Abstract
We studied the digestive and respiratory tract motor responses in 10 chronically instrumented dogs during eructation activated after feeding. Muscles were recorded from the cervical area, thorax, and abdomen. The striated muscles were recorded using EMG and the smooth muscles using strain gauges. We found eructation in three distinct functional phases that were composed of different sets of motor responses: gas escape, barrier elimination, and gas transport. The gas escape phase, activated by gastric distension, consists of relaxation of the lower esophageal sphincter and diaphragmatic hiatus and contraction of the longitudinal muscle of the thoracic esophagus and rectus abdominis. All these motor events promote gas escape from the stomach. The barrier elimination phase, probably activated by rapid gas distension of the thoracic esophagus, consists of relaxation of the pharyngeal constrictors and excitation of dorsal and ventral upper esophageal sphincter distracting muscles, as well as rapid contraction of the diaphragmatic dome fibers. These motor events allow esophagopharyngeal air movement by promoting retrograde airflow and opening of the upper esophageal sphincter. The transport phase, possibly activated secondary to diaphragmatic contraction, consists of a retrograde contraction of the striated muscle esophagus that transports the air from the thoracic esophagus to the pharynx. We hypothesize that the esophageal reverse peristalsis is mediated by elementary reflexes, rather than a coordinated peristaltic response like secondary peristalsis. The phases of eructation can be activated independently of one another or in a different manner to participate in physiological events other than eructation that cause gastroesophageal or esophagogastric reflux.
Keywords: belching, gastrointestinal motor activity, pharynx, larynx, esophagus
some of the motor events associated with eructation are known, i.e., upper and lower esophageal sphincter (UES and LES) relaxation (13, 15, 16, 23–25, 31), glottal closure (26), changes in respiration (11, 22), and retrograde esophageal peristalsis (11), but the specific muscles that accomplish these functions are unknown or incompletely known. Other functions during eructation, e.g., opening of the UES, have not been investigated. Moreover, while some of the motor responses during eructation have been identified, as noted above, most studies focused on only one muscle or function, and the relationship among functions and motor responses producing the effects of eructation has not been investigated. Therefore, while it has been determined that transient LES relaxation (TLESR), as well as relaxation of the UES, is associated with eructation (23), the relationship between TLESR and UES relaxation during eructation is unknown.
Some studies (3, 10, 24, 31) have suggested that the reflexes associated with eructation may contribute to gastroesophageal reflux (GER) or esophagopharyngeal reflux (EPR), but this concept has not been investigated.
The aims of this study were to determine 1) the motor responses of the respiratory and digestive tracts associated with eructation, 2) the manner in which the motor responses associated with eructation are organized to cause gastroesophagopharyngeal reflux of gas, and 3) whether the reflexes associated with eructation could contribute to GER or EPR.
METHODS
Animal Preparation
The experiments were performed at the Zablocki Veterans Affairs Medical Center and were approved by the Animal Care and Use Committee of the Zablocki Veterans Affairs Medical Center. Aseptic techniques were used to implant recording devices on the muscles of the digestive and respiratory tracts in 10 mixed-breed dogs (17–25 kg body wt) of either sex, as described previously (14, 17). Recording devices were placed on appropriate muscles in the thorax, abdomen, and cervical region during separate surgeries to minimize risk of infection and stress to the animals. To obtain sufficient data, yet minimize the number of surgeries performed on each dog, we used two groups of animals: those with cervical and abdominal devices (n = 6) and those with cervical, abdominal, and thoracic devices (n = 4). The surgeries were performed in the order listed. Recovery time of ≥20 days was allowed between surgeries, and experiments were begun ≥10 days after surgery.
Abdominal surgery.
A midventral laparotomy was performed to expose the abdominal cavity. Bipolar silver wire electrodes were sewn on the diaphragmatic dome (DD, n = 6) and diaphragmatic hiatus (DH, n = 4) fibers and rectus abdominis (RA, n = 4). Strain gauge force transducers (n = 6) were sewn onto the seromuscular layer of the gastric fundus, corpus, antrum, duodenum, jejunum, and ileum. The lead wires from these recording devices were brought out of the abdomen through a stainless steel cannula, as described previously (14, 17).
Cervical surgery.
A midline incision was placed on the ventral surface of the neck, and the esophageal, pharyngeal, laryngeal, and hyoid muscles were carefully exposed as described previously (14, 17). Bipolar silver wire electrodes were sewn onto the mylohyoideus (MH, n = 3), geniohyoideus (GH, n = 5), thyrohyoideus (TH, n = 10), hyopharyngeus (HP, n = 4), hyoglossis (HG, n = 3), stylopharyngeus (StP, n = 3), thyropharyngeus (TP, n = 9), cricopharyngeus (CP, n = 10), esophagus (n = 9), sternohyoideus (SH, n = 3), sternothyroideus (STh, n = 3), cricothyroideus (n = 4), cricoaretynoideus dorsalis (n = 3), thyroaretynoideus (TA, n = 3), and esophagus (n = 10). A strain gauge was sewn onto the cervical esophagus (n = 2) along its longitudinal axis. The lead wires from these recording devices were tunneled subcutaneously to the intrascapular region and brought out of the neck through a dental acrylic cannula, as described previously (14, 17). Because the cannula could hold only enough wire to serve nine devices, not every muscle was recorded in every animal.
Thoracic surgery.
The chest was opened between the eighth and ninth ribs on the right side, and the following recording devices were implanted on the esophagus: a bipolar silver wire electrode (n = 2) or a strain gauge force transducer (n = 2) sewn along the longitudinal axis of the esophagus and a strain gauge force transducer sewn under tension along the circular axis of the LES (n = 4). The LES strain gauge was sewn under tension to allow for recording of LES relaxation. The lead wires from these recording devices were brought out through a stab wound in the chest wall between the sixth and seventh ribs as close as possible to the vertebral column. The wires were then tunneled subcutaneously to the intrascapular region, where they exited the skin through a dental acrylic cannula, as described previously (14, 17).
Data Acquisition, Storage, and Analysis
Electromyography.
The implanted bipolar electrodes consisted of 18-gauge silver wires spaced 5 mm apart and exposed for a length of 3 mm embedded in a thin (2-mm) Silastic rubber backing. The electrodes were sutured to the muscle along the long axis of the muscle fibers. This electrode design resulted in the esophageal electrodes being oriented in the longitudinal direction and situated in the longitudinal muscle. The electrodes were connected to an amphenol plug by Teflon-coated silver-plated copper wires, and the plug was embedded in a dental acrylic base. For EMG recording, electrical activity was band-pass filtered (0.1–1.0 kHz) and then amplified (1,000×) by an alternating-current amplifier (model 7P3, Grass).
Contractile activity.
The strain gauge force tranducers consisted of precision strain gauge elements (EA-06-031DE-120, Micromeasurements Group, Raleigh, NC) glued onto a copper-beryllium shim, waterproofed using polysulfide coating (M coat JL, Micromeasurements Group), and embedded in Silastic rubber. The shims of the strain gauges used for recording LES tone were made thinner and more flexible than other strain gauges to allow for recording of relaxation. The strain gauges were connected to an amphenol plug by Teflon-coated silver-plated copper wires. Each strain gauge was connected electrically to a quarter Wheatstone bridge circuit before amplification by a direct-current amplifier with a high-frequency filter set at 15 Hz (model 7P122, Grass).
Data recording storage.
The amplified signals were stored on tape (model D, Vetter) and later transferred to a computer using CODAS (Dataq Instruments) hardware and software.
