Abstract
Kisspeptin signaling via its cognate receptor G protein-coupled receptor 54 (GPR54) in gonadotropin-releasing hormone (GnRH) neurons plays a critical role in regulating pituitary secretion of luteinizing hormone and thus reproductive function. GPR54 is Gq-coupled to activation of phospholipase C and multiple second messenger signaling pathways. Previous studies have shown that kisspeptin potently depolarizes GnRH neurons through the activation of canonical transient receptor potential channels and inhibition of inwardly rectifying K+ channels to generate sustained firing. Since the initial studies showing that kisspeptin has prolonged effects, the question has been why is there very little spike frequency adaption during sustained firing? Presently, we have discovered that kisspeptin reduces spike frequency adaptation and prolongs firing via the inhibition of a calcium-activated slow afterhyperpolarization current (IsAHP). GnRH neurons expressed two distinct IsAHP, a kisspeptin-sensitive and an apamin-sensitive IsAHP. Essentially, kisspeptin inhibited 50% of the IsAHP and apamin inhibited the other 50% of the current. Furthermore, the kisspeptin-mediated inhibition of IsAHP was abrogated by the protein kinase C (PKC) inhibitor calphostin C, and the PKC activator phorbol 12,13-dibutyrate mimicked and occluded any further effects of kisspeptin on IsAHP. The protein kinase A (PKA) inhibitors H-89 and the Rp diastereomer of adenosine 3′,5′-cyclic monophosphorothioate had no effect on the kisspeptin-mediated inhibition but were able to abrogate the inhibitory effects of forskolin on the IsAHP, suggesting that PKA is not involved. Therefore, in addition to increasing the firing rate through an overt depolarization, kisspeptin can also facilitate sustained firing through inhibiting an apamin-insensitive IsAHP in GnRH neurons via a PKC.
Keywords: calcium-activated potassium current, apamin, gonadotropin-releasing hormone, calcium-activated slow afterhyperpolarization current
hypothalamic gonadotropin-releasing hormone (GnRH) neurons control fertility by integrating hormonal and neural input to generate rhythmic pulsatile activity, which in turn stimulates pulsatile secretion of luteinizing hormone (LH) from the anterior pituitary. One of the critical neural inputs to GnRH neurons arises from kisspeptin neurons (9, 24, 56). In fact kisspeptin, encoded by the Kiss-1 gene, is a critical peptide controlling reproductive function (15, 27, 43, 53). Targeted deletion of the Kiss-1 gene in mice prevents sexual development and causes reproductive failure (11, 28). At the cellular level, nanomolar concentrations of kisspeptin induce a strong depolarization and intensive firing of GnRH neurons (18, 31, 42, 59). Indeed, kisspeptin neurons provide the most potent excitatory input to GnRH neurons, and in vivo administration of kisspeptins induces surge-like LH secretion in several species (16, 20, 36, 45).
GnRH is released in an episodic or pulsatile manner, and it is thought that neurosecretory (e.g., GnRH) neurons fire in a sustained bursting mode to augment peptide release such as during a “surge” (2, 12, 21, 23, 33, 37). Recently, It was reported that the calcium-activated, slow afterhyperpolarization currents (IsAHP) are critical for the expression of burst firing in GnRH neurons (29).
There are three kinds of calcium-activated potassium channels that mediate afterhyperpolarizations based on their kinetics: the fast AHP mediated by large-conductance Ca2+-activated K+ channels; the medium AHP (mAHP) mediated by small-conductance Ca2+-activated K+ (SK) channels; and the slow AHP (sAHP) mediated by a yet unidentified potassium channel that is regulated by many neurotransmitters (4, 46, 47, 54). GnRH neurons express a mAHP current and both an apamin-sensitive and an apamin-insensitive IsAHP (5, 8, 22, 29, 30, 51). In most central neurons, the mAHP controls action potential discharge frequency, whereas the sAHP is largely responsible for producing spike frequency adaptation, i.e., decreased firing in the face of a sustained depolarizing stimulus (47). However, in GnRH neurons the IsAHP have been reported to regulate both action potential discharge frequency and spike frequency adaptation (29).
Kisspeptin depolarizes GnRH neurons via the coupling of G protein-coupled receptor 54 (GPR54) to a phospholipase C (PLC) signaling pathway that activates nonselective canonical transient receptor potential (TRPC) channels that allow influx of calcium ions (59). Because the calcium influx would activate the IsAHP and attenuate firing, we hypothesized that kisspeptin would inhibit the calcium-activated IsAHP and stimulate burst firing and ultimately peptide release. Indeed, we have found that kisspeptin inhibits an apamin-insensitive IsAHP in GnRH neurons through a protein kinase C (PKC)-dependent signaling pathway that affects bursting activity.
MATERIALS AND METHODS
Animals and treatments.
All animal treatments described in this study are in accordance with institutional guidelines based on National Institutes of Health standards and were performed with institutional Animal Care and Use Committee approval at the Oregon Health and Science University. Transgenic female mice expressing enhanced green fluorescent protein under the control of the GnRH promoter were used in these studies (52). Animals were group-housed until surgery at which time they were housed individually. All animals were maintained under controlled temperature and photoperiod (lights on at 0600 and off at 1800) and given free access to food and water. Adult (2- to 5-mo-old) females were ovariectomized under isoflurane inhalation anesthesia. After ∼7 days, the animals were treated with 17β-estradiol benzoate to induce positive feedback as described (6). Animals were killed at 1000–1100 at which time the uterus was removed and weighed as a measure of plasma 17β-estradiol levels (7). Recordings were done from 1300 to 1800 during the afternoon.
Peparation of preoptic area GnRH slices.
Mice were killed quickly by decapitation. The brain was rapidly removed from the skull, and a block containing the diagonal band (DB)-preoptic area (POA) was immediately dissected. The DB-POA block was submerged in cold (4°C) oxygenated (95% O2-5% CO2) high-sucrose cerebrospinal fluid (CSF, in mM): 208 sucrose, 2 KCl, 26 NaHCO3, 10 glucose, 1.25 NaH2PO4, 2 MgSO4, 1 MgCl2, and 10 HEPES, pH 7.4, 290 mosm. Coronal slices (200 μm) from the DB-POA were cut on a vibratome during which time (10 min) the slices were bathed in high-sucrose CSF at 4°C. The slices were then transferred to an auxiliary chamber where they were kept at room temperature (25°C) in artificial CSF (aCSF) consisting of (in mM): 124 NaCl, 5 KCl, 2.6 NaH2PO4, 2 MgCl2, 2 CaCl2, 26 NaHCO3, 10 HEPES, and 10 glucose, pH 7.4, 310 mosm until recording (recovery for 2 h). A single slice was transferred to the recording chamber at a time and was kept viable by continually perfusing with warm (35° C), oxygenated aCSF at 2 ml/min.
