severe (6), particularly compared with the lethal outcome in the two reported cases of children with the Cx26-A88V mutation (15, 20), suggesting that severity of KID syndrome may correlate with the relative increase in hemichannel activity caused by the respective mutation.
Differences in the amplitude of hemichannel currents induced by Cx26-D50A and Cx26-A88V mutations are likely due to divergent ways that these two amino acid substitutions have altered the voltage gating and/or open probability of wild-type Cx26 hemichannels. Cx26-A88V hemichannels showed high activity over the entire range of tested voltages (±110 mV), whereas Cx26-D50A channels were most active at membrane potentials greater than or equal to +20 mV. Human keratinocytes typically have a resting membrane potential between −20 and −30 mV (25, 48). In this voltage range, the hemichannel activity of Cx26-A88V would be expected to be much greater than that of Cx26-D50A and thus could lead to a more severe (lethal) disease phenotype. This idea is supported by biophysical analysis of the other lethal KID mutation, Cx26-G45E, which also showed high levels of hemichannel activity across a broad range of positive and negative membrane potentials (13, 29, 37). Robust Cx26-G45E hemichannel activity has also been documented in primary keratinocytes derived from transgenic mice expressing this mutation, and these animals also developed a severe lethal form of epidermal pathology (29). The definitive determination of whether disease severity correlates with differences in the biophysical properties of the mutant proteins is currently limited by the small number of human cases linked to each of the mutations.
Mutations in other connexin genes have also been associated with hereditary skin disease, and analysis of mutant proteins from these disorders has also implicated a putative role for increased hemichannel activity in epidermal pathology. Two mutations in Cx30 (GJB6) linked to hidrotic ectodermal dysplasia, Cx30-G11R and Cx30-A88V, were reported to induce large voltage-activated currents and cause cell death when expressed in Xenopus oocytes. Transfection of HeLa cells with these Cx30 mutants resulted in increased ATP leakage into the extracellular medium, further suggesting enhanced hemichannel activity (12). Similar observations were made in a study of a mutation in Cx31 (GJB3) associated with erythrokeratodermia variabilis. Expression of the mutant Cx31-R42P in HeLa cells facilitated dye uptake and induced cell death, outcomes that could be blocked by treatment with a connexin channel inhibitor or elevated extracellular calcium (5). In the present work, we have characterized the functional activity of two Cx26 mutations that cause KID syndrome and found that expression of either mutant, in three different in vitro systems, resulted in significantly increased membrane currents consistent with active hemichannels. Comparison of our results with published data from previous studies documents that aberrant hemichannel activity is a shared feature among KID-associated Cx26 mutations, as eight of the nine mutants characterized thus far have shown increased hemichannel function (Table 1). Taken together, it is clear that increased hemichannel activity is a common feature of connexin mutations associated with KID syndrome and other epidermal disorders. While the precise mechanism(s) whereby this hemichannel activity disrupts epidermal homeostasis is unknown, the identification of a possible role for it in pathogenesis opens a new avenue for development of potential treatments for these disorders.
Table 1.
Documentation of increased hemichannel activity in Cx26 mutations linked to keratitis-ichthyosis-deafness syndrome
| Mutation | Oocyte Voltage Clamp | Mammalian Cell Patch Clamp | Dye-Based Methods | References |
|---|---|---|---|---|
| G11R | — | — | Yes | (42) |
| G12R | Yes | — | Yes | (9, 21) |
| N14K | Yes | — | — | (21) |
| S17F | No | — | — | (21) |
| A40V | Yes | Yes | — | (23, 31, 37) |
| G45E | Yes | Yes | Yes | (13, 29, 37) |
| D50N | Yes | — | Yes | (9, 21) |
| D50A | Yes | Yes | — | This study |
| A88V | Yes | Yes | — | This study |
Cx26, connexin 26.
GRANTS
This work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant R01-AR-59505 (to T. W. White).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: P.V.M., N.A.L., L.L., H.-Z.W., J.R.L., and Z.S. performed experiments; P.V.M., N.A.L., L.L., H.-Z.W., and T.W.W. prepared figures; P.V.M. drafted manuscript; N.A.L., H.-Z.W., J.R.L., Z.S., and P.R.B. analyzed data; P.R.B. interpreted results of experiments; P.R.B. and T.W.W. edited and revised manuscript; T.W.W. conception and design of research; T.W.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Caterina Sellitto for constructive comments on the manuscript, Dr. Marcia Simon for providing primary human keratinocytes, and Chris Gordon for assistance with tissue culture.