Experimental Protocol
Animals were fasted overnight (15–18 h), and eructation was stimulated by feeding canned dog food. The dogs invariably had episodes of eructation during and after feeding. Eructation was initially noted by characteristic audible sounds and later by the characteristic and unique motor responses.
Statistics
Differences between mean values were tested using Student's t-test. P ≤ 0.05 was considered significant.
RESULTS
Eructation Responses
The digestive and respiratory tract responses were assessed in groups associated with the different motor responses that occur during eructation: TLESR, transient UES relaxation (TUESR), and esophageal transit.
Responses associated with TLESR.
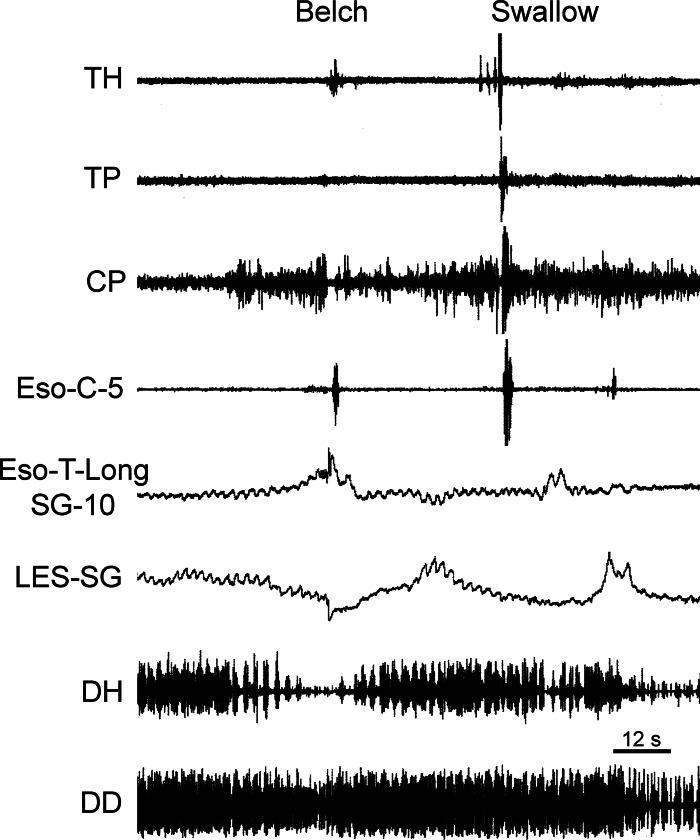
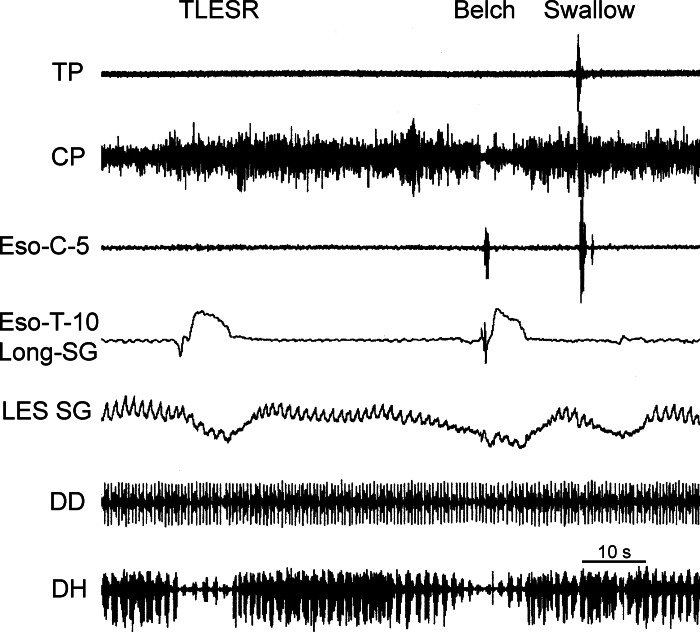
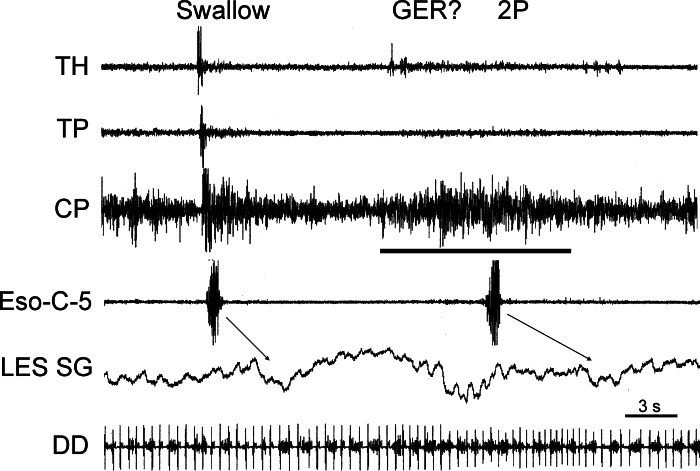
The first observed motor event of the digestive tract associated with eructation was TLESR (Figs. 1–3), which began 5.0 ± 1.0 s before inhibition of the CP EMG and lasted for 12.0 ± 0.4 s (Table 1). Concomitant with the TLESR was an increase in longitudinal tension of the thoracic esophagus (Fig. 1, Table 1) and inhibition of spontaneous respiratory-related EMG activity (Figs. 1–3, Table 1) of the DH. There was no significant difference (P > 0.05) in the time delay from CP EMG inhibition or duration of the decrease in LES tone and DH EMG inhibition (Table 1). The time delay from CP EMG inhibition (4.5 ± 1.3 s, n = 2) and duration of the esophageal longitudinal contraction (11.4 ± 2.5 s, n = 2) were similar to LES tone decrease and DH inhibition, but the number of observations was too small for statistical comparison of these values (Table 1). The RA EMG increased during the initial period of TLESR but ended abruptly with the rapid contraction of the DD (see below).
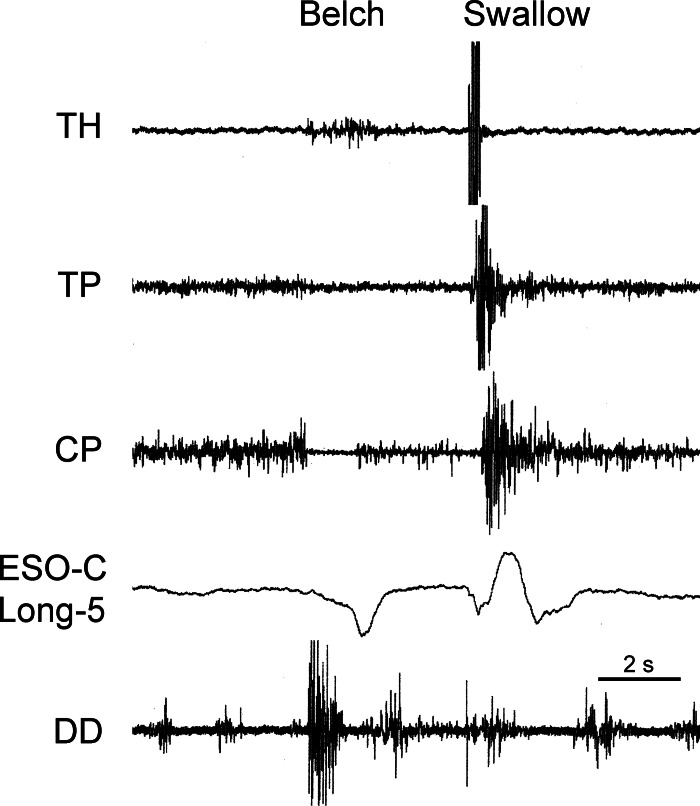
Fig. 1.
