Abstract
Introduction
Lisdexamfetamine dimesylate (LDX) is a long-acting prodrug stimulant for the treatment of attention-deficit/hyperactivity disorder (ADHD). Post hoc subgroup analyses were performed from two studies in children with ADHD to compare the efficacy of LDX in participants who had received prior methylphenidate (MPH) treatment with that of the overall study populations.
Methods
Study 1 (7-week; open-label design) and study 2 (randomized, double-blind, placebo-controlled, crossover, laboratory school design) enrolled children aged 6–12 years with ADHD and baseline ADHD Rating Scale IV (ADHD-RS-IV) total score ≥28. Both studies excluded children whose prestudy ADHD treatment provided effective control of ADHD symptoms with an acceptable safety profile. Post hoc efficacy analyses were performed in children who had received MPH within 6 months of study enrollment. Efficacy measures included the following scales: ADHD-RS-IV, Clinical Global Impressions-Improvement (CGI-I), Expression and Emotion Scale for Children (EESC), Behavior Rating Inventory of Executive Function (BRIEF), Swanson, Kotkin, Agler, M-Flynn, and Pelham (SKAMP), and Permanent Product Measure of Performance (PERMP).
Results
In studies 1 and 2, 83/318 (26%) and 67/129 (52%) participants, respectively, had received MPH within 6 months and were not adequately controlled on current medication with acceptable tolerability; most of these participants had received long-acting MPH. In prior MPH participants, efficacy assessments demonstrated improvements from baseline (study 1) and versus placebo (study 2) that were comparable with those seen in the respective overall study population. Safety profiles were consistent with long-acting stimulant use.
Conclusion
In two studies, children who had received prior MPH treatment improved during treatment with LDX and experienced similar improvements in their symptoms as the overall study populations. For children with ADHD who were previously treated with MPH, LDX may, therefore, be an efficacious treatment option.
Keywords: Attention-deficit/hyperactivity disorder, Children, Efficacy, Lisdexamfetamine dimesylate, Methylphenidate, Safety, Stimulants, Psychiatry
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a common neurobehavioral disorder in children [1]. As reviewed by Rader et al., stimulants have been used for decades to treat ADHD symptoms [2, 3] and remain a first-line option [2, 4]. Methylphenidate (MPH) and amphetamine (AMP) psychostimulants have similar subjective effects [5], but somewhat different mechanisms of action [6–8].
The selection of an AMP- or MPH-based medication as the first-choice treatment should be left to the physician, in consultation with the patient and family [9]. ADHD treatment guidelines recommend that, if treatment with one stimulant is ineffective, an alternative stimulant should be attempted before considering second-line therapy [2]. This is supported by crossover trials which suggest that the outcome of treatment with one stimulant is not predictive of that with the other [10, 11]. However, a greater understanding of the response to treatment in patients who have previously received a different stimulant will further assist prescribers in making informed clinical choices.
Lisdexamfetamine dimesylate (LDX) is a long-acting prodrug stimulant approved for the treatment of ADHD in the United States and Canada for children 6–12 years of age, adolescents 13–17 years of age, and adults. In Europe, LDX is indicated as part of a comprehensive treatment program for ADHD in children aged 6 years and over, when response to previous MPH treatment is considered clinically inadequate. After oral ingestion, therapeutically inactive LDX is converted to l-lysine and active d-AMP in the blood [12]. LDX was designed to have an extended duration, without the need for multiple daily dosing [13, 14]. Clinical trials of LDX have demonstrated short- and long-term efficacy [13–17].
In a 7-week, open-label, dose-optimization study (study 1) of children with ADHD [16], LDX (20–70 mg/day) was effective, as assessed by a clinician-rated symptom scale and by clinician- and parent-rated global measures. Since many children with ADHD also experience impairments in executive function (EF) [18] and in emotional function across settings [19], this study measured these impairments at baseline and posttreatment. The Behavior Rating Inventory of Executive Function (BRIEF) scale [20, 21] examined real-world parent-assessed EF behaviors and the Expression and Emotion Scale for Children (EESC) [22] evaluated parent-rated negative/positive aspects of emotional expression in children before and during treatment. Participants significantly improved versus baseline in BRIEF and EESC total and subscale scores following LDX treatment [16].
Children with ADHD exhibit impairments in the school setting, due to inattention and hyperactivity/impulsivity; therefore, laboratory school models have been used to assess ADHD impact and treatments. A randomized, placebo-controlled, crossover study (study 2) [14] evaluated the impact of LDX treatment from dosing to 13 h post-dose using a laboratory school setting. The Swanson, Kotkin, Agler, M-Flynn, and Pelham (SKAMP) scale, a clinician-rated assessment of classroom behavior with subscales for deportment (SKAMP-D) and attention (SKAMP-A) [14, 23], and the Permanent Product Measure of Performance (PERMP), a series of skill-adjusted, timed (10-min) math tests that measure ability to attend to work and effortful performance [24], were used. Children receiving LDX treatment had significantly improved SKAMP and PERMP scores at all time points versus placebo (1.5–13 h post-dose) [14]. In studies 1 and 2, treatment-emergent adverse events (TEAEs) were consistent with other pediatric studies of LDX [14, 16].
Given the paucity of empirical evidence on the response to stimulant medication in patients who have previously received another stimulant, the current post hoc analyses examined the effects of LDX treatment in subgroups of children who had taken MPH in the 6-month period before enrollment in studies 1 and 2, without knowledge of the outcome of this prior therapy. All participants in these two studies [14, 16] had at least moderately symptomatic ADHD at baseline. The study outcomes analyzed were clinician-rated ADHD symptoms and global severity, parent-rated EF behaviors and emotional expression, and investigator-assessed behavior and effortful performance. The results may help clinicians determine whether LDX is an appropriate option for patients who have recently been exposed to MPH.