Footnotes
This article is the topic of an Editorial Focus by Michael Koval (20a).
REFERENCES
- 1. Barrio LC, Suchyna T, Bargiello T, Xu LX, Roginski RS, Bennett MV, Nicholson BJ. Gap junctions formed by connexins 26 and 32 alone and in combination are differently affected by applied voltage. Proc Natl Acad Sci USA 88: 8410–8414, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bruzzone R, Haefliger JA, Gimlich RL, Paul DL. Connexin40, a component of gap junctions in vascular endothelium, is restricted in its ability to interact with other connexins. Mol Biol Cell 4: 7–20, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bruzzone R, White TW, Paul DL. Connections with connexins: the molecular basis of direct intercellular signaling. Eur J Biochem 238: 1–27, 1996 [DOI] [PubMed] [Google Scholar]
- 4. Chandrasekhar A, Bera AK. Hemichannels: permeants and their effect on development, physiology and death. Cell Biochem Funct 30: 89–100, 2012 [DOI] [PubMed] [Google Scholar]
- 5. Chi J, Li L, Liu M, Tan J, Tang C, Pan Q, Wang D, Zhang Z. Pathogenic connexin-31 forms constitutively active hemichannels to promote necrotic cell death. PLos One 7: e32531, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cushing SL, MacDonald L, Propst EJ, Sharma A, Stockley T, Blaser SL, James AL, Papsin BC. Successful cochlear implantation in a child with Keratosis, Icthiosis and Deafness (KID) Syndrome and Dandy-Walker malformation. Int J Pediatr Otorhinolaryngol 72: 693–698, 2008 [DOI] [PubMed] [Google Scholar]
- 7. del Castillo FJ, del Castillo I. The DFNB1 subtype of autosomal recessive non-syndromic hearing impairment. Front Biosci 16: 3252–3274, 2011 [DOI] [PubMed] [Google Scholar]
- 8. DeVries SH, Schwartz EA. Hemi-gap-junction channels in solitary horizontal cells of the catfish retina. J Physiol 445: 201–230, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Donnelly S, English G, de Zwart-Storm EA, Lang S, van Steensel MA, Martin PE. Differential susceptibility of Cx26 mutations associated with epidermal dysplasias to peptidoglycan derived from Staphylococcus aureus and Staphylococcus epidermidis. Exp Dermatol 21: 592–598, 2012 [DOI] [PubMed] [Google Scholar]
- 10. Duman D, Tekin M. Autosomal recessive nonsyndromic deafness genes: a review. Front Biosci 17: 2213–2236, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ebihara L, Steiner E. Properties of a nonjunctional current expressed from a rat connexin46 cDNA in Xenopus oocytes. J Gen Physiol 102: 59–74, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Essenfelder GM, Bruzzone R, Lamartine J, Charollais A, Blanchet-Bardon C, Barbe MT, Meda P, Waksman G. Connexin30 mutations responsible for hidrotic ectodermal dysplasia cause abnormal hemichannel activity. Hum Mol Genet 13: 1703–1714, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Gerido DA, DeRosa AM, Richard G, White TW. Aberrant hemichannel properties of Cx26 mutations causing skin disease and deafness. Am J Physiol Cell Physiol 293: C337–C345, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Gonzalez D, Gomez-Hernandez JM, Barrio LC. Species specificity of mammalian connexin-26 to form open voltage-gated hemichannels. FASEB J 20: 2329–2338, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Haruna K, Suga Y, Oizumi A, Mizuno Y, Endo H, Shimizu T, Hasegawa T, Ikeda S. Severe form of keratitis-ichthyosis-deafness (KID) syndrome associated with septic complications. J Dermatol 37: 680–682, 2010 [DOI] [PubMed] [Google Scholar]
- 16. Horton RM, Cai ZL, Ho SN, Pease LR. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. Biotechniques 8: 528–535, 1990 [PubMed] [Google Scholar]