Responses associated with transient lower esophageal sphincter (LES) relaxation (TLESR) during eructation. Responses of esophagus, diaphragm, and pharynx associated with eructation and swallowing are shown. Note simultaneous increase in tone of longitudinal thoracic esophagus (Eso-T-Long), decrease in tone of LES (LES-SG), and decrease in respiratory-related phasic activity of diaphragmatic hiatus (DH) during eructation and comparison with the subsequent swallow. Numbers with Eso-C and Eso-T-Long (i.e., 5 and 10) indicate distance (cm) of the recording device from the cricopharyngeus [CP (for a cervical device)] or LES (for a thoracic device). Also ECG activity is often imposed on diaphragmatic and thoracic EMG electrodes. All recordings are EMG, except those designated SG, which are strain gauge recordings. All strain gauge recordings are in the circular direction, unless indicated by the notation Long (i.e., longitudinally oriented). TH, thyrohyoideus; TP, thyropharyngeus; Eso, esophagus; C, cervical; T, thoracic; DD, diaphragmatic dome.
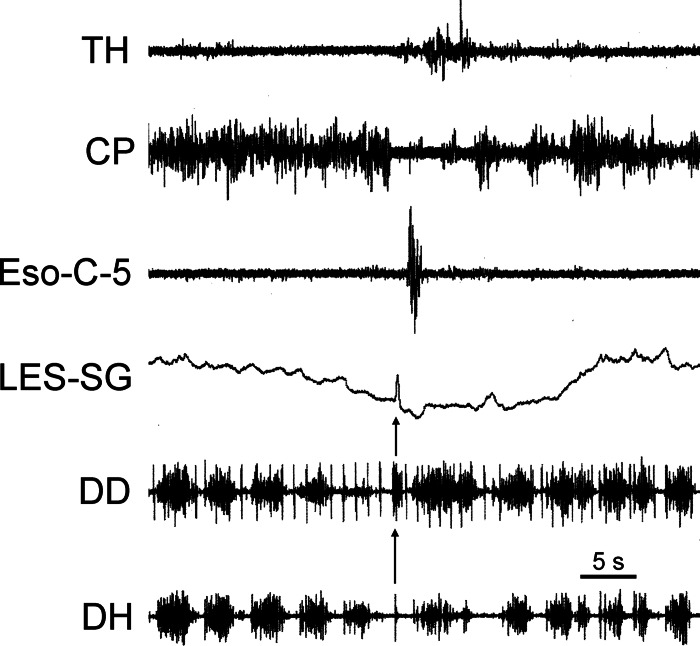
Fig. 2.
Responses of the diaphragm during eructation: activation of a rapid short-duration activation of DD and DH fibers (arrow) during inhibition of ventilation and CP relaxation associated with eructation. Note longer duration of DD than DH response. Simultaneous with these diaphragmatic responses was a deflection of the LES strain gauge.
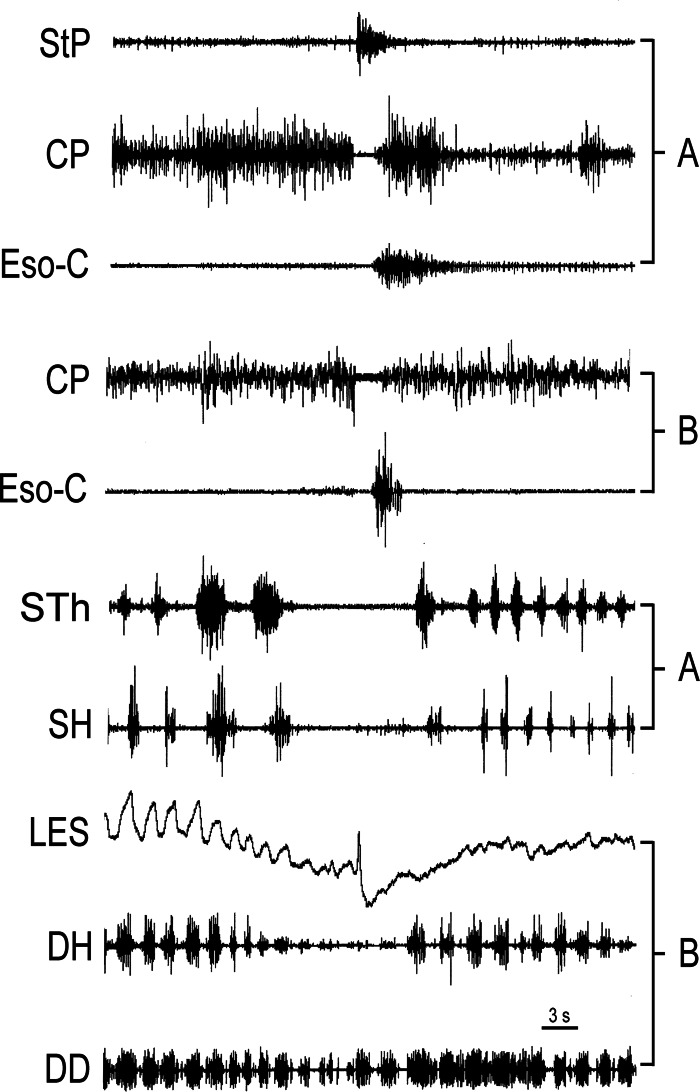
Fig. 3.
Responses of sternal muscles during eructation. Data from two different animals, A and B, relate sternal muscle activity with the diaphragm during eructation, since no one animal had electrodes on all these muscles. Decrease in activity of the sternohyoideus (SH) and sternothyroideus (STh) temporally corresponds to a decrease in DH EMG and LES tone. StP, stylopharyngeus.
Table 1.
Timing characteristics of reponses during eructation
| n | Time From CP EMG Inhibition, s | Duration, s | |
|---|---|---|---|
| LES tone decrease | 4 | 5.0 ± 1.0 | 12.0 ± 0.4 |
| DH EMG inhibition | 4 | 4.4 ± 0.9 | 10.8 ± 0.7 |
| Esophageal longitudinal contraction | 2 | 4.5 ± 1.2 | 11.4 ± 1.5 |
| DD EMG activation | 4 | 0.09 ± 0.005 | 0.36 ± 0.04 |
| DH EMG activation | 4 | 0.08 ± 0.004 | 0.05 ± 0.01 |
Values are means ± SE. CP, cricopharyngeus; LES, lower esophageal sphincter; DH, diaphragmatic hiatus; DD, diaphragmatic dome.
Rapid responses of the diaphragm (Figs. 2, 4, and 5; see Fig. 7), LES (Figs. 1–4), and thoracic esophageal longitudinal tension (Fig. 1) occurred concomitantly. The DD EMG response occurred rapidly over a short duration (0.36 ± 0.04 s, n = 4) and very soon (0.09 ± 0.005 s, n = 4) after the beginning of CP EMG inhibition. The coefficient of variation of the time delay between CP EMG inhibition and the DD EMG rapid response, i.e., 10.9%, was much less than that of the time delay between the beginning of TLESR and CP EMG inhibition, i.e., 38.8.3%. The duration of the EMG activation (Fig. 2) of the DD was longer (P < 0.05, n = 4) than that of the DH (0.05 ± 0.01 s).