Visualized whole cell patch recording.
Whole cell patch recordings were made with a Zeiss Axioskop FS upright microscope equipped with fluorescence (fluorescein isothiocyanate filter set) and infrared differential interference contrast imaging devices. GnRH neurons were identified by the method described in our previous papers (57–59). Patch pipettes (1.5 mm OD borosilicate glass; A-M Systems) were pulled on a Brown/Flaming puller (model P-97; Sutter Instrument) and were filled with the following internal solution (in mM): 130 KMeSO4, 5 Na2 phosphocreatine, 10 KCl, 1 MgCl2, 0.025 EGTA, 10 HEPES, 2.5 ATP, and 0.25 GTP adjusted to pH 7.3 with KOH; 290 mosm. Pipette resistances were 2–3 MΩ when filled with the above pipette solution. In the whole cell configuration, access resistance was 10–25 MΩ. Voltage-clamp experiments were performed with an Axopatch 1D amplifier (2–10 kHz lowpass filter; Axon Instruments, Foster City, CA). Under voltage-clamp configuration, cells were voltage clamped at a holding potential of −60 mV. IsAHP was elicited once every 60 s by 600-ms-long depolarizing step commands to +40 mV. The medium AHP current (ImAHP) was elicited once every 30 s by 100-ms-long depolarizing step commands. In current clamp, the sAHP following a train of action potentials was induced by 20 pulses of 300 pA in amplitude and 3 ms in duration delivered at 20 Hz. Each measurement was repeated 10 times, and the signals were averaged. The mean resting membrane potential of GnRH neurons measured with low-EGTA (25–50 μM) internal solution was −65.9 ± 1.0 mV (n = 17), which is about 3 mV more negative than that measured with high EGTA (11 mM) internal solution (57). The reported membrane potentials have been corrected for the liquid junction potential of 10 mV. Under current-clamp configuration, the spike frequency adaptation properties before and after kisspeptin application were evaluated by examining the current injection-induced firing from the “clamped” initial membrane potential of −65 mV. First, the amount of current required to depolarize the cell membrane to a threshold level of action potential firing (rheobase) was examined by current pulses with step increments of 5 pA. Next, repeated firing was induced by current pulses of 2× rheobase amplitude. Electrophysiological signals were digitized with Digidata 1322A (Axon Instruments), and the data were analyzed using Clampfit 9.2 software (Axon Instruments).
Electrophysiological solutions/drugs.
aCSF was used in all cases for electrophysiological recording. In whole cell voltage-clamp recordings, tetrodotoxin (TTX) was used to isolate the effect of presynaptic input. Different drug stock solutions were diluted 1,000-fold in aCSF to their final concentrations in 20-ml syringes and were delivered by a Gilson Mini-Plus Pump with a perfusion rate of 2 ml/min. Kisspeptin-10 [Mouse Kiss-1(110–119)-NH2; Kp-10] was purchased from Phoenix Pharmaceuticals (Belmont, CA) and dissolved in water to a stock concentration of 100 μM. Apamin (240 μM in stock) and TTX (1 mM in stock) were purchased from Alomone Laboratories (Jerusalem, Israel) and were dissolved in water. Forskolin (10 mM in stock), phorbol 12,13-dibutyrate (PDBu, 1 mM in stock), calphostin C (500 μM stock solution), H-89 (10 mM stock solution), and 3-(triphenylmethylaminomethyl)pyridine, (UCL-2077, 10 mM stock solution) were purchased from Tocris (Ellisville, MO) and dissolved in dimethyl sulfoxide. The Rp diastereomer of adenosine 3′,5′-cyclic monophosphorothioate (Rp-cAMPS; Sigma-Aldrich, St. Louis, MO) was dissolved in the internal solution.
Electrophysiology data measurement and analysis.
The IsAHP was measured at its peak. Only cells with stable initial IsAHP greater than 15 pA were included in the study. The control IsAHP for each recording was the average of two to three pulses before drug application. The after-drug IsAHP was the average of two to three pulses after 5–8 min drug application. Data were analyzed and plotted using Clampfit 9.2, Macromedia Freehand 10, GraphPad Prism 4, and Sigma Plot 8.0 software. Comparisons between different treatments were performed using an unpaired Student's t-test or a one-way or two-way ANOVA with Bonferroni posttests. Differences were considered significant if the probability of error was <5%. All data were presented as means ± SE.
RESULTS
Kisspeptin reduces spike frequency adaptation in GnRH neurons.