- 17. Iossa S, Marciano E, Franze A. GJB2 gene mutations in syndromic skin diseases with sensorineural hearing loss. Curr Genomics 12: 475–785, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jonard L, Feldmann D, Parsy C, Freitag S, Sinico M, Koval C, Grati M, Couderc R, Denoyelle F, Bodemer C, Marlin S, Hadj-Rabia S. A familial case of Keratitis-Ichthyosis-Deafness (KID) syndrome with the GJB2 mutation G45E. Eur J Med Genet 51: 35–43, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Kar R, Batra N, Riquelme MA, Jiang JX. Biological role of connexin intercellular channels and hemichannels. Arch Biochem Biophys 524: 2–15, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Koppelhus U, Tranebjaerg L, Esberg G, Ramsing M, Lodahl M, Rendtorff ND, Olesen HV, Sommerlund M. A novel mutation in the connexin 26 gene (GJB2) in a child with clinical and histological features of keratitis-ichthyosis-deafness (KID) syndrome. Clin Exp Dermatol 36: 142–148, 2011 [DOI] [PubMed] [Google Scholar]
- 20a. Koval M. Drowning out communication. Focus on “The human Cx26-D50A and Cx26-A88V mutations causing keratitis-ichthyosis-deafness syndrome display increased hemichannel activity.” Am J Physiol Cell Physiol (April 10, 2013). doi: 10.1152/ajpcell.00087.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee JR, Derosa AM, White TW. Connexin mutations causing skin disease and deafness increase hemichannel activity and cell death when expressed in Xenopus oocytes. J Invest Dermatol 129: 870–878, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee JR, White TW. Connexin-26 mutations in deafness and skin disease. Exp Rev Mol Med 11: e35, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Levit NA, Mese G, Basaly MG, White TW. Pathological hemichannels associated with human Cx26 mutations causing Keratitis-Ichthyosis-Deafness syndrome. Biochim Biophys Acta 1818: 2014–2019, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Malchow RP, Qian H, Ripps H. Evidence for hemi-gap junctional channels in isolated horizontal cells of the skate retina. J Neurosci Res 35: 237–245, 1993 [DOI] [PubMed] [Google Scholar]
- 25. Mauro TM, Pappone PA, Isseroff RR. Extracellular calcium affects the membrane currents of cultured human keratinocytes. J Cell Physiol 143: 13–20, 1990 [DOI] [PubMed] [Google Scholar]
- 26. Mazereeuw-Hautier J, Bitoun E, Chevrant-Breton J, Man SY, Bodemer C, Prins C, Antille C, Saurat JH, Atherton D, Harper JI, Kelsell DP, Hovnanian A. Keratitis-ichthyosis-deafness syndrome: disease expression and spectrum of connexin 26 (GJB2) mutations in 14 patients. Br J Dermatol 156: 1015–1019, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Mese G, Londin E, Mui R, Brink PR, White TW. Altered gating properties of functional Cx26 mutants associated with recessive non-syndromic hearing loss. Hum Genet 115: 191–199, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Mese G, Richard G, White TW. Gap junctions: basic structure and function. J Invest Dermatol 127: 2516–2524, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Mese G, Sellitto C, Li L, Wang HZ, Valiunas V, Richard G, Brink PR, White TW. The Cx26–G45E mutation displays increased hemichannel activity in a mouse model of the lethal form of keratitis-ichthyosis-deafness syndrome. Mol Biol Cell 22: 4776–4786, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mese G, Valiunas V, Brink PR, White TW. Connexin26 deafness associated mutations show altered permeability to large cationic molecules. Am J Physiol Cell Physiol 295: C966–C974, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Montgomery JR, White TW, Martin BL, Turner ML, Holland SM. A novel connexin 26 gene mutation associated with features of the keratitis-ichthyosis-deafness syndrome and the follicular occlusion triad. J Am Acad Dermatol 51: 377–382, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Pfahnl A, Dahl G. Gating of cx46 gap junction hemichannels by calcium and voltage. Pflügers Arch 437: 345–353, 1999 [DOI] [PubMed] [Google Scholar]