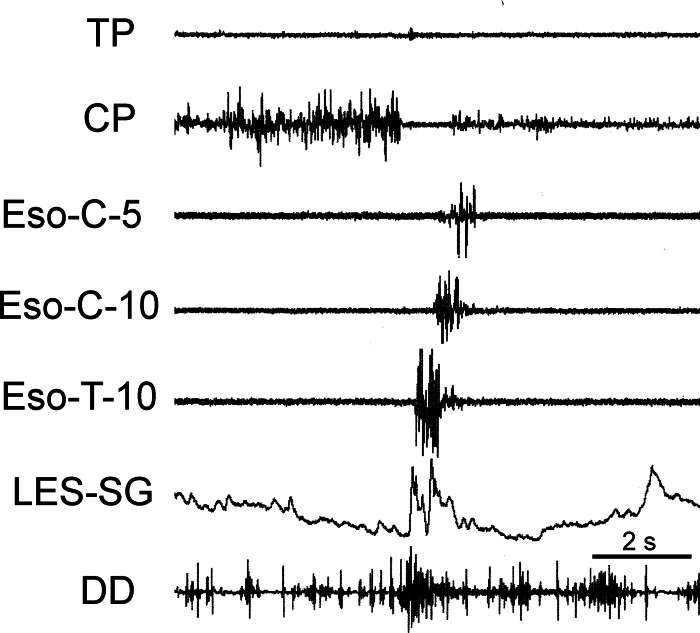
Fig. 4.
Retrograde esophageal contraction during eructation. Phasic activation of esophageal EMG activity begins on the most distal thoracic esophageal electrode and progress rapidly to the most orad cervical esophageal electrode, but not the CP. Note biphasic nature of the esophageal, as well as LES, response but monophasic nature of the DD response.
Fig. 5.
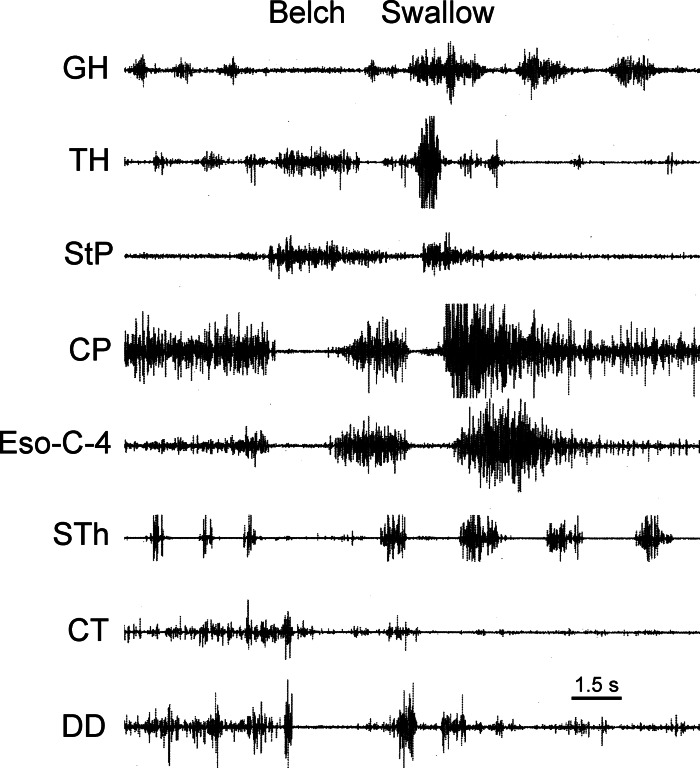
Motor responses associated with opening of the upper esophageal sphincter (UES) during eructation. EMG activity of the primary constrictor of the UES, i.e., CP, as well as the two primary muscles, i.e., StP and TH, that impart distracting force on the UES, is shown. An eructation is followed by a swallow. Simultaneous inhibition of the pharyngeal constrictors, e.g., CP, and activation of the UES distracting muscles, i.e., StP and TH, would act to open the UES during eructation. GH, geniohyoideus; CT, cricothyroideus.
Fig. 7.
Role of the longitudinal fibers of the cervical esophagus associated with eructation. Decrease in tone of the longitudinal fibers of the cervical esophagus associated with eructation, as well as its response during swallowing, is shown. Note that since this strain gauge was not sewn under tension, this apparent decrease in tone must represent stretch of the esophagus by forces outside the cervical esophagus, e.g., shortening of the thoracic esophagus or distraction by the bolus.
Muscles of the larynx/pharynx, i.e., the SH and STh, were also inhibited during eructation in association with TLESR (Fig. 3). Since no one animal had electrodes implanted on the diaphragm, as well as the sternal muscles, we combined responses from two different animals to illustrate the relationship between responses of these sets of muscles during eructation (Fig. 3). The timing of events of these two responses was synchronized to the occurrence of CP EMG inhibition. The relaxation of the sternal muscles was concomitant with inhibition of the diaphragm.
During eructation, no responses were observed from the distal stomach to the ileum. However, occasionally a short-duration low-magnitude change in tone was observed in the gastric fundus that was concomitant with activation of the DD EMG.
Responses associated with TUESR.
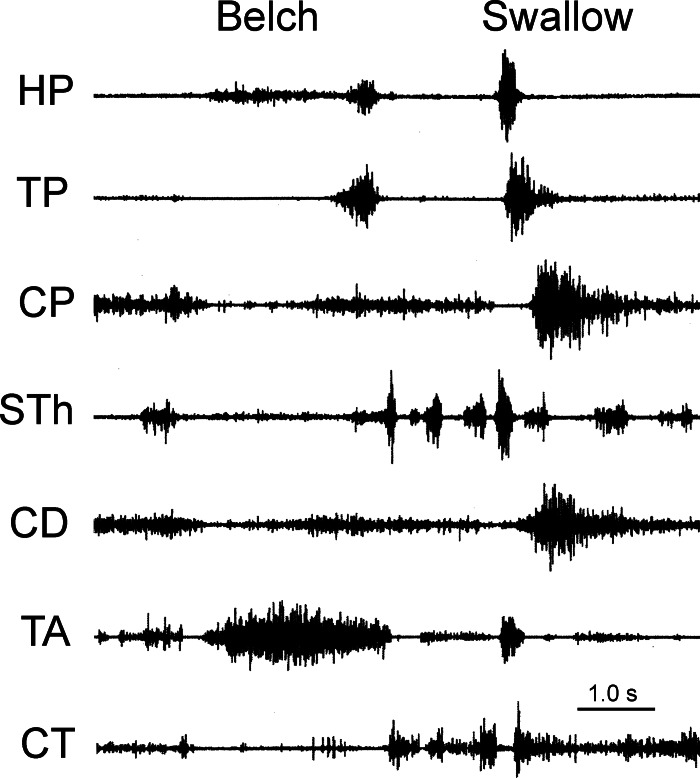
The CP and TP relaxed in association with eructation (Figs. 1–11). The CP relaxed for 1.22 ± 0.11 s (n = 9), but since the TP does not have a constant tone, the duration of TP inhibition could not be accurately quantified. For comparison, the duration of CP EMG inhibition during swallowing in these animals was 0.34 ± 0.02 s (n = 9). The TH and StP were activated (Fig. 5) during CP inhibition in a relatively constant manner, 1.23 ± 0.14 s (n = 9) and 1.20 ± 0.06 s (n = 3), respectively, and at an intensity lower than during swallowing. Muscles superior to the hyoid bone, i.e., HP (Fig. 6) and HG, were also activated during this time period for 1.33 ± 0.14 s (n = 3). These muscles were also activated in a relatively constant and low-level manner. The MH and GH (Fig. 5) did not respond in association with eructation. During TUESR, the TA was strongly activated for 1.23 ± 0.09 s (n = 3), whereas the CD and CT were inhibited (Fig. 6).
