Abstract
Reductions in estrogen function lead to adiposity and peripheral insulin resistance. Significant metabolic changes have been found in adipocytes and skeletal muscle with disruptions in the estrogen-signaling axis; however, it is unclear if intercellular communication exists between these tissues. The purpose of this study was to examine the impact of isolated adipocytes cocultured with single adult skeletal muscle fibers (SMF) collected from control female (SHAM) and ovariectomized female (OVX) mice. In addition, a second purpose was to compare differential effects of primary adipocytes from omental and inguinal adipose depots on SMF from these same groups. OVX SMF displayed greater lipid content, impaired insulin signaling, and lower insulin-induced glucose uptake compared with SHAM SMF without coculture. In the SHAM group, regardless of the adipose depot of origin, coculture induced greater intracellular lipid content compared with control SHAM SMF. The increased lipid in the SMF was associated with impaired insulin-induced glucose uptake when adipocytes were of omental, but not inguinal, origin. Coculture of OVX SMF with omental or inguinal adipocytes resulted in higher lipid content but no further reduction in insulin-induced glucose uptake compared with control OVX SMF. The data indicate that, in the OVX condition, there is a threshold for lipid accumulation in skeletal muscle beyond which there is no further impairment in insulin responsiveness. These results also demonstrate depot-specific effects of adipocyte exposure on skeletal muscle glucose uptake and further implicate a role for increased intracellular lipid storage in the pathogenesis of insulin resistance when estrogen levels are reduced.
Keywords: adipocyte, ovariectomy
risk of type 2 diabetes and cardiovascular disease significantly increases with disruptions in ovary function or the estrogen-signaling axis. A primary outcome of lost estrogen function is a significant increase in fat mass (i.e., adiposity) (13–15), particularly in the visceral region (27, 29, 35, 60). Current dogma suggests that the increased adipose tissue mass is a primary contributor to the development of the metabolic syndrome in women; however, few studies have directly assessed the impact of visceral or subcutaneous adiposity on peripheral tissue function.
Anatomical location of adiposity can dictate the impact of obesity on metabolic health. For example, visceral (i.e., omental) fat mass is strongly associated with insulin resistance independent of total body weight (52), which is likely a result of the adipocytes being more lipolytically active than subcutaneous adipocytes (24, 28, 73). Dogma suggests that a consequence of increases in visceral adipose tissue mass is greater lipid exposure to peripheral tissue, resulting in the development of insulin resistance. However, others have argued that increases in upper body subcutaneous adipose tissue are more detrimental to insulin function in peripheral tissue (33). In addition, the release of endocrine factors termed “adipokines” offers an alternative explanation for the risk differences based on the anatomical location of the adipose tissue. Specifically, greater amounts of the insulin-sensitizing adipokines are released from subcutaneous adipose tissue than from visceral adipose tissue (73), whereas visceral adipose tissue secretes more proinflammatory cytokines, contributing to the onset of insulin resistance (51, 75). Collectively, a number of potential cellular mechanisms could link increases in adipose tissue mass to the development of peripheral insulin resistance in women with disrupted estrogen signaling.
Not only is the anatomical location of fat storage important but also the mechanism responsible for expansion of the tissue mass (73). For example, an increase in adipocyte size is correlated with increased risk of metabolic disease (16, 49), whereas enlargement due to an increase in adipocyte number is often associated with enhanced insulin sensitivity (8). Significant increases in adipocyte size often lead to lipid overflow into circulation, contributing to the storage of excess lipid in insulin-sensitive tissues such as skeletal muscle and liver. We have previously shown that reductions in circulating estrogens lead to visceral adiposity coupled with elevations in basal lipolysis and increased circulating free fatty acids (77), which was associated with significant increases in intramuscular lipid in skeletal muscle (31), and a nonsignificant increase in stored hepatic triacylglycerol (TAG) (31).
In sedentary individuals, large amounts of stored intracellular lipid in skeletal muscle are associated with impaired insulin-stimulated glucose uptake (37, 40). Skeletal muscle accounts for ∼80% of postprandial glucose uptake; therefore, reduced insulin-stimulated glucose uptake by skeletal muscle is a significant factor contributing to the onset of peripheral insulin resistance (11, 12). Thus, it is logical to hypothesize that, under conditions of disrupted estrogen signaling, a significant interaction exists between the adipocyte and skeletal muscle cell that mediates the development of peripheral insulin resistance. However, to the best of our knowledge, this hypothesis has never been directly tested, creating a gap in our understanding of the relationship between adiposity and insulin function in skeletal muscle with respect to women's biology.
To this end, we developed a novel coculture system that allows placement of primary mature adipocytes and isolated single adult skeletal muscle fibers in the same culture dish without physical contact. This coculture approach is unique because both the adipocytes and single skeletal muscle fibers isolated from the animal express an adult phenotype in culture, rather than a developmental phenotype found in myotubes derived from satellite cells or preadipocytes from the stromal vascular fraction. Up to this point, myotubes and/or preadipocytes have been most commonly employed in coculture approaches. In this approach, the culture conditions are more applicable to physiological function of adult cells. We hypothesized that single muscle fibers from ovariectomized (OVX) mice would exhibit greater lipid content and associated impairments in insulin-induced glucose uptake compared with muscle fibers from age-matched control female mice (SHAM). In response to adipocyte coculture, we hypothesized that exposure of muscle fibers from OVX mice would result in greater lipid accumulation and increased development of insulin resistance as indicated by attenuated insulin-induced glucose uptake and insulin signaling compared with muscle fibers from SHAM mice. We predict this effect will be greatest when the adipocytes are derived from the visceral (i.e., omental) region compared with the subcutaneous (i.e., inguinal) region. By elucidating the intercellular signaling mechanisms that link adipocyte function to the regulation of skeletal muscle metabolism, it may be possible to develop new interventions for women experiencing metabolic conditions that arise due to ovary dysfunction.
METHODS
Animals.
For these experiments, we used a bilateral ovariectomy surgical approach (OVX) in adult female virgin mice (C57Bl/6; age of mice at OVX surgery 10 wk) to disrupt ovarian function and reduce circulating estrogen levels. The SHAM group was subjected to a SHAM surgery as previously described (36). Our laboratory has previously shown that this model decreases the levels of circulating estrogens by ∼70% within 48 h (63) and results in uterine tissue atrophy of 72% (31) albeit we did not measure estrogen levels in the media from the various cell culture conditions (see below). In addition, we have previously shown that the OVX mouse model exhibits numerous metabolic similarities to humans experiencing disruptions in their estrogen-signaling axis (66, 77) that is not a result of hyperphagia (4, 31, 57, 76). Mice were given ad libitum access to water and standard rodent chow (Purina Laboratory Rodent Diet 5001: 23% protein, 4.5% fat, 6% fiber) and were housed in a temperature-controlled room on a 12:12-h light-dark cycle. Daily food intake was measured in a subset (see below) of mice until 8 wk after surgery. Due to tissue requirements and time constraints, three sets of age-matched animals were used for this study. The first set of mice was used to determine the time course and depot-specific changes in adipocyte size and adipocyte number in female mice following OVX (n = 9 SHAM; n = 9 OVX). Groups of mice were killed, and adipocytes were isolated 2, 4, or 8 wk postsurgery. Based on the results of set one, the second set of mice (n = 10 SHAM; n = 10 OVX for biochemical and Western blot analyses) and third set (n = 5 SHAM; n = 5 OVX for skeletal muscle fiber image analyses) were killed 8 wk postsurgery, and tissue was used for skeletal muscle fiber and adipocyte coculture experiments. All aspects of this study were approved by the University of Maryland Institutional Animal Care & Use Committee Review Board.
Adipocyte isolation.
Primary adipocytes were isolated from omental (visceral fat pad) and inguinal (subcutaneous fat pad) adipose tissue according to the Rodbell technique with slight modifications (56). Briefly, freshly prepared Krebs Ringer Buffer (KRB, pH 7.4) (24.6 mM NaHCO3, 1.1 mM KH2PO4, 130.2 mM NaCl, 4.7 mM KCl, 2.54 mM MgSO4, 3.27 mM CaCl2, 5 mM dextrose, 0.02 μM adenosine, and 4 mg/ml BSA) was equilibrated by bubbling with 95% O2-5% CO2 for 10 min. Adipose tissue was removed and rinsed with KRB and then placed in a conical tube with 1 ml KRB plus 10 μl of liberase blendzyme (Roche Applied Science, Indianapolis, IN) per gram of tissue. The conical tube containing the adipose tissue was then incubated in a shaking-water bath at 37°C and 80 rpm for 45–60 min or until adipose tissue was fully digested. Following digestion, fat was strained through 250 μm mesh, and then the adipocytes were rinsed with fresh KRB buffer three times. Finally, the cells were left suspended in ∼2 ml of KRB, and an aliquot was removed for adipocyte size determination.
Adipocyte size and number.
Two separate aliquots of the adipocyte suspension were imaged under a standard light microscope at ×10 magnification and then analyzed using Image J software by a blinded investigator to measure adipocyte diameter [National Institutes of Health (NIH), Bethesda, MD]. The distribution of adipocyte sizes in each sample was determined and graphed by counting the number of cells from 30 to >100 μm. A minimum of 300 adipocytes/sample from each animal was measured for cell size determination. Adipocyte number was calculated by using the radius of each cell to determine the adipocyte cell volume, which was then divided by the corresponding fat pad weight and multiplied by 0.948 g/ml (known adipocyte density) according to previously described methods (3, 57, 59).
Adipocyte BODIPY staining.
Adipocytes were incubated in 5 ml of KRB with 10 μl of BODIPY 493/503 (1 μg/ml in dimethyl sulfoxide) for 15 min. The adipocytes were rinsed three times with fresh KRB buffer before imaging using a Nikon Eclipse Ti-U fluorescent microscope (Nikon Instruments, Melville, NY).
Adult single skeletal muscle fiber isolation.
Intact adult skeletal muscle fibers were isolated from the flexor digitorum brevis (FDB) muscle according to previously described techniques (46, 55). Utilizing cultured single skeletal muscle fibers is advantageous because the fibers express an adult cellular phenotype compared with the commonly used C2C12 or L6 myotubes that express a cellular makeup that is dominated by an embryonic phenotype (2). Briefly, surgically excised FDB muscles were incubated in dissociation media (DM) containing DMEM, 1% penicillin/streptomycin (pen/strep), and 2% FBS. FDB muscles were placed in a 40-mm dish with 3 ml of DM plus 100 μl of liberase blendzyme (2.5 mg/ml stock; Roche Applied Science) and then in an incubator (37°C, 5% CO2) for 2 h. Following the 2-h incubation, muscles were placed in a new 40-mm dish with fresh coculture media (DMEM + 1% pen/strep, 2% FBS, 0.5% BSA, and 2 mmol/l l-carnitine) and triturated with a 1-ml pipette to dissociate the muscle into single FDB fibers. Following the trituration process, fibers were plated on extracellular matrix (ECM; Sigma Aldrich, St. Louis, MO)-coated six-well plates such that each well contained a total of 3 ml of coculture media plus fibers. Fibers were incubated for 2 h to allow adherence to the ECM before beginning the coculture experiment.