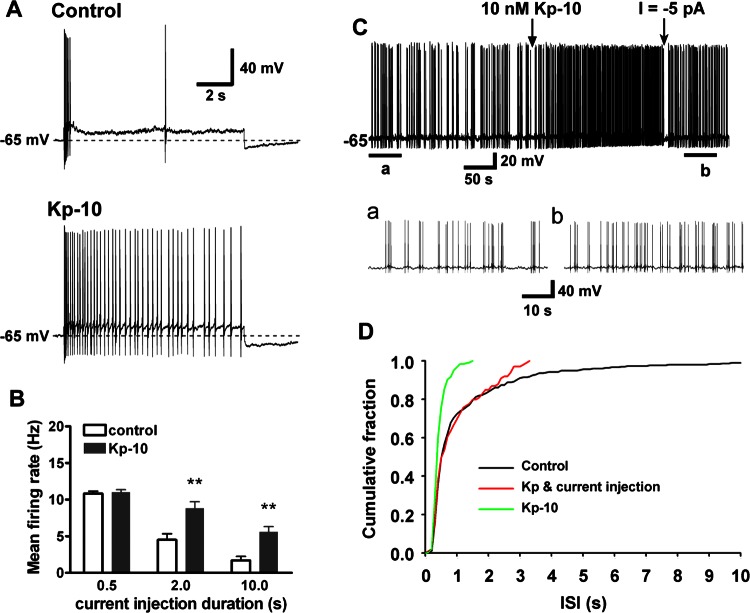
Kisspeptin excites GnRH neurons through the activation of TRPC channels and inhibition of inwardly rectifying K+ (Kir) channels, which robustly depolarizes GnRH neurons to cause continuous firing (59). Because most central nervous system (CNS) neurons exhibit spike frequency adaptation, which is the decreased firing with continued stimulus, we decided to measure the effects of kisspeptin on spike frequency adaptation. For these experiments, we current clamped the membrane potential to −65 mV, which is the resting membrane potential of GnRH neurons, and we established the rheobase current for generating firing (Fig. 1). A 10-s current injection of 2× rheobase induced early burst firing followed by quiescence or reduced firing after 0.5 s, indicative of robust spike frequency adaptation. However, with bath application of kisspeptin (Kp-10, 10 nM), an identical depolarizing stimulus (2× rheobase current) caused a significantly longer burst of action potentials lasting for the duration of the pulse (Fig. 1A). In the presence of kisspeptin, the remaining sAHP at the end of the pulse most likely resulted from the kisspeptin-insensitive component. Figure 1B summarizes the effect of kisspeptin on the induced firing rate over several stimulation durations (0.5, 2, and 10 s) at 2× rheobase. Kisspeptin had no effect on the induced firing rate when the current injection duration was shorter than 0. 5 s, but it dramatically increased the firing rate for longer durations (2 and 10 s). Therefore, kisspeptin reduces spike frequency adaptation and prolongs burst firing duration in GnRH neurons. Figure 1C shows an example recording of kisspeptin's ability to increase the firing rate and decrease the interspike interval (ISI) even when the membrane potential was brought back to the prekisspeptin level by current injection to offset TRPC channel activation. The ISI analysis in Fig. 1D clearly shows that kisspeptin increased the firing rate via depolarization and elimination of the long ISI or interburst interval.
Fig. 1.
Kisspeptin reduces spike frequency adaptation in gonadotropin-releasing hormone (GnRH) neurons. A: representative current-clamp recordings showing that kisspeptin-10 (Kp-10) reduces spike frequency adaptation and prolongs the burst firing induced by a 10-s, 2× rheobase current pulse. B: summary of the effects of kisspeptin on the mean firing rate for different depolarizing durations (0.5, 2, and 10 s). **P < 0.01 compared with control conditions (n = 6 for each group). Two-way ANOVA with Bonferroni posttest. C: an example recording showing that kisspeptin increased the firing rate even when the membrane potential was brought back to the prekisspeptin level by current injection (trace b vs. trace a). The time of application of kisspeptin or current injection is indicated by an arrow. Segments a and b are exhibited below in an expanded time scale. D: interspike interval (ISI) analysis of trace C showing that kisspeptin increased the firing rate via depolarization and elimination of the long ISI or interburst interval.
Kisspeptin inhibits IsAHP through a PKC signaling pathway.
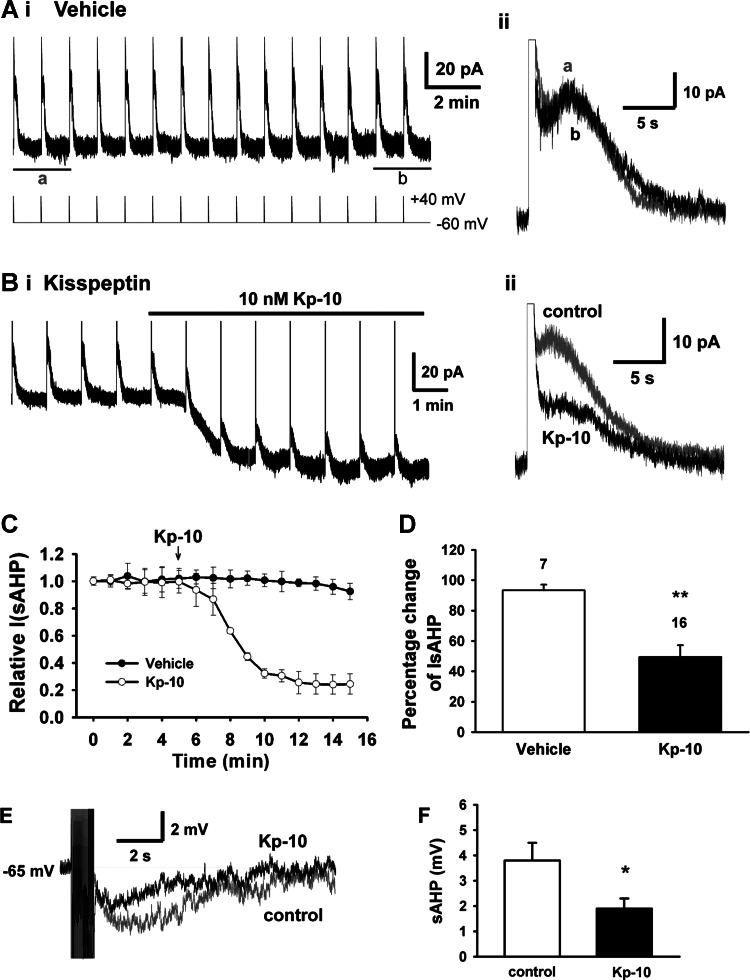
Because the IsAHP governs spike frequency adaptation in CNS neurons (13, 32), we assessed the effects of kisspeptin on the IsAHP in GnRH neurons. To quantify the effects of kisspeptin on the IsAHP, a voltage-clamp protocol that steps from −60 to +40 mV for 600 ms was used since this is needed to fully activate the IsAHP in GnRH neurons (30). We found that this protocol, which was delivered every minute, induced a reproducible response (Fig. 2A). Cells that exhibited an IsAHP greater than 15 pA were examined for the effects of kisspeptin. As shown in Fig. 2B, the GnRH neuron exhibited stable peak IsAHP currents of 23 pA before kisspeptin application. After 3 min kisspeptin application, the IsAHP was strongly inhibited by 54%, and concurrently kisspeptin induced an inward current of 37 pA, mainly because of the activation of TRPC channels (59). Figure 2C shows the time course of the kisspeptin inhibition of the peak IsAHP and the lack of an effect of the vehicle control over the same time period. Kisspeptin reduced the mean IsAHP by 50.8% (from 25.2 ± 1.6 to 12.4 ± 2.1 pA, n = 16) within 10 min, whereas the IsAHP only decreased slightly (6.7%) with vehicle perfusion over the same time period (Fig. 2D). We also examined the effect of kisspeptin on the IsAHP in current-clamp recordings. As shown in Fig. 2E, a train of 20 action potentials at 20 Hz induced an sAHP that lasted about 10 s. Kisspeptin application inhibited the sAHP. The summary data in Fig. 2F show that kisspeptin significantly decreased the sAHP by 50% (from 3.8 ± 0.7 to 1.9 ± 0.4 mV, n = 5, P < 0.05).