Fig. 6.
Laryngeal responses associated with eructation. Strong activation of the thyroarytenoideus (TA) and relaxation of the cricoarytenoideus dorsalis (CD) during the period of CP relaxation associated with eructation, as well as their responses during swallowing, are shown. The TA acts to close the glottis, and the CD acts to open the glottis; therefore, actions of these muscles would provide significant protection of the airway. Also note low-level activation of the hyopharyngeus (HP), which occurs during CP inhibition.
Fig. 8.
TLESR without transient UES relaxation (TUESR). Two TLESRs of similar magnitude are shown: one with TUESR and one without; swallow is shown for comparison. Traces indicate that decrease in DH is associated with TLESR, not TUESR, and rapid deflection of the LES, EMG response of the DD, and reverse peristalsis are related to TUESR, not TLESR. Note also activation of secondary peristalsis of the thoracic esophagus in both cases of TLESR.
Fig. 9.
A possible GER event. A TLESR associated with increased tone of the CP (horizontal bar) followed by activation of secondary peristalsis (2P) is shown; a swallow is shown for comparison. Arrows indicate progression of peristalsis from the cervical esophagus to the associated relaxation of the LES. It is likely that the TLESR caused reflux of material that distended the esophagus, activating the esophago-UES contractile reflex and then secondary peristalsis.
Fig. 10.
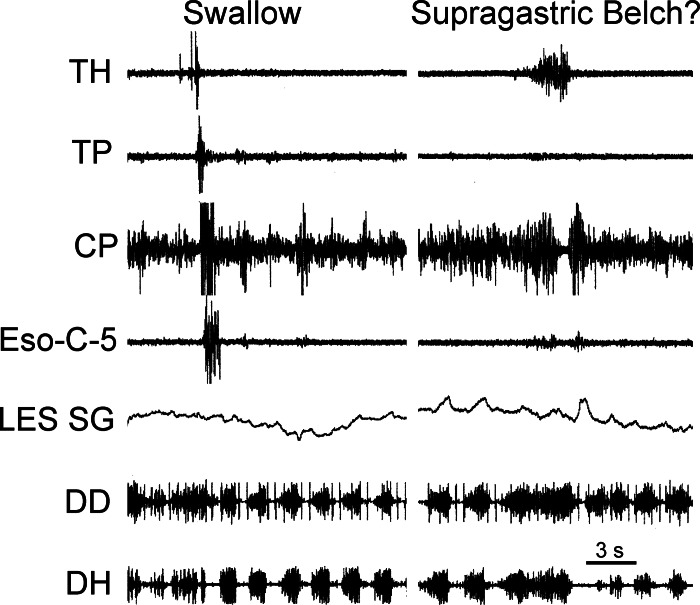
A possible supragastric belch. A swallow and a response that has the characteristics of a supragastric belch are shown (3, 10, 12). Note that during the possible supragastric belch, relaxation of the CP was concomitant with TH activation without relaxation of the LES or retrograde esophageal peristalsis. While the TH contracted strongly, as during eructation, the DD and DH contracted, rather than relaxed. This motor event must have opened the UES, but there was no eructation or swallow. Prolonged activation of the diaphragm would have resulted in prolonged inflation and decreased intrathoracic pressure during the TUESR. For comparison with eructation in the same animal see Fig. 3.
Fig. 11.
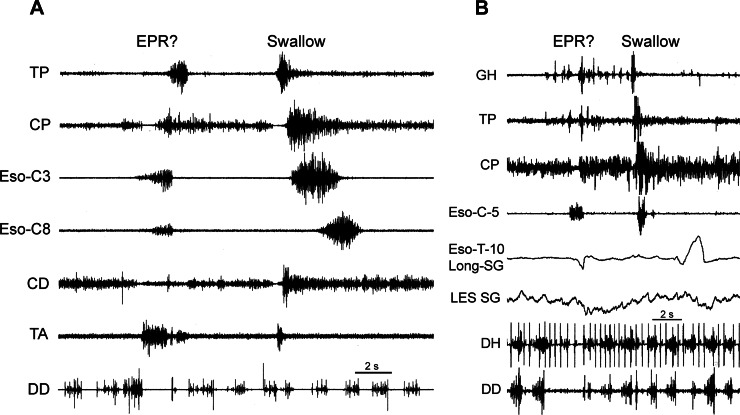
A possible esophagopharyngeal reflux (EPR) event. Two examples (A and B) of a unique eructation-like event that may cause EPR recorded from two different animals are shown. Both examples have a very unique event of a TUESR concomitant with activation of the cervical esophagus. In A, esophageal contraction seemed to propagate orad, as well as caudad; in B, peristaltic wave did not progress to the thoracic esophagus. In A, the airway strongly closed, as indicated by strong activation of the TA, similar to eructation; this suggests that an EPR event occurred during this time. In B, a TLESR occurred but, unlike eructation, after the beginning of the TUESR. The DD and DH were inhibited for a short duration, and rapid activation of the DD did not occur as in eructation. We hypothesize that this is an EPR event.
A decrease in tension of the longitudinal fibers of the cervical esophagus occurred concomitant with TUESR (Fig. 7), which is in contrast to contraction of the longitudinal muscle of the thoracic esophagus (Fig. 1), which occurred concomitant with TLESR. This decrease in tension had two phases: an initial low-amplitude slow rate decrease and a larger and more rapid tension decrease at the end of the response.
Responses associated with esophageal transit.
We found that, during CP EMG inhibition, an EMG burst starting in the thoracic esophagus propagated orad at 34.4 ± 4.9 cm/s (n = 5; Fig. 4). The time delay from the beginning of CP EMG inhibition to the beginning of the EMG burst of the cervical esophageal retrograde EMG response, i.e., 0.69 ± 0.01 s (n = 4), had a very low coefficient of variation of 3.7%, which was similar to that of the delay between CP EMG inhibition and the rapid EMG burst of the DD. The retrograde esophageal EMG response usually did not progress to and include the CP muscle (Figs. 1–4). Also, the particular esophageal retrograde EMG response shown in Fig. 4, as opposed to those in Figs. 1–3, was biphasic, whereas the DD response was monophasic. The deflection of the LES strain gauge of the response depicted in Fig. 4 also occurred in a biphasic fashion, unlike this response in other eructations (Figs. 1–3 and 8). In addition, a secondary peristaltic wave was sometimes observed (Fig. 8) in the thoracic esophagus after the retrograde esophageal EMG response had passed.
Eructation-Related Responses
Two sets of responses occurred: 1) one or two, but not all three, groups of responses associated with eructation and 2) an apparent initial reflux event that was not GER but, rather, EPR.
TLESR responses without TUESR responses.
On 27 occasions in 2 animals, we recorded TLESRs, similar to those during eructation, that were not accompanied by TUESRs (Figs. 8 and 9). The duration of these TLESRs was 12.1 ± 0.8 s. These decreases in LES tone were usually accompanied by decreases in DH EMG activity (Fig. 8) and secondary peristalsis of the thoracic esophagus (Fig. 8), as occurred during eructation (Fig. 8). On the other hand, unlike eructation, we did not observe the rapid deflection of LES tone or activation of the diaphragm during these events (Figs. 8 and 9). We also found concomitant increases in resting tone of CP EMG in 16 of the 27 TLESRs, and 5 of these were followed by secondary peristalsis (Fig. 9).
TUESR responses without TLESR and esophageal transit responses.