Coculture conditions.
Single skeletal muscle fibers were isolated and adhered to the bottom of the well with ECM as described above. Coculture inserts were then placed in the wells with the muscle fibers. Subsequently, freshly isolated adipocytes were placed in the coculture inserts (300,000 cells/insert), thus preventing direct contact of two different cell types (Millipore, Billerica, MA). The base of the coculture inserts contained a permeable membrane with a pore size of 0.4 μm, allowing for movement of circulating factors. The coculture combinations consisted of SHAM skeletal muscle fibers without adipocytes, OVX skeletal muscle fibers without adipocytes, SHAM skeletal muscle fibers with SHAM omental adipocytes, OVX skeletal muscle fibers with OVX omental adipocytes, SHAM skeletal muscle fibers with SHAM inguinal adipocytes, and OVX skeletal muscle fibers with OVX inguinal adipocytes. The number of adipocytes placed in the coculture well was based on results from preliminary experiments in which glycerol and nonesterified fatty acid (NEFA) release into the media did not exceed concentrations measured in mouse serum (77). Adenosine was not added to the KRB buffer to allow for lipolysis to occur in the adipocytes. The appropriate volume of packed adipocytes was added to the coculture wells after the fibers were allowed to adhere to the ECM for 2 h, and the coculture was maintained for 24 h.
Insulin stimulation.
Following the overnight coculture, coculture inserts containing the adipocytes were removed from the six-well plates, and fibers were placed in serum-free media and returned to the incubator for 5 h. After 5 h, one well from each condition was stimulated with insulin (50 nM), with the other well from each condition serving as an unstimulated (basal) control. The 50 nM concentration was chosen based on values reported in the literature (41, 42, 67), as well as from pilot experiments in which we found that doses higher than 50 nM did not elicit a further increase in protein kinase B (Akt) phosphorylation after 30 min (data not shown). Fibers were returned to the incubator for 30 min before isolation of protein. We chose 30 min of treatment, since maximal GLUT-4 translocation in skeletal muscle has been observed after 20–30 min of insulin exposure (43) and we found in pilot experiments that phosphorylation of insulin signaling targets peaked at 30 min (data not shown).
Protein isolation.
Following the coculture and insulin stimulation, protein was isolated from the skeletal muscle fibers to assess the activation of the insulin-signaling pathway. Immediately after insulin stimulation, media was removed, and 300 μl of ice-cold Mueller buffer (0.1% Triton X-100, 4 mM EGTA, 10 mM EDTA, 15 mM Na4P2O7H2O, 100 mM β-glycerophosphate, 25 mM NaF, 5 mM Na3VO4, and 1 protease inhibitor tablet/10 ml; Roche) were placed in the well with the fibers. The cells were scraped, and the Mueller buffer containing the fibers was drawn off and placed in ice-cold microcentrifuge tubes. To ensure complete cellular disruption, the fibers were homogenized with a plastic dounce (Kimble & Kontes, Vernon Hills, IL) and then placed in a −80°C freezer overnight. The following morning, the homogenates were thawed on ice and then vortexed and rocked at 4°C for 1 h, with additional vortexing every 15 min. Protein concentration was determined for each sample according to the BCA protein assay method (Thermo Fischer Scientific, Rockford, IL).
Western blotting.
Equal amounts of total protein were resolved on either 10% or 4–15% SDS-PAGE criterion gels (Bio-Rad, Hercules, CA) according to previously published methods (77). Membranes were probed with antibodies for phospho (p)-Akt (Ser473), total Akt, p-glycogen synthase kinase (GSK)-3β (Ser9), total GSK-3β, hexokinase (HK) II (Cell Signaling, Danvers, MA), and GLUT-4 (Abcam, Cambridge, MA). Images were quantified with Image J software (NIH), and all phosphorylated targets were normalized to their respective total protein. In addition, HKII and GLUT-4 were normalized to myosin as previously described (47).
Glycerol and NEFA.
Media samples were collected after 1 and 24 h of coculture. Media levels of glycerol were measured using a free glycerol determination kit from Sigma (Sigma Aldrich), and NEFA was measured using a kit from Wako Diagnostics (Wako Diagnostics, Richmond, VA).
Glucose uptake.
Glucose uptake was measured using the fluorescent d-glucose analog 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxy-d-glucose (2-NBDG) (Invitrogen, Grand Island, NY). 2-NBDG has been successfully used as an alternative to 2-deoxyglucose in the measurement of glucose uptake in multiple cell types, including skeletal muscle (26, 42, 78, 80). In preliminary experiments, treatment with either the GLUT inhibitor cytochalasin B or the phosphatidylinositol 3-kinase inhibitor LY-294002 completely inhibited insulin-induced glucose uptake in the skeletal muscle fibers (data not shown). For measurement of 2-NBDG uptake, coculture was conducted as described above. Following the 24-h coculture period, adipocytes were removed, and fibers were placed in serum-free DMEM for a serum starvation period of 5 h. After serum starvation, fibers were rinsed three times with KRB (in mM: 135 NaCl, 10 NaHCO3, 5 KCl, 3 CaCl2, 2 MgSO4, and 1.2 NaH2PO4), and one-half of the wells underwent a 30-min pretreatment with 50 nM insulin, whereas the other one-half served as the unstimulated (basal) wells. During these 30 min, the plate was returned to the incubator. After the 30-min pretreatment, all wells were treated with 50 μM 2-NBDG for 30 min, with the insulin-treated wells receiving 50 nM insulin treatment along with the 2-NBDG. During the 2-NBDG treatment, the plate was covered in foil and underwent gentle agitation at room temperature. After 2-NBDG treatment, wells were washed with 3× Ringer buffer. After rinsing, all Ringer buffer was removed, and 100 μl of ice-cold cell buffer (40 mM KCl, 20 mM Tris-base, and 1% Nonidet P-40; pH 7.4) were added to each well and gently agitated for 10 min, at which point the cells and buffer were transferred to a black 96-well plate. The 2-NBDG fluorescence was quantified on a fluorescent plate reader (BioTek, Winooski, VT) at an excitation of 438 nm and emission of 535 nm. The 2-NBDG values were normalized to total myosin as previously described (47).
Lipid droplet staining in the skeletal muscle fibers.
Fluorescent staining of the skeletal muscle fibers was conducted according to previously published techniques (65) using BODIPY 493/503 to stain neutral lipid droplets (LD) within the muscle fibers (Invitrogen, Cambridge, MA) and 4,6-diamidino-2-phenylindole (DAPI) to label myonuclei (Invitrogen). Following the 24-h coculture period, fibers from each condition were placed on an ECM-coated glass bottom plate (MatTek, Ashland, MA). Cells were incubated with Ringer buffer containing BODIPY 493/503 (1:1,000) and DAPI (1:5,000) for 30 min. After 30 min, the fibers were rinsed three times with fresh Ringer buffer and imaged using a Zeiss AxioObserver Z1 fluorescent microscope (Carl Zeiss MicroImaging, Jena, Germany). A total of five fibers/muscle/animal/coculture condition were imaged. All fibers were z-stacked to determine fluorescent distribution of LD and nuclei throughout the fiber. As previously described (54), LDs were quantified on the surface and 6 μm within each fiber at three randomized areas using Image J software (NIH). No quantifiable differences in BODIPY 493/503 signal were detected between surface and 6-μm sections in any muscle fiber; therefore, the two were averaged for the final data analysis for all muscle fibers and groups.
Cytokine/adipokine analysis of media.
Measurement of interleukin (IL)-10, IL-6, monocyte chemotactic protein (MCP)-1, tumor necrosis factor (TNF)-α, adiponectin, and leptin in the coculture media was conducted using a multianalyte enzyme-linked immunosorbent assay system according to the manufacturer's directions (Luminex, Austin, TX). All samples were measured in duplicate on the same plate with the coefficient of variation within samples being <5%. Briefly, 200 μl of assay buffer were added to each well of a 96-well filter plate (Millipore). After 10 min of shaking, the plate was vacuumed, and 25 μl of assay buffer were added to each well, followed by 25 μl of standard/sample. Next, 25 μl of a mixture containing the appropriate cytokine (1:50 dilution) conjugated to beads was added to the wells, and the plate was placed on a shaker at 4°C overnight. The following day, the plate was vacuumed, and 200 μl of wash buffer were added to each well. The wash buffer was vacuumed off, and the washes were repeated two more times. Following the last vacuum of wash buffer, 25 μl of detection antibody were added to each well, and the plate was then placed back on the shaker for 30 min. After the 30 min, three more rounds of washing were performed, followed by the addition of 150 μl of Sheath Fluid to each well. The plate was read using a Luminex 100 plate reader and Softmax Pro Software, and then the data were calculated using Bio-Rad software (Bio-Rad). All data were normalized to the total protein detected in the sample.
Statistics.
Statistical analysis was conducted using two-way ANOVA, a one-way ANOVA, or t-tests where appropriate. When post hoc tests were appropriate, Student-Newman-Keuls was used. All analyses were completed with Sigma Stat statistical software (Systat Software, San Jose, CA). Statistical significance was accepted at an α-level of P ≤ 0.05.
RESULTS
Anatomical characteristics.
Following 8 wk of ovariectomy, the body weight of the OVX mice was significantly greater than the SHAM mice (Table 1). The mass of the omental and inguinal adipose tissue was significantly greater in the OVX mice compared with the SHAM mice (Table 1). In addition, while no differences were detected between the omental and inguinal adipose tissue masses in the SHAM mice, omental fat mass was significantly greater than inguinal fat mass in the OVX mice. The average daily food intake was significantly reduced by ∼20% in the OVX mice compared with the SHAM mice over the 8 wk (Table 1).
Table 1.
OVX mice exhibit a significantly higher body weight and fat mass despite consuming slightly less food per day
| SHAM (n =15) | OVX (n =15) | |
|---|---|---|
| Body wt, g | 22.18 ± 0.44 | 25.33 ± 0.57* |
| Omental fat mass, g | 0.237 ± 0.02 | 0.848 ± 0.06*† |
| Inguinal fat mass, g | 0.221 ± 0.01 | 0.501 ± 0.04* |
| Food intake, g·day−1 · 10 g body mass−1 | 2.395 ± 0.015 | 1.88 ± 0.006* |
Data are presented as means ± SE; n = 15 mice/group, except food intake results (n = 5/group).