Methods
These post hoc subanalyses examined the efficacy measures from two multicenter studies of the efficacy and safety of LDX conducted in children with ADHD aged 6–12 years, herein referred to as study 1 [16] and study 2 [14]. Study 1 was a prospective, 7-week, open-label dose-optimization study; and study 2 was a laboratory school study incorporating a 4-week dose-optimization phase, followed by a 2-week, randomized, double-blind, placebo-controlled, crossover period. Both studies enrolled children with a baseline ADHD Ratings Scale IV [25] (ADHD-RS-IV) total score ≥28, but excluded patients whose pre-study ADHD treatment provided effective control of ADHD symptoms with acceptable tolerability, and patients who had failed to respond to a course of AMP therapy of adequate dose and duration.
The subgroups for the present analyses comprised children who had been treated with MPH (MPH hydrochloride, MPH, or dexmethylphenidate hydrochloride) at any time within the 6-month period immediately prior to study enrollment. If on treatment at screening, participants underwent a washout period of at least 7 days prior to baseline.
Study 1
Study 1 evaluated LDX (20–70 mg/day) efficacy in children (6–12 years) with ADHD with baseline ADHD-RS-IV total score ≥28 and was described in full previously [16]. The primary efficacy assessment was the change in ADHD-RS-IV total score from baseline to endpoint. Secondary efficacy measures included the Clinical Global Impressions (CGI) scale [26], the EESC [22], and the BRIEF-Parent Form [20].
ADHD-RS-IV is an 18-item, clinician-rated scale based on criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR), with symptoms grouped into two subscales (inattention and hyperactivity/impulsivity) [25, 27]. Each symptom item was scored from 0 (never or rarely) to 3 (very often); total scores ranged from 0 to 54. The CGI global assessments evaluated baseline severity and improvement over time. At baseline, the CGI-Severity (CGI-S) scale rated ADHD severity from 1 (normal/not at all ill) to 7 (among the most extremely ill). At all subsequent visits, the CGI-Improvement (CGI-I) assessed improvement from 1 (very much improved) to 7 (very much worse). The EESC is a 29-item validated measure of emotional expression; total scores ranged from 29 to 145, higher scores indicating greater impairment [22]. The EESC was administered at baseline and at the final study week. The BRIEF-Parent Form is an 86-item validated assessment of EF in children (5–18 years) [20, 21]. Global Executive Composite (GEC) scores were transformed to T-scores. T-scores of 50 represent the mean for the normative group distribution [20]. T-scores ≥65 [≥1.5 standard deviation (SD) above the mean] on BRIEF clinical scales and indices were considered potentially clinically significant scores.
Study 2
Study 2, in children (6–12 years) with ADHD and a baseline ADHD-RS-IV score ≥28, evaluated LDX (30–70 mg/day) efficacy and was described in full previously [14]. Study 2 included a 4-week, open-label, dose-optimization phase, followed by a crossover phase where each participant took LDX and placebo for 1 week each in randomized order. The primary efficacy measure was the mean SKAMP-D subscore over the course of a laboratory school day. Secondary efficacy measures included SKAMP-A, PERMP math scores, ADHD-RS-IV, and CGI scores. All efficacy assessments reported here are for the crossover phase.
The SKAMP scale [23] is a 13-item validated rating scale used to evaluate ADHD manifestations in a laboratory school setting. In addition to a total score, subscores are calculated for deportment and attention [14, 23]. PERMP consists of a 5-page, 80-problem math test, and participants are scored according to the number of problems attempted and the number solved correctly in a 10-min period [24]. SKAMP and PERMP assessments were made 0.5 h pre-dose and 1.5–13.0 h post-dose. ADHD-RS-IV and CGI scores were measured at baseline and at all subsequent weeks, including the two crossover weeks (visits 5/6).
Studies 1 and 2 Analyses
Efficacy outcomes for the overall group were analyzed according to the efficacy population, defined as all randomized participants who received ≥1 dose of study treatment with ≥1 available post-randomization measure of the primary efficacy variable. Efficacy outcomes for the study 1 post hoc analysis were for all LDX dose groups combined from baseline to endpoint, defined as the last valid efficacy assessment (i.e., ADHD-RS-IV) post-baseline. For study 2, efficacy outcomes were reported from baseline to weeks 5 and 6 (visit 5/6), the two crossover phase assessments, for participants taking LDX (all doses) and placebo.
Clinical Response Criteria
A child may exhibit considerable clinical response to treatment from baseline, using the ADHD-RS-IV scale, yet still be symptomatic. Inclusion of the CGI-I criteria may clinically define how well a participant improved with treatment from baseline, although this child still exhibited ADHD symptoms. A stringent definition for clinical response that combines the two criteria may provide more insight into treatment options for clinicians [28]. For this analysis, clinical responders were classified as participants who achieved at least a 30% reduction in ADHD-RS-IV total score from baseline and a CGI-I score of 1 or 2.
Symptomatic Remission Criteria
There are varying definitions of thresholds to describe ADHD symptomatic remission, which may include clinical response to a degree that the participant no longer exhibits symptoms sufficient to meet DSM-IV-TR criteria [29]. Here, the authors used a conservative definition of symptomatic remission with no symptom item on the ADHD-RS-IV endorsed as more severe than mild [28]. Thus, symptomatic remission was defined as ADHD-RS-IV item scores of ≤1, for each of the 18 items, at endpoint in study 1 or during the crossover phase of study 2. Symptomatic remission, as defined in this analysis, may be considered a more stringent definition than that defined in prior LDX studies and analyses (ADHD-RS-IV total score of ≤18 at endpoint) [30–32]. An overall score of ≤18 does not give specific information on the effects of treatment on each individual item, where the participant may still exhibit symptom severity greater than mild on some items.
Written informed consent was obtained from each patient’s legal guardian, and assent was obtained from each child prior to study-related procedures being performed. The study protocol was approved by the institutional review board at each study center, and the studies were performed in accordance with the International Conference on Harmonisation of Good Clinical Practice, 18th World Medical Assembly (Helsinki 1964), and amendments of the 29th (Tokyo 1975), the 35th (Venice 1983), the 41st (Hong Kong 1989), and the 48th (South Africa 1996) World Medical Assemblies.
Results
Study 1
Baseline characteristics and demographics of the overall study population have been previously presented [16]. Of 318 enrolled participants, 83 (26.1%) had taken MPH within 6 months of study initiation, 67/83 (80.7%) were treated with long-acting MPH, and 18/83 took ≥1 mg/kg/day (a dose considered “generally effective” [33, 34]. Table 1 shows summary statistics for the dosage and duration of this previous MPH treatment. Children in the prior MPH group had a mean age (SD) of 9.2 (1.88) years, a mean (SD) weight of 33.6 (8.58) kg, and the majority were male (65 of 83, 78.3%), similar to the overall study population. The mean (SD) ADHD-RS-IV baseline total score in this subgroup was 42.6 (6.81) and was similar for males and females with mean (SD) ADHD-RS-IV total scores of 43.0 (6.94) and 41.3 (6.34), respectively.
Table 1.
Prior methylphenidate (MPH) dosage summary statistics for Study 1 (n = 83)
| Mean (SD) | Median (range) | |
|---|---|---|
| Duration of prior MPH treatment, days | 357.9 (505.36) | 202.0 (1–3.060) |
| MPH dose, mg/day | 24.6 (20.35) | 20.0 (0–144) |
| MPH dose, mg/kg/day | 0.8 (0.63) | 0.6 (0–4) |
The mean (SD) change from baseline to endpoint with LDX treatment for ADHD-RS-IV total score was similar for the overall study population and the prior MPH group (Fig. 1). The mean (SD) relative improvement from baseline to endpoint in ADHD-RS-IV total score for children in the prior MPH group was 64.9% (23.88). Improvement with LDX was numerically greater for males versus females; the relative improvement was 66.3% (23.02) with an endpoint ADHD-RS-IV total score of 14.1 (9.08) for males versus 59.7% (26.80) with an endpoint ADHD-RS-IV total score of 16.6 (11.69) for females. At endpoint, inattention and hyperactivity/impulsivity subscale scores for prior MPH participants were improved overall by 62.7% (26.97) and 67.2% (24.39) from baseline, respectively.
Fig. 1.
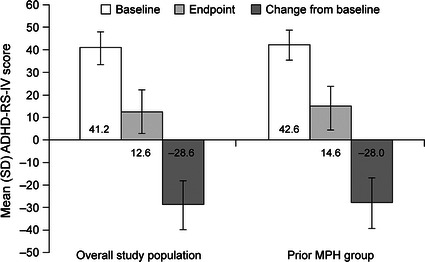
Study 1 ADHD-RS-IV total scores for overall study population and prior MPH group. ADHD-RS-IV Attention-Deficit/Hyperactivity Disorder Rating Scale IV, MPH methylphenidate, SD standard deviation
Other secondary efficacy assessments also demonstrated improvement with LDX. Mean (SD) BRIEF GEC scores at baseline and endpoint were similar for the overall study population and prior MPH group (Fig. 2). Mean (SD) CGI-I scores at endpoint, and EESC total scores, and BRIEF index subscale scores (Behavioral Recognition Index and Metacognition Index) at baseline and endpoint, were similar between the overall study population and prior MPH group (Table 2). Moreover, with LDX treatment the BRIEF index subscale scores were normalized at endpoint (Fig. 2; Table 2).
Fig. 2.
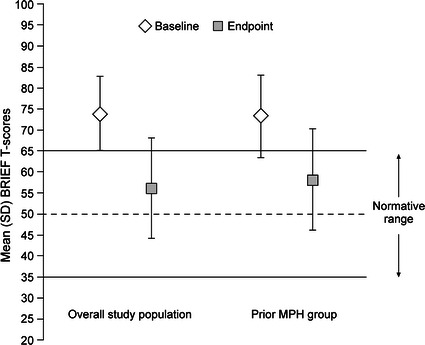
Study 1 BRIEF GEC T-scores for overall study population and prior MPH group. BRIEF Behavior Rating Inventory of Executive Function, GEC Global Executive Composite, MPH methylphenidate, SD standard deviation
Table 2.
Study 1: CGI-I, EESC, and BRIEF index subscales for the overall study population and prior methylphenidate (MPH) group
| Overall study population | Prior MPH group | |||
|---|---|---|---|---|
| Baseline | Endpoint | Baseline | Endpoint | |
| CGI-I | – | 1.5 (0.8) | – | 1.6 (0.8) |
| EESC | 63.4 (18.0) | 55.9 (17.7) | 63.3 (17.1) | 55.4 (17.3) |
| BRIEF indexes | ||||
| Behavioral regulation index | 71.0 (11.8) | 55.7 (12.5) | 71.6 (11.4) | 57.9 (12.3) |
| Metacognition index | 73.1 (8.4) | 55.5 (11.5) | 71.6 (9.7) | 57.7 (11.6) |
CGI-I Clinical Global Impressions-Improvement, BRIEF Behavior Rating Inventory of Executive Function, EESC Expression and Emotion Scale for Children
Rates of symptomatic remission in the overall study population (49.1%) and prior MPH group (42.2%) were similar at endpoint of study 1, although the prior MPH group had numerically lower symptomatic remission rates compared with the overall group, including the subgroups of prior MPH participants with MPH doses <1 or ≥1 mg/kg/day (Fig. 3). Moreover, 283 of 316 (89.6%) participants in the overall study population and 72 of 83 (86.7%) prior MPH participants at endpoint achieved clinical response (≥30% reduction from baseline in ADHD-RS-IV total score and CGI-I rating of 1 or 2) with LDX.
Fig. 3.
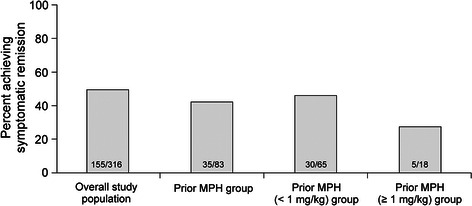
Study 1 rates of symptomatic remission* for overall study population and prior MPH group. ADHD-RS-IV Attention-Deficit/Hyperactivity Disorder Rating Scale IV, MPH methylphenidate. *Symptomatic remission defined as all ADHD-RS-IV item scores ≤1
Study 2
Baseline characteristics and demographics of the overall study population have been previously presented [14]. Of the 129 enrolled participants, 67 (51.9%) had taken MPH within 6 months prior to study entry; all but two were treated with long-acting MPH and 17/67 took ≥1 mg/kg/day. Table 3 shows summary statistics for the dosage and duration of this previous MPH treatment. In the crossover phase, 113 children including all 67 prior MPH participants were included in the overall study population. Children in the prior MPH group had a mean age (SD) of 10.0 (1.60) years, a mean (SD) weight of 31.6 (6.98) kg, and the majority were male (54 of 67, 80.6%), similar to the overall study population. The mean (SD) ADHD-RS-IV baseline total score in this subgroup was 43.1 (7.22) and was similar for males and females with mean (SD) ADHD-RS-IV total scores of 42.6 (7.27) and 45.2 (6.87), respectively.
Table 3.
Prior methylphenidate (MPH) dosage summary statistics for Study 2 (n = 67)
| Mean (SD) | Median (range) | |
|---|---|---|
| Duration of prior MPH treatment, days | 458.1 (455.57) | 323.0 (4–2,335) |
| MPH dose, mg/day | 27.1 (15.10) | 24.0 (0–90) |
| MPH dose, mg/kg/day | 0.9 (0.54) | 0.8 (0–3) |
Change from baseline in mean (SD) ADHD-RS-IV total scores for participants when taking LDX and placebo during the crossover phase were similar for the overall study population (n = 113) and prior MPH group (n = 67) (Fig. 4). The mean (SD) relative improvement from baseline to endpoint in ADHD-RS-IV total score for children who had previously received MPH was 57.1% (26.11) in the LDX treatment group and 18.1% (28.85) in the placebo group. For prior MPH participants who had received average MPH dose ≥1 mg/kg/day (n = 17), from a mean (SD) baseline score of 43.6 (7.71), the mean (SD) relative improvement in ADHD-RS-IV total scores was 58.0% (21.16) with LDX and 16.4% (25.58) with placebo.
Fig. 4.
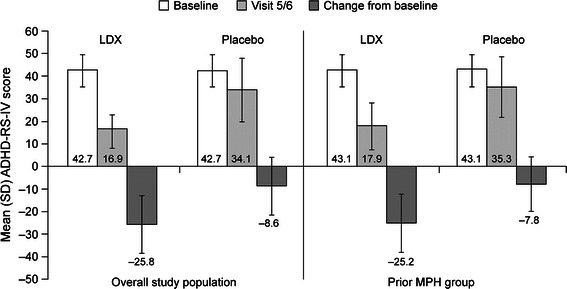
Study 2 ADHD-RS-IV total scores for overall study population and prior MPH group. ADHD-RS-IV Attention-Deficit/Hyperactivity Disorder Rating Scale IV, LDX lisdexamfetamine dimesylate, MPH methylphenidate, SD standard deviation
For prior MPH males, from a mean (SD) baseline score of 42.6 (7.27), the mean (SD) relative improvement in ADHD-RS-IV total scores was 55.3% (27.07) with LDX and 17.6% (29.38) with placebo; for prior MPH females, from a mean (SD) baseline score of 45.2 (6.87), the mean (SD) relative improvement in ADHD-RS-IV total scores was 64.8% (20.79) with LDX and 19.9% (27.59) with placebo. Least squares (LS) mean [95% confidence interval (CI)] difference (LDX minus placebo) was −17.1 (−20.41, −13.78; P<0.0001) and −17.1 (−21.38, −12.85) for the overall study population and prior MPH participants, respectively. The overall LS [standard error (SE)] effect size of LDX was −1.4 (0.16) and −1.4 (0.21) for the overall study population and prior MPH participants, respectively.
For prior MPH participants, mean (SD) relative improvement in ADHD-RS-IV inattention subscale scores was 56.3% (27.54) with LDX and 17.0% (28.61) with placebo from a mean (SD) baseline score of 21.4 (4.14). The mean (SD) relative improvement in ADHD-RS-IV hyperactivity/impulsivity subscale scores was 57.9% (28.22) with LDX and 18.2% (33.14) with placebo from a mean (SD) baseline score of 21.7 (4.32).
Improvements in SKAMP-D were similar in the overall study population and prior MPH participants. For both, SKAMP-D scores were improved at all post-dose time points from 1.5 to 13 h with LDX versus placebo (P ≤ 0.0046 and P ≤ 0.0284 for all time points in the overall study population and prior MPH group, respectively) (Fig. 5a). LS mean (95% CI) difference was −0.74 (−0.85, −0.63; P < 0.0001) and −0.83 (−1.03, −0.64; P < 0.0001) for the overall study population and prior MPH participants, respectively. The overall LS (SE) effect size of LDX was −1.73 (0.18) and −1.77 (0.28) for the overall study population and prior MPH participants, respectively. Similarly for SKAMP-A, improvements with LDX were similar in the overall study population and prior MPH participants improvements seen from 1.5 h, up to and including 13 h post-dose with LDX versus placebo (P < 0.0001 and P ≤ 0.0114 for all time points in the overall study population and prior MPH group, respectively) (Fig. 5b). LS mean (95% CI) difference (LDX minus placebo) was −0.78 (−0.87, −0.70; P < 0.0001) and −0.6 (−0.76, −0.44; P < 0.0001) for the overall study population and prior MPH participants, respectively. The overall LS (SE) effect size of LDX was −2.41 (0.21) and −1.6 (0.27) for the overall study population and prior MPH participants, respectively.
Fig. 5.
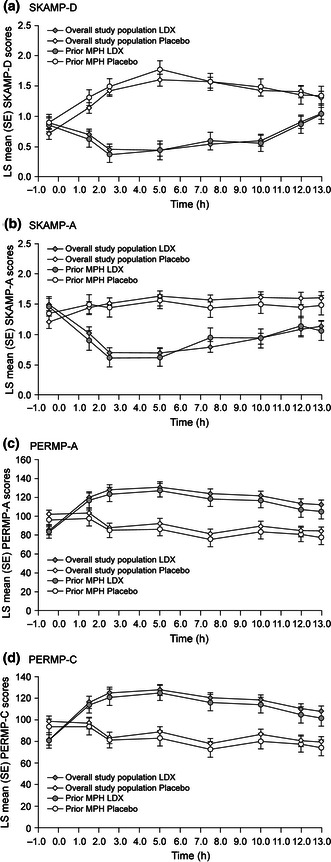
Study 2 LS Mean (SE) SKAMP-D (a), SKAMP-A (b), PERMP-A (c), and PERMP-C (d) scores for overall study population and prior MPH group. LDX lisdexamfetamine dimesylate, MPH methylphenidate, PERMP Permanent Product Measure of Performance Attempted (PERMP-A) and Correct (PERMP C), SE standard error, SKAMP Swanson, Kotkin, Agler, M-Flynn, and Pelham Deportment (SKAMP-D) and Attention (SKAMP-A) scale
Both the overall study population and the prior MPH groups performed similarly on the PERMP (Fig. 5c, d). PERMP-A and PERMP-C scores were improved at all post-dose time points from 1.5 to 13 h with LDX versus placebo (P < 0.0001 for all time points in the overall study population and prior MPH participants, respectively, for both PERMP-A and PERMP-C).
At visit 5/6 of the crossover period, mean (SD) CGI-I scores for participants taking LDX and placebo, respectively, were 1.7 (0.9) and 3.5 (1.2) for the overall study population, 1.7 (0.96) and 3.6 (1.14) for prior MPH participants, and 1.7 (0.85) and 3.7 (0.99) for prior MPH participants who had received ≥1 mg/kg/day MPH.
At visit 5/6 of the crossover phase, 54 of 67 (80.6%) achieved clinical response with LDX and 10 of 67 (14.9%) participants with placebo. Rates of symptomatic remission were similar in the overall study population (LDX 31.9%, placebo 9.7%) and the prior MPH group (LDX 28.4%, placebo 10.4%), including the subgroups of prior MPH participants with MPH doses <1 or ≥1 mg/kg/day (Fig. 6).
Fig. 6.
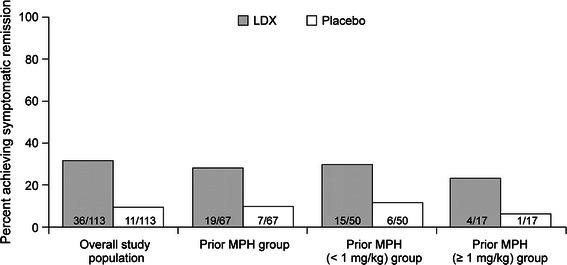
Rates of symptomatic remission* for the overall study population and the prior MPH groups. ADHD-RS-IV Attention-Deficit/Hyperactivity Disorder Rating Scale IV, MPH methylphenidate. *Symptomatic remission defined as all ADHD-RS-IV item scores ≤1
Summary of Safety Findings in Study 1 and Study 2
Safety data from both studies have been previously reported [14, 16] and are briefly summarized here for reference. In study 1, the majority of treated participants [269/317 (84.9%)] experienced TEAEs with LDX. TEAEs reported by ≥10% of participants included decreased appetite (43.2%), decreased weight (17.0%), insomnia (16.1%), irritability (16.1%), headache (13.9%), upper abdominal pain (13.2%), and initial insomnia (11.4%); the majority were mild to moderate in severity. There were no deaths; two serious AEs (i.e., syncope, sinus arrest) were reported [16]. In study 2, the majority of participants [110/129 (85.3%)] experienced TEAEs with LDX during the open-label phase. TEAEs reported by ≥10% of participants included decreased appetite (47.3%), insomnia (27.1%), headache (17.1%), irritability (16.3%), upper abdominal pain (15.5%), and affect lability (10.1%); the majority were mild or moderate in intensity [14]. There were no deaths or serious AEs. In both studies, small increases in blood pressure and pulse were observed consistent with AMP treatment, and there were no clinically meaningful trends observed in electrocardiogram (ECG) interval data [14, 16].
Discussion
The present analyses did not specifically identify patients whose previous MPH treatment had failed, but did comprise patients who had received any prior MPH treatment within 6 months, and were not excluded on the grounds of adequate symptomatic control and acceptable tolerability with current medication. In studies 1 and 2 [14, 16], LDX effectively reduced ADHD symptoms in children previously treated with MPH within 6 months of study initiation, most of whom were previously treated with long-acting MPH. For prior MPH participants, ADHD-RS-IV total scores decreased at endpoint by a mean of 64.9% in study 1. At the end of the crossover phase of study 2, mean ADHD-RS-IV total scores were lower for prior MPH participants receiving LDX (57.1% reduction) and placebo (18.1% reduction). The results were comparable with the overall populations for both studies; however, a trend that suggests a slightly lower reduction in ADHD-RS-IV scores with LDX in the prior MPH group versus the respective overall study populations exists. CGI-I results confirm the efficacy of LDX in prior MPH participants. In study 1, the mean CGI-I for prior MPH participants at endpoint was 1.6. At the end of the crossover phase of study 2, the mean CGI-I for prior MPH participants receiving LDX was 1.7 versus 3.6 when receiving placebo. These values are comparable to those of the overall study populations of study 1 (1.5) and study 2 (1.7 LDX, 3.5 placebo).
In study 2, LDX demonstrated efficacy versus placebo in children with prior MPH treatment by improving attention, behavior, and math scores as assessed with SKAMP-A, SKAMP-D, and PERMP scores, respectively. This effect was sustained from 1.5 to 13.0 h post-dose (last time point assessed) for SKAMP-A, SKAMP-D, and PERMP scores. Overall, the results of these analyses are in line with a similar post hoc analysis of data from a 4-week, parallel-group, placebo-controlled study of LDX efficacy in children with ADHD that found improvements in ADHD symptoms and global illness were comparable between prior MPH users and the overall study population [32]. Clinical trials of ADHD generally focus on core ADHD symptom of inattention and hyperactivity/impulsivity. Nevertheless, ADHD is associated with impairment in EF, as reviewed by Edward Brown, which is considered by many as an essential component of ADHD etiology [35]. Study 1 examined BRIEF scores as a measure of EF. LDX treatment resulted in improvements in BRIEF scores among prior MPH children, indicating that parents perceived improved EF in daily life. The magnitude of the improvement seen in BRIEF GEC score in this post hoc analysis is comparable to that in the overall study population. All BRIEF subscale scores were normalized with LDX treatment. For example, improved BRIEF subscale score for emotional control may demonstrate improvements in the child’s ability to better manage emotional responses and, therefore, core EF behaviors such as emotional regulation and frustration modulation [18, 20, 21].
The EESC results from study 1 suggest that LDX treatment in prior MPH children with ADHD does not negatively affect overall emotional expression. Children with ADHD have significantly worse scores on measures of emotional well-being than children without ADHD [19]. Although this has not been established in clinical trials, emotional flattening resulting from stimulants is a concern of clinicians and parents [22]. The magnitude of improvement in the EESC total score for prior MPH children in this analysis was comparable to the overall study.
Differential clinical response rates to stimulant treatment are common [10, 11]. Due to suboptimal treatment of ADHD, alternative stimulants should be evaluated to improve patient outcomes [2]. Clinical response rate can be improved to an estimated 92% when stimulants are tried sequentially, after one has failed [10]. There has been limited systematic evaluation of patient outcomes after switching stimulants. The authors’ findings suggest that LDX may provide effective symptom control in children with ADHD who have had prior MPH exposure. A substantial proportion of participants in both studies achieved symptomatic remission, suggesting that LDX treatment in children previously treated with MPH had improved ADHD symptoms. Based on the study data, many patients previously treated with MPH may respond well to LDX and achieve symptomatic remission, although overall remission rates may be slightly lower in prior MPH-treated patients. These results agree with those of a recent post hoc analysis which found that children with significant ADHD symptoms despite MPH treatment improved on LDX to a similar degree to the overall study population [32].
Both studies were not prospectively designed to examine the effects of LDX on MPH nonresponders; hence, the interpretation of the findings from this manuscript was confounded by the post hoc nature of the analyses. Study 1 was limited by the lack of a placebo group for comparison. In contrast, study 2 was strengthened by the utilization of the placebo-controlled laboratory school setting and the blinded design of the crossover phase. The dose-optimization phase of both studies allowed assessment of participants at optimal doses, closely approximating clinical practice in medication dosing. Another limitation was that participants were not necessarily taking MPH immediately before screening (only had taken MPH within 6 months of study), which limits extrapolation of these findings. There were no comparable reports of symptom control on prior MPH using the ADHD-RS-IV or other study measures, and often precise dates for initiation/termination of prior treatment were missing. Nor was there control over patient compliance/adherence to the prior MPH regimen. Since participants who were responding well to, and tolerating, their prior medication were excluded from both studies of the overall study populations, it is presumed that enrolled participants were those who may have discontinued MPH therapy due to poor efficacy or tolerability.
Conclusion
For children with ADHD who were previously treated with MPH, LDX may be an efficacious treatment option, by significantly reducing ADHD symptoms, improving EF and emotional expression as well as attention and deportment in the laboratory school setting.
Acknowledgments
Clinical research and the article processing charges were funded by the sponsor, Shire Development LLC under the direction of the authors. Huda Ismail Abdullah, PhD, and Michael Pucci, PhD, employees of SCI Scientific Communications and Information (SCI); Kira Belkin and Debbi Gorman, employees of Excerpta Medica; and Eric Southam, an employee of Oxford Pharmagenesis, provided writing assistance for this publication. Editorial assistance in formatting, proofreading, copy editing, and fact checking was also provided by SCI and Oxford Pharmagenesis. Louise Boulet and Danny Germain from Shire Development LLC also reviewed and edited the manuscript for scientific accuracy. Shire Development LLC provided funding to SCI for support in writing and editing this manuscript. Although the Sponsor was involved in the design, collection, analysis, interpretation, and fact checking of information, the content of this manuscript, the ultimate interpretation, and the decision to submit it for publication in Advances in Therapy were made by the authors independently. Rakesh Jain is the guarantor for this article, and takes responsibility for the integrity of the work as a whole.
Conflict of interest
Rakesh Jain has received speaker’s honoraria and grant/research support from Shire and participates or has participated in speakers’ bureaux for Shire. Thomas Babcock is an employee of Shire Development LLC and holds stock and/or stock options in Shire. Bryan Dirks is an employee of Shire Development LLC and holds stock and/or stock options in Shire. Ben Adeyi is an employee of Shire Development LLC and holds stock and/or stock options in Shire. Brian Scheckner is an employee of Shire Development LLC and holds stock and/or stock options in Shire. Robert Lasser is an employee of Shire Development LLC and holds stock and/or stock options in Shire. John Renna is an employee of Shire Development LLC and holds stock and/or stock options in Shire. Teodor Burtea is a former employee of Shire Canada, Inc. and a current employee of Ferring Pharmaceuticals USA, Inc. Don Duncan has acted as a consultant to Shire, Janssen-Ortho, Purdue Pharma and Eli Lilly, has served as an expert witness for Janssen-Ortho, and receives grant/research support from Janssen-Ortho.
Compliance with Ethics Guidelines
Written informed consent was obtained from each patient’s legal guardian, and assent was obtained from each child prior to study-related procedures being performed. The study protocol was approved by the institutional review board at each study center, and the studies were performed in accordance with the International Conference on Harmonisation of Good Clinical Practice, 18th World Medical Assembly (Helsinki 1964), and amendments of the 29th (Tokyo 1975), the 35th (Venice 1983), the 41st (Hong Kong 1989), and the 48th (South Africa 1996) World Medical Assemblies.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
References
- 1.Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007;164:942–948. doi: 10.1176/appi.ajp.164.6.942. [DOI] [PubMed] [Google Scholar]
- 2.Rader R, McCauley L, Callen EC. Current strategies in the diagnosis and treatment of childhood attention-deficit/hyperactivity disorder. Am Fam Physician. 2009;79:657–665. [PubMed] [Google Scholar]
- 3.Goldman LS, Genel M, Bezman RJ, Slanetz PJ. Diagnosis and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Council on Scientific Affairs, American Medical Association. JAMA. 1998;279:1100–1107. doi: 10.1001/jama.279.14.1100. [DOI] [PubMed] [Google Scholar]
- 4.Dopheide JA. ASHP therapeutic position statement on the appropriate use of medications in the treatment of attention-deficit/hyperactivity disorder in pediatric patients. Am J Health Syst Pharm. 2005;62:1502–1509. doi: 10.2146/ajhp040600. [DOI] [PubMed] [Google Scholar]
- 5.Heishman SJ, Henningfield JE. Discriminative stimulus effects of d-amphetamine, methylphenidate, and diazepam in humans. Psychopharmacology (Berl) 1991;103:436–442. doi: 10.1007/BF02244241. [DOI] [PubMed] [Google Scholar]
- 6.Heron C, Costentin J, Bonnet JJ. Evidence that pure uptake inhibitors including cocaine interact slowly with the dopamine neuronal carrier. Eur J Pharmacol. 1994;264:391–398. doi: 10.1016/0014-2999(94)00502-8. [DOI] [PubMed] [Google Scholar]
- 7.Hess EJ, Collins KA, Wilson MC. Mouse model of hyperkinesis implicates SNAP-25 in behavioral regulation. J Neurosci. 1996;16:3104–3111. doi: 10.1523/JNEUROSCI.16-09-03104.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heal DJ, Cheetham SC, Smith SL. The neuropharmacology of ADHD drugs in vivo: insights on efficacy and safety. Neuropharmacology. 2009;57:608–618. doi: 10.1016/j.neuropharm.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 9.Pliszka SR, Crismon ML, Hughes CW, et al. The Texas Children’s Medication Algorithm Project: revision of the algorithm for pharmacotherapy of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2006;45:642–657. doi: 10.1097/01.chi.0000215326.51175.eb. [DOI] [PubMed] [Google Scholar]
- 10.Arnold LE. Methylphenidate vs. amphetamine: comparative review. J Atten Disord. 2000;3:200–211. doi: 10.1177/108705470000300403. [DOI] [Google Scholar]
- 11.Elia J, Borcherding BG, Rapoport JL, Keysor CS. Methylphenidate and dextroamphetamine treatments of hyperactivity: are there true nonresponders? Psychiatry Res. 1991;36:141–155. doi: 10.1016/0165-1781(91)90126-A. [DOI] [PubMed] [Google Scholar]
- 12.Pennick M. Absorption of lisdexamfetamine dimesylate and its enzymatic conversion to d-amphetamine. Neuropsychiatr Dis Treat. 2010;6:317–327. doi: 10.2147/NDT.S9749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biederman J, Krishnan S, Zhang Y, McGough JJ, Findling RL. Efficacy and tolerability of lisdexamfetamine dimesylate (NRP-104) in children with attention-deficit/hyperactivity disorder: a phase III, multicenter, randomized, double-blind, forced-dose, parallel-group study. Clin Ther. 2007;29:450–463. doi: 10.1016/S0149-2918(07)80083-X. [DOI] [PubMed] [Google Scholar]
- 14.Wigal SB, Kollins SH, Childress AC, Squires L. A 13-hour laboratory school study of lisdexamfetamine dimesylate in school-aged children with attention-deficit/hyperactivity disorder. Child Adolesc Psychiatry Ment Health. 2009;3:17. doi: 10.1186/1753-2000-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biederman J, Boellner SW, Childress A, Lopez FA, Krishnan S, Zhang Y. Lisdexamfetamine dimesylate and mixed amphetamine salts extended-release in children with ADHD: a double-blind, placebo-controlled, crossover analog classroom study. Biol Psychiatry. 2007;62:970–976. doi: 10.1016/j.biopsych.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 16.Findling RL, Ginsberg LD, Jain R, Gao J. Effectiveness, safety, and tolerability of lisdexamfetamine dimesylate in children with attention-deficit/hyperactivity disorder: an open-label, dose-optimization study. J Child Adolesc Psychopharmacol. 2009;19:649–662. doi: 10.1089/cap.2008.0165. [DOI] [PubMed] [Google Scholar]
- 17.Findling RL, Childress AC, Krishnan S, McGough JJ. Long-term effectiveness and safety of lisdexamfetamine dimesylate in school-aged children with attention-deficit/hyperactivity disorder. CNS Spectr. 2008;13:614–620. doi: 10.1017/s1092852900016898. [DOI] [PubMed] [Google Scholar]
- 18.Mares D, McLuckie A, Schwartz M, Saini M. Executive function impairments in children with attention-deficit hyperactivity disorder: Do they differ between school and home environments? Can J Psychiatry. 2007;52:527–534. doi: 10.1177/070674370705200811. [DOI] [PubMed] [Google Scholar]
- 19.Strine TW, Lesesne CA, Okoro CA, et al. Emotional and behavioral difficulties and impairments in everyday functioning among children with a history of attention-deficit/hyperactivity disorder. Prev Chronic Dis. 2006;3:A52. [PMC free article] [PubMed] [Google Scholar]
- 20.Gioia GA, Isquith PK, Guy SC, Kenworthy L. BRIEF: Behavior Rating Inventory of Executive Function: professional manual. Lutz, FL: Psychological Assessment Resources Inc, 2000. Child Neuropsychol. 2000;6:235–238. doi: 10.1076/chin.6.3.235.3152. [DOI] [PubMed] [Google Scholar]
- 21.Gioia GA, Isquith PK, Retzlaff PD, Espy KA. Confirmatory factor analysis of the Behavior Rating Inventory of Executive Function (BRIEF) in a clinical sample. Child Neuropsychol. 2002;8:249–257. doi: 10.1076/chin.8.4.249.13513. [DOI] [PubMed] [Google Scholar]
- 22.Kratochvil CJ, Faries D, Vaughan B, et al. Emotional expression during attention-deficit/hyperactivity disorders treatment: initial assessment of treatment effects. J Child Adolesc Psychopharmacol. 2007;17:51–62. doi: 10.1089/cap.2006.0018. [DOI] [PubMed] [Google Scholar]
- 23.Wigal SB, Gupta S, Guinta D, et al. Reliability and validity of the SKAMP rating scale in a laboratory school setting. Psychopharmacol Bull. 1998;34:47–53. [PubMed] [Google Scholar]
- 24.Wigal SB, Wigal TL. The laboratory school protocol: its origin, use, and new applications. J Atten Disord. 2006;10:92–111. doi: 10.1177/1087054705286049. [DOI] [PubMed] [Google Scholar]
- 25.DuPaul GJ, Power T, Anastopoulos A, Reid R. ADHD Rating Scale-IV: checklists, norms, and clinical interpretation. New York: Guilford Press; 1998. [Google Scholar]
- 26.Guy W. Clinical global impressions. In: ECDEU Assessment Manual for Psychopharmacology. US Department of Health, Education, and Welfare; Public Health Service, Alcohol, Drug Abuse and Mental Health Administration, NIMH Psychopharmacology Research Branch, Rockville, MD; 1976. p. 218–22.
- 27.American Psychiatric Association. Attention-deficit and disruptive behavior disorders. In: diagnostic and statistical manual of mental disorders DSM-IV-TR, 4th ed. Washington, DC: American Psychiatric Association; 2000. p. 85–93.
- 28.Steele M, Jensen PS, Quinn DMP. Remission versus response as the goal of therapy in ADHD: a new standard for the field? Clin Ther. 2006;28:1892–1908. doi: 10.1016/j.clinthera.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Swanson JM, Kraemer HC, Hinshaw SP, et al. Clinical relevance of the primary findings of the MTA: success rates based on severity of ADHD and ODD symptoms at the end of treatment. J Am Acad Child Adolesc Psychiatry. 2001;40:168–179. doi: 10.1097/00004583-200102000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Wigal T, Brams M, Gasior M, Gao J, Giblin J. Effect size of lisdexamfetamine dimesylate in adults with attention-deficit/hyperactivity disorder. Postgrad Med. 2011;123:169–176. doi: 10.3810/pgm.2011.03.2275. [DOI] [PubMed] [Google Scholar]
- 31.Ginsberg L, Katic A, Adeyi B, et al. Long-term treatment outcomes with lisdexamfetamine dimesylate for adults with attention-deficit/hyperactivity disorder stratified by baseline severity. Curr Med Res Opin. 2011;27:1097–1107. doi: 10.1185/03007995.2011.567256. [DOI] [PubMed] [Google Scholar]
- 32.Jain R, Babcock T, Burtea T, et al. Efficacy of lisdexamfetamine dimesylate in children with attention-deficit/hyperactivity disorder previously treated with methylphenidate: a post hoc analysis. Child Adolesc Psychiatry Ment Health. 2011;5:35. doi: 10.1186/1753-2000-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pelham WE, Gnagy EM, Burrows-Maclean L, et al. Once-a-day Concerta® methylphenidate versus three-times-daily methylphenidate in laboratory and natural settings. Pediatrics. 2001;107:E105. doi: 10.1542/peds.107.6.e105. [DOI] [PubMed] [Google Scholar]
- 34.Steele M, Weiss M, Swanson J, Wang J, Prinzo RS, Binder CE. A randomized, controlled effectiveness trial of OROS-methylphenidate compared to usual care with immediate-release methylphenidate in attention-deficit/hyperactivity disorder. Can J Clin Pharmacol. 2006;13:e50–e62. [PubMed] [Google Scholar]
- 35.Brown TE. ADD/ADHD and impaired executive function in clinical practice. Curr Psychiatry Rep. 2008;10:407–411. doi: 10.1007/s11920-008-0065-7. [DOI] [PubMed] [Google Scholar]