In five cases in one animal, we found an inhibition of the CP, along with strong contraction of the TH very similar to that during eructation, but the duration of the CP inhibition was only 0.66 ± 0.05 s. However, in these cases, a TLESR, peristalsis of the thoracic esophagus, or inhibition of the DH did not occur (Fig. 10). Other significant differences between this event and eructation were the very strong activation of the CP after the CP inhibition and activation of the DD and DH, which began before CP relaxation.
EPR.
In two animals, we found six cases of a very unique event in which a cervical esophageal phasic EMG response occurred throughout CP inhibition (Fig. 11). This esophageal activation also propagated orally through the CP and TP and caudally in the esophagus (Fig. 11A), but it did not propagate to the thoracic esophagus (Fig. 11B). A TLESR did occur, but after initiation of TUESR, not before as during eructation. The duration of CP EMG inhibition during this event was 0.73 ± 0.09 s. In addition, concomitant with CP relaxation was strong activation of the TA and relaxation of the CD very similar to our findings during eructation (Fig. 11A). The DD and DH were inhibited for one or two inhalations (Fig. 11).
DISCUSSION
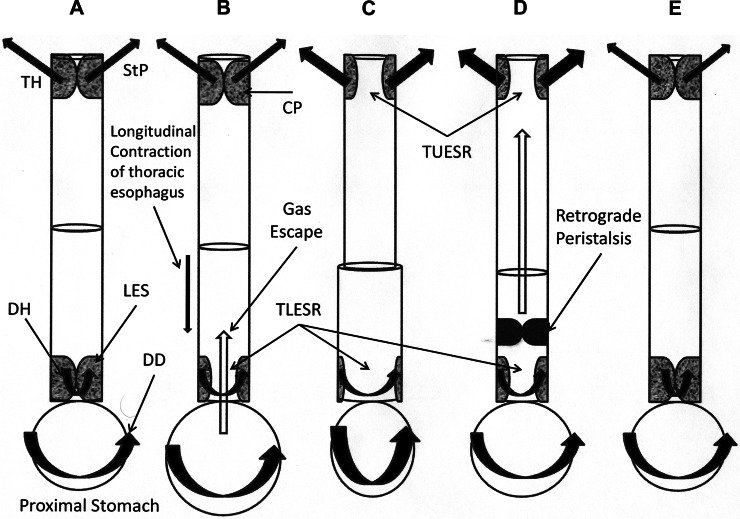
We found that eructation, a complex set of motor responses of the digestive and respiratory tracts, is organized in three phases that are independent of each other. These phases are gas escape, barrier elimination, and gas transport.
The gas escape phase consists of activations and inhibitions of various muscles designed to allow gas escape from the stomach (Fig. 12B). It is likely that this first phase of eructation is activated by slow air distension of the stomach, as it has been found in humans (20, 28) and animals (8) that distension of the stomach with air causes TLESR or eructation. Aerophagia usually occurs during feeding in dogs, which readily activates eructation (11).
Fig. 12.
Phases of eructation. A–E: timing of phases. A: initial condition with UES and LES closed. B: gas escape phase. Gastric distension causes the TLESR, which is accompanied by longitudinal contraction of the thoracic esophagus and inhibition of the DH, resulting in gas escape from the stomach to the esophagus. C: barrier elimination phase. The thoracic esophagus is filled with air from the prior phase, which causes TUESR by relaxation of the CP and activation of the UES distracting muscles, i.e., TH and StP, and then DD briefly contracts. D: gas transport phase. Possibly because of DD contraction of the prior phase, the retrograde peristaltic contraction is activated and moves rapidly orad but usually does not continue all the way to the UES. E: LES, UES, and DH regain normal tone.
The primary muscles involved in this phase are the LES, DH, and longitudinal esophageal muscles. The LES (24, 25, 31), as well as the DH (19, 21), provides the barrier between the proximal stomach and esophagus that prevents GER, and we found, as others have reported (22–24, 28), that both are inhibited during the first phase of eructation. Not only are the LES and DH inhibited, but there is a very close temporal relationship between these inhibitions, suggesting a functional and mechanistic relationship.
We found that contraction of the longitudinal muscle of the thoracic esophagus during eructation or TLESR is very closely temporally related to LES relaxation and DH inhibition. Others have also found, under other situations, a similar close relationship among these responses. The TLESR has been found to be associated with esophageal shortening (24) and esophageal longitudinal muscle contraction (1). In addition, longitudinal contraction of the esophagus has been associated with inhibition of the DH (18) during esophageal distension, and axial stretch of the LES causes a decrease in LES tone (5). On the basis of this close association of longitudinal esophageal contraction and LES relaxation, others have suggested that LES relaxation occurs secondary to longitudinal contraction of the thoracic esophagus (1). Our studies provide additional strong evidence of a very close relationship between longitudinal contraction of the thoracic esophagus and LES relaxation and DH inhibition suggestive of a reflex response.
Interestingly, the respiratory rhythm of the SH and STh are inhibited in close association with DH inhibition during eructation. While the function of this effect is unknown, these muscles may be inhibited as part of a general inhibition of respiration during this phase of eructation. After all, increased intrathoracic pressure during expiration would inhibit gas movement from the abdomen to the thorax. In addition, the SH and STh provide tension inferiorly to the larynx and hyoid; therefore, inhibition of these muscles would allow for increased superior movement of the larynx during this phase of the eructation.
The gas escape phase of eructation, therefore, is composed of temporally closely related contractions or inhibitions of the LES, longitudinal muscles of the esophagus, DH, STh, and SH, all designed to promote gas escape from the stomach. Given this close relationship and common function, it is likely that a common central neural pathway controls these responses.
The barrier elimination phase of eructation is composed of activation of TUESR and brief contraction of the DD (Fig. 12C). The brief contraction of the DD is related to TUESR, not TLESR. The timing of DD EMG activation relative to the beginning of TUESR was much less variable, i.e., coefficient of variation of ∼11%, than its time delay from TLESR, i.e., coefficient of variation of ∼39%. In addition, we found that when TLESR occurred without TUESR, the rapid excitation of DD did not occur. Therefore, we conclude that this rapid excitation of the DD EMG is part of the phase of eructation that controls TUESR.
While we did not record gas movement during eructation, others have found that the initial gastric gas escape during eructation distends the thoracic esophagus (11), and restriction of distension of the first 5 cm of the thoracic esophagus in dogs prevents eructation (29). In humans (13, 26) and animals (15, 16), rapid distension of the esophagus has been found to activate UES relaxation. In addition, in humans (23) it has been found that when UES relaxation accompanies TLESR, a common cavity occurs 84% of the time, and UES relaxation usually (92% of the time) occurs during gastroesophageal reflux of air when the individual is in the upright position (2). Therefore, while the mechanism that leads to activation of the TUESR during eructation has not been conclusively defined, it is highly likely related to gastric gas escape.
The brief contraction of the DD occurs after activation of the TUESR; therefore, we hypothesize that the DD response is a result, not a cause, of gas escape. Radiographic studies in the dog (11) found that the first gas escape during eructation distends the thoracic esophagus, and this is quickly followed by a brief inspiratory movement. These findings suggest the existence of an esophago-DD contractile reflex that is activated by rapid distension of the esophagus. This contraction is unlikely to be involved in the initial gas escape, but it may help initiate the transport phase of eructation (see below) by decreasing intrathoracic pressure, thereby promoting gastroesophageal air movement. More studies are needed to confirm this hypothesis.