SHAM, control; OVX, ovariectomized.
Significantly different from SHAM (P ≤ 0.05).
Significantly different from OVX inguinal (P ≤ 0.05).
Adipocyte size.
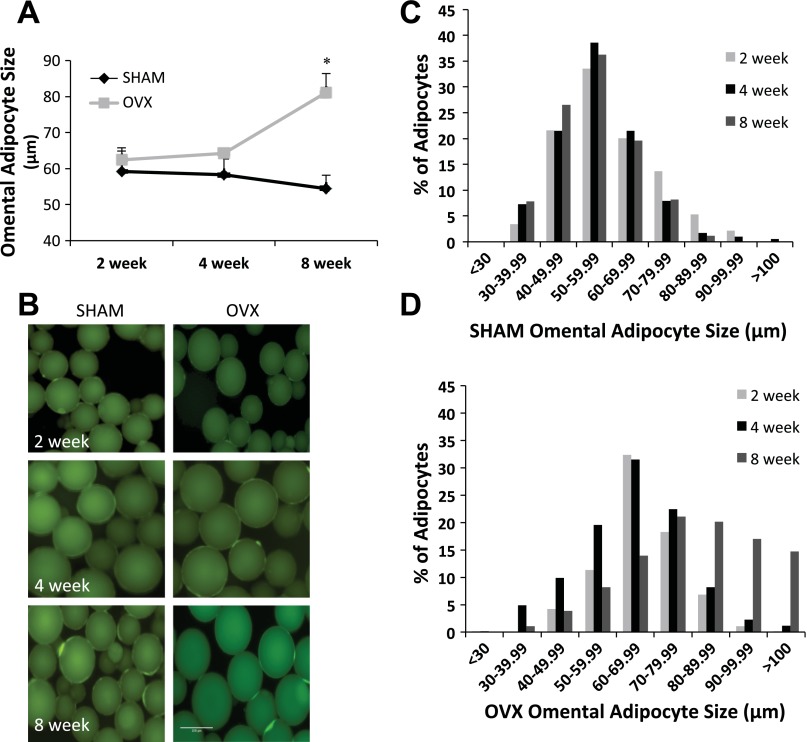
It is well recognized that OVX results in increased visceral fat mass; however, there is little documentation concerning changes in individual adipocyte size and/or cell number across fat pads after the OVX surgery. Thus, changes in adipocyte size and number were investigated in omental and inguinal adipose tissue from SHAM and OVX mice at 2, 4, and 8 wk after surgery. Average omental adipocyte size was significantly greater in OVX mice compared with SHAM mice 8 wk after OVX surgery (Fig. 1A). When adipocyte size was plotted as a frequency histogram (Fig. 1, C and D), the data revealed a rightward shift in the distribution of adipocytes from OVX animals, indicating an increasing percentage of cells with a large diameter, which was most pronounced at the 4- and 8-wk time points. In contrast, there was no difference in the omental adipocyte size distribution in the SHAM animals across all time points (Fig. 1C).
Fig. 1.
A–D: adipocytes isolated from the omental fat pad in ovariectomized (OVX) mice are significantly larger than omental adipocytes from age-matched control (SHAM) mice. A: average omental adipocyte diameter at 2, 4, and 8 wk post-OVX or SHAM surgery. B: example images of omental adipocytes isolated from SHAM and OVX mice. C: omental adipocyte size distribution for SHAM mice. D: omental adipocyte size distribution for OVX mice. Time points are defined as the weeks post OVX or SHAM surgery (light gray bars, 2 wk post; black bars, 4 wk; dark gray bars, 8 wk mice); n = 9 animals/condition. *Significantly greater than SHAM (P ≤ 0.05).
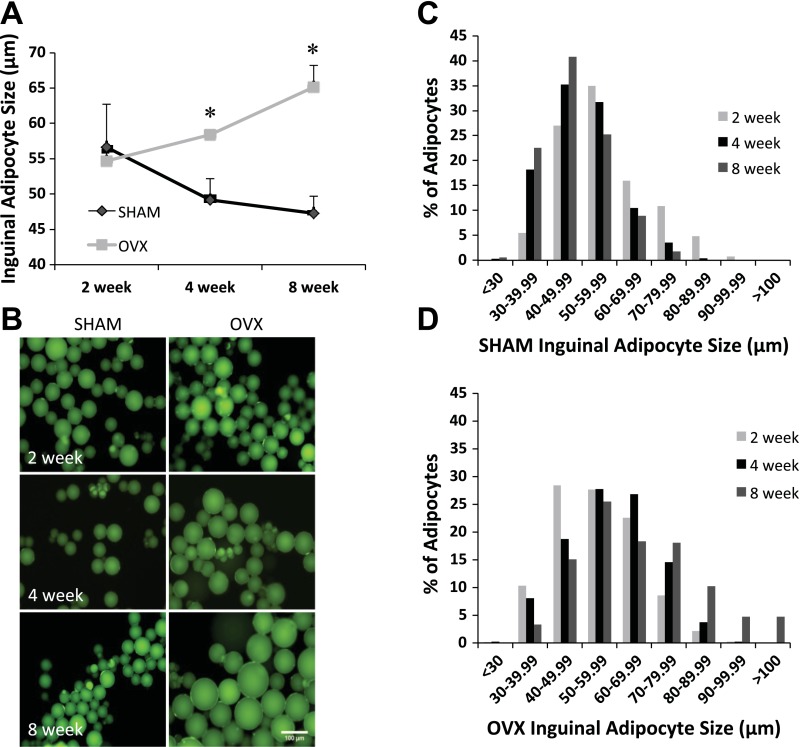
Average inguinal adipocyte size was significantly greater in the OVX animals compared with the SHAM animals at both the 4- and 8-wk time points (Fig. 2A). At the 2-wk time point, no difference in average inguinal adipocyte size was observed between SHAM and OVX animals. When adipocyte size was plotted as a frequency histogram (Fig. 2, C and D), the data revealed a rightward shift in the distribution of adipocytes from OVX animals that was most apparent at the 4- and 8-wk time points. In contrast, there was no difference in the inguinal adipocyte size distribution in the SHAM animals across all time points.
Fig. 2.
A–D: adipocytes isolated from the inguinal fat pad in OVX mice are significantly larger than inguinal adipocytes from age-matched SHAM mice. A: average inguinal adipocyte diameter at 2, 4, and 8 wk post-OVX or SHAM surgery. B: example images of inguinal adipocytes isolated from SHAM and OVX mice. C: inguinal adipocyte size distribution for SHAM mice. D: inguinal adipocyte size distribution for OVX mice. Time points are defined as the weeks post OVX or SHAM surgery (light gray bars, 2 wk post; black bars, 4 wk; dark gray bars, 8 wk mice); n = 9 animals/condition. *Significantly greater than SHAM (P ≤ 0.05).
No differences in omental or inguinal total adipocyte number were detected between SHAM and OVX animals at any time point (Fig. 3, A and B). Thus the increase in adipose tissue mass is largely due to an increase in adipocyte size rather than an increase in number of adipocytes.
Fig. 3.
A and B: no differences in total omental (A) or total inguinal (B) adipocyte number were detected between OVX and SHAM animals at any of the time points. Black lines, SHAM animals; gray lines, OVX animals; n = 9 animals/condition.
We previously had found that adipose tissue in the OVX mice exhibited loss of regulatory control over basal lipolysis, resulting in elevations of NEFA in the circulation (77). To directly test the possibility that skeletal muscle in the OVX mouse is susceptible to excess lipid storage under conditions of increased NEFA exposure, we developed a novel coculture model that employs mature adipocytes and intact single skeletal muscle fibers. Because we determined that 8 wk post-OVX surgery resulted in a detectable significant increase in adipocyte size, all coculture experiments were conducted at this time point.
Intracellular lipid content in single fibers.
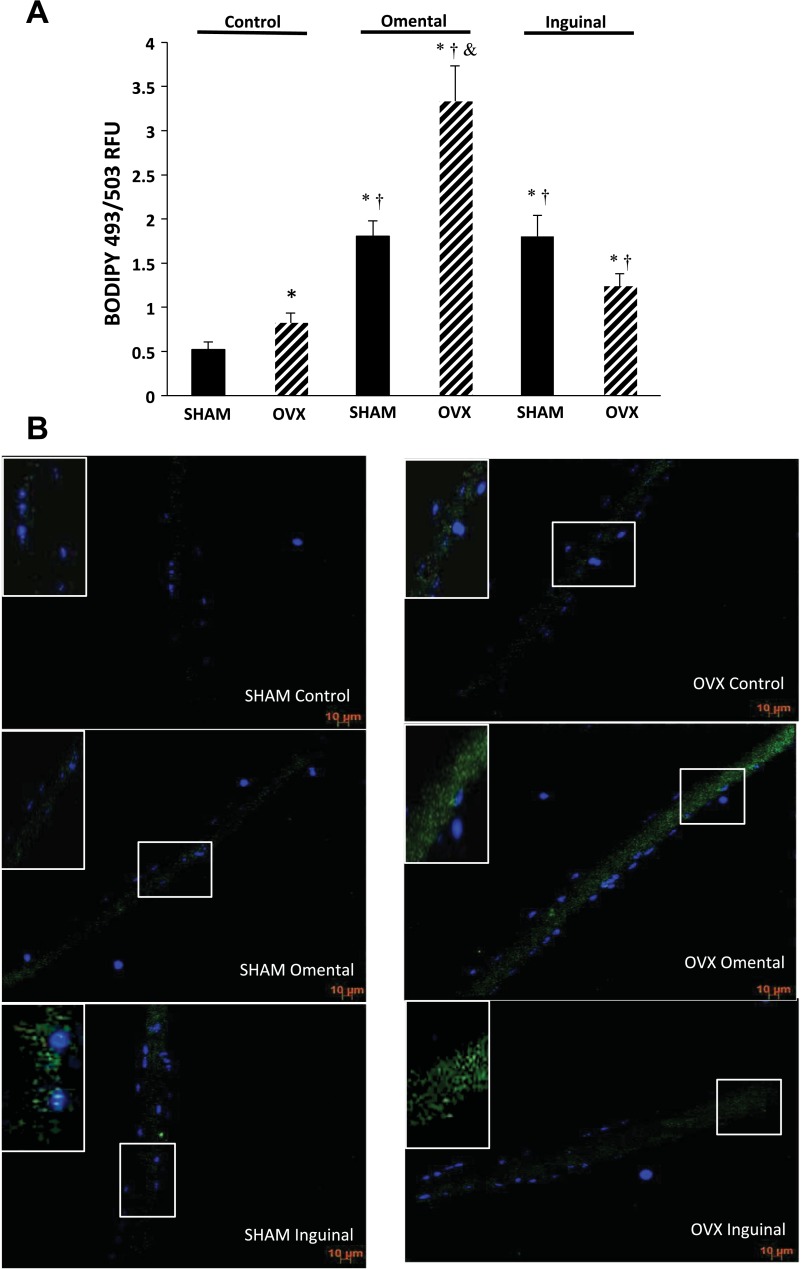
In single fibers that were not exposed to adipocytes (i.e., control condition), lipid content was significantly greater by 57% in the OVX mice compared with SHAM (Fig. 4, A and B). Coculture of SHAM skeletal muscle fibers with omental or inguinal adipocytes resulted in significantly more lipid content compared with the SHAM and OVX control condition (Fig. 4, A and B). Coculture of OVX skeletal muscle fibers with omental or inguinal adipocytes resulted in significantly greater lipid content than OVX control fibers. Furthermore, OVX omental coculture was significantly greater by 85% than the SHAM omental coculture. Interestingly, unlike SHAM coculture, OVX omental coculture resulted in significantly greater skeletal muscle fiber lipid content than inguinal coculture (Fig. 4, A and B).