- 33. Pfenniger A, Wohlwend A, Kwak BR. Mutations in connexin genes and disease. Eur J Clin Invest 41: 103–116, 2011 [DOI] [PubMed] [Google Scholar]
- 34. Richard G. Connexin disorders of the skin. Clin Dermatol 23: 23–32, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Richard G, Rouan F, Willoughby CE, Brown N, Chung P, Ryynanen M, Jabs EW, Bale SJ, DiGiovanna JJ, Uitto J, Russell L. Missense mutations in GJB2 encoding connexin-26 cause the ectodermal dysplasia keratitis-ichthyosis-deafness syndrome. Am J Hum Genet 70: 1341–1348, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ripps H, Qian H, Zakevicius J. Properties of connexin26 hemichannels expressed in Xenopus oocytes. Cell Mol Neurobiol 24: 647–665, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sanchez HA, Mese G, Srinivas M, White TW, Verselis VK. Differentially altered Ca2+ regulation and Ca2+ permeability in Cx26 hemichannels formed by the A40V and G45E mutations that cause keratitis ichthyosis deafness syndrome. J Gen Physiol 136: 47–62, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sbidian E, Feldmann D, Bengoa J, Fraitag S, Abadie V, de Prost Y, Bodemer C, Hadj-Rabia S. Germline mosaicism in keratitis-ichthyosis-deafness syndrome: pre-natal diagnosis in a familial lethal form. Clin Genet 77: 587–592, 2010 [DOI] [PubMed] [Google Scholar]
- 39. Scott CA, Kelsell DP. Key functions for gap junctions in skin and hearing. Biochem J 438: 245–254, 2011 [DOI] [PubMed] [Google Scholar]
- 40. Skinner BA, Greist MC, Norins AL. The keratitis, ichthyosis, and deafness (KID) syndrome. Arch Dermatol 117: 285–289, 1981 [PubMed] [Google Scholar]
- 41. Stong BC, Chang Q, Ahmad S, Lin X. A novel mechanism for connexin 26 mutation linked deafness: cell death caused by leaky gap junction hemichannels. Laryngoscope 116: 2205–2210, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Terrinoni A, Codispoti A, Serra V, Didona B, Bruno E, Nistico R, Giustizieri M, Alessandrini M, Campione E, Melino G. Connexin 26 (GJB2) mutations, causing KID Syndrome, are associated with cell death due to calcium gating deregulation. Biochem Biophys Res Commun 394: 909–914, 2010 [DOI] [PubMed] [Google Scholar]
- 43. Turner DL, Weintraub H. Expression of achaete-scute homolog 3 in Xenopus embryos converts ectodermal cells to a neural fate. Genes Dev 8: 1434–1447, 1994 [DOI] [PubMed] [Google Scholar]
- 44. van Steensel MA, van Geel M, Nahuys M, Smitt JH, Steijlen PM. A novel connexin 26 mutation in a patient diagnosed with keratitis-ichthyosis-deafness syndrome. J Invest Dermatol 118: 724–727, 2002 [DOI] [PubMed] [Google Scholar]
- 45. White TW. Functional analysis of human Cx26 mutations associated with deafness. Brain Res Brain Res Rev 32: 181–183, 2000 [DOI] [PubMed] [Google Scholar]
- 46. White TW, Bruzzone R, Goodenough DA, Paul DL. Mouse Cx50, a functional member of the connexin family of gap junction proteins, is the lens fiber protein MP70. Mol Biol Cell 3: 711–720, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. White TW, Paul DL. Genetic diseases and gene knockouts reveal diverse connexin functions. Annu Rev Physiol 61: 283–310, 1999 [DOI] [PubMed] [Google Scholar]
- 48. Wohlrab D, Wohlrab J, Markwardt F. Electrophysiological characterization of human keratinocytes using the patch-clamp technique. Exp Dermatol 9: 219–223, 2000 [DOI] [PubMed] [Google Scholar]
- 49. Xu J, Nicholson BJ. The role of connexins in ear and skin physiology–functional insights from disease-associated mutations. Biochim Biophys Acta 1828: 167–178, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhao HB, Kikuchi T, Ngezahayo A, White TW. Gap junctions and cochlear homeostasis. J Membr Biol 209: 177–186, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]