We found that opening of the UES during eructation is governed by two factors: relaxation of the pharyngeal constrictors, i.e., the TP and CP, and activation of UES distracting muscles, i.e., the TH and StP. The TP and CP relaxed during eructation, and the CP relaxed for ∼1.2 s. During CP EMG inhibition, the StP and TH were activated in a constant manner that was much lower in amplitude than during swallowing. The StP connects the dorsal pharynx with the styloid process of the hyoid bone, thereby pulling the dorsal wall of the pharynx dorsally. The TH connects the thyroid cartilage with the hyoid bone, thereby pulling the larynx ventrally and, thus, the ventral wall of the UES ventrally. The combination of TH and StP contraction would have the effect of opening the UES. However, given the rather constant and low level of activation, it is unlikely that contraction of these muscles imparts a large force or movement of the larynx or pharynx cranially. The hyoid bone is not in a fixed position, and simultaneously with the contraction of the TH and StP during pharyngeal inhibition, the superior hyoid muscles, i.e., the HG and HP, were activated. The HG and HP, similar to the TH and StP, were activated in a rather constant and low-level manner. It is hypothesized that the low-level contraction of the HG and HP maintains the superior-inferior position of the hyoid bone, thereby fixing it in position to allow the TH and StP to have full effects on the UES.
Prior studies in humans (26) have found that, during belching, the hyoid bone moves ventrally, but not superiorly, and its movement is less during swallowing. This observation in humans is consistent with our EMG findings in dogs, as the activated muscles that could cause cranial movement, i.e., the StP, TH, HG, and HP, were activated in a constant low-level manner consistent with maintenance of position, rather than active movement, as during swallowing.
The gas transport phase of eructation is characterized by activation of a retrograde esophageal peristaltic contraction (Fig. 12D). Immediately after the brief contraction of the DD fibers, a phasic EMG response of the thoracic esophagus that propagates toward the CP at the rate of ∼35 cm/s was initiated. Throughout propagation of this esophageal retrograde contraction, the CP EMG is inhibited. Considering that orthograde esophageal peristalsis causes contraction of the CP muscle (15, 16) or UES (4) due to activation of the esophago-UES contractile reflex (EUCR), we conclude that the EUCR must be inhibited during eructation.
The mechanism of propagation of the retrograde esophageal contraction associated with eructation is unknown; however, this retrograde contraction in the dog (11) is similar to the retrograde esophageal contractions observed during rumination or eructation in ruminants (27, 30) and eructation in decerebrate cats (16). That is, these retrograde contractions occurred in the striated muscle portion of the esophagus (11, 16, 27, 30), had a very rapid propagation velocity (11, 15, 27, 30), and propagated in both directions from the point of initiation (16). Such retrograde esophageal contractions have been found to be centrally mediated, as they are blocked by vagotomy (7).
Given the unique characteristics of the retrograde esophageal contraction associated with eructation, we hypothesize that it is controlled more like a series of elementary reflexes (6) than the well-organized peristaltic response of secondary peristalsis. These unique characteristics include the following. 1) The retrograde contraction is ∼10 times faster than secondary peristalsis (9). 2) The shape of the retrograde contraction, as opposed to secondary peristalsis, depends on the stimulus. That is, secondary peristalsis is a monophasic pressure wave, regardless of the bolus (9), but retrograde peristalsis can be monophasic or biphasic, depending on the nature of the stimulating bolus. 3) In most cases, the peristaltic wave completes its journey to the LES (9), but the retrograde peristaltic wave never fully reaches the CP. That is, when the air bolus nears the open UES, it will escape the esophagus, thereby ending its distension of the esophagus and the esophageal reaction to this distension. 4) The eructation-related peristaltic response propagates in either direction from the point of initiation, but secondary peristalsis propagates in only one direction, no matter where it is initiated in the esophagus (8). Therefore, the eructation-related peristalsis reacts much more to the stimulus than does secondary peristalsis, and the successive activation of elementary reflexes of the esophagus by an air bolus would produce the type of esophageal response observed during eructation. Further studies are needed to confirm this hypothesis.
We found two events in which the phases of eructation were activated independently of the other phases and one event in which all phases were activated, but in a different manner to produce a different effect.
We found that sometimes the gas escape phase occurred without the barrier elimination and transport phases of eructation. During these events, the spontaneous tone of the CP increased concomitant with LES relaxation, and this was followed by initiation of secondary peristalsis. Esophageal distension (15, 16) can activate the EUCR and secondary peristalsis. Therefore, a likely explanation for this set of responses was that the decrease in LES tone allowed GER to occur, and the refluxed material distended the esophagus, activating the EUCR and secondary peristalsis. However, we did not monitor reflux; therefore, more studies are needed to confirm this hypothesis. It has been suggested that TLESRs are among the main causes of GER (24, 25) and may also participate in eructation (23). Our current studies support these concepts.
In another type of eructation-like event, the barrier elimination phase occurred with no evidence of the gas escape or gas transport phases of eructation. That is, TUESR occurred with strong contraction of a distracting muscle, i.e., the TH, but there was no evidence of TLESR or retrograde esophageal peristalsis. These events would have resulted in strong opening of the UES. In addition, rather than inhibition of DH EMG, this event was accompanied by prolonged excitation of DH and DD EMG prior to and during inhibition of CP EMG. This diaphragmatic contraction would have caused prolonged inhalation, decreasing intrathoracic pressure. These responses are very similar to the responses that occur during a supragastric belch, i.e., inspiration, lack of LES relaxation and esophageal contraction, and UES relaxation, as described previously in humans (3, 10, 12). Therefore, our studies suggest that the barrier elimination phase of eructation can be activated independent of the gas escape and transport phases of eructation and used in a separate physiological event.
In a third type of eructation-like event, all phases occurred, but in a different manner to produce a different effect. In this event, the barrier elimination phase, i.e., TUESR, occurred, but the gas transport phase began in the cervical, rather than thoracic, esophagus. This cervical esophageal response began ∼3 cm from the CP and propagated orad through the CP and TP, as well as caudad in the esophagus. However, in another example, the orthograde peristaltic wave did not progress to the thoracic esophagus. This cervical retrograde contraction would have promoted EPR. The duration of CP EMG inhibition during this event was about half of that during eructation but about twice that during a swallow. This short duration of CP inhibition may be related to the very proximal location of the response, as the bolus would not have had to travel very far, so there would be no need for a long duration of CP inhibition. While a TLESR occurred during this event, it began after the TUESR began; thus, as opposed to eructation, the TLESR, and thus GER, was not the generator of the response. This was further supported by the lack of rapid activation of DD EMG, which is a hallmark of eructation and an index of GER. Therefore, we hypothesize that this eructation-like event is the motor event that causes EPR. More studies are needed to confirm this hypothesis.
In summary, we have defined the motor responses associated with the three phases of eructation: gas escape, barrier elimination, and gas transport. The gas escape phase, activated by gastric distension, primarily consists of relaxation of the LES and DH and contraction of the longitudinal muscle of the thoracic esophagus, which promote gas escape from the stomach. The barrier elimination phase, probably activated by rapid gas distension of the thoracic esophagus, consists of relaxation of the pharyngeal constrictors and excitation of dorsal and ventral UES distracting muscles, as well as rapid contraction of the DD fibers. The gas transport phase, possibly activated by a thoracic esophageal pressure pulse generated by contraction of the diaphragm, consists of retrograde contraction of the striated muscle esophagus, which transmits the air from the thoracic esophagus to the pharynx. The esophageal reverse peristalsis might be caused by elementary reflexes, rather than a coordinated peristaltic response. The phases of eructation can be activated independently of one another to participate in physiological events that can cause GER, the supragastric belch, and EPR.