Fig. 4.
A and B: intracellular lipid content was higher in single skeletal muscle fibers from OVX animals compared with SHAM. Intracellular lipid content in single muscle fibers was significantly elevated further under coculture conditions regardless of the depot in both SHAM and OVX animals. A: average relative intracellular fluorescent intensity of single muscle fibers from both SHAM and OVX animals for each condition. Data are presented as means ± SE; n = 5 animals/condition and 25 fibers/group (5 fibers/animal for each condition). *Significantly different from SHAM control (P ≤ 0.05). †Significantly different from OVX control (P ≤ 0.05). &Significantly different from SHAM omental (P ≤ 0.05). B: representative images of BODIPY 493/503 (green) and 4,6-diamidino-2-phenylindole (blue) staining in fibers from each group and condition. Images shown are maximum intensity projections obtained from z-stacks of the fibers. Insets in top left corner are zoomed-in areas taken from within the muscle fiber as marked by the white box within the fiber.
Coculture media analysis.
Glycerol and free fatty acid concentrations were measured after 1 and 24 h of coculture to assess the media that single muscle fibers were exposed to (Table 2). For omental and inguinal adipocytes isolated from both the SHAM and OVX groups, 24 h of coculture resulted in higher glycerol levels compared with media collected at the 1-h time point. After 24 h, glycerol in the OVX omental coculture was significantly greater than the OVX inguinal coculture, with a similar trend observed in the SHAM omental coculture compared with media from SHAM inguinal coculture; however, it did not reach statistical significance (P = 0.06) (Table 2). At the 1-h time point, NEFA levels were undetectable in SHAM omental and inguinal media, with levels still undetectable in the SHAM omental media after 24 h (Table 2). NEFA was detectable in coculture media of both OVX conditions at the 24-h time point but not the 1-h time point. NEFA levels were significantly different between the SHAM and OVX groups in the inguinal condition at 24 h, with no other differences detected between SHAM and OVX.
Table 2.
Glycerol and NEFA levels from coculture media after 1 and 24 h
| Glycerol, μg/ml |
NEFA, mmol/l |
|||
|---|---|---|---|---|
| 1 h | 24 h | 1 h | 24 h | |
| SHAM omental | 20.69 ± 10.36 | 72.17 ± 13.08* | UD | UD |
| SHAM inguinal | UD | 40.36 ± 15.78 | UD | 1.1E-05 ± 0.01 |
| OVX omental | 21.2 ± 14.18 | 85.41 ± 13.53* | 0.02 ± 0.01 | 0.009 ± 0.008 |
| OVX inguinal | UD | 41.08 ± 12.57† | 0.021 ± 0.01 | 0.041 ± 0.01§ |
Data are presented as means ± SE; n = 10/group. NEFA, nonesterified fatty acid; UD, undetectable. All values are presented as the difference from the respective control condition.
Significantly different from respective 1-h value.
Significantly different from respective OVX omental time point (P ≤ 0.05).
Significantly different from 24 h SHAM inguinal (P ≤ 0.05).
Insulin-induced glucose uptake.
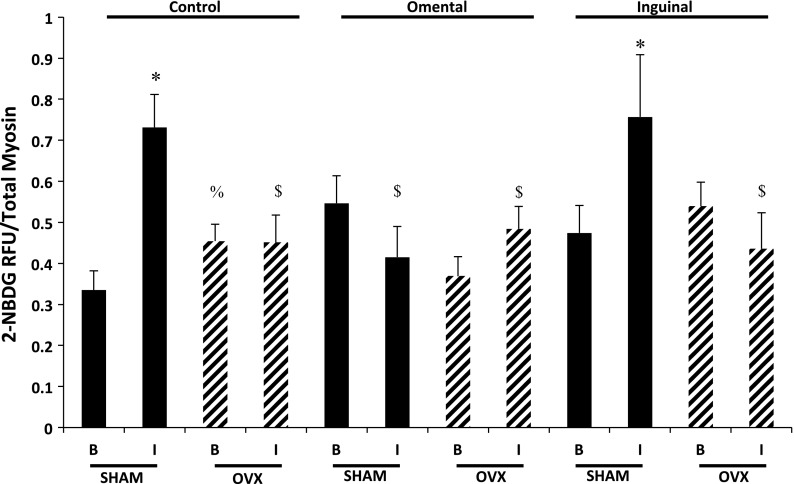
In the control fibers (no adipocyte exposure), glucose uptake in OVX fibers in the basal state (no insulin treatment) was significantly greater than that of SHAM fibers (Fig. 5). In response to insulin stimulation, SHAM control fibers displayed significantly greater glucose uptake compared with the basal state. However, in the control condition, the muscle fibers from the OVX animals did not demonstrate a significant increase in glucose uptake in response to insulin stimulation compared with the OVX basal condition. In addition, insulin stimulation of single skeletal muscle fibers from the OVX animals resulted in significantly lower glucose uptake compared with the insulin-stimulated fibers from the SHAM animals. Coculture of single skeletal muscle fibers with omental adipocytes resulted in a significantly lower insulin-induced glucose uptake response in the SHAM skeletal muscle fibers. However, in SHAM skeletal muscle fibers cocultured with inguinal adipocytes, the response to insulin was preserved. Compared with the basal values, exposure of either omental or inguinal adipocytes did not have a significant impact on basal or insulin-induced glucose uptake in OVX fibers (Fig. 5).
Fig. 5.
Insulin-induced glucose uptake in the single skeletal muscle fibers was significantly reduced in the OVX animals compared with the SHAM animals. Coculture of omental, but not inguinal, adipocytes reduced insulin responsiveness in single fibers from SHAM animals. Black bars, SHAM animals; hatched bars, OVX animals. Basal (B) and insulin-stimulated (I; 50 nM) glucose uptake; n = 5 animals/condition, and data are expressed as 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxy-d-glucose (2-NBDG)/total myosin content (means ± SE). *Significantly different from respective basal condition (P ≤ 0.05). %Significantly different from SHAM control basal condition (P ≤ 0.05). $Significantly different from SHAM control + I (P ≤ 0.05).
Insulin signaling.
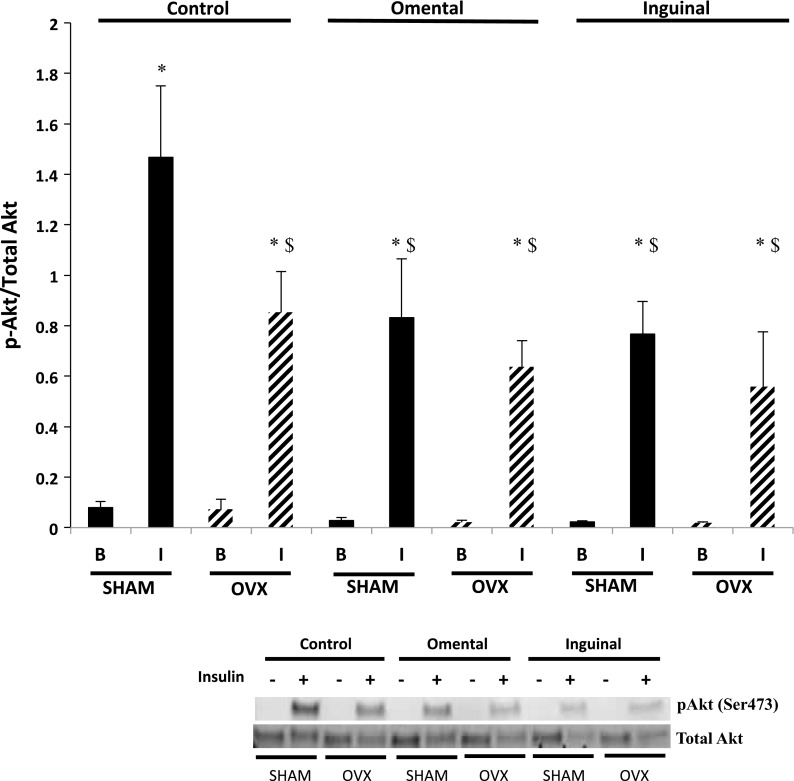
Phosphorylation of residue Ser473 Akt is a critical step in insulin-stimulated GLUT-4 translocation (21) and required for complete activation of Akt (64). Insulin exposure (50 nM; 30 min) resulted in significantly greater Akt phosphorylation (Ser473) compared with the basal condition in SHAM control fibers (Fig. 6). A significant increase in Akt phosphorylation (Ser473) was also observed in response to insulin in the OVX fibers; however, insulin-stimulated Akt phosphorylation was significantly attenuated by 58% compared with insulin-treated SHAM control fibers (Fig. 6). Insulin induced a significant increase in Akt phosphorylation in fibers from both SHAM and OVX animals cocultured with adipocytes from either the omental or inguinal depot; however, the magnitude of the response was blunted by ∼40–50% in the coculture conditions compared with insulin-treated SHAM control fibers. In OVX fibers, insulin-induced Akt phosphorylation in both omental and inguinal adipocyte coculture conditions was not different from the insulin response of the OVX control fibers.
Fig. 6.
Insulin-induced protein kinase B (Akt) phosphorylation (p) (Ser473) was significantly lower in single skeletal muscle fibers from the OVX animals compared with the SHAM animals. Coculture of omental and inguinal adipocytes resulted in lower insulin-induced Akt phosphorylation in single fibers from SHAM animals but had no further effect on fibers from OVX animals. Basal and insulin-stimulated conditions (50 nM for 30 min). Data are presented as phosphorylated Akt divided by total Akt content (means ± SE). Black bars, SHAM fibers; hatched bars, OVX fibers. Representative blots are depicted for each condition; n = 10 animals/condition. *Significantly different from respective basal condition (P ≤ 0.05). $Significantly different from SHAM control + I (P ≤ 0.05).
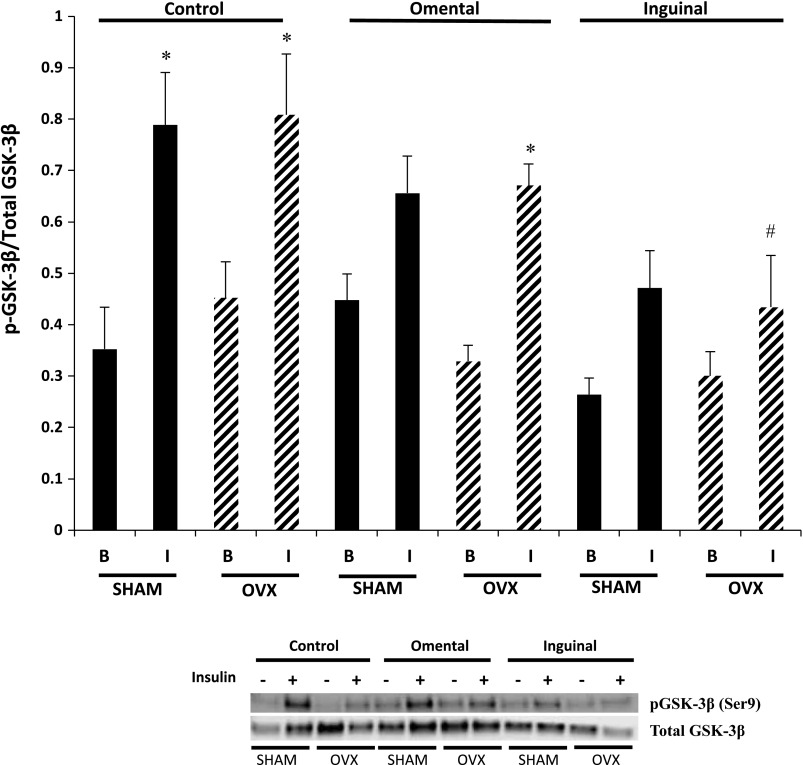
In the control condition, muscle fibers isolated from SHAM and OVX animals displayed significantly greater phosphorylation of GSK-3β (Ser9) in response to insulin compared with their corresponding basal condition (Fig. 7). Fibers from the SHAM group that were cocultured with either omental or inguinal adipocytes exhibited no difference in phosphorylation of GSK-3β in response to insulin stimulation compared with the basal condition (P = 0.07). However, in the SHAM group, the magnitude of GSK-3β phosphorylation in response to insulin appeared to be lower following inguinal coculture compared with the control condition (P = 0.07). In OVX control fibers and fibers cocultured with omental adipocytes, there was a significant increase in GSK-3β phosphorylation in response to insulin stimulation. However, inguinal adipocyte coculture completely abolished the insulin-induced GSK-3β phosphorylation in the OVX group (Fig. 7).
Fig. 7.
Insulin-induced glycogen synthase kinase (GSK)-3β phosphorylation (Ser9) was not different in single skeletal muscle fibers from the OVX animals compared with the SHAM animals. Coculture of omental and inguinal adipocytes resulted in moderate to minimal reductions in insulin-induced GSK-3β phosphorylation in single muscle fibers from SHAM and OVX animals. Basal and insulin-stimulated conditions (50 nM for 30 min). Data are presented as phosphorylated GSK-3β divided by total GSK-3β content (means ± SE). Black bars, SHAM fibers; hatched bars, OVX fibers. Representative blots are depicted for each condition; n = 10 animals/condition. *Significantly different from respective basal condition (P ≤ 0.05). #Significantly different from OVX control + I (P ≤ 0.05).
In skeletal muscle, the primary glucose transporter is GLUT-4 (79). No difference in GLUT-4 protein content was detected between SHAM and OVX control fibers, and there was no effect of adipocyte coculture on GLUT-4 protein content in either SHAM or OVX fibers (Fig. 8A). HK catalyzes the conversion of glucose to glucose 6-phosphate upon glucose entry into the cell. Multiple isoforms of HK exist, with HKII the primary isoform detected in skeletal muscle (110, 141). No difference in HKII content was detected between SHAM and OVX control skeletal muscle fibers (Fig. 8B). In fibers isolated from SHAM animals, neither omental nor inguinal adipocyte coculture affected muscle fiber HKII protein content compared with muscle fibers in the control condition. However, muscle fibers isolated from OVX animals exhibited significantly lower HKII content in both the omental and inguinal cocultured conditions compared with muscle fibers in the control condition (Fig. 8B).
Fig. 8.
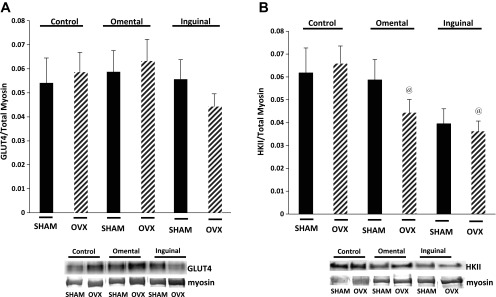
A and B: no difference in GLUT-4 (A) or hexokinase (HK) II (B) content was detected between SHAM and OVX control muscle fibers; however, coculture resulted in significantly lower HKII protein content in the single muscle fibers from the OVX group. Data are presented as GLUT-4 or HKII divided by total myosin content (means ± SE). Black bars, SHAM fibers; hatched bars, OVX fibers. Representative blots are depicted for each condition. All presented images are derived from the same immunoblot; the lanes are noncontiguous to allow for continuity with the figure above; n = 10 animals/condition. @Significantly different from respective OVX control condition (P ≤ 0.05).
Adipokine concentrations.
To assess the release of paracrine factors from the adipocytes in the coculture system, IL-6, MCP-1, interferon-γ-induced protein-10, TNF-α, adiponectin, and leptin levels were assessed in the media (Table 3). These factors were selected because of their reported roles as adipokines acting to facilitate or inhibit insulin signaling. Only adiponectin and IL-6 were readily detectable above baseline in the coculture media, with no differences in concentrations found between SHAM and OVX coculture conditions with either inguinal or omental adipocytes (Table 3).
Table 3.
Cytokine concentrations in media following 24 h of coculture
| SHAM Omental | SHAM Inguinal | OVX Omental | OVX Inguinal | |
|---|---|---|---|---|
| IL-6 | 17.13 ± 4.2 | 15.4 ± 3.7 | 19.72 ± 6.8 | 12.64 ± 4.5 |
| Adipo | 63.12 ± 3.0 | 52.82 ± 9.3 | 60.51 ± 8.7 | 55.32 ± 9.34 |
Data are presented as means ± SE; n = 5/target. Adipo, adiponectin. Units are pg/mg protein.
DISCUSSION
Here we demonstrate that, compared with SHAM animals, OVX animals exhibit greater intracellular lipid content, impaired insulin-induced glucose uptake, and lower insulin-induced phosphorylation of Akt in isolated single adult skeletal muscle fibers. The use of the coculture model with primary adipocytes did not further exacerbate the decrement in insulin-induced glucose uptake in the OVX group. However, omental and inguinal adipocytes from SHAM animals cocultured with single skeletal muscle fibers from SHAM animals resulted in greater skeletal muscle fiber lipid content, but only the omental coculture exhibited impaired insulin-induced glucose uptake. Both omental and inguinal adipocyte coculture resulted in lower insulin-induced phosphorylation of Akt compared with SHAM control fibers. No changes in GLUT-4 protein were detected across groups; however, both omental and inguinal coculture in the OVX group resulted in lower total HKII protein content compared with OVX control fibers. These data suggest that disruptions to the estrogen-signaling axis (i.e., ovary removal) result in increased skeletal muscle intracellular lipid accumulation and reduced insulin action in skeletal muscle that was not exacerbated by further lipid accumulation. In addition, the data demonstrate the use of a novel coculture approach for studying paracrine-signaling factors that enable intercellular communication between adipocytes and skeletal muscle.
A disruption in ovarian function, regardless of insult, results in concurrent alterations in body composition and body fat distribution that place women at an increased risk for the development of obesity-related conditions. Using the well-described OVX model to disrupt ovary function, we observed a higher body weight compared with SHAM mice that was largely mediated by greater adipose tissue mass (i.e., omental and inguinal). These data are in agreement with previous reports from our laboratory and that of others (10, 57, 77). This accumulation of omental adipose tissue is also observed in human females experiencing alterations in the estrogen-signaling axis (6, 29), which is of particular concern given the relationship between omental adiposity and the onset of the metabolic syndrome, insulin resistance, and cardiovascular disease (5). Interestingly, the increase in adipose tissue mass occurs in the OVX group even though food consumption is significantly lower compared with the SHAM group. The reduced energy consumption is in agreement with other groups using the OVX mouse (72). This suggests the adiposity is likely the result of reduced energy expenditure, which is evident in the OVX mouse model in the form of reduced cage activity and low voluntary running distances (32, 57).
In OVX mice, we found that the expansion of both omental and inguinal adipose tissue is mediated by adipocyte hypertrophy without changes in adipocyte number. Others have reported similar adipocyte hypertrophy in the OVX model; however, these studies only investigated a single time point after ovary removal, and focused on different fat depots (10, 57). Because omental (i.e., visceral) fat mass is a significant contributor to the relationship between adiposity and metabolic disease in postmenopausal women (38), investigation of this depot in the OVX model is important. Thus, our findings provide novel insight into the impact of lost ovarian function on the time course of adipocyte hypertrophy across multiple fat depots. As a point of reference, while adipocytes from OVX animals in our study were significantly larger than SHAM adipocytes, the adipocytes on average were smaller than values found in the ob/ob mouse model or mice on a high-fat (60% fat) diet. Libinaki et al. report average epididymal adipocyte diameter of 138 μm in mice following 32 wk of high-fat feeding and an average diameter of 127 μm in ob/ob mice (45).
Others have previously proposed using various obesity models that adipocyte hypertrophy results in fatty acid overflow and the development of lipotoxicity in insulin-sensitive tissues (71). Our previous results demonstrated a loss of lipolytic control in the adipocytes of OVX animals (77), which is likely the result of the significant adipocyte hypertrophy. We also found that the OVX model exhibits enhanced intracellular lipid accumulation in skeletal muscle (31), which suggests that changes in adipocyte function may be responsible for increasing lipid deposition in skeletal muscle. To address the concept of adipocyte overflow in the OVX model, we developed a coculture system whereby primary adult adipocytes and primary adult single skeletal muscle fibers were placed in the same culture well, separated physically by a permeable membrane. In agreement with our previous work and that of others, we found evidence for increased fatty acid levels in the culture media from the adipocytes isolated from the OVX mice compared with the SHAM animals (10, 77). Our results would suggest a form of incomplete hydrolysis of the TAG occurred due to differences in the NEFA between SHAM and OVX groups that are not matched in glycerol content. These results are in agreement with our previous findings of increased ATGL function in adipose tissue of the OVX group. Our data also suggest fatty acid content in the culture media differed depending on which fat pad the adipocytes were derived from before the coculture. In addition, inguinal adipocytes from OVX animals exhibited higher NEFA media content compared with inguinal adipocytes from SHAM, which concurs with previous results from another model of obesity (28). However, it should be noted that these results should not be interpreted as measures of lipolytic rate due to the potential for fatty acids to be either reesterified by adipocytes or to be sequestered by the skeletal muscle fibers.
Under control conditions, the OVX mice exhibit a significantly higher intracellular lipid content in single muscle fibers compared with muscle fibers from SHAM mice. The higher intracellular lipid content of muscle fibers from the OVX mice in the control condition compared with muscle fibers from the SHAM mice is in line with observations from our laboratory and has recently been reported by another group (31, 34). Exposure of skeletal muscle fibers from the SHAM mice to omental and inguinal coculture resulted in higher intracellular lipid content compared with control fibers from SHAM and OVX mice. Interestingly, the single muscle fibers from the OVX mice that were exposed to omental coculture exhibited lipid content that was significantly higher than all other conditions. These data suggest that muscle fibers from OVX mice are either more likely to incorporate fatty acid species released from the omental region or that omental adipocytes are more lipolytically active than inguinal adipocytes when cultured in vitro. With respect to the former, this would imply that there are differences in the species of fatty acids released from the omental region, which is possible, since previous studies have reported depot-specific differences in fatty acid composition (19, 20). However, it is more likely that the latter possibility would explain the difference since previous research has shown that omental adipocytes are more lipolytically active than inguinal adipocytes (48). Collectively, these findings demonstrate that skeletal muscle fibers from OVX mice have greater intracellular lipid content compared with SHAM mice and that muscle fibers from OVX mice are more susceptible to lipid overload than muscle fibers from SHAM mice. Finally, the data also demonstrate the value of this coculture model for studying skeletal muscle and adipocyte cellular interactions outside of the OVX model. However, it should be noted that this coculture approach does not account for immune cells within the adipose tissue of the OVX model, which previous work has shown to be an important regulator of metabolic function as well (72).
Excessive intracellular lipid accumulation in skeletal muscle in sedentary individuals has been shown to impair insulin function and potentially contribute to insulin resistance. However, it is unclear if a similar relationship exists under conditions where estrogen levels are reduced. Our data indicate that, in the control conditions, the skeletal muscle fibers from SHAM mice exhibited low lipid content and a significant insulin-induced glucose uptake response. However, the insulin response was significantly impaired in control fibers from OVX animals that had significantly higher lipid content. Our finding of lower insulin-induced glucose uptake in skeletal muscle fibers from OVX mice is in line with other studies reporting reduced glucose uptake in whole skeletal muscle of OVX animals compared with SHAM (22, 53, 58). In SHAM skeletal muscle fibers, the omental adipocyte coculture blunted the insulin-induced glucose uptake response, which was associated with significantly higher lipid content. In contrast, SHAM skeletal muscle fibers exposed to inguinal coculture did not demonstrate any deficiency in insulin-induced glucose uptake even though the coculture resulted in increased intracellular lipid content. In the mouse, the inguinal fat pad is subcutaneous fat, and previous work has suggested that increases in subcutaneous fat mass do not correlate with the development of insulin resistance (1). Thus, it possible that there are specific effects of adipocyte exposure on the muscle cell that are dependent upon the depot origin of the adipocytes. These data clearly suggest that increases in lipid content within a muscle fiber do not always correlate with reduced insulin responsiveness in skeletal muscle. The theory of lipotoxicity proposes accumulation of lipid may concurrently result in the accumulation of lipid products such as diacylglycerol and ceramide (30, 44), which can impair insulin function in skeletal muscle (7, 30). However, recent data have questioned this theory (40), and our data would suggest that the relationship is indeed more complicated than lipid accumulation alone, and more factors need to be considered to accurately interpret this relationship. Increasing the lipid exposure of the fibers from the OVX animals did not exacerbate the insulin response, in that the coculture approach resulted in substantially more lipid content without a further decrement in the insulin response. These data may suggest that, in the OVX condition, there is a threshold point for lipid accumulation that is associated with impaired insulin function in skeletal muscle.
Based on the alterations in glucose uptake observed in SHAM and OVX fibers, we investigated the phosphorylation status of key regulators of insulin-induced glucose uptake in skeletal muscle. In control OVX fibers, the magnitude of Akt phosphorylation in response to insulin was reduced compared with SHAM fibers, corresponding with our observations for insulin-induced glucose uptake. These results are similar to others reporting reductions in insulin-stimulated Akt phosphorylation and glucose uptake in OVX skeletal muscle (53). In SHAM fibers, insulin-induced Akt phosphorylation was lower following omental coculture compared with SHAM control fibers, corresponding to lower insulin-induced glucose uptake values. Interestingly, insulin-induced phosphorylation of Akt was lower in the SHAM inguinal coculture compared with SHAM control despite normal insulin-induced glucose uptake. Thus, it is possible that inguinal adipocyte exposure results in activation of an undefined alternative insulin-sensitive pathway in SHAM skeletal muscle fibers, or the exposure of fibers to insulin-sensitizing adipokines plays a role. Unfortunately, at this point, we have yet to identify a signaling protein that could explain this response, and we found no differences in insulin-sensitizing adipokine (i.e., adiponectin) concentrations across conditions.
Another insulin-sensitive signaling protein, GSK-3β, plays an important role in the cellular fate of glucose. In response to insulin stimulation, GSK-3β is phosphorylated by Akt at Ser9, thus removing cellular inhibition of glycogen synthase and facilitating glycogen storage (68). In our study, we found minimal differences in basal and insulin-stimulated GSK-3β phosphorylation between SHAM and OVX groups in the control and omental coculture conditions. In contrast, the inguinal coculture condition, for reasons we have yet to define, OVX fibers displayed lower insulin-induced GSK-3β phosphorylation than control fibers, which agrees with findings of reduced GSK-3β phosphorylation in skeletal muscle when exposed to excess circulating lipid (17). GSK-3β is often thought of as a substrate for Akt, thus it is a bit surprising that we did not see greater reductions in the phosphorylation content of GSK-3β. However, using an adipocyte-conditioned media approach, others have found significant decreases in Akt phosphorylation that did not translate into large decreases in GSK-3β phosphorylation (61). The disconnect between the phosphorylation status of Akt and GSK-3β is not uncommon in the literature, with another publication finding significant reductions in Akt phosphorylation and no to minor effects on GSK-3β phosphorylation (62). Because glucose may either be stored or metabolized upon uptake, it is possible that coculture impacted the fate of glucose in a depot-specific fashion; however, further investigation is necessary to determine the impact of coculture on the cellular metabolism of glucose in skeletal muscle fibers.
Glucose uptake in skeletal muscle is ultimately mediated by induction of GLUT-4 translocation, which facilitates the uptake of circulating glucose (74). Here we have found no effect of either OVX or of adipocyte coculture on total GLUT-4 protein content. Some studies have reported reduced GLUT-4 protein content in OVX skeletal muscle (58), whereas others have found no effect of OVX (25, 50). Reduced skeletal muscle GLUT-4 translocation in rats following ovary removal has been reported by Rincon et al. (55); thus, it is possible that impaired GLUT-4 translocation, rather than total protein content, may explain alterations in glucose uptake in OVX fibers and in SHAM fibers following omental adipocyte coculture. Thus, a critical future direction for this research will be assessing the regulation of GLUT-4 translocation in the OVX animals.
HKII also plays a critical role in skeletal muscle glucose uptake, phosphorylating glucose to glucose 6-phosphate upon entry in the cell and thus enhancing glucose uptake. Increased fatty acid exposure can impair glucose flux through glycolysis by reducing HK activity, suggesting a sensitivity to lipid-based insults (70). We found significant reductions in total HKII protein content in OVX fibers following coculture with both omental and inguinal adipocytes, but no effect of OVX alone, or of adipocyte coculture on SHAM fibers. This finding is likely important since heterozygous ablation of HKII in mice suppresses whole body insulin action and insulin-stimulated skeletal muscle glucose uptake (18). Additionally, in both obese and type 2 diabetic states, skeletal muscle expression of HKII is reduced in humans (9). In another coculture model, reduced HKII mRNA expression in primary myotubes following adipocyte coculture has also been reported (39). Interestingly, we only found significantly lower HKII protein content in the OVX coculture condition, suggesting an increased susceptibility of the OVX fibers to lipid insult compared with skeletal muscle fibers from the SHAM animals. This difference in the HKII response to coculture could be dictated by differential fatty acid species (e.g., high levels of palmitic acid) released from the OVX adipocytes or increased skeletal muscle susceptibility to lipid exposure in the OVX group. It should be pointed out that the loss of HKII did not translate into a further decrease in insulin-induced glucose uptake; however, it is unclear what the implications for a lipid-induced decrease in HKII function would mean over a longer duration of time, since our culture duration was short in duration.
Many of our findings suggest that the in vitro effects of adipocyte exposure on skeletal muscle are dependent upon the in vivo environment from which adipocytes were derived, which is in agreement with results from others using a similar coculture approach (39). Taken together, OVX results in skeletal muscle lipid deposition and impaired insulin responsiveness, suggesting a role for lipotoxicity in mediating the onset of insulin resistance when the estrogen-signaling axis is disrupted due to surgical ovary removal. These data have important implications for women, independent of age, electing to undergo oophorectomy or who experience loss of estrogen function resulting in a significant increase in the risk of metabolic disease. Based on studies utilizing aromatase knockout and estrogen knockout mice, as well as in vivo stimulation of the estrogen receptor, there is evidence that reduced estrogens may be the link between ovarian dysfunction and skeletal muscle insulin resistance (23, 27, 35, 69). However, because OVX results in multiple hormonal changes (i.e., follicle-stimulating hormone, inhibin, androstenedione, etc.), we cannot rule out the contribution of other circulating factors to impaired insulin responsiveness in our model. Future studies that we are currently conducting using this approach are expected to elucidate some of these answers by matching adipocytes from OVX animals to single skeletal muscle fibers from SHAM animals and vice versa. Further elucidation of the interaction between ovarian hormones, adiposity, and the skeletal muscle response to insulin is crucial considering the increased prevalence of insulin resistance and type 2 diabetes and the absence of a widely accepted treatment for females with reduced ovarian function.
GRANTS
This study was funded by a Pilot and Feasibility grant from the Baltimore Diabetes Research Training Center (DRTC-P60DK079637) (E. E Spangenburg); L. M. Wohlers was funded by National Institute on Aging Grant AG-000268 and the University of Maryland Kinesiology Graduate Research Initiative Fund.
DISCLOSURES
The authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
Author contributions: L.M.W., E.R.C., and E.E.S. conception and design of research; L.M.W. and B.L.P. performed experiments; L.M.W., B.L.P., and E.E.S. analyzed data; L.M.W. and E.E.S. interpreted results of experiments; L.M.W. and E.E.S. prepared figures; L.M.W. drafted manuscript; L.M.W., E.R.C., and E.E.S. edited and revised manuscript; L.M.W., E.R.C., and E.E.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Carole Stzalryd and Urmila Sreenivasan for helpful advice in the adipocyte isolation process and Ana Valencia, Kathryn Jackson, and Samantha Goldklang for technical assistance. Furthermore, the authors thank the University of Maryland Cytokine Core Laboratory for adipokine measurement.
REFERENCES
- 1. Amati F, Pennant M, Azuma K, Dube JJ, Toledo FG, Rossi AP, Kelley DE, Goodpaster BH. Lower thigh subcutaneous and higher visceral abdominal adipose tissue content both contribute to insulin resistance. Obesity (Silver Spring) 20: 1115–1117, 2012 [DOI] [PubMed] [Google Scholar]
- 2. Beylkin DH, Allen DL, Leinwand LA. MyoD, Myf5, and the calcineurin pathway activate the developmental myosin heavy chain genes. Dev Biol 294: 541–553, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Bourgeois F, Alexiu A, Lemonnier D. Dietary-induced obesity: effect of dietary fats on adipose tissue cellularity in mice. Br J Nutr 49: 17–26, 1983 [DOI] [PubMed] [Google Scholar]
- 4. Brown M. Skeletal muscle and bone: effect of sex steroids and aging. Adv Physiol Educ 32: 120–126, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Canoy D, Boekholdt SM, Wareham N, Luben R, Welch A, Bingham S, Buchan I, Day N, Khaw KT. Body fat distribution and risk of coronary heart disease in men and women in the European Prospective Investigation Into Cancer and Nutrition in Norfolk cohort: a population-based prospective study. Circulation 116: 2933–2943, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab 88: 2404–2411, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Chavez JA, Knotts TA, Wang LP, Li G, Dobrowsky RT, Florant GL, Summers SA. A role for ceramide, but not diacylglycerol, in the antagonism of insulin signal transduction by saturated fatty acids. J Biol Chem 278: 10297–10303, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Combs TP, Pajvani UB, Berg AH, Lin Y, Jelicks LA, Laplante M, Nawrocki AR, Rajala MW, Parlow AF, Cheeseboro L, Ding YY, Russell RG, Lindemann D, Hartley A, Baker GR, Obici S, Deshaies Y, Ludgate M, Rossetti L, Scherer PE. A transgenic mouse with a deletion in the collagenous domain of adiponectin displays elevated circulating adiponectin and improved insulin sensitivity. Endocrinology 145: 367–383, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Cusi KJ, Pratipanawatr T, Koval J, Printz R, Ardehali H, Granner DK, Defronzo RA, Mandarino LJ. Exercise increases hexokinase II mRNA, but not activity in obesity and type 2 diabetes. Metabolism 50: 602–606, 2001 [DOI] [PubMed] [Google Scholar]
- 10. D'Eon TM, Souza SC, Aronovitz M, Obin MS, Fried SK, Greenberg AS. Estrogen regulation of adiposity and fuel partitioning. Evidence of genomic and non-genomic regulation of lipogenic and oxidative pathways. J Biol Chem 280: 35983–35991, 2005 [DOI] [PubMed] [Google Scholar]
- 11. DeFronzo RA, Gunnarsson R, Bjorkman O, Olsson M, Wahren J. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J Clin Invest 76: 149–155, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 32, Suppl 2: S157–S163, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Despres JP, Nadeau A, Tremblay A, Ferland M, Moorjani S, Lupien PJ, Theriault G, Pinault S, Bouchard C. Role of deep abdominal fat in the association between regional adipose tissue distribution and glucose tolerance in obese women. Diabetes 38: 304–309, 1989 [DOI] [PubMed] [Google Scholar]
- 14. Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet 365: 1415–1428, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Emery EM, Schmid TL, Kahn HS, Filozof PP. A review of the association between abdominal fat distribution, health outcome measures, and modifiable risk factors. Am J Health Promot 7: 342–353, 1993 [DOI] [PubMed] [Google Scholar]
- 16. Franck N, Stenkula KG, Ost A, Lindstrom T, Stralfors P, Nystrom FH. Insulin-induced GLUT4 translocation to the plasma membrane is blunted in large compared with small primary fat cells isolated from the same individual. Diabetologia 50: 1716–1722, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Frangioudakis G, Cooney GJ. Acute elevation of circulating fatty acids impairs downstream insulin signalling in rat skeletal muscle in vivo independent of effects on stress signalling. J Endocrinol 197: 277–285, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Fueger PT, Lee-Young RS, Shearer J, Bracy DP, Heikkinen S, Laakso M, Rottman JN, Wasserman DH. Phosphorylation barriers to skeletal and cardiac muscle glucose uptakes in high-fat fed mice: studies in mice with a 50% reduction of hexokinase II. Diabetes 56: 2476–2484, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Garaulet M, Hernandez-Morante JJ, Lujan J, Tebar FJ, Zamora S. Relationship between fat cell size and number and fatty acid composition in adipose tissue from different fat depots in overweight/obese humans. Int J Obes (Lond) 30: 899–905, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Garaulet M, Perez-Llamas F, Perez-Ayala M, Martinez P, de Medina FS, Tebar FJ, Zamora S. Site-specific differences in the fatty acid composition of abdominal adipose tissue in an obese population from a Mediterranean area: relation with dietary fatty acids, plasma lipid profile, serum insulin, and central obesity. Am J Clin Nutr 74: 585–591, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Gonzalez E, McGraw TE. Insulin signaling diverges into Akt-dependent and -independent signals to regulate the recruitment/docking and the fusion of GLUT4 vesicles to the plasma membrane. Mol Biol Cell 17: 4484–4493, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gorres BK, Bomhoff GL, Gupte AA, Geiger PC. Altered estrogen receptor expression in skeletal muscle and adipose tissue of female rats fed a high-fat diet. J Appl Physiol 110: 1046–1053, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gorres BK, Bomhoff GL, Morris JK, Geiger PC. In vivo stimulation of oestrogen receptor alpha increases insulin-stimulated skeletal muscle glucose uptake. J Physiol 589: 2041–2054, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J 29: 2959–2971, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Hansen PA, McCarthy TJ, Pasia EN, Spina RJ, Gulve EA. Effects of ovariectomy and exercise training on muscle GLUT-4 content and glucose metabolism in rats. J Appl Physiol 80: 1605–1611, 1996 [DOI] [PubMed] [Google Scholar]
- 26. Hassanein M, Weidow B, Koehler E, Bakane N, Garbett S, Shyr Y, Quaranta V. Development of high-throughput quantitative assays for glucose uptake in cancer cell lines. Mol Imaging Biol 13: 840–852, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci USA 97: 12729–12734, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hoffstedt J, Arner P, Hellers G, Lonnqvist F. Variation in adrenergic regulation of lipolysis between omental and subcutaneous adipocytes from obese and non-obese men. J Lipid Res 38: 795–804, 1997 [PubMed] [Google Scholar]
- 29. Howard BV, Kuller L, Langer R, Manson JE, Allen C, Assaf A, Cochrane BB, Larson JC, Lasser N, Rainford M, Van Horn L, Stefanick ML, Trevisan M. Risk of cardiovascular disease by hysterectomy status, with and without oophorectomy: the Women's Health Initiative Observational Study. Circulation 111: 1462–1470, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes 51: 2005–2011, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Jackson KC, Wohlers LM, Lovering RM, Schuh RA, Maher AC, Bonen A, Koves TR, Ilkayeva O, Thomson DM, Muoio DM, Spangenburg EE. Ectopic lipid deposition and the metabolic profile of skeletal muscle in ovariectomized mice. Am J Physiol Regul Integr Comp Physiol 304: R206–R217, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jackson KC, Wohlers LM, Valencia AP, Cilenti M, Borengasser SJ, Thyfault JP, Spangenburg EE. Wheel running prevents the accumulation of monounsaturated fatty acids in the liver of ovariectomized mice by attenuating changes in SCD-1 content. Appl Physiol Nutr Metab 36: 798–810, 2011 [DOI] [PubMed] [Google Scholar]
- 33. Jensen MD. Is visceral fat involved in the pathogenesis of the metabolic syndrome? Human model Obesity (Silver Spring) 14, Suppl 1: 20S–24S, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Jeong S, Yoon M. Swimming's prevention of ovariectomy-induced obesity through activation of skeletal-muscle PPARalpha. Int J Sport Nutr Exerc Metab 22: 1–10, 2012 [DOI] [PubMed] [Google Scholar]
- 35. Jones ME, Thorburn AW, Britt KL, Hewitt KN, Misso ML, Wreford NG, Proietto J, Oz OK, Leury BJ, Robertson KM, Yao S, Simpson ER. Aromatase-deficient (ArKO) mice accumulate excess adipose tissue. J Steroid Biochem Mol Biol 79: 3–9, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Keck M, Romero-Aleshire MJ, Cai Q, Hoyer PB, Brooks HL. Hormonal status affects the progression of STZ-induced diabetes and diabetic renal damage in the VCD mouse model of menopause. Am J Physiol Renal Physiol 293: F193–F199, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Koonen DP, Sung MM, Kao CK, Dolinsky VW, Koves TR, Ilkayeva O, Jacobs RL, Vance DE, Light PE, Muoio DM, Febbraio M, Dyck JR. Alterations in skeletal muscle fatty acid handling predisposes middle-aged mice to diet-induced insulin resistance. Diabetes 59: 1366–1375, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Koster A, Stenholm S, Alley DE, Kim LJ, Simonsick EM, Kanaya AM, Visser M, Houston DK, Nicklas BJ, Tylavsky FA, Satterfield S, Goodpaster BH, Ferrucci L, Harris TB. Body fat distribution and inflammation among obese older adults with and without metabolic syndrome. Obesity (Silver Spring) 18: 2354–2361, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kovalik JP, Slentz D, Stevens RD, Kraus WE, Houmard JA, Nicoll JB, Lea-Currie YR, Everingham K, Kien CL, Buehrer BM, Muoio DM. Metabolic remodeling of human skeletal myocytes by cocultured adipocytes depends on the lipolytic state of the system. Diabetes 60: 1882–1893, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Lopaschuk GD, Muoio DM. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 7: 45–56, 2008 [DOI] [PubMed] [Google Scholar]
- 41. Lanner JT, Bruton JD, Assefaw-Redda Y, Andronache Z, Zhang SJ, Severa D, Zhang ZB, Melzer W, Zhang SL, Katz A, Westerblad H. Knockdown of TRPC3 with siRNA coupled to carbon nanotubes results in decreased insulin-mediated glucose uptake in adult skeletal muscle cells. FASEB J 23: 1728–1738, 2009 [DOI] [PubMed] [Google Scholar]
- 42. Lanner JT, Katz A, Tavi P, Sandstrom ME, Zhang SJ, Wretman C, James S, Fauconnier J, Lannergren J, Bruton JD, Westerblad H. The role of Ca2+ influx for insulin-mediated glucose uptake in skeletal muscle. Diabetes 55: 2077–2083, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Lauritzen HP, Ploug T, Prats C, Tavare JM, Galbo H. Imaging of insulin signaling in skeletal muscle of living mice shows major role of T-tubules. Diabetes 55: 1300–1306, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Lee JS, Pinnamaneni SK, Eo SJ, Cho IH, Pyo JH, Kim CK, Sinclair AJ, Febbraio MA, Watt MJ. Saturated, but not n-6 polyunsaturated, fatty acids induce insulin resistance: role of intramuscular accumulation of lipid metabolites. J Appl Physiol 100: 1467–1474, 2006 [DOI] [PubMed] [Google Scholar]
- 45. Libinaki R, Heffernan M, Jiang WJ, Ogru E, Ignjatovic V, Gianello R, Trickey L, Taylor M, Ng F. Effects of genetic and diet-induced obesity on lipid metabolism. IUBMB Life 48: 109–113, 1999 [DOI] [PubMed] [Google Scholar]
- 46. Lovering RM, Michaelson L, Ward CW. Malformed mdx myofibers have normal cytoskeletal architecture yet altered EC coupling and stress-induced Ca2+ signaling. Am J Physiol Cell Physiol 297: C571–C580, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mackrell JG, Cartee GD. A novel method to measure glucose uptake and Myosin heavy chain isoform expression of single fibers from rat skeletal muscle. Diabetes 61: 995–1003, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Marin P, Andersson B, Ottosson M, Olbe L, Chowdhury B, Kvist H, Holm G, Sjostrom L, Bjorntorp P. The morphology and metabolism of intraabdominal adipose tissue in men. Metabolism 41: 1242–1248, 1992 [DOI] [PubMed] [Google Scholar]
- 49. O'Connell J, Lynch L, Cawood TJ, Kwasnik A, Nolan N, Geoghegan J, McCormick A, O'Farrelly C, O'Shea D. The relationship of omental and subcutaneous adipocyte size to metabolic disease in severe obesity. PLoS One 5: e9997, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Park SM, Park CH, Wha JD, Choi SB. A high carbohydrate diet induces insulin resistance through decreased glucose utilization in ovariectomized rats. Korean J Intern Med 19: 87–92, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Phillips SA, Ciaraldi TP, Oh DK, Savu MK, Henry RR. Adiponectin secretion and response to pioglitazone is depot dependent in cultured human adipose tissue. Am J Physiol Endocrinol Metab 295: E842–E850, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pouliot MC, Despres JP, Nadeau A, Moorjani S, Prud'Homme D, Lupien PJ, Tremblay A, Bouchard C. Visceral obesity in men. Associations with glucose tolerance, plasma insulin, and lipoprotein levels. Diabetes 41: 826–834, 1992 [DOI] [PubMed] [Google Scholar]
- 53. Prasannarong M, Vichaiwong K, Saengsirisuwan V. Calorie restriction prevents the development of insulin resistance and impaired insulin signaling in skeletal muscle of ovariectomized rats. Biochim Biophys Acta 1822: 1051–1061, 2012 [DOI] [PubMed] [Google Scholar]
- 54. Prats C, Donsmark M, Qvortrup K, Londos C, Sztalryd C, Holm C, Galbo H, Ploug T. Decrease in intramuscular lipid droplets and translocation of HSL in response to muscle contraction and epinephrine. J Lipid Res 47: 2392–2399, 2006 [DOI] [PubMed] [Google Scholar]
- 55. Rincon J, Holmang A, Wahlstrom EO, Lonnroth P, Bjorntorp P, Zierath JR, Wallberg-Henriksson H. Mechanisms behind insulin resistance in rat skeletal muscle after oophorectomy and additional testosterone treatment. Diabetes 45: 615–621, 1996 [DOI] [PubMed] [Google Scholar]
- 56. Rodbell M. Metabolism of isolated fat cells. I. Effects of hormones on glucose metabolism and lipolysis. J Biol Chem 239: 375–380, 1964 [PubMed] [Google Scholar]
- 57. Rogers NH, Perfield JW, 2nd, Strissel KJ, Obin MS, Greenberg AS. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology 150: 2161–2168, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Saengsirisuwan V, Pongseeda S, Prasannarong M, Vichaiwong K, Toskulkao C. Modulation of insulin resistance in ovariectomized rats by endurance exercise training and estrogen replacement. Metabolism 58: 38–47, 2009 [DOI] [PubMed] [Google Scholar]
- 59. Sakai T, Sakaue H, Nakamura T, Okada M, Matsuki Y, Watanabe E, Hiramatsu R, Nakayama K, Nakayama KI, Kasuga M. Skp2 controls adipocyte proliferation during the development of obesity. J Biol Chem 282: 2038–2046, 2007 [DOI] [PubMed] [Google Scholar]
- 60. Schneider JG, Tompkins C, Blumenthal RS, Mora S. The metabolic syndrome in women. Cardiol Rev 14: 286–291, 2006 [DOI] [PubMed] [Google Scholar]
- 61. Sell H, Eckardt K, Taube A, Tews D, Gurgui M, Van Echten-Deckert G, Eckel J. Skeletal muscle insulin resistance induced by adipocyte-conditioned medium: underlying mechanisms and reversibility. Am J Physiol Endocrinol Metab 294: E1070–E1077, 2008 [DOI] [PubMed] [Google Scholar]
- 62. Sitnick M, Bodine SC, Rutledge JC. Chronic high fat feeding attenuates load-induced hypertrophy in mice. J Physiol 587: 5753–5765, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sitnick M, Foley AM, Brown M, Spangenburg EE. Ovariectomy prevents the recovery of atrophied gastrocnemius skeletal muscle mass. J Appl Physiol 100: 286–293, 2006 [DOI] [PubMed] [Google Scholar]
- 64. Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med 9: 59–71, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Spangenburg EE, Pratt SJ, Wohlers LM, Lovering RM. Use of BODIPY (493/503) to visualize intramuscular lipid droplets in skeletal muscle. J Biomed Biotechnol 2011: 598358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Spangenburg EE, Wohlers LM, Valencia AP. Metabolic dysfunction under reduced estrogen levels: looking to exercise for prevention. Exerc Sport Sci Rev 40: 195–203, 2012 [DOI] [PubMed] [Google Scholar]
- 67. Steinberg GR, Michell BJ, van Denderen BJ, Watt MJ, Carey AL, Fam BC, Andrikopoulos S, Proietto J, Gorgun CZ, Carling D, Hotamisligil GS, Febbraio MA, Kay TW, Kemp BE. Tumor necrosis factor alpha-induced skeletal muscle insulin resistance involves suppression of AMP-kinase signaling. Cell Metab 4: 465–474, 2006 [DOI] [PubMed] [Google Scholar]
- 68. Sutherland C, Leighton IA, Cohen P. Inactivation of glycogen synthase kinase-3 beta by phosphorylation: new kinase connections in insulin and growth-factor signalling. Biochem J 296: 15–19, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Takeda K, Toda K, Saibara T, Nakagawa M, Saika K, Onishi T, Sugiura T, Shizuta Y. Progressive development of insulin resistance phenotype in male mice with complete aromatase (CYP19) deficiency. J Endocrinol 176: 237–246, 2003 [DOI] [PubMed] [Google Scholar]
- 70. Thompson AL, Cooney GJ. Acyl-CoA inhibition of hexokinase in rat and human skeletal muscle is a potential mechanism of lipid-induced insulin resistance. Diabetes 49: 1761–1765, 2000 [DOI] [PubMed] [Google Scholar]
- 71. Unger RH. Lipid overload and overflow: metabolic trauma and the metabolic syndrome. Trends Endocrinol Metab 14: 398–403, 2003 [DOI] [PubMed] [Google Scholar]
- 72. Vieira Potter VJ, Strissel KJ, Xie C, Chang E, Bennett G, Defuria J, Obin MS, Greenberg AS. Adipose tissue inflammation and reduced insulin sensitivity in ovariectomized mice occurs in the absence of increased adiposity. Endocrinology 153: 4266–4277, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev 21: 697–738, 2000 [DOI] [PubMed] [Google Scholar]
- 74. Wasserman DH, Kang L, Ayala JE, Fueger PT, Lee-Young RS. The physiological regulation of glucose flux into muscle in vivo. J Exp Biol 214: 254–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wiest R, Moleda L, Farkas S, Scherer M, Kopp A, Wonckhaus U, Buchler C, Scholmerich J, Schaffler A. Splanchnic concentrations and postprandial release of visceral adipokines. Metabolism 59: 664–670, 2010 [DOI] [PubMed] [Google Scholar]
- 76. Witte MM, Resuehr D, Chandler AR, Mehle AK, Overton JM. Female mice and rats exhibit species-specific metabolic and behavioral responses to ovariectomy. Gen Comp Endocrinol 166: 520–528, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wohlers LM, Spangenburg EE. 17beta-estradiol supplementation attenuates ovariectomy-induced increases in ATGL signaling and reduced perilipin expression in visceral adipose tissue. J Cell Biochem 110: 420–427, 2010 [DOI] [PubMed] [Google Scholar]
- 78. Yamada K, Saito M, Matsuoka H, Inagaki N. A real-time method of imaging glucose uptake in single, living mammalian cells. Nat Protoc 2: 753–762, 2007 [DOI] [PubMed] [Google Scholar]
- 79. Zisman A, Peroni OD, Abel ED, Michael MD, Mauvais-Jarvis F, Lowell BB, Wojtaszewski JF, Hirshman MF, Virkamaki A, Goodyear LJ, Kahn CR, Kahn BB. Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance. Nat Med 6: 924–928, 2000 [DOI] [PubMed] [Google Scholar]
- 80. Zou C, Wang Y, Shen Z. 2-NBDG as a fluorescent indicator for direct glucose uptake measurement. J Biochem Biophys Methods 64: 207–215, 2005 [DOI] [PubMed] [Google Scholar]