Fig. 2.
Kisspeptin inhibits the calcium-activated slow afterhyperpolarization current (IsAHP) in GnRH neurons. Ai: the trace on top is a representative recording showing that the IsAHP recorded in aCSF/vehicle is very stable over 15 min of recording. The trace on bottom shows the voltage-clamp protocol for generating the IsAHP, which consisted of a series of 600-ms depolarization steps from −60 to +40 mV and back to −60 mV that was delivered every 60 s. Aii: the superimposition of the average IsAHP of the first two (a, gray) and the last two (b, black) steps. Bi: an example recording showing that Kp-10 strongly inhibited the IsAHP by 54% after 6–7 min of application. Bii: the superimposition of the average IsAHP of the two recordings right before Kp-10 application and the last two recordings after 5 min of Kp-10 application. C: the mean time course of the effects of vehicle and Kp-10 on the peak IsAHP values from three representative recordings. The start of the kisspeptin (10 nM) perfusion is indicated by the arrow. D: summary of the percent changes in the peak IsAHP after vehicle control or Kp-10. **P < 0.01, kisspeptin vs. vehicle control (Student's t-test). Cell numbers are indicated. E: kisspeptin inhibited the slow afterhyperpolarization (sAHP) induced by a train of 20 action potentials delivered at 20 Hz in current clamp. The current pulse width and amplitude for each action potential induction was 3 ms and 300 pA, respectively. F: summary of the effects of kisspeptin on the sAHP. The cell number was 5 for each group in each experiment. *P < 0.05 compared with control (Student's t-test).
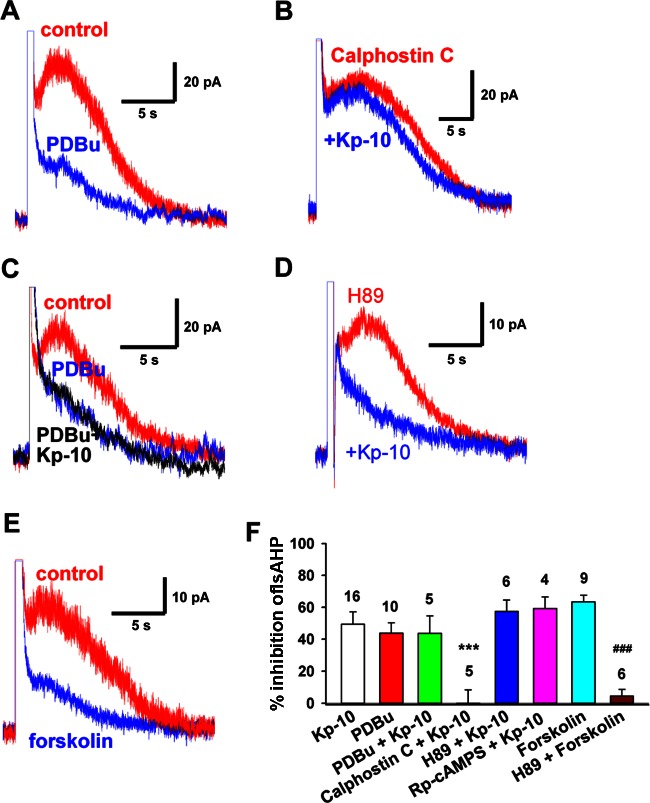
Next we examined the signaling pathway through which kisspeptin inhibited the IsAHP in GnRH neurons. In central neurons, IsAHP is inhibited by neurotransmitters that activate protein kinase A (PKA) or PKC signaling pathways (35, 41, 54). The kisspeptin receptor GRP54 is a Gq-coupled receptor that activates PKC (25, 31, 59). Therefore, we asked whether kisspeptin inhibits IsAHP through a PKC signaling pathway and examined the effects of both PKC activators and PKC inhibitors. As shown in Fig. 3, the PKC activator PDBu (1 μM) mimicked the effects of kisspeptin and further occluded any effects of kisspeptin on the IsAHP (Fig. 3, A, C, and F). Moreover, the PKC inhibitor calphostin C abrogated the inhibitory effects of kisspeptin on the IsAHP (Fig. 3, B and F). We also examined the effects of a PKA activator and two inhibitors on the IsAHP. Following pretreatment with the PKA catalytic subunit inhibitor H-89 (10 μM for 1–2 h) or whole cell dialysis with the regulatory subunit inhibitor Rp-cAMP for 10 min, kisspeptin still inhibited the IsAHP by 57.5 ± 7.2% (n = 6) and 59.3 ± 7.3% (n = 4), respectively, which was not significantly different from the effects of kisspeptin alone (Fig. 3, D and F). As a positive control for H-89 (10 μM), the same pretreatment abrogated the effects of the PKA activator forskolin (10 μM) to inhibit IsAHP (4.5 ± 4.1% for H-89 + forskolin vs. 63.5 ± 4.2% for forskolin alone, P < 0.001) (Fig. 3F). Therefore, these pharmacological experiments indicate that kisspeptin inhibits IsAHP through activating a PKC but not a PKA signaling pathway.
Fig. 3.
Kisspeptin inhibits IsAHP in GnRH neurons via a protein kinase C (PKC) signaling pathway. A: phorbol 12,13-dibutyrate (PDBu, 1 μM), a PKC activator, inhibited the IsAHP in GnRH neurons. B: calphostin C (500 nM), a PKC inhibitor, blocked the effects of Kp-10 on the IsAHP after the cell was pretreated with calphostin C for 1 h. C: PDBu occluded the effects of kisspeptin. D: the protein kinase A (PKA) inhibitor H-89 (10 μM) did not block the effects of Kp-10 on the IsAHP. E: the PKA activator forskolin (10 μM) inhibited the IsAHP. This effect was blocked by H-89 (F). F: summary of the effects of Kp-10, the PKC activator PDBu, the PKC inhibitor calphostin C, the PKA activator forskolin, and the PKA inhibitors H-89 and Rp-cAMPS on the IsAHP. Cell numbers are indicated. ***P < 0.001 compared with the Kp-10 group. ###P < 0.001 compared with the forskolin group. One-way ANOVA with Bonferroni posttest.
Kisspeptin inhibits the apamin-insensitive IsAHP.
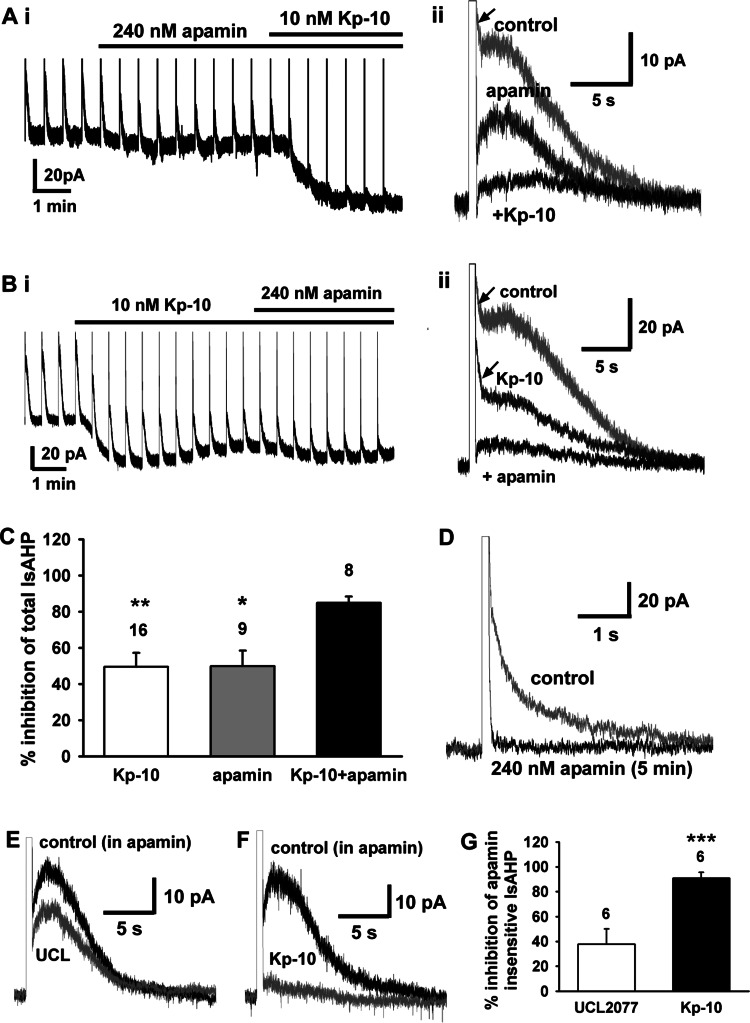
It has been reported that GnRH neurons express both an apamin-sensitive IsAHP and an apamin-insensitive IsAHP (22, 29). Therefore, to examine whether kisspeptin inhibits the apamin-insensitive IsAHP, we first examined the effects of kisspeptin on the apamin-insensitive currents after apamin application. As shown in Fig. 4A, a concentration of apamin (240 nM) that generates a maximum response (22, 29) inhibited the total IsAHP by ∼50%, and Kp-10 inhibited the residual current such that the combined inhibition was 87%. We next examined the effects of apamin in the presence of kisspeptin. As shown in Fig. 4B, a concentration of kisspeptin (10 nM) that generates a maximum depolarization (59) inhibited the total IsAHP by ∼50%, and the residual IsAHP was inhibited by apamin (240 nM) such that the combined inhibition was 86%. The summary data in Fig. 4C document that kisspeptin and apamin inhibited the total IsAHP by 49.5 ± 7.7% (n = 16) and 49.8 ± 8.6% (n = 9), respectively, and the combination of kisspeptin and apamin inhibited the IsAHP by 84.9 ± 3.4% (n = 8). Interestingly, an ImAHP was clearly evident (Fig. 4, Aii and 4Bii) and, as expected, was inhibited by apamin (Fig. 4Aii), whereas kisspeptin did not affect the ImAHP (Fig. 4Bii). In addition, we found that GnRH neurons exhibited a clear ImAHP when a shorter (100 ms) voltage-step protocol was used. We used this protocol to document that 240 nM apamin completely inhibited the medium AHP within 5 min (95.7 ± 2.2%, n = 3) (Fig. 4D). Therefore, because apamin (240 nM) was fully efficacious to inhibit the medium AHP but only partially inhibits the IsAHP, an apamin-insensitive IsAHP must be expressed in GnRH neurons. UCL-2077 has been found to inhibit an apamin-insensitive IsAHP in hippocampal and GnRH neurons (29, 48). Thus, we examined its effects on the apamin-insensitive IsAHP. GnRH neurons were pretreated with apamin (240 nM) in the recording chamber for 20–30 min before UCL-2077 application. As shown in Fig. 4, E and F, the apamin-insensitive IsAHP activated slowly. The mean time to reach the peak of the IsAHP was 2.1 ± 0.5 s (n = 7), and the time constant for decay was 7,242.5 ± 6.8 ms (n = 9). UCL-2077 (10 μM) only partially inhibited the apamin-insensitive IsAHP with a mean inhibition of 37.8 ± 12.2% (n = 6). In contrast, 10 nM kisspeptin almost completely inhibited the apamin-insensitive IsAHP with a mean inhibition of 90.8 ± 4.6% (n = 6, P < 0.001 vs. UCL-2077) (Fig. 4, F and G). Therefore, 10 nM Kp-10 fully inhibits an apamin-insensitive IsAHP in GnRH neurons. Moreover, to examine if kisspeptin preferentially inhibits IsAHP over activating TRPC channels at lower concentrations, we examined the effects of 3 nM Kp-10 (an EC50 concentration for kisspeptin to induce an inward current). Kp-10 (3 nM) also inhibited the apamin-insensitive IsAHP by about 50% (57.0 ± 8.6%, n = 3). Therefore, the EC50 values for kisspeptin's inhibition of the apamin-insensitive IsAHP and activation of TRPC channels are similar.
Fig. 4.
Kisspeptin inhibits an apamin-insensitive IsAHP in GnRH neurons. Ai: a representative recording showing that apamin (240 nM) inhibited the peak IsAHP by 50%, and the residual current was inhibited by Kp-10 (10 nM; total inhibition 87%). Aii: superimposition of the IsAHP traces before (control) and after apamin and Kp-10 applications. The medium AHP current is indicated by the arrow. Bi: a representative recording showing that kisspeptin inhibited 50% of the peak IsAHP, and the remaining component was inhibited by apamin (total inhibition 86%). Bii: superimposition of the IsAHP traces before (control) and after Kp-10 and apamin applications. The medium AHP currents are indicated by the arrows. C: summary of the percent inhibition by Kp-10, apamin, and the combined application of Kp-10 and apamin (Kp-10 + apamin) on the total IsAHP. *P < 0.05 and **P < 0.01 compared with the Kp-10 + apamin group (1-way ANOVA). Cell numbers are indicated. D: a representative recording of a GnRH neuron demonstrating the efficacy of 240 nM apamin to completely inhibit the medium AHP current (ImAHP) within 5 min of bath application. The ImAHP was elicited by a 100-ms step command to +40 mV from the holding potential of −60 mV. E: a representative recording showing that UCL-2077 (UCL, 10 μM) partially inhibited the apamin-insensitive IsAHP. F: a representative recording showing that Kp-10 (10 nM) almost completely inhibited the apamin-insensitive IsAHP. G: summary of the inhibitory effects of UCL-2077 and Kp-10 on the apamin-insensitive IsAHP. ***P < 0.001; Student's t-test.
DISCUSSION
Since the initial studies showing that kisspeptin has long-lasting effects to prolong firing of GnRH neurons (18, 31, 42, 59), the question has been why is there little spike frequency adaption? In this study, we show for the first time that kisspeptin inhibits an apamin-insensitive IsAHP in GnRH neurons, which reduces spike frequency adaptation and prolongs burst firing. These effects of kisspeptin on IsAHP are mediated via the activation of a PKC signaling pathway. Therefore, in addition to depolarizing GnRH neurons through activating TRPC channels and inhibiting Kir channels, kisspeptin prolongs burst firing of GnRH neurons by inhibiting the IsAHP.
The IsAHP is a Ca2+-activated K+ current that follows a long burst of action potentials. It can also be elicited by depolarizing pulses of hundreds of milliseconds that activate high-voltage-gated calcium channels. This IsAHP current peaks in several hundred milliseconds, decays with a time constant from 1 to 10 s, and is not sensitive to apamin inhibition in most neurons (4, 46, 47, 54). However, GnRH neurons have been found to express both an apamin-insensitive IsAHP and an apamin-sensitive IsAHP (22, 29, 30, 51) (present findings). It is most likely that the SK channel mediates the apamin-sensitive IsAHP (and ImAHP) in GnRH neurons (1). Indeed, a relatively high level of expression of SK3 transcripts is found in native GnRH neurons (6).
The molecular identity of the apamin-insensitive IsAHP has not been ascertained, but it is modulated by a plethora of neurotransmitters and neuropeptides via activation of PKA, PKC, or calmodulin kinase II (13, 19, 38–41, 46, 54). For monoamine neurotransmitters (e.g., norepinephrine, serotonin, and dopamine), the inhibition of the IsAHP involves upregulation of cAMP and the subsequent activation of PKA (41). Some neuropeptides such as corticotropin-releasing hormone and vasoactive intestinal peptide also inhibit the IsAHP through activating a cAMP-PKA signaling pathway (19). In thalamic paraventricular nucleus neurons, the IsAHP is modulated by both PKA and PKC activity (60). However, in GnRH neurons, the selective PKA inhibitors Rp-cAMPS and H-89 did not attenuate the effects of kisspeptin. In contrast, the broad-spectrum PKC inhibitor calphostin C abrogated the effects of kisspeptin, and PDBu occluded the effects of kisspeptin. This is analogous to the kainate receptor-mediated inhibition of IsAHP in CA1 pyramidal neurons via activation of PKC (17, 35). Our findings are also consistent with the pathway elucidated in both heterologous cell expression systems and in native GnRH neurons in which kisspeptin has been shown to signal via the Gq-coupled GPR54 to stimulate PLC, activate PKC, and increase cytosolic calcium oscillations (10, 25, 26, 31, 59).
At the cellular level, the IsAHP limits the firing that underlies the late phase of spike frequency adaptation and is a major player in controlling neuronal excitability (13, 32). Inhibition of IsAHP by neurotransmitters reduces spike frequency adaptation and increases the duration and firing rate during burst firing (13, 14, 44, 60). Our results clearly show that kisspeptin inhibited an apamin-insensitive IsAHP and reduced spike frequency adaptation in GnRH neurons. The pyridine UCL-2077 is thought to inhibit the apamin-insensitive IsAHP in GnRH neurons (29). However, we found that UCL-2077 (10 μM) only partially inhibited the apamin-insensitive IsAHP, which is consistent with the effects reported by Shah et al. (48) in hippocampal CA1 neurons. Also, UCL-2077 is not selective for the IsAHP since it potentiates KCNQ 5 channels that underlie the M current (50), which confounds the effects of UCL on GnRH neurons since they express KCNQ 5 channels and a robust M current (55). Therefore, the endogenous neurotransmitter kisspeptin is the most efficacious inhibitor of the apamin-insensitive IsAHP in GnRH neurons.
It is well known that GnRH is released in a pulsatile manner, and the hypothalamic surge of GnRH and subsequent pituitary release of LH are required for triggering ovulation in the female. Although single action potential-induced calcium influx is enough to spark the release of classical transmitters, burst firing or tetanic stimulation is required for the release of neuropeptides such as vasopressin, oxytocin, substance P, and atrial natriuretic factor (3, 34, 49). Previous studies have shown that kisspeptin robustly depolarizes and increases the firing of GnRH neurons through a combination of activating TRPC channels and attenuating Kir conductances (31, 42, 58, 59). In the present study, we show for the first time that kisspeptin can also modulate burst firing duration by inhibiting an apamin-insensitive IsAHP. Therefore, the combination of the depolarization by activating TRPC and inhibiting Kir channels and an apamin-insensitive IsAHP would greatly facilitate burst firing that would contribute to the preovulatory surge of GnRH peptide.
GRANTS
This research was supported by National Institutes of Health Grants NS-38809, NS-43330, and DK-68098.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: C.Z., O.K.R., and M.J.K. conception and design of research; C.Z. performed experiments; C.Z. analyzed data; C.Z., O.K.R., and M.J.K. interpreted results of experiments; C.Z. prepared figures; C.Z., O.K.R., and M.J.K. drafted manuscript; C.Z., O.K.R., and M.J.K. edited and revised manuscript; C.Z., O.K.R., and M.J.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Martha A. Bosch and Marina V. Rulevskaya for excellent technical support. Also, we would like to thank Dr. John P. Adelman for his comments on an earlier draft of this manuscript.
REFERENCES
- 1. Adelman JP, Maylie J, Sah P. Small-conductance Ca2+-activated K+ channels: form and function. Annu Rev Phys 74: 1–25, 2011 [DOI] [PubMed] [Google Scholar]
- 2. Andrew RD, Dudek FE. Burst discharge in mammalian neuroendocrine cells involves an intrinsic regenerative mechanism. Science 221: 1050–1052, 1983 [DOI] [PubMed] [Google Scholar]
- 3. Bicknell RJ. Optimizing release from peptide hormone secretory nerve terminals. J Exp Biol 139: 51–65, 1988 [DOI] [PubMed] [Google Scholar]
- 4. Bond CT, Maylie J, Adelman JP. Small-conductance calcium-activated potassium channels. Ann NY Acad Sci 868: 370–378, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Bosch MA, Kelly MJ, Rønnekleiv OK. Distribution, neuronal co-localization and 17β-E2 modulation of small conductance calcium-activated K+ channel (SK3) mRNA in the guinea pig brain. Endocrinology 143: 1097–1107, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Bosch MA, Tonsfeldt KJ, Ronnekleiv OK. mRNA expression of ion channels in GnRH neurons: subtype-specific regulation by 17β-estradiol. Mol Cell Endocrinol doi: 10.1016/j.mce.2012.12.021: 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bronson FH, Vom Saal FS. Control of the preovulatory release of luteinizing hormone by steroids in the mouse. Endocrinology 104: 1247–1255, 1979 [DOI] [PubMed] [Google Scholar]
- 8. Chu Z, Andrade J, Shupnik MA, Moenter SM. Differential regulation of gonadotropin-releasing hormone neuron activity and membrane properties by acutely applied estradiol: dependence on dose and estrogen receptor subtype. J Neurosci 29: 5616–5627, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology 147: 5817–5825, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Constantin S, Caligioni CS, Stojilkovic S, Wray S. Kisspeptin-10 facilitates a plasma membrane-driven calcium oscillator in gonadotropin-releasing hormone-1 neurons. Endocrinology 150: 1400–1412, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. d'Anglemont de Tassigny X, Fagg LA, Dixon JPC, Day K, Leitch HG, Hendrick AG, Zahn D, Franceschini I, Caraty A, Carlton MBL, Aparicio SAJR, Colledge WH. Hypogonadotropic hypogonadism in mice lacking a functional KiSS 1 gene. Proc Natl Acad Sci USA 104: 10714–10719, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dutton A, Dyball REJ. Phasic firing enhances vasopressin release from the rat neurohypophysis. J Physiol 290: 433–440, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Faber ESL, Sah P. Physiological role of calcium-activated potassium currents in the rat lateral amygdala. J Neurosci 22: 1618–1628, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ghamari-Langroudi M, Bourque CW. Muscarinic receptor modulation of slow afterhyperpolarization and phasic firing in rat supraoptic nucleus neurons. J Neurosci 24: 7718–7726, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gottsch ML, Clifton DK, Steiner RA. Kisspepeptin-GPR54 signaling in the neuroendocrine reproductive axis. Mol Cell Endocrinol 254–255: 91–96, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 145: 4073–4077, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Grabauskas G, Lancaster B, O'Connor V, Wheal HV. Protein kinase signalling requirements for metabotropic action of kainate receptors in rat CA1 pyramidal neurones. J Physiol 579: 363–373, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci 25: 11349–11356, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haug T, Storm JF. Protein kinase A mediates the modulation of the slow Ca(2+)-dependent K(+) current, I(sAHP), by the neuropeptides CRF, VIP, and CGRP in hippocampal pyramidal neurons. J Neurophysiol 83: 2071–2079, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology 80: 264–272, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Jasoni CL, Romano N, Constantin S, Lee K, Herbison AE. Calcium dynamics in gonadotropin-releasing hormone neurons. Front Neuroendocrinol 31: 259–269, 2010 [DOI] [PubMed] [Google Scholar]
- 22. Kato M, Tanaka N, Usui S, Sakuma Y. SK channel blocker apamin inhibits slow afterhyperpolarization currents in rat gonadotropin-releasing hormone neurones. J Physiol 574.2: 431–442, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kelly MJ, Rønnekleiv OK. Electrophysiological analysis of neuroendocrine neuronal activity in hypothalamic slices. In: Methods in Neurosciences: Pulsatility in Neuroendocrine Systems, edited by Levine JE. San Diego, CA: Academic, 1994, p. 47–67 [Google Scholar]
- 24. Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, Yamada S, Inoue K, Ohtaki T, Matsumoto H, Maeda KI. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology 146: 4431–4436, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, Brezillon S, Tyldesley R, Suarez-Huerta N, Vandeput F, Blanpain C, Schiffmann SN, Vassart G, Parmentier M. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem 276: 34631–34636, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Kroll H, Bolsover S, Hsu J, Kim SH, Bouloux PM. Kisspeptin-evoked calcium signals in isolated primary rat gonadotropin-releasing hormone neurones. Neuroendocrinology 93: 114–120, 2011 [DOI] [PubMed] [Google Scholar]
- 27. Kuohung W, Kaiser UB. GPR54 and KiSS-1: role in the regulation of puberty and reproduction. Rev Endocr Metab Disord 7: 257–263, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, Le WW, Hoffman GE, Seminara SB. Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology 148: 4927–4936, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Lee K, Duan W, Sneyd J, Herbison AE. Two slow calcium-activated afterhyperpolarization currents control burst firing dynamics in gonadotropin-releasing hormone neurons. J Neurosci 30: 6214–6224, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu X, Herbison AE. Small-conductance calcium-activated potassium channels control excitability and firing dynamics in gonadotropin-releasing hormone (GnRH) neurons. Endocrinology 149: 3598–3604, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu X, Lee K, Herbison AE. Kisspeptin excites gonadotropin-releasing hormone (GnRH) neurons through a phospholipase C/calcium-dependent pathway regulating multiple ion channels. Endocrinology 149: 4605–4614, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Madison DV, Nicoll RA. Control of the repetitive discharge of rat CA1 pyramidal neurones in vitro. J Physiol (Lond) 354: 319–331, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Marrero HG, Lemos JR. Frequency-dependent potentiation of voltage-activated responses only in the intact neurohypohysis of the rat. Eur J Physiol 450: 96–110, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Masterson SP, Li J, Bickford ME. Frequency-dependent release of substance P mediates heterosynaptic potentiation of glutamatergic synaptic responses in the rat visual thalamus. J Neurophysiol 104: 1758–1767, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Melyan Z, Wheal HV, Lancaster B. Metabotropic-mediated kainate receptor regulation of IsAHP and excitability in pyramidal cells. Neuron 34: 107–114, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MBL, Colledge WH, Caraty A, Aparicio SAJR. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA 102: 1761–1766, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moenter SM. Identified GnRH neuron electrophysiology: a decade of study. Brain Res 1364: 10–24, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nicoll RA. The coupling of neurotransmitter receptors to ion channels in the brain. Science 241: 545–551, 1988 [DOI] [PubMed] [Google Scholar]
- 39. Pedarzani P, Storm JF. Dopamine modulates the slow Ca2+-activated K+ current IAHP via cyclic AMP-dependent protein kinase in hippocampal neurons. J Neurophysiol 74: 2749–2753, 1995 [DOI] [PubMed] [Google Scholar]
- 40. Pedarzani P, Storm JF. Protein kinase A-independent modulation of ion channels in the brain by cyclic AMP. Proc Natl Acad Sci USA 92: 11716–11720, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pedarzani P, Storm JF. PKA mediates the effects of monoamine transmitters on the K+ current underlying the slow spike frequency adaptation in hippocampal neurons. Neuron 11: 1023–1035, 1993 [DOI] [PubMed] [Google Scholar]
- 42. Pielecka-Fortuna J, Chu Z, Moenter SM. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology 149: 1979–1986, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Plant TM. The role of KiSS-1 in the regulation of puberty in higher primates. Eur J Endocrinol 155: S11–S16, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Power JM, Sah P. Competition between calcium-activated K+ channels determines cholinergic action on firing properties of basolateral amygdala projection neurons. J Neurosci 28: 3209–3220, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Roseweir AK, Kauffman AS, Smith JT, Guerriero KA, Morgan K, Pielecka-Fortuna J, Pineda R, Gottsch ML, Tena-Sempere M, Moenter SM, Terasawa E, Clarke IJ, Steiner RA, Millar RP. Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci 29: 3920–3929, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sah P. Ca2+-activated K+ currents in neurones: types, physiological roles and modulation. Trends Neurosci 19: 150–154, 1996 [DOI] [PubMed] [Google Scholar]
- 47. Sah P, Faber ESL. Channels underlying neuronal calcium-activated potassium currents. Prog Neurobiol 66: 345–353, 2002 [DOI] [PubMed] [Google Scholar]
- 48. Shah MM, Javadzadeh-Tabatabaie M, Benton DCH, Ganellin CR, Haylett DG. Enhancement of hippocampal pyramidal cell excitability by the novel selective slow-afterhyperpolarization channel blocker 3-(triphenylmethylaminomethyl)pyridine (UCL2077). Mol Pharmacol 70: 1494–1502, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shakiryanova D, Tully A, Hewes RS, Deitcher DL, Levitan ES. Activity-dependent liberation of synaptic neuropeptide vesicles. Nat Neurosci 8: 173–178, 2005 [DOI] [PubMed] [Google Scholar]
- 50. Soh H, Tzingounis AV. The specific slow afterhyperpolarization inhibitor UCL2077 is a subtype-selective blocker of the epilepsy associated KCNQ channels. Mol Pharmocol 78: 1088–1095, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Spergel DJ. Calcium and small-conductance calcium-activated potassium channels in gonadotropin-releasing hormone neurons before, during, and after puberty. Endocrinology 148: 2383–2390, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Suter KJ, Song WJ, Sampson TL, Wuarin JP, Saunders JT, Dudek FE, Moenter SM. Genetic targeting of green fluorescent protein to gonadotropin-releasing hormone neurons: characterization of whole-cell electrophysiological properties and morphology. Endocrinology 141: 412–419, 2000 [DOI] [PubMed] [Google Scholar]
- 53. Tena-Sempere M. Kiss-1 and reproduction: focus on its role in the metabolic regulation of fertility. Neuroendocrinology 83: 275–281, 2006 [DOI] [PubMed] [Google Scholar]
- 54. Vogalis F, Storm JF, Lancaster B. SK channels and the varieties of slow after-hyperpolarizations in neurons. Eur J Neurosci 18: 3155–3166, 2003 [DOI] [PubMed] [Google Scholar]
- 55. Xu C, Roepke TA, Zhang C, Rønnekleiv OK, Kelly MJ. GnRH activates the M-current in GnRH neurons: an autoregulatory negative feedback mechanism? Endocrinology 149: 2459–2466, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yeo SH, Herbison AE. Projections of arcuate nucleus and rostral periventricular kisspeptin neurons in the adult female mouse brain. Endocrinology 152: 2387–2399, 2011 [DOI] [PubMed] [Google Scholar]
- 57. Zhang C, Bosch MA, Levine JE, Rønnekleiv OK, Kelly MJ. Gonadotropin-releasing hormone neurons express KATP channels that are regulated by estrogen and responsive to glucose and metabolic inhibition. J Neurosci 27: 10153–10164, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhang C, Bosch MA, Rønnekleiv OK, Kelly MJ. GABAB receptor mediated inhibition of GnRH neurons is suppressed by kisspeptin-GPR54 signaling. Endocrinology 150: 2388–2394, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhang C, Roepke TA, Kelly MJ, Rønnekleiv OK. Kisspeptin depolarizes gonadotropin-releasing hormone neurons through activation of TRPC-like cationic channels. J Neurosci 28: 4423–4434, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhang L, Kolaj M, Renaud LP. Ca2+-dependent and Na+-dependent K+ conductances contribute to a slow AHP in thalamic paraventricular nucleus neurons: a novel target for orexin receptors. J Neurophysiol 104: 2052–2062, 2010 [DOI] [PubMed] [Google Scholar]