GRANTS
This study was supported in part by Veterans Affairs Merit Review Grant 5120-02P (I. M. Lang) and National Institutes of Health Grant DC-00669 (R. Shaker).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
I.M.L. is responsible for conception and design of the research; I.M.L. performed the experiments; I.M.L. analyzed the data; I.M.L., B.K.M., and R.S. interpreted the results of the experiments; I.M.L. prepared the figures; I.M.L. drafted the manuscript; I.M.L., B.K.M., and R.S. edited and revised the manuscript; I.M.L., B.K.M., and R.S. approved the final version of the manuscript.
ACKNOWLEDGMENTS
The technical assistance of M. Steensrud is greatly appreciated.
REFERENCES
- 1. Babaei A, Bhargava V, Korsapati H, Zheng WH, Mittal RK. A unique longitudinal muscle contraction associated with transient lower esophageal sphincter relaxation. Gastroenterology 134: 1322–1331, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Babaei A, Bhargaca V, Mittal RK. Upper esophageal sphincter during transient lower esophageal sphincter relaxation: effects of reflux and posture. Am J Physiol Gastrointest Liver Physiol 298: G601–G607, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bredenoord AJ, Smout AJ. Physiolgic and pathologic belching. Clin Gastroenterol Hepatol 5: 772–775, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Creamer B, Schelegel J. Motor responses of the esophagus to distension. J Appl Physiol 10: 498–504, 1957 [DOI] [PubMed] [Google Scholar]
- 5. Dogen I, Bhargava V, Liu J, Mittal RK. Axial stretch: a novel mechanism of the lower esophageal sphincter relaxation. Am J Physiol Gastrointest Liver Physiol 292: G329–G334, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Doty RW, Bosma JF. An electromyograhic analysis of reflex deglutition. J Neurophysiol 19: 44–60, 1956 [DOI] [PubMed] [Google Scholar]
- 7. Duncan DL. The effects of vagotomy and splanchnectomy on gastric motility in sheep. J Physiol 119: 156–169, 1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Franzi SJ, Martin CJ, Cox MR, Dent J. Response of canine lower esophageal sphincter to gastric distension. Am J Physiol Gastrointest Liver Physiol 259: G380–G385, 1990 [DOI] [PubMed] [Google Scholar]
- 9. Goyal RK, Paterson WG. Esophageal motility. In: Handbook of Physiology. The Gastrointestinal System. Motility and Circulation. Bethesda, MD: Am. Physiol. Soc., 1989, sect. 6, vol. I, p. 865–908 [Google Scholar]
- 10. Hemmink GJM, Bredenoord AJ, Weusten BL, Timmer R, Smout AJ. Supragastric belching in patients with reflux symptoms. Am J Gastroenterol 104: 1992–1997, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Heywood LY, Wood AK. Retrograde oesophageal contractions in the dog. Q J Exp Physiol 73: 87–94, 1988 [DOI] [PubMed] [Google Scholar]
- 12. Kessing BF, Bredenoord AJ, Smout AJ. Mechanisms of gastric and supragastric belching: a study using concurrent high-resolution manometry and impedence monitoring. Neurogastroenterol Motil 24: e573–e579, 2012 [DOI] [PubMed] [Google Scholar]
- 13. Kharilas PJ, Dodds WJ, Dent J, Wyman JB, Hogan WJ, Arndorfer RC. Upper esophageal sphincter function during belching. Gastroenterology 91: 133–140, 1986 [DOI] [PubMed] [Google Scholar]
- 14. Lang IM, Dana N, Medda BK, Shaker R. Mechanisms of airway protection during retching, vomiting, and swallowing. Am J Physiol Gastrointest Liver Physiol 283: G529–G536, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Lang IM, Medda BK, Jadcherla S, Shaker R. The role of the superior laryngeal nerve in esophageal reflexes. Am J Physiol Gastrointest Liver Physiol 302: G1445–G1457, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lang IM, Medda BK, Shaker R. Mechanisms of reflexes induced by esophageal distension. Am J Physiol Gastrointest Liver Physiol 281: G1246–G1263, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Lang IM, Sarna SK, Dodds WJ. Pharyngeal, esophageal, and proximal gastric responses associated with vomiting. Am J Physiol Gastrointest Liver Physiol 265: G963–G972, 1993 [DOI] [PubMed] [Google Scholar]
- 18. Liu J, Puckett JL, Takeda T, Jung HY, Mittal RK. Crural diaphragm inhibition during esophageal distension correlates with contraction of the esophageal longitudinal muscles in cats. Am J Physiol Gastrointest Liver Physiol 288: G927–G932, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Martin CJ, Dodds WJ, Liem H, Dantas RO, Layman RD, Dent J. Diaphragmatic contribution to gastroesophageal competence and reflux in dogs. Am J Physiol Gastrointest Liver Physiol 263: G551–G557, 1992 [DOI] [PubMed] [Google Scholar]
- 20. McNally EF, Kelly JE, Ingelfinger FJ. Mechanisms of belching: effects of gastric distension with air. Gastroenterology 64: 254–259, 1964 [PubMed] [Google Scholar]
- 21. Mittal RK. The crural diaphragm, an external lower esophageal sphincter, a definitive study. Gastroenterology 105: 1565–1567, 1993 [DOI] [PubMed] [Google Scholar]
- 22. Monges H, Salducci J, Naudy B. Dissociation between the electrical activity of the diaphragmatic dome and crura muscular fibers during esophageal distension, vomiting, and eructation. An electromyographic study in the dog. J Physiol (Paris) 74: 541–554, 1978 [PubMed] [Google Scholar]
- 23. Pandolfino JE, Ghosh SK, Zhang Q, Han A, Kharilas PJ. Upper sphincter function during transient lower oesophageal sphincter relaxation (tLOSR): it is mainly about microburps. Neurogastroenterol Motil 19: 203–210, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Pandolfino JE, Zhang Q, Ghosh SK, Han A, Boniquit C, Kharilas PJ. Transient lower esophageal sphincter relaxations and reflux: mechanistic analysis using concurrent fluoroscopy and high-resolution manometry. Gastroenterology 131: 1725–1733, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Patrikios J, Martin CJ, Dent J. Relationship of transient lower esophageal sphincter relaxation to postprandial gastroesophageal reflux and belching in dogs. Gastroenterology 90: 545–551, 1986 [DOI] [PubMed] [Google Scholar]
- 26. Shaker R, Ren J, Kern M, Dodds WJ, Hogan WJ, Li Q. Mechanisms of airway protection and upper esophageal sphincter opening during belching. Am J Physiol Gastrointest Liver Physiol 262: G621–G628, 1992 [DOI] [PubMed] [Google Scholar]
- 27. Stevens CE, Sellers AF. Pressure events in bovine esophagus and reticulorumen associated with eructation, deglutition and regurgitation. Am J Physiol 199: 598–602, 1960 [Google Scholar]
- 28. Straathof JW, Ringers J, Lamers CB, Masclee AA. Provocation of transient lower esophageal sphincter relaxation by gastric distension with air. Am J Gastroenterol 96: 2317–2323, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Strombeck DR, Griffin DW, Harrold D. Eructation of gas through the gastroesophageal sphincter before and after limiting distension of the gastric cardia or infusion of a β-adrenergic amine in dogs. Am J Vet Res 50: 751–753, 1989 [PubMed] [Google Scholar]
- 30. Winship DH, Zboralske FF, Weber WN, Soergel KH. Esophagus in rumination. Am J Physiol 207: 1189–1194, 1964 [DOI] [PubMed] [Google Scholar]
- 31. Wyman JB, Dent J, Heddle R, Dodds WJ, Toouli J, Downton J. Control of belching by the lower oesophageal sphincter. Gut 31: 639–646